Abstract
Purpose
To determine the maximum tolerated dose (MTD) of perifosine (NSC 639966), an alkylphospholipid modulator of signal transduction, using different oral loading and maintenance regimens in an effort to avoid gastrointestinal toxicity while seeking maximal sustained plasma concentrations.
Methods
Thirty-one patients with advanced neoplasms were treated with monthly cycles of perifosine loading doses of 300, 600, 900, 1,200 and 1,500 mg (dose levels 1 through 5, respectively) on days 1–2 depending on the actual dose of the initial cycle. For subsequent cycles, perifosine loading doses were reduced to 100, 200, 300, 400 and 1,000 mg at the respective corresponding dose levels. Daily perifosine “maintenance” doses of 50, 100, 150, 200 and 250 mg for levels 1 through 5, respectively, commenced on days 2 or 3 and continued for a total of 21 days. No treatment was given for days 22–27. The pharmacokinetics of perifosine with these schedules was characterized.
Results
Dose-limiting diarrhea developed at or above dose level 4. The MTD and recommended phase II dose was dose level 3B, with a loading dose of 900 mg on day 1 divided into two doses of 450 mg administered 6 h apart and a maintenance dose of 150 mg on day 2 through 21. On subsequent cycles, the loading dose was reduced to 300 mg. Non-gastrointestinal toxicities included three episodes of gout or gout-like syndromes observed at doses above the MTD. The median peak plasma concentration of perifosine achieved at the MTD was approximately 8.3 μg/mL. Four patients had stable disease ranging from 167 to 735 days.
Conclusions
Perifosine given according to a loading and maintenance schedule can safely sustain concentrations of drug, approaching concentrations achieved in preclinical models with evidence of anti-tumor effect.
Keywords: Perifosine, Loading dose, Maintenance dose, Drug development, Phase I
Introduction
Antitumor ether lipids, including alkyllysophospholipids and alkyl phospholipids, were originally designed as analogs of naturally occurring ether lipids or as antimetabolites to modulate phospholipids metabolism [l,2]. Pharmacological effects of initially studied compounds included anti-proliferative [3], pro-apoptotic [4] and immunomodulatory effects [5]. Subsequent mechanistic studies have shown that these molecules can interfere with key events in signal transduction, including growth-factor-induced activation of phospholipase C and phosphatidylinositol turnover [6–8] and lipid metabolism [9]. These findings have further encouraged interest in their development as candidate antineoplastic agents. Rac-l-O-octadecyl-2–0-methylglycero-3-phosphocholine (ET-18–0CH3, edelfosine) was the prototypical compound of this series. Subsequently, the glycerol backbone was shown to be dispensable with retention of antitumor activity, and hexadecylphosphocholine (miltefosine) was identified as an alkylphospholipid for initial clinical evaluation. Perifosine (octadecyl-(1, l-dimethylpiperidino-4-yl) phosphate; D-21266; NSC 639966) is a structurally related molecule, having a piperidine head group (instead of choline) and a longer alkyl chain [10].
Miltefosine had emerged from the NCI in vitro anticancer drug screen as active, with a unique signature of antiproliferative activity, and in vivo activity in prostate cancer xenografts was evident. The COMPARE computational algorithm identified perifosine as an alkylphospholipid with a similar spectrum of activity to miltefosine, but very different from that of conventional cytotoxic agents (data not shown). Mechanistic studies have recently suggested that among the basis for the anti-proliferative effect of perifosine was a capacity to induce the cell cycle regulatory molecule p21WAF1/CIP1, an endogenous cyclin-dependent kinase inhibitor, in a p53-dependent fashion [11]. In more recent studies, potent ability to down-regulate signaling through the Akt pathway [12, 13] accompanies induction of p21WAP1/CIP1. In fact, the most consistent pharmacodynamics activity that has been observed in most tumor types analyzed is the inhibition of Akt, inhibiting the translocation of Akt to the plasma membrane thus blocking subsequent Akt activation. The clinical contribution of Akt inhibition, however, remains to be assessed [14].
Miltefosine has an activity in several tumor models including human breast carcinoma xenografts [15, 16]. Based in part on these observations, phase I and phase II studies were initiated in patients using an oral formulation [17–19]. Due to gastrointestinal intolerance, only limited dose escalation was possible. Owing to hemolysis as a dose-limiting toxicity, intravenous application of miltefosine was not possible. Nonetheless, miltefosine is licensed for topical use against breast cancer metastases and cutaneous lymphoma in Europe [20, 21]. Recently, low oral doses of miltefosine have been found active against visceral leishmaniasis [22].
Perifosine was developed and selected for improved gastrointestinal tolerability in animals. Preclinical schedule optimization studies revealed that loading doses of perifosine of 200 mg/kg on day 1 followed by 30–60 mg/kg on days 2 through 9 were associated with substantial tumor growth delays in human prostate cancer xenografts in athymic mice. Likewise, dimethylbenzanthracene-induced mammary carcinoma in female Sprague-Dawley rats treated with perifosine, using a high loading dose followed by a lower daily maintenance dose regimen, also elicited a pronounced antitumor effect [23]. Therefore, clinical experience with an analogous schedule would be of interest to compare initial human experience with a chronic schedule, without a loading dose, which again revealed gastrointestinal toxicity to be problematic [24].
Based on these new preclinical findings and the pre ceding animal studies, our trial was designed to use the combination of a large initial (loading) dose followed by daily lower (maintenance) doses, with the hope that an optimal loading (to rapidly achieve sustained plasma concentrations) and maintenance (to assess tolerability over a protracted interval of dose) regimen could be developed. Focused approaches to supportive care of gastrointestinal side effects might therefore ameliorate these side effects while achieving sustained plasma concentrations.
Patients and methods
Patients were eligible for entry onto the study if they were at least 18 years of age and had a histologic diagnosis of a solid tumor (excluding lymphoma), effective standard salvage or primary therapy was not available, and they had an Eastern Cooperative Oncology Group performance status of 0–2. Laboratory requirements included ALT and AST ≤2.5 times normal, total bilirubin ≤1.5 mg/dL, serum creatinine ≤1.5 mg/dL or if ≥1.5 mg/dL, measured creatinine clearance ≥60 mL/min, hemoglobin ≥9 g/dL, platelets ≥100,000/mm3 absolute granulocyte count ≥1,500/mm3. Patients had to be ≥4 weeks removed from radiation or chemotherapy and recovered from associated toxicities, and ≥6 weeks from nitrosoureas, mitomycin C, or boneseeking radioisotopes prior to entry. Patients treated with suramin had to be >3 months from the last day of treatment. Patients treated with UCN-01 had to be ≥2 months from the last day of infusion. Prostate cancer patients must have had tumor progression during blockade of testicular and adrenal androgens, with anti-androgens discontinued for at least 4 weeks without disease improvement prior to study entry. GnRH analogs were to be maintained in prostate cancer patients without orchiectomy, and all prostate cancer patients had to have serum testosterone concentrations in the castrate range. Patients with breast carcinoma receiving hormonal therapy also were to have discontinued these medications ≥4 weeks prior to study entry and have shown evidence of disease progression. Patients were excluded if they had a history of CNS neoplasms, positive HIV serology, recent acute or chronic gastrointestinal conditions, preexisting retinal disease, presence of cataracts interfering with normal vision or requiring medical intervention or other significant medical problems including unstable or newly diagnosed angina, myocardial infarction within 6 months, or NYHA class II to IV congestive heart failure. The study was conducted in accord with all applicable federal regulations, and the National Cancer Institute Institutional Review Board approved the protocol. All patients provided signed informed consent prior to enrolling and were eligible to participate in the study.
Baseline evaluation
Before entry, all patients underwent a complete history and physical examination. Chest X-rays and electrocardiograms performed within the previous month were required for all patients, as were computed tomographic scans of the chest, abdomen and pelvis within the preceding 30 days. Laboratory studies included complete blood count with differential and platelet count, prothrombin time, partial thromboplastin time, thrombin time, fibrinogen, hepatic panel (alkaline phosphatase, total bilirubin, AST and ALT), creatine phosphokinase, acute care panel (electrolytes, glucose, creatinine and blood urea nitrogen), uric acid, lactate dehydrogenase, total protein, direct bilirubin, mineral panel (albumin, calcium, phosphorus and magnesium) and urinalysis. Baseline and every 3-month ophthalmologic evaluations were included to monitor for retinal abnormalities and cataract formation, toxicities reported in certain preclinical safety studies. Pulmonary function tests within 4 weeks of study entry and subsequently every 3 months were performed. At dose level 5, after gout occurred in association with perifosine, 24-h urinary uric acid collection was added.
