ABSTRACT
Chorea-acanthocytosis (ChAc) is a rare neurodegenerative disease associated with mutations in the human VPS13A gene. The mechanism of ChAc pathogenesis is unclear. A simple yeast model was used to investigate the function of the single yeast VSP13 orthologue, Vps13. Vps13, like human VPS13A, is involved in vesicular protein transport, actin cytoskeleton organisation and phospholipid metabolism. A newly identified phenotype of the vps13Δ mutant, sodium dodecyl sulphate (SDS) hypersensitivity, was used to screen a yeast genomic library for multicopy suppressors. A fragment of the MYO3 gene, encoding Myo3-N (the N-terminal part of myosin, a protein involved in the actin cytoskeleton and in endocytosis), was isolated. Myo3-N protein contains a motor head domain and a linker. The linker contains IQ motifs that mediate the binding of calmodulin, a negative regulator of myosin function. Amino acid substitutions that disrupt the interaction of Myo3-N with calmodulin resulted in the loss of vps13Δ suppression. Production of Myo3-N downregulated the activity of calcineurin, a protein phosphatase regulated by calmodulin, and alleviated some defects in early endocytosis events. Importantly, ethylene glycol tetraacetic acid (EGTA), which sequesters calcium and thus downregulates calmodulin and calcineurin, was a potent suppressor of vps13Δ. We propose that Myo3-N acts by sequestering calmodulin, downregulating calcineurin and increasing activity of Myo3, which is involved in endocytosis and, together with Osh2/3 proteins, functions in endoplasmic reticulum-plasma membrane contact sites. These results show that defects associated with vps13Δ could be overcome, and point to a functional connection between Vps13 and calcium signalling as a possible target for chemical intervention in ChAc. Yeast ChAc models may uncover the underlying pathological mechanisms, and may also serve as a platform for drug testing.
This article has an associated First Person interview with the first author of the paper.
KEY WORDS: Yeast, Chorea-acanthocytosis, Vps13, Myo3, Calcium signalling, Endocytosis
Summary: Using the vps13Δ strain, a yeast model of the neurodegenerative disorder chorea-acanthocytosis, we found that its defects can be overcome by reduction of calcineurin activity and/or type-I-myosin activation.
INTRODUCTION
The VPS13 (vacuolar protein sorting 13) proteins are conserved among eukaryota. In humans, the VPS13 protein family consists of four members encoded by the VPS13A, B, C and D genes (Velayos-Baeza et al., 2004). Mutations in VPS13A lead to a rare, fatal neurodegenerative disease: chorea-acanthocytosis (ChAc; OMIM 200150) (Rampoldi et al., 2001; Rubio et al., 1997; Ueno et al., 2001). ChAc is characterised by many neurological symptoms, including: chorea; dystonia and twitches; and, often, the presence of acanthocytes (erythrocytes with a spiked morphology) (Hardie et al., 1991). Several studies have shown a role of the VPS13 proteins in cytoskeletal organisation (De Franceschi et al., 2011; Föller et al., 2012), vesicular transport (Honisch et al., 2015; Schmidt et al., 2013), autophagy (Muñoz-Braceras et al., 2015) and phosphatidylinositol metabolism (Park et al., 2015); however, the molecular functions remain unclear. Mutations that cause ChAc usually result in a reduction or absence of VPS13A, but several cases of patients with amino acid substitutions have been described (reviewed in Rzepnikowska et al., 2017b). Mutations in the other VPS13 genes are also associated with various neurological, mental and developmental disorders and intellectual disabilities (Fromer et al., 2014; Kolehmainen et al., 2003; Lesage et al., 2016). Studies have also reported links between mutations in the VPS13 genes with diabetes (Grarup et al., 2011; Saxena et al., 2010) and with cancer (Furukawa et al., 2011; Morisaki et al., 2014). Currently, there is not an effective therapy for neurodegenerative disorders linked to VPS13 mutations.
There is a single Vps13 protein of 3144 amino acid residues (aa) in the yeast Saccharomyces cerevisiae. This yeast Vps13 protein shares the highest degree of similarity (in terms of domain structure) with human VPS13A. The yeast VPS13 gene was initially identified in a screen for mutants that secrete the vacuolar enzyme carboxypeptidase Y (CPY; EC 3.4.16.1), which implies a role in protein targeting to the vacuole (Bankaitis et al., 1986). Further studies, including those modelling mutations identified in patients, showed the importance of Vps13 in vesicular transport, particularly for Golgi-to-vacuole transport (Brickner and Fuller, 1997; De et al., 2017; Redding et al., 1996; Rzepnikowska et al., 2017a), endosomal trafficking (Dalton et al., 2017; Luo and Chang, 1997; Rzepnikowska et al., 2017a) and mitochondrial DNA maintenance (Park et al., 2016). Furthermore, it was recently shown that Vps13 is present at membrane contact sites – zones of physical contact between two organelles or between an organelle and a plasma membrane, which mediate direct transport of lipids, ions and metabolites. So far, Vps13 has been identified at the nuclear-vacuolar junction (NVJ), at the endosomal-mitochondrial junction (EMJ) and at the vacuolar-mitochondrial junction (v-CLAMP) (Lang et al., 2015; Park et al., 2016; reviewed in Rzepnikowska et al., 2017b). Vps13 is able to bind to phosphatidylinositol lipids via four different sites: N-terminal; C-terminal; and internal SHR-BD and APT1 domains (De et al., 2017; Rzepnikowska et al., 2017a). In addition, the null mutant exhibits a severe sporulation defect (Brickner and Fuller, 1997) due to involvement of Vps13 in formation of the prospore membrane (Nakanishi et al., 2007; Park and Neiman, 2012). Finally, Vps13 interacts with actin and actin cytoskeleton proteins, and has an impact on the actin cytoskeleton organisation (Michelot et al., 2010; Rzepnikowska et al., 2017a). Since actin patches are sites of endocytosis, defects in the functioning of the actin cytoskeleton are accompanied by a defect in endocytosis in vps13Δ cells (Luo and Chang, 1997; Rzepnikowska et al., 2017a), as observed in several other actin cytoskeleton mutants (Huckaba et al., 2004).
Endocytosis is a process that enables the uptake of extracellular materials and is involved in the regulation of plasma membrane composition. After initiation of endocytosis, an invagination of the plasma membrane is formed, followed by scission and formation of an endocytic vesicle. The force needed for membrane invagination is mainly produced during assembly of actin filaments at the endocytic site. One of the key players during this step is the Arp2/3 complex (reviewed in Goode et al., 2015). This complex is an actin nucleation factor and gives rise to branched actin filaments, facilitating a burst of actin assembly. The action of Arp2/3 is supported by a group of proteins called nucleation-promoting factors; among them are Las17, type I myosins and Abp1 (reviewed in Goode et al., 2015). In S. cerevisiae there are two type I myosins encoded by the homologous MYO3 and MYO5 genes. A single deletion of either MYO3 or MYO5 results in minor defects; however, the double-knockout mutant shows severe defects in actin polymerisation that result in impaired endocytosis and growth (Geli and Riezman, 1996; Goodson et al., 1996). In Myo3/5, several regions can be identified: a motor head domain; a linker region; and a tail, which promotes actin nucleation (Anderson et al., 1998). Recruitment of Myo5 to endocytic sites is regulated by calmodulin, a highly conserved, calcium-binding, regulatory protein of 147 aa (Grötsch et al., 2010). Calmodulin binds to IQ motifs found in the linker region of Myo5 (Geli et al., 1998). Upon binding of calmodulin, Myo5 changes its conformation from open to closed, in which form it is unable to bind to membranes and perform its functions (Geli et al., 1998). At endocytic sites, Myo5 facilitates membrane internalisation via both motor and nucleation functions (Sun et al., 2006). Calmodulin also binds to the Arc35 subunit of the Arp2/3 complex (Schaerer-Brodbeck and Riezman, 2003), and to the Rvs167 endocytic protein involved in vesicle scission (Myers et al., 2016). Thus, endocytosis and the actin cytoskeleton organisation are regulated by calmodulin in several ways.
Calcium ions (Ca2+) are one of the most crucial signalling molecules that enable cells to adapt to changes in internal and external conditions. Cytosolic Ca2+ concentration is maintained at low levels, 50-200 nM (Iida et al., 1990; Nakajima-Shimada et al., 1991), and is regulated by a system of calcium pumps present in the membranes. In yeast, Ca2+ is stored mainly in vacuoles (Dunn et al., 1994), but also in the Golgi apparatus and endoplasmic reticulum (ER) (Pezzati et al., 1997). Changes in cytosolic Ca2+ concentration influences the state of calmodulin, which can be present in Ca2+-free or Ca2+-bound forms. The different forms have different protein targets (reviewed in Cyert, 2001; Yap et al., 1999). One of the Ca2+-independent targets is Myo5. Among Ca2+-dependent targets, there is a protein phosphatase calcineurin (reviewed in Cyert, 2001) and Arc35 (Schaerer-Brodbeck and Riezman, 2003). Calcineurin is a heterodimer consisting of catalytic and calmodulin-binding subunit A – which, in S. cerevisiae, is encoded by two redundant genes, CNA1 and CMP2 (CNA2) – and a regulatory and calcium-binding subunit B, encoded by the CNB1 gene (Cyert et al., 1991; Cyert and Thorner, 1992; Liu et al., 1991). Upon activation by calmodulin, calcineurin mediates the response to changes in Ca2+ concentration through its main target, Crz1 (Matheos et al., 1997; Stathopoulos and Cyert, 1997). Crz1 is a transcription factor, which, after dephosphorylation by calcineurin, translocates to the nucleus and binds to calcineurin-dependent response elements (CDREs), enabling calcineurin-driven gene expression (Stathopoulos-Gerontides et al., 1999; Stathopoulos and Cyert, 1997). Calmodulin and calcineurin are highly conserved in eukaryotes, are crucial for calcium signalling (reviewed in Cyert, 2001), and are essential for the function and development of the central nervous system. Expression of calcineurin catalytic subunits in the brain is significantly higher than in other organs (Bond et al., 2017) and mutations in these subunits or changes in activity, both down- and upregulation, result in neurological disorders (reviewed in Kipanyula et al., 2016; Mizuguchi et al., 2018). It was also reported recently that calcium signalling is defective in cells isolated from ChAc patients (Pelzl et al., 2017a,b). Thus, components of the calcium signalling pathway are potential targets in therapy for neurodegenerative diseases.
Studies of yeast vps13 mutant, a model for ChAc and other diseases connected with VPS13 genes, are ongoing. Recently, missense mutations identified in ChAc patients were modelled in yeast, giving insight into possible mechanisms of pathogenesis (Park et al., 2016; Rzepnikowska et al., 2017a). Here, we describe a new growth phenotype of vps13 mutants, which we found suitable for genetic screens and drug testing. Our genomic library screen revealed a MYO3 gene fragment as a multicopy suppressor of vps13-I2749R and vps13Δ phenotypes, proving that both point mutations in, and deletions of, VPS13 could be overcome. Further genetic and biochemical analyses pointed to calcium signalling, with the involvement of calmodulin and calcineurin, as an important factor for the alleviation of vps13 defects. Our new phenotype could be useful for drug screening, as demonstrated by testing with calcineurin inhibitors. These findings can help to better understand the mechanisms of ChAc and will help to develop new treatments in the future.
