Abstract
Sulfur containing amino acids contribute substantially to the maintenance and integrity of cellular systems by influencing cellular redox state and cellular capacity to detoxify toxic compounds, free radicals and reactive oxygen species. Methionine and cysteine are the two primary sulfur-containing amino acids in mammals. Methionine is an essential amino acid, obtained by dietary intake while cysteine is non-essential and a metabolite of methionine metabolism. Each of these amino acids contributes significantly to the cellular pool of organic sulfur and generally to sulfur homeostasis as well as playing a significant role in regulation of one carbon metabolism. Genetic defects in the enzymes regulating sulfur pools produce a variety of human pathologies, including homo- and cystinuria, homo- and cysteinemia, and neural tube defects. In addition, thiol imbalance has been associated with multiple disorders, including vascular disease, Alzheimer’s, HIV and cancer. Possible treatments to restore the thiol balance are also discussed.
Keywords: Cysteine, Methionine, Sulfuration, One carbon metabolism
1. Biosynthesis and metabolism of methionine and cysteine
1.1. Methionine → SAM → SAH → homocysteine
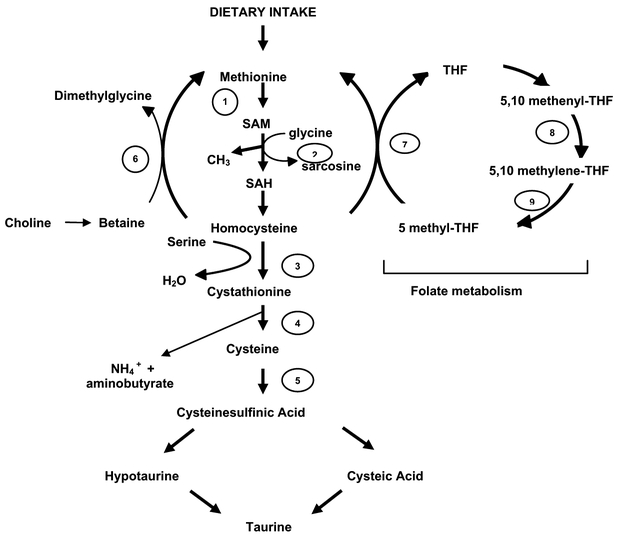
Methionine is required for protein synthesis while its activated form, S-adenosylmethionine (SAM), serves as a methyl donor in numerous biological reactions. Initially,ATP is attached to the sulfur atom of methionine to form SAM, a reaction catalyzed by methionine adenosyl transferase (MAT), Fig. 1. In the next step, SAM condenses with glycine, resulting in the release of a methyl group and sarcosine, to form S-adenosylhomocysteine (SAH). This reaction is catalyzed by the cytosolic enzyme glycine N-methyltransferase (GNMT) [1]. GNMT, highly expressed in the liver, acts to regulate the ratio of SAM and SAH. Where the methyl group supply is limiting, GNMT activity is reduced in order to provide a greater supply for other SAM dependent methyltransferases.
Fig. 1.
Pathways for the conversion of dietary methionine to cysteine and the intersection of this pathway with folate and one carbon metabolism. The enzymes in each step are as follows: (1) Methionine adenosyl-transferase (MAT); (2) Glycine N-methyltransferase (GNMT); (3) Cystathionine β-synthase (CBS); (4) γ-cystathionine (γ-CYS); (5) Cysteine sulfinic acid decarboxylase (CDO); (6) Betaine homocysteine methyltransferase (BHMT); (7) Methionine synthase (MS); (8) 5,10 methylene tetrahydrofolate dehydrogenase; (9) 5,10 methylene tetrahydrofolate reductase (MTHFR).
Conversion of SAH to homocysteine is the intersection of the transulfuration pathway and the folic acid cycle. As such it is worth digressing from the pathway of conversion of methionine to cysteine and discuss the role of GNMT, a folate binding protein, as a sensor to maintain cellular thiol balance.
1.2. Folic acid metabolism
A functional link between sulfur amino acid metabolism and the metabolism of methyl groups is provided by the conversion of homocysteine to methionine, resulting in the formation of tetrahydrofolate (THF), the active form of the water-soluble vitamin folic acid (Fig. 1). Folic acid is synthesized by two sequential reductions both requiring NADPH and catalyzed by dihydrofolate reductase (DHFR). 5,10-Methenyl-THF while also involved in the metabolism of glycine and serine can be thought of as a central compound with respect to the various folate derivatives. It can be generated from THF either through conversion of serine to glycine or through the decarboxlyation of glycine. Its reduction to 5-methyl-THF is essentially irreversible and with the possible exception of some central nervous system functions, 5-methyl-THF is of limited direct biological consequence. This compound is the predominant extracellular form of folic acid and once transported into cells it must be demethylated to THF. Because of the irreversibility of the reaction catalyzed by DHFR it cannot be oxidized to 5,10-methylene-THF. Demethylation may be achieved where homocysteine accesses the methyl acceptor and is converted back to methionine. As outlined above, this is a reaction in which both folic acid and vitamin B-12 participate. While this reaction is of central significance to folic acid homeostasis, it is relatively minor in terms of regeneration of methionine, a characteristic that contributes to methionine being an essential amino acid.
Inhibition of GNMT is achieved by SAM induced allosteric inhibition of 5,10-methylene tetrahydrofolate reductase (MTHFR) with concomitant decreased levels of 5-methyl-THF [2]. GNMT is a folate-binding protein and is subject to inhibition by 5-methyl-THF [3]. Thus, under conditions of low availability of methyl groups, low SAM levels prevail and this leads to an increase in 5-methyl-THF with subsequent inhibition of GNMT [4]. Conversely, with excess methyl groups, SAM levels increase, with subsequent decrease in MTHFR and 5-methyl-THF and enhancement of GNMT activity.