Drug supply and administration
Perifosine was supplied to NCI by ASTA Medica, Dresden, Germany, as 50-mg capsules. The IND for perifosine (NSC 639966) is held by the Cancer Therapy Evaluation Program, Division of Cancer Treatment and Diagnosis, NCI. The initial cycle’s loading dose was administered as an inpatient, while maintenance dosing occurred as an outpatient. Second and subsequent cycle loading and maintenance doses were given as an outpatient. Following the administration of the loading dose over the first one to 2 days of a treatment cycle, daily maintenance doses of the study medication were administered at approximately the same time each day with a meal up to day 21, followed by 1 week without treatment to complete a 28-day course of therapy. The loading dose was administered with antiemetic prophylaxis. Maintenance dosing was intended to be administered without anti-emetic prophylaxis, but anti-emetics could be used on an as-needed basis. Nausea prophylaxis was administered 30–60 min before the loading dose and included granisetron (1 mg oral or 10 µg/kg intravenous), metoclopramide (20 mg oral or 1–2 mg/kg intravenous), diphenhydramine (25 mg, intravenous or oral) and dexamethasone (8 mg, intravenous or oral). At dose level 3 and higher (see Table 1), the loading dose was administered as divided doses, separated by 6 h, and additional anti-emetic therapy [metoclopramide (20–40 mg intravenous or oral) and diphenhydramine (25 mg intravenous or oral)] were given 4 h after the second half of a divided loading dose, if needed.
Table 1.
Perifosine dosing schema
| Day 1 loading dose (mg) |
Day 2–21 maintenance dose (mg) |
||
|---|---|---|---|
| Cycle 1 | Subsequent cycles | Cycle 1 | Subsequent cycles |
| Level 1 | Level 1 | ||
| I(a) 300 × 1 dose | 100 | 50 | 50 |
| I(b) 150 × 2 doses | 100 | ||
| I(c) 100 × 3 doses | 100 | ||
| Level II | Level II | ||
| II(a) 600 × 1 dose | 200 | 100 | 100 |
| II(b) 300 × 2 doses | 200 | ||
| II(c) 200 × 3 doses | 200 | ||
| Level III | Level III | ||
| III(a) 900 × 1 dose | 300 | 150 | 150 |
| III(b) 450 × 2 doses | 300 | ||
| III(c) 300 × 3 doses | 300 | ||
| Level IV | Level IV | ||
| IV(a) 1,200 × 1 dose | 400 | 200 | 200 |
| IV(b) 600 × 2 doses | 400 | ||
| IV(c) 400 × 3 doses | 400 | ||
| Level V | Level V | ||
| V(a) 1,500 × 1 dose | 1,000 | 250 | 250 |
| V(b) 500 × 3 doses | 1,000 | ||
| V(c) 250 × 6 doses | 1,000 | ||
In order to better tolerate the large number of capsules per dose (50 mg/pill), the indicated loading doses are divided in two parts and administered every 6 h on day 1
Study design
A phase I trial [15] of perifosine in Europe suggested that daily single doses of 200 mg achieved a Cmax of 2–4 µg/mL (4.3–8.6 µM). We utilized these data to design a pharmacokinetic directed dose escalation schedule consisting of two components: a loading phase and a maintenance phase. Table 1 shows the dosing schema from dose level 1 to dose level 5. Cycle 1 loading doses have up to three cohorts, indicated as a, b or c, where the loading dose was to be divided if necessary. Subsequent loading doses were split to facilitate outpatient administration, and these were indicated by an asterisk. Since a relatively long half-life was also suggested in the initial study [8], loading doses at the start of subsequent cycles were reduced in comparison with the cycle 1 loading dose.
Dose-limiting toxicity (DLT) was defined as ≥grade 2 irreversible non-hematologic toxicity (except nausea and vomiting, see below), grade 4 granulocytopenia or thrombocytopenia of at least 4 days in duration, and reversible grade 3 or 4 non-hematologic toxicity (excluding nausea, vomiting, alopecia and fever). Nausea and vomiting were not considered dose-limiting unless refractory to maximal antiemetic supportive care and still ≥grade 3. At least three and as many as eleven patients were entered on to sequential dose levels. If one of the first three patients experienced a DLT, at least three additional patients were entered at that dose level. If any of these additional patients experienced DLTs, the MTD was considered to have been exceeded and additional patients were entered at one dose level below. If only one of the six patients at the expanded dose level had a DLT, the MTD had not been defined, and the trial accrued to the next dose level. The dose recommended for phase II evaluation was defined as a dose associated with no more than one of the six patients experiencing a DLT, and the dose below which ≥2 of 6 patients experience a DLT. Escalation of loading doses proceeded sequentially to a higher dose level if level (a) was tolerated (i.e., la–2a–3a). In the event of DLT at any loading dose level (a), the loading dose was divided in subsequent patients into “split” doses. If a given split dose level was subsequently tolerated, dose escalation occurred within the same subgroup (e.g., 2b–3b–4b).
For a patient to receive subsequent courses, it was necessary for drug-related toxicities to resolve and any laboratory abnormalities to recover to meet study entry criteria. Patients could continue therapy in the absence of progressive disease or unacceptable toxicity. Acute toxicity on cycle 1 was used to identify DLT for both loading and maintenance schemes. Toxicity within the first 2 days was considered to be due to the loading dose, while all subsequent toxicities during cycle 1 were considered related to the maintenance dose. Intra-patient dose escalation to a dose level one below the accruing dose level was allowed but did not occur in this study. Dose de-escalation following completion of cycle 1 occurred in eight patients.
Response and progression of disease
Imaging studies for restaging were performed after every three cycles, unless the patient had symptoms or signs suggesting progression of disease or severe toxicity. The revised National Cancer Institute Common Toxicity Criteria, version 2.0, was used to grade and categorize adverse events. Complete response was defined as disappearance of all evidence of disease for at least 1 month. Partial response required a 50 % decrease in the sum of the products of the largest perpendicular diameters for at least 1 month. Progression of disease was defined as an increase in 25 % or greater in the sum of the products of the tumor diameters, or the appearance of new lesions. Patients with stable disease did not meet criteria for either response or progression.
Pharmacokinetic studies
During the first cycle, plasma was collected before treatment and at 8 and 48 h, and at days 15 and 21 after initiation of perifosine. When the loading dose was divided, sampling occurred at these time points following administration of the first portion of the loading dose. On subsequent cycles, plasma was collected preloading dose, day 15 and day 21. All samples were stored at −70 °C until analyzed at the conclusion of the study. Perifosine plasma concentrations were determined using a validated LC-MS assay [25]. Briefly, sample preparation utilized simple acetonitrile precipitation without an evaporation step. With a Develosil UG-30 column, perifosine and the internal standard, hexadecylphosphocholine, were separated at retention times of 2.2 and 1.1 min, respectively. The mobile phase consisted of ammonium formate and acetonitrile. The detection utilized selected ion monitoring in the positivemode at m/z 462.4 and 408.4 for the protonated molecular ions of perifosine and the internal standard, respectively. The lower limit of quantitation of perifosine was 8.7 nM. The data were analyzed using ADAPT II version 4 (Biomedical Stimulation Resource, University of Southern California, Los Angeles, CA). A one-compartment linear model fits the data best. Simulation was performed using WinNonlin version 4.0 (Pharsight Corporation, Mountain View, CA) with the use of a one-compartment linear model. The pharmacokinetic parameters obtained from dose level 3 were used as priors for the simulation of subsequent regimens.
Results
Patient demographics
Thirty-one patients were entered onto the trial between November 1999 and April 2003. Patient characteristics are summarized in Table 2. The majority of patients were male, and the median Eastern Cooperative Oncology Group performance status was 1.
Table 2.
