Abstract
Cisplatin, a commonly used chemotherapeutic agent, is nephrotoxic. The mechanism by which cisplatin selectively kills the proximal tubule cells was heretofore unknown. Recent studies in mice and rats have shown that the nephrotoxicity of cisplatin can be blocked by acivicin or (aminooxy)acetic acid, the same enzyme inhibitors that block the metabolic activation of a series of nephrotoxic halogenated alkenes. In this study, it was hypothesized that cisplatin is activated in the kidney to a toxic metabolite through the same pathway that has been shown to activate the halogenated alkenes. This activation begins with the formation of a glutathione-conjugate that is metabolized to a cysteinyl-glycine-conjugate, to a cysteineconjugate, and finally to a reactive thiol. In this study, a protocol was developed in which confluent monolayers of LLC-PK1 cells were exposed to clinically relevant concentrations of cisplatin or cisplatin-conjugate for 3 h. Cell viability was assayed at 72 h. The role of gamma-glutamyl transpeptidase (GGT) and cysteine-S-conjugate beta-lyase in the metabolism of each of the cisplatin-conjugates was investigated. Pre-incubation of cisplatin with glutathione, cysteinyl-glycine, or N-acetyl-cysteine to allow for the spontaneous formation of cisplatin-conjugates increased the toxicity of cisplatin toward LLC-PK1 cells. Inhibition of GGT activity showed that GGT was necessary only for the toxicity of the cisplatin-glutathione-conjugate. Inhibition of cysteine-S-conjugate beta-lyase reduced the toxicity of each of the cisplatin-conjugates. These data demonstrate that metabolism of cisplatin in proximal tubule cells is required for its nephrotoxicity. The elucidation of this pathway provides new targets for the inhibition of cisplatin nephrotoxicity.
Cisplatin is a potent antitumor agent currently used in the treatment of germ cell tumors, head and neck tumors, cervical cancer, and as a salvage treatment for other solid tumors (1). The dose of cisplatin that can be administered is limited by its nephrotoxicity (2). The mechanism by which cisplatin kills the proximal tubule cells in the kidney has been the focus of intense investigation for many years. In tumors and other dividing cells, cisplatin-DNA crosslinks are thought to be the cytotoxic lesion (3). Nonproliferating cells are less sensitive to the toxicity of DNA-damaging agents, yet the quiescent proximal tubule cells are selectively killed by cisplatin. High concentrations of cisplatin induce necrotic cell death in confluent monolayers of proximal tubule cells, whereas lower concentrations of cisplatin induce apoptosis through a caspase-9 – dependent pathway (4,5). The molecular mechanism by which cisplatin kills these nonproliferating cells has been unclear.
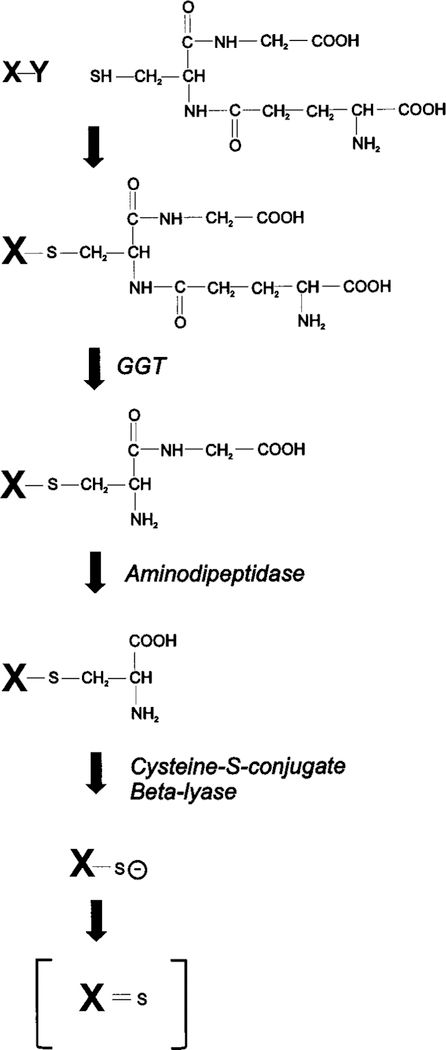
Our studies in rats and mice have shown that the nephrotoxicity of cisplatin can be blocked by inhibiting either of two enzymes expressed in the proximal tubules, gamma-glutamyl transpeptidase (GGT) or cysteine-S-conjugate beta-lyase (6–8). Data from these in vivo studies have lead to the hypothesis that the nephrotoxicity of cisplatin is the result of the metabolic activation of the cisplatin in the kidney to a more potent toxin. We propose that this activation is through a pathway that includes GGT and cysteine-S-conjugate beta-lyase. These two enzymes have been shown to be required for the nephrotoxicity of a series of halogenated alkenes that include hexachloro-1,3-butadiene, trichloroethylene, and tetrafluoroethylene (9 –13). The metabolism of these compounds is initiated by their conjugation to glutathione (Figure 1). As the glutathione-conjugates pass through the kidney, they are cleaved to cysteinylglycine-conjugates by GGT expressed on the surface of the proximal tubule cells (14). GGT cleaves gamma-glutamyl bonds in extracellular glutathione and glutathione-conjugates (15). The cysteinyl-glycine-conjugates are further metabolized to cysteine-conjugates by aminodipeptidases, also expressed on the surface of the proximal tubule cells (16). Both the GGT and aminodipeptidase catalyzed reactions take place extracellularly. The cysteine-conjugates are transported into the proximal tubule cells, where they are further metabolized by cysteine-S-conjugate beta-lyase to the highly reactive thiols (17). The toxicity of N-acetyl-cysteine-conjugates of the halogenated alkenes is similar to the cysteine-conjugates, and both are metabolized by cysteine-S-conjugate beta-lyase to reactive thiols (18). The reactive electrophilic metabolites bind to cellular macromolecules, triggering an increase in cytosolic free calcium and ultimately cell death (19). The toxicity caused by the nephrotoxic halogenated-alkenes is localized to the proximal convoluted tubules in the outer renal cortex, the same cells killed by cisplatin (10,20 –22).
Figure 1.
Metabolic activation of glutathione-conjugates to reactive thiols. Halogenated alkenes (X represents the alkene and Y a halogen molecule: fluorine, chlorine, or bromine) have been shown to be metabolized to nephrotoxins via this pathway (9,12,60). The haloge-nated alkenes form glutathione-S-conjugates, which are cleaved to cysteinyl-glycine-conjugates by GGT. Aminodipeptidase cleaves the cysteinyl-glycine-conjugates to cysteine-conjugates. Cysteine-S-con-jugate beta-lyase catalyzes the production of unstable reactive thiols, which are toxic. We propose that cisplatin is metabolized through this same pathway. In the proposed pathway, Y would represent one of the chlorines in cisplatin and X would represent the remainder of the cisplatin molecule. In the proposed pathway, the sulfur of the gluta-thione molecule binds to the platinum displacing the chlorine.
Cisplatin is not a substrate for either GGT or cysteine-S-conjugate betalyase. However, cisplatin has been shown to form glutathione-conjugates spontaneously in solution (23,24). Dissociation of one of the chlorines from the cisplatin molecule results in a positive charge on the platinum that will attract the negatively charged sulfur on the cysteine moiety of the glutathione molecule. Cisplatin-glutathione-conjugates have been isolated from cells treated with cisplatin and from the serum of cisplatin-treated rats (24,25). Pretreating rats with an inhibitor of glutathione-S-transferases reduced the nephrotoxicity of cisplatin, suggesting that in vivo glutathione-S-transferases may catalyze the conjugation of cisplatin to glutathione (26). Conjugation to glutathione is a means of detoxifying electrophilic compounds. However, as noted above, the glutathione-conjugates of some compounds can be further metabolized to nephrotoxins. We propose cisplatin is bioactivated in the kidney by the same pathway that activates the halogenated alkenes. Our hypothesis is that GGT cleaves the gamma-glutamyl group of the glutathione-conjugate, and aminodipeptidase cleaves the cysteinyl-glycine bond, resulting in a platinum-cysteine-conju-gate. Finally the cysteine-conjugate is metabolized by cysteine-S-conjugate beta-lyase to a reactive thiol.