1.3. Transulfuration pathway: SAH + serine → cystathione → cysteine
Homocysteine is a sulfur containing amino acid that plays a significant role in one carbon metabolism and methylation reactions. In humans, the sole source of homocysteine is though dietary methionine intake and subsequent metabolism. As discussed more in detail, the inability of certain individuals to metabolize homocysteine via methylation and/or transulfuration can result in its systemic buildup in the circulation. In the initial step of the transulfuration pathway, serine is combined with homocysteine in a reaction catalyzed by the B-6 dependent enzyme cystathionine β-synthase (CBS) to form cystathionine, which is converted to cysteine via γ-cystathionine.
Subsequent metabolism of cysteine may eventually lead to taurine, a non-standard amino acid that has significant consequences in fetal and childhood development. While high plasma concentrations of cysteine can prove to be toxic, the amino acid can be limiting in the synthesis and maintenance of cellular glutathione pools. As discussed in other chapters in this series [5], glutathione is a primary source of cellular nucleophiles and critical to the maintenance of a reduced environment by maintaining a balanced cellular redox potential. Of equal biological importance, sulfur-amino acids contribute to critical regulatory pathways that involve one carbon metabolism.
2. One carbon metabolism
One carbon metabolism is the transfer of one-carbon groups from one compound to another in a complex array of inter-related biochemical reactions that in its most simple view serve two critical functions: methylation of DNA/RNA and synthesis of nucleotides and thymidylate [6,7]. THF can function as a coenzyme in the transfer of one-carbon units, critical in a number of important cellular synthetic reactions, perhaps the most important of which is the synthesis of thymidylate. The conversion of 5-methyl-THF to THF pro vides the one-carbon group that is used in the remethylation of homocysteine to produce methionine, a reaction catalyzed by the B-12 dependent enzyme methionine synthase. It is also worth noting that an oxidized metabolite of choline, betaine (trimethylglycine) can also serve as a methyl donor. Its participation in the remethylation of homocysteine to methionine produces dimethylglycine, which is not itself a methyl donor. However, when the methyl groups are removed as formaldehyde, oxidation to formate can lead to the formation of 10-formyl-THF. Although the major significance of this pathway probably relates to the biosynthesis of phospholipids, a conversion of choline to glycine can be thought of as a salvage pathway for one-carbon units.
One carbon metabolism has been attributed to greater than 80 reactions and utilizes folate and the B vitamins as coenzymes. Modest dietary deficiencies of these coenzymes are associated with important diseases, including neural tube defects (NTD), cardiovascular disease and cancer.
What is apparent from the pathway outlined in Fig. 1 is that although cysteine is not directly involved in one carbon metabolism, it can be synthesized from serine and methionine and these two amino acids are directly involved. Thus, overall, serine and methionine together with glycine, homocysteine and various THF moieties are major contributors to one carbon metabolism.
3. Defects in the transulfuration pathway
3.1. Homocysteinuria
Previous studies have suggested that homocysteine is a specific risk factor and/or a marker for human pathologies such as cardiovascular disease [8–10]. Whether homocysteine is a cause or effect of the increased incidence of vascular disease is not entirely clear, however, there is a meaningful correlation between elevated plasma homocysteine and mortality from all causes [11].
Both methionine and homocysteine accumulate within cells and bodily fluids of individuals with homocysteinuria with the concomitant result that cysteine biosynthesis is impaired, hence reducing the availability of this amino acid. The buildup of homocysteine has numerous pathological effects, many of which are still under intense study. Excess homocystine has been shown to alter the normal synthesis of collagen crosslinking [12]. Interference with normal collagen formation may contribute to ocular, skeletal, and vascular complications in patients. In the eye, changes in the ligaments of the optic lens can contribute towards lens stability. In bones, the matrix may be compromised accounting for osteoporosis. In a similar manner, interference with vascular wall formation and maintenance may contribute towards arterial and venous thrombotic disease. Increased homocysteine accumulation may also contribute towards enhanced platelet adhesiveness thereby providing the baseline for thrombotic occlusive disease.
In addition to homocysteinuria, an elevated plasma concentration of homocysteine is common among patients with cardiovascular disease. While treatments to solve the underlying metabolic malfunction are sought, there is evidence that lowering homocysteine levels pharmacologically may also have therapeutic advantages. Several studies have shown an inverse relationship between homocysteine levels and folic acid and/or the B vitamins [13]. Hence, dietary supplementation with the corresponding vitamin deficiency is beneficial. Numerous studies have shown that folic acid supplementation (dietary or supplements) can reduce plasma homocysteine levels [14,15].
3.2. Homocystinuria
Homocystinuria is a metabolic disorder that was discovered in the 1960s independently in the US and Ireland by observations of elevated homocystine (the disulfide of homocysteine) levels in the urine of mentally retarded individuals [16,17]. While the worldwide incidence is 1 in 344,000, Celtic regions have a significantly higher incidence of 1 in 65,000. Homocystinuria affects the eyes, central nervous system, skeletal and vascular system. The disorder is characterized by seven biochemically distinct alterations, all resulting in the increase in concentrations of homocystine and methionine in body fluids (Table 1). The most prevalent form of the disease is characterized by decreased activity of CBS, while other forms have resulted from impaired conversions of homocysteine to methionine [18]. Decreased CBS activity has been classified in three categories: (1) no residual activity; (2) decreased activity with normal affinity for the cofactor pyridoxal phosphate and (3) decreased activity with low affinity for the cofactor [19].
Table 1.
Biochemical features of homocystinuria
| Defect inVitamin B-12 metabolism |
| Deficiency of N(5,10)-methylenetetrahydrofolate reductase |
| Selective intestinal malabsorption of B-12 |
| Vitamin B-12 responsive homocystinuria, clb E type |
| Methylcobalamin deficiency, cbl g type |
| Vitamin B-12 metabolic defect, type 2 |
| Transcobalamin II deficiency |
CBS is a 63 kDa heme containing enzyme that forms a homotetramer and catalyzes the condensation of homocysteine with serine to form cystathionine (Fig. 1). CBS requires both pyridoxal phosphate and heme and its activity can be stimulated by SAM. The locus for the enzyme has been matched to the q21 region of chromosome 21 [20,21]. The gene contains 23 exons and has an unusually high number of Alu repeats that may predispose it to deleterious rearrangement [22]. In fact, nearly 92 disease-associated mutations in the CBS gene have been identified in laboratories around the world [23]. The most common mutation in the Celtic region is the G307S mutation [24]. Individuals homozygous for this allele have been shown not to respond to pyridoxine supplementation [23] whereas individuals with an I278T mutation respond favorably to dietary supplementation [23].
