Abstract
The specific posttranslational modification of protein cysteine residues by the addition of the tripeptide glutathione is termed S-glutathionylation. This process is promoted by oxidative and nitrosative stress but also occurs in unstressed cells. Altered levels of S-glutathionylation in some proteins have been associated with numerous pathologies, many of which have been linked to redox stress in the endoplasmic reticulum (ER). Proper protein folding is dependent upon controlled redox conditions within the ER, and it seems that ER conditions can in turn affect rates of S-glutathionylation. This article seeks to bring together the ways through which these processes are interrelated and considers the implications of these interrelationships upon therapeutic approaches to disease.
Introduction
Cellular homeostasis is an intricate balance of survival and death signals, many of which are mediated by the posttranslational modification of proteins. Protein phosphorylation is probably the most widely appreciated example of such modification, whereby an interplay of kinases and phosphatases determines which hydroxyl-bearing side chains (i.e., Ser, Thr, and Tyr) of any given target protein are phosphorylated. New observations have made clear that the specific posttranslational modification of the sulfhydryl-bearing side chains (i.e., Cys) can likewise participate in the relay of cell signals. In particular, the S-glutathionylation of proteins, whereby a disulfide bond is established between a protein cysteinyl residue and glutathione (GSH), is, like protein phosphorylation, a reversible mechanism of protein regulation (Figure 1). Significantly, S-glutathionylation can be promoted by physiological levels of reactive oxygen species (ROS) and reactive nitrogen species (RNS), so that posttranslational modification of target proteins in this case is directly linked to the redox status of the cell. Indeed, the reaction can serve to protect proteins from oxidative or nitrosative damage and can also effect changes in protein structure that may be relevant to function or subcellular localization.
Figure 1. Reactivity of cysteinyl residues and the process of S-glutathionylation.
A. The cysteinyl residues of proteins (Cys; Cys–SH to stress the thiol form) of cells under oxidative/nitrosative stress can be oxidized to various acidic forms, including cysteine sulfenic acid (Cys–SOH), which is fairly labile and can be readily reduced back to the thiol (Cys–SH) or further oxidized to the more stable cysteine sulfinic acid form (Cys–SOOH). It is becoming increasingly clear that such oxidation may be essential to normal deactivation-and-reactivation cycles of proteins and enzymes. B. The more familiar reaction of protein cysteinyl residues, namely, in the formation of disulfide bonds (e.g., as a manifestation of protein secondary structure), is related to the process of protein S-glutathionylation, which is increasingly recognized as essential to cellular behavior in health and in disease states. In the presence of physiological concentrations of glutathione (GSH), specific cysteinyl residues, by virtue of their position and reactivity within protein microenvironments, can undergo such modification through reactions with oxidized glutathione disulfide (GSSG) or by glutathione-utilizing enzymes such as glutathione S-transferases (GST). This posttranslational modification becomes reversible under catalysis that involves small redox proteins such as sulfiredoxin and glutaredoxin. (Oxidation is indicated in blue; reduction is indicated in green.)
The reversibility of protein S-glutathionylation, like the reversibility of protein phosphorylation, is critical to the regulatory role of the modification in cell signaling. In the case of S-glutathionylation, small cysteine-rich proteins such as glutare doxin and sulfiredoxin function in catalytic reactions to provide a modification cycle that is suited to the control of regulatory pathways (1–3). Moreover, a number of phosphatases are subject to S-glutathionylation; in this way, the two modification pathways are interrelated.
Glutathione S-Transferases and Kinase Regulation
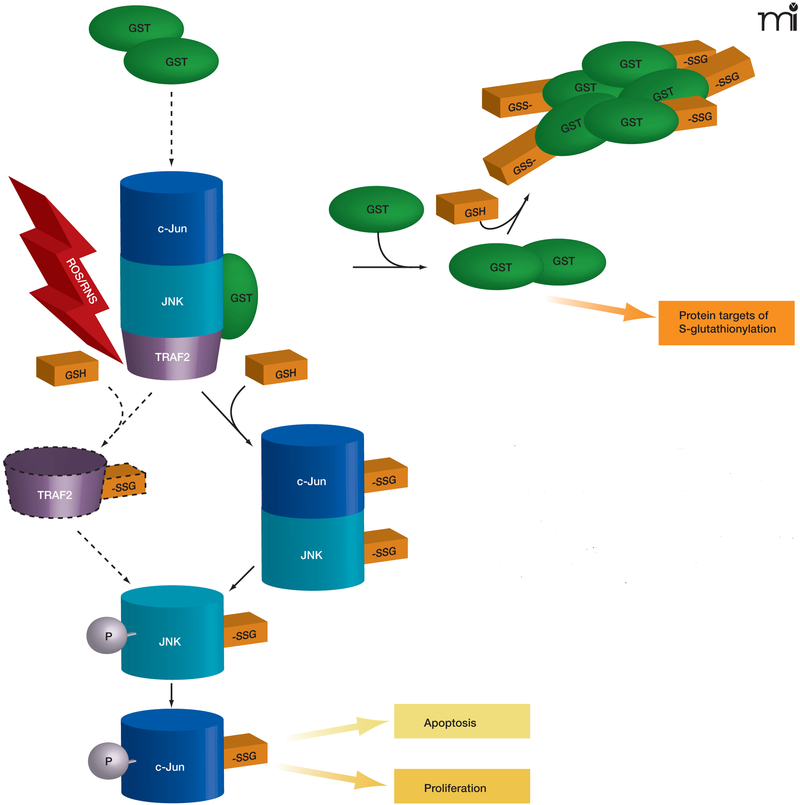
Glutathione S-transferases (GSTs) are a family of phase II detoxification enzymes that catalyze the conjugation of GSH to electrophiles through thioether linkages (4). GSTs also play a regulatory role in cellular signaling by associating with critical kinases involved in cellular responses to stress, apoptosis, and proliferation (5, 6). The soluble human GSTs can be divided into classes based on structural analysis (i.e., alpha, mu, pi, and theta classes); the first reported example of kinase regulation by a GST was in the inhibition of c-Jun aminoterminal kinase (JNK) by a pi class GST (pi GST) (5). JNK, a stress-activated kinase, has been implicated in pro-apoptotic signaling and may mediate the cytotoxicity of a variety of chemotherapeutic agents (7). The phosphorylation of JNK activates c-Jun, resulting in subsequent activation of downstream effectors. In unstressed cells, low JNK catalytic activity is maintained through its sequestration within a protein complex that includes pi GST, JNK, and c-Jun (Figure 2). Under conditions of oxidative or nitrosative stress, however [during which all three of these proteins are S-glutathionylated (8–10)], the pi GST·JNK complex dissociates, so that JNK is free to act on downstream gene targets, whereas the pi GST undergoes oligomerization. Other GST isozymes seem capable of mediating similar regulatory interactions. For example, GSTA1–1 inhibits JNK signaling caused by either inflammatory cytokines or oxidative stress (11). The authors invoke a mechanism similar to that shown for the pi GST·JNK interaction (Figure 2) and offer the premise that GST isozymes may overlap in substrate specificity.
Figure 2. The interplay of protein phosphorylation and protein S-glutathionylation pathways in cell signaling.