RESULTS
Inactivation of VPS13 causes hypersensitivity to sodium dodecyl sulphate in yeast cells
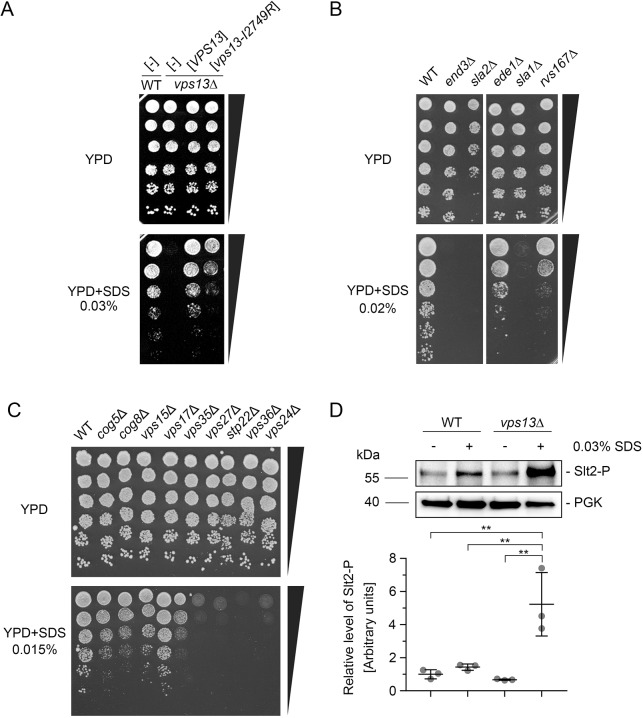
In our previous study we discovered that vps13 mutant cells, either vps13Δ or vps13-I2749R, with a single amino acid substitution in the APT1 domain of Vps13 corresponding to the I2771R mutation found in a ChAc patient, exhibit defects in actin cytoskeletal organisation and endocytosis (Rzepnikowska et al., 2017a). Various mutants that exhibit defects in the actin cytoskeleton, in protein transport, in cell wall integrity or have altered calcium homeostasis are hypersensitive to low concentrations of sodium dodecyl sulphate (SDS) (Ammons et al., 2015; Chen et al., 2012; Fokina et al., 2012; Varelas et al., 2006), a small amphiphilic detergent. Therefore, we tested both vps13Δ and vps13-I2749R mutants for growth in the presence of this compound. Both vps13 mutants appeared hypersensitive to the addition of 0.03% SDS to the rich medium (Fig. 1A), and vps13Δ was most sensitive. In addition, we found that other mutants that are defective in actin cytoskeleton organisation and endocytosis (end3Δ, sla2Δ, ede1Δ, sla1Δ and rvs167Δ), or defective in Golgi transport or endocytic sorting (cog5Δ, cog8Δ, vps15Δ, vps35Δ, vps27Δ, stp22Δ, vps36Δ and vps24Δ), are also hypersensitive to low concentrations of SDS (Fig. 1B,C). Under SDS stress conditions, Golgi and endocytic traffic are probably crucial for the effective replacement of plasma membrane proteins that have been damaged by SDS.
Fig. 1.
The vps13 mutants are hypersensitive to SDS but cell wall integrity is not compromised. (A) vps13 mutants are hypersensitive to SDS. (B) Mutants defective in endocytosis are hypersensitive to SDS. (C) Mutants defective in Golgi transport or endocytic sorting are hypersensitive to SDS. (D) Slt2 is hyperphosphorylated in vps13 cells following SDS stress. Overnight yeast cultures were shifted to fresh YPD or YPD+0.03% SDS and incubated for 3 h. Western blot was performed and analysed by densitometry. The phosphorylation levels of Slt2 in relation to Pgk1 were compared by one-way ANOVA followed by Tukey's multiple comparison test (n=3); **P<0.01. Error bars indicate s.d.
It was previously documented that SDS damages the cell wall and plasma membrane in Pichia pastoris yeast cells (reviewed in Levin, 2005; Zhang et al., 2015); therefore, we investigated whether the cell wall integrity is compromised in vps13Δ. Cellular integrity in yeast is mainly controlled by the cell wall integrity (CWI) pathway (reviewed in Levin, 2005), which employs one branch of a mitogen-activated protein kinase (MAPK) cascade for signal transduction. This MAPK signalling pathway involves protein kinase C, Bck1, Mkk1/2 and Slt2/Mpk1 kinase, which further phosphorylates and activates transcription factors to produce proteins involved in cell wall biogenesis and other processes. Increased phosphorylation of Slt2 usually indicates a defect in cell wall integrity. Investigation of the level of phosphorylated Slt2 by western blotting using anti-phospho-p44/42 MAPK antibody in vps13Δ cell extracts revealed that levels were similar to the wild type. This finding supports the view that the CWI pathway is not activated and cell wall integrity is not compromised in vps13Δ cells grown in regular growth medium (Fig. 1D). Addition of SDS to the medium activated the CWI pathway in vps13Δ cells with approximately 5-fold higher phosphorylation of Slt2 kinase compared to wild type (Fig. 1D). This activation is apparently not sufficient to compensate for the damage, caused by SDS, to vps13Δ mutant cells.
A fragment of the MYO3 gene suppresses the SDS hypersensitivity of vps13-I2749R and vps13Δ cells
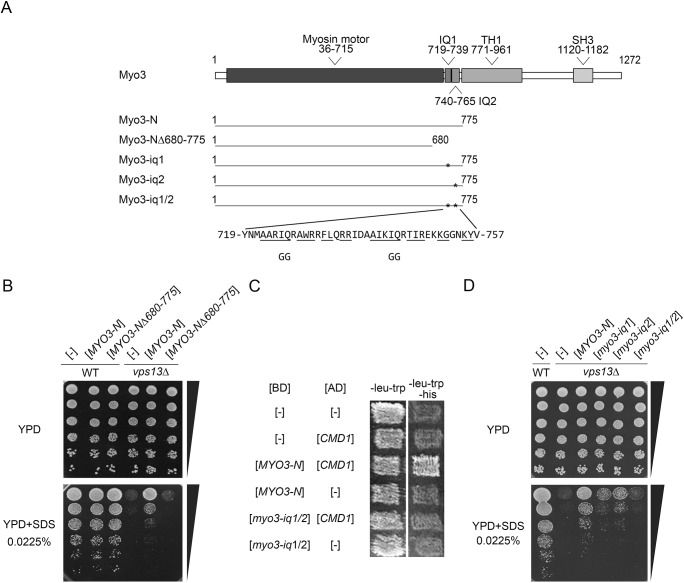
To determine whether vps13 mutations can be overcome, and to elucidate the importance of Vps13-dependent processes in SDS tolerance, we performed a screen for multicopy suppressors of vps13-I2749R. The vps13Δ strain carrying the vps13-I2749R allele on a plasmid was transformed with a multicopy genomic library. Several clones, bearing various none-overlapping genomic fragments, were able to grow on SDS-containing medium, and plasmids from these clones were isolated, retested for suppression and sequenced. One plasmid contained a fragment of the MYO3 gene (MYO3-N) encoding the N-terminal 1-775 aa of type I myosin (Myo3-N), an actin cytoskeleton protein involved in endocytosis (Geli and Riezman, 1996; Goodson and Spudich, 1995), which was responsible for suppression. The Myo3-N fragment consists of: a motor domain (aa 36-715); a linker (aa 719-771), containing two IQ motifs, indicating ability to bind calmodulin (Ho et al., 2002); and five amino acids of a tail lipid-binding TH1 domain (aa 771-775) (Fig. 2A). Subsequently, we found that the MYO3-N fragment also suppresses the vps13Δ deletion mutation (Fig. 2B). This suggests that the Myo3-N protein does not suppress defects by directly interacting with Vps13-I2479R, but instead may involve another factor. Furthermore, we showed that a fragment of Myo3-N containing the IQ motifs (aa 680-775) was necessary for suppression (Fig. 2B), suggesting that calmodulin could be this other factor.
Fig. 2.
The MYO3-N fragment encoding the motor domain and calmodulin-binding motifs from Myo3 suppresses hypersensitivity of vps13Δ to SDS. (A) Schematic representation of Myo3 domain structure and Myo3 variants studied. Myo3 domains and motifs are based on the Uniprot database (http://www.uniprot.org/uniprot/P36006, 02.08.2018). *, position of mutations introduced in vitro. Amino acids conforming to the consensus of the calmodulin-binding motif as defined in the SMART database (http://smart.embl-heidelberg.de, 02.08.2018). IQ motifs are underlined and amino acid substitutions are shown. (B) MYO3-N suppresses vps13Δ but MYO3-NΔ680-775 does not. (C) Myo3-N interacts with calmodulin in the two-hybrid system and mutations in IQ motifs abolish this interaction. BD, DNA-binding domian; AD, activating domain. (D) IQ motifs of Myo3 are required for suppression of vps13Δ.
To verify the hypothesis that calmodulin binding by Myo3-N is required for suppression, three myo3-N alleles with one (myo3-iq1, myo3-iq2) or both (myo3-iq1/2) IQ motifs disrupted were generated by in vitro mutagenesis (Fig. 2A). To test the interaction of Myo3-N and Myo3-iq1/2 with calmodulin, we used a two-hybrid system, which was previously shown to be appropriate for the detection of the interaction between Myo5 (a homologue of Myo3) and calmodulin (Geli et al., 1998). As predicted, Myo3-N interacted with calmodulin but Myo3-iq1/2 protein did not (Fig. 2C). Most importantly, the myo3-iq1/2 allele was not able to suppress the SDS-hypersensitivity phenotype of vps13Δ (Fig. 2D), although it was similarly expressed as MYO3-N, when the cellular levels of hemagglutinin (HA)-tagged versions of protein products were compared (Fig. S1). The myo3-iq1 and myo3-iq2 mutant alleles, each with a single IQ motif removed, were less efficient in suppression than MYO3-N (Fig. 2D). The additive effects of IQ deletions were previously observed for MYO5 (Geli et al., 1998). Our results indicate that the mechanism by which MYO3-N suppresses vps13Δ requires binding of Myo3-N to calmodulin and that calmodulin signalling affects the vps13Δ phenotype.
Crz1-dependent transcriptional response is increased in vps13Δ
Calmodulin has several targets in yeast, including the protein phosphatase calcineurin, which is important in the response of cells to stress (reviewed in Cyert, 2001). In response to increased cytoplasmic calcium upon stress, calmodulin binds and activates calcineurin, which subsequently dephosphorylates and activates the transcription factor Crz1 (Matheos et al., 1997; Stathopoulos and Cyert, 1997) to promote cell survival. To compare activity of calcineurin in wild-type and vps13Δ cells, and in these cells upon expression of MYO3-N, we used a plasmid containing a calcineurin-dependent response elements (CDREs) fused to the lacZ open reading frame encoding β-galactosidase (Stathopoulos and Cyert, 1997) (Fig. 3A). Activity of β-galactosidase was measured in cell extracts derived from respective transformants. Results show that the activity of calcineurin is significantly increased in vps13Δ when compared to the wild-type strain (Fig. 3B) and it is reduced by the expression of MYO3-N (Fig. 3B). These results imply that cytoplasmic calcium concentration could be increased in vps13Δ and that Myo3-N might act by binding and sequestering calmodulin, which in turn causes deactivation of calcineurin. This suggests that deactivation of calcineurin by genetic manipulation or by chemical inhibitors might possibly cause suppression.
Fig. 3.
