Abstract
Reactive oxygen species (ROS) and oxidative damage to biomolecules have been postulated to be involved in the causation and progression of several chronic diseases, including cancer and cardiovascular diseases, the two major causes of morbidity and mortality in Western world. Consequently dietary antioxidants, which inactivate ROS and provide protection from oxidative damage are being considered as important preventive strategic molecules. Carotenoids have been implicated as important dietary nutrients having antioxidant potential, being involved in the scavenging of two of the ROS, singlet molecular oxygen (1O2) and peroxyl radicals generated in the process of lipid peroxidation. Carotenoids are lipophilic molecules which tend to accumulate in lipophilic compartments like membranes or lipoproteins. Chronic ethanol consumption significantly increases hydrogen peroxide and decreases mitochondrial glutathione (GSH) in cells overexpressing CYP2E1. The depletion of mitochondrial GSH and the rise of hydrogen peroxide are responsible for the ethanol-induced apoptosis. Increased intake of lycopene, a major carotenoid in tomatoes, consumed as the all-trans-isomer attenuates alcohol induced apoptosis in 2E1 cells and reduces risk of prostate, lung and digestive cancers. Cancer-preventive activities of carotenoids have been associated as well as with their antioxidant properties and the induction and stimulation of intercellular communication via gap junctions which play a role in the regulation of cell growth, differentiation and apoptosis. Gap junctional communication between cells which may be a basis for protection against cancer development is independent of the antioxidant property.
Keywords: Tetraterpenes, Carotenoids, Lycopene, Antioxidants, Cardiovascular, Cancers
1. Introduction
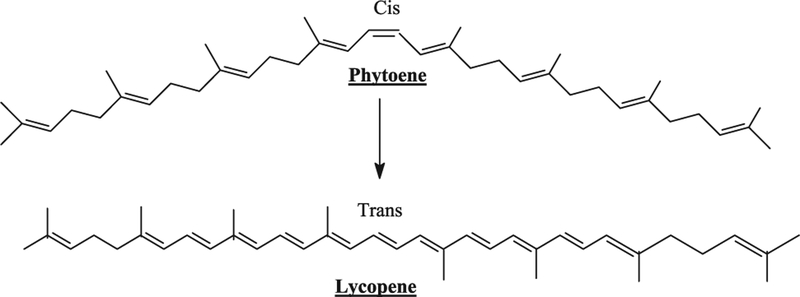
Carotenoids belong to the tetraterpenes family and are represented by more than 600 known natural sructural variants. Carotenoids are synthesized in plants, fungi, bacteria and algae whereas in animals and human they are not and are incorporated from their diet. The formation of the tetraterpene skeleton (phytoene), results from a loss of a proton, generating a double bond in the center of the molecule. Carotenoids are divided in two classes, carotenes containing only carbon and hydrogen atoms and oxocarotenoids (xanthophylls) which carry at least one oxygen atom. According to the number of double bonds, several cis/trans (E/Z) configurations are possible for a given molecule. In bacteria the double bond has trans-configuration whereas in plants and fungi this double bond has the cis-configuration and an additional isomerization step is involved to change the configuration of the central double bond giving eventually lycopene.
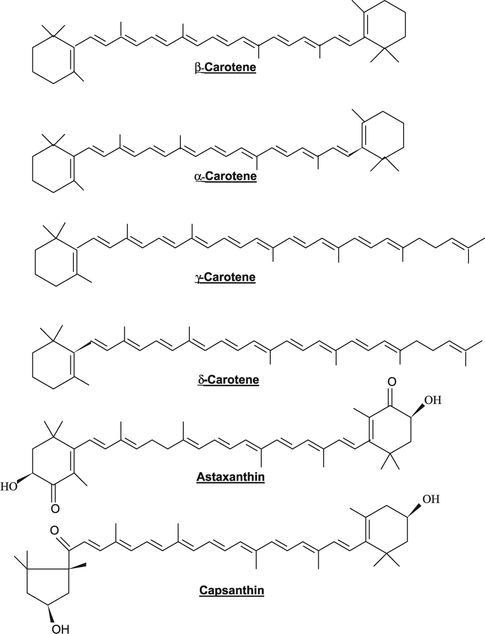
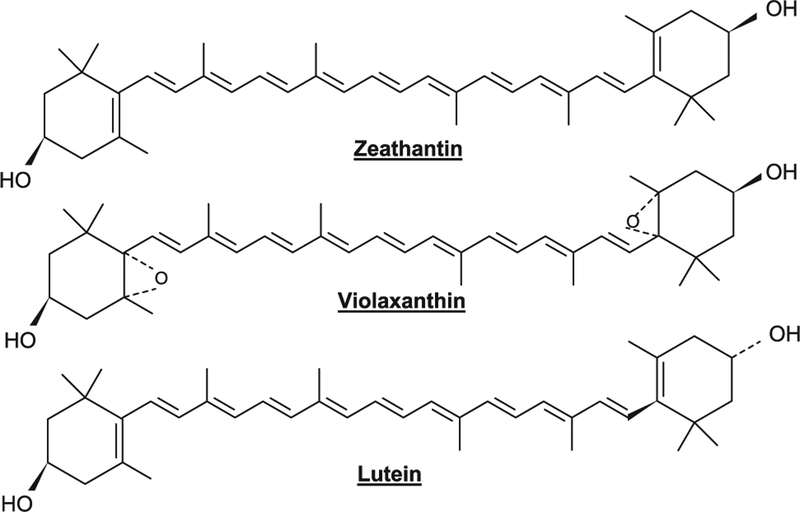
Lycopene is an open chain hydrocarbon containing 11 conjugated and 2 non-conjugated double bonds arranged in a linear array (Fig. 1). During chemical reactions, light or thermoenergy, these bonds can undergo isomerization from trans to mono or poly-cis isomers and the most commonly identified are all trans, 5-cis, 9-cis, 13-cis and 15-cis isomeric forms of lycopene. α-Carotene has a β-ring at one end of the chain and an ɛ-type at the other. γ-Carotene, a precursor of β-carotene and δ-carotene, a precursor of α-carotene are carotenoids where only one end of the chain has become cyclized (Fig. 2). Xanthophylls, oxygenated, green leaf carotenoids such as zeathanthin, lutein and violaxanthin are also widely distributed (Fig. 3). Carotenoids are natural pigments contributing to yellow, orange (yellow and orange fruits and vegetables contain hydrocarbon carotenes with substantial levels of cryptoxanthins and xanthophylls) and red pigmentations to plant tissues (red fruits and vegetables contain mainly lycopene). The green vegetables had high contents of both xanthophylls and hydrocarbon carotenes. Lycopene is a characteristic lipophilic red pigment in ripe tomato fruit (Lycopersicon esculente; Solanaceae). Since it lacks β-ionone ring structure, it lacks provitamin A activity. The orange color of carrots (Daucus carota; Umbelliferae/Apiaceae) is caused by β-carotene, widespread in higher plants and the brilliant red pigment of pepper (Capsicum annuum; Solanaceae) is due to capsanthine. Astaxanthine, is commonly found in marine animals and is responsible for the pink/red coloration of crustaceans. Shellfish and fish such as salmon are unable to synthesize carotenoids; hence, astaxanthin is produced by modification of plant carotenoids obtained in the diet. Carotenoids function along with chlorophylls in photosynthesis and serve as important protectants for plants and algae against photooxidative damage, quenching toxic oxygen species. Some herbicides (bleaching herbicides) act by inhibiting carotenoids biosynthesis and the unprotected plant is subsequently killed by photooxidation.
Fig. 1.
In plants and fungi, the central double bond of phytoene has the cis-configuration and an isomerization step is involved to change the central double bond to trans configuration which is subsequently metabolized to lycopene.
Fig. 2.
Carotenoids are natural pigments contributing to yellow, orange and red pigmentations found in plant tissues. Lycopene (see Fig. 1) is the characteristic carotenoid pigment in ripe tomato fruit (Lycopersicon esculente; Solanaceae). The orange color of carrots (Daucus carota; Umbelliferae/Apiaceae) is caused by β-Carotene, a widespread in higher plants. The brillant red pigment of peppers (Capsicum annuum; Solanaceae) is due to capsanthine and those commonly found in marine animals and is responsible for the pink/red coloration of crustaceans, shellfish and fish such as salmon is caused by astaxanthin. Astaxanthin is produced by modification of plant carotenoids obtained in the diet.
Fig. 3.
Xanthophylls, oxygenated carotenoids, such as zeathanthin, lutein and violaxanthin are widely distributed in green leaf carotenoids.
In animals and human, carotenoids particularly β-carotene and lycopene, play a role in the protection against photooxidative processes by acting as singlet molecular oxygen and peroxyl radicals scavengers and can interact synergistically with other antioxidants. They have been implicated in the inhibition of cancer cells in vitro [1–3], in animal models [4–8] and in human, as important dietary phytonutrients having cancer preventive activity for lung, colon, breast and prostate cancer [9–11].