Patient characteristics
| Total number of patients | 31 |
| Number assessable for response (%) | 24(77) |
| Median age (range) in years | 59(21–85) |
| Male/female | 21/10 |
| ECOG PS | |
| 0 | 4 |
| 1 | 25 |
| 2 | 2 |
| Tumor by type | |
| Prostate | 10 |
| Colorectal | 8 |
| Melanoma | 2 |
| Sarcoma (leiomyosarcoma) | 3(2) |
| Renal carcinoma | 2 |
| Other | 6 |
ECOG Eastern Cooperative Oncology Group, PS performance status
Dose escalation and toxicity
Table 1 describes the dosing scheme for the loading and maintenance portions of the cycle. All treatment cycles were planned for 21 days of drug treatment with 7-day rest from treatment to allow assessment of adverse events or symptom resolution. Repeat loading on day 1 of subsequent cycles occurred according to a reduced dosing scheme also detailed in Table 1. This design accommodated the expected prolonged half-life of the drug. The mean number of cycles per patient was 2.8, and the number of cycles ranged from 1 to 19. Eight of the 31 patients received reduced doses following cycle 1.
Toxicities within the first 2 days of the cycle were attributed to the loading dose, and subsequent toxicities until the end of the first cycle were attributed to maintenance dosing. No DLT was experienced during the loading phase at dose level I, II and the first patients on level III. The loading dose at dose level IV was split [dose level IV(b)], with administration separated by 6 h. This was not done in response to any specific toxicity but in order to decrease the number of pills to be ingested at this loading dose. Each “half ‘ of the loading dose was preceded by the anti-emetic prophylaxis regimen detailed above.
There was no DLT during loading at dose level IV(b). However, the first patient at loading dose level V(b) had DLT (grade 3 diarrhea) on the first day of cycle 1. Subsequently, the loading dose (1,500 mg) was further divided into 6 doses of 250 mg separated by 6 h [dose level V(c)] with anti-emetic prophylaxis. A third patient at dose level V(c) had grade 3 nausea despite maximal anti-emetic support during the loading phase. Therefore, the MTD (maximum tolerated dose) had been exceeded. Further enrollment was at the previous dose level, level IV(b), at which two patients overall developed grade 3 diarrhea during the loading phase, and again the MTD was determined to have been exceeded. The loading dose was lowered to dose level III(a). At this loading dose, one patient had grade 3 diarrhea that required us to enroll at dose level III(b). Only one out of six patients at dose level III(b) had dose-limiting toxicity, and therefore, dose level III(b) was defined as the “maximum tolerated dose” and recommended phase II dose. Gastrointestinal toxicities were the most common adverse events and the incidence increased at higher dose levels. Tables 3 and 4 list drug-related toxicities encountered during the loading and maintenance phases, respectively. Table 5 lists all drug related adverse events at dose level III(b), the proposed MTD and recommended phase II dose.
Table 3.
Loading phase toxicity (number of occurrence during cycle 1 of perifosinea)
| Toxicity | Dose level 1 |
Dose level II |
Dose level III |
Dose level IV |
Dose level V |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (N = 3) |
(N = 3) |
(N = 3) |
(N = 3) |
(N = 3) |
|||||||||||
| Gr1 | Gr2 | Gr3 | Gr1 | Gr2 | Gr3 | Gr1 | Gr2 | Gr3 | Gr1 | Gr2 | Gr3 | Gr1 | Gr2 | Gr3 | |
| Albumin | 1 | ||||||||||||||
| Anorexia | 1 | 4 | 1 | 4 | 2 | ||||||||||
| Dehydration | 1 | ||||||||||||||
| Diarrhea | 1 | 2 | 3 | 2 | 2 | 5 | 1 | 2 | 2 | 1 | |||||
| Dizziness | 1 | ||||||||||||||
| Dyspepsia | 1 | ||||||||||||||
| Dyspnea | 1 | 3 | |||||||||||||
| Fatigue | 1 | 3 | 2 | 1 | 2 | ||||||||||
| Fever | 1 | 1 | |||||||||||||
| Flatulence | 2 | 1 | 2 | ||||||||||||
| GI-other | 1 | ||||||||||||||
| Hiccups | 1 | ||||||||||||||
| Hyperglycemia | 1 | 1 | 1 | ||||||||||||
| Nausea | 2 | 1 | 3 | 3 | 6 | 3 | 1 | 1 | 1 | ||||||
| Tumor flare | 1 | ||||||||||||||
| Vision | 1 | ||||||||||||||
| Vomiting | 1 | 1 | 3 | 1 | 5 | 3 | 1 | 1 | 1 | ||||||
Toxicities from all arms (a–c) have been combined. Anemia, lymphopenia not included
Table 4.
Maintenance phase toxicity (number of occurrences during cycle 1 of perifosinea)
| Toxicity | Dose level 1 |
Dose level II |
Dose level III |
Dose level IV |
Dose level V |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (N = 3) |
(N = 3) |
(N = 3) |
(N = 3) |
(N = 3) |
||||||||||||
| Gr1 | Gr2 | Gr3 | Gr1 | Gr2 | Gr3 | Gr1 | Gr2 | Gr3 | Gr4 | Gr1 | Gr2 | Gr3 | Gr1 | Gr2 | Gr3 | |
| Albumin | 3 | 3 | 1 | 1 | ||||||||||||
| Alk Phos | 1 | |||||||||||||||
| Anemia | 1 | 1 | 2 | 2 | 5 | 4 | 1 | |||||||||
| Anorexia | 1 | 1 | 2 | 1 | ||||||||||||
| Arthralgia | 2 | 1 | ||||||||||||||
| Cough | 1 | |||||||||||||||
| Creatinine | 1 | |||||||||||||||
| Dehydration | 1 | 1 | ||||||||||||||
| Diarrhea | 1 | 1 | 1 | 1 | ||||||||||||
| Dizziness | 2 | |||||||||||||||
| Dyspepsia | 1 | 2 | ||||||||||||||
| Dyspnea | 1 | |||||||||||||||
| Fatigue | 1 | 1 | 1 | |||||||||||||
| Fever | 1 | |||||||||||||||
| Headache | 2 | 1 | ||||||||||||||
| Hyperglycemia | 1 | 1 | 1 | 1 | 3 | 6 | 2 | |||||||||
| Hot flash | 1 | |||||||||||||||
| Hypoxia | 1 | 1 | ||||||||||||||
| Infection | 2 | 1 | ||||||||||||||
| Lymphopenia | 2 | |||||||||||||||
| Myalgia | 1 | |||||||||||||||
| Nausea | 1 | 4 | 2 | 2 | 1 | |||||||||||
| Neutropenia | 1 | 1 | 1 | |||||||||||||
| Stomatitis | 1 | |||||||||||||||
| SVT | 1 | |||||||||||||||
| Taste | 1 | |||||||||||||||
| Transaminase | 3 | |||||||||||||||
| Tumor flare | 1 | |||||||||||||||
| Vision | 1 | |||||||||||||||
| Vomiting | 3 | 1 | 2 | 1 | ||||||||||||
Toxicities from all arms (a–c) have been combined. Anemia, lymphopenia not included.
Table 5.
All adverse events at the maximal tolerated dose (MTD) dose level III(b) (N = 6 patients, 11 cycles of treatment
| Toxicity | Toxicity grade |
||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Albumin | 1 | 1 | |||
| Anorexia | 3,2 | 3 | 1a | ||
| Anxiety | 1 | ||||
| Bilirubin | 1 | ||||
| Bladder spasm | 1 | ||||
| Bone pain | 1 | ||||
| Constipation | 1 | ||||
| Cough | 1,2 | ||||
| Creatinine | 1a | ||||
| Dehydration | 1a | ||||
| Diarrhea | 4,1 | 1 | |||
| Dizziness/vertigo | 2 | ||||
| Dyspnea | 1 | 1,1b | 1a | ||
| Edema | 1a | ||||
| Fatigue | 1,2 | ||||
| Fever/infection | 1,3 | 3 | |||
| Headache | 1 | 1 | |||
| Hyperglycemia | 3 | 6 | |||
| Hemoglobin | 1 | ||||
| Hot flash | 1 | ||||
| Hypoglycemia | 1 | 2 | |||
| Hyperkalemia | 1 | ||||
| Hypoglycemia | 1 | ||||
| Hypomagnesemia | 1 | ||||
| Hyponatremia | 1 | ||||
| Hypotension | 1 | ||||
| Hypoxia | 1b | 1a | |||
| Lymphopenia | 1 | ||||
| Nausea | 9 | 4 | |||
| Pleural effusion | 1 | ||||
| Pneumothorax | 1b | ||||
| PT/PTT | 4 | 1 | |||
| SGOT/SGPT | 2 | ||||
| SVT | 1a | ||||
| Sweating | 1 | ||||
| Taste disturbance | 3 | ||||
| Transfusion, RBC | 3 | ||||
| Tumor flare | 2 | ||||
| Urinary frequency | 1 | ||||
| Vertigo | 1 | ||||
| Vision change | 1 | ||||
| Vomiting | 7,2 | 3 | |||
| Weight loss | 2 | ||||
Relation to drug: italics: unrelated or unlikely; bold: possible, probable or likely
One patient with sarcoma and pulmonary metastases experienced grade 3 anorexia, increased creatine, dehydration, edema, grade 4 hypotension with dyspnea and hypoxia with radiographic evidence of progression of disease
One patient without widespread metastases to liver, lung and pleural effusions also felt to have disease progression
At dose level III, the maintenance dose was generally well tolerated except for intermittent mild nausea and fatigue. Anecdotally, there appeared to be better tolerance of the maintenance dose if consumed with the evening meal. Two patients in this level had grade 3 infections without neutropenia. The first patient presented with fever and low back pain 5 days into the first cycle. Diagnostic workup revealed blood cultures positive for alpha hemolytic streptococcus with radiological evidence of fluid collection around the right psoas muscle. The patient improved with intravenous antibiotics. A second patient diagnosed with bronchoalveolar carcinoma was treated for bronchitis with intravenous antibiotics during the last week of the first cycle of perifosine. One patient with metastatic sarcoma to lung experienced azotemia and deterioration of respiratory function with evidence of progression of disease.