Greater than 80% of homozygous individuals for complete synthase deficiency have optical defects. Mental retardation can occur in approximately 50% of the patients and this is sometimes accompanied by behavioral symptoms. It is interesting to note the wide range of cognitive abilities in individuals with homocystinuria. Nearly 1/3 of individuals with this disorder have normal intelligence [25]. Of the individuals that show altered capabilities, two categories have been defined; those that respond to B-6 supplementation (mean IQ of 79) and B-6 non-responsive (mean IQ of 57) [18]. Approximately one quarter of the patients die from vascular occlusive disease before the age of 30. Heterozygote patients may also be at increased risk of premature peripheral and cerebral occlusive vascular disease. It is proposed that heterozygocity shows a dominant negative effect as enzyme levels are ~25–30% of normal, rather than 50%. This phenomenon may be attributed to the formation of the homodimer.
Effective treatment is greatly enhanced by early diagnosis. Newborn screening for decreased CBS activity began in Ireland in 1971. Infants diagnosed have been successfully treated with methionine restricted cystine supplemented diets. In addition, oral supplementation with pyridoxine can provide a reduction in urinary methionine and homocystine. This benefit perhaps reflects the capacity of residual enzyme activity to be enhanced by the presence of the cofactor.
3.3. Cystathioninuria
Cystathioninuria is an autosomal recessive disorder that is attributed to a defect in the cystathionase gamma-lyase (CTH) gene that involves the cleavage of cystathione to cysteine. Consequently, there is an increase in urinary excretion of cystathione due to the elevated plasma concentrations. Mutations in the CTH gene result in a decreased capacity for the enzyme to bind its cofactor, pyridoxal phosphate [26]. Cystathioninuria is considered to be a benign biochemical anomaly that has a low occurrence of 1 per 14,000 live births [27]. Cystathioninuria is not clinically associated with consistent or striking pathologic features; however some individuals may have developmental defects, convulsions, thrombocytopenia and mental retardation.
4. Inherited defects in membrane transport
4.1. Methionine malabsorption
At least 10 disorders of amino acid transport have been described. Both cysteine and methionine are represented in these disease states of methionine malabsorption, folate mal-absorption and cystinuria. Frequently, these conditions affect transport in the kidney and the gastrointestinal tract, or both. Only rarely is there an impact upon other tissues. For example, the autosomal recessive methionine malabsorption trait has direct effects upon jejunal mucosa. The clinical manifestations include, mental retardation, convulsions, hyperpneic attacks, white hair, α-hydroxybutyricaciduria [28]. The disorder is diagnosable primarily as a consequence of urinary excretion of α-hydroxybutyric acid that is a byproduct of the breakdown of unabsorbed methionine by intestinal flora and adds an odor reminiscent of malt to the urine. Treatment of such individuals with a methionine-restricted diet can produce improvement in the symptoms.
4.2. Folate malabsorption
Folate malabsorption is a hereditary defect in transport of folic acid in the intestine as well as the blood–brain barrier [29]. Individuals with this disorder experience megaloblastic anemia, mental retardation, convulsions, and movement disorders. Some studies show that treatment of such individuals with folic acid reduces seizures while others show the condition to be aggravated [30,31].
4.3. Cystinuria
Cystinuria, one of the most common inborn errors of amino acid transport, is inherited as an autosomal recessive trait resulting from mutations in membrane transport proteins for structurally related amino acids. In the early 1800s, Wollaston [32] identified yellow stones in the urine of some patients that he proposed were composed of sulfur containing amino acids, and termed the substance cystic oxide, and referred to the syndrome as cystinuria. The disease is characterized by excessive urinary excretion of lysine, arginine, ornithine, and cystine and is a consequence of restricted tubular reabsorption of these amino acids. Although a similarly impaired absorption also occurs in the intestinal tract, clinical symptoms of cystinuria manifest as a buildup of cystine stones in renal, ureteral, and bladder calculi. These calculi occur primarily because cystine is one of the least water soluble amino acids and as such is more likely to precipitate in target organs. Characteristic of the disease is the fact that at physiological pH, the solubility of cysteine is approximately 300 mg per liter. Individuals who suffer from the disease frequently excrete 600–1800 mg of cysteine per day, producing an environment conducive to the formation of stones. While these stones are characteristically found in the second or third decade of life, in some individuals they can occur in the first year.
Three types of cystinuria have been described, type I, II, and III. These designations are based on the excretion of cystine and dibasic amino acids in the urine of heterozygous individuals. Specifically, type I refers to heterozygotes who excrete normal amounts of cystine and dibasic amino acids; in type II, heterozygotes excrete 9–15 times more; in type III, heterozygotes excrete twice the normal range. Type III patients respond to cystine supplementation whereas Type I and II do not. The underlying mechanism leading to this disorder has been attributed to mutations in at least two amino acid transporters. Type I cystinuria has been attributed to the SLC3A1 amino acid transport gene localized to chromosome 2 [33,34]. Mutations in a second amino acid transporter that contains a heavy and light chain and is encoded by two genes (SLC7A9 and SLC7A3) was identified in Type II and III patients [34]. Mutations in SCL7A9 have been identified that are characteristic of mild (A182T allele) to severe (G105R, V170M and R33W alleles) cystinuria [35].
Medical treatment of the disease includes a high fluid ingestion usually in excess of 4 l per day. Ideal urinary cysteine excretion should be less than 250–300 mg per liter. These high levels of water intake can serve either to prevent crystal formation, or to dissolve existing crystals. While alkalinization of urine can also positively impact on stone formation, such a treatment modality must be balanced with the possibility of inducing calcium based stones and other nephrological complications. A further treatment modality involves the use of penicillamine which can redox exchange with cysteine to form mixed disulfides of penicillamine and cysteine. This disulfide is significantly more soluble than cystine and can therefore help to reduce the physiological concentrations of amino acids.