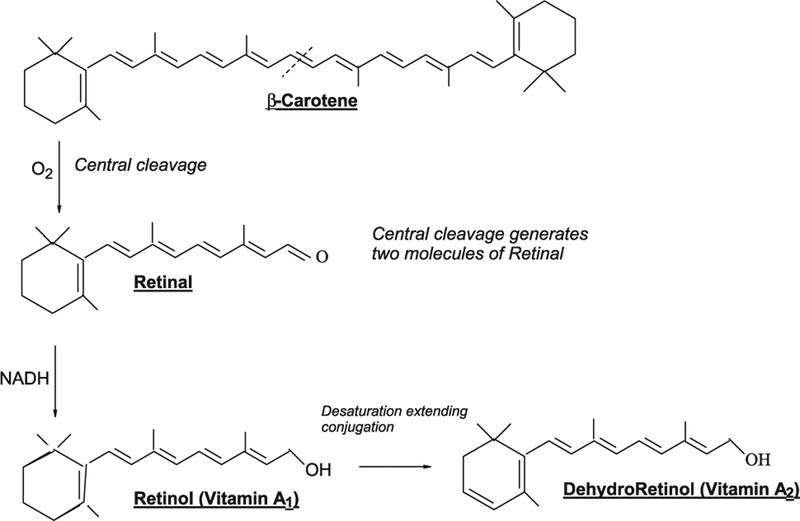
The A group of vitamins are important metabolites of carotenoids. Vitamin A1 (retinol) has a diterpene structure but it is derived in mammals by oxidative metabolism of tetraterpenoid, mainly β-carotene, taken in the diet. Cleavage occurs in the mucosal cells of the intestine and is catalysed by an O2-dependent dioxygenase, probably via an intermediate peroxide. Vitamin A2 (dehydroretinol) is an analog of retinol containing a cyclohexadiene ring system. Retinol and its derivatives are found only in animal products and these provide some of our dietary need (Fig. 4).
Fig. 4.
Vitamin A1 (retinol) is derived in mammals by oxidative metabolism of β-Carotene, taken in the diet. Cleavage is catalysed by an O2-dependent dioxygenase, probably via an intermediate peroxide. Vitamin A2 (dehydroretinol) is an analog of retinol containing a cyclohexadiene ring system. Retinol and its derivatives are found only in animal products.
2. Bioavailability of carotenoids
Among the 600 known carotenoids in nature, only about 20 are found in human plasma and tissues. Lycopene is the most predominant carotenoid in human plasma and has a half life of about 2–3 days. Owing to its lipophilic nature, lycopene was found to concentrate in LDL and VLDL fractions and not in HDL fraction of the serum [29]. The strong association with plasma cholesterol is most likely due to the fact that lycopene is predominantly transported in LDL which carries the bulk of cholesterol in the plasma [30–32].
Lycopene is a non-provitamin A and one of the major carotenoids in western diets accounting for more than 50% of carotenoids in human serum [12]. Tomato products (juice, ketchup, soup, sauce) are the major contributors of lycopene in the diet [16–18]. While lycopene is concentrated in tomatoes its content varies from 0.85 mg to 13.6 mg/100 g acording to fruit ripening and to the tomato variety. In raw tomatoes, all-trans is the predominant isomeric form of lycopene but isomerization to cis occurs during cooking, food processing or storage [13,14]. trans-lycopene and trans-β-carotene were stable for up to 3 years of storage in liquid frozen serum at −80 °C [15]. Consumption of tomato products with olive oil but not with sunflower oil improves the antioxidant activity of the plasma [19]. Other sources rich in lycopene include watermelon, pink grapefruit, pink guava and papaya [20,21]. Lycopene is synthesized via a series of four desaturation reactions from phytoene. These reactions occur in the plastids of higher plants and are catalyzed by two membrane bound desaturases [22]. Lycopene itself is cyclized to ɛ and β-carotenes both of which are precursors of xanthophylls, found in the photosynthetic apparatus. The genes for virtually all these enzymes have been cloned and can now be used for plant transformations, particularly to elevate the level of lycopene in tomatoes [23].
Animals maintained on lycopene diet, consumed on average 142 μg of lycopene per day of which 104 μg was absorbed by the body (which corresponds to 73% net dietary uptake) calculated as the difference between lycopene intake and fecal output [24]. Ingested carotenoids including lycopene are incorporated into dietary lipid micelles, absorbed into the intestinal mucosal lining via passive diffusion. They are incorporated into chylomicrons and released into lymphatic system for transport to the liver. Lycopene accumulates in hepatocytes and to a lesser extent in spleen. It tends to accumulate in tissues such as testes, adrenal glands and prostate [25] Carotenoids are transported by the lipoproteins into the plasma for distribution to different organs [26–28].
Although trans-lycopene constitutes the predominant isomer in food sources, in human plasma, 50% of the total lycopene has been found as cis isomers [25]. Whether this is due to in vivo isomerization or preferential absorption of cis-lycopene is still unclear. Very little is known about in vivo metabolism of lycopene. A number of oxygenated metabolites have been found in plasma and tissues such as 2,6-cyclolycopene-1,5-diols [33]. It can undergo in-vivo oxidation to form epoxides that can be converted to 5,6-dihydroxy-5,6-dihydrolycopene [34]. These oxygenated lycopenes are products of in vivo oxidation and may have physiological roles per se.
Carotenoids are lipophilic molecules which tend to accumulate in lipophilic compartments, like membranes or lipoproteins. They are often solubilized in organic solvents such as tetrahydrofuran (THF) or dimethylsulfoxide (DMSO). However, an uncontrolled precipitation process occurs upon addition of these solutions to aqueous media. In this process, carotenoid crystals are formed with a non-controllable particle size. The solubility and uptake of these large crystals in the cells is quite limited and there is almost no protection against chemical degradation. Alternative ways of delivering lipid-soluble compounds include micelles, microemulsions, nanoparticles, water-dispersible beadlets, artificial liposomes, or specialized formulations, each of which has an influence on the uptake and stability of the compound [35–38]. Synthetic lycopene is a red crystalline powder that is soluble in fat and most organic solvents, but insoluble in water. It is sensitive to light and oxygen and is not suitable for commercial use. The market material contains 5–10% lycopene in beadlet formulations [39]. It has a low order of acute toxicity and no teratogenic effects were noted in rats with 1000 mg/kg body weight/day.
The high concentration of lycopene in blood correlates with reduced risk of prostate cancer [40–43], digestive tract cancers [44–46], pancreatic cancer [47] cervical intraepithelial neoplasia [48] and myocardial infarction [17,49]. It is assumed that these effects are associated to its high antioxidant activity and singlet oxygen quenching capacity [29]. In addition, lycopene levels are shown to be inversely associated with age [50,51]. Blood lycopene level may differ from blood β-carotene level. In smokers and in alcohol consumers the blood level of β-carotene is lower than that of non-smokers [52–55,57] which does not seem to be the case for lycopene [50,51,56,57].
3. Cellular and molecular processes
3.1. The carotenoid antioxidative effects
Oxidative stress has been widely postulated to be involved in the causation and progression of several chronic diseases. ROS are generated endogenously through normal metabolic activity, life style activities, and diet. They react with critical cellular biomolecules such as lipids, proteins and DNA and initiate events that lead to increased risk of chronic disease such as cancer, cardiovascular disease, and osteoporosis. Consequently, dietary antioxidants which inactivate ROS and provide protection from oxidative damage are being considered as important preventive strategic molecules [58–63]. The total antioxidant capacity of plasma is due to the relative concentration in antioxidant compounds and to their synergism. In particular an interaction exists between aqueous and lipophilic antioxidants in defending lipoproteins against oxidative damage [64,65]. Carotenoids are most likely involved in the scavenging of two ROS, singlet molecular oxygen (1O2) and peroxyl radicals. They are also effective deactivators of electronically excited sensitizer molecules which are involved in the generation of radicals and singlet oxygen [66]. Dietary carotenoids protect human lymphocytes from damage by singlet oxygen 1O2, and lower the risk for several degenerative disorders, including various types of cancer, cardiovascular or ophtalmological diseases. The interaction of carotenoids with 1O2 depends on physical quenching which involves direct energy transfer between both molecules. The efficacy of carotenoids for physical quenching is related to the number of conjugated double bonds present in the molecule which determines their lowest triplet energy level. β-Carotene, zeaxanthine cryptoxanthin, and α-carotene, which are detected in human serum and tissues belong to the group of highly active quenchers of 1O2. The most efficient carotenoid is the open ring carotenoid lycopene, which contributes up to 30% to total carotenoids in humans [67–70]. Scavenging of peroxyl radicals generated in the process of lipid peroxidation interrupts the reaction sequence which finally leads to damage in lipophilic compartments. Moreover, induction of phase II enzymes, conjugate reactive electrophiles and act as indirect antioxidants, achieving protection against a variety of carcinogens in animals and humans. Transcriptional control of the expression of these enzymes is mediated at least in part through antioxidant responsive elements (ARE). The transcription factor Nrf2, which binds to ARE, appears to be essential for the induction of glutathione-S-transferases (GSTs), NAD-(P)H:quinone oxidoreductase (NQO1) as well as the thiol-containing reducing factor, thioredoxin [71,72]. In rodents, lycopene resulted in increased levels of GSH and the phase II GSTs [73,74]. Canthaxanthin and astaxanthin (not lutein and lycopene) were active in inducing phase II metabolizing enzymes, p-nitrophenol-UDP-glucuronosyl transferase and NQO1 [73,74]. Lycopene was reported to be more effective than β-Carotene in cell protection against hydrogen peroxide (H2O2) and nitrogen dioxide radical (NO•2) components that can arise from cigarette smoke [75–79]. Clinical trials on the incidence and mortality of cancer and cardiovascular disease in smokers have shown that β-Carotene supplemental is either non-protective or even detrimental [80,81]. One of the reasons for this is that the antioxidant activity of carotenoids depends on the oxygen tension present in the system. At low partial pressures of oxygen such as those found in most tissues under physiological conditions, β-Carotene was found to inhibit oxidation. The initial antioxidant activity of β-Carotene is followed by a prooxidant action at high oxygen tension. Thus, in thymocytes, β-Carotene is an antioxidant at low oxygen pressure but a pro-oxidant at high oxygen concentrations [82]. In addition, lycopene may have also prooxidant activities depending on the type of oxidants used. Thus, it can be either antioxidant or prooxidant at normal oxygen tension in human foreskin fibroblasts cells (Hs68) [83]. The prooxidant effects of β-Carotene may be related to adverse effects observed under the supplementation of high doses of β-Carotene [84,85]. However, it has been suggested that some of the degradation products of β-Carotene rather than β-Carotene itself may be prooxidant or procarcinogenic [86,87]. Cu(II)-initiated LDL oxidation was inhibited in purified LDL by β-Carotene but not by lycopene or lutein [88]. Furthermore, in vitro enrichment of LDL with β-Carotene inhibited endothelial cell-mediated oxidation, while enrichment with lycopene or lutein enhanced cell-mediated oxidation [90]. In liposomes oxidized by peroxyl radicals, the ineffectiveness of lycopene was probably due to the rapid degradation of β-Carotene [89]. However, it remains unclear how β-Carotene and lycopene may enhance lipid peroxidation. In smokers it has been suggested that β-Carotene forms radical cations by regenerating a vitamin E radical [91,92]. Vitamin C plays an important role in reducing the radical cation of β-Carotene [91,92], however, because the high lipophilicity of β-Carotene and its location at the interior membrane, the hydrosoluble vitamin C cannot efficiently reduce the carotene radicals. Another possibility is that β-Carotene may react with lipid peroxyl radicals and form radical cations, which can then be reduced by vitamin E. Vitamin C in turn can reduce vitamin E radical to regenerate vitamin E to prevent the damaging effects of β-Carotene cation [93]. Analysis of the antioxidant status of blood in rats, revealed that some antioxidant enzymes, such as superoxide dismutase, glutathione reductase and glutathione peroxidase can be induced by lycopene [94]. Recently, it has been reported that lycopene prevents cataractogenesis in vivo and in vitro by virtue of its antioxidant properties [95].