A potential for exacerbation of joint-related symptoms was noted at dose levels higher than that proposed for further clinical study. During the initial dose escalation, a patient enrolled on dose level IV(b), with asymptomatic hyperuricemia prior to enrollment, developed sudden onset severe pain and swelling of the right foot on day 16 of cycle 1. The adverse event required hospital admission for evaluation and thus constituted a grade 3 arthralgia/arthritis. On evaluation by arthrocentesis, acute gout was confirmed. Indomethacin was prescribed and symptoms resolved. The first patient on dose level V(b) also reported pain and swelling of the right great toe on day 7 of cycle 1 (reported as grade 2 arthralgia/arthritis during maintenance phase). The second patient had a history of gout, with the last episode 2 years prior to starting perifosine. Prophylactic medications were not in use. This patient was clinically diagnosed as having active gout and had a prompt and complete response to colchicine. Subsequently, allopurinol was added, and the remainder of the treatment course was uneventful. Subsequently, when the MTD had been exceeded on dose level V and patients were enrolled at dose level IV(b), a third case of arthralgia occurred during a patient’s cycle 1. This patient had no history of gout or hyperuricemia, but had arthralgia involving the left great toe, right knee and right ankle, and became febrile on day 5 of cycle 1. Arthrocentesis of the right knee was suggestive of inflammatory arthritis, and radiographs of the right knee and ankle showed chondrocalcinosis of the joints. Findings on X-ray and arthrocentesis were suggestive of a calcium pyrophosphate deposition disease, i.e., pseudogout. The patient required 1 week of hospitalization and treatment with NSAIDs and narcotics. This adverse event was reported as grade 3 arthralgia/arthritis.
After the second episode of gout, the protocol was amended to perform more extensive evaluation of serum uric acid levels prior to and during treatment, with 12-h urinary uric acid excretion evaluated 1 day prior to therapy, and on day 3 of the initial cycle. When uric acid values on day 3 are compared in 23 patients to their respective baselines, the post-dosing day 3 uric acid was statistically significantly higher than prior to dosing (p < 0.0001). This difference was not related to dose or schedule (data not shown). Similar analysis for urinary uric acid on seven patients did not reveal a statistically significant difference in uric acid excretion (p < 0.381). Gout or gout-like syndromes have not yet been recorded as an adverse event at the recommended phase II dose.
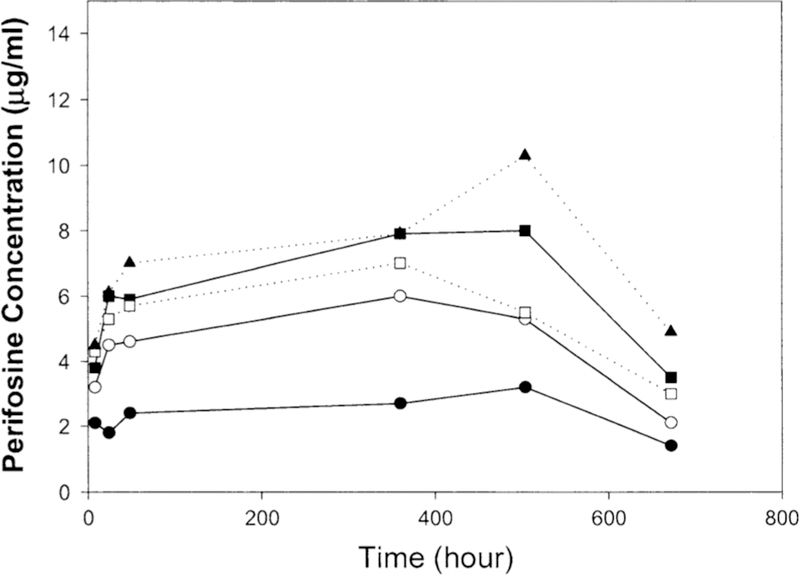
Pharmacokinetics
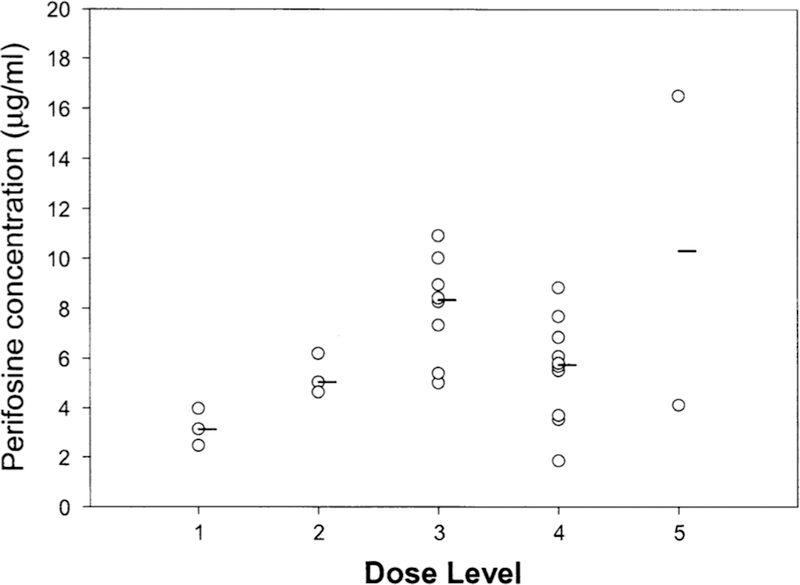
Pharmacokinetic analysis was performed in 24 patients. The perifosine plasma concentration–time profile for each dose level is presented in Fig. 1. At the end of the 21-day treatment, the perifosine concentration appeared to be approaching or reaching steady-state concentration. The AUC (0–28 day) value, although not calculated, appeared to increase linearly in dose levels IV and V based on the concentration–time profiles (Fig. 1). Perifosine concentrations at day 21 increased linearly with dose at level I to level III (Fig. 2). However, at higher dose levels (level IV and V), perifosine concentrations at day 21 did not increase proportionally with dose.
Fig. 1.
Perifosine plasma concentration–time profile. The data were from 24 patients who received perifosine from dose level l to level 5 at cycle 1. Filled circle level l (n = 3), open circle level 2 (n = 3), filled square level 3 (n = 8), open square level 4 (n = 7), filled triang le level 5 (n = 3). Median concentrations are shown
Fig. 2.