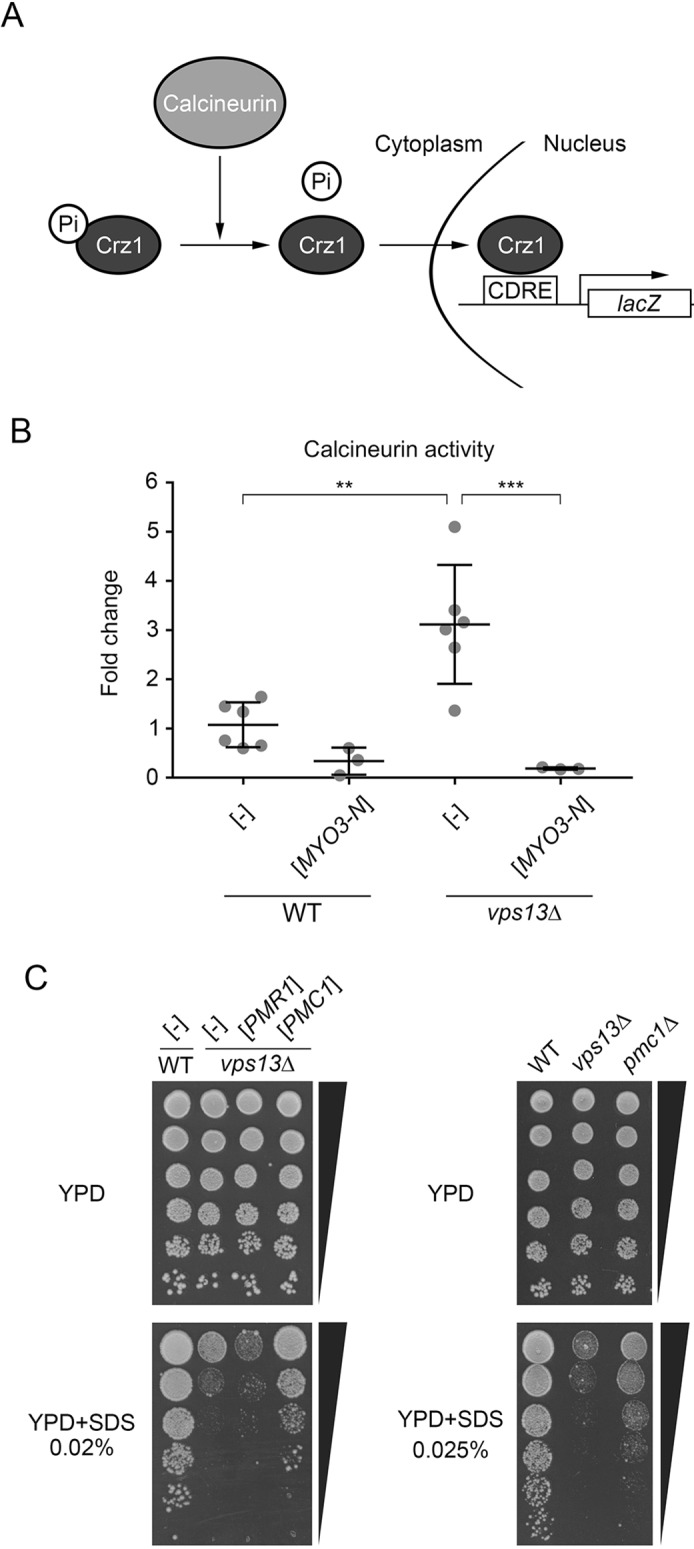
Activity of calcineurin is increased in vps13Δ and reduced by MYO3-N, and PMC1 suppresses the vps13Δ growth defect. (A) Schematic representation of the calcineurin-Crz1 signalling system used to measure the activity of calcineurin. (B) Activity of calcineurin is increased in vps13Δ and is diminished by MYO3-N. Results were analysed statistically by one-way ANOVA followed by Tukey's multiple-comparisons test (n=6 for WT [-] and vps13 [-], n=3 for WT [MYO3-N] and vps13Δ [MYO3-N]; **P<0.01, ***P<0.001). Error bars indicate s.d. (C) PMC1 is a multicopy suppressor of vps13Δ hypersensitivity to SDS and pmc1Δ is hypersensitive to SDS. Growth of wild-type and vps13Δ transformed with plasmids bearing PMR1 or PMC1, or with empty vector (left panel). Growth of wild-type, vps13Δ and pmc1Δ strains on SDS medium (right panel).
PMC1 gene, encoding vacuolar calcium pump, is a multicopy suppressor of vps13Δ
To address the question of whether an increased cytoplasmic calcium level is the reason for hyperactivation of calcineurin in vps13Δ, we tested the effect of overexpression of PMR1 and PMC1 genes for growth of vps13Δ on SDS medium. These genes encode ER/Golgi or vacuolar calcium pumps, respectively, and transport calcium out of the cytoplasm. We found that PMC1, but not PMR1, is a potent suppressor (Fig. 3C). We analysed further whether the defect of vacuolar transport of calcium in pmc1Δ is alone sufficient to decrease yeast cell tolerance to SDS and found that pmc1Δ is hypersensitive to SDS but less than vps13Δ (Fig. 3C). Since vps13Δ shows defects in Golgi-to-vacuole transport, the pathway needed for normal Pmc1 targeting, the Pmc1 pump could possibly be mislocalised in these cells. Thus, mislocalisation of Pmc1 could be one of the reasons for vps13Δ hypersensitivity to SDS. However, we were unable to prove this since we could not get PMC1-mCherry vps13Δ clones. This suggests that such cells might be unviable.
The fine-tuned activity of calcineurin is important for vps13Δ growth on SDS-containing medium
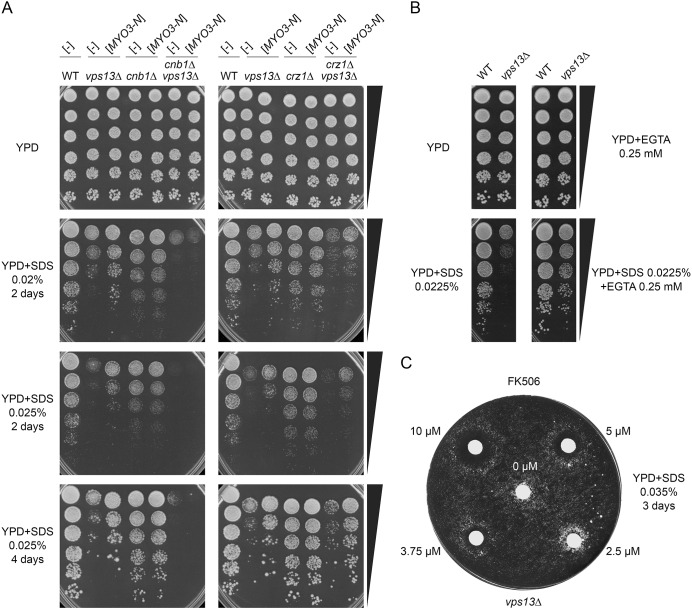
The Cnb1 regulatory subunit of calcineurin is required for its activity (Cyert and Thorner, 1992). It has been reported that the cnb1Δ strain is more sensitive to a variety of stresses (Mulet et al., 2006), but there is no data relating to the sensitivity to SDS. Therefore, to analyse how calcineurin deactivation affects the response to SDS in vps13Δ cells and to establish whether active calcineurin is required for vps13Δ suppression by MYO3-N, the growth of wild-type, cnb1Δ and cnb1Δ vps13Δ mutants on SDS-containing medium was compared in the presence or absence of MYO3-N. We found that the cnb1Δ strain is more sensitive to SDS than the wild-type strain and that cnb1Δ mutation negatively interacts genetically with vps13Δ (Fig. 4A), indicating that vps13Δ cells rely on active calcineurin to cope with SDS stress. Interestingly, the cnb1Δ vps13Δ mutant was not suppressed by MYO3-N, indicating that Myo3-N requires the Cnb1 protein and functional calcineurin for vps13Δ suppression (Fig. 4A). Thus, it seems that a certain level of calcineurin activity is required for normal growth in the presence of SDS. Sequestration of calmodulin, the activator of calcineurin, by MYO3-N results in a reduction in calcineurin activity, thus fine tuning calcineurin activity to this required level.
Fig. 4.
A normal/intermediate activity of calmodulin-calcineurin pathway is required to cope with SDS stress in vps13Δ. (A) Growth of wild-type, vps13Δ, cnb1Δ, crz1Δ, vps13Δ cnb1Δ and vps13Δ crz1Δ cells expressing, or not, MYO3-N on YPD+SDS. (B) Addition of EGTA improves growth of vps13Δ on SDS-containing medium. (C) FK506 affects growth of vps13Δ on SDS-containing medium. Strains were grown to early exponential phase. Cells (0.05 OD) were plated on YPD+0.035% SDS, and 2 µl of 0-10 µM FK506 (HY-13756, MedChemExpress, Monmouth Junction, USA) was applied on paper discs as indicated.
The contribution of Crz1 to the SDS stress response and to the MYO3-N suppression of vps13Δ was also studied. Comparison of the growth of isogenic crz1Δ and crz1Δ vps13Δ mutants with vps13Δ and with the wild-type strain revealed that the crz1Δ strain exhibits a similar sensitivity to SDS as the wild-type strain in conditions tested. It also revealed that crz1Δ vps13Δ grows more slowly than vps13Δ but suppression by MYO3-N is still observed (Fig. 4A). These results show that the Crz1 transcription factor positively contributes to SDS stress tolerance in vps13Δ and, most importantly, that vps13Δ suppression by MYO3-N involves a mechanism that is independent of Crz1 activity.
To investigate the possibility of using chemical intervention in the vps13Δ strain, we tested the effects of: ethylene glycol tetraacetic acid (EGTA), a chelator of divalent cations that is more specific for Ca2+ than for other cations; and FK506, a calcineurin inhibitor. EGTA reduces the concentration of calcium in the cytoplasm, thus inhibiting the Ca2+-dependent action of calmodulin and inhibiting calcineurin. Interestingly, addition of EGTA significantly improved growth of vps13Δ on SDS plates (Fig. 4B). Thus, a partial inhibition of the calcium-dependent functions of calmodulin and the inhibition of calcineurin is beneficial for vps13Δ cells in the presence of SDS stress. FK506 improved growth of vps13Δ at low concentrations (2.5 μM spotted onto filter paper), but higher concentrations exhibited an adverse effect (Fig. 4C). Thus, calcineurin cannot be inhibited completely, but its downregulation can be fine-tuned in order to improve the growth of vps13 cells. This result points to FK506 as a potential drug and to calcineurin as a useful target to select drugs for ChAc therapy.
The cmd1-226 mutant allele, encoding a calmodulin variant with the F92A amino acid substitution, suppresses vps13Δ
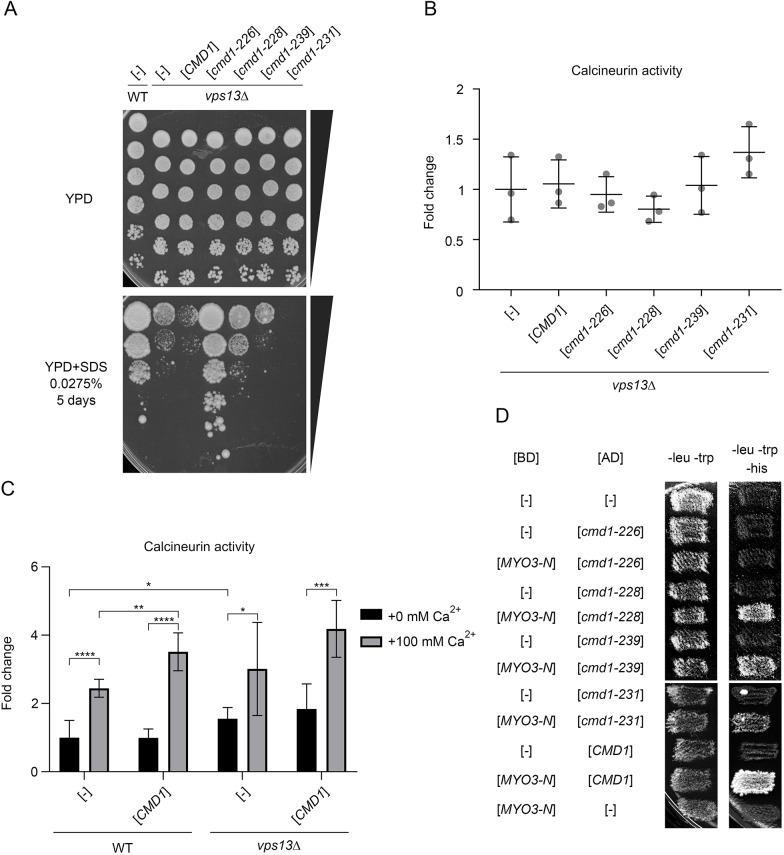
The importance of calmodulin binding by Myo3-N for the suppression of vps13 suggests that a calmodulin sequestration mechanism is involved. To verify this assumption, we analysed whether expression of multiple copies of CMD1 alleles encoding wild-type or mutant calmodulin have an effect on the growth of vps13Δ cells in the presence of SDS. The various cmd1 alleles were selected to represent different groups of mutants with the same phenotype: defective actin cytoskeleton, altered localisation of calmodulin, defective karyokinetic spindle or defective budding, as previously described (Geli et al., 1998; Ohya and Botstein, 1994). The wild-type CMD1 negatively affected growth of vps13Δ cells, implying that the control of the calmodulin pool and/or accessibility to different substrates is important for regulation of the cell response to SDS stress in vps13Δ, supporting the sequestration mechanism for MYO3-N (Fig. 5A). The cmd1-231 allele, which causes a defect in budding, affected growth similarly to wild-type CMD1, while the mutant cmd1-228 and cmd1-239 alleles essentially did not affect growth of vps13Δ. Surprisingly, the cmd1-226 allele, which results in the F92A substitution in calmodulin and represents a group of mutants that show defects in the organisation of the actin cytoskeleton and in endocytosis, was a potent suppressor of vps13Δ (Fig. 5A).