4. Effect on gap junctional communication
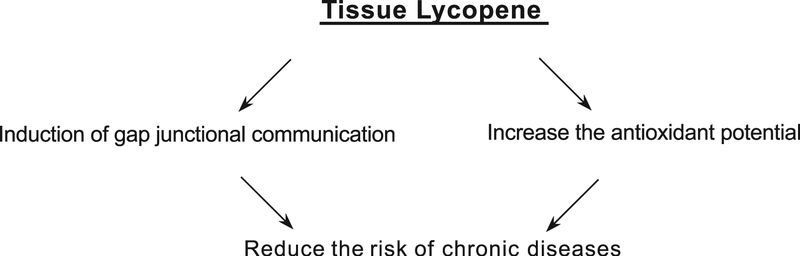
Gap junctions are cell-to-cell channels which enable connecting cells to exchange low-molecular weight compounds like nutrients and signaling molecules [96]. One feature of carcinogenesis is the loss of gap junctional communication (GJC) [97]. Induction of intercellular communication via gap junctions can be achieved with carotenoids and retinoids and is correlated with inhibited cell growth of chemically transformed cells [98]. Non-tumorous cells communicate via GJC, whereas most tumor cells have dysfunctional homologous or heterologous GJC [99]. Carotenoids, in addition to their antioxidant properties, stimulate GJC in a differential and dose dependent manner [100–102]. Stimulatory effects on GJC were described for lycopene which also affects cell growth [100–103]. The biochemical mechanism underlying the activation of GJC is not yet well understood. Carotenoids have been reported to react with almost any radical species. The products of such reactions are frequently short-lived radical species. In the majority of interactions with radicals, carotenoids break down to degradation products similar to what is seen with oxidative degradation [104]. There is evidence from cell culture studies that central cleavage products of carotenoids are ultimately active components triggering GJC. Oxidation products such as the dialdehyde 2,7,11-trimethyl-tetradecahexaene-1,14-dial obtained from the oxidation of lycopene, stimulates GJC in WB-F344 cells, rat liver epithelial cells [104] but other oxidation products formed upon chemical oxidation of lycopene were not active. Lycopene, increases GJC between cells and enhances the expression of connexin 43, a gene encoding major gap junction protein, and thereby upregulated GJC and acts as anti-carcinogen. However, its effect is less pronounced than β-Carotene or canthaxanthin [105–107]. Other mechanisms including posttranslational modification, protein trafficking or changes in pH or calcium levels may be also relevant in the stimulation of GJC [108–110]. Loss of GJC may be important for malignant transformation, and its restoration may reverse the malignant process [99,100]. The GJC ability of lycopene and its singlet oxygen quenching abilities or anti-oxidant properties are independent of each other (Fig. 5) [101,102].
Fig. 5.
Cellular processes of lycopene.
5. Role of carotenoids in human health
Since carotenoids are highly hydrophobic, their interaction with ROS is expected to occur in a lipophilic environment, such as in cell membranes and lipoprotein components. The carotenoids found in cells and tissues are selectively absorbed by membranes depending on the structural carotenoid features (size, shape and polarity), as well as on membrane characteristics (composition and fluidity) [111]. These properties determine the incorporation yield and the carotenoid’s ability to fit into the membrane bilayer [28]. Controversial data are reported about location and distribution of carotenoids in the membrane bilayers. Specific interactions with membranes can occur in the presence of non-polar carotenoids (β-Carotene and lycopene) or polar carotenoids (zeaxanthin and lutein). The antioxidant capacity of these two classes of carotenoids proved to be dependent on their different location in the bilayer. While β-Carotene and lycopene were able to quench radicals in the hydrophobic part of the membrane, zeaxanthin was effective as an antioxidant in the polar region, exposed to an aqueous environment. Polar carotenoids can regulate the membrane fluidity in a way similar to cholesterol, although they locate differently within the lipid bilayer membrane [112–114].
6. Lycopene effects in alcohol-induced liver injury
Oxidative stress has been implicated in the pathogenesis of alcohol-induced liver injury [115]. Induction of cyto-chrome P4502E1 (CYP2E1) by ethanol is one of the main mechanisms through which ethanol generate oxidative stress [116]. Several pathways have been shown to contribute to the pathogenesis of alcoholic liver disease [117]. One of these is the induction of CYP2E1 by ethanol with the generation of oxidative stress and lipid peroxidation upon oxidation of ethanol [118]. When rats were fed ethanol with diets containing polyunsaturated fatty acid (PUFA), this toxicity is increased possibly because the presence of PUFA and enhanced lipid peroxidation after induction of CYP2E1 by ethanol [119]. Oxidative stress generated in the HepG2 human hepatic cells transfected with CYP2E1 cDNA (2E1 cells), increased H2O2 production that is associated with depletion of mitochondrial GSH are responsible for the ethanol induced apoptosis. In 2E1 cells that kept some liver characteristics, without expressing cytochrome P450 activity, lycopene significantly attenuates alcohol-induced apoptosis [120]. Moreover, arachidonic acid (AA), is an important constituent of membrane phospholipids. The double bonds found in AA of the cellular lipids are available targets for ROS. After conversion to prostaglandins (PG) leukotrienes and generation of oxygen-derived free radicals, it mediates a variety of pathological processes [121–123]. Because of the oxidative stress, AA is toxic to HepG2 and 2E1 cells. Although, lycopene at 10 μM attenuated AA toxicity in HepG2, the most significant effect was observed in 2E1 cells, suggesting that lycopene acts as an anti-oxidant thereby preventing the severe oxidative stress induced by AA in 2E1 cells [124].
7. Photoprotective potential of carotenoids
Photooxidative processes play a role in the pathobiochemistry of light exposed tissues including the eye and the skin. Age-related macular degeneration which affects the macula lutea of the retina, the area of maximal visual acuity is a major cause for irreversible blindness among the elderly in the Western world [125]. Macular pigments protect against the photooxidative processes which may be related to the antioxidant activities of the macular carotenoids and/or to their light filtering effects [126]. While lutein and zeaxanthin are responsible for coloration of the macula lutea, lycopene, α-carotene or β-Carotene are not found in this tissue.
Ultraviolet (UV) irradiation is associated with oxidative processes involved in photoaging, which induce photodamage and lead to premature skin aging [127]. Following UV irradiation, a cascade of genes are induced, including metal-loproteinase 1 (MMP-1) [128] and heme oxygenase 1 (HO-1) [129,130], respectively, the interstitial collagenase and the oxidative stress marker gene. Singlet oxygen is strongly implicated in the induction of these two genes [131,132]. The physiological consequence of increased MMP-1 expression is increased skin collagen degradation and subsequent formation of wrinkles [127,133]. Protection against MMP-1 UV-induction is obtained with low concentrations of vitamin C, vitamin E or carnosic acid. In contrast, β-Carotene or lycopene stimulated MMP-1 expression. This effect was completely suppressed when vitamin E was included in a nanoparticle formulation [134]. Thus, depending on the experimental conditions, such as oxygen tension, carotenoid concentration and interactions with other antioxidants influencing the cellular and molecular responses, β-Carotene and lycopene can act as as antioxidants or prooxidants [135,136]. HO-1, a general stress gene is induced following UVA radiation and is highly implicated in skin aging [129,130]. UV-induced HO-1 mRNA expression was not affected by vitamin C, vitamin E or carnosic acid at the same concentrations that were effective for inhibition of MMP-1 expression. HO-1 expression was stimulated by β-Carotene when given alone, but the combination of β-Carotene, vitamin C and vitamin E suppressed this induction. Physiologically HO-1 represents an early response to stress and is induced in a similar way to heat shock proteins as part of the cellular defense to environmental and chemical stress. Thus, although HO-1 mRNA induction indicates higher oxidative stress, it is not clear whether its expression should be stimulated or inhibited.