5. Pathologies associated with folic acid metabolizing enzymes
5.1. Methionine synthase reductase deficiency
Methylation of homocysteine via methionine synthase, results in the formation of methionine. Methionine synthase requires the cofactor cob(I)alamin, which becomes oxidized to cob(II)alamin, resulting in the inactivation of the enzyme. Hence, a second enzyme, methionine synthase reductase (MTRR) is required to maintain methionine synthase in a functional state [36]. MTRR is a 77.7 kDa protein containing 698 amino acids and is a member of the electron transferase family of enzymes [36]. RT-PCR analyses have identified a variety of mutations in the MTRR gene that are associated with homocystinuria–megaloblastic anemia and spina bifida [36–41].
5.2. Methylenetetrahydrofolate reductase deficiency
5,10 Methylenetetrahydrofolate reductase (MTHFR) is the enzyme that catalyzes the conversion of 5,10-methylene-THF to 5-methyl-THF, the co-substrate for remethylation of homocysteine to methionine. A 7.2 kb transcript of MTHFR was identified in all tissues; however a 9 kb transcript has been identified in brain, muscle, placenta and the stomach [42]. The tissue specific transcript has been shown to be the product of an alternate transcriptional start site and polyadenylation signal.
A number of polymorphisms exist within the MTHFR gene and some are associated with a decrease in enzyme activity that leads to MTHFR deficiency. MTHFR deficiency alters folate metabolism and is associated with a variety of disorders, including homocystinuria, homocysteinemia, NTD and coronary heart disease. While 24 point mutations have been identified that alter enzyme activity [43], a high degree of MTHFR deficiency has been causally associated with nine of these [44]. The C559T mutation which gives rise to a termination codon was identified in native Americans who lack MTHFR activity and have severe homocystinuria [45]. A second mutation (G482A), which is also associated with this disorder, introduces a Pst1 site with decreased enzyme capacity [45]. This allele is associated with a milder disorder.
Perhaps one of the most widely studied allelic variants of MTHFR is the thermolabile C667T allele that has decreased enzyme activity [45]. This allele has been identified in all populations studied and ranges in frequency of 0.1–0.38, as illustrated in Table 2 [46–48]. Chromatographic studies showed that the distribution of RBC folates is altered in individuals carrying the C667T allele [49]. High pressure liquid chromatography and mass spectrum analysis of DNA derived from the RBC of homozygous individuals for the C667T allele showed a positive correlation of DNA methylation and an inverse correlation with plasma homocysteine levels [50,46] was the first to show that folate acts to stabilize the thermolabile enzyme. Subsequent studies have shown that increasing serum folate levels >15.4 nM appear to neutralize the clinical manifestations due to the thermolabile allele [51].
Table 2.
Frequency of the C667T allele of the MTHFR gene in various populations
| French–Canadian | 0.38 |
| Caucasian | 0.30 |
| African American | 0.10 |
Homozygous individuals carrying the C667T allele have a threefold increased risk of developing premature cardiovascular disease [52,53] confirmed the risk factor of the thermolabile allele with coronary disease in a meta-analysis of dozens of independent studies [53]. The risk was particularly enhanced when serum folate levels were decreased.
5.3. Neural tube defects and MTHFR deficiency
NTD are developmental abnormalities of the spinal cord that are recessively inherited. NTD include a wide variety of disorders, such as spina bifida occulta, diastematomyelia, and intradural or extradural lipoma.
It is not known whether the majority of mothers who have children with NTD do have elevated homocysteine levels and are therefore likely to have disturbed homocysteine metabolism. In fact, the C667T allele of MTHFR was characterized as the first genetic risk factor for NTD at the molecular level[54]. Ou et al. [55] showed that C667T homozygosity was associated with a 7.2-fold increased risk for neural tube disorders. The literature shows conflicting results with the thermolabile allele and NTD [55–57]. These studies provide stronger evidence that a number of factors contribute to the development of NTD.
Folic acid can serve to lower homocysteine levels by providing the factors necessary for the remethylation of homocysteine to cysteine. Evidence from the Centers for Disease Control suggest that administration of folic acid prior to conception and during the first 4 weeks of pregnancy can prevent ~50% of NTD [58]. In 1998, the US mandated that grain products were to be fortified with folic acid (140 μg/100 g) to prevent NTD in pregnant women. It is important to note that NTD do not arise from a nutritional deficiency of folate but from metabolic defects that can be corrected by significantly large doses of folic acid at developmentally critical times.
5.4. Polymorphisms in MTHFR and MTRR as risk factors for spina bifida
Spina bifida is one of the disorders associated with NTD and has a prevalence in the general population of 0.14%. Polymorphism of the MTHFR (C677T), MTRR (A66G) and methionine synthase (A2756G) genes have been studied extensively in families who have members with spina bifida and compared to individuals from unaffected families [36,39,40,41,59]. Determining the significance of these enzymes to the disease is complicated by consideration of other maternal and embryonic risk factors. Pietrzyk et al. [59] concluded from a study of 106 mothers and 104 children from affected families and a control group of 100 adults that maternal homozygosity for polymorphisms in MTHFR and methionine synthase genes confer a high risk. In prior studies, Doolin et al. [41] showed this same causal relationship and suggested that the risk of having a child with spina bifida increased with the number of maternal alleles. From these and numerous other studies, enzymes involved in the homocysteine-folate metabolic axis should be considered as independent risk factors for spina bifida.
6. Down syndrome
Down syndrome, trisomy 21, is one of the most common human disorders associated with chromosomal imbalance. Clearly the location of the CBS gene on chromosome 21 has a significant impact on affected individuals who have an additional chromosome. The CBS protein has been shown to be overexpressed in individuals with Down syndrome and is shown to alter homocysteine metabolism resulting in a metabolic imbalance such that folate-dependent resynthesis of methionine is compromised [60].