Under oxidative/nitrosative stress (indicated by ROS/ RNS), pi GST monomers associate with the target proteins c-Jun, JNK, and TRAF2, which are consequently S-glutathionylated (as indicated by –SSG). The immediate effect of S-glutathionylation could be TRAF2 activation, as well as the phosphorylative activation (indicated by –P) of c-Jun and JNK, culminating in the deployment of apoptotic or proliferative pathways. In addition, S-glutathionylation of pi GST results in its aggregation and concomitant inactivation. Dashed lines and arrows indicate hypothetical, rather than experimentally established, steps.
In addition, pi GST appears to regulate enzyme activities that otherwise manage cellular redox reactions. 1-Cys peroxiredoxin (1-cysPrx), which prevents oxidative damage to membrane constituents, can directly associate with pi GST (12, 13). During its catalytic cycle, a specific Cys residue within 1cysPrx undergoes oxidation to the sulfenic acid form, and the enzyme is thereby rendered inactive. Reactivation of oxidized 1cysPrx is effected by its heterodimerization with GSH-bound pi GST, leading first to the S-glutathionylation of 1cysPrx at its reactive Cys residue and subsequently to an intersubunit disulfide bond within the heterodimer. Finally, the GSH-mediated reduction of the disulfide bond regenerates the active, sulfhydryl-bearing form of 1cysPrx. Interestingly, the overexpression of 1cysPrx has been associated with cellular resistance to radiation as well as with suppression of JNK activation and apoptosis (14). Mutation of the catalytic Cys52 residue of 1cysPrx to Ser obliterates peroxidase activity and inhibits JNK activation; nevertheless, both wild-type and mutant proteins can be co-immunoprecipitated with pi GST and JNK. The authors concluded that 1cysPrx was able to suppress apoptosis through inhibition of JNK activation.
A further regulatory role for pi GST has been defined in the context of tumor necrosis factor-alpha (TNF-α) signaling. Wu and colleagues (15) showed that pi GST heterodimerizes with TNF receptor–associated factor 2 (TRAF2) and inhibits TRAF2-induced activation of both JNK and p38 (but not of NFκB). Class pi GST also interferes with the interaction between TRAF2 and apoptosis signal–regulating kinase 1 (ASK1), thereby inhibiting the autophosphorylation of ASK1 and apoptosis. Significantly, these effects, which are independent of the catalytic activity of pi GST, represent connectivity between S-glutathionylation and endoplasmic reticulum (ER) stress, because TRAF2/ASK1 activation mediates an ER stress–induced apoptotic pathway (see below).
Glutathione S-Transferases in Posttranslational Modification
In addition to its role in reactivating 1cysPrx (12, 13), pi GST may also catalyze the forward reaction of the protein S-glutathionylation cycle (Figure 1B) (16). The rate and extent of protein S-glutathionylation is significantly reduced in pi GST-deficient animals, and in contrast to its inhibitory associations with 1cysPrx and TRAF2 (see above), the catalytic activity of pi GST is required for protein S-glutathionylation. HEK293 cells expressing catalytically inactive pi GST manifest diminished S-glutathionylation of cellular proteins in response to oxidative and nitrosative stress.
Interestingly, two Cys residues of pi GST itself are subject to S-glutathionylation (Cys47 and Cys101) (16), and the S-glutathionylation of pi GST reduces its enzyme activity against chemical substrates and promotes its multimerization. It is not known whether S-glutathionylation plays a role in the dissociation of pi GST from the kinases described above. However, Cys47 and Cys101 reside in distinct effector domains, both crucial for interaction with JNK, and Cys47 is critical for heterodimerization with 1cysPrx. In any event, a realistic model for pi GST in cell signaling will have to address both its enzymatic and inhibitory ligand roles in oxidative or nitrosative stress.
Targets of S-Glutathionylation
Although the S-glutathionylation of proteins was generally described in the 1990s, the identification of protein substrates was only made possible by the more recent advent of proteomic approaches. It is important to note that the actual number of cellular S-glutathionylated proteins is not large, relative to the proteome. Cluster analysis suggests that the modification may affect very specific cellular functions. In Table 1, for example, six general categories of cell physiology appear to be affected by S-glutathionylation, each of which is basic to the biology of the cell: 1) the cytoskeleton; 2) metabolism and energy; 3) signaling proteins—particularly kinases and phosphatases; 4) calcium homeostasis; 5) protein folding and stability; and 6) redox homeostasis.
Table 1.
Cluster analysis of proteins susceptible to S-glutathionylation.
| Proteina | Cluster | Function | Notes | Reference |
|---|---|---|---|---|
| Actin | 1 | Cytoskeleton | Effects are multiple: see text | (56) |
| Spectrin | 1 | Cytoskeleton | Cell morphology, molecular traffic | (57) |
| Tubulin | 1 | Cytoskeleton | Mitosis, cell movement, intracellular movement | (58) |
| Vimentin | 1 | Cytoskeleton | Intermediate cytoskeletal filament | (59) |
| Glyceraldehyde-3- phosphate dehydrogenase (GAPDH) | 2 | Energy metabolism, glycolysis | Inactivated at Cys149 | (60) |
| Phosphoglycerate kinase | 2 | Energy metabolism, glycolysis. | Inhibited by S-glutathionylation | (59) |
| Triose phosphate isomerase | 2 | Energy metabolism, glycolysis. | Inhibited by S-glutathionylation | (59) |
| Pyruvate kinase | 2 | Energy metabolism, glycolysis. | Inhibited by S-glutathionylation | (59) |
| Aldolase | 2 | Energy metabolism, glycolysis. | Inhibited by S-glutathionylation | (59) |
| α-ketoglutarate dehydrogenase | 2 | Energy metabolism, glycolysis. | Inhibited by S-glutathionylation | (61) |
| Mitochondrial isocitrate dehydrogenase | 2 | Energy metabolism, mitochondrial redox balance | Inhibited by S-glutathionylation | (61) |
| Complex 1 | 2 | Energy metabolism, glycolysis | Mitochondrial redox balance | (62) |
| NADPH | 2 | Energy metabolism, glycolysis | Inhibited by S-glutathionylation | (63) |
| GAPDH | 2 | Energy metabolism, glycolysis | Carbohydrate metabolism | (64) |
| ATPase | 2 | Energy metabolism, glycolysis | Mitochondrial redox balance | (37) |
| NADH ubiquinone reductase | 2 | Energy metabolism, glycolysis | Mitochondrial redox balance | (65) |
| Carbonic anhydrase III | 2 | Energy metabolism, glycolysis | Enzyme | (66) |
| Catechol-O-methyltransferase | 2 | Energy metabolism, glycolysis | Enzyme | (67) |
| Pyruvate dehydrogenase | 2 | Energy metabolism, glycolysis | Inhibited by S-glutathionylation | (68) |
| MEKK1 (JNK) | 3 | Signaling | Kinase | (8) |
| Protein tyrosine phosphatase 1B | 3 | Signaling | Phosphatase | (37) |
| PTEN | 3 | Signaling | Phosphatase | (69) |
| Pyrophosphatase 2A | 3 | Signaling | Phosphatase | (59) |
| NFκB, subunits 65 and 50 | 3 | Signaling | Transcription factor | (70, 71) |
| PKC | 3 | Signaling | Kinase | (72) |
| PKG | 3 | Signaling | Kinase | (73) |
| cAMP dependent PKA | 3 | Signaling | Kinase | (74) |
| Creatinine kinase | 3 | Signaling | Kinase | |
| c-able | 3 | Signaling | Kinase | (75) |
| p53 | 3 | Signaling | Transcription factor | (76) |
| Caspase 3 | 3 | Signaling | Apoptosis | (66) |
| GTPase p21 ras | 3 | Signaling | Activated by S-glutathionylation with impact on downstream kinases | (77) |
| S100A1 and S100B | 4 | Calcium homeostasis | Ca2+ affinity enhanced by S-glutathionylation | (78) |
| SERCA | 4 | Calcium homeostasis | Inhibition of Ca2+ uptake linked to S-glutathionylation | (79) |
| Ryanodine receptor I and II | 4 | Calcium homeostasis | Activates receptor and stimulates Ca2+ release | (80) |
| CTFR | 4 | Calcium homeostasis | Ion channel | (81) |
| PDI | 5 | Protein folding | Activity inhibited by S-glutathionylation | (37) |
| HSP 65, 70 | 5 | Protein folding | Protein chaperone | (37) |
| 20S Proteosome | 5 | Protein folding | Protein stability | (82) |
| Ubiquitin conjugating enzyme | 5 | Protein folding | Protein stability | (59) |
| Thioredoxin 1 | 6 | Redox | (83) | |
| Glutathione S-transferase | 6 | Redox | Phase II detoxification, JNK inhibitor | (16) |
| Peroxiredoxin | 6 | Redox | (84) |
The proteins listed here were identified as targets in vitro or in cell culture.