In this study, we have used LLC-PK1 cells, a porcine proximal tubule cell line, to test our hypothesis. LLC-PK1 cells were used to investigate the bioactivation of halogenated alkenes to nephrotoxins (12,27–30). Confluent monolayers of LLC-PK1 cells have the morphologic and metabolic characteristics of proximal tubules (31,32). There have been several studies of cisplatininduced toxicity that have used dividing LLC-PK1 cells (33–35). Cisplatin binds DNA, forming inter-strand and intrastrand crosslinks that inhibit cell division (36,37). Dividing cells can be killed by low concentrations of cisplatin. These studies have not addressed the mechanism of cisplatin nephrotoxicity because in vivo the proximal tubule cells are not dividing. They form a confluent monolayer of epithelial cells lining the tubules. The proximal tubules are exposed to cisplatin during its biphasic excretion into the urine (38). The highest levels of exposure are during the first 3 h after administration. The toxicity is dose-and time-dependent (22). Damage to the proximal tubules is first observed 3 to 4 d after the administration of the drug. Montine and Borch (39) evaluated the toxicity of cisplatin and its non-nephrotoxic derivative, carboplatin, in confluent monolayers of LLC-PK1 cells. They showed that cisplatin toxicity was dose-and timedependent, whereas carboplatin was not toxic even at tenfold the LC50 dose of cisplatin.
We began our studies by developing a treatment protocol that closely mimicked the in vivo exposure of proximal tubule cells to cisplatin. Confluent monolayers of cells were exposed to cisplatin for 3 h. The cisplatin was removed and replaced with tissue culture media. The cells were maintained in culture for 3 d and then assayed for viability. With this protocol, we observed toxicity at 50 µM cisplatin. The serum concentrations of cisplatin in patients and experimental animals treated with nephrotoxic levels of cisplatin reaches 30 to 50 µM (40,41); whereas, in non-nephrotoxic regimens, the serum concentra-tion rarely exceeds 10 µM platinum (42). We incubated cisplatin with glutathione, cysteinylglycine, or NAC to allow for the formation of cisplatin-conjugates. Previous studies have shown that cisplatin will spontaneously form conjugates with glutathione and cysteine (23,43,44). These conjugates are the intermediates along the proposed pathway of metabolic activation to a nephrotoxin. The enzymes in the proposed pathway, GGT and cysteine-S-conjugate beta-lyase, were inhibited. The effect of this inhibition on the toxicity of each conjugate was evaluated.
Materials and Methods
Cell Line
LLC-PK1 (ATCC CRL 1392), a proximal tubule cell line isolated from pig kidney, was purchased from American Type Culture Collection (Rockville, MD). Cells were maintained in Dulbecco Modified Eagle’s Medium (DMEM; Life Technologies/BRL, Grand Island, NY), 5% fetal bovine serum (FBS; Hyclone Laboratories, Logan, UT), 50 units penicillin G, and 50 µg streptomycin/ml (Life Technologies/BRL) at 37°C in a 5% CO2 atmosphere. Subconfluent cultures were passaged every 3 to 4 d. For experiments, LLC-PK1 cells were seeded in 96-well plates at 1 × 104 cells/well. On the third day after plating, confluent monolayers formed and the media was re-placed with fresh media. Cells were used for experiments on day 7.
Time Course of Cisplatin Toxicity
A fresh stock solution of 3.33 mM cis-platinum(II)-diamine dichloride (cisplatin, Sigma Chemical Co.) was prepared in 0.9% sodium chloride solution the day of the experiment. For the experiments in which cells were exposed continuously to cisplatin for up to 3 d, the cisplatin was diluted in DMEM medium containing 10% FBS, 50 units penicillin G, and 50 µg streptomycin/ml. At the time points indicated, the number of viable cells was determined by the MTT assay (45). Wells with untreated cells served as controls. A standard curve was developed relating cell number to the OD570 value obtained in the MTT assay.
For experiments in which cells were exposed to cisplatin for 3 h, the cisplatin was diluted in Hanks Balanced Salt Solution (HBSS, Cat. No. 11201, Life Technologies) with 5 mM N-[2-Hydroxyethyl]piperazine-N-[2-ethane sulfonic acid] (HEPES, Sigma Chemical Co.), pH 7.2. Medium was removed from the cells and replaced with cisplatin diluted in HBSS with HEPES, pH 7.2. The cells were incubated at 37°C. After 3 h, the cisplatin solution was removed from the cells and replaced with DMEM medium containing 5% FBS, penicillin, and streptomycin. The cells were incubated at 37°C in 5% CO2. In the time course experiments, the MTT assay was started at the time indicated. In all other experiments, cell viability was determined 72 h after the start of the experiment (69 h after the drug was removed).
Treatment with Glutathione, Cysteinyl-Glycine, and N-Acetyl Cysteine-Glutathione Solutions
Solutions containing 50 mM cisplatin and equimolar glutathione, cysteinyl-glycine (Bachem, King of Prussia, PA) or N-acetyl cysteine (NAC) in HBSS with 5 mM HEPES, pH 7.2, were incubated at 37°C for 30 min to allow for the spontaneous formation of cisplatin-conjugates (23,43). The medium was removed from the cells, and 150 ml of the incubation mixture was added to each well. The cells were incubated at 37°C. The mixture was removed after 3 h and replaced with fresh DMEM medium containing 5% FBS and penicillin and streptomycin. The cells were incubated for an additional 69 h at 37°C in 5% CO2. The number of viable cells was determined 72 h after the start of the experiment by the MTT assay.
Kidney Homogenates
Kidneys from 5-mo-old female Balb/c mice were harvested and stored at 80°C. Kidneys were thawed, homogenized in 0.9% sodium chloride, and assayed for GGT and aminopeptidase N activity. Kid-neys to be assayed for cysteine-S-conjugate beta-lyase activity were homogenized in 3 vol of 10 mM Tris-HCl, 0.25 M sucrose (pH 7.5) in a Potter-Elvehjem homogenizer, 4°C. The homogenate was freeze-thawed twice, followed by sonication twice for 10 s with a 30-s cooling interval, then centrifuged at 3000 × g for 5 min. The supernatant was used to assay enzyme activity.
GGT Assay
GGT activity was assayed as described previously (14). LLC-PK1 cells were trypsinized off the plates and assayed for GGT activity. One unit of GGT activity was defined as the amount of enzyme that released 1 mmol of p-nitroaniline per min at 25°C. The protein concentrations of the kidney homogenates and LLC-PK1 monolayers were determined with the BCA assay (Pierce, Rockford, IL).
Aminopeptidase Assay
The activity of aminopeptidase N (EC 3.4.11.2), previously re-ferred to as aminopeptidase M, was measured by the method of Hughey and co-workers with S-benzylglycine-p-nitroanilide as the substrate as described previously (16,46). To determine the level of aminopeptidase N activity in confluent monolayers of LLC-PK1 cells, the media was removed, the intact monolayers were rinsed with phosphate-buffered saline (PBS) and the reaction mixture for the assay was added directly to the cells. The plates were incubated at 37°C. The reaction was stopped by removing the assay solution and boiling it. One unit of aminopeptidase N activity is defined as the amount of enzyme that released 1 mmol of p-nitroaniline per min at 37°C. The protein concentrations of the kidney homogenates and LLC-PK1 monolayers were determined with the BCA assay.
Cysteine-S-Conjugate Beta-Lyase Assay
Confluent monolayers of LLC-PK1 cells were trypsinized off the plates. The cells were rinsed with PBS, resuspended in 10 mM Tris-HCl, 0.25 M sucrose (pH 7.5), freeze-thawed twice, sonicated twice for 10 s with a 30 s cooling interval, then centrifuged at 3000 × g for 5 min as described previously (47). The supernatant was used to assay enzyme activity. S-(1,2-Dichlorovinyl)-L-cysteine (DCVC) was synthesized by the method of McKinney et al. (48). Cysteine-S-conjugate beta-lyase activity was measured by a method developed by Dr. Authur Cooper (personal communication), which is a modification of a previously published assay (13). Briefly, 20 µl of reaction mixture was prepared containing 100 mM potassium phosphate buffer (pH 7.2), 5 mM DCVC, 10 mM PLP, and the kidney or cell supernatant. The reaction mixture was incubated at 37°C. Addition of 20 µl of 5 mM 2,4-dinitrophenylhydrazine (DNP) in 2 M HCl stopped the reaction and produced a quantifiable reaction with the pyruvate re-leased during the reaction. The solution was incubated at 37°C for an additional 5 min; then 160 ml of 1 M KOH was added. The mixture was rapidly transfered to a 96-well plate, and the absorbance was measured at 450 nm within 2 min. The background absorbance level was determined by adding the supernatant immediately after the DNP. One unit of activity was defined as the amount of enzyme that released 1 mmol of pyruvate per min at 37°C. Protein concentrations were determined by BCA protein assay.