Fig. 5.
The cmd1-226 mutant allele, but not wild-type CMD1, restores growth of vps13Δ on YPD+SDS plates. (A) Different CMD1 alleles variously affect the growth of vps13Δ. (B) Wild-type and mutant calmodulin do not influence calcineurin activity in vps13Δ cells. Results were analysed by one-way ANOVA (n=3); P=0.196. Error bars indicate s.d. (C) Effects of calcium addition and CMD1 overexpression on calcineurin activity in wild-type and vps13Δ cells. Results were analysed statistically by two-tailed Student's t-test (n=6; *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001. Error bars indicate s.d. (D) Cmd1-226 does not interact with Myo3-N. Results from a two-hybrid experiment are shown. BD, DNA-binding domain; AD, activating domain.
To test how overproduction of wild-type or mutant calmodulin affects activity of calcineurin in vps13Δ cells, the CDRE-lacZ reporter was used. Interestingly, neither wild-type nor mutant calmodulin genes, including the cmd1-226 suppressor allele, significantly affected the increased activity of calcineurin in vps13Δ cells (Fig. 5B). Since additional calmodulin did not have an effect in vps13Δ, we checked whether calcineurin could be stimulated by addition of calcium to the medium in this strain. Wild-type and vps13Δ cells showed higher activity of calcineurin after calcium addition, indicating that calcineurin activity in vps13Δ in regular conditions is not maximal (Fig. 5C). Moreover, in wild-type cells, the effect of calcium and expression of additional copies of CMD1 was additive. However, vps13Δ cells expressing CMD1 from the plasmid did not significantly increase calcineurin activity in the presence or absence of additional calcium. Thus, even when calcium is not a limiting factor, further activation of calcineurin by Cmd1 requires Vps13. These results indicate that reduction of calcineurin activity is probably not a mechanism for restoring growth of vps13 cells on SDS plates mediated by cmd1-226. The Cmd1-226 mutant protein might target proteins other than calcineurin. One possibility is that Cmd1-226 is different in terms of its binding with Myo3, as is the case for Myo2 (Sekiya-Kawasaki et al., 1998). Therefore, we analysed how selected cmd1 mutations affect the interaction of calmodulin with Myo3 using a two-hybrid system. This analysis shows that the cmd1-226 mutation, in contrast to other mutations tested, results in total loss of interaction between calmodulin and Myo3-N (Fig. 5D). Thus, a complete loss of interaction between mutant calmodulin and Myo3 is required for cmd1-226-based suppression, the opposite to MYO3-N-based suppression of vps13Δ. Cmd1-226 may act by diluting wild-type calmodulin and activating full-length Myo3, or by other mechanisms.
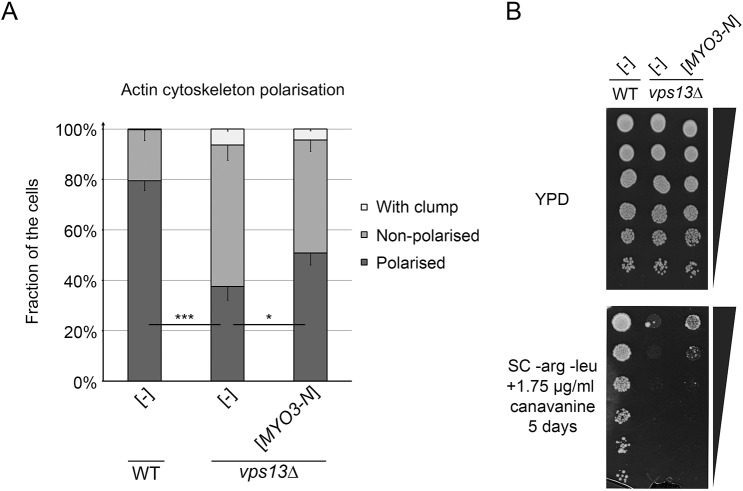
Expression of MYO3-N suppresses the actin cytoskeleton polarisation and endocytosis defects of vps13Δ
We have previously reported that vps13Δ shows defects in the organisation of the actin cytoskeleton that are accompanied by an endocytosis defect, observed as canavanine hypersensitivity (Rzepnikowska et al., 2017a). We found that MYO3-N suppresses the actin cytoskeleton polarisation defect of vps13Δ, since a greater number of cells with properly polarised actin patches in the bud, visible actin cables in the mother, and without actin clumps, were found when MYO3-N was expressed (Fig. 6A). The vps13Δ cells expressing MYO3-N were less sensitive to canavanine than vps13Δ cells bearing empty vector (Fig. 6B), also indicating that endocytosis is improved.
Fig. 6.
MYO3-N suppresses the actin-cytoskeleton polarisation defect and the canavanine hypersensitivity of vps13 cells. (A) MYO3-N suppresses the actin-cytoskeleton polarisation defect of vps13Δ. Quantification of actin polarisation was analysed using the two-tailed Student's t-test (n=3; cells counted per replicate: 105; 184; 148 for WT [-], 130; 103; 195 for vps13Δ [-], 138; 111; 121 for vps13Δ [MYO3-N]); *P<0.05, ***P<0.001. Error bars indicate s.d. (B) MYO3-N suppresses canavanine hypersensitivity of vps13Δ.
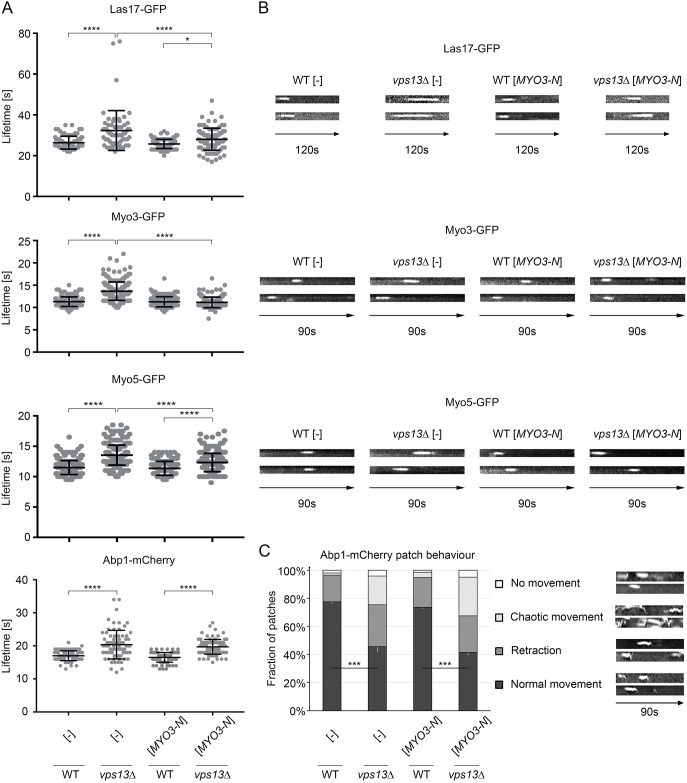
Actin patches are sites where endocytosis takes place (Huckaba et al., 2004). Vps13 has been found in artificial actin patches assembled on microbeads covered with the nucleation-promoting factor Las17 (yeast WASP) (Michelot et al., 2010). Therefore, it is possible that Vps13 is directly involved in the early steps of endocytosis and/or that the early steps of endocytosis in vps13Δ cells are disturbed by the actin cytoskeleton malfunctioning. To test this possibility, the lifetimes of endocytic reporters fused with a particular fluorescent protein (GFP or mCherry) in cortical patches were determined in the wild-type and vps13Δ strains. Kymographs, indicating the behaviour of individual patches, were also obtained. The reporter proteins analysed represent actin cytoskeleton components that associate temporarily with the endocytic vesicle as it forms. They are thus used for the visualisation of specific stages of endocytosis. Myo3/5 work together with Las17 in the myosin/WASP module to activate actin polymerisation (reviewed in Lu et al., 2016). Abp1 acts later in the actin module and is a marker that tracks invagination of the plasma membrane and inward endocytic vesicle movement. The actin cytoskeleton regulators Las17, Myo3, Myo5 and Abp1 were monitored by fluorescence microscopy. In vps13Δ cells, the lifetimes of the patches formed by all of these proteins were longer than in respective control cells, 32.32 for Las17-GFP, 13.66 for Myo3-GFP, 13.55 for Myo5-GFP and 20.37 s for Abp1-mCherry as opposed to 26.38, 11.29, 11.49 and 17.01 s, respectively (Fig. 7A). Lifetimes of Las17-GFP and Myo5-GFP were partially, but significantly, shortened when MYO3-N was expressed in vps13Δ (28.05 and 12.32 s, respectively) (Fig. 7A). MYO3-N did not affect the lifetime of Abp1-mCherry (19.7 s) (Fig. 7A). Interestingly, the lifetime of Myo3-GFP patches in vps13Δ cells expressing MYO3-N was shortened to a normal value (11.14 s) and was statistically not different from the control strain (Fig. 7A).
Fig. 7.
The vps13Δ mutation impairs plasma membrane events involved in endocytosis and MYO3-N suppresses some of the defects. (A) Lifetimes of Las17-GFP, Myo3-GFP, Myo5-GFP and Abp1-mCherry patches. Results were compared using one-way ANOVA followed by Tukey's multiple-comparisons test (n=87; 63; 103; 97 for Las17-GFP, n=163; 212; 207; 192 for Myo3-GFP, n=302; 264; 296; 251 for Myo5-GFP, n=83; 75; 104; 100 for Abp1-mCherry for WT [-], vps13 [-], WT [MYO3-N] and vps13 [MYO3-N], respectively); *P<0.05, ****P<0.0001. Error bars indicate s.d. (B) Kymographs of Las17-GFP, Myo3-GFP and Myo5-GFP patches. Two representative kymographs for each strain, as in A, are shown. Las17-GFP patches were observed for 120 s using an Olympus IX-81 microscope and imaging was performed via 1 s time lapse. Myo3-GFP and Myo5-GFP were observed for 90 s using an OMX DeltaVision V4 microscope and imaging was performed via 0.5 s time lapse. (C) Kymographs of Abp1-mCherry patches. Two representative kymographs for each class of patch behaviour are shown. Abp1-mCherry patches were viewed for 90 s using an Olympus IX-81 microscope and imaging was performed via 1 s time lapse. Quantification of the patches was analysed using a two-tailed Student's t-test (n=5, patches counted per replicate: 44; 45; 54; 55; 65 for WT [-], 50; 47; 60; 47; 60 for vps13Δ [-], 51; 57; 64; 37; 61 for WT [MYO3-N], 54; 69; 59; 67; 59 for vps13Δ [MYO3-N]); ***P<0.001. Statistical significances and error bars indicating s.d. are shown for patches with normal behaviour.
Kymographs suggested that the immobile phase of Las17-, Myo3-, Myo5- and Abp1-associated patches was affected – these proteins were retained for longer at the plasma membrane in vps13Δ than in the wild-type cells (Fig. 7B). What is more, the Apb1m-Cherry patch tracks indicate that the movement of some of the patches was affected in vps13Δ mutant cells (Fig. 7C). At least three abnormal behaviours of the patches were observed: the patch remained near the plasma membrane and did not move toward the centre of the cell (no movement); it moved inward but then retracted; or it moved in a direction other than towards the cell centre (chaotic movement). MYO3-N expression did not influence the defective patch movement of vps13Δ (Fig. 7C).
In summary, the results suggest that Vps13 is involved in the early steps of endocytosis and it affects the duration of association of several actin cytoskeleton proteins with endocytic sites. It also affects the movement of endocytic vesicles, as observed by Abp1-mCherry, in terms of occurrence, distance travelled and direction. Production of Myo3-N in vps13Δ improves endocytosis by correcting a number of steps in the process, in particular restoring the functioning of full-length Myo3.