Previously in this review the maternal polymorphisms in the MTHFR (C667T) and MTRR (A66G) genes were discussed as potential risk factors for spina bifida. The same polymorphisms were examined and have been linked to the etiology of Down syndrome [61]. Wisniewski et al. [62] reported the presence of senile plaques and neurofibrillary tangles in the brains of Down syndrome patients. These findings are neuropathologic hallmarks of Alzheimer dis ease, however, the presence of plaques appear at an earlier age. It is interesting to note that polymorphisms in the MTHFR gene, as well as increased plasma homocysteine levels have been associated with Alzheimer’s disease in some populations [63]. In general, MTHFR polymorphisms have been linked to a wide variety of disorders that are associated with impaired mental dysfunction, including Alzheimer’s disease [64], Down syndrome [62], and Spina bifida [60,42]. Studies are on-going to clarify the impact of allelic variation for folate metabolizing enzymes in individuals who have Down syndrome.
7. Cancer
Methionine is an essential amino acid that plays a major role in DNA synthesis and one carbon metabolism. Some cancer cell primary cultures and cell lines express an unusual dependence on methionine for growth [64]. Conversely, most non-transformed cells are methionine independent [65]. Initially, a defect in the enzyme methionine synthase was considered a viable explanation for the methionine dependence. Indeed, decreased activity of this enzyme has been demonstrated in some tumor cell lines [66], but not all [67]. Furthermore, some methionine dependent cells have defects in cobalamin metabolism [68], while others are associated with defective expression of methylthioadenosine phosphorylase [69]. Thus, it is apparent that methionine dependent tumor cell growth is determined by a plurality of factors.
While it is difficult to analyze methionine dependence for tumors in cancer patients, there have been a number of clinical trials that have studied the enzymes involved in methionine metabolism. Matsuo et al. [70] examined polymorphisms of methionine synthase reductase (MTRR), methionine synthase and MTHFR in 72 colon and 70 rectal cancer patients and compared the incidence to 241 non-cancer patients. These studies showed that individuals homozygous for the A66G polymorphism in MTRR had a significantly higher risk of colorectal cancer than other geno-types in Japanese populations [70]. These same three genes were analyzed in Caucasians with non-Hodgkin’s lymphoma, multiple myeloma and non-cancer patients. The methionine synthase A2756G polymorphism was shown to confer a 2.4-fold lower risk in lymphoma patients.
Alterations in one carbon metabolism have also been associated with the pathology of cancer. In fact, it has been shown that aberrant DNA-methylation is common phenotype in human neoplasias. It is plausible that decreased SAM levels in tumor cells could alter DNA methylation. The underlying cause could be a consequence of nutritional imbalance or allelic variation in genes governing SAM metabolism. Paz et al. [71] analyzed 233 individuals with a wide range of cancer types for the methylation status of three genes involved in methyl group metabolism. In this study, a positive correlation was found among the C677T allele of MTHFR and the 2756G allele of methionine synthase [71]. Efforts continue to unravel what role, if any, these enzymes may have in tumor progression. Whether therapeutic targeting of these pathways could be used to achieve anticancer effects remains to be established.
5,10-methylene-THF and its 5,10 precursors have critical roles to play in purine and pyrimidine biosynthesis. The pool of 5,10-methylene-THF increases with diminished activity of MTHFR. Skibola et al. [72] hypothesized that the enhanced 5,10-methylene-THF pools could result in decreased misincorporation of uracil into DNA, thereby resulting in fewer double strand breaks. Hence, folate status would be critical in the development of rapidly proliferating cancers (colorectal and leukemias) that had high requirements for DNA synthesis. The 667TT genotype was shown to confer a 4.3-fold decreased risk among acute lymphocytic leukemia (ALL) patients whereas folate insufficiency may play a key role in development of ALL [72]. Further studies in pediatric leukemia patients showed a significant association with the C667T genotype when compared to healthy newborns [73].
5-Formyl-THF is also known as leucovorin, and can be used pharmaceutically as a rescue agent in combination with cancer drugs of the antimetabolite class. The role that folate metabolizing enzymes play in the etiology of cancer is not clearly understood. However, a better understanding of the intricate relationship between cancer risk and thiol status may unravel new initiatives that could incorporate folate supplementation as a means of chemoprevention in high risk individuals.