Inhibition of GGT and Cysteine-S-Conjugate Beta-Lyase in LLC-PK1 Cells
To inhibit GGT activity in confluent monolayers of LLC-PK1 cells, the medium was removed 2 h before treatment with cisplatin or its conjugates and replaced with HBSS with 5 mM HEPES, pH 7.2, containing 250 µM acivicin. During the 3 h treatment of the cells with the cisplatin mixtures, the treatment solution also contained 250 mM acivicin. To inhibit cysteine-S-conjugate beta-lyase activity, media was removed from the cells 30 min before treatment and replaced with HBSS with 5 mM HEPES, pH 7.2, containing 100 mM (aminooxy-)acetic acid (AOAA) (12,27–29). During the 3 h treatment, the treatment solution also contained 100 mM AOAA.
Statistical Analyses
All cell culture experiments were repeated at least three times. In each experiment, all points were done in triplicate. The SD from the mean was computed for each treatment. Statistically significant differences among the mean values were detected by a one-way ANOVA. A Tukey test was used to determine which mean values were significantly different from the control value (T0 or untreated cells) (49). Statistically significant differences in cell survival due to treatment with acivicin or AOAA were detected by a t test.
Results
Cisplatin Toxicity: Continuous Exposure of LLC-PK1 Cells to Cisplatin
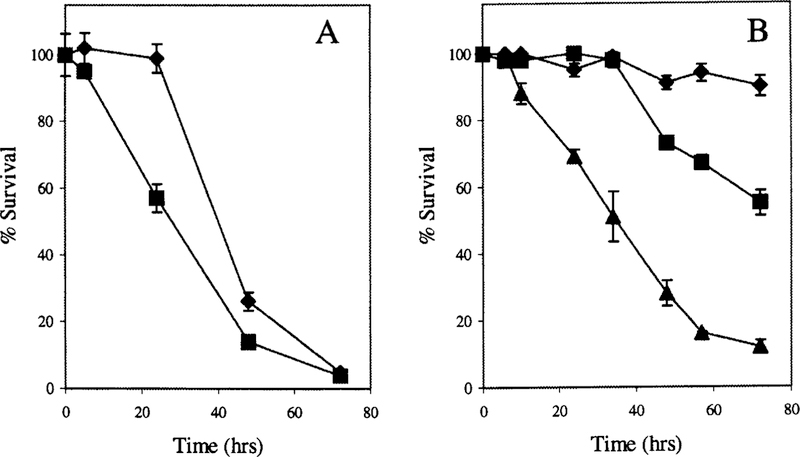
In vivo nephrotoxicity of cisplatin is not apparent until 3 to 5 d after administration of cisplatin (50). We analyzed the viability of LLC-PK1 cells with time during continuous expo-sure to cisplatin. Confluent monolayers of LLC-PK1 cells were treated with 50 µM or 100 µM cisplatin. Cells that were incubated in 50 µM cisplatin did not show any significant decrease in viability during the first 24 h of exposure (Figure 2A). However, there was a statistically significant reduction in cell survival by 48 h when compared with untreated controls (P < 0.05). Toxicity increased with time. At 72 h, only 5% of the cells were still alive. The toxicity was both dose- and time-dependent. Cells incubated in 100 µM cisplatin showed significantly reduced survival by 24 h (P < 0.05).
Figure 2.
Survival of confluent monolayers of LLC-PK1 cells after continous exposure to cisplatin (A) or a 3 h pulse (B). (A) Confluent monolayers of LLC-PK1 cells were incubated in DMEM containing 50 mM cisplatin (diamonds) or 100 mM cisplatin (squares). At each time point, cell survival was determined relative to untreated cells. Each point represents the mean of three points ± SD. (B) Confluent monolayers of LLC-PK1 cells were incubated for 3 h in a balanced salt solution containing 50 mM cisplatin (diamonds), 100 mM cisplatin (squares), or 150 mM cisplatin (triangles). The cisplatin was removed at the end of the 3 h exposure and fresh medium added to the cells. At each time point, cell survival was determined relative to the number of viable cells in control wells. Each point represents the average of three means SEM.
Cisplatin Toxicity: 3 h Exposure of LLC-PK1 Cells to Cisplatin
Confluent monlayers of LLC-PK1 cells were incubated in cisplatin for 3 h. The cisplatin was removed and fresh medium was added to the cells. Cell survival was assayed with time after the cisplatin treatment (Figure 2B). As was observed with continuous exposure to cisplatin, there was time- and dose-dependent toxicity. Cells treated with 50 µM or 100 µM cisplatin for 3 h did not show any significant reduction in viability until 48 h (P < 0.05). Treatment with 150 µM cisplatin resulted in significant toxicity within 10 h (P < 0.05). Continuous incubation in the presence of cisplatin was more toxic to LLC-PK1 cells than the limited 3 h exposure (Figure 2, A and B). Exposure of the cells to 50 µM cisplatin for 3 h resulted in a low but significant amount of toxicity at 72 h. This limited exposure to cisplatin closely mimics the in vivo exposure.
Enzyme Activity in LLC-PK1 Cells
GGT activity is induced in LLC-PK1 cells as they become confluent and form monolayers (27). With the plating conditions used for these experiments, the cells became confluent on day three, and the GGT activity then increased approximately fourfold, reaching a maximum level by day 7 (data not shown).
The specific activity of GGT in the LLC-PK1 cells on day 7 is shown in Table 1. A comparison of GGT activity in LLC-PK1 cells with the activity in mouse kidney showed that the LLC-PK1 cells have 74% of the level of activity in the kidney. Aminopeptidase N activity was also assayed in both LLC-PK1 cells and mouse kidney. The specific activity of aminopeptidase N activity was 1.4-fold higher in the LLC-PK1 cells than the kidney. The largest difference in enzyme activity between LLC-PK1 cells and kidney was the level of cysteine-S-conju-gate beta-lyase activity. The cysteine-S-conjugate beta-lyase activity was measured in low-speed supernatants that included both cytosol and mitochondria. LLC-PK1 cells had only 16% of the activity measured in mouse kidney. These data demon-strate that all of the enzymes in the proposed pathway are expressed in LLC-PK1 cells, although the cysteine-S-conjugate beta-lyase activity is lower than in the kidney.
Table 1.
Specific activity of enzymes in confluent monolayers of LLC-PK1 cells and in mouse kidney
| Enzyme | LLC-PK1 Cells | Mouse Kidney |
|---|---|---|
| GGT | 446 ± 37a | 603 ± 89 |
| Aminopeptidase N | 12.6 ± 0.88 | 9.07 ± 1.63 |
| Cysteine-S-conjugate beta-lyase | 0.244 ± 0.05 | 1.52 ± 0.30 |
All enzyme units are expressed as mU/mg protein ± SD.
Potentiation of Cisplatin Toxicity: Formation of Toxic Cisplatin Derivatives
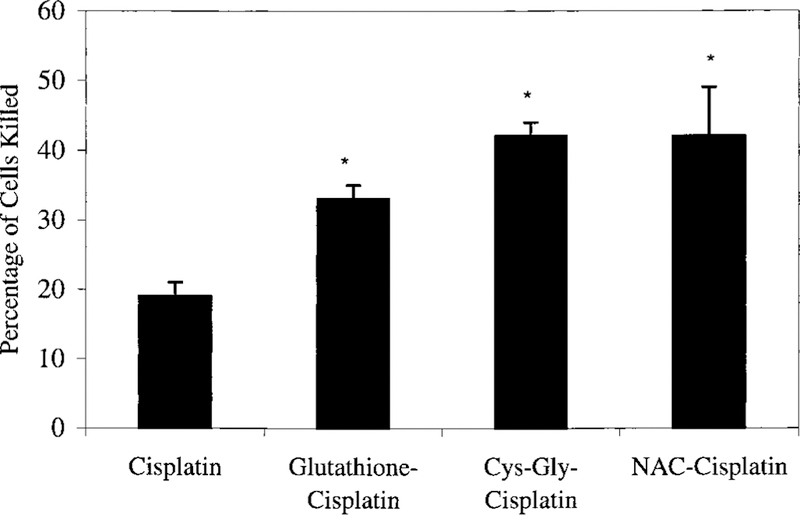
Preincubating cisplatin with equimolar glutathione for 30 min at 37°C significantly increased the toxicity of cisplatin (Figure 3). Confluent monolayers of LLC-PK1 cells were ex-posed to 50 µM cisplatin-glutathione or 50 µM cisplatin. When assayed for cell viability at 72 h, the data show that 50 µM cisplatin-glutathione killed 33% 2 of the cells versus 19% 2 of the cells killed by 50 µM cisplatin (P < 0.05). Preincubating cisplatin with equimolar cysteinyl-glycine or N-acetyl cysteine at 37°C for 30 min also potentiated the toxicity of cisplatin. Treatment for 3 h with 50 µM cisplatin-cysteinyl-glycine killed 42% 2 of the cells versus 19% 2 of the cisplatin-treated cells (P < 0.05). Treatment with 50 µM cisplatin-N-acetyl cysteine killed 41% 7 of the cells, significantly more than killed by cisplatin (P < 0.05).