Perifosine plasma concentration versus doses. Perifosine concentrations at day 21 from dose level l to level 5 of cycle l were determined. Median values are represented by a line
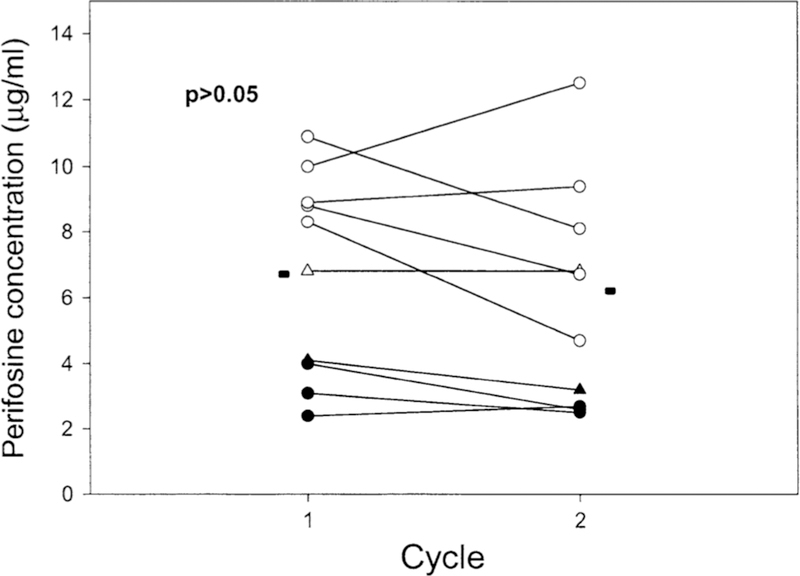
After the first cycle, median perifosine concentrations on day 28 before the second cycle were ranged from 1.4 to 5.1 µg/mL for dose levels I and V, respectively. To examine whether perifosine might have a tendency to accumulate with repeated treatment courses, perifosine concentrations at day 21 from cycle I and cycle II were compared. Figure 3 shows in data from 10 patients that there was no significant difference between perifosine plasma concentrations achieved at these time points (paired t test, p = 0.18). Likewise, perifosine plasma concentrations at day 21 from cycle 3 and subsequent cycles from several patients were also evaluated. There was no significant change of perifosine concentration (data not shown). A one-compartment linear model fits the data well. The elimination half-life of perifosine was quite long, with a median halflife from 75 to 150 h from dose level I to level V (Table 6). The drug was highly distributed into tissues with a volume of distribution of 108.1 liters (median, dose level I-III) and a low clearance of 0.77 L/h (median, dose level I-III). At higher dose levels (IV and V), increased clearance (1.24 and 0.98 L/h median, respectively) and volume of distribution (183 and 194 L, respectively) were observed, which may be due to the incomplete drug absorption. Perifosine follows linear pharmacokinetics from dose levels I to III. The disproportional increase in perifosine concentration at dose levels IV and V may be due to solubility-limited oral absorption, i.e., at higher doses, the drug might exceed its GI solubility and might not be absorbed any faster.
Fig. 3.
Perifosine plasma concentration at cycle l versus cycle 2. Data were perifosine concentrations at day 21 of each cycle from 10 patients. Filled cycle level l, open circle level 3, open triangle level 4,filled triangle level 5. Median values are represented by a line
Table 6.
Summary of pharmacokinetic parameters of perifosine in patients with refractory neoplasms
| Dose level | LD (mg) | MD (mg) | N | Ka (l/h) | CL/F (L/h) | Vd/F(L) | Tl/2 (h) | Day 21a (µg/mL) | |
|---|---|---|---|---|---|---|---|---|---|
| I | 300 | 50 | 3 | Median | 0.28 | 0.56 | 136.3 | 168.4 | 3.12 |
| Mean | 0.31 | 0.68 | 152.3 | 150.8 | 3.17 | ||||
| SD | 0.13 | 0.20 | 74.9 | 38.7 | 0.76 | ||||
| II | 600 | 100 | 3 | Median | 0.10 | 0.77 | 84.2 | 76.5 | 5.03 |
| Mean | 0.07 | 0.81 | 79.9 | 75.1 | 5.28 | ||||
| SD | 0.05 | 0.08 | 16.5 | 19.2 | 0.81 | ||||
| III | 900 | 150 | 8 | Median | 0.07 | 0.76 | 115.5 | 98.5 | 8.32 |
| Mean | 0.19 | 0.78 | 120.6 | 111.5 | 8.02 | ||||
| SD | 0.26 | 0.19 | 31.9 | 34.9 | 2.06 | ||||
| Total (I-III) | Median | 0.10 | 0.77 | 108.1 | 98.8 | ||||
| Mean | 0.19 | 0.76 | 118.6 | 112.1 | |||||
| SD | 0.21 | 0.17 | 45.4 | 40. l | |||||
| IV | 1,200 | 200 | 7 | Median | 0.20 | 1.24 | 185.0 | 108.5 | 5.73 |
| Mean | 0.41 | l.37 | 225.8 | 112.3 | 5.53 | ||||
| SD | 0.53 | 0.45 | 121.2 | 29. l | 2.07 | ||||
| V | 1,500 | 250 | 3 | Median | 0.08 | 0.98 | 193.6 | 117.l | 10.30 |
| Mean | 0.22 | l.14 | 192.l | 143.4 | 10.30 | ||||
| SD | 0.24 | 0.66 | 25. l | 79.8 | 8.77 |
One compartmental model analysis was used to calculate the pharmacokinetic parameters. Data from cycle I were used LD loading dose, MD maintenance dose
Perifosine concentration at day 21 of cycle 1
Response
There were no complete or partial responses on this study. Four patients had stable disease ranging from 167 to 735 days. One patient each at dose levels I(a) and III(a) had stable disease, and two patients at dose level II(a) had stable disease. Three of the four patients with stable disease had castrate-resistant prostate cancer. The fourth patient with stable disease for 167 days had metastatic malignant melanoma. A noteworthy experience was that of an 82-year-old male with metastatic prostate cancer with predominantly soft tissue disease involving the left supraclavicular fossa, whose disease had progressed despite hormonal ablation and docetaxel. This patient had stable disease by periodic whole-body CT scans and bone scans for a period of 2 years.
Discussion
In this phase I trial, perifosine was administered according to different oral loading and maintenance regimens. The MTD and recommended Phase II dose was reached at dose level III (loading dose of 900 mg divided into two doses for cycle 1 and 300 mg for subsequent cycles, and a maintenance dose of 150 mg). Gastrointestinal signs were the dose-limiting toxicity, observed at or above dose level IV. Three episodes of gout or gout-like syndromes at dose levels higher than the MTD were also seen. At 48 h after administration of the loading dose, the median perifosine concentration was >70 % of the concentration observed on day 21, thus confirming the loading and maintenance strategy to achieve sustained plasma concentrations relatively quickly and safely, despite the oral route of administration. The median peak concentration of perifosine achieved at the MTD was 8.3 μg/mL. Four patients had stable disease ranging from 167 to 735 days.
Hexadecylphosphocholine (miltefosine) is the only alkylphospholipid for which there is substantial prior clinical experience. Initially evaluated in phase I and II studies, miltefosine caused substantial gastrointestinal toxicities, although even at low, tolerated doses, miltefosine has shown noteworthy activity against visceral leishmaniasis [22]. Eight phase I-II studies, including 443 patients using topically applied miltefosine (from 2 to 8 %) for skin metastases in patients with breast cancer, showed a median response rate of 38 %, and activity in this form was confirmed in a randomized controlled trial [21]. Evidence from this experience led to the approval of miltefosine in Europe for the treatment of cutaneous breast cancer metastases, and there had been prior documentation of activity of the topically applied drug in cutaneous lymphoma [20]. Perifosine was developed in the hope of achieving better gastrointestinal tolerance than seen with oral miltefosine.
The first Phase I perifosine trial demonstrated that 350 mg once weekly was tolerated in humans [26]. This trial did not include anti-emetic prophylaxis. Another study suggested that oral perifosine was tolerable up to 600 mg/week in cancer patients when administered with meal and prophylactic antiemetics [27]. A European Phase I trial [24] examined the tolerability of 3-week courses of daily oral administration followed by a break, similar to our trial but without the loading doses. The MTD was established at 200 mg/day with toxicities consisting mainly of nausea, vomiting and diarrhea. Fatigue was reported in 43 % of the study subjects. The concentrations achieved in that trial at the MTD were 3.2 µg/mL (day 4, predose) to 5.6 µg/mL (day 21) and appeared to require at least two to three cycles to reach a steady state. The results of a third Phase I perifosine trial have recently been reported [28]. Similar to our study, this study investigated a loading dose and maintenance dose schedule of administration with prophylactic anti-emetics. The MTD and recommended Phase II dose was determined to be a loading dose of 150 mg × 6 (every 6 h) followed by a maintenance phase of 100 mg once daily. DLTs consisted of nausea, diarrhea, dehydration and fatigue. Biologically active concentrations, based on preclinical data, were achieved. There was one partial response and multiple patients with stable disease beyond cycle 2.