While topical application of sunscreen provides a barrier protection to the skin epithelium, protection of the more profound dermal layers may be offered by dietary antioxidants [137]. Protection by β-Carotene is enhanced when combined with vitamin E [138]. Ingestion of tomato paste daily for 10 weeks, protected against UV light-induced erythema on the dorsal skin [139]. Lycopene present in skin can act as antioxidant, protecting against UV radiation. However, it is quickly depleted from skin upon exposure to solar radiation [140] and undergoes oxidative or enzymatic cleavage to form apo-carotenoids [141]. Therefore, it has been suggested that lycopene should be delivered as powdered nanoparticles together with vitamin C and or vitamin E [134].
8. Carotenoids and cancer
Before malignancy detection, high blood levels of insulin-like growth factor (IGF-1) predicts an increased risk of breast, prostate, colo-rectal and lung cancers [142–145]. In mammary cancer cells, lycopene treatment markedly reduced IGF-I stimulation of both tyrosine phosphorylation of insulin receptor substrate and DNA binding capacity of the AP-1 transcription factor [146]. These effects were associated with an increase in membrane-associated IGF-binding proteins (IGFBPs). Moreover, lycopene induced delay in progression through G1 and S phases [3,146]. A similar effect was demonstrated with α-carotene in GOTO human neuroblastoma cells [147] without apoptotic or necrotic cell death. Cell cycle transition through a late G1 is governed by a retinoblasma protein pathway (pRb) [148], a tumor suppressor that prevents premature G1/S transition via physical interaction with transcription factors of the E2F family. The activity of pRb is regulated by an assembly of cyclins, cyclin dependent kinases (Cdks) and Cdk inhibitors. Cdk activity is modulated in both a positive and a negative manner by cyclins and Cdk inhibitors respectively. The D-type cyclins are the main elements acting as growth factor sensors [149]. Cyclin D1 is an oncogene expressed in many breast cancer cells as well as in primary tumors [150].
The anticancer activity of carotenoid derivatives is mediated by the activation of retinoid receptors [151]. However, it can also be mediated by several cleavage products of β-Carotene [152] and lycopene [146]. Lycopene was shown to be a powerful inhibitor of endometrial, mammary (MCF-7) and lung (NCI-H226) human cancer cells proliferation and suppress insulin-like growth factor-I-stimulated growth [153]. Moreover, it inhibits growth and development of KB-1 human oral tumor cells [154] and C-6 glioma cells transplanted into rats [155].
An early epidemiological study on elderly Americans indicated that high intake of tomatoes was associated with a significant reduction of mortality from cancers. The estimated intakes of total carotenoid, β-Carotene, α-carotene, lutein and β-cryptoxanthin were not associated with the risk factor. Higher estimated lycopene intake was inversely related to risk of prostate cancer [156]. Among all dietary carotenoid intake and serum carotenoid concentrations, only lycopene showed an inverse association with the risk of cervical intraepithelial neoplasia as compared to control [157]. Serum lycopene levels were also found to be inversely related to the risk of bladder cancer [158], and breast cancer [159]. Lower serum lycopene levels were also reported in human immunodeficiency virus (HIV) positive women and children [160,161]. An inverse relationship between dietary intake of lycopene-rich food and the risk of prostate cancer has been reported while β-Carotene, α-carotene, lutein and β-cryptoxanthin did not show the same correlation [156]. However, lycopene alone is not a potent inhibitor of prostate carcinoma cell proliferation. The simultaneous addition of lycopene together with α-tocopherol, at physiological concentrations (less than 1 μM and 50 μM, respectively) resulted in a strong inhibitory effect of prostate carcinoma cell proliferation. The effect of lycopene with α-tocopherol was synergistic and was not shared by β-tocopherol, ascorbic acid and probucol [2].
9. Carotenoids in the prevention of cardio-vascular diseases
Coronary heart disease is the major cause of morbidity and mortality in the Western world. There is extensive evidence that oxidatively modified low-density lipoproteins (LDL) are involved in the initiation and promotion of atherosclerosis [61]. Cigarette smoking is a well-known risk factor for coronary atherosclerosis [162]. Atherogenesis may be due to foam cell production by the introduction of a source of free radicals that cause LDL oxidation [163]. Thus, protection from LDL oxidation by antioxidants may lead to protection against human coronary heart disease. Smokers have significantly lower blood levels of β-Carotene than non-smokers [50–55,164], this does not seem to be the case for lycopene [56,57,164,165]. Since β-Carotene and lycopene are transported primarily in LDL, it has been suggested that they are in prime position to protect LDL from oxidation [166]. In a multicenter-case-control study of antioxidant nutrients in adipose tissue and risk of myocardial infarction, it was concluded that lycopene and not β-Carotene contribute to the protective effect. The protective potential of lycopene was maximum among individuals with highest polyunsatu-rated fat stores [49,167].
In conclusion, the beneficial effects of carotenoids in human disease prevention have been widely reported particularly in alcoholic liver injury, cancer, cardiovascular diseases or as photoprotective. However, it remains to identify and characterize the active carotenoid derivatives and to determine whether this potential is due to a synergistic action of various carotenoids and antioxidant micronutrients.
References
- [1].Levy J, Bosin E, Feldman B, Giat Y, Miinster A, Danilenko M, et al. Lycopene is a more potent inhibitor of human cancer cell proliferation than either α-carotene or β-Carotene. Nutr Cancer 1995;24:257–67. [DOI] [PubMed] [Google Scholar]
- [2].Pastori M, Pfander H, Boscoboinik D, Azzi A. Lycopene in association with α-tocopherol inhibits at physiological concentrations proliferation of prostate carcinoma cells. Biochem Biophys Res Commun 1998;250:582–5. [DOI] [PubMed] [Google Scholar]
- [3].Amir H, Karas M, Giat J, Danilenko M, Levy R, Yermiahu T, et al. Lycopene and 1,25-dihydroxyvitamin-D3 cooperate in the inhibition of cell cycle progression and induction of differentiation in HL-60 leukemic cells. Nutr Cancer 1999;33:105–12. [DOI] [PubMed] [Google Scholar]
- [4].Nagasawa H, Mitamura T, Sakamoto S, Yamamoto K. Effect of lycopene on spontaneous mammary development in SHN virgin mice. Anticancer Res 1995;15:1173–8. [PubMed] [Google Scholar]
- [5].Narisawa T, Fukaura Y, Hasebe M, Ito M, Aizawa R, Murakoshi M, et al. Inhibitory effects of natural carotenoids, α-carotene, β-Carotene, lycopene and lutein on colonic aberrant crypt foci formation in rats. Cancer lett 1996;107:137–42. [DOI] [PubMed] [Google Scholar]
- [6].Giron YE, Rise M, Levy J. Effects of lycopene enriched tomato oleoresin on 7,12-dimethyl-benz[a]anthracene-induced rat mammary tumors. Cancer Detect Prevent 1997;21:118–23. [PubMed] [Google Scholar]
- [7].Okajima E, Tsutsumi M, Ozono S, Akai H, Denda A, Nishino H, et al. Inhibitory effect of tomato juice on rat urinari bladder carcinogenesis after N-butyl-N-(4-hydroxybutyl)nitrosamine initiation. Jpn J Cancer Res 1998;89:22–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kim YC, Araki S, Kim DJ, Park CB, Takasuka N, Baba-Toriyama H, et al. Chemopreventive effects of carotenoids and curcumins on mouse colon carcinogenesis after 1,2-dimethylhydrazine initiation. Carcinogenesis 1998;19:81–5. [DOI] [PubMed] [Google Scholar]
- [9].Van Poppel G Carotenoids and cancer: an update with emphasis on human intervention studies. Eur J Cancer 1993;29A:1335–44. [DOI] [PubMed] [Google Scholar]
- [10].Van Pooppel G, Gooldbohm RA. Epidemiologic evidence for β-Carotene and cancer prevention. Am J Clin Nutr 1995;62:1393S–402S. [DOI] [PubMed] [Google Scholar]