The most prevalent S-glutathionylated protein, and therefore most readily detected, is actin. When cells are stimulated with growth factors, S-glutathionylation of actin alters the ratio of soluble:polymerized protein. Consequently, there can be changes in the cellular architecture and membrane ruffling with concomitant changes in intracellular trafficking of many types of molecules. The S-glutathionylation of actin influences cellular adhesion and cell–cell interactions (17) as well as protein–protein interactions; for example, S-glutathionylated actin, relative to the unmodified protein, manifests a weaker affinity for tropomyosin (18).
Further elucidation of protein functions that are regulated through S-glutathionylation will likely carry clinical implications. For example, the constitutive activation of kinases has been intimately linked to cancer, with major impact upon drug development efforts. Similarly, the accumulation of S-glutathionylated proteins is emerging as a key factor in multiple diseases. Especially intriguing is the concurrence of increased protein S-glutathionylation rate and ER stress in the diseases highlighted in Table 2. This overlap likely reflects signals emanating from the ER as it processes proteins that respond (e.g., by undergoing S-glutathionylation) to cellular redox conditions.
Table 2:
S-glutathionylated proteins identified in human diseases.
| Protein | Diseasea | UPR activation |
|---|---|---|
| Actin (85)b | Ischemia/Cardiovascular disease | Yes (86) |
| Friedreich’s ataxia | No | |
| Tau (87) | Alzheimer’s disease | Yes (88) |
| Hemoglobin (89) | Type 2 diabetes | Yes (90) |
| CTFR (81) | Cystic fibrosis | Yes (91) |
| γ-S-crystallin (92) | Cataract disease | Yes (93) |
| Spectrin (57) | Sickle cell anemia | No |
Only those diseases in which S-glutathionylated proteins have been identified are shown.
Numbers in parentheses indicate cited references.
The Redox Environment of the Endoplasmic Reticulum
The ER is the first intracellular compartment for processing secretory and transmembrane proteins. Such processing may include a series of posttranslational modifications, notably glycosylation and disulfide bond formation, and depends on the distinct redox conditions provided within the ER. In contrast to the reducing conditions of the cytosol, where molar amounts of GSH may exceed those of GSSG by 100-fold, a highly oxidizing environment exists within the ER (GSH:GSSG ~ 3:1) to allow protein disulfide bond formation. This unique ER environment also provides the platform to sense oxidative and nitrosative stress. Protein folding within the ER is catalytically mediated, and chaperone proteins exist to prevent the aggregation of proteins as they undergo maturation within the ER (19). The folding process can be complex, involving multiple folding intermediates and the isomerization of disulfide bonds. (Proteins may also undergo reductive unfolding and subsequent degradation.) In yeast, molecular oxygen is the electron acceptor in the formation of protein disulfide bonds, which thus brings a risk for oxidative damage (20). In mammalian cells, it is well established that ER stress and the unfolded protein response (UPR; see below) are components of the hypoxic stress response in tumors (21). Clinical studies with the use of oxygen electrodes and markers of hypoxia have shown that O2 concentration heterogeneity occurs within individual tumors, ranging from zero to 100% (22). Translational attenuation occurs during hypoxia through the activation of pancreatic ER kinase (PERK), an ER resident kinase, and phosphorylation of eukaryotic initiation factor eIF2α. Cells derived from PERK-deficient mice are defective in eIF2α phosphorylation during hypoxia (23).
Multiple signaling pathways have evolved to ensure quality control in protein folding within the ER. Indeed, stress upon the ER results in the accumulation of malfolded proteins, leading to cellular deployment of the unfolded protein response (UPR). Specifically, the ER membrane contains three signal-transducing proteins that modulate the UPR: PERK, activating transcription factor (ATF), and IRE1 (Figure 3). Regulation of these three proteins is contingent upon interactions with BiP [binding protein; also known as glucose related protein 78 (GRP78)]. The accumulation of malfolded proteins results in the dissociation of BiP and elicits the UPR, which culminates in three processes: 1) the inhibition of protein synthesis; 2) the increased expression of ER resident chaperones; and 3) the degradation of terminally malfolded proteins.
Figure 3. The Unfolded Protein Response (UPR) and pro-apoptotic pathways.
The UPR is a complex signaling cascade that can be induced by stress and the accumulation of malfolded proteins in the ER. During homeostasis (upper schematic; blue), protein disulfide isomerase (PDI) promotes the proper folding of immature proteins (yellow strings). The ER-resident protein BiP associates with properly folded proteins and concomitantly inhibits three ER transmembrane proteins, namely, PERK, ATF6, and IRE1. Under conditions of oxidative and nitrosative stress (indicated by ROS/RNS and red background in lower ER schematic), PDI is modified (see text for discussion) and rendered inactive, and unfolded proteins (yellow strings) accumulate in the ER. BiP dissociates from improperly folded proteins and concomitantly surrenders negative regulatory interactions with PERK, ATF6, and IRE1. PERK thereby phosphorylates and inactivates (blunt arrow) eukaryotic translation initiation factor eIF2α, the phosphorylation of which is also associated with transcriptional activation of genes involved in the UPR. This transcriptional activation, which is also promoted by the activation of ATF6 and IRE1, can drive pro-apoptotic signaling, particularly through activation of CHOP (see text for details). IRE1 can also interact with TRAF2, which can function pro-apoptotically through association with ASK1 and JNK (see text; also see Figure 2). A third route to apoptosis is offered by caspase activity; intriguingly, regulation of caspase activity (particularly that of CASP3) may also be a function of GST.