Osh2 and Osh3 proteins bridging cortical ER with plasma membrane at endocytic sites are essential for suppression of vps13Δ by MYO3-N
We found that the defects of vps13Δ cells – in endocytosis, in the extended lifetime of Las17-GFP and in abnormal Abp1 patch behaviour – are similar to the defects observed in osh2Δ osh3Δ cells (Encinar del Dedo et al., 2017). Osh2 and Osh3 are components of ER-plasma membrane contact sites important for endocytosis (Encinar del Dedo et al., 2017). Therefore, we tested osh2Δ osh3Δ and other mutants defective in various membrane contact sites for growth in the presence of SDS and whether Osh2/3 proteins are required for MYO3-N suppression of vps13Δ. The nvj1Δ, vps39Δ and mmm1Δ mutants, lacking the proteins involved in the formation of nuclear-vacuolar junctions, v-CLAMPs and ER-mitochondria encounter structures (ERMES), respectively, grew as wild type on SDS-containing medium, showing that these membrane contact sites are not important in this condition (Fig. S2). The analysis of osh2Δ osh3Δ double mutants, and osh2Δ osh3Δ vps13Δ triple mutants, transformed with MYO3-N plasmid or empty vector, revealed that: the osh2Δ osh3Δ strain is much more sensitive to SDS than vps13Δ; the osh2Δ osh3Δ vps13Δ is similarly sensitive to osh2Δ osh3Δ; and neither is suppressed by MYO3-N (Fig. 8). Therefore, Osh2/3 are crucial for SDS tolerance and, under this stress condition, act with Vps13 in the same pathway. Finally, Osh2/3 are important for Myo3-N to act as a suppressor of vps13Δ. Thus, calmodulin-binding motifs of Myo3-N and the presence of full-length myosin type I linked with Osh2/3 at ER-plasma membrane endocytic contact sites, but not other membrane contact sites, are important for growth of the vps13Δ strain on SDS-containing medium.
Fig. 8.
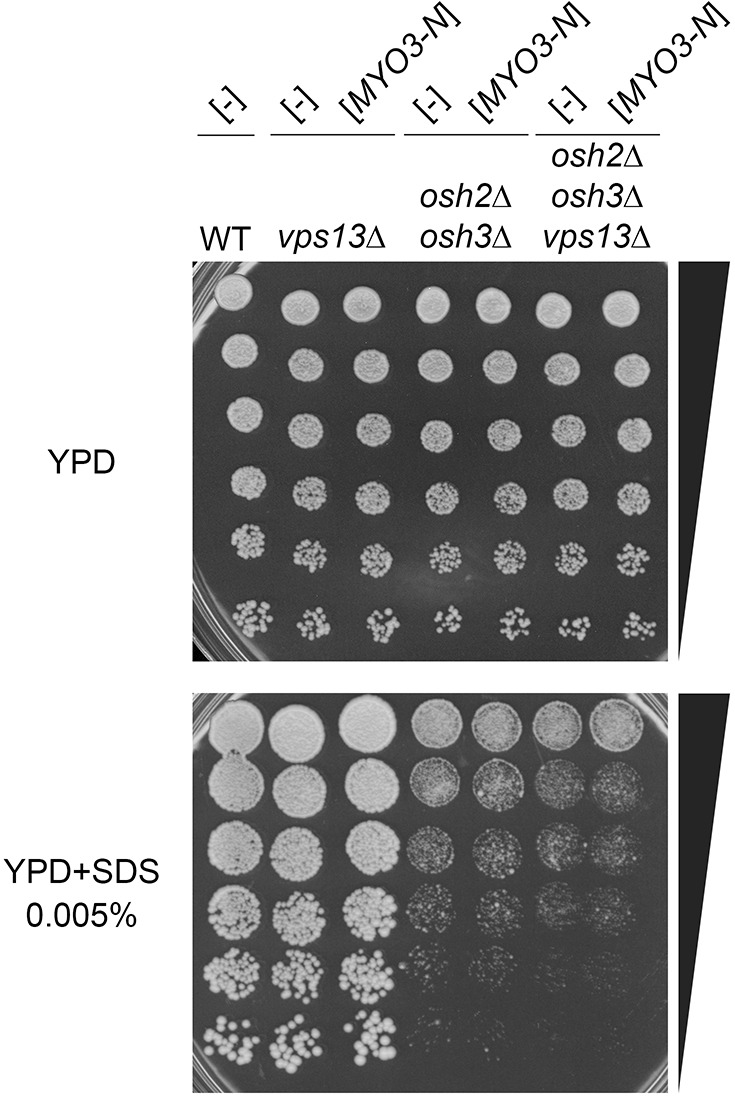
The osh2Δ osh3Δ double mutant is hypersensitive to SDS and Osh2/3 are important for vps13Δ suppression by MYO3-N.
DISCUSSION
Vps13 proteins are important for neuronal cell physiology, as mutations in VPS13 genes cause genetic neurodegenerative disorders in humans. Despite this importance, the molecular function of Vps13 and pathogenesis of associated diseases are still unclear. Using a yeast model for ChAc, a vps13Δ mutant and its newly discovered SDS-hypersensitivity phenotype, we have screened for genetic suppressors to see whether the Vps13 deficiency can be overcome and to learn more about the pathological cellular processes that could be the basis for the disease. Here, we show that vps13Δ defects can be suppressed by: MYO3-N, which encodes the Myo3-N myosin fragment that binds and sequesters calmodulin; expression of the cmd1-226 allele encoding a mutant calmodulin that is unable to bind myosin; or by PMC1, encoding a vacuolar calcium pump. We also show that calcium signalling is activated in vps13Δ and that Myo3-N downregulates calcineurin activity and acts as a suppressor via a Crz1-independent mechanism. Furthermore, we show that vps13Δ can also be suppressed using chemical compounds, EGTA and FK506, which affect calcium signalling and inhibit calcineurin. Our studies of early endocytosis events in vps13Δ and genetic analysis point to the possibility that Vps13 is a component of ER–plasma-membrane contact sites.
Discovering a new phenotype of vps13Δ cells, hypersensitivity to SDS, allowed us to perform a successful screen for multicopy suppressors, but also has potential implications for ChAc patients. SDS is one of the most popular detergents worldwide. It is used as an additive to a wide range of cosmetics, pharmaceuticals, and household and industrial cleaning products. This amphiphilic compound easily accumulates in living organisms (reviewed in Cserháti et al., 2002), inhibits growth of bacteria, algae, fishes and yeasts (reviewed in Lechuga et al., 2016; Sandbacka et al., 2000), and negatively affects mammalian cells. The hypersensitivity of vps13Δ to SDS and the abundance of SDS in many everyday products suggest that ChAc patients may be more vulnerable to a danger posed by SDS. It could be one of the environmental factors that influence the time of onset and progression of the disease. However, sensitivity of human cells to SDS requires further study.
We used vps13Δ yeast as a ChAc model to better understand its pathophysiology. Our results show that the CWI signalling pathway was not activated in vps13Δ cells, suggesting that there is no defect in the cell wall in these cells. Rather, plasma membrane composition might be altered due to defects in endocytosis or plasma membrane stiffness/rigidity affected due to the defect in actin cytoskeleton organisation in vps13Δ. This may be the reason for low tolerance to SDS, as supported by the finding that several endocytic mutants with defects in the actin cytoskeleton and in endocytosis, and mutants defective in Golgi and endosomal sorting, were also found to be hypersensitive to SDS. We have found that the CWI pathway responds to SDS with a much greater level of phosphorylation in vps13Δ, when compared to wild-type cells, to compensate for damage caused by the detergent.
Changes in calcineurin activity are connected with several diseases, including neurodegeneration. Calcineurin signalling was elevated in vps13Δ, and this is detrimental, as shown for yeast models for other neurodegenerative diseases (Caraveo et al., 2014). One possible explanation is that a lack of Vps13 protein causes a defect in protein trafficking that triggers a cellular response involving calcineurin upregulation. Our data of vps13Δ suppression by PMC1 suggest that Pmc1 could be mislocalised in vps13Δ and inefficient in pumping calcium to the vacuole, resulting in cytoplasmic calcium increase and calcineurin activation. Such a view is also supported by negative genetic interaction of vps13Δ with pmr1Δ (Costanzo et al., 2010). It is also possible that Vps13 is more directly involved in calcium signalling, since, in high-throughput studies, Vps13 has been shown to bind calmodulin (Ho et al., 2002). However, we were not able to confirm this interaction by other methods. Although detrimental in excess, the activities of calcineurin and of its Crz1 effector are important for the growth of vps13Δ cells under SDS stress, similarly to as observed for Candida glabrata yeast (Chen et al., 2012). Calcium signalling and calcineurin activity could be upregulated in vps13 cells by at least two mechanisms: through secondary effects of disrupted transport processes or through the removal of potential direct interactions.
We have shown that the effects of the vps13Δ mutation, monitored through growth on SDS medium, can be overcome in several ways: titration of calmodulin by Myo3-N; expression of additional copies of PMC1; additional production of mutant calmodulin Cmd1-226; or addition of calcineurin inhibitors. Expressed Myo3-N binds to and sequesters free calmodulin, which activates Myo3/5 and downregulates the activity of calcineurin. The growth defect of the cnb1Δ vps13Δ mutant is not suppressed by MYO3-N; however, the crz1Δ vps13Δ mutant was still suppressed. This suggests that active calcineurin, but not Crz1, is required for the suppression and only partial downregulation is beneficial. This effect could be also achieved by lowering cytoplasmic calcium via overproduction of the Pmc1 vacuolar calcium pump. This notion is further supported by the finding that chemical calcineurin inhibitors, EGTA and FK506, suppressed the vps13Δ growth defect. FK506 suppressed vps13Δ only at a very low concentration, while at higher concentrations FK506 enhanced the vps13Δ hypersensitivity to SDS. Our results are in line with observations that the moderate calcineurin activity is protective for cells, but elevated or inhibited activity is toxic (Caraveo et al., 2014, reviewed in Kipanyula et al., 2016). In agreement with a calmodulin-sequestering model, overproduction of wild-type calmodulin is harmful to vps13Δ cells in spite of it not affecting calcineurin activity in vps13Δ cells. The cmd1-226 also did not alter the calcineurin activity, which suggests that the mechanism of the suppression in this case is calcineurin independent. One possibility is that Cmd1-226 dilutes or somehow negatively affects wild-type calmodulin, what in turn increases the pool of the active forms of Myo3/5, which contributes to suppression by enhancing endocytosis. The other possible explanation of cmd1-226 action is by interacting with Arc35, a subunit of the Arp2/3 complex that activates the polymerisation of actin filaments. Calmodulin encoded by cmd1-226 binds to Arc35 protein, while that encoded by cmd1-231, which was the most toxic to vps13Δ, does not (Schaerer-Brodbeck and Riezman, 2003). We cannot exclude the possibility that SDS stress somehow causes cell division defects in vps13Δ and Cmd1-226 helps to overcome this defect by acting on other targets, such as Spc110, a spindle-pole-body calmodulin-binding protein (Geiser et al., 1993).