References
- [1].Wagner C, Decha-Umphai W, Corbin J. Phosphorylation modulates the activity of glycine N-methyltransferase, a folate binding protein. In vitro phosphorylation is inhibited by the natural folate ligand. J Biol Chem 1989;264(16):9638–42. [PubMed] [Google Scholar]
- [2].Jencks DA, Mathews RG. Allosteric inhibition of methylenetetrahydrofolate reductase by adenosylmethionine. Effects of adenosylmethionine and NADPH on the equilibrium between active and inactive forms of the enzyme and on the kinetics of approach to equilibrium. J Biol Chem 1987;262(6):2485–93. [PubMed] [Google Scholar]
- [3].Wagner C, Briggs WT, Cook RJ. Inhibition of glycine N-methyltransferase activity by folate derivatives: implications for regulation of methyl group metabolism. Biochem Biophys Res Commun 1985;127(3):746–52. [DOI] [PubMed] [Google Scholar]
- [4].Kutzbach C, Stokstad EL. Feedback inhibition of methylenetetrahydrofolate reductase in rat liver by S-adenosylmethionine. Biochim Biophys Acta 1967;139(1):217–20. [DOI] [PubMed] [Google Scholar]
- [5].Townsend DM, Tew KD, Tapiero H. The importance of glutathione in human disease. Biomed Pharmacother 2003;57(3–4):145–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Choi SW, Mason JB. Folate and carcinogenesis: an integrated scheme. J Nutr 2000;130(2):129–32. [DOI] [PubMed] [Google Scholar]
- [7].Mason JB. Biomarkers of nutrient exposure and status in one-carbon (methyl) metabolism. J Nutr 2003;133(Suppl 3):941S–7S. [DOI] [PubMed] [Google Scholar]
- [8].McCully KS. Vascular pathology of homocysteinemia: implications for the pathogenesis of arteriosclerosis. Am J Pathol 1969;56(1):111–28. [PMC free article] [PubMed] [Google Scholar]
- [9].Clarke R, Daly L, Robinson K, Naughten E, Cahalane S, Fowler B, et al. Hyperhomocysteinemia: an independent risk factor for vascular disease. N Engl J Med 1991;324(17):1149–55. [DOI] [PubMed] [Google Scholar]
- [10].Kang SS, Wong PWK, Malinow MR. Hyperhomocyst(e)inemia as a risk factor for occlusive vascular disease. Ann Rev Nutr 1992;12:279–98. [DOI] [PubMed] [Google Scholar]
- [11].Vollset SE, Refsum H, Tverdal A, Nygard O, Nordrehaug JE, Tell GS, et al. Plasma total homocysteine and cardiovascular and noncardiovascular mortality: the Hordaland Homocysteine Study.Am J Clin Nutr 2001;74(1):130–6. [DOI] [PubMed] [Google Scholar]
- [12].McKusick VA. Heritable disorders of connective tissue. 3rd ed St. Louis: C.V. Mosby; 1966. p. 155. [Google Scholar]
- [13].Ubbink JB, Vermaak WJ, van der Merwe A, Becker PJ. Vitamin B-12, vitamin B-6, and folate nutritional status in men with hyperhomocysteinemia. Am J Clin Nutr 1993;57(1):47–53. [DOI] [PubMed] [Google Scholar]
- [14].Jacques PF, Selhub J, Bostom AG, Wilson PW, Rosenberg IH. The effect of folic acid fortification on plasma folate and total homocysteine concentrations. N Engl J Med 1999;340(19):1449–54. [DOI] [PubMed] [Google Scholar]
- [15].Riddell LJ, Chisholm A, Williams S, Mann JI. Dietary strategies for lowering homocysteine concentrations. Am J Clin Nutr 2000;71(6): 1448–54. [DOI] [PubMed] [Google Scholar]
- [16].Carson NA. Metabolic abnormalities detected in a survey of mentally backward individuals in Northern Ireland. Arch Dis Child 1962;37: 505–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Gerritsen T, Vaughn JG, Waisman HA. The identification of homocystine in the urine. Biochem Biophys Res Commun 1962;9:493–6. [DOI] [PubMed] [Google Scholar]
- [18].Mudd SH, Skovby F, Levy HL, Pettigrew KD, Wilcken B, Pyeritz RE, et al. The natural history of homocystinuria due to cystathionine beta-synthase deficiency. Am J Hum Genet 1985;37(1):1–31. [PMC free article] [PubMed] [Google Scholar]
- [19].Fowler B, Kraus J, Packman S, Rosenberg LE. Homocystinuria. Evidence for three distinct classes of cystathionine beta-synthase mutants in cultured fibroblasts. J Clin Invest 1978;61(3):645–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Skovby F, Krassikoff N, Francke U. Assignment of the gene for cystathionine beta-synthase to human chromosome 21 in somatic cell hybrids. Hum Genet 1984;65(3):291–4. [DOI] [PubMed] [Google Scholar]
- [21].Munke M, Kraus JP, Ohura T, Francke U. The gene for cystathionine beta-synthase (CBS) maps to the subtelomeric region on human chromosome 21q and to proximal mouse chromosome 17. Am J Hum Genet 1988;42(4):550–9. [PMC free article] [PubMed] [Google Scholar]
- [22].Kraus JP, Oliveriusova J, Sokolova J, Kraus E, Vlcek C, de Fran-chis R, et al. The human cystathionine beta-synthase (CBS) gene: complete sequence, alternative splicing, and polymorphisms. Genomics 1998;52(3):312–24. [DOI] [PubMed] [Google Scholar]
- [23].Kraus JP, Janosik M, Kozich V, Mandell R, Shih V, Sperandeo MP, et al. Cystathionine beta-synthase mutations in homocystinuria. Hum Mutat 1999;13(5):362–75. [DOI] [PubMed] [Google Scholar]
- [24].Kraus JP. Komrower lecture. Molecular basis of phenotype expression in homocystinuria. J Inherit Metab Dis 1994;17(4):383–90. [DOI] [PubMed] [Google Scholar]
- [25].Abbott MH, Folstein SE, Abbey H, Pyeritz RE. Psychiatric manifestations of homocystinuria due to cystathionine beta-synthase deficiency: prevalence, natural history, and relationship to neurologic impairment and vitamin B6-responsiveness. Am J Med Genet 1987; 26(4):959–69. [DOI] [PubMed] [Google Scholar]
- [26].Frimpter GW. Cystathioninuria: nature of the defect. Science 1965; 149(688):1095–6. [DOI] [PubMed] [Google Scholar]
- [27].Wong LT, Hardwick DF, Applegarth DA, Davidson AG. Review of metabolic screening program of children’s hospital, Vancouver, British Columbia. 1971–1977. Clin Biochem 1979;12(5):167–72. [DOI] [PubMed] [Google Scholar]