Our experience with the loading and maintenance regimen is noteworthy because at our MTD and recommended Phase II dose, we sustained a perifosine plasma concentration of 4.6–10.2 µg/mL, with a median of 8.3 µg/mL. This exceeds that needed for anti-proliferative activity in many, but not all cell lines in vitro [1]. Non-tumor-bearing mice received 216 mg/kg PO x 2 loading, and 34 mg/kg PO daily on days 2–21 as a maintenance dose, a regimen modeled on the level IV dose studied here (actually one level above the MTD, and similar to a dose level producing efficacy in animal models achieved 10.7 and 8.3 µg/mL, respectively, on days 3 and 15 (M. Hollingshead, unpublished results)). Therefore, plasma concentrations achieved in our clinically relevant MTD level 3 are relevant to those displaying antitumor activity in model systems.
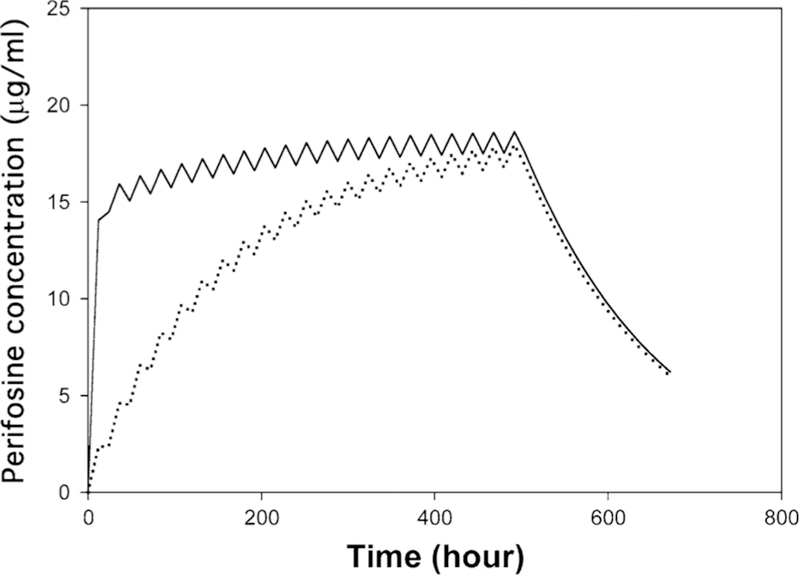
Is a loading dose necessary or useful to rapidly achieve potentially active plasma concentrations with this drug, which has a relatively long half-life and potentially slow approach to steady state owing to its gastrointestinal absorption? The DLT during the loading dose was the limiting factor in our study. The MTD and recommended Phase II maintenance dose in our study was 150 mg, compared to 200 mg in the European trial. The peak plasma concentrations achieved during the initial courses did appear to approach a stable level more rapidly in our trial with somewhat lower plasma concentrations after one cycle. While not directly evaluated, the schedules with and without loading might be perfectly comparable to ours assuming equally attentive supportive care measures for nausea are maintained. On the other hand, when the pharmacokinetic parameters from the dose level 3 obtained here are used in a model without loading and only daily dosing, perifosine concentration continues to increase in the absence of a loading dose (Fig. 4). With a loading dose, perifosine concentrations at 48 and 120 h are approximately 81 and 86 %, respectively, of the peak concentration achieved at day 21. Perifosine concentrations at 48 and 120 h without loading doses simulate to 25 and 52 %, respectively, of the day 21 peak. Further studies attempting the use of protracted daily dosing without breaks will further shed light on the most appropriate dosing schedule for perifosine.
Fig. 4.
Simulated perifosine plasma concentration–time profile at dose level 3. A one-compartment linear model was applied. The solid line is predicted perifosine concentration–time profile in the presence of a loading dose. The dotted line is predicted perifosine concentration–time profile without a loading dose
Although a dose-proportional increase in Cmax was seen up to level III, only a minor increase was seen in levels IV and V. While this is compatible with a barrier to gastrointestinal absorption, it is also possi ble that binding to plasma protein is being saturated, with subsequent rapid clearance of the less tightly bound drug. Disproportional increase due to interpatient variability may not be completely excluded. Further studies on perifosine protein binding are necessary, and, in addition, studies are planned in patients with accessible tumor cells in the blood and marrow compartments to better understand the relationship of pharmacology to distribution to tumor cell sites and effect on specific molecular targets including p21WAF1/CIP1 [11] and the Akt activation scale [12, 13].
Perifosine-related toxicity was easily managed at the recommended phase II dose: Dyspnea in the setting of underlying infection or underlying cancer was the only relevant dose-limiting toxicity, and this was of uncertain relation to drug. Gastrointestinal toxicity was grade 1 or 2. Unexpected toxicities included articular symptoms with two frank exacerbations of gout and one pseudo-gout, all at higher doses than the MTD. In several patients across multiple dose levels, there was evidence of increased serum uric acid while on treatment. The mechanism of this effect is not known and could reflect unanticipated actions of the drug on the renal tubule, or in the peripheral tissues, with arthralgias merely secondary to altered uric acid concentrations in susceptible patients. While not experienced at the recommended phase II dose level in our patients, the small numbers treated here might underestimate the occurrence of this toxicity in a larger population. Although patients were screened for worsening vision and the development of cataracts based on the occurrence of the latter toxicity in animal toxicity studies, no evidence of worsening vision was observed.
Since our study, there have been 2 additional Phase II trials in metastatic melanoma and androgen-independent prostate cancer that have used a similar regimen with a loading dose followed by maintenance dosing [29, 30]. The Phase II study in androgen-independent prostate cancer used a loading dose of 900 mg on day 1 followed by a maintenance dose of 150 mg daily for 20 days, followed by a loading dose of 600 mg on subsequent cycles with the same maintenance dose. Pharmacokinetic studies showed an average minimum concentration at steady state of 4,059 ng/mL. Unfortunately, there were no radiographic responses or PSA declines >50 %, with a median time to progression of 4 weeks. A similar loading dose of 900 mg and maintenance of 150 mg daily followed by a loading dose of 300 mg on subsequent cycles was maintained in the melanoma trial. Unfortunately, there were no objective responses in 14 evaluable patients, but gastrointestinal toxicity was of low grade with minimal hematologic toxicity. Nonetheless, these studies suggested that there was no further development of single-agent perifosine recommended in either the metastatic melanoma or castration resistant prostate cancer populations [29, 30].
In renal cell carcinoma, the therapeutic relevance of the kinase mammalian target of rapamycin (mTOR) has been established by the clinical efficacy of allosteric mTOR inhibitors in patients with advanced RCC, including temsirolimus and everolimus. Efforts to improve these mTOR inhibitors have focused on overcoming mechanisms of resistance, including targeting PI3 K or Akt upstream of mTOR. Two trials were conducted comparing these mTOR inhibitors versus perifosine in patients who progressed on VEFG-targeted therapy and found that it was not superior to currently available second-line agents. However, there was a small subset of patients that derived substantial clinical benefit, and as such further studies are warranted to define biomarkers for patient selection and/or measure response to therapy [31].
There have been several studies in myeloma evaluating the combination of perifosine with FDA-approved agents. A phase I trial of perifosine was shown to have promising activity and excellent tolerability when combined with lenalidomide and dexamethasone in relapsed/refractory myeloma [32]. Perifosine was combined with bortezomib and dexamethasone in patients with relapsed/refractory multiple myeloma who were previously treated with bortezomib based on preclinical data indicating that perifosine enhances dexamethasone-, doxorubicin-, melphalan- and bortezomib-induced cytotoxicity in MM cells [33] and phase I/II clinical data showing an ORR of 65 % in bortezomib-relapsed patients and 32 % in bortezomib refractory patients [34]. Unfortunately, the phase III trial showed no benefit in PFS or ORR when adding perifosine, which was dosed at 50 mg daily, to bortezomib and dexamethasone, although OS appeared to be greater in favor of the perifosine arm at the interim analysis with no safety differences [35]. However, the study was discontinued early following the recommendation of the monitoring committee due to limited study logistics and slow accrual, resulting in a small sample size and making the ability to interpret these results further difficult given no obvious clinical benefit in terms of PFS.