- [11].Giovannucci E Tomatoes tomato-based products, lycopene and cancer: review of the epidemiologic literature. J Natl Cancer Inst 1999;91:317–31. [DOI] [PubMed] [Google Scholar]
- [12].Gerster H The potential role of lycopene for human health. J Am Coll Nutr 1997;16:109–26. [DOI] [PubMed] [Google Scholar]
- [13].Tonucci LH, Holden JM, Beecher GR, Khachik F, Davis CS, Mulokozi G. Carotenoid contents of thermally processed tomato-based food products. J Agric Food Chem 1995;43:579–86. [Google Scholar]
- [14].Mangels AR, Holden JM, Beecher GR, Forman MR, Lanza E. Carotenoid contents of fruits and vegetables: an evaluation of analytical data. J Am Diet Assoc 1993;93:284–96. [DOI] [PubMed] [Google Scholar]
- [15].Thomas JB, Duewer DL, Kline MC, Sharpless KE. The stability of retinol, α-tocopherol, trans-lycopene, and trans β-Carotene in liquid-frozen and lyophilized serum. Clinica Chimica Acta 1998;276:75–87. [DOI] [PubMed] [Google Scholar]
- [16].Rao AV, Agarwal S. Role of lycopene as antioxidant carotenoid in the prevention of chronic diseases: a review. Nutr Res 1999;19:199–203. [Google Scholar]
- [17].Clinton SK. Lycopene chemistry, biology and implications for human health and disease. Nutr Rev 1998;56:35–51. [DOI] [PubMed] [Google Scholar]
- [18].Hadley CW, Clinton SK, Schwartz SJ. The consumption of processed tomato products enhances plasma lycopene concentrations in association with a reduced lipoprotein sensitivity to oxidative damage. J Nutr 2003;133:727–32. [DOI] [PubMed] [Google Scholar]
- [19].Lee A, Thurnham DI, Chopra M. Consumption of tomato products with olive oil but not sunflower oil increases the antioxidant activity of plasma. Free Rad Biol Med 2000;29:1051–5. [DOI] [PubMed] [Google Scholar]
- [20].Nguyen ML, Schwartz SJ. Lycopene: chemical and biological properties. Food Technol 1999;53:38–45. [Google Scholar]
- [21].Edwards AJ, Vinyard BT, Wiley ER, Brown ED, Collins JK, Perkins-Veazie P, et al. Consumption of watermelon juice increases plasma concentrations of lycopene and β-Carotene in humans. J Nutr 2003; 133:1043–50. [DOI] [PubMed] [Google Scholar]
- [22].Harker M, Hirschberg J. Molecular biology of carotenoid biosynthesis in photosynthetic organisms. Methods Enzymol 1998;297:244–63. [Google Scholar]
- [23].Bramley PM. The regulation and genetic manipulation of carotenoid biosynthesis in tomato fruit. Pure Appl Chem 1997;69:2159–62. [Google Scholar]
- [24].Jain CK, Agarwal S, Rao AV. The effect of dietary lycopene on bioavailability, tissue distribution, in vivo antioxidant properties and colonic preneoplasia in rats. Nutr Res 1999;19:1383–91. [Google Scholar]
- [25].Clinton SK, Emenhiser C, Schwartz SJ, Bostwick DG, Williams AW, Moore BJ, et al. Cis–trans lycopene isomers, carotenoids, and retinol in the human prostate. Cancer Epidemiol Biomark Prev 1996;5:823–33. [PubMed] [Google Scholar]
- [26].Bjornson L, Kayden H, Miller E, Moshell A. The transport of α-tocopherol and β-Carotene in human blood. J lipid Res 1976;17: 343–52. [PubMed] [Google Scholar]
- [27].Romanchik J, Morel D, Harrison E. Distribution of carotenoids and α-tocopherol among lipoproteins do not change when human plasma is incubated in vitro. J Nutr 1995;125:2610–7. [DOI] [PubMed] [Google Scholar]
- [28].Parker RS. Absorption, metabolism and transport of carotenoids. FASEB J 1996;10:542–51. [PubMed] [Google Scholar]
- [29].Stahl W, Sies H. Lycopene: a biologically important carotenoid for humans? Arch Biochem Biophys 1996;336:1–9. [DOI] [PubMed] [Google Scholar]
- [30].Campbell DR, Gross MD, Martini MC, Grandits GA, Slavin JL, Potter JD. Plasma carotenoids as biomarkers of vegetable and fruit intake. Cancer Epidemiol Biomarkers Prev 1994;3:493–500. [PubMed] [Google Scholar]
- [31].Vogel S, Contois JH, Tucker KL, Wilson PWF, Scaefer EJ, Lammi-Keefe CJ. Plasma retinol, tocopherol and carotenoids concentrations in healthy elderly participants of the Framingham Heart Study. Am J Clin Nutr 1997;66:950–8. [DOI] [PubMed] [Google Scholar]
- [32].Michaud DS, Giovannucci EL, Ascherio A, Rimm EB, Forman MR, Sampson L, et al. Associations of plasma carotenoid concentrations and dietary intake of specific carotenoids in samples of two prospective cohort studies using a new carotenoid database. Cancer Epidemiol Biomarkers Prev 1998;7:283–90. [PubMed] [Google Scholar]
- [33].Khachik F, Beecher GR, Goli MB, Lusby WR, Smith JC. Separation and identification of carotenoids and their oxidative products in extracts of human plasma. Anal Chem 1992;64:2111–22. [DOI] [PubMed] [Google Scholar]
- [34].Khachik F, Beecher GR, Smith JC Jr. Lutein, lycopene and their oxidative metabolite in chemoprevention of cancer. J Cell Biochem Suppl 1995;22:236–46. [DOI] [PubMed] [Google Scholar]
- [35].Xu X, Wang Y, Constantinou AI, Stacewicz-Sapuntzakis M, Bowen PE, Van Breemen RB. Solubilization and stabilization of carotenoids using micelles: delivery of lycopene to cells in culture. Lipids 1999;34:1031–6. [DOI] [PubMed] [Google Scholar]
- [36].Williams AW, Boileau TW, Clinton SK, Erdman JW Jr. β-Carotene stability and uptake by prostate cancer cells are dependent of delivery vehicles. Nutr Cancer 2000;36:185–90. [DOI] [PubMed] [Google Scholar]
- [37].Pfitzner I, Francz PI, Biesalski HK. Carotenoid: methyl-beta-cyclodextrin formula, an improved method for supplementation of cultured cells. Biochim Biophys Acta 2001;1474:163–8. [DOI] [PubMed] [Google Scholar]
- [38].Junghans A, Sies H, Stahl W. Carotenoid-containing unilamellar liposomes loaded with glutathione: a model to study hydrophobic-hydrophilic antioxidant interactions. Free Rad Res 2001;33:801–8. [DOI] [PubMed] [Google Scholar]
- [39].McClain RM, Bausch J. Summary of safety studies conducted with synthetic lycopene. Regulatory Toxicol Pharmacol 2003;37:274–85. [DOI] [PubMed] [Google Scholar]
- [40].Gann PH, Ma J, Giovannucci E, Willett WC, Sacks FM, Hennekens CH, et al. Lower prostate cancer risk in men with elevated plasma lycopene levels: results of a prospective analysis. Cancer Res 1999; 59:1225–30. [PubMed] [Google Scholar]
- [41].Giovannucci E, Ascherio A, Rimm EB, Stampfer MJ, Colditz GA, Willett WC. Intake of carotenoids and retinol in relation to risk of prostate cancer. J Natl Cancer Inst 1995;87:1767–76. [DOI] [PubMed] [Google Scholar]
- [42].Giovannucci E Tomatoes tomato-based products, lycopene and cancer: review of the epidemiologic literature. J Natl Cancer Inst 1999;91:317–31. [DOI] [PubMed] [Google Scholar]
- [43].Liu QY, Hhung JC, Heber D, Go VL, Reuter VE, Cordon-Cardo C, et al. Inverse associations between plasma lycopene and other carotenoids and prostate cancer. Cancer Epidemiol Biomarkers Prev 2001;10:749–56. [PubMed] [Google Scholar]
- [44].Franceschi S, Bidoli E, La Vecchia C, Talamini R, D’Avanzo B, Negri E. Tomatoes and risk of digestive-tract cancers. Int J Cancer 1994;59:181–4. [DOI] [PubMed] [Google Scholar]
- [45].Tsugane S, Tsuda N, Gey F, Watanabe S. Cross-sectional study with multiple measurements of biological markers for assessing stomach cancer risks at the population level. Environ Health Perspect 1992;98: 207–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].De Stefani E, Oreggia F, Boffetta P, Deneo-Pellegrini H, Ronco A, Mendilaharsu M. Tomatoes, Tomato rich foods, lycopene and cancer of the upper aerodigestive tract: a case control in Uruguay. Oral Oncol 2000;36:47–53. [DOI] [PubMed] [Google Scholar]
- [47].Burney PG, Comstock GW, Morris JS. Serologic precursors of cancer: serum micronutrients and the subsequent risk of pancreatic cancer. Am J Clin Nutr 1989;49:895–900. [DOI] [PubMed] [Google Scholar]
- [48].VanEenwyk J, Davis FG, Bowen PE. Dietary and serum carotenoids and cervical intraepithelial neoplasia. Int J Cancer 1991;48:34–8. [DOI] [PubMed] [Google Scholar]
- [49].Kohlmeier L, Kark JD, Gomez-Garcia E, Martin BC, Steck SE, Kardinaal AF, et al. Lycopene and myocardial infarction risk in the EURAMIC study. Am J Epidemiol 1997;146:618–26. [DOI] [PubMed] [Google Scholar]
- [50].Peng YM, Peng YS, Lin Y, Moon T, Roe DJ, Ritenbaugh C. Concentrations and plasma-tissue diet relationships of carotenoids, retinoids and tocopherol in humans. Nutr Cancer 1995;23:233–46. [DOI] [PubMed] [Google Scholar]