- [28].Smith AJ, Strang LB. An inborn error of metabolism with the urinary excretion of alpha-hydroxy-butyric acid and phenylpyruvic acid.Arch Dis Child 1958;33:109–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Tapiero H, Tew KD, Gaté L, Machover D. Prevention of pathologies associated with oxidative stress and dietary intake deficiencies: folate deficiency and requirements. Biomed Pharmacother 2001;55:381–90. [DOI] [PubMed] [Google Scholar]
- [30].Luhby AL, Cooperman JM, Pesci-Bourel A. A new inborn error of metabolism: folic acid responsive megaloblastic anemia, ataxia, mental retardation, and convulsions. J Pediat 1965;67:1052 [Google Scholar]
- [31].Lanzkowsky P, Erlandson ME, Bezan AI. Isolated defect of folic acid absorption associated with mental retardation and cerebral calcification. Blood 1969;34(4):452–65. [PubMed] [Google Scholar]
- [32].Wollaston WH. On cystic oxide, a new species of urinary calculus. Phil Trans Roy Soc Lond 1810;100:223–30. [Google Scholar]
- [33].Calonge MJ, Gasparini P, Chillaron J, Chillon M, Gallucci M, Rou-saud F, et al. Cystinuria caused by mutations in rBAT, a gene involved in the transport of cystine. Nat Genet 1994;6(4):420–5. [DOI] [PubMed] [Google Scholar]
- [34].Lee WS, Wells RG, Sabbag RV, Mohandas TK, Hediger MA. Cloning and chromosomal localization of a human kidney cDNA involved in cystine, dibasic, and neutral amino acid transport. J Clin Invest 1993; 91(5):1959–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Feliubadalo L, Font M, Purroy J, Rousaud F, Estivill X, Nunes V, et al. Non-type I cystinuria caused by mutations in SLC7A9, encoding a subunit (bo+AT) of rBAT. International Cystinuria Consortium. Nat Genet 1999;23(1):52–7. [DOI] [PubMed] [Google Scholar]
- [36].Leclerc D, Wilson A, Dumas R, Gafuik C, Song D, Watkins D, et al. Cloning and mapping of a cDNA for methionine synthase reductase, a flavoprotein defective in patients with homocystinuria. Proc Natl Acad Sci USA 1998;95(6):3059–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Wilson A, Leclerc D, Rosenblatt DS, Gravel RA. Molecular basis for methionine synthase reductase deficiency in patients belonging to the cblE complementation group of disorders in folate/cobalamin metabolism. Hum Mol Genet 1999;8(11):2009–16. [DOI] [PubMed] [Google Scholar]
- [38].Wilson A, Platt R, Wu Q, Leclerc D, Christensen B, Yang H, et al. A common variant in methionine synthase reductase combined with low cobalamin (vitamin B12) increases risk for spina bifida. Molec Genet Metab 1999;67:317–23. [DOI] [PubMed] [Google Scholar]
- [39].Hobbs CA, Sherman SL, Yi P, Hopkins SE, Torfs CP, Hine RJ, et al. Polymorphisms in genes involved in folate metabolism as maternal risk factors for Down syndrome. Am J Hum Genet 2000;67(3):623–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].James SJ, Pogribna M, Pogribny IP, Melnyk S, Hine RJ, Gibson JB, et al. Abnormal folate metabolism and mutation in the methylenetetrahydrofolate reductase gene may be maternal risk factors for Down syndrome. Am J Clin Nutr 1999;70(4):495–501. [DOI] [PubMed] [Google Scholar]
- [41].Doolin MT, Barbaux S, McDonnell M, Hoess K, Whitehead AS, Mitchell LE. Maternal genetic effects, exerted by genes involved in homocysteine remethylation, influence the risk of spina bifida. Am J Hum Genet 2002;71(5):1222–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Gaughan DJ, Barbaux S, Kluijtmans LA, Whitehead AS. The human and mouse methylenetetrahydrofolate reductase (MTHFR) genes: genomic organization, mRNA structure and linkage to the CLCN6 gene. Gene 2000;257(2):279–89. [DOI] [PubMed] [Google Scholar]
- [43].Sibani S, Christensen B, O’Ferrall E, Saadi I, Hiou-Tim F, Rosenblatt DS, et al. Characterization of six novel mutations in the methylenetetrahydrofolate reductase (MTHFR) gene in patients with homocystinuria. Hum Mutat 2000;15(3):280–7. [DOI] [PubMed] [Google Scholar]
- [44].Rozen R Molecular genetics of methylenetetrahydrofolate reductase deficiency. J Inherit Metab Dis 1996;19(5):589–94. [DOI] [PubMed] [Google Scholar]
- [45].Goyette P, Sumner JS, Milos R, Duncan AM, Rosenblatt DS, Matthews RG, et al. Human methylenetetrahydrofolate reductase: isolation of cDNA, mapping and mutation identification. Nat Genet 1994; 7(2):195–200. [DOI] [PubMed] [Google Scholar]
- [46].Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA, Matthews RG, et al. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet 1995;10(1):111–3. [DOI] [PubMed] [Google Scholar]
- [47].Schneider JA, Rees DC, Liu YT, Clegg JB. Worldwide distribution of a common methylenetetrahydrofolate reductase mutation. Am J Hum Genet 1998;62(5):1258–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].McAndrew PE, Brandt JT, Pearl DK, Prior TW. The incidence of the gene for thermolabile methylene tetrahydrofolate reductase in African Americans. Thromb Res 1996;83(2):195–8. [DOI] [PubMed] [Google Scholar]
- [49].Bragley PJ, Selhub J. A common mutation in the methylenetetrahydrofolate reductase gene is associated with an accumulation of formylated tetrahydrofolates in red blood cells. Proc Natl Acad Sci USA 1998;95:13217–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Friso S, Choi SW, Girelli D, Mason JB, Dolnikowski GG, Bagley PJ, et al. A common mutation in the 5,10-methylenetetrahydrofolate reductase gene affects genomic DNA methylation through an interaction with folate status. Proc Natl Acad Sci USA 2002;99(8):5606–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Jacques PF, Bostom AG, Williams RR, Ellison RC, Eckfeldt JH, Rosenberg IH, et al. Relation between folate status, a common mutation in methylenetetrahydrofolate reductase, and plasma homocysteine concentrations. Circulation 1996;93(1):7–9. [DOI] [PubMed] [Google Scholar]