Figure 3.
Toxicity of a 3 h treatment with cisplatin and cisplatin-conjugates toward confluent monolayers of LLC-PK1 cells. LLC-PK1 cells were exposed for 3 h to 50 mM cisplatin or 50 mM cisplatin preincubated with equimolar glutathione, cysteinyl-glycine, or N-acetyl-cysteine. The cisplatin solutions were removed at the end of the 3 h exposure, and fresh medium was added to the cells. At 72 h, the number of cells killed was determined as a percentage of the number of cells in control wells. Each point represents mean of three points ± SD. * differed significantly from the cisplatin-treated cells (P < 0.05)
Inhibition of GGT
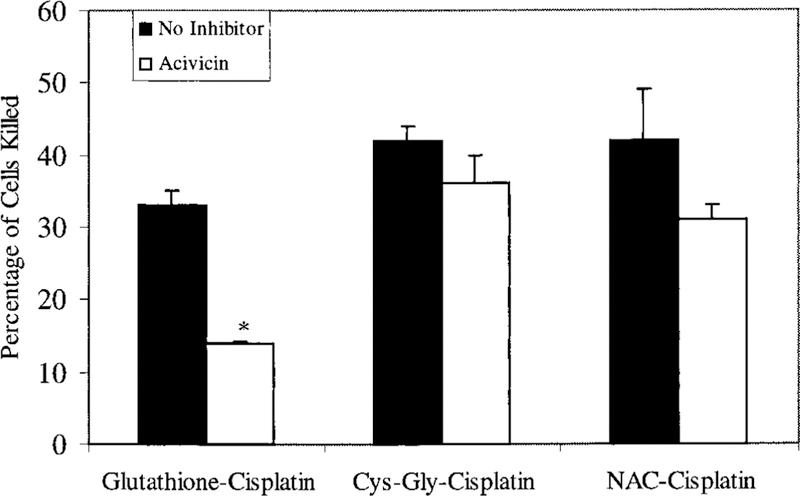
GGT is the first enzyme in the proposed metabolic pathway for activation of cisplatin-glutathione-conjugates. We inhibited GGT activity and determined the effect of this inhibition on the toxicity of cisplatin-glutathione-conjugates and on the toxicity of the two conjugates that are downstream of the GGT reac-tion, the cisplatin-cysteinyl-glycine-conjugate and the cisplatin-cysteine-conjugate. Preliminary experiments showed that acivicin inhibited GGT in monolayers of LLC-PK1 cells in a time- and dose-dependent manner (data not shown). For these studies, the cell monolayers were treated with 250 µM acivicin for 2 h before treatment with the cisplatin mixtures. The acivicin inhibited GGT activity by 89%, from 446 ± 37 mU/mg protein to 53 ± 8 mU/mg protein. The toxicity of the cisplatin-glutathione-conjugates was dose-dependent (Table 2). A 3 h treatment of LLC-PK1 cells with 25 µM cisplatin-glutathione killed 17% 5 of the cells, 50 µM killed 33 %± 4 of the cells. Inhibition of GGT reduced the total toxicity to 2% ± 5 and 14% ± 2, respectively. Inhibiting GGT activity eliminated all of the toxicity of the cisplatin-glutathione-con-jugate. In the presence of acivicin, the toxicity of 50 µM cisplatin-glutathione-conjugate was less than the toxicity of 50 µM cisplatin (Figure 3). In contrast, inhibition of GGT had no significant effect on the toxicity of the cisplatin-cysteinyl-glycine-conjugate or the cisplatin-NAC-conjugate (Figure 4). These data are consistent with our hypothesis as both the cysteinyl-glycine-platinum-conjugate and cysteine-conjugate are downstream of the GGT reaction. Therefore inhibiting GGT should have no effect on their toxicity.
Table 2.
Toxicity of a 3 h treatment with cisplatin-glutathione-conjugates towards LLC-PK1 cells
| Inhibitor | Dose of Cisplatin-Glutathione-Conjugates |
||
|---|---|---|---|
| 0 | 25 µM | 50 µM | |
| None | 0 ± 3a | 17 ± 5 | 33 ± 4 |
| Acivicin | 0 ± 1 | 2 ± 5b | 14 ± 2c |
| AOAA | 0 ± 3 | 0 ± 6b | 14 ± 6c |
Confluent monolayers of LLC-PK1 cells were exposed to cisplatin-glutathione-conjugates for 3 h in the presence or absence of acivicin or AOAA. Toxicity was assessed at 72 h. Data are the percentage of cells killed.
Values differ significantly from percentage of cells killed in the absence of inhibitor; P < 0.05.
Values differ significantly from percentage of cells killed in the absence of inhibitor; P < 0.01.
Figure 4.
Effect of acivicin on the toxicity of a 3 h treatment of cisplatin-conjugates. LLC-PK1 cells were exposed for 3 h to 50 mM cisplatin preincubated with equimolar glutathione, cysteinyl-glycine, or N-acetyl-cysteine. Cells were either treated with no inhibitor (black bars) or were treated with acivicin (white bars) to inhibit GGT activity. The cisplatin solutions and acivicin were removed at the end of the 3 h exposure and fresh medium added to the cells. At 72 h, the number of cells killed by the cisplatin-conjugates was determined as a percentage of the number of cells in control wells. Each point represents mean of three points ± SD. * treatment with acivicin significantly reduced the percentage of cells killed (P < 0.001)
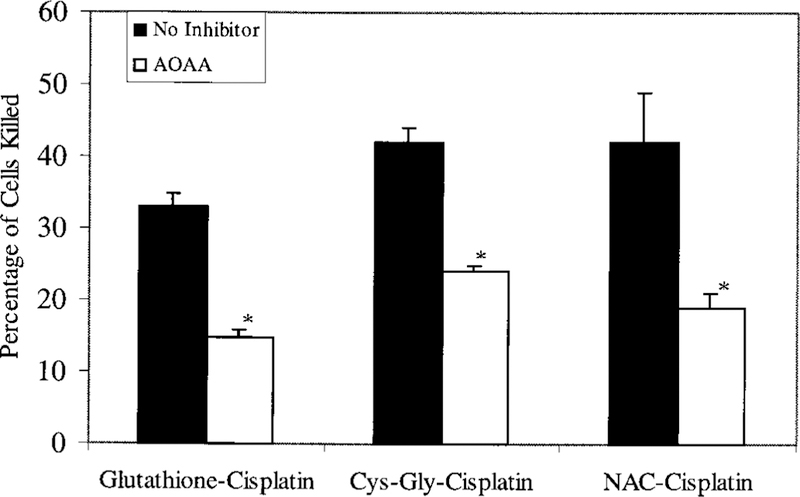
Inhibition of Cysteine-S-Conjugate Beta-Lyase
The beta-lyase reaction is the final reaction in the proposed metabolic activation of a cisplatin-glutathione-conjugate to a nephrotoxin. We inhibited cysteine-S-conjugate beta-lyase activity by pretreating the LLC-PK1 cells with 100 µM AOAA for 30 min before exposure to the cisplatin-conjugates (12,28,29,51). Inhibiting cysteine-S-conjugate beta-lyase significantly inhibited the toxicity of each of the cisplatin-conjugates in the proposed pathway (Figure 5). Pretreatment with AOAA reduced the toxicity of the cisplatin-glutathione-conjugate from 33% ± 2 of LLC-PK1 cells killed to 15% ± 1 (P < 0.001). The toxicity of both the cisplatin-cysteinyl-glycine and cisplatin-cysteine-conjugate was also significantly reduced by AOAA (P < 0.005). These data are consistent with our hypothesis,which predicts that each of the conjugates would be metabolized to a cisplatin-cysteine-conjugate then activated by cysteine-S-conjugate beta-lyase to a nephrotoxin. Controls within these experiments showed that pretreating cells with acivicin and AOAA resulted in a small but significant inhibition of the toxicity of 50µM cisplatin from 25% ± 1 of LLC-PK1 cells killed to 16% ± 5 (P < 0.05). The toxicity of cisplatin in the presence of the inhibitors is equivalent to the toxicity of the cisplatin-glutathione adducts with the inhibitors (Table 2). These data suggest that in the absence of the inhibitors the LLC-PK1 cells are conjugating a small portion of the cisplatin to glutathione and metabolizing it through the proposed pathway.