The UPR can be viewed as a cascade of transcriptional and translational events that sense the capacity of the ER to manage the cellular demands of protein maturation. When the ER is unable to meet these demands, the UPR evokes apoptotic pathways (24). Three pathways (Figure 4) for ER-induced apoptosis are known and can be triggered by ROS and RNS. The first involves activation of JNK and dissociation of the TRAF2–ASK1 complex. This pathway is redox-sensitive, and multiple proteins within this cascade are targets of S-glutathionylation. The second pathway involves transcriptional activation of the gene that encodes the C/EBP homologous protein [CHOP; also known as growth arrest– and DNA damage–inducible gene 153 (GADD153)]. The ER membrane proteins IRE1 and ATF6 are transcriptional activators of genes that encode ER-resident proteins and specifically activate the promoter of the CHOP-encoding gene. CHOP can be phosphorylated by the p38 MAP kinase family, leading to cell cycle arrest (25). Overexpression of CHOP leads to the proapoptotic translocation of BAX from the cytosol into mitochondria, whereas IRE1 has been shown to interact, via its cytoplasmic domain, with BAX as well as the related proapoptotic Bcl-2 member BAK and thereby to become activated (26). BAX and BAK have been shown to localize to mitochondria and the ER, where they presumably contribute to membrane permeability (27, 28). The third ER-induced apoptotic pathway involves caspase activation, including CASP3, a target of S-glutathionylation.
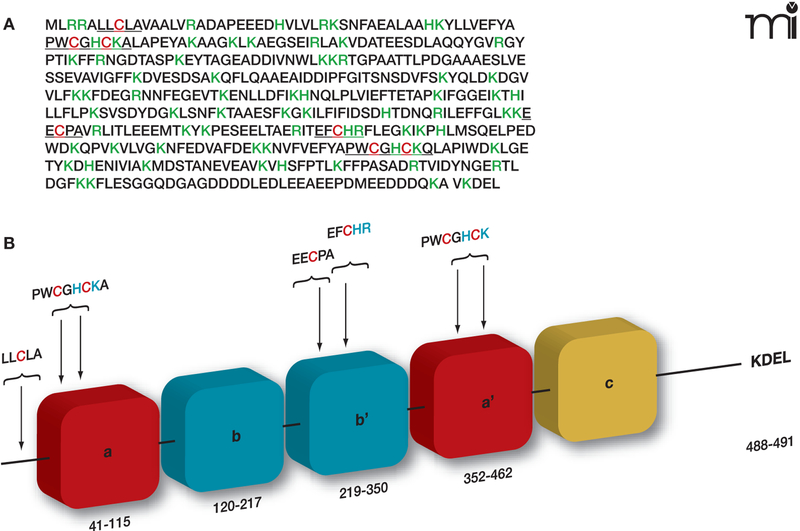
Figure 4. Regulation of PDI by its specific S-glutathionylation.
A. PDI is a member of the thioredoxin superfamily of small thiol-rich proteins. The protein sequence of PDI includes seven cysteine residues (highlighted in red). Basic amino acids that flank cysteine residues (green) may act to lower the pKa of the thiol group, making the relevant Cys more likely to be susceptible to S-glutathionylation. B. A cartoon structure of the domains in PDI shows a single cysteine in the N-terminal domain, four in the catalytic domains, and two in the b’ domain.
Despite insights into the crucial roles of ER transmembrane proteins and BiP in the UPR, upstream events—especially the deregulation of chaperones that allows the accumulation of malfolded proteins—are not well characterized. Human pathology and cancer drug discovery efforts have focused on protein disulfide isomerase (PDI), the most abundant ER chaperone, and its redox regulation as an upstream signaling event leading to the UPR (Figure 3).
Protein Disulfide Isomerase
PDI is the most abundant chaperone in the ER. At 57 kDa, PDI is a large member of the thioredoxin superfamily and is organized into five domains (a, b, b’, a’, and c); it also contains a C-terminal KDEL sequence that targets it to the ER (Figure 4) (29). PDI contains two active sites, in the a and a’ thioredoxin domains, each having two conserved cysteine residues that cycle between oxidized (disulfide) and reduced (dithiol) states (30). The crystal structure of yeast PDI suggests that the four thioredoxin domains (a, b, b’, a’) form a twisted U shape with the catalytic domains facing each other and an internal hydrophobic surface that interacts with malfolded proteins (31). Similar to pi GST, PDI has both enzymatic and protein binding functions. PDI facilitates the folding and correct S-S disulfide bond formation of its protein substrates. PDI is found in multimeric proteins such as prolyl-4-hydroxylase and microsomal triglyceride transfer protein (32, 33). PDI also interacts with the estrogen receptor to modulate its association with DNA (34, 35). PDI is also specifically and potently inhibited by estrogen, and PDI shares sequence similarity with the estrogen receptor. Numerous studies have demonstrated that PDI is key to ER homeostasis. PDI is regulated by the endoplasmic reticulum oxidase (ERO1), which restores reduced PDI to an oxidized state through disulfide exchange with ERO1. Interestingly, ERO1 activity is also regulated through modulation of noncatalytic cysteine residues (94). ERO1 activity is attenuated under oxidized conditions in the ER.
Posttranslational modifications that alter PDI function have recently been described. Nitrosylation of cysteine residues in the active sites of PDI occurs in the brains of patients manifesting sporadic Parkinson’s and Alzheimer’s disease (36), both of which involve ER stress and activation of the UPR. Nitrosylated PDI cannot function as a folding catalyst and thereby leads to the accumulation of malfolded proteins. In addition, PDI undergoes S-glutathionylation upon treatment with the anti-cancer agent PABA/NO (37), and we have shown that S-glutathionylation occurs at active-site Cys residues, leading to enzyme inactivation, activation of the UPR, and cancer cell death. Collectively, these studies provide evidence that redox-regulation of PDI is an upstream signaling event in the UPR.
Nitrosylation and S-glutathionylation of PDI are important posttranslational modifications, the deregulation of which can lead to tissue injury, with implications for cancer therapeutics. Proteomic analysis in a wide variety of cancer cell lines has shown alterations in the expression pattern of numerous PDI family members that correlate with differential drug response (38–41). The role of PDI in the cancer phenotype is not well characterized. A study with human breast ductal carcinoma tissue and histologically normal tissue concluded that a subset of approximately thirty proteins, which included PDI and six related proteins, were characteristic of epithelial neoplasia (42). A clinical correlation requires further confirmation; however, the UPR has been shown as a novel component of the hypoxic stress response in tumors and correlates with a poor drug response and more aggressive disease (42).
Drugs that Affect S-Glutathionylation
Early studies characterizing S-glutathionylated proteins used hydrogen peroxide as an inducer of oxidative stress. In subsequent studies, it became clear that a number of agents can produce oxygen or nitrogen radicals, and the definitions of ROS and NOS are quite broad. For example, ROS is associated with many radicals, including superoxide (O2·-), peroxyl (O2·), and hydroxyl (OH·) radicals and also encompass hydrogen peroxide and singlet oxygen (1O2). The definition of ROS is also broad as a result of the use of oxidized glutathione (GSSG) as a marker of an oxidizing cellular environment. Definitions of RNS include nitric oxide (NO·) and nitrogen dioxide (NO2·) radicals as well as non-radicals such as nitrous acid, dinitrogen tetroxide (N2O4), and peroxinitrite (ONOO−). Endogenous ROS can be by-products of lipid peroxidation and the electron transport chain or caused by γ- and UV-irradiation. Elevated levels of nitric oxide (NO) provide the primary source of RNS. NO is an endogenous diffusible messenger shown to participate in survival and death pathways (43) and can alter protein function through direct modification or indirectly by generating products that ultimately lead to S-glutathionylation. Deregulation of endogenous NO production can lead to the release of RNS and ROS, each of which have been implicated in a number of human pathologies, including neurodegenerative disorders, cystic fibrosis, aging, and cancer (44–46). This observation has led to investigations of the capacity of NO to induce cytotoxicity, with particular reference to antitumor activities (47), and resonates with the results and implications from work on GST (see above). It was the coalescence of NO and GST biology that led to the design and synthesis of the novel anticancer pro-drug PABA/NO that has been discussed above in the context of PDI inactivation (48).