A phase III trial (X-PECT) of perifosine was also evaluated in colorectal cancer. The X-PECT study was based on a randomized phase II trial examining P (perifosine)CAP (capecitabine) versus CAP in patients with secondline or third-line metastatic colorectal cancer (mCRC), which found an OS of 17.7 months for patients receiving perifosine/capecitabine versus 7.6 months for those receiving capecitabine only (p = 0.0052) [36]. The randomized phase III trial combined 50 mg of perifosine with 1,000 mg/m2 of capecitabine twice daily days 1–14 versus placebo + capecitabine. Unfortunately, this trial did not show such promising results, as there was no overall survival or PFS advantage in adding perifosine to capecitabine in the refractory colorectal cancer setting [37]. Biomarker analysis is pending to see whether there is a subgroup that may have a potential benefit, as approximately 40 % of patients with colorectal cancer show deregulation of the PI3 K/AKT/mTOR pathway.
The disappointing results of both phase III perifosine trials in myeloma and colorectal cancer could be attributed to dosing strategy as both studies evaluated perifosine at 50 mg based upon the respective phase II results. This suggests that dosing schedules need to be explored and further studies with higher doses may be warranted. Currently, the only active trial is evaluating perifosine as a single agent in recurrent pediatric solid tumors (www.ClinicalTrials.gov Identifier NCT00776867). This dose escalation study employs a loading dose followed by a maintenance dose based on BSA until progression to determine the MTD of perifosine as the primary end point. Secondary measures include determining PK-toxicity correlations and defining predictive molecular biomarkers of response in tissue samples.
In conclusion, we have defined a maximum tolerated dose and recommended phase II dose of orally administered perifosine that caused mild gastrointestinal toxicity and which maintains a sustained plasma concentration of perifosine. It represents a novel approach to modulating important signal transduction pathways influenced by lipid intermediates or their downstream targets in tumor tissue. Perifosine overall appears to be well tolerated with demonstrated clinical benefit in certain subsets of patients, and through biomarker studies, it may be possible that we can better identify this subpopulation.
Acknowledgments
We thank the nursing staff of National Cancer Institute and the fellows of the Medical Oncology Branch at National Cancer Institute for their care of our patients; Cancer Therapy and Evaluation Program for sponsoring the trial; Eunhee W. Woo (deceased, and to whose memory we dedicate this paper), Suoping Zhai, Kyung Hwang and Nicola Smith for their assistance in conducting this study; and Victoria Giffi for editorial assistance. This work was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health. This is a US Government work. There are no restrictions on its use. The views expressed within this paper do not necessarily reflect those of the US Government.
Contributor Information
William D. Figg, Medical Oncology Branch, National Cancer Institute, Bldg 10/Room 5A01, 9000 Rockville Pike, Bethesda, MD 20892, USA, figgw@helix.nih.gov
Manish Monga, Medical Oncology Branch, National Cancer Institute, Bldg 10/Room 5A01, 9000 Rockville Pike, Bethesda, MD 20892, USA.
Donna Headlee, Medical Oncology Branch, National Cancer Institute, Bldg 10/Room 5A01, 9000 Rockville Pike, Bethesda, MD 20892, USA.
Avni Shah, Medical Oncology Branch, National Cancer Institute, Bldg 10/Room 5A01, 9000 Rockville Pike, Bethesda, MD 20892, USA.
Cindy H. Chau, Medical Oncology Branch, National Cancer Institute, Bldg 10/Room 5A01, 9000 Rockville Pike, Bethesda, MD 20892, USA
Cody Peer, Medical Oncology Branch, National Cancer Institute, Bldg 10/Room 5A01, 9000 Rockville Pike, Bethesda, MD 20892, USA.
Richard Messman, Developmental Therapeutics Program, National Cancer Institute, Bethesda, MD, USA.
Yusri A. Elsayed, Medical Oncology Branch, National Cancer Institute, Bldg 10/Room 5A01, 9000 Rockville Pike, Bethesda, MD 20892, USA
Anthony J. Murgo, Cancer Therapy Evaluation Program, National Cancer Institute, Bethesda, MD, USA
Giovanni Melillo, Science Applications International Corp., Frederick, MD, USA.
Qin C. Ryan, Medical Oncology Branch, National Cancer Institute, Bldg 10/Room 5A01, 9000 Rockville Pike, Bethesda, MD 20892, USA
Mikhail Kalnitskiy, Medical Oncology Branch, National Cancer Institute, Bldg 10/Room 5A01, 9000 Rockville Pike, Bethesda, MD 20892, USA.
Adrian M. Senderowicz, National Institute for Dental and Craniofacial Research, Bethesda, MD 20892, USA
Melinda Hollingshead, Developmental Therapeutics Program, National Cancer Institute, Bethesda, MD, USA.
Susan G. Arbuck, Cancer Therapy Evaluation Program, National Cancer Institute, Bethesda, MD, USA
Edward A. Sausville, Developmental Therapeutics Program, National Cancer Institute, Bethesda, MD, USA, esausville@umm.edu
References
- 1.Arthur G, Bittman R (1998) The inhibition of cell signaling pathways by antitumor ether lipids. Biochim Biophys Acta Lipids Lipid Metab 1390(1):85–102 [DOI] [PubMed] [Google Scholar]
- 2.Herrmann DBJ ( 1985) Changes in cellular lipid-synthesis of normal and neoplastic-cells during cytolysis induced by alkyl lysophospholipid analogs. J Natl Cancer Inst 75(3):423–430 [PubMed] [Google Scholar]
- 3.Modolell M, Andreesen R, Pahlke W, Brugger U, Munder PG (1979) Disturbance of phospholipid metabolism during the selective destruction of tumor cells induced by alkyl-lysophospholipids. Cancer Res 39(11):4681–4686 [PubMed] [Google Scholar]
- 4.Ruiter GA, Verheij M, Zerp SF, van Blitterswijk WJ (2001) Alkyl-lysophospholipids as anticancer agents and enhancers of radiation-induced apoptosis. Int J Radiat Oncol 49(2):415–419 [DOI] [PubMed] [Google Scholar]
- 5.Vehmeyer K, Scheurich P, Eibl H, Unger C (1991) Hexadecylphosphocholine-mediated enhancement of T-cell responses to interleukin-2. Cell Immunol 137(1):232–238 [DOI] [PubMed] [Google Scholar]
- 6.Seewald MJ, Olsen RA, Sehgal I, Melder DC, Modest EJ, Powis G (1990) Inhibition of growth factor-dependent inositol phosphate Ca-2 + signaling by antitumor ether lipid analogs. Cancer Res 50(15):4458–4463 [PubMed] [Google Scholar]
- 7.Strassheim D, Shafer SH, Phelps SH, Williams CL (2000) Small cell lung carcinoma exhibits greater phospholipase C-beta l expression and edelfosine resistance compared with non-small cell lung carcinoma. Cancer Res 60(10):2730–2736 [PubMed] [Google Scholar]
- 8.Uberall F, Oberhuber H, Maly K, Zaknun J, Demuth L, Grunicke HH (1991) Hexadecylphosphocholine inhibits inositol phosphate formation and protein-kinase-C activity. Cancer Res 51(3):807–812 [PubMed] [Google Scholar]
- 9.van der Luit AH, Budde M, Ruurs P, Verheij M, van Blitterswijk WJ (2002) Alkyl-Iysophospholipid accumulates in lipid rafts and induces apoptosis via raft-dependent endocytosis and inhibition of phosphatidylcholine synthesis. J Biol Chem 277(42):39541–39547 [DOI] [PubMed] [Google Scholar]
- 10.Ruiter GA, Zerp SF, Bartelink H, van Blitterswijk WJ (1999) Alkyl-Iysophospholipids activate the SAPK/JNK signaling pathway and enhance radiation-induced apoptosis. Eur J Cancer 35:S179. [PubMed] [Google Scholar]