As we have documented here, vps13Δ exhibits defects in an early endocytic processes. These defects include an extended lifetime of several endocytic actin-cytoskeleton proteins and abnormal Abp1 patch behaviour, which is a marker of endocytic vesicle internalisation. Myo3-N production in vps13Δ results in restoration of the lifetime of Myo3 patches engaged in invagination and in release of endocytic vesicles, and in partially shortened lifetimes of Las17 and Myo5. Myo3-N production may also have other effects and it should also be noted that the vesicle internalisation defect of vps13Δ is not corrected. It was recently documented that endocytic plasma membrane invaginations associate with the cortical ER and this contact facilitates actin polymerisation and endocytic vesicle scission (Encinar del Dedo et al., 2017). Myo5 is a protein that establishes the contact between the ER and endocytic sites by binding Osh2 and Osh3. Osh2/3 in turn bind Scs2 and Scs22, ER membrane proteins, and the Osh2 sterol transfer domain is required for the onset of actin polymerisation at endocytic sites (Encinar del Dedo et al., 2017). Myo3 exhibits a similar domain structure to Myo5 and can possibly perform similar functions at endocytic sites. Previous results are consistent with the view that type I myosin could induce formation of ER-plasma membrane contact sites and this capacity is required for myosin-Osh2/3-Scs2/22 link and the sterol transfer activity of Osh2, actin polymerisation and endocytic vesicle scission (Encinar del Dedo et al., 2017). In the osh2Δ osh3Δ and scs2Δ scs22Δ strains, the lifespan of Abp1 patches was increased, with a high fraction of patches initiating the inward movement and retracting back to the plasma membrane (Encinar del Dedo et al., 2017), similar to what we observed for vps13Δ. Our results showing that vps13Δ defects in endocytosis are suppressed by MYO3-N in an Osh2/3-dependent way suggest that one mechanism of suppression could be by promoting the formation of ER-plasma membrane connections at endocytic sites, which involve type I myosin. Also, a recent report suggests that Vps13 could be a binding partner of Osh2 (Bean et al., 2018). This implies a possible Vps13 function in the formation of ER-plasma membrane contact sites, which are important for maintaining the lipid and protein composition of plasma membrane and are a prerequisite for SDS-stress survival. A more efficient action of myosin in promoting sterol transfer by Osh2/3 at endocytic sites helps to overcome the defects caused by the lack of Vps13. This suggests that: Vps13 may be important for the transfer of some lipids to plasma membranes at some ER-lasma membrane contact sites; a lack of Vps13 participation in the formation of contact sites affects endocytosis; and this defect could be overcome by activation of endocytic ER-plasma membrane contact sites. This model is supported by a recent study in which the N-terminal fragment of Vps13 was shown to be able to transfer the bulk of glycerolipids between liposomes (Kumar et al., 2018), implying that Vps13 might relocate lipids between membranes. Such transfer could possibly be required for growth of plasma membrane invagination or vesicle scission during the internalisation step of endocytosis. It requires further study to find out whether the role of Vps13 in endocytosis is direct, since the SDS growth and endocytosis defect of vps13Δ may result also from activation of calcium signalling. Since the growth defect of vps13Δ on SDS medium is more severe than that of pmc1Δ, the activation of calcium signalling is probably not the only reason and other mechanisms may contribute.
In this study, we demonstrate the relationship between Vps13 and calcium signalling, and the importance of Osh2/3-dependent membrane contact sites for bypassing the vps13Δ growth defect. Our results will be helpful for studies to better understand the pathogenesis of ChAc in higher eukaryotes and in cell lines. The multicopy suppressor that we selected can suppress the other phenotypes of vps13Δ, not just the SDS hypersensitivity. In addition, chemical compounds such as EGTA or FK506 can suppress the SDS hypersensitivity. Thus, we believe that this phenotype is useful to learn about pathways that could be potential targets for chemical intervention, and could be used directly to screen for drugs to treat ChAc.
MATERIALS AND METHODS
Strains, media and growth conditions
Escherichia coli strain DH5α was used for plasmid propagation. The yeast S. cerevisiae strains used in this study are listed in Table S1. For genetic analysis, strains BY4741 cnb1Δ vps13Δ and BY4741 crz1Δ vps13Δ were constructed by deletion of VPS13 using the vps13::URA3 cassette (pKA475) in the single-deletion strains BY4741 cnb1Δ and BY4741 crz1Δ. Gene disruptions were performed by transformation of yeast cells with a PCR product containing the URA3 selection marker flanked by 50 bp of homology to the 5′ and 3′ regions of VPS13. The vps13::URA3 cassette was also used to construct strains encoding Las17-mRFP, Myo3-GFP, Myo5-GFP or Abp1-mCherry endocytic markers in the genome and devoid of Vps13 protein. Genomic integrations were confirmed by PCR on genomic DNA.
Yeast were grown at 30°C in liquid YPD complete medium (1% yeast extract, 2% peptone, 2% glucose) or in synthetic SC medium (0.067% yeast nitrogen base without amino acids, 2% glucose) with desired supplements (adenine, uracil, amino acids) (all media ingredients: DB, Sparks, USA). For growth tests, cells were grown overnight in liquid media and cultures were diluted with water to obtain a cell density equal to OD600∼1. Subsequently, aliquots of 4-fold serial dilutions of cells were spotted on plates with solid media. Media used for growth tests were YPD with, or without, addition of SDS (Sigma-Aldrich, St Louis, USA), and SC-leu-arg with, or without, addition of canavanine (Sigma-Aldrich), as described in the figure legends. Plates were incubated at 30°C for 2 (YPD) or 3 (YPD+SDS) days, or as indicated. Mutant cells were very sensitive to small changes in SDS concentration and final SDS concentration in the medium strongly depends on the lot of medium and lot of SDS stock solution, and various physical factors; therefore, each growth experiment was performed using several dilutions of SDS stock solution and a representative result is shown. For two-hybrid analysis, the PJ69-4A strain was used and respective transformants were grown on SC-trp-leu plates, replicated on SC-trp-leu-his supplemented with 1.5 mM aminotriazol (Sigma-Aldrich) and incubated for 3-5 days. Liquid cultures were inoculated with a mix of several yeast colonies obtained after transformation. Growth tests were repeated at least twice. Plates were scanned by HP Scanjet G4010 or Epson Perfection 2480 Photo scanner. Imagines were processed with Adobe Photoshop 6.0.
Plasmids
Plasmids used are described in Table S2. To subclone the MYO3 gene fragment, the BamHI and SalI DNA fragment from the original clone from the genomic bank was transferred into pRS425-PGPD vector to obtain pRS425-PGPD-MYO3-N. Further transferring a 3.2 kb BamHI HindIII fragment of pRS425-PGPD-MYO3-N to pRS425-PGPD generated pRS425-PGPD-MYO3-NΔ680-775. To destroy calmodulin-binding motifs IQ1 and IQ2 in Myo3-N, mutations were introduced in the MYO3-N fragment, after subcloning into pUC19 using PstI and SalI enzymes, by one-step site-directed mutagenesis. Three plasmids were obtained: pUC19-MYO3-iq1, encoding myosin fragment with I725G and Q726G substitutions; pUC19-MYO3-iq2, encoding protein with I743G and Q744G substitutions; and pUC19-MYO3-iq1/2, encoding protein with all four substitutions. Next, the PstI-SalI fragments were transferred back to create pRS425-PGPD-myo3-iq1, pRS425-PGPD-myo3-iq2 and pRS425-PGPD-myo3-iq1/2. pRS425-PGPD-MYO3-N-HA and pRS425-PGPD-myo3-iq1/2-HA were generated by introducing the HA tag into the SalI polylinker restriction site. Sequences of oligonucleotides used in this study are available upon request.
To construct plasmids for two-hybrid systems, the CMD1 gene and mutated alleles were amplified by PCR using YEplac181lac bearing CMD1, cmd1-226, cmd1-228, cmd1-231 or cmd1-239 alleles as the templates. PCR products were digested with PstI and BamHI and were cloned into pGAD424. To obtain pGBT9-MYO3-N or pGBT9-myo3-iq1/2, the appropriate fragments were amplified by PCR with pRS425-PGPD-MYO3-N or pRS425-PGPD-myo3-iq1/2 as the templates. PCR products were cloned into EcoRI and SalI sites of pGBT9.
Screen for multicopy suppressors of vps13-I2749R mutation
The vps13Δ [vps13-I2749R] cells were transformed with pFL44-based genomic bank (Kabir et al., 2005), plated on SC-ura-leu plates and incubated at 30°C for 3 days. Then, transformants were replicated on YPD plates with addition of 0.03% SDS and incubated at 30°C for 3 days. Positive clones were isolated and grown in SC-ura liquid medium for plasmid isolation. Plasmids were retransformed and suppression of the vps13Δ [vps13-I2749R] growth defect was confirmed.
Actin staining and fluorescence microscopy
For actin staining, cells were grown in SC-leu medium to log-phase, fixed with formaldehyde (Sigma-Aldrich for 2 h, washed and stained with Alexa-Fluor-546-conjugated phalloidin (Thermo Fisher Scientific, Waltham, USA). Cells were viewed with an Eclipse E800 (Nikon, Tokyo, Japan) fluorescence microscope equipped with a DS-5Mc camera (Nikon, Tokyo, Japan). Images were collected using Lucia General 5.1 software (Laboratory Imaging Ltd, Prague, Czech Republic). The same fields were viewed by differential interference contrast (DIC) optics. All samples in the experiment were encoded and the microscope observations and quantifications were performed blindly. Charts and statistical analyses were done using Microsoft Excel.
For live-cell imaging, cells expressing tagged proteins were visualised after growing to early log-phase in synthetic medium with appropriate supplements. Epifluorescence microscopy was performed using Olympus IX-81 inverted microscope (Olympus, Tokyo, Japan) with a DeltaVision RT Restoration Microscopy System (using a 100×/1.40 NA oil objective) and Photometrics Coolsnap HQ camera, with imaging and image capture performed using SoftWoRx™ image analysis and model-building application (Applied Precision Instruments, Seattle, USA). Live-cell imaging of GFP-tagged Las17 and mCherry-tagged Abp1 proteins was performed with 1 s time lapse. All image data sets were deconvolved, using the SoftWoRx application. Live-cell images of Myo3-GFP and Myo5-GFP were acquired using OMX DeltaVision V4 microscope (GE Healthcare Life Sciences, Chicago, USA) and a 60× USPLAPO (1.42 NA) objective with refractive index 0.1514 immersion oil (Cargille, Cedar Grove, USA) with 0.5 s time lapse. Samples were illuminated using Insight Solid State Illuminator (10%), and images were taken simultaneously on separate scientific complementary metal oxide semiconductor (sCMOS) cameras (30 ms exposure). Seven 250 nm sections were acquired every 500 ms (181 time points). The stacks were then deconvolved and processed, using SoftWorx, to produce a movie composed of maximum intensity projections at each time point. The Myo3-/Myo5-GFP lifetime was analysed from those projections. Movies and kymographs were assembled using Fiji software. Charts and statistical analyses were done in GraphPad Prism (https://www.graphpad.com/scientific-software/prism/, 02.08.2018).
Western blot analysis
Yeast cells were grown at 30°C in SC-ura-leu to the log-phase. Protein extracts were prepared after disrupting cells with glass beads as described (Rzepnikowska et al., 2017a) or alkaline lysis (Yaffe and Schatz, 1984). Samples were analysed by standard SDS-PAGE followed by western blotting using mouse monoclonal anti-Pgk1 (dilution 1:20,000, Molecular Probes Cat# A-6457, RRID:AB_221541, Thermo Fisher Scientific), monoclonal anti-HA epitope (dilution 1:2000, Cat# 901503, BioLegend, San Diego, USA), rabbit anti-phospho-p44/42 MAPK (Erk1/2) (Thr202/Thr204; dilution 1:1000, Antibody #9101, Cell Signalling Technology, Danvers, USA; validation: Pérez et al., 2016), recognising phospho-Slt2, antibodies. Secondary anti-mouse or anti-rabbit IgG horseradish peroxidase (HRP)-conjugated antibodies (dilution 1:2000, Dako, Glostrup, Denmark) were also used. Signal was detected by enhanced chemiluminescence (Millipore, Darmstadt, Germany). Densitometry was performed with ImageJ (https://imagej.nih.gov/ij/, 02.08.2018). Chart and statistical analyses were done in GraphPad Prism.
β-galactosidase activity
For the β-galactosidase activity assay, cells were grown overnight in SC-ura or SC-ura-leu, collected and extracts prepared with glass beads. Activity of β-galactosidase was analysed as described (Kamińska et al., 2005). In each of three experiments, at least three independent transformants of respective yeast strains were inoculated, and their extracts were assayed in duplicates.