- [51].Brady WE, Mares-Perlman JA, Bowen P, Stacewicz-Sapuntzakis M. Human serum carotenoid concentrations are related to physiologic and lifestyle factors. J Nutr 1996;126:129–37. [DOI] [PubMed] [Google Scholar]
- [52].Stryker WS, Kaplan LA, Stein EA, Stampfer MJ, Sober A, Wyllett WC. The relation of diet, cigarette smoking, and alcohol consumption to plasma β-Carotene and alpha-tocopherol levels. Am J Epidemiol 1988;127:283–96. [DOI] [PubMed] [Google Scholar]
- [53].Pamuk ER, Byers T, Coates RJ, Vann JW, Sowell AL, Gunter EW, et al. Effect of smoking on serum nutrient concentrations in African-American women. Am J Clin Nutr 1994;59:891–5. [DOI] [PubMed] [Google Scholar]
- [54].Fukao A, Tsubono Y, Kawamura M, Ido T, Akazawa N, Tsuji I, et al. The independent association of smoking and drinking with serum β-Carotene levels among males in Miyagi, Japan. Int J Epidemiol 1996;25:300–6. [DOI] [PubMed] [Google Scholar]
- [55].Margetts BM, Jackson AA. The determinants of plasma β-Carotene: interaction between smoking and other life style factors. Eur J Clin Nutr 1996;50:236–8. [PubMed] [Google Scholar]
- [56].Ross MA, Crosley LK, Brown KM, Duthie SJ, Collins AC, Arthur JR, et al. Plasma concentrations of carotenoids and antioxidant vitamins in Scotish males: influences of smoking. Eur J Clin Nutr 1995;49:861–5. [PubMed] [Google Scholar]
- [57].Tsubono Y, Tsugane S, Gey KF. Differential effects of cigarette smoking and alcohol consumption on the plasma levels of carotenoids in middle-aged Japanese men. Jpn J Cancer Res 1996;87:563–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Ames BN, Gold LS, Willett WC. Causes and prevention of cancer. Proc Natl Acad Sci USA 1995;92:5258–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Pincemail J Free radicals and antioxidants in human disease In: Favier AE, Cadet J, Kalyanaraman B, Fontecave M, Pierre J-L, editors. Analysis of free radicals in biological systems. Basel, Switzerland: Birkhäuser Verlag; 1995. p. 83–98. [Google Scholar]
- [60].Stadtman ER. Protein oxidation and aging. Science 1992;257:1220–4. [DOI] [PubMed] [Google Scholar]
- [61].Witztum JL. The oxidation hypothesis of atherosclerosis. Lancet 1994;344:793–5. [DOI] [PubMed] [Google Scholar]
- [62].Ames BN, Shigenaga MK, Hagan TM. Oxidants, antioxidants and the degenerative diseases of aging. Proc Natl Acad Sci USA 1993;90: 7915–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Halliwell B Free radicals, antioxidants and human disease: curiosity, cause or consequence? Lancet 1994;344:721–4. [DOI] [PubMed] [Google Scholar]
- [64].Harats D, Chevion S, Nahir M, Norman Y, Sagee O, Berry E. Citrus fruit supplementation reduces lipoprotein oxidation in young men ingesting a diet high in saturated fat: presumptive evidence for an interaction between vitamin C and E in vivo. Am J Clin Nutr 1998;67: 240–5. [DOI] [PubMed] [Google Scholar]
- [65].Oshima S, Ojima F, Sakamoto H, Ishiguro Y, Terao J. Supplementation with carotenoids inhibit singlet oxygen mediated oxidation of human plasma low-density lipoprotein. J Agric Food Chem 1996;44: 2306–9. [Google Scholar]
- [66].Young AJ, Lowe GM. Antioxidant and prooxidant properties of carotenoids. Arch Biochem Biophys 2001;385:20–7. [DOI] [PubMed] [Google Scholar]
- [67].DiMascio P, Kaiser S, Sies H. Lycopene as the most effective biological carotenoid singlet oxygen quencher.Arch Biochem Biophys 1989; 274:532–8. [DOI] [PubMed] [Google Scholar]
- [68].Mayne ST. Beta carotene, carotenoids and disease prevention in humans. FASEB J 1996;10:690–701. [PubMed] [Google Scholar]
- [69].Miller NJ, Sampson J, Candeias LP, Bramley PM, Rice-Evans CA. Antioxidant activities of carotenes and xanthophylls. FEBS Lett 1996; 384:240–6. [DOI] [PubMed] [Google Scholar]
- [70].Sundquist A, Briviba K, Sies H. Singlet oxygen quenching of carotenoids. Methods Enzymol 1994;234:384–8. [DOI] [PubMed] [Google Scholar]
- [71].Ramos-Gomez M, Kwak MK, Dolan PM, Itoh K, Yamamoto M, Talalay P, et al. Sensitivity to carcinogenesis is increased and chemo-protective efficacy of enzyme inducers is lost in Nrf2 transcription factor-deficient mice. Proc Natl Acad Sci USA 2001;98:3410–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Kim YC, Masutani H, Yamaguchi Y, Itoh K, Yamamoto M, Yodoi J. Hemin-induced activation of thioredoxin gene by Nrf2. A differential regulation of the antioxidant responsive element by a switch of its binding factors. J Biol Chem 2001;276:18399–406. [DOI] [PubMed] [Google Scholar]
- [73].Bhuvaneswari V, Velmurugan B, Balasenthil S, Ramachandran CR, Nagini S. Chemopreventive efficacy of lycopene on 7,12-dimethylbenz[a]anthracene-induced hamster buccal pouch carcino-genesis. Fitoterapia 2001;72:865–74. [DOI] [PubMed] [Google Scholar]
- [74].Gradelet S, Astorg P, Leclerc J, Chevalier J, Vernevaut MF, Siess MH. Effects of canthaxanthin, astaxanthin, lycopene and lutein on liver xenobiotic-metabolizing enzymes in the rat. Xenobiotica 1996;26: 49–63. [DOI] [PubMed] [Google Scholar]
- [75].Böhm F, Edge R, Burke M, Truscott TG. Dietary uptake of lycopene protects human cells from singlet oxygen and nitrogen dioxide—ROS components from cigarette smoke. J Photochem Photobiol 2001;64: 176–8. [DOI] [PubMed] [Google Scholar]
- [76].Handelman G, Packer L, Cross C. Destruction of tocopherols, carotenoids, and retinul in human plasma by cigarette smoke. Am J Clin Nutr 1996;63:559–65. [DOI] [PubMed] [Google Scholar]
- [77].Böhm F, Tinkler JH, Truscott TG. Carotenoids protect against cell membrane damage by nitrogen dioxide radical. Nature Med 1995;1: 98–9. [DOI] [PubMed] [Google Scholar]
- [78].Mortensen A, Skibsted LH, Sampson J, Rice-Evans C, Everett SA. Comparative mechanisms and rates of free radical scavenging by carotenoid antioxidants. FEBS Lett 1997;418:91–7. [DOI] [PubMed] [Google Scholar]
- [79].Tinkler JH, Böhm F, Schalch W, Truscott TG. Dietary carotenoids protect human cells from damage. J Photochem Photobiol 1994;26: 283–5. [DOI] [PubMed] [Google Scholar]
- [80].Hennekens C, Buring J, Manson J, Stampfer M, Rosner B, Belanger C, et al. Lack of effect of long-term supplementation with betα-carotene on the incidence of malignant neoplasms and cardiovascular disease. N Engl J Med 1996;334:1145–9. [DOI] [PubMed] [Google Scholar]
- [81].Omenn GS, Goodman GE, Thoenquist MD, Balmes J, Cullen MR, Glass A, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med 1996;334: 1150–5. [DOI] [PubMed] [Google Scholar]
- [82].Lowe GM, Booth LA,Young AJ, Bilton RF. Lycopene and β-Carotene protect against oxidative damage in HT29 cells at low concentrations but rapidly loose this capacity at higher doses. Free Radic Res 1999; 30:141–51. [DOI] [PubMed] [Google Scholar]
- [83].Yeh S-L, Hu M-L. Antioxidant and prooxidant effects of lycopene in comparison with β-Carotene on oxidant-induced damage in Hs68 cells. J Nutr Biochem 2000;11:548–54. [DOI] [PubMed] [Google Scholar]
- [84].Palozza P Prooxidant actions of carotenoids in biological systems. Nutr Rev 1998;56:257–65. [DOI] [PubMed] [Google Scholar]
- [85].Palozza P, Luberto C, Calviello G, Ricci P, Bartoli GM. Antioxidant and prooxidant role of β-Carotene in murine normal and tumor thymocytes: Effects of partial pressure. Free Rad Biol Med 1997;22: 1065–73. [DOI] [PubMed] [Google Scholar]
- [86].Wang XD, Russel RM. Procarcinogenic and anticarcinogenic effects of β-Carotene. Nutr Rev 1999;57:263–72. [DOI] [PubMed] [Google Scholar]
- [87].Salgo MG, Cueto R, Winston GW, Pryor WA. Beta carotene and its oxidation products have diffeent effects on microsome mediated binding of benzo[a]pyrene to DNA. Free Radic Biol Med 1999;26:162–73. [DOI] [PubMed] [Google Scholar]
- [88].Romanchik J, Harrison E, Morel D. Addition of lutein, lycopene or β-Carotene to LDL or serum in vitro: effects on carotenoid distribution, LDL composition and LDL oxidation. J Nutr Biochem 1997;8: 681–8. [Google Scholar]
- [89].Woodall A, Britton G, Jackson M. Carotenoids and protection of phospholipids in solution or in liposomes against oxidation by peroxyl radicals—relationship between carotenoid structure and protective ability. Biochim Biophys Acta 1997;1336:575–86. [DOI] [PubMed] [Google Scholar]