Figure 5.
Effect of AOAA on the toxicity of a 3 h treatment of cisplatin-conjugates. LLC-PK1 cells were exposed for 3 h to 50 µM cisplatin preincubated with equimolar glutathione, cysteinyl-glycine, or N-acetyl-cysteine. Cells were either treated with no inhibitor (black bars) or were treated with AOAA (white bars) to inhibit cysteine-S-conjugate beta-lyase activity. The cisplatin solutions and AOAA were removed at the end of the 3 h exposure and fresh medium added to the cells. At 72 h, the number of cells killed was determined as a percentage of the number of cells in control wells. Each point represents mean of three points ± SD. * treatment with AOAA significantly reduced the percentage of cells killed (P < 0.005)
Discussion
The development of a protocol for short-term exposure of a monolayer of kidney cells to clinically relevant concentrations of cisplatin has allowed us to test our hypothesis regarding the metabolic activation of cisplatin to a nephrotoxin. Incubating cisplatin in a balanced salt solution containing equimolar glutathione, cysteinyl-glycine or N-acetyl-cysteine increased the toxicity of the cisplatin. Cisplatin reacts spontaneously in solution with glutathione or cysteine to form cisplatin-glutathione or cisplatin-cysteine-conjugates (23,24,44). Inhibition of GGT blocked the toxicity of the cisplatin-glutathione-conjugate. These data support our hypothesis that cleavage of the cisplatin- glutathione-conjugate to a cisplatin-cysteinyl-glycine-conjugate by GGT is the first step in the metabolism of the cisplatin-glutathione-conjugate to a nephrotoxin. The inhibition of GGT had no effect on the toxicity of the cisplatincysteinyl- glycine-conjugate or the cisplatin-cysteine-conjugate, both of which are downstream of the GGT reaction in the proposed pathway. Inhibition of cysteine-S-conjugate betalyase reduced the toxicity of the cisplatin-glutathione- conjugate,the cisplatin-cysteinyl-glycine-conjugate, and the cisplatin-cysteine-conjugate. These data are also consistent with our hypothesis that cysteine-S-conjugate beta-lyase catalyzes the final step in the pathway converting the cisplatin-cysteineconjugate to a reactive thiol.
Data from in vivo studies support the hypothesis that the formation of a cisplatin-glutathione-conjugate is an essential component of the nephrotoxicity of cisplatin. Cisplatin-glutathione- conjugates have been identified in rat kidneys (25). Buthionine-sulfoximine, a glutathione-depleting agent, diminished the nephrotoxic effects of cisplatin in rats when injected 2 hrs before treatment (52,53). Several studies have presented data indicating that in vivo the formation of cisplatin-glutathi-one-conjugates is catalyzed by glutathione-S-transferases. Sad-zuka et al. treated rats with ketoprofen, an inhibitor of gluta-thione-S-transferase pi, before cisplatin treatment and found a significant decrease in nephrotoxicity (26). Decreased levels of glutathione or glutathione-S-transferase activity would reduce the formation of the cisplatin-glutathione-conjugate, the sub-strate for GGT in the first step of the proposed activation pathway. We are characterizing the cisplatin-adducts that are formed during the incubation of cisplatin with glutathione, cysteinyl-glycine, or NAC. The incubation mixtures are being analyzed by high-pressure liquid chromatography and mass spectrometry to determine the relative abundance and structure of each adduct. An abstract describing the preliminary results of those studies has been published (54).
Administration of high doses of glutathione within 30 min of cisplatin administration has been shown to protect against cisplatin-induced nephrotoxicity (55,56). The amount of glutathione that is necessary to achieve this protective effect is 83-fold higher than the cisplatin concentration. Whereas these data may appear to contradict our hypothesis, that the formation of a cisplatin-glutathione-conjugate activates cisplatin to a nephrotoxin, we have proposed that the high concentration of glutathione protects against cisplatin nephrotoxicity by serving as a competitive inhibitor of GGT activity (8). Glutathione is the major physiologic substrate for GGT (57). GGT is localized to the cell surface and would be inhibited by high levels of glutathione in the extracellular fluid. By inhibiting GGT activity, glutathione would reduce the metabolism of the cisplatin-glutathione- to a cisplatin-cysteinyl-glycine-conjugate.
Several additional studies indicate that cisplatin is activated to a nephrotoxin via metabolism through a series of interme-diate-compounds. Daley-Yates and McBrien reported that within 15 min after injecting rats with a single dose of cisplatin, seven platinum-containing species were present in plasma that could be separated via HPLC (58). The mixture of platinum containing species was more nephrotoxic than cisplatin. Cisplatin-cysteine-conjugates have been identified in the kidneys of cisplatin-treated rats (25). Maines incubated cysteine and cisplatin in a 2:1 molar ratio for 30 min at 37°C (59). He reported that in the kidney the cysteine-platinum incubation mixture was a more potent inhibitor of hemometabolism and glutathione synthesis than cisplatin.
The final step in the proposed activation pathway is the metabolism of the cysteine-S-conjugate to a reactive thiol. Cysteine-S-conjugate beta-lyases catalyze a beta-elimination reaction of the halogenated alkene-cysteine-S-conjugates (60). Several proteins have been identified in kidney that can cata-lyze this reaction. All are PLP-dependent and are therefore inhibited by AOAA (61). Mitochondria appear to be the primary target of the cysteine-S-conjugate beta-lyase–mediated toxicity of the haloalkenes (62,63). A high–molecular weight protein complex in kidney mitochondria has been shown to have cysteine-S-conjugate beta-lyase activity (13,64). This high-molecular weight complex may contain the dominant enzyme that catalyzes the conversion of the cisplatin-cysteine-conjugate to a reactive thiol in vivo. The enzyme has not yet been identified.
Possible alternative mechanisms of action by the inhibitors have been ruled out by our in vivo studies. In our original study of the role of GGT in cisplatin nephrotoxicity, we used acivicin to inhibit GGT in vivo (8). Acivicin is a glutamine analogue. It blocks L-glutamine–requiring enzymes and at high concentrations inhibits de novo synthesis of purine and pyrimidines (65). To determine whether acivicin affected cisplatin nephrotoxicity by inhibiting GGT or through an alternative mechanism, we assessed the nephrotoxicity of cisplatin in GGT knockout mice (7). The data from both the acivicin studies and the GGT-knockout mice showed that renal cisplatin toxicity is dependent on GGT activity. Inhibition of cysteine-S-conjugate beta-lyase with AOAA in mice demonstrated that inhibition of this enzyme blocked the nephrotoxicity of cisplatin but had no effect on the uptake of platinum into the kidney (6).
While conjugation of cisplatin to glutathione is the first step in the activation of cisplatin to a nephrotoxin, it renders cisplatin inactive as an antitumor drug. Conjugation of cisplatin with glutathione reduces the formation of interstrand and intrastrand platinum DNA adducts, resulting in decreased toxicity of cisplatin in dividing cells (66). This contradictory role of glutathione conjugation in the nephrotoxicity and antitumor activity of the drug is further confirmed by data showing that depletion of glutathione by buthionine-sulfoximine or reduction of glutathione-S-transferase activity potentiated the anti-tumor activity of cisplatin (66,67). Increased levels of intracellular glutathione or glutathione-S-transferase have been associated with cisplatin resistance in tumor cells (68 –72). Expression of GGT also has opposing roles in the nephrotoxicity and antitumor activity of cisplatin. GGT expression is necessary for the metabolism of cisplatin to a nephrotoxin (7,8). However, GGT expression in tumors decreases the antitumor activity of the drug (73). These contradictory effects may be due to differences among tissues in the uptake of the cisplatin-cysteine-conjugates or in expression of the cysteine-conjugate beta-lyase that converts the cisplatin-cysteine-conjugate to a reactive thiol. These issues will be investigated in our future studies.