Supplementary Material
Acknowledgements
We are grateful to H. Riezman (University of Geneva) and R. Kucharczyk (Institute of Biochemistry and Biophysics PAS) for plasmids, and M. I. Geli (Institute for Molecular Biology of Barcelona) for strains.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: J.K., T.Z.; Methodology: P.S., I. S.-de R., J.K.; Formal analysis: P.S., I. S.-de R., J.K.; Investigation: P.S., D.K., I. S.-de R., W.R., J.K.; Resources: P.S., I.S.-de R., J.K.; Writing - original draft preparation: P.S., J.K., T.Z.; Writing - review & editing: D.K., W.R., K.R.A.; Supervision: J.K., T.Z.; Project administration: T.Z.; Funding acquisition: K.R.A., T.Z.
Funding
This work was supported by the National Science Centre Poland [grant UMO-2015/19/B/NZ3/01515 to T.Z.]; and by the Biotechnology and Biological Sciences Research Council [grant BB/N007581/1 to K.R.A.].
Supplementary information
Supplementary information available online at http://dmm.biologists.org/lookup/doi/10.1242/dmm.036830.supplemental
References
- Ammons L. M., Bingham L. R., Callery S., Corley E., Katherine A., Lipton J. K., Mendez C. A., Morrison T., Rallis C. and Mcclellan A. J. (2015). Yeast require an intact tryptophan biosynthesis pathway and exogenous tryptophan for resistance to sodium dodecyl sulfate. J. Student Res. 4, 74-82. [Google Scholar]
- Anderson B. L., Boldogh I., Evangelista M., Boone C., Greene L. A. and Pon L. A. (1998). The Src homology domain 3 (SH3) of a yeast type I myosin, Myo5p, binds to verprolin and is required for targeting to sites of actin polarization. J. Cell Biol. 141, 1357-1370. 10.1083/jcb.141.6.1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankaitis V. A., Johnson L. M. and Emr S. D. (1986). Isolation of yeast mutants defective in protein targeting to the vacuole. Proc. Natl. Acad. Sci. USA 83, 9075-9079. 10.1073/pnas.83.23.9075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean B. D. M., Dziurdzik S. K., Kolehmainen K. L., Fowler C. M. S., Kwong W. K., Grad L. I., Davey M., Schluter C. and Conibear E. (2018). Competitive organelle-specific adaptors recruit Vps13 to membrane contact sites. J. Cell Biol. 217, 3593 10.1083/jcb.201804111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond R., Ly N. and Cyert M. S. (2017). The unique C terminus of the calcineurin isoform CNAβ1 confers non-canonical regulation of enzyme activity by Ca2+ and calmodulin. J. Biol. Chem. 292, 16709-16721. 10.1074/jbc.M117.795146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickner J. H. and Fuller R. S. (1997). SOI1 encodes a novel, conserved protein that promotes TGN-endosomal cycling of Kex2p and other membrane proteins by modulating the function of two TGN localization signals. J. Cell Biol. 139, 23-36. 10.1083/jcb.139.1.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caraveo G., Auluck P. K., Whitesell L., Chung C. Y., Baru V., Mosharov E. V., Yan X., Ben-Johny M., Soste M., Picotti P. et al. (2014). Calcineurin determines toxic versus beneficial responses to -synuclein. Proc. Natl. Acad. Sci. USA 111, E3544-E3552. 10.1073/pnas.1413201111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.-L., Konieczka J. H., Springer D. J., Bowen S. E., Zhang J., Silao F. G. S., Bungay A. A. C., Bigol U. G., Nicolas M. G., Abraham S. N. et al. (2012). Convergent evolution of calcineurin pathway roles in thermotolerance and virulence in Candida glabrata. G3 2, 675-691. 10.1534/g3.112.002279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanzo M., Baryshnikova A., Bellay J., Kim Y., Spear E. D., Sevier C. S., Ding H., Koh J. L. Y., Toufighi K., Mostafavi S. et al. (2010). The genetic landscape of a cell. Science 327, 425-431. 10.1126/science.1180823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cserháti T., Forgács E. and Oros G. (2002). Biological activity and environmental impact of anionic surfactants. Environ. Int. 28, 337-348. 10.1016/S0160-4120(02)00032-6 [DOI] [PubMed] [Google Scholar]
- Cyert M. S. (2001). Genetic analysis of calmodulin and its targets in Saccharomyces cerevisiae. Annu. Rev. Genet. 35, 647-672. 10.1146/annurev.genet.35.102401.091302 [DOI] [PubMed] [Google Scholar]
- Cyert M. S. and Thorner J. (1992). Regulatory subunit (CNB1 gene product) of yeast Ca2+/calmodulin-dependent phosphoprotein phosphatases is required for adaptation to pheromone. Mol. Cell. Biol. 12, 3460-3469. 10.1128/MCB.12.8.3460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyert M. S., Kunisawa R., Kaim D. and Thorner J. (1991). Yeast has homologs (CNA1 and CNA2 gene products) of mammalian calcineurin, a calmodulin-regulated phosphoprotein phosphatase. Proc. Natl. Acad. Sci. USA 88, 7376-7380. 10.1073/pnas.88.16.7376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton L. E., Bean B. D. M., Davey M. and Conibear E. (2017). Quantitative high-content imaging identifies novel regulators of Neo1 trafficking at endosomes. Mol. Biol. Cell 28, 1539-1550. 10.1091/mbc.e16-11-0772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De M., Oleskie A. N., Ayyash M., Dutta S., Mancour L., Abazeed M. E., Brace E. J., Skiniotis G. and Fuller R. S. (2017). The Vps13p-Cdc31p complex is directly required for TGN late endosome transport and TGN homotypic fusion. J. Cell Biol. 216, 425-439. 10.1083/jcb.201606078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Franceschi L., Tomelleri C., Matte A., Brunati A. M., Bovee-Geurts P. H., Bertoldi M., Lasonder E., Tibaldi E., Danek A., Walker R. H. et al. (2011). Erythrocyte membrane changes of chorea-acanthocytosis are the result of altered Lyn kinase activity. Blood 118, 5652-5663. 10.1182/blood-2011-05-355339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn T., Gable K. and Beeler T. (1994). Regulation of cellular Ca2+ by yeast vacuoles. J. Biol. Chem. 269, 7273-7278. [PubMed] [Google Scholar]
- Encinar del Dedo J., Idrissi F.-Z., Fernandez-Golbano I. M., Garcia P., Rebollo E., Krzyzanowski M. K., Grötsch H. and Geli M. I. (2017). ORP-mediated ER contact with endocytic sites facilitates actin polymerization. Dev. Cell 43, 588-602.e6. 10.1016/j.devcel.2017.10.031 [DOI] [PubMed] [Google Scholar]
- Fokina A., Sokolov S., Kang H. A., Ter-Avanesyan M. D. and Agaphonov M. (2012). Inactivation of Pmc1 vacuolar Ca 2+ ATPase causes G 2 cell cycle delay in Hansenula polymorpha. Cell Cycle 11, 778-784. 10.4161/cc.11.4.19220 [DOI] [PubMed] [Google Scholar]
- Föller M., Hermann A., Gu S., Alesutan I., Qadri S. M., Borst O., Schmidt E.-M., Schiele F., Müller vom Hagen J., Saft C. et al. (2012). Chorein-sensitive polymerization of cortical actin and suicidal cell death in chorea-acanthocytosis. FASEB J. 26, 1526-1534. 10.1096/fj.11-198317 [DOI] [PubMed] [Google Scholar]
- Fromer M., Pocklington A. J., Kavanagh D. H., Williams H. J., Dwyer S., Gormley P., Georgieva L., Rees E., Palta P., Ruderfer D. M. et al. (2014). De novo mutations in schizophrenia implicate synaptic networks. Nature 506, 1-16. 10.1038/nature12929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa T., Kuboki Y., Tanji E., Yoshida S., Hatori T., Yamamoto M., Shibata N., Shimizu K., Kamatani N. and Shiratori K. (2011). Whole-exome sequencing uncovers frequent GNAS mutations in intraductal papillary mucinous neoplasms of the pancreas. Sci. Rep. 1, 161 10.1038/srep00161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiser J. R., Sundberg H. A., Chang B. H., Muller E. G. D. and Davis T. N. (1993). The essential mitotic target of calmodulin is the 110-kilodalton component of the spindle pole body in Saccharomyces cerevisiae. Mol. Cell. Biol. 13, 7913-7924. 10.1128/MCB.13.12.7913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geli M. I. and Riezman H. (1996). Role of type I myosins in receptor-mediated endocytosis in yeast. Science 272, 533-535. 10.1126/science.272.5261.533 [DOI] [PubMed] [Google Scholar]
- Geli M. I., Wesp A. and Riezman H. (1998). Distinct functions of calmodulin are required for the uptake step of receptor-mediated endocytosis in yeast: the type I myosin Myo5p is one of the calmodulin targets. EMBO J. 17, 635-647. 10.1093/emboj/17.3.635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietz R. D. and Sugino A. (1988). New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene 14, 527-534. 10.1016/0378-1119(88)90185-0 [DOI] [PubMed] [Google Scholar]
- Goode B. L., Eskin J. A. and Wendland B. (2015). Actin and endocytosis in budding yeast. Genetics 199, 315-358. 10.1534/genetics.112.145540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson H. V. and Spudich J. A. (1995). Identification and molecular characterization of a yeast myosin I. Cell Motil. Cytoskelet. 30, 73-84. 10.1002/cm.970300109 [DOI] [PubMed] [Google Scholar]
- Goodson H. V., Anderson B. L., Warrick H. M., Pon L. A. and Spudich J. A. (1996). Synthetic lethality screen identifies a novel yeast myosin I gene (myo5) - myosin I proteins are required for polarization of the actin cytoskeleton. J. Cell Biol. 133, 1277-1291. 10.1083/jcb.133.6.1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grarup N., Overvad M., Sparsø T., Witte D. R., Pisinger C., Jørgensen T., Yamauchi T., Hara K., Maeda S., Kadowaki T. et al. (2011). The diabetogenic VPS13C/C2CD4A/C2CD4B rs7172432 variant impairs glucose-stimulated insulin response in 5722 non-diabetic Danish individuals. Diabetologia 54, 789-794. 10.1007/s00125-010-2031-2 [DOI] [PubMed] [Google Scholar]
- Grötsch H., Giblin J. P., Idrissi F.-Z., Fernández-Golbano I.-M., Collette J. R., Newpher T. M., Robles V., Lemmon S. K. and Geli M.-I. (2010). Calmodulin dissociation regulates Myo5 recruitment and function at endocytic sites. EMBO J. 29, 2899-2914. 10.1038/emboj.2010.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie R. J., Pullon H. W. H., Harding A. E., Owen J. S., Pires M., Daniels G. L., Imai Y., Misra V. P., King R. H. M., Jacobs J. M. et al. (1991). Neuroacanthacytosis. A clinical, haematological and pathological study of 19 cases. Brain 114, 13-49. [PubMed] [Google Scholar]
- Ho Y., Gruhler A., Heilbut A., Bader G. D., Moore L., Adams S.-L., Millar A., Taylor P., Bennett K., Boutilier K. et al. (2002). Systematic identification of protein complexes in Saccharomyces cerevisiae by mass spectrometry. Nature 415, 180-183. 10.1038/415180a [DOI] [PubMed] [Google Scholar]
- Honisch S., Fehrenbacher B., Lebedeva A., Alesutan I., Castor T., Alkahtani S., Alarifi S., Schaller M., Stournaras C. and Lang F. (2015). Chorein sensitive dopamine release from pheochromocytoma (PC12) cells. NeuroSignals 23, 1-10. 10.1159/000442599 [DOI] [PubMed] [Google Scholar]
- Huckaba T. M., Gay A. C., Pantalena L. F., Yang H.-C. and Pon L. A. (2004). Live cell imaging of the assembly, disassembly, and actin cable-dependent movement of endosomes and actin patches in the budding yeast, Saccharomyces cerevisiae. J. Cell Biol. 167, 519-530. 10.1083/jcb.200404173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida H., Yagawa Y. and Anraku Y. (1990). Essential role for induced Ca2+influx followed by [Ca2+] i rise in maintaining viability of yeast cells late in the mating pheromone response pathway. J. Biol. Chem. 265, 13391-13399. [PubMed] [Google Scholar]