- [90].Dugas TR, Morel DW, Harrison EH. Dietary supplementation with β-Carotene but not with lycopene, inhibits endothelial cell-mediated oxidation of low-density lipoprotein. Free Rad Biol Med 1999;26: 1238–44. [DOI] [PubMed] [Google Scholar]
- [91].Truscott TG. β-Carotene and disease: a suggested pro-oxidant and anti-oxidant mechanism and speculations concerning its role in cigarette smoking. J Photochem Photobiol 1996;35:233–5. [DOI] [PubMed] [Google Scholar]
- [92].Böhm F, Edge R, McGarvey DJ, Truscott TG. β-Carotene with vita-min E and C offers synergistic cell protection against NO2. FEBS Lett 1998;436:387–9. [DOI] [PubMed] [Google Scholar]
- [93].Niki E, Noguchi N, Tsuchihashi H, Gotoh N. Interaction among vitamin C, vitamin E, and β-Carotene. Am J Clin Nutr 1995;62: 1322S–6S. [DOI] [PubMed] [Google Scholar]
- [94].Lauridsen VST, Daneshvar B, Jakobsen J. Dose–response effects of lycopene on selected drug-metabolizing and antioxidant enzymes in the rat. Cancer Lett 2000;154:201–10. [DOI] [PubMed] [Google Scholar]
- [95].Gupta SK, Trivedi D, Srivastava S, Joshi S, Halder N, Verma SD. Lycopene attenuates oxidative stress induced experimental cataract development: an in vitro and in vivo study. Nutrition 2003;19:794–9. [DOI] [PubMed] [Google Scholar]
- [96].Donaldson P, Eckert R, Green C, Kistler J. Gap junction channels: new roles in disease. Histol Histopathol 1997;12:219–31. [PubMed] [Google Scholar]
- [97].Yamasaki H, Mesnil M, Omori Y, Mironov N, Krutovskikh V. Inter-cellular communication and cancerogenesis. Mutat Res 1995;333: 181–8. [DOI] [PubMed] [Google Scholar]
- [98].Bertram JS, Pung A, Churley M, Kappock TJ, Wilkins LR, Cooney RV. Diverse carotenoids protect against chemically induced neoplastic transformation. Carcinogenesis 1991;12:671–8. [DOI] [PubMed] [Google Scholar]
- [99].Trosko JE, Chang CC. Mechanism of up-regulated gap junctional intercellular communication during chemoprevention and chemo-therapy of cancer. Mutat Res 2001;480/481:219–29. [DOI] [PubMed] [Google Scholar]
- [100].Hotz-Wagenblatt A, Shalloway D. Gap junctional communication and neoplastic transformation. Crit Rev Oncogenesis 1993;4:541–58. [PubMed] [Google Scholar]
- [101].Stahl W, von Laar J, Martin HD, Emmerich T, Sies H. Stimulation of gap junctional communication: comparison of acyclo-retinoic acid and lycopene. Arch Biochem Biophys 2000;373:271–4. [DOI] [PubMed] [Google Scholar]
- [102].Stahl W, Nicolai S, Briviba K, Hanusch M, Broszeit G, Peters M, et al. Biological activities of natural and synthetic carotenoids: Induction of gap junctional communication and singlet oxygen quenching. Carcinogenesis 1997;18:89–92. [DOI] [PubMed] [Google Scholar]
- [103].Bertram JS. Carotenoids and gene regulation. Nutr Rev 1999;57:182–91. [DOI] [PubMed] [Google Scholar]
- [104].Krutovskikh V, Asamoto M, Takasuka N, Murakoshi M. Differential dose-dependent effects of alpha-carotene and lycopene on gap juctional intercellular communication in rat liver in vivo. Jpn J Cancer Res 1997;88:1121–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Krinsky NI,Yeum K-J. Carotenoid-radical interactions. Biochem Biophys Res Comm 2003;305:754–60. [DOI] [PubMed] [Google Scholar]
- [106].Aust O, Ale-Agha N, Zhang L, Wollersen H, Sies H, Stahl W. Lycopene oxidation product enhances gap junctional communication. Food and Chemical Toxicol 2003;41:1399–407. [DOI] [PubMed] [Google Scholar]
- [107].Zhang LX, Cooney RV, Bertram J. carotenoids enhance gap junctional communication and inhibit lipid peroxidation in C3H/10T1/2 cells: relationship to their cancer preventive action. Carcinogenesis 1991; 12:2109–14. [DOI] [PubMed] [Google Scholar]
- [108].Zhang LX, Cooney RV, Bertram JS. Carotenoids up-regulate connexin 43 gene expression independent of their provitamin A or anti-oxidant properties. Cancer Res 1992;52:5707–12. [PubMed] [Google Scholar]
- [109].Stock A, Sies H, Stahl W. Enhancement of gap junctional communication and connexin43-expression by thyroid hormones. Biochem Pharmacol 1998;55:475–9. [DOI] [PubMed] [Google Scholar]
- [110].Evans HW, Martin PEM. Gap junctions: structure and function. Mol Membrane Biol 2002;19:121–36. [DOI] [PubMed] [Google Scholar]
- [111].Gruszecki WI. Carotenoids in membranes In: Frank HA, Young AJ, Britton G, editors. The photochemistry of carotenoids. Dordrecht/Boston/London: Kluwer Acad Publ; 1999. p. 363–79. [Google Scholar]
- [112].Sujak A, Gabrielska J, Grudzecki W, Borc R, Mazurec P, Gruszeki WI. Lutein and zeaxanthin as protectors of lipid membranes against oxidative damage: the structural aspects. Arch Biochem Biophys 1999; 371:301–7. [DOI] [PubMed] [Google Scholar]
- [113].Woodall A, Britton G, Jackson M. Carotenoids and protection of phospholipids in solution or in liposomes against oxidation by peroxyl radicals—relationship between carotenoid structure and protective ability. Biochim Biophys Acta 1997;1336:575–86. [DOI] [PubMed] [Google Scholar]
- [114].Junghans A, Sies H, Stahl W. Carotenoid-containing unilamellar liposomes loaded with glutathione: a model to study hydrophobic–hydro-philic antioxidant interaction. J Free Rad Res 2000;33:801–8. [DOI] [PubMed] [Google Scholar]
- [115].Lieber CS. Alcohol and the liver: 1994 update. Gastroenterology 1994;106:1085–105. [DOI] [PubMed] [Google Scholar]
- [116].Lieber CS. Cytochrome P-4502E1: its physiological and pathological role. Physiol Rev 1997;77:517–43. [DOI] [PubMed] [Google Scholar]
- [117].Lieber CS. Ethanol metabolism, cirrhosis and alcoholism. Clin Chim Acta 1997;257:59–84. [DOI] [PubMed] [Google Scholar]
- [118].Hirano T, Kaplowitz N, Tsukamoto H, Kamimura S, Fernandez-Checa JC. Hepatic mitochondrial glutathione depletion and progression of experimental alcoholic liver disease in rats. Hepatology 1992; 16:1423–7. [DOI] [PubMed] [Google Scholar]
- [119].Takahashi H, Johansson H, French SW. Effect of dietary fat composition on activities of the microsomal ethanol oxidizing system and ethanol-inducible cytochrome P450 (CYP2E1) in the liver of rat chronically fed ethanol. Pharmacol Toxicol 1992;70:347–51. [DOI] [PubMed] [Google Scholar]
- [120].Xu Y Leo MA, Lieber CS. Lycopene attenuates alcoholic apoptosis in HepG2 cells expressing CYP2E1. Biochem Biophys Res Comm 2003;308:614–8. [DOI] [PubMed] [Google Scholar]
- [121].Seeds MC, Bass DA. Regulation and metabolism of arachidonic acid. Clin Rev Allergy Immunol 1999;17:5–22. [DOI] [PubMed] [Google Scholar]
- [122].Brash AR. Arachidonic acid as a bioactive molecule. J Clin Invest 2001;107:1339–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Tapiero H, Nguyen Ba G, Couvreur P, Tew KD. Polyunsaturated fatty acids (PUFA) and eicosanoids in human health and pathologies. Biomed Pharmacother 2002;56:215–22. [DOI] [PubMed] [Google Scholar]
- [124].Xu Y, Leo MA, Lieber CS. Lycopene attenuates arachidonic acid toxicity in HepG2 cells overexpressing CYP2E1. Biochem Biophys Res Comm 2003;303:745–50. [DOI] [PubMed] [Google Scholar]
- [125].Landrum JT, Bone RA. Lutein, zeaxanthin and the macular pigment. Arc Biochem Biophys 2001;385:28–40. [DOI] [PubMed] [Google Scholar]
- [126].Beatty S, Murray IJ, Henson DB, Carden D, Koh H, Boulton ME. Macular pigment and risk for age-related macular degeneration in subjects from a Northern European population. Invest Ophthalmol Vis Sci 2001;42:439–46. [PubMed] [Google Scholar]
- [127].Fisher GJ, Wang ZQ, Datta SC, Varani J, Kang S, Voorhees JJ. Pathophysiology of premature skin aging induced by ultraviolet light. N Engl J Med 1997;337:1419–28. [DOI] [PubMed] [Google Scholar]
- [128].Scharffetter K, Wlaschek M, Hogg A, Bolsen K, Schothorst A, Goerz G, et al. UVA irradiation induces collagenase in human dermal fibroblasts in vitro and in vivo. Arch Dermatol Res 1991;283:506–11. [DOI] [PubMed] [Google Scholar]
- [129].Keyse SM, Tyrell RM. Heme oxygenase is the major 32-kDa stress protein induced in human skin fibroblasts by UVA radiation, hydrogen peroxide and sodium arsenite. Proc Natl Acad Sci USA 1989;86:99–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Applegate LA, Luscher P, Tyrell RM. Induction of heme oxygenase: a general response to oxidant stress in cultured mammalian cells. Cancer Res 1991;51:974–8. [PubMed] [Google Scholar]