- [52].Kluijtmans LA, van den Heuvel LP, Boers GH, Frosst P, Stevens EM, van Oost BA, et al. Molecular genetic analysis in mild hyperhomocysteinemia: a common mutation in the methylenetetrahydrofolate reductase gene is a genetic risk factor for cardiovascular disease. Am J Hum Genet 1996;58(1):35–41. [PMC free article] [PubMed] [Google Scholar]
- [53].Klerk M, Verhoef P, Clarke R, Blom HJ, Kok FJ, Schouten EG. MTHFR 677C → T polymorphism and risk of coronary heart disease: a meta-analysis. JAMA 2002;288(16):2023–31. [DOI] [PubMed] [Google Scholar]
- [54].Christensen B, Arbour L, Tran P, Leclerc D, Sabbaghian N, Platt R, et al. Genetic polymorphisms in methylenetetrahydrofolate reductase and methionine synthase, folate levels in red blood cells, and risk of neural tube defects. Am J Med Genet 1999;84(2):151–7. [DOI] [PubMed] [Google Scholar]
- [55].Ou CY, Stevenson RE, Brown VK, Schwartz CE, Allen WP, Khoury MJ, et al. 5,10 Methylenetetrahydrofolate reductase genetic polymorphism as a risk factor for neural tube defects. Am J Med Genet 1996;63(4):610–4. [DOI] [PubMed] [Google Scholar]
- [56].Mornet E, Muller F, Lenvoise-Furet A, Delezoide AL, Col JY, Simon-Bouy B, et al. Screening of the C677T mutation on the methylenetetrahydrofolate reductase gene in French patients with neural tube defects. Hum Genet 1997;100(5–6):512–4. [DOI] [PubMed] [Google Scholar]
- [57].Speer MC, Worley G, Mackey JF, Melvin E, Oakes WJ, George TM. The thermolabile variant of methylenetetrahydrofolate reductase (MTHFR) is not a major risk factor for neural tube defect in American Caucasians. The NTD Collaborative Group. Neurogenetics 1997; 1(2):149–50. [DOI] [PubMed] [Google Scholar]
- [58].Motulsky AG. Nutritional ecogenetics: homocysteine-related arteriosclerotic vascular disease, neural tube defects, and folic acid. Am J Hum Genet 1996;58(1):17–20. [PMC free article] [PubMed] [Google Scholar]
- [59].Pietrzyk JJ, Bik-Multanowski M, Sanak M, Twardowska M. Polymorphisms of the 5,10-methylenetetrahydrofolate and the methionine synthase reductase genes as independent risk factors for spina bifida. J Appl Genet 2003;44(1):111–3. [PubMed] [Google Scholar]
- [60].Pogribna M, Melnyk S, Pogribny I, Chango A, Yi P, James SJ. Homocysteine metabolism in children with Down syndrome: in vitro modulation. Am J Hum Genet 2001;69(1):88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].O’Leary VB, Parle-McDermott A, Molloy AM, Kirke PN, Johnson Z, Conley M, et al. MTRR and MTHFR polymorphism: link to Down syndrome? Am J Med Genet 2002;107(2):151–5. [DOI] [PubMed] [Google Scholar]
- [62].Wisniewski KE, Wisniewski HM, Wen GY. Occurrence of neuropathological changes and dementia of Alzheimer’s disease in Down’s syndrome. Ann Neurol 1985;17(3):278–82. [DOI] [PubMed] [Google Scholar]
- [63].Religa D, Styczynska M, Peplonska B, Gabryelewicz T, Pfeffer A, Chodakowska M, et al. Homocysteine, apolipoproteine E and methylenetetrahydrofolate reductase in Alzheimer’s disease and mild cognitive impairment. Dement Geriatr Cogn Disord 2003;16(2):64–70. [DOI] [PubMed] [Google Scholar]
- [64].Hoffman RM. Methionine dependence in cancer cells—a review. In Vitro 1982;18(5):421–8. [DOI] [PubMed] [Google Scholar]
- [65].Hoffman RM. Altered methionine metabolism and transmethylation in cancer. Anticancer Res 1985;5(1):1–30. [PubMed] [Google Scholar]
- [66].Liteplo RG, Hipwell SE, Rosenblatt DS, Sillaots S, Lue-Shing H. Changes in cobalamin metabolism are associated with the altered methionine auxotrophy of highly growth autonomous human melanoma cells. J Cell Physiol 1991;149(2):332–8. [DOI] [PubMed] [Google Scholar]
- [67].Tautt JW,Anuszewska EL, Koziorowska JH. Methionine regulation of N-5-methyltetrahydrofolate: homocysteine methyltransferase and its influence on the growth and protein synthesis in normal, neoplastic, and transformed cells in culture. J Natl Cancer Inst 1982;69(1):9–14. [PubMed] [Google Scholar]
- [68].Fiskerstrand T, Christensen B, Tysnes OB, Ueland PM, Refsum H. Development and reversion of methionine dependence in a human glioma cell line: relation to homocysteine remethylation and cobalamin status. Cancer Res 1994;54(18):4899–906. [PubMed] [Google Scholar]
- [69].Tang B, Li YN, Kruger WD. Defects in methylthioadenosine phosphorylase are associated with but not responsible for methionine-dependent tumor cell growth. Cancer Res 2000;60(19):5543–7. [PubMed] [Google Scholar]
- [70].Matsuo K, Hamajima N, Hirai T, Kato T, Inoue M, Takezaki T, et al. Methionine synthase reductase gene A66G polymorphism is associated with risk of colorectal cancer.Asian Pac J Cancer Prev 2002;3(4): 353–9. [PubMed] [Google Scholar]
- [71].Paz MF, Avila S, Fraga MF, Pollan M, Capella G, Peinado MA, et al. Germ-line variants in methyl-group metabolism genes and susceptibility to DNA methylation in normal tissues and human primary tumors. Cancer Res 2002;62(15):4519–24. [PubMed] [Google Scholar]
- [72].Skibola CF, Smith MT, Kane E, Roman E, Rollinson S, Cart-wright RA, et al. Polymorphisms in the methylenetetrahydrofolate reductase gene are associated with susceptibility to acute leukemia in adults. Proc Natl Acad Sci USA 1999;96(22):12810–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Wiemels JL, Smith RN, Taylor GM, Eden OB, Alexander FE, Greaves MF. Methylenetetrahydrofolate reductase (MTHFR) polymorphisms and risk of molecularly defined subtypes of childhood acute leukemia. Proc Natl Acad Sci USA 2001;98(7):4004–9. [DOI] [PMC free article] [PubMed] [Google Scholar]