The pi GST isozyme is expressed at elevated levels in a variety of human tumors and is linked with the development of resistance to a number of anticancer agents (46, 49). Catalytic activation of PABA/NO by pi GST releases NO that elicits antitumor activity both in vitro and in vivo (50–51). The pharmacology of PABA/NO predicts that NO will be quite rapidly released to result in extensive modification of target proteins. In fact, only two markedly nitrosated proteins were identified (3), whereas approximately twenty S-glutathionylated proteins were apparent (37). Although reactive cysteine residues are subject to both nitrosation and glutathionylation (e.g., PDI; see above), and although the identity of the direct glutathionyl donor may either be S-glutathione or a donor intermediate, the very fact that an NO-releasing drug causes such marked S-glutathionylation is in itself interesting. Nitrosated Cys residues may be susceptible to subsequent substitution by glutathione, which could help to explain the relatively small number of nitrosated proteins in PABA/ NO treated cells.
Adriamycin is an antitumor agent that is clinically used to treat solid and hematological tumors. The therapeutic value of the drug is tempered by dose-limiting toxicity that impairs macrophages and limits wound healing. Using cultured human macrophages, Asmis et al. showed that adriamycin causes S-glutathionylation of cellular proteins prior to caspase-independent death (52). These observations were validated in mouse models. Since anthracyclines such as adriamycin are known to cycle through redox reactions with quinone intermediates, it seems probable that free radical by-products of this drug may cause oxidative stress and thereby lead to S-glutathionylation. Whether these events have a bearing on the cytotoxicity of adriamycin is not firmly established.
Induction of oxidative stress may also occur by manipulation of the GSH:GSSG ratio. Recently, the novel cancer therapeutic NOV-002 was introduced to early clinical trial status in the US. The drug is a 1000:1 mix of GSSG and cisplatin, where the latter is not present in sufficient quantities to have a pharmacological impact. In common with a number of other sulfur-based therapeutics (53), NOV-002 has a stimulating effect upon myeloproliferation, a characteristic that underlies its therapeutic utility. In an in vivo setting, the drug can affect the GSH:GSSG ratio in blood and can stimulate S-glutathionylation, particularly of actin, although cell surface proteins are also S-glutathionylated in the presence of NOV-002 (54). Although NOV-002 does not induce apoptosis, the drug leads to ER stress. Neither the reduced nor the disulfide form of glutathione can passively cross the cell membrane; however, γ-glutamyl transpeptidase (γ-GGT) is an outer membrane–associated enzyme that catalyzes the cleavage of the GSH tripeptide from GSSG and glutathione conjugates and effects recycling of the constituent amino acids. A series of papers have proposed a mechanism whereby GSSG stimulates γ-GGT activity to result in the liberation of H2O2 into the intracellular environment [for review, see (55)]. Thus, extracellular exposure to excess GSSG could raise intracellular concentrations of ROS and thereby stimulate the S-glutathionylation of actin. Given the crosstalk between protein S-glutathionylation and phosphorylation pathways outlined earlier, GSSG (the active component of NOV-002) and γ-GGT could trigger the enhanced phosphorylation of regulatory kinase cascades that are involved in cell proliferation.
Conclusions
A variety of critical cellular processes are subject to regulation by protein S-glutathionylation. The list of modified proteins is not yet complete, nor is the structural and functional significance of the modification. Nevertheless, distinct crossover areas link phosphatase and kinase activity with S-glutathionylation, implying a control point between phosphorylation and thiol-mediated recognition of stress conditions. Certain drugs can promote S-glutathionylation and thereby affect a number of cellular functions, including proliferation and apoptosis. It is likely that the pathways affected are tissue-specific; in particular, aberrations of redox balance that have been identified in cancer may represent opportunities for therapeutic intervention. In cancer drug discovery, the UPR is an evolving target, and the indication that redox conditions in the ER may determine protein folding may have important implications for UPR-based clinical strategies. New approaches to diseases that involve alterations of cellular redox may benefit from the growing recognition of the role of S-glutathionylation in protein modification and the concomitant signaling events that elaborate the UPR.
Biography
Danyelle M. Townsend, PhD, is Assistant Professor in Pharmaceutical and Biomedical Sciences and Director of the Drug Metabolism and Pharmacokinetics Facility at the Hollings Cancer Center. She is interested in the molecular targets of ROS and RNS and cellular pathways that counteract stress. Her current work seeks to unite mechanistic and translational studies in the preclinical development of anticancer agents that target redox signaling. E-mail townsed@musc.edu; fax 843-792-9588.

References
- 1.Beer SM, Taylor ER, Brown SE, Dahm CC, Costa NJ, Runswick MJ, and Murphy MP Glutaredoxin 2 catalyzes the reversible oxidation and glutathionylation of mitochondrial membrane thiol proteins: Implications for mitochondrial redox regulation and antioxidant DEFENSE. J. Biol. Chem 279, 47939–47951 (2004). [DOI] [PubMed] [Google Scholar]
- 2.Shelton MD, Chock PB, and Mieyal JJ Glutaredoxin: Role in reversible protein S-glutathionylation and regulation of redox signal transduction and protein translocation. Antioxid. Redox. Signal 7, 348–366 (2005). [DOI] [PubMed] [Google Scholar]
- 3.Findlay VJ, Townsend DM, Saavedra JE, Buzard GS, Citro ML, Keefer LK, Ji X, and Tew KD Tumor cell responses to a novel glutathione S-transferase-activated nitric oxide-releasing prodrug. Mol. Pharmacol 65, 1070–1079 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tew KD Glutathione-associated enzymes in anticancer drug resistance. Cancer Res. 54, 4313–4320 (1994). [PubMed] [Google Scholar]
- 5.Adler V, Yin Z, Fuchs SY et al. Regulation of JNK signaling by GSTp. EMBO J. 18, 1321–1334 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang T, Arifoglu P, Ronai Z, and Tew KD Glutathione S-transferase P1–1 (GSTP1–1) inhibits c-Jun N-terminal kinase (JNK1) signaling through interaction with the C terminus. J. Biol. Chem 276, 20999–21003 (2001).This work, along with reference (5), establishes that pi GST is more than a Phase II detoxification enzyme. The non-catalytic properties of pi GST encompass kinase regulation and mediation of S-glutathionylation reactions. As such, GST is a regulator of multiple signaling pathways that govern survival and apoptotic pathways.