- 11.Patel V, Lahusen T, Sy T, Sausville EA, Gutkind JS, Senderowicz AM (2002) Perifosine, a novel alkylphospholipid, induces p21(WAF1) expression in squamous carcinoma cells through a p53-independent pathway, leading to loss in cyclin-dependent kinase activity and cell cycle arrest. Cancer Res 62(5): 1401–1409 [PubMed] [Google Scholar]
- 12.Kondapaka SB, Singh SS, Dasmahapatra GP, Sausville EA, Roy KK (2003) Perifosine, a novel alkylphospholipid, inhibits protein kinase B activation. Mol Cancer Ther 2(11):1093–1103 [PubMed] [Google Scholar]
- 13.Ruiter GA, Zerp SF, Bartelink H, van Blitterswijk WJ, Verheij M (2003) Anti-cancer alkyl-Iysophospholipids inhibit the phosphatidylinositol 3-kinase-Akt/PKB survival pathway. Anticancer Drugs 14(2):167–173 [DOI] [PubMed] [Google Scholar]
- 14.Fensterle J, Aicher B, Seipelt I, Teifel M, Engel J (2014) Current view on the mechanism of action of perifosine in cancer. Anticancer Agents Med Chem 14(4):629–635 [DOI] [PubMed] [Google Scholar]
- 15.Naundorf H, Rewasowa EC, Fichtner I, Buttner B, Becker M, Gorlich M (1992) Characterization of two human mammary carcinomas, MT-1 and MT-3, suitable for in vivo testing of ether lipids and their derivatives. Breast Cancer Res Treat 23(1–2):87–95 [DOI] [PubMed] [Google Scholar]
- 16.Safa O, Parkin SM, Matthew AM, Bibby MC (1997) Morphological and immunological observations on the effects of hexadecylphosphocholine (HPC) in nude mice bearing MT-1 breast cancer xenografts. Anticancer Res 17(1A):37–43 [PubMed] [Google Scholar]
- 17.Planting AST, Stoler G, Verweij J (1993) Phase-II study of daily oral miltefosine (hexadecylphosphocholine) in advanced colorectal-cancer. Eur J Cancer 29A(4):518–519 [DOI] [PubMed] [Google Scholar]
- 18.Verweij J, Planting A, Vanderburg M, Stoler G (1992) A dose finding study of miltefosine (hexadecylphosphocholine) in patients with metastatic solid tumors. J Cancer Res Clin Oncol 118(8):606–608 [DOI] [PubMed] [Google Scholar]
- 19.Verweij J, Krzemieniecki K, Kok T et al. (1993) Phase ll study of miltefosine (hexadecylphosphocholine) in advanced soft tissue sarcomas of the adult-an EORTC Soft Tissue and Bone Sarcoma Group Study. Eur J Cancer 29A(2):208–209 [DOI] [PubMed] [Google Scholar]
- 20.Dummer R, Krasovec M, Roger J, Sindermann H, Burg G (1993) Topical administration of hexadecylphosphocholine in patients with cutaneous lymphomas—results of a phase-I/II study. J Am Acad Dermatol 29(6):963–970 [DOI] [PubMed] [Google Scholar]
- 21.Leonard R, Hardy J, van Tienhoven G et al. (2001) Randomized, double-blind, placebo-controlled, multicenter trial of 6% miltefosine solution, a topical chemotherapy in cutaneous metastases from breast cancer. J Clin Oncol 19(21):4150–4159 [DOI] [PubMed] [Google Scholar]
- 22.Jha TK, Sundar S, Thakur CP et al. (1999) Miltefosine, an oral agent, for the treatment of Indian visceral leishmaniasis. N Engl J Med 341(24):1795–1800 [DOI] [PubMed] [Google Scholar]
- 23.Hilgard P, Klenner T, Stekar J, Nossner G, Kutscher B, Engel J (1997) D-2I266, a new heterocyclic alkylphospholipid with antitumour activity. Eur J Cancer 33(3):442–446 [DOI] [PubMed] [Google Scholar]
- 24.Crul M, Rosing H, de Klerk GJ et al. (2002) Phase I and pharmacological study of daily oral administration of perifosine (D-21266) in patients with advanced solid tumours. Eur J Cancer 38(12): 1615–1621 [DOI] [PubMed] [Google Scholar]
- 25.Woo EW, Messmann R, Sausville EA, Figg WD (2001) Quantitative determination of perifosine, a novel alkylphosphocholine anticancer agent, in human plasma by reversed-phase liquid chromatography-electrospray mass spectrometry. J Chromatogr B 759(2):247–257 [DOI] [PubMed] [Google Scholar]
- 26.Traiser M, Reichert S, Yoss A (1998) Current development status of the second generation alkylphosphocholine analog perifosine. Drugs Today 34:67–71 [Google Scholar]
- 27.Unger C, Berdel W, Hanauske AR, Sindermann H, Engel J, Mross K (2010) First-time-in-man and pharmacokinetic study of weekly oral perifosine in patients with solid tumours. Eur J Cancer 46(5):920–925 [DOI] [PubMed] [Google Scholar]
- 28.Van Ummersen L, Binger K, Volkman J et al. (2004) A phase I trial of perifosine (NSC 639966) on a loading dose/maintenance dose schedule in patients with advanced cancer. Clin Cancer Res 10(22):7450–7456 [DOI] [PubMed] [Google Scholar]
- 29.Posadas EM, Gulley J, Arlen PM et al. (2005) A phase II study of perifosine in androgen independent prostate cancer. Cancer Biol Ther 4(10): 1133–1137 [DOI] [PubMed] [Google Scholar]
- 30.Ernst DS, Eisenhauer E, Wainman N et al. (2005) Phase ll study of perifosine in previously untreated patients with metastatic melanoma. Invest New Drugs 23(6):569–575 [DOI] [PubMed] [Google Scholar]
- 31.Cho DC, Hutson TE, Samlowski W et al. (2012) Two phase 2 trials of the novel Akt inhibitor perifosine in patients with advanced renal cell carcinoma after progression on vascular endothelial growth factor-targeted therapy. Cancer 118(24):6055–6062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jakubowiak AJ, Richardson PG, Zimmerman T, Alsina M, Kaufman JL, Kandarpa M, Kraftson S, Ross CW, Harvey C, Hideshima T, Sportelli P, Poradosu E, Gardner L, Giusti K, Anderson KC (2012) Perifosine plus lenalidomide and dexamethasone in relapsed and relapsed/refractory multiple myeloma: a Phase I Multiple Myeloma Research Consortium study. Br J Haematol 158(4):472–480 [DOI] [PubMed] [Google Scholar]
- 33.Hideshima T, Catley L, Yasui H, Ishitsuka K, Raje N, Mitsiades C, Podar K, Munshi NC, Chauhan D, Richardson PG, Anderson KC (2006) Perifosine, an oral bioactive novel alkylphospholipid, inhibits Akt and induces in vitro and in vivo cytotoxicity in human multiple myeloma cells. Blood 107(10):4053–4062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Richardson PG, Wolf J, Jakubowiak A et al. (2011) Perifosine plus bortezomib and dexamethasone in patients with relapsed/refractory multiple myeloma previously treated with bortezomib: results of a multicenter phase I/II trial. J Clin Oncol 29(32):4243–4249 [DOI] [PubMed] [Google Scholar]
- 35.Richardson PG, Nagler A, Ben-Yehuda D, Badros AZ, Hari P, Hajek R, Spicka I, Kaya H, Le Blanc R, Yoon S-S, Kim K, Martinez-Lopez J, Mittelman M, Shpilberg O, Tothova E, Laubach JP, Ghobrial IM, Leiba M, Gatt ME, Sportelli P, Chen M, Anderson KC (2013) Randomized placebo-controlled phase Ill study of perifosine combined with bortezomib and dexamethasone in relapsed, refractory multiple myeloma patients previously treated with bortezomib. ASH 2013 annual meeting abstract 3189 (poster presentation) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bendell JC, Nemunaitis J, Vukelja SJ, Hagenstad C, Campos LT, Hermann RC, Sportelli P, Gardner L, Richards DA (2011) Randomized placebo-controlled phase II trial of perifosine plus capecitabine as second- or third-line therapy in patients with metastatic colorectal cancer. J Clin Oncol 29(33):4394–4400 [DOI] [PubMed] [Google Scholar]
- 37.Bendell JC, Senzer NN, Richards DA, Firdaus I, Lockhart AC, Cohn AL, Saleh MN, Gardner LR, Sportelli P (2012) Cathy eng. Results of the X-PECT study: a phase Ill randomized doubleblind, placebo-controlled study of perifosine plus capecitabine (P-CAP) versus placebo plus capecitabine (CAP) in patients (pts) with refractory metastatic colorectal cancer (mCRC). J Clin Oncol 30(suppl: abstr LBA350l) [Google Scholar]