- [131].Scharffetter-Kochanek K, Wlaschek M, Briviba K, Sies H. Singlet oxygen induces collagenase expression in human skin fibroblasts. FEBS Lett 1993;331:304–6. [DOI] [PubMed] [Google Scholar]
- [132].Basu-Modak S, Tyrell RM. Singlet oxygen: a primary effector in the ultra violet A/near visible light induction of the human heme oxygenase gene. Cancer Res 1993;53:4505–10. [PubMed] [Google Scholar]
- [133].Fisher GJ, Datta SC, Talwar HS, Wang ZQ, Varani J, Kang S, et al. Molecular basis of of sun-induced premature skin ageing and retinoid antagonism. Nature 1996;379:335–9. [DOI] [PubMed] [Google Scholar]
- [134].Offord EA, Gauthier JC, Avanti O, Scaletta C, Runge F, Krämer K, et al. Photoprotective potential of lycopene, β-Carotene, vitamin E, vitamin C and carnosic acid in UVA-irradiated human skin fibroblasts. Free Rad Biol Med 2002;32:1293–303. [DOI] [PubMed] [Google Scholar]
- [135].Lowe GM, Booth LA,Young AJ, Bilton RF. Lycopene and β-Carotene protect against oxidative damage in HT29 cells at low concentrations but rapidly loose this capacity at higher doses. Free Radic Res 1999; 30:141–51. [DOI] [PubMed] [Google Scholar]
- [136].Palozza P Prooxidant actions of carotenoids in biological systems. Nutr Rev 1998;56:257–65. [DOI] [PubMed] [Google Scholar]
- [137].Fuchs J Potential and limitation of the natural antioxidants α-tocopherol, L-ascorbic acid and β-Carotene in cutaneous photoprotection. Free Radic Biol Med 1998;25:848–73. [DOI] [PubMed] [Google Scholar]
- [138].Stahl W, Heinrich U, Jungmann H, Sies H, Tronnier H. Carotenoids and carotenoids plus vitamin E protect against ultraviolet light-induced erythema in humans. Am J Clin Nutr 2000;71:795–8. [DOI] [PubMed] [Google Scholar]
- [139].Stahl W, Heinrich U, Wiseman S, Eichler O, Sies H, Tronnier H. Dietary tomato paste protects against ultraviolet light-induced erythema in humans. J Nutr 2001;131:1449–51. [DOI] [PubMed] [Google Scholar]
- [140].Ribaya-Mercado JD, Garmyn M, Gilchrest BA, Russel RM. Skin lycopene is destroyed preferentially over β-Carotene during ultraviolet irradiation in humans. J Nutr 1995;125:1854–8. [DOI] [PubMed] [Google Scholar]
- [141].Tang G, Wang XD, Russel RM, Krinsky NI. Characterization of β-apo-13-carotenone and β-apo-14’-carotenal as enzymatic products of the excentric cleavage of β-Carotene. Biochemistry 1991;30:9829–34. [DOI] [PubMed] [Google Scholar]
- [142].Mantzoros CS, Tzonou A, Signorello LB, Stampfer M, Trichopoulos D, Adami HO. Insulin-like growth factor I in relation to prostate cancer and begnin prostatic hyperplasia. Br J Cancer 1997;76:1115–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Hankinson SE, Willett WC, Colditz GA, Hunter DJ, Michaud DS, Deroo B, et al. Circulating concentrations of insulin-like growth factor I and risk of breast cancer. Lancet 1998;351:1393–6. [DOI] [PubMed] [Google Scholar]
- [144].Ma J, Pollak MN, Giovannucci E, Chan JM, Tao Y, Hennekens CH, et al. Prospective study of colorectal cancer risk in men and plasma levels of insulin-like growth factor (IGF)-I and IGF-binding protein-3. J Natl Cancer Inst 1999;91:620–5. [DOI] [PubMed] [Google Scholar]
- [145].Yu H, Spitz MR, Mistry J, Gu J, Hong WK, Wu X. Plasma levels of insulin-like growth factor-I and lung cancer risk: a case control analysis. J Natl Cancer Inst 1999;91:151–6. [DOI] [PubMed] [Google Scholar]
- [146].Karas M, Amir H, Fishman D, Danilenko M, Segal S, Nahum A, et al. Lycopene interferes with cell cycle progression and insulin-like growth factor I signaling in mammary cancer cells. Nutr Cancer 2000;36:101–11. [DOI] [PubMed] [Google Scholar]
- [147].Murakoshi M, Takayasu J, Kimura O, Kohmura E, Nishino H, Okuzumi J, et al. Inhibitory effect of α-carotene on proliferation of the human neuroblastoma cell line GOTO. J Natl Cancer Inst 1989;81: 1649–52. [DOI] [PubMed] [Google Scholar]
- [148].Bartek J, Bartkova J, Lukas J. The retinoblastoma protein pathway in cell cycle control and cancer. Exp Cell Res 1997;237:1–6. [DOI] [PubMed] [Google Scholar]
- [149].Sherr CJ. D-type cyclins. Trends Biochem Sci 1995;20:187–90. [DOI] [PubMed] [Google Scholar]
- [150].Buckley MF, Sweeney KJ, Hamilton JA, Sini RL, Manning DL, Nicholson RI, et al. Expression and amplification of cyclin genes in human breast cancer. Oncogene 1993;8:2127–33. [PubMed] [Google Scholar]
- [151].Ben-Dor A, Nahum A, Danilenko M, Giat Y, Stahl W, Martin HD, et al. Effects of acyclo-retinoic acid and lycopene on activation of the retinoic acid receptor and proliferation of mammary cancer cells. Arch Biochem Biophys 2001;391:295–302. [DOI] [PubMed] [Google Scholar]
- [152].Tibaduiza EC, Fleet JC, Russel RM, Krinsky NI. Excentric cleavage products of betα-carotene inhibit estrogen receptor positive and negative breast tumor cell growth in vitro and inhibit activator protein–1-mediated transcriptional activation. J Nutr 2002;132:1368–75. [DOI] [PubMed] [Google Scholar]
- [153].Levy J, Bosin E, Feldmen B, Giat Y, Miinster A, Danilenko M, et al. Lycopene is a more potent inhibitor of human cancer cell proliferation than either α-carotene or β-Carotene. Nutr Cancer 1995;24:257–66. [DOI] [PubMed] [Google Scholar]
- [154].Livny O, Kaplan I, Reifen R, Polak-Charcon S, Madar Z, Scwartz B. Lycopene inhibits proliferation and enhances gap-junction communication of KB-1 human oral tumor cells. J Nutr 2002;132:3754–9. [DOI] [PubMed] [Google Scholar]
- [155].Wang CJ, Chou MY, Lin JK. Inhibition of growth and development of the transplantable C-6 glioma cells inoculated in rats by retinoids and carotenoids. Cancer Lett 1989;48:135–42. [DOI] [PubMed] [Google Scholar]
- [156].Giovannucci E, Ascherio A, Rimm EB, Stampfer MJ, Colditz GA, Willett WC. Intake of carotenoids and retinol in relation to risk of prostate cancer. J Natl Cancer Inst 1995;87:1767–76. [DOI] [PubMed] [Google Scholar]
- [157].Van Eewyck J, Davis FG, Bowen PE. Dietary and serum carotenoids and cervical intraepithelial neoplasia. Int J Cancer 1991;48:34–8. [DOI] [PubMed] [Google Scholar]
- [158].Helzlsouer KJ, Comstock GW, Morris JS. Selenium, lycopene, α-tocopherol, β-Carotene, retinol and subsequent bladder cancer. Cancer Res 1989;49:6144–8. [PubMed] [Google Scholar]
- [159].Potischman N, McCulloch CE, Byers T, Nemoto T, Stubbe N, Milch R, et al. Breast cancer and dietary and plasma concentrations of carotenoids and vitamin A. Am J Clin Nutr 1990;52:909–15. [DOI] [PubMed] [Google Scholar]
- [160].Coodley GO, Coodley MK, Nelson HD. Micronutrients in HIV-infected women. J Womens Health 1995;4:303–11. [Google Scholar]
- [161].Periquet BA, Jammes NM, Lambert WE, Tricoire J, Moussa MM, Garcia J, et al. Micronutrients levels in HIV-1-infected children.AIDS 1995;9:887–93. [DOI] [PubMed] [Google Scholar]
- [162].VanAntwerpen VL, Theron AJ, Richard G, Van Der Merwe C, Walt R, Anderson R. Relationship between the plasma levels of betα-carotene and functions in cigarette smokers. Int J Vitam Nutr Res 1995;65: 231–5. [PubMed] [Google Scholar]
- [163].Salonen JT, Korpela H, Salonen R, Nyyssonen K, Ylaherttuala S. Autoantibody against oxidized LDL and progression of carotid atherosclerosis. Lancet 1992;339:883–7. [DOI] [PubMed] [Google Scholar]
- [164].Rao AV,Agarwal S. Effect of diet and smoking on serum lycopene and lipid peroxidation. Nutr Res 1998;18:713–21. [Google Scholar]
- [165].Hininger I, Chopra M, Thurnham D, Laporte F, Richard M. Effect of increased fruit and vegetable intake on the susceptibility of lipoprotein to oxidation in smokers. Eur J Clin Nutr 1997;51:601–6. [DOI] [PubMed] [Google Scholar]
- [166].Goulinet S, Chapman MJ. Plasma LDL and HDL subspecies are heterogenous in particle content of tocopherols and oxygenated and hydrocarbon carotenoids. Relevance to oxidative resistance and atherogenesis. Arterioscler Thromb Vasc Biol 1997;17:786–96. [DOI] [PubMed] [Google Scholar]
- [167].Sesso HD, Liu S, Gaziano M, Buring JE. Dietary lycopene, Tomato-based food products and cardiovascular disease in women. J Nutr 2003;133:2336–41. [DOI] [PubMed] [Google Scholar]