- 7.Yin Z, Ivanov VN, Habelha H, Tew K, and Ronai Z Glutathione S-transferase p elicits protection against H2O2-induced cell death via coordinated regulation of stress kinases. Cancer Res. 60, 4053–4057 (2000). [PubMed] [Google Scholar]
- 8.Cross JV and Templeton DJ Oxidative stress inhibits MEKK1 by site-specific glutathionylation in the ATP-binding domain. Biochem J. 381, 675–683 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klatt P and Lamas S c-Jun regulation by S-glutathionylation. Methods Enzymol. 348, 157–174 (2002). [DOI] [PubMed] [Google Scholar]
- 10.Townsend DM, Keefer LK, Saavedra JE, Pazoles CJ, and Tew KD (Unpublished results.) [Google Scholar]
- 11.Romero L, Andrews K, Ng L, O’Rourke K, Maslen A, and Kirby G Human GSTA1–1 reduces c-Jun N-terminal kinase signaling and apoptosis in Caco-2 cells. Biochem. J 400, 135–141 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manevich Y, Feinstein SI, and Fisher AB Activation of the antioxidant enzyme 1-CYS peroxiredoxin requires glutathionylation mediated by heterodimerization with pi GST. Proc. Natl. Acad. Sci. USA 101, 3780–3785 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ralat LA, Manevich Y, Fisher AB, and Colman RF Direct evidence for the formation of a complex between 1-cysteine peroxiredoxin and glutathione S-transferase pi with activity changes in both enzymes. Biochemistry 45, 360–372 (2006). [DOI] [PubMed] [Google Scholar]
- 14.Kim YJ, Lee WS, Ip C, Chae HZ, Park EM, and Park YM Prx1 suppresses radiation-induced c-Jun NH2-terminal kinase signaling in lung cancer cells through interaction with the glutathione S-transferase Pi/c-Jun NH2-terminal kinase complex. Cancer Res. 66, 7136–7142 (2006). [DOI] [PubMed] [Google Scholar]
- 15.Wu Y, Fan Y, Xue B, Luo L, Shen J, Zhang S, Jiang Y, and Yin Z Human glutathione S-transferase P1–1 interacts with TRAF2 and regulates TRAF2-ASK1 signals. Oncogene 25, 5787–5800 (2006). [DOI] [PubMed] [Google Scholar]
- 16.Townsend DM. (Unpublished results.) [Google Scholar]
- 17.Fiaschi T, Cozzi G, Raugei G, Formigli L, Ramponi G, and Chiarugi P Redox regulation of beta-actin during integrin-mediated cell adhesion. J. Biol. Chem 281, 22983–22991 (2006). [DOI] [PubMed] [Google Scholar]
- 18.Chen FC and Ogut O Decline of contractility during ischemia-reperfusion injury: actin glutathionylation and its effect on allosteric interaction with tropomyosin. Am. J. Physiol. Cell Physiol. 290, C719–727 (2006). [DOI] [PubMed] [Google Scholar]
- 19.Hartl FU and Hayer-Hartl M Molecular chaperones in the cytosol: from nascent chain to folded protein. Science 295, 1852–1858 (2002). [DOI] [PubMed] [Google Scholar]
- 20.Tu BP and Weissman JS Oxidative protein folding in eukaryotes: mechanisms and consequences. J. Cell Biol. 164, 341–346 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feldman DE, Chauhan V and Koong AC The unfolded protein response: A novel component of the hypoxic stress response in tumors. Mol. Cancer Res. 3, 597–605 (2005).ER stress is a molecular signature of tumor hypoxia. Proteins critical to UPR-induced apoptosis are novel targets for chemotherapeutic strategies.
- 22.Evans SM and Koch CJ Prognostic significance of tumor oxygenation in humans. Cancer Lett. 195, 1–16 (2003). [DOI] [PubMed] [Google Scholar]
- 23.Brewer JW and Diehl JA PERK mediates cell-cycle exit during the mammalian unfolded protein response. Proc. Natl. Acad. Sci. USA 97, 12625–12630 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rao RV and Bredesen DE Misfolded proteins, endoplasmic reticulum stress and neurodegeneration. Curr. Opin. Cell Biol. 16, 653–662 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang XZ and Ron D Stress-induced phosphorylation and activation of the transcription factor CHOP (GADD153) by p38 MAP kinase. Science 272, 1347–1349 (1996). [DOI] [PubMed] [Google Scholar]
- 26.Hetz C, Bernasconi P, Fisher J et al. Proapoptotic BAX and BAK modulate the unfolded protein response by a direct interaction with IRE1alpha. Science 312, 572–576 (2006). [DOI] [PubMed] [Google Scholar]
- 27.Zong WX, Li C, Hatzivassiliou G, Lindsten T, Yu QC, Yuan J, and Thompson CB Bax and Bak can localize to the endoplasmic reticulum to initiate apoptosis. J. Cell Biol. 162, 59–69 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scorrano L, Oakes SA, Opferman JT, Cheng EH, Sorcinelli MD, Pozzan T, and Korsmeyer SJ BAX and BAK regulation of endoplasmic reticulum Ca2+: A control point for apoptosis. Science 300, 135–139 (2003). [DOI] [PubMed] [Google Scholar]
- 29.Pihlajaniemi T, Helaakoski T, Tasanen K, Myllylä R, Huhtala ML, Koivu J, and Kivirikko KI Molecular cloning of the beta-subunit of human prolyl 4-hydroxylase. This subunit and protein disulphide isomerase are products of the same gene. EMBO J. 6, 643–6649 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferrari DM and Soling HD The protein disulphide-isomerase family: Unravelling a string of folds. Biochem. J 339, 1–10 (1999). [PMC free article] [PubMed] [Google Scholar]
- 31.Tian G, Xiang S, Noiva R, Lennarz WJ, and Schindelin H The crystal structure of yeast protein disulfide isomerase suggests cooperativity between its active sites. Cell 124, 61–73 (2006). [DOI] [PubMed] [Google Scholar]
- 32.Farjanel J, Perier C, Szymanovicz G, and Frey J Simultaneous characterizations of 3-prolylhydroxylase and 4-prolylhydroxylase activities by ion exchange chromatography. Biochimie 62, 195–199 (1980). [DOI] [PubMed] [Google Scholar]
- 33.Pirneskoski A, Ruddock LW, Klappa P, Freedman RB, Kivirikko KI, and Koivunen P Domains b’ and a’ of protein disulfide isomerase fulfill the minimum requirement for function as a subunit of prolyl 4-hydroxylase. The N-terminal domains a and b enhances this function and can be substituted in part by those of ERp57. J. Biol. Chem 276, 11287–11293 (2001). [DOI] [PubMed] [Google Scholar]
- 34.Landel CC, Kushner PJ and Greene GL The interaction of human estrogen receptor with DNA is modulated by receptor-associated proteins. Mol. Endocrinol 8, 1407–1419 (1994). [DOI] [PubMed] [Google Scholar]
- 35.Schultz-Norton JR, McDonald WH, Yates JR, and Nardulli AM Protein disulfide isomerase serves as a molecular chaperone to maintain estrogen receptor alpha structure and function. Mol. Endocrinol 20, 1982–1995 (2006). [DOI] [PubMed] [Google Scholar]
- 36.Uehara T, Nakamura T, Yao D, Shi ZQ, Gu Z, Ma Y, Masliah E, Nomura Y, and Lipton SA S-nitrosylated protein disulphide isomerase links protein misfolding to neurodegeneration. Nature 441, 513–517 (2006).Nitrosylation of PDI in the brains of patients with Parkinson’s and Alzheimer’s disease underscores the importance of this protein to normal cellular function.
- 37.Townsend DM, Findlay VJ, Fazilev F, Ogle M, Fraser J, Saavedra JE, Ji X, Keefer LK, and Tew KD A glutathione S-transferase pi-activated prodrug causes kinase activation concurrent with S-glutathionylation of proteins. Mol. Pharmacol 69, 501–508 (2006).Exploitation of PDI through drug-induced S-glutathionylation is a unique strategy to target cancer cells.