Thiol compounds are used in clinical practice to mitigate cisplatin-induced nephrotoxicity. Some thiol compounds such as diethyldithiocarbamate bind to cisplatin and inactive it, reducing both the antitumor and nephrotoxic activity of the drug (74). We propose that these compounds block both the binding of cisplatin to DNA and the binding of cisplatin to glutathione, preventing its further metabolism to a nephrotoxin. A large number of sulfur-containing compounds have been shown to reduce the nephrotoxicity of cisplatin without inhib-iting its antitumor effect (75). Some of these agents, such as the prodrug Amifostine, are used in the clinic to protect against cisplatin nephrotoxicity (76). Procainamide, an antiarrhythmic drug, also protects against the nephrotoxicity of cisplatin with-out altering its antitumor activity (77). The formation of the procainamide-cisplatin complex increases the amount of plat-inum bound to DNA, which would explain the maintenance of the antitumor activity of cisplatin in the presence of procainamide (78). The binding of procainamide to the cisplatin may prevent the formation of a cisplatin-glutathione complex and thereby protect against the metabolism of cisplatin to a nephrotoxin. The thiol agents may be working by the same mechanism as procainamide, forming complexes with cisplatin that do not prevent the binding of the platinum to DNA but do prevent the formation of a glutathione-cisplatin-conjugate.
In this study, we have demonstrated that preincubating cisplatin with glutathione, cysteinyl-glycine, or NAC potentiates its toxicity toward LLC-PK1 cells. In all of these assays, the cells were incubated in HBSS buffered with HEPES during the 3 h exposure to cisplatin or cisplatin adducts. Preliminary experiments had shown that the toxicity of cisplatin is modu-lated if the cells are incubated in tissue culture medium during the cisplatin exposure. Cisplatin was less toxic to LLC-PK1 cells when they were incubated in DMEM, rather than HBSS, during the 3 h cisplatin exposure. However, cisplatin was more toxic when the cells were incubated in RPMI-1640 medium during the exposure period. In a separate study, we have found that the modulation of cisplatin toxicity by the media is due to the interplay of several media components. We are analyzing the effect of each component and determining the mechanism by which it influences cisplatin toxicity. Those data will be presented in a forthcoming paper. To eliminate the confound-ing variables in the media, all of the studies in this paper have been done with the cells in HBSS, a balanced salt solution, during the 3 h exposure to cisplatin.
It is a common assumption that conjugation to glutathione is a detoxification mechanism; but, as has been shown for the halogenated-alkenes and now cisplatin, conjugation to glutathione is the first step in the pathway that activates some compounds to potent nephrotoxins. The data in this study further define the steps in the metabolism of cisplatin to a nephrotoxin. Delineation of this pathway provides insights into the distinct nephrotoxic and antitumor activity of cisplatin. Strategic inhibition of critical components of this pathway could reduce the nephrotoxicity of cisplatin while potentiating its antitumor effect.
Acknowledgments
This work was supported by Grant R01CA57530 to MHH from the National Cancer Institute.
References
- 1.Giaccone G: Clinical perspectives on platinum resistance. Drugs 59[Suppl 4]: 9–17, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Pinzani V, Bressolle F, Haug IJ, Galtier M, Blayac JP: Cisplatin induced renal toxicity and toxicity-modulating strategies: A re- view. Cancer Chemother Pharmacol 35: 1–9, 1994 [DOI] [PubMed] [Google Scholar]
- 3.Fink D, Howell SB: How does cisplatin kill cells? In: Platinum-based drugs in cancer therapy edited by Kelland LR, Favilli F, Totowa, New Jersey, Humana Press, 2000, pp 149–167 [Google Scholar]
- 4.Lieberthal W, Triaca V, Levine J: Mechanisms of death induced by cisplatin in proximal tubular epithelial cells: apoptosis vs. necrosis. Am J Physiol 270: F700–F708, 1996 [DOI] [PubMed] [Google Scholar]
- 5.Park MS, De Leon M, Devarajan P: Cisplatin induces apoptosis in LLC-PK1 cells via activation of mitochondrial pathways. J Am Soc Nephrol 13: 858–865, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Townsend DM, Hanigan MH: Inhibition of gamma-glutamyl transpeptidase or cysteine S-conjugate beta-lyase activity blocks the nephrotoxicity of cisplatin in mice. J Pharmacol Exp Ther 300 142–148, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanigan MH, Lykissa ED, Townsend DM, Ou C, Barrios R, Lieberman MW: Gamma-glutamyl transpeptidase-deficient mice are resistant to the nephrotoxicity of cisplatin. Am J Pathol 159: 1889–1894, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanigan MH, Gallagher BC, Taylor PT Jr, Large MK: Inhibition of gamma-glutamyl transpeptidase activity by acivicin in vivo protects the kidney from cisplatin-induced toxicity. Cancer Res 54 5925–5929, 1994 [PubMed] [Google Scholar]
- 9.Lash LH, Parker JC: Hepatic and renal toxicities associated with perchloroethylene. Pharmacol Rev 53: 177–208, 2001 [PubMed] [Google Scholar]
- 10.Dekant W, Vamvakas S, Anders MW: Formation and fate of nephrotoxic and cytotoxic glutathione S-conjugates: Cysteine- conjugate beta-lyase pathway. In: Advances in Pharmacology, Vol. 27, edited by Anders MW, Dekant W, New York, Academic Press Inc., 1995, pp 115–162 [DOI] [PubMed] [Google Scholar]
- 11.Elfarra AA, Jakobson I, Anders MW: Mechanism of S-(1,2-dichlorovinyl)glutathione-induced nephrotoxicity. Biochem Pharmacol 35: 283–288, 1986 [DOI] [PubMed] [Google Scholar]
- 12.Finkelstein MB, Baggs RB, Anders MW: Nephrotoxicity of the glutathione and cysteine-conjugates of 2-bromo-2-chloro-1,1-di-fluoroethene. J Pharmacol Exp Ther 261: 1248–1252, 1992 [PubMed] [Google Scholar]
- 13.Cooper AJ, Wang J, Gartner CA, Bruschi SA: Co-purification of mitochondrial HSP70 and mature protein disulfide isomerase with a functional rat kidney high-M(r) cysteine S-conjugate beta-lyase. Biochem Pharmacol 62: 1345–1353, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Hanigan MH, Frierson HF Jr.: Immunohistochemical detection of gamma-glutamyl transpeptidase in normal human tissue. J Histochem Cytochem 44: 1101–1108, 1996 [DOI] [PubMed] [Google Scholar]
- 15.Hanigan MH: Gamma-glutamyl transpeptidase, a glutathionease: Its expression and function in carcinogenesis. Chem Biol Inter-actions 111: 112:333–342, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Hughey RP, Rankin BB, Elce JS, Curthoys NP: Specificity of a particulate rat renal paptidase and its localization along with other enzymes of mercapturic acid synthesis. Arch Biochem Biophys 186: 211–217, 1978 [DOI] [PubMed] [Google Scholar]
- 17.Schaeffer VH, Stevens JL: The transport of S-cysteine-conju-gates in LLC-PK1 cells and its role in toxicity. Mol Pharmacol 31: 506–512, 1987 [PubMed] [Google Scholar]
- 18.Wolfgang GHI, Gandolfi AJ, Stevens JL, Brendel K: N-acetyl S-(1,2-dichlorovinyl)-l-cysteine produces a similar toxicity to s-(1,2-dichlorovinyl)-l-cysteine in rabbit renal slices: Differential transport and metabolism. Toxicol Appl Pharmacol 101: 205– 219, 1989 [DOI] [PubMed] [Google Scholar]
- 19.Chen Q, Yu K, Stevens JL: Regulation of the cellular stress response by reactive electrophiles. The role of covalent binding and cellular thiols in transcriptional activation of the 70-kilodal-ton heat shock protein gene by nephrotoxic cysteine-conjugates. J Biol Chem 267: 24322–24327, 1992 [PubMed] [Google Scholar]
- 20.Jaffe DR, Hassall CD, Brendel K, Gandolfi AJ: In vivo and in vitro nephrotoxicity of the cysteine-conjugate of hexachlorobuta-diene. J Toxicol Environ Health 11: 857–867, 1983 [DOI] [PubMed] [Google Scholar]
- 21.Choie DD, Longnecker DS, del Campo AA: Acute and chronic cisplatin nephropathy in rats. Lab Invest 44: 397–402, 1981 [PubMed] [Google Scholar]
- 22.Levine BS, Henry MC, Port CD, Richter WR, Urbanek MA: Nephrotoxic potential of cis-diamminedichloroplatinum and four analogs in male Fischer 344 rats. JNCI 67: 201–206, 1981 [PubMed] [Google Scholar]
- 23.Bernareggi A, Torti L, Facino RM, Carini M, Depta G, Casetta B, Farrell N, Spadacini S, Ceserani R, Tognella S: Characteriza- tion of cisplatin-glutathione adducts by liquid chromatography-mass spectrometry: Evidence for their formation in vitro but not in vivo after concomitant administration of cisplatin and gluta-thione to rats and cancer patients. J Chromatogr 669: 247–263, 1995 [DOI] [PubMed] [Google Scholar]
- 24.Ishikawa T, Ali-Osman F: Glutathione-associated cis-diam-minedichloroplatinum(II) metabolism and ATP-dependent efflux from leukemia cells. J Biol Chem 268: 20116–20125, 1993 [PubMed] [Google Scholar]
- 25.Mistry P, Lee C, McBrien DC: Intracellular metabolites of cis-platin in the rat kidney. Cancer Chemother Pharmacol 24: 73–79, 1989 [DOI] [PubMed] [Google Scholar]
- 26.Sadzuka Y, Shimizu Y, Takino Y, Hirota S: Protection against cisplatin-induced nephrotoxicity in the rat by inducers and an inhibitor of glutathione S-transferase. Biochem Pharmacol 48: 453–459, 1994 [DOI] [PubMed] [Google Scholar]
- 27.Stevens J, Hayden P, Taylor G: The role of glutathione-conjugate metabolism and cysteine-conjugate B-lyase in the mechanism of S-cysteine-conjugate toxicity in LLC-PK1 cells. J Biol Chem 261 3325–3332, 1986 [PubMed] [Google Scholar]
- 28.Otieno MA, Anders MW: Stable transfection of LLC-PK1 cells with human microsomal glutathione s-transferase gene increases haloalkene glutathione s-conjugate formation and cytotoxicity. Biochem Biophys Res Comm 234: 481–484, 1997 [DOI] [PubMed] [Google Scholar]
- 29.Mertens JJWM, Weijnen JGJ, Van Doorn WJ, Spenkelink B, Temmink JHM, Van Bladeren PJ: Differential toxicity as a result of apical and basolateral treatment of LLC-PK1 monolayers with S-(1,2,3,4,4-pentachlorobutadienyl) glutathione and N-acetyl-S-(1,2,3,4,4-pentachlorobutadienyl)-L-cysteine. Chem Biol Inter-actions 65: 283–293, 1988 [DOI] [PubMed] [Google Scholar]
- 30.Elfarra AA, Anders MW: Renal processing of glutathione-conjugates, role in nephrotoxicity. Biochem Pharmacol 33: 3729–3732, 1984 [DOI] [PubMed] [Google Scholar]
- 31.Sepulveda FV, Burton KA, Pearson JD: The development of gamma-glutamyltransferase in a pig renal-epithelial- cell line in vitro. Relationship to amino acid transport. Biochem J 208: 509–512, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rabito CA, Kreisberg JI, Wight D: Alkaline phosphatase and t-glutamyl transpeptidase as polarization markers during the or-ganization of LLC-PK1 cells into an epithelial membrane. J Biol Chem 259: 574–582, 1984 [PubMed] [Google Scholar]
- 33.Komatsuda A, Wakui H, Oyama Y, Imai H, Miura AB, Itoh H, Tashima Y: Overexpression of the human 72 kDa heat shock protein in renal tubular cells confers resistance against oxidative injury and cisplatin toxicity. Nephrol Dial Transplant 14: 1385–1390, 1999 [DOI] [PubMed] [Google Scholar]
- 34.Uozumi J, Koikawa Y, Yasumasu T, Tokuda N, Ueda T, Kumazawa J: The effect of methylprednisolone on platinum kinetics and urinary enzyme excretion following intravenous cisplatin in vivo and on the growth inhibition of LLC-PK1 cells by cisplatin in vitro. Res Exp Med 196: 211–217, 1996 [DOI] [PubMed] [Google Scholar]
- 35.Kawabe T, Chen ZS, Wada M, Uchiumi T, Ono M, Akiyama S, Kuwano M: Enhanced transport of anticancer agents and leuko-triene C4 by the human canalicular multispecific organic anion transporter (cMOAT/MRP2). FEBS Lett 456: 327–331, 1999 [DOI] [PubMed] [Google Scholar]
- 36.Kelland LR: Preclinical perspectives on platinum resistance. Drugs 59[Suppl 4]: 1–8, 2000 [DOI] [PubMed] [Google Scholar]
- 37.Trimmer EE, Essigmann JM: Cisplatin. Essays Biochem 34: 191–211, 1999 [DOI] [PubMed] [Google Scholar]
- 38.Cornelison TL, Reed E: Nephrotoxicity and hydration manage-ment for cisplatin, carboplatin, and ormaplatin. Gynecol Oncol 50 147–158, 1993 [DOI] [PubMed] [Google Scholar]
- 39.Montine TJ, Borch RF: Quiescent LLC-PK1 cells as a model for cis-diamminedichloroplatinum (II) nephrotoxicity and modula-tion by thiol rescue agents. Cancer Res 48: 6017–6024, 1988 [PubMed] [Google Scholar]
- 40.DeConti RC, Toftness BR, Lange RC, Creasey WA: Clinical and pharmacological studies with cis-diamminedichloroplatinum(II). Cancer Res 33: 1310–1315, 1973 [PubMed] [Google Scholar]
- 41.Lelieveld P, Van der Vijgh WJ, Veldhuizen RW, Van Velzen D, Van Putten LM, Atassi G, Danguy A: Preclinical studies on toxicity, antitumour activity and pharmacokinetics of cisplatin and three recently developed derivatives. Eur J Cancer Clin Oncol 20: 1087–1104, 1984 [DOI] [PubMed] [Google Scholar]
- 42.Nagai N, Kinoshita M, Ogata H, Tsujino D, Wada Y, Someya K, Ohno T, Masuhara K, Tanaka Y, Kato K, Nagai H, Yokoyama A, Kurita Y: Relationship between pharmacokinetics of unchanged cisplatin and nephrotoxicity after intravenous infusions of cis-platin to cancer patients. Cancer Chemother Pharmacol 39: 131–137, 1996 [DOI] [PubMed] [Google Scholar]
- 43.Bose RN, Ghosh SK, Moghaddas S: Kinetic analysis of the cis-diamminedichloroplatinum(II)– cysteine reaction: Implica-tions to the extent of platinum–DNA binding. J Inorg Biochem 65 199–205, 1997 [DOI] [PubMed] [Google Scholar]
- 44.Berners-Price SJ, Kuchel PW: Reaction of cis- and trans-[PtCl2(NH3)2] with reduced glutathione studied by 1H, 13C, 19Pt, and 15N-1HDEPT NMR. J Inorg Biochem 38: 305–326, 1990 [DOI] [PubMed] [Google Scholar]
- 45.Mosmann T: Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods 65: 55–63, 1983 [DOI] [PubMed] [Google Scholar]
- 46.Hanigan MH, Ricketts WA: Extracellular glutathione is a source of cysteine for cells that express gamma-glutamyl transpeptidase. Biochemistry 32: 6302–6306, 1993 [DOI] [PubMed] [Google Scholar]
- 47.Perry SJ, Schofield MA, MacFarlane M, Lock EA, King LJ, Gibson GG, Goldfarb PS: Isolation and expression of a cDNA coding for rat kidney cytosolic cysteine-conjugate beta-lyase. Mol Pharmacol 43: 660–665, 1993 [PubMed] [Google Scholar]
- 48.McKinney LL, Picken JC Jr., Weakley FB, Eldridge AC, Camp-bell RE, Cowan JC, Biester HE: Possible toxic factor of trichlo-roethylene-extracted soybean oil meal. J Am Chem Soc 81: 909–915, 1959 [Google Scholar]