- 38.Chen CY, Jia JH, Zhang MX, Meng YS, Kong DX, Pan XL, and Yu XP. Proteomic analysis on multi-drug resistant cells HL-60/DOX of acute myeloblastic leukemia. Chin. J. Physiol 48,. 115–120 (2005). [PubMed] [Google Scholar]
- 39.Persson S, Rosenquist M, Knoblach B, Khosravi-Far R, Sommarin M, and Michalak M Diversity of the protein disulfide isomerase family: identification of breast tumor induced Hag2 and Hag3 as novel members of the protein family. Mol. Phylogenet. Evol 36, 734–740 (2005). [DOI] [PubMed] [Google Scholar]
- 40.Shin BK, Wang H, Yim AM et al. Global profiling of the cell surface proteome of cancer cells uncovers an abundance of proteins with chaperone function. J. Biol. Chem 278, 7607–7616 (2003). [DOI] [PubMed] [Google Scholar]
- 41.Chen ST, Pan TL, Tsai YC, and Huang CM Proteomics reveals protein profile changes in doxorubicin-treated MCF-7 human breast cancer cells. Cancer Lett. 181, 95–107 (2002). [DOI] [PubMed] [Google Scholar]
- 42.Bini L, Magi B, Marzocchi B et al. Protein expression profiles in human breast ductal carcinoma and histologically normal tissue. Electrophoresis 18, 2832–2841 (1997). [DOI] [PubMed] [Google Scholar]
- 43.Moncada S Nitric oxide in the vasculature: Physiology and pathophysiology. Ann. NY Acad. Sci 811, 60–67 (1997). [DOI] [PubMed] [Google Scholar]
- 44.Ilic TV, Jovanovic M, Jovicic A, and Tomovic M Oxidative stress indicators are elevated in de novo Parkinson’s disease patients. Funct. Neurol 14, 141–147 (1999). [PubMed] [Google Scholar]
- 45.Tieu K, Ischiropoulos H, and Przedborski S Nitric oxide and reactive oxygen species in Parkinson’s disease. IUBMB Life 55, 329–335 (2003). [DOI] [PubMed] [Google Scholar]
- 46.Townsend D and Tew K Cancer drugs, genetic variation and the glutathione-S-transferase gene family. Am. J. Pharmacogenomics 3, 157–172 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cui S, Reichner JS, Mateo RB, and Albina JE Activated murine macrophages induce apoptosis in tumor cells through nitric oxide-dependent or -independent mechanisms. Cancer Res. 54, 2462–2467 (1994). [PubMed] [Google Scholar]
- 48.Saavedra JE, Billiar TR, Williams DL, Kim YM, Watkins SC, and Keefer LK. Targeting nitric oxide (NO) delivery in vivo. Design of a liver-selective NO donor prodrug that blocks tumor necrosis factor-alpha-induced apoptosis and toxicity in the liver. J. Med. Chem 40, 1947–1954 (1997). [DOI] [PubMed] [Google Scholar]
- 49.O’Brien M, Kruh GD and Tew KD The influence of coordinate over-expression of glutathione phase II detoxification gene products on drug resistance. J. Pharmacol. Exp. Ther 294, 480–487 (2000). [PubMed] [Google Scholar]
- 50.Townsend DM. (Unpublished results.) [Google Scholar]
- 51.Findlay VJ, Townsend DM, Morris TE, Fraser JP, He L, and Tew KD A novel role for human sulfiredoxin in the reversal of glutathionylation. Cancer Res. 66, 6800–6806 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Asmis R, Wang Y, Xu L, Kisgati M, Begley JG, and Mieyal JJ A novel thiol oxidation-based mechanism for adriamycin-induced cell injury in human macrophages. FASEB J. 19, 1866–1868 (2005). [DOI] [PubMed] [Google Scholar]
- 53.Tew KD and Ali-Osman F Redox pathways in cancer drug discovery. Curr. Opin. Pharmacol 7, 353–354 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Townsend DM, et al. NOV-002, a glutathione disulfide mimetic,as a modulator of cellular redox balance. Cancer Res. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pompella A, Corti A, Paolicchi A Giommarelli C, and Zunino F γ-Glutamyltransferase, redox regulation and cancer drug resistance. Curr. Opin. Pharmacol 7, 360–366 (2007). [DOI] [PubMed] [Google Scholar]
- 56.Wang J, Boja ES, Tan W, Tekle E, Fales HM, English S, Mieyal JJ, and Chock PB Reversible glutathionylation regulates actin polymerization in A431 cells. J. Biol. Chem 276, 47763–47766 (2001). [DOI] [PubMed] [Google Scholar]
- 57.Rossi R, Giustarini D, Milzani A, and Dalle-Donne I Membrane skeletal protein S-glutathionylation and hemolysis in human red blood cells. Blood Cells Mol. Dis 37, 180–187 (2006). [DOI] [PubMed] [Google Scholar]
- 58.Landino LM, Moynihan KL, Todd JV, Kennett KL Modulation of the redox state of tubulin by the glutathione/glutaredoxin reductase system. Biochem. Biophys. Res. Commun 314, 555–560 (2004). [DOI] [PubMed] [Google Scholar]
- 59.Fratelli M, Demol H, Puype M et al. Identification by redox proteomics of glutathionylated proteins in oxidatively stressed human T lymphocytes. Proc. Natl. Acad. Sci. USA 99, 3505–3510 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cotgreave IA, Gerdes R, Schuppe-Koistinen I, and Lind C S-glutathionylation of glyceraldehyde-3-phosphate dehydrogenase: role of thiol oxidation and catalysis by glutaredoxin. Methods Enzymol. 348, 175–182 (2002). [DOI] [PubMed] [Google Scholar]
- 61.Nulton-Persson AC Starke DW, Mieyal JJ, and Sweda LI Reversible inactivation of alpha-ketoglutarate dehydrogenase in response to alterations in the mitochondrial glutathione status. Biochemistry 42, 4235–4242 (2003). [DOI] [PubMed] [Google Scholar]
- 62.Taylor ER, Hurrell F, Shannon RJ, Lin TK, Hirst J, and Murphy MP Reversible glutathionylation of complex I increases mitochondrial superoxide formation. J. Biol. Chem 278, 19603–19610 (2003). [DOI] [PubMed] [Google Scholar]
- 63.Kil IS and Park JW Regulation of mitochondrial NADP+-dependent isocitrate dehydrogenase activity by glutathionylation. J. Biol. Chem 280, 10846–10854 (2005). [DOI] [PubMed] [Google Scholar]
- 64.Mohr S, Hallak H, de Boitte A, Lapetina EG, and Brüne B Nitric oxide-induced S-glutathionylation and inactivation of glyceraldehyde-3-phosphate dehydrogenase. J. Biol. Chem 274, 9427–94230 (1999). [DOI] [PubMed] [Google Scholar]
- 65.Chen CL, Zhang L, Yeh A, Chen CA, Green-Church KB, Zweier JL, and Chen YR Site-specific S-glutathiolation of mitochondrial NADH ubiquinone reductase. Biochemistry 46, 5754–5765 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Klatt P, Pineda Molena E, Perez-Sala D, and Lamas S Novel application of S-nitrosoglutathione-Sepharose to identify proteins that are potential targets for S-nitrosoglutathione-induced mixed-disulphide formation. Biochem J. 349, 567–578 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cotton NJ, Stoddard B and Parson WW Oxidative inhibition of human soluble catechol-O-methyltransferase. J. Biol. Chem 279, 23710–23718 (2004). [DOI] [PubMed] [Google Scholar]