- 49.Glantz SA: Primer of Bio-Statics, 3rd edition. New York, McGraw-Hill, Inc., 1992 [Google Scholar]
- 50.Dobyan DC, Levi J, Jacobs C, Kosek J, Weiner MW: Mechanism of cis-platinum nephrotoxicity: II. Morphological observations. J Pharmacol Exp Ther 213: 551–556, 1980 [PubMed] [Google Scholar]
- 51.Stevens J, Hayden P, Taylor G: The role of glutathione-conjugate metabolism and cysteine-conjugate b-lyase in the mechanism of S-cysteine-conjugate toxicity in LLC-PK1 cells. J Biol Chem 261 3325–3332, 1986 [PubMed] [Google Scholar]
- 52.Mayer RD, Lee K, Cockett ATK: Inhibition of cisplatin-induced nephrotoxicity in rats by buthionine sulfoximine, a glutathione synthesis inhibitor. Cancer Chemother Pharmacol 20: 207–210, 1987 [DOI] [PubMed] [Google Scholar]
- 53.Mayer RD, Lee K, Cockett AT: Improved use of buthionine sulfoximine to prevent cisplatin nephrotoxicity in rats. J Cancer Res Clin Oncol 115: 418–422, 1989 [DOI] [PubMed] [Google Scholar]
- 54.Townsend DM, Marto JA, Macdonald TL, Hanigan MH: Char-acterization of a glutathione-conjugate of cisplatin that is metab-olized by gamma-glutamyl transpeptidase to a nephrotoxin. Proceedings from the American Association for Cancer Research 42 201, 2001 [Google Scholar]
- 55.Cozzaglio L, Doci R, Colla G, Zunino F, Casciarri G, Gennari L: A feasibility study of high-dose cisplatin and 5-fluorouracil with glutathione protection in the treatment of advanced colorectal cancer. Tumori 76: 590–594, 1990 [DOI] [PubMed] [Google Scholar]
- 56.Zunino F, Pratesi G, Micheloni A, Cavalletti E, Sala F, Tofanetti O Protective effect of reduced glutathione against cisplatin-induced renal and systemic toxicity and its influence on the therapeutic activity of the antitumor drug. Chem Biol Interac-tions 70: 89–101, 1989 [DOI] [PubMed] [Google Scholar]
- 57.Hanigan MH, Pitot HC: Gamma-glutamyl transpeptidase — Its role in hepatocarcinogenesis. Carcinogenesis 6: 165–172, 1985 [DOI] [PubMed] [Google Scholar]
- 58.Daley-Yates PT, McBrien DCH: Cisplatin metabolites in plasma: A study of their pharmacokinetics and importance in the neph-rotoxic and antitumour activity of cisplatin. Biochem Pharmacol 33 3063–3070, 1984 [DOI] [PubMed] [Google Scholar]
- 59.Maines MD: Differential effect of cis-platinum (cis-diamminedi-chloroplatinum) on regulation of liver and kidney haem and haemoprotein metabolism. Biochem J 237: 713–721, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Anders MW, Dekant W: Glutathione-dependent bioactivation of haloalkenes. Annu Rev Pharmacol Toxicol 38: 501–537, 1998 [DOI] [PubMed] [Google Scholar]
- 61.Cooper AJ: Mechanisms of cysteine S-conjugate beta-lyases. Adv Enzymol Relat Areas Mol Biol 72: 199–238, 1998 [DOI] [PubMed] [Google Scholar]
- 62.Bruschi SA, West KA, Crabb JW, Gupta RS, Stevens JL: Mito-chondrial HSP60 (P1 protein) and a HSP70-like protein (mor-talin) are major targets for modification during S-(1,1,2,2-tet-rafluoroethyl)- L-cysteine-induced nephrotoxicity. J Biol Chem 268 23157–23161, 1993 [PubMed] [Google Scholar]
- 63.Chen Y, Cai J, Anders MW, Stevens JL, Jones DP: Role of mitochondrial dysfunction in S-(1,2-dichlorovinyl)-l-cysteine-induced apoptosis. Toxicol Appl Pharmacol 170: 172–180, 2001 [DOI] [PubMed] [Google Scholar]
- 64.Abraham DG, Thomas RJ, Cooper AJ: Glutamine transaminase K is not a major cysteine S-conjugate beta-lyase of rat kidney mitochondria: Evidence that a high-molecular weight enzyme fulfills this role. Mol Pharmacol 48: 855–860, 1995 [PubMed] [Google Scholar]
- 65.Earhart RH, Neil GL: Acivicin in 1985. In: Advances in Enzyme Regulation, Vol. 24, edited by Weber G, New York, Pergamon Press, 1985, pp 179–205 [DOI] [PubMed] [Google Scholar]
- 66.Gosland M, Lum B, Schimmelpfennig J, Baker J, Doukas M: Insights into mechanisms of cisplatin resistance and potential for its clinical reversal. Pharmacotherapy 16: 16–39, 1996 [PubMed] [Google Scholar]
- 67.Shoieb AM, Hahn KA, Van Laack RL, Barnhill MA: In vitro reversal of glutathione-s-transferase-mediated resistance in ca-nine osteosarcoma (COS31) cells. In Vivo 12: 455–462, 1998 [PubMed] [Google Scholar]
- 68.Godwin AK, Meister A, O’Dwyer PJ, Huang CS, Hamilton TC, Anderson ME: High resistance to cisplatin in human ovarian cancer cell lines is associated with marked increase of glutathi-one synthesis. Proc Natl Acad Sci USA 89: 3070–3074, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nishimura T, Newkirk K, Sessions RB, Andrews PA, Trock BJ, Rasmussen AA, Montgomery EA, Bischoff EK, Cullen KJ: Immunohistochemical staining for glutathione S-transferase pre-dicts response to platinum-based chemotherapy in head and neck cancer. Clin Cancer Res 2: 1859–1865, 1996 [PubMed] [Google Scholar]
- 70.Ban N, Takahashi Y, Takayama T, Kura T, Katahira T, Sakamaki S, Niitsu Y: Transfection of glutathione S-transferase (GST)-pi antisense complementary DNA increases the sensitivity of a colon cancer cell line to adriamycin, cisplatin, melphalan, and etoposide. Cancer Res 56: 3577–3582, 1996 [PubMed] [Google Scholar]
- 71.Goto S, Iida T, Cho S, Oka M, Kohno S, Kondo T: Overexpres-sion of glutathione S-transferase pi enhances the adduct forma-tion of cisplatin with glutathione in human cancer cells. Free Radic Res 31: 549–558, 1999 [DOI] [PubMed] [Google Scholar]
- 72.Puchalski RB, Fahl WE: Expression of recombinant glutathione S-transferase pi, Ya, or Yb1 confers resistance to alkylating agents. Proc Natl Acad Sci USA 87: 2443–2447, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hanigan MH, Gallagher BC, Townsend DM, Gabarra V: Gam-ma-glutamyl transpeptidase accelerates tumor growth and in-creases the resistance of tumors to cisplatin in vivo. Carcinogen-esis 20: 553–559, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Borch RF, Katz JC, Lieder PH, Pleasants ME: Effect of dieth-yldithiocarbamate rescue on tumor response to cis-platinum in a rat model. Proc Natl Acad Sci USA 77: 5441–5444, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jones MM, Basinger MA, Holscher MA: Control of the nephro-toxicity of cisplatin by clinically used sulfur-containing com-pounds. Fundam Appl Toxicol 18: 181–188, 1992 [DOI] [PubMed] [Google Scholar]
- 76.Korst AE, Boven E, van der Sterre ML, Fichtinger-Schepman AM, Van der Vijgh WJ: Pharmacokinetics of cisplatin with and without amifostine in tumour-bearing nude mice. Eur J Cancer 34: 412–416, 1998 [DOI] [PubMed] [Google Scholar]
- 77.Esposito M, Viale M, Vannozzi MO, Zicca A, Cadoni A, Merlo F, Gogioso L: Effect of the antiarrhythmic drug procainamide on the toxicity and antitumor activity of cis-diamminedochloroplati-num(II). Toxicol Appl Pharmacol 140: 370–377, 1996 [DOI] [PubMed] [Google Scholar]
- 78.Viale M, Vannozzi MO, Pastrone I, Mariggio MA, Zicca A, Cadoni A, Cafaggi S, Tolino G, Lunardi G, Civalleri D, Lindup WE, Esposito M: Reduction of cisplatin nephrotoxicity by pro-cainamide: does the formation of a cisplatin-procainamide com-plex play a role? J Pharmacol Exp Ther 293: 829–836, 2000 [PubMed] [Google Scholar]