- 68.Odin JA, Huebert RC, Casciola-Rosen L, LaRusso NF, and Rosen A Bcl-2-dependent oxidation of pyruvate dehydrogenase-E2, a primary biliary cirrhosis autoantigen, during apoptosis. J. Clin. Invest 108, 223–232 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yu CX, Li S, and Whorton AR Redox regulation of PTEN by S-nitrosothiols. Mol. Pharmacol 68, 847–854 (2005). [DOI] [PubMed] [Google Scholar]
- 70.Reynaert NL, van der Vilet A, Guala AS et al. Dynamic redox control of NF-kappaB through glutaredoxin-regulated S-glutathionylation of inhibitory kappaB kinase beta. Proc. Natl. Acad. Sci. USA 103, 13086–13091 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shelton MD, Kern TS, and Mieyal JJ Glutaredoxin regulates nuclear factor kappa-B and intercellular adhesion molecule in Muller cells: Model of diabetic retinopathy. J. Biol. Chem 282, 12467–12474 (2007). [DOI] [PubMed] [Google Scholar]
- 72.Ward NE, Stewart JR, Ioannides CG, and O’Brian CA Oxidant-induced S-glutathiolation inactivates protein kinase C-alpha (PKC-alpha): a potential mechanism of PKC isozyme regulation. Biochemistry 39, 10319–10329 (2000). [DOI] [PubMed] [Google Scholar]
- 73.Burgoyne JR, Madhani M, Cuello F, Charles RL, Brennan JP, Schröder E, Browning DD, and Eaton P Cysteine redox sensor in PKGIa enables oxidant-induced activation. Science 317, 1393–1397 (2007). [DOI] [PubMed] [Google Scholar]
- 74.Humphries KM, Deal MS and Taylor SS Enhanced dephosphorylation of cAMP-dependent protein kinase by oxidation and thiol modification. J. Biol. Chem 280, 2750–2758 (2005). [DOI] [PubMed] [Google Scholar]
- 75.Leonberg AK and Chai YC The functional role of cysteine residues for c-Abl kinase activity. Mol. Cell. Biochem 304, 207–212 (2007). [DOI] [PubMed] [Google Scholar]
- 76.Velu CS, Niture SK, Doneanu CE, Pattabiraman N, and Srivenugopal KS Human p53 is inhibited by glutathionylation of cysteines present in the proximal DNA-binding domain during oxidative stress. Biochemistry 46, 7765–7780 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Adachi T, Pinentel DR, Heilbeck T, Hou X, Lee YJ, Jiang B Ido Y, and Cohen RA S-glutathiolation of Ras mediates redox-sensitive signaling by angiotensin II in vascular smooth muscle cells. J. Biol. Chem 279, 29857–29862 (2004). [DOI] [PubMed] [Google Scholar]
- 78.Goch G, Vdovenko S, Kozlowska H, and Bierzynski A Affinity of S100A1 protein for calcium increases dramatically upon glutathionylation. FEBS J 272, 2557–2565 (2005). [DOI] [PubMed] [Google Scholar]
- 79.Adachi T, Weisbrod RM, Pimental DR, Ying J, Sharov VS, Schoneich C, and Cohen RA S-Glutathiolation by peroxynitrite activates SERCA during arterial relaxation by nitric oxide. Nat. Med 10, 1200–1207 (2004). [DOI] [PubMed] [Google Scholar]
- 80.Hidalgo C, Sanchez G, Barrientos G, and Aracena-Parks P A trans-verse tubule NADPH oxidase activity stimulates calcium release from isolated triads via ryanodine receptor type 1 S-glutathionylation. J. Biol. Chem 281, 26473–26482 (2006). [DOI] [PubMed] [Google Scholar]
- 81.Wang W, Oliva C, Li G, Holmgren A, Lillig CH, and Kirk KL Reversible silencing of CFTR chloride channels by glutathionylation. J. Gen. Physiol 125, 127–141 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Demasi M, Shringarpure R, and Davies KJ Glutathiolation of the proteasome is enhanced by proteolytic inhibitors. Arch. Biochem. Biophys 389, 254–263 (2001). [DOI] [PubMed] [Google Scholar]
- 83.Haendeler J Thioredoxin-1 and posttranslational modifications. Antioxid. Redox Signal. 8, 1723–1728 (2006). [DOI] [PubMed] [Google Scholar]
- 84.Noguera-Mazon V, Lemoine J, Walker O, Rouhier N Salvador A, Jacquot JP, Lancelin JM, and Krimm I Glutathionylation induces the dissociation of 1-Cys D-peroxiredoxin non-covalent homodimer. J. Biol. Chem 281, 31736–31742 (2006). [DOI] [PubMed] [Google Scholar]
- 85.Pastore A, Tozzi G, Gaeta LM et al. Actin glutathionylation increases in fibroblasts of patients with Friedreich’s ataxia: a potential role in the pathogenesis of the disease. J. Biol. Chem 278, 42588–42595 (2003). [DOI] [PubMed] [Google Scholar]
- 86.Toth A, Nickson P, Mandl A, Bannister ML, Toth K, and Erhardt P Endoplasmic reticulum stress as a novel therapeutic target in heart diseases. Cardiovasc. Hematol. Disord. Drug Targets 7, 205–218 (2007). [DOI] [PubMed] [Google Scholar]
- 87.Dinoto L, Deture MA, and Purich DL Structural insights into Alzheimer filament assembly pathways based on site-directed muta-genesis and S-glutathionylation of three-repeat neuronal Tau protein. Microsc. Res. Tech 67, 156–163 (2005). [DOI] [PubMed] [Google Scholar]
- 88.Unterberger U, Höftberger R, Gelpi E, Flicker H, Budka H, and Voigtländer T Endoplasmic reticulum stress features are prominent in Alzheimer disease but not in prion diseases in vivo. J. Neuropathol. Exp. Neurol 65, 348–357 (2006). [DOI] [PubMed] [Google Scholar]
- 89.Sampathkumar R, Balasubramanyam M, Sudarslal S, Rema M, Mohan V, and Balaram P Increased glutathionylated hemoglobin (HbSSG) in type 2 diabetes subjects with microangiopathy. Clin. Biochem 38, 892–899 (2005). [DOI] [PubMed] [Google Scholar]
- 90.Allen JR, Nguyen LX, Sargent KE, Lipson KL, Hackett A, and Urano F High ER stress in beta-cells stimulates intracellular degradation of misfolded insulin. Biochem. Biophys. Res. Commun 324, 166–170 (2004). [DOI] [PubMed] [Google Scholar]
- 91.Nanua S, Sajjan U, Keshavjee S, and Hershenson MB Absence of typical unfolded protein response in primary cultured cystic fibrosis airway epithelial cells. Biochem. Biophys. Res. Commun 343, 135–143 (2006). [DOI] [PubMed] [Google Scholar]
- 92.Craghill J, Cronshaw AD and Harding JJ The identification of a reaction site of glutathione mixed-disulphide formation on gammaS-crystallin in human lens. Biochem J. 379, 595–600 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shinohara T, Ikesugi K, and Mulhern ML Cataracts: Role of the unfolded protein response. Med. Hypotheses 66, 365–370 (2006). [DOI] [PubMed] [Google Scholar]
- 94.Sevier CS, Qu H, Heldman N, Gross E, Fass D, and Kaiser CA Modulation of cellular disulfide bond formation and the ER redox environment by feedback regulation of ERO1. Cell 129, 333–344 (2007). [DOI] [PubMed] [Google Scholar]