- James P., Halladay J. and Craig E. A. (1996). Genomic libraries and a host strain designed for highly efficient two-hybrid selection in yeast. Genetics 144, 1425-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabir M. A., Kaminska J., Segel G. B., Bethlendy G., Lin P., Della Seta F., Blegen C., Swiderek K. M., Zoładek T., Arndt K. T. et al. (2005). Physiological effects of unassembled chaperonin Cct subunits in the yeast Saccharomyces cerevisiae. Yeast 22, 219-239. 10.1002/yea.1210 [DOI] [PubMed] [Google Scholar]
- Kamińska J., Wysocka-Kapcińska M., Smaczyńska-de Rooij I., Rytka J. and Żołądek T. (2005). Pan1p, an actin cytoskeleton-associated protein, is required for growth of yeast on oleate medium. Exp. Cell Res. 310, 482-492. 10.1016/j.yexcr.2005.08.018 [DOI] [PubMed] [Google Scholar]
- Kipanyula M. J., Kimaro W. H. and Etet P. F. S. (2016). The emerging roles of the calcineurin-nuclear factor of activated T-lymphocytes pathway in nervous system functions and diseases. J. Aging Res. 2016, 5081021 10.1155/2016/5081021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolehmainen J., Black G. C. M., Saarinen A., Chandler K., Clayton-Smith J., Träskelin A.-L., Perveen R., Kivitie-Kallio S., Norio R., Warburg M. et al. (2003). Cohen syndrome is caused by mutations in a novel gene, COH1, encoding a transmembrane protein with a presumed role in vesicle-mediated sorting and intracellular protein transport. Am. J. Hum. Genet. 72, 1359-1369. 10.1086/375454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucharczyk R., Dupre S., Avaro S., Haguenauer-tsapis R., Slonimski P. P. and Rytka J. (2000). The novel protein Ccz1p required for vacuolar assembly in Saccharomyces cerevisiae functions in the same transport pathway as Ypt7p. J. Cell Sci. 113, 4301-4311. [DOI] [PubMed] [Google Scholar]
- Kumar N., Leonzino M., Hancock-Cerutti W., Horenkamp F. A., Li P. Q., Lees J. A., Wheeler H., Reinisch K. M. and De Camilli P. (2018). VPS13A and VPS13C are lipid transport proteins differentially localized at ER contact sites. J. Cell Biol. 217, 3625-3639. 10.1083/jcb.201807019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang A. B., Peter A. T. J., Walter P. and Kornmann B. (2015). ER-mitochondrial junctions can be bypassed by dominant mutations in the endosomal protein Vps13. J. Cell Biol. 210, 883-890. 10.1083/jcb.201502105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechuga M., Fernández-Serrano M., Jurado E., Núñez-Olea J. and Ríos F. (2016). Acute toxicity of anionic and non-ionic surfactants to aquatic organisms. Ecotoxicol. Environ. Saf. 125, 1-8. 10.1016/j.ecoenv.2015.11.027 [DOI] [PubMed] [Google Scholar]
- Lesage S., Drouet V., Majounie E., Deramecourt V., Jacoupy M., Nicolas A., Cormier-Dequaire F., Hassoun S. M., Pujol C., Ciura S. et al. (2016). Loss of VPS13C function in autosomal-recessive Parkinsonism causes mitochondrial dysfunction and increases PINK1/Parkin-dependent mitophagy. Am. J. Hum. Genet. 98, 500-513. 10.1016/j.ajhg.2016.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin D. E. (2005). Cell wall integrity signaling in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 69, 262-291. 10.1128/MMBR.69.2.262-291.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Ishii S., Tokai M., Tsutsumi H., Ohki O., Akada R., Tanaka K., Tsuchiya E., Fukui S. and Miyakawa T. (1991). The Saccharomyces cerevisiae genes (CMP1 and CMP2) encoding calmodulin-binding proteins homologous to the catalytic subunit of mammalian protein phosphatase 2B. Mol. Gen. Genet. 227, 52-59. 10.1007/BF00260706 [DOI] [PubMed] [Google Scholar]
- Lu R., Drubin D. G. and Sun Y. (2016). Clathrin-mediated endocytosis in budding yeast at a glance. J. Cell Sci. 129, 1531-1536. 10.1242/jcs.182303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W.-J. and Chang A. (1997). Novel genes involved in endosomal traffic in yeast revealed by suppression of a targeting-defective plasma membrane ATPase mutant. J. Cell Biol. 138, 731-746. 10.1083/jcb.138.4.731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheos D. P., Kingsbury T. J., Ahsan U. S. and Cunningham K. W. (1997). Tcn1p/Crz1p, a calcineurin-dependent transcription factor that differentially regulates gene expression in Saccharomyces cerevisiae. Genes Dev. 11, 3445-3458. 10.1101/gad.11.24.3445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelot A., Costanzo M., Sarkeshik A., Boone C., Yates J. R. and Drubin D. G. (2010). Reconstitution and protein composition analysis of endocytic actin patches. Curr. Biol. 20, 1890-1899. 10.1016/j.cub.2010.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuguchi T., Nakashima M., Kato M., Okamoto N., Kurahashi H., Ekhilevitch N., Shiina M., Nishimura G., Shibata T., Matsuo M. et al. (2018). Loss-of-function and gain-of-function mutations in PPP3CA cause two distinct disorders. Hum. Mol. Genet. 27, 1421-1433. 10.1093/hmg/ddy052 [DOI] [PubMed] [Google Scholar]
- Morisaki T., Yashiro M., Kakehashi A., Inagaki A., Kinoshita H., Fukuoka T., Kasashima H., Masuda G., Sakurai K., Kubo N. et al. (2014). Comparative proteomics analysis of gastric cancer stem cells. PLoS ONE 9, e110736 10.1371/journal.pone.0110736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulet J. M., Martin D. E., Loewith R. and Hall M. N. (2006). Mutual antagonism of target of rapamycin and calcineurin signaling. J. Biol. Chem. 281, 33000-33007. 10.1074/jbc.M604244200 [DOI] [PubMed] [Google Scholar]
- Muñoz-Braceras S., Calvo R. and Escalante R. (2015). TipC and the chorea-acanthocytosis protein VPS13A regulate autophagy in Dictyostelium and human HeLa cells. Autophagy 11, 918-927. 10.1080/15548627.2015.1034413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers M. D., Ryazantsev S., Hicke L., Payne G. S., Myers M. D., Ryazantsev S., Hicke L. and Payne G. S. (2016). Calmodulin promotes N-BAR domain-mediated membrane constriction and endocytosis. Dev. Cell 37, 162-173. 10.1016/j.devcel.2016.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima-Shimada J., Iida H., Tsuji F. I. and Anraku Y. (1991). Monitoring of intracellular calcium in Saccharomyces cerevisiae with an apoaequorin cDNA expression system. Proc. Natl. Acad. Sci. USA 88, 6878-6882. 10.1073/pnas.88.15.6878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi H., Suda Y. and Neiman A. M. (2007). Erv14 family cargo receptors are necessary for ER exit during sporulation in Saccharomyces cerevisiae. J. Cell Sci. 120, 908-916. 10.1242/jcs.03405 [DOI] [PubMed] [Google Scholar]
- Ohya Y. and Botstein D. (1994). Structure-based systematic isolation of conditional-lethal mutations in the single yeast calmodulin gene. Genetics 138, 1041-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer S. E., Smaczynska-de Rooij I. I., Marklew C. J., Allwood E. G., Mishra R., Johnson S., Goldberg M. W. and Ayscough K. R. (2015). A dynamin-actin interaction is required for vesicle scission during endocytosis in yeast. Curr. Biol. 25, 868-878. 10.1016/j.cub.2015.01.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.-S. and Neiman A. M. (2012). VPS13 regulates membrane morphogenesis during sporulation in Saccharomyces cerevisiae. J. Cell Sci. 125, 3004-3011. 10.1242/jcs.105114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.-S., Halegoua S., Kishida S. and Neiman A. M. (2015). A conserved function in phosphatidylinositol metabolism for mammalian Vps13 family proteins. PLoS ONE 10, e0124836 10.1371/journal.pone.0124836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.-S., Thorsness M. K., Policastro R., McGoldrick L. L., Hollingsworth N. M., Thorsness P. E. and Neiman A. M. (2016). Yeast Vps13 promotes mitochondrial function and is localized at membrane contact sites. Mol. Biol. Cell 27, 2435-2449. 10.1091/mbc.e16-02-0112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelzl L., Elsir B., Sahu I., Bissinger R., Singh Y., Sukkar B., Honisch S., Schoels L., Jemaà M., Lang E. et al. (2017a). Lithium sensitivity of store operated Ca2+ entry and survival of fibroblasts isolated from chorea-acanthocytosis patients. Cell. Physiol. Biochem. 42, 2066-2077. 10.1159/000479901 [DOI] [PubMed] [Google Scholar]
- Pelzl L., Hauser S., Elsir B., Sukkar B., Sahu I., Singh Y., Höflinger P., Bissinger R., Jemaà M., Stournaras C. et al. (2017b). Lithium sensitive ORAI1 expression, store operated Ca2+ entry and suicidal death of neurons in chorea-acanthocytosis. Sci. Rep. 7, 1-10. 10.1038/s41598-017-06451-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez J., Arcones I., Gómez A., Casquero V. and Roncero C. (2016). Phosphorylation of Bni4 by MAP kinases contributes to septum assembly during yeast cytokinesis. FEMS Yeast Res. 16, fow060 10.1093/femsyr/fow060 [DOI] [PubMed] [Google Scholar]
- Pezzati R., Bossi M., Podini P., Meldolesi J. and Grohovaz F. (1997). High-resolution calcium mapping of the endoplasmic reticulum-Golgi-exocytic membrane system. Electron energy loss imaging analysis of quick frozen-freeze dried PC12 cells. Mol. Biol. Cell 8, 1501-1512. 10.1091/mbc.8.8.1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampoldi L., Dobson-Stone C., Rubio J. P., Danek A., Chalmers R. M., Wood N. W., Verellen C., Ferrer X., Malandrini A., Fabrizi G. M. et al. (2001). A conserved sorting-associated protein is mutant in chorea-acanthocytosis. Nat. Genet. 28, 119-120. 10.1038/88821 [DOI] [PubMed] [Google Scholar]
- Redding K., Brickner J. H., Marschall L. G., Nichols J. W. and Fuller R. S. (1996). Allele-specific suppression of a defective trans-Golgi network (TGN) localization signal in Kex2p identifies three genes involved in localization of TGN transmembrane proteins. Mol. Cell. Biol. 16, 6208-6217. 10.1128/MCB.16.11.6208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio J. P., Danek A., Stone C., Chalmers R., Wood N., Verellen C., Ferrer X., Malandrini A., Fabrizi G. M., Manfredi M. et al. (1997). Chorea-acanthocytosis: genetic linkage to chromosome 9q21. Am. J. Hum. Genet. 61, 899-908. 10.1086/514876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rzepnikowska W., Flis K., Kaminska J., Grynberg M., Urbanek A., Ayscough K. R. and Zoladek T. (2017a). Amino acid substitution equivalent to human choreaacanthocytosis I2771R in yeast Vps13 protein affects its binding to phosphatidylinositol 3-phosphate. Hum. Mol. Genet. 26, 1497-1510. 10.1093/hmg/ddx054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rzepnikowska W., Flis K., Muñoz-Braceras S., Menezes R., Escalante R. and Zoladek T. (2017b). Yeast and other lower eukaryotic organisms for studies of Vps13 proteins in health and disease. Traffic 18, 711-719. 10.1111/tra.12523 [DOI] [PubMed] [Google Scholar]
- Sandbacka M., Christianson I. and Isomaa B. (2000). The acute toxicity of surfactants on fish cells, Daphnia magna and fish - a comparative study. Toxicol. Vitr. 14, 61-68. 10.1016/S0887-2333(99)00083-1 [DOI] [PubMed] [Google Scholar]
- Saxena R., Hivert M.-F., Langenberg C., Tanaka T., Pankow J. S., Vollenweider P., Lyssenko V., Bouatia-Naji N., Dupuis J., Jackson A. U. et al. (2010). Genetic variation in GIPR influences the glucose and insulin responses to an oral glucose challenge. Nat. Genet. 42, 142-148. 10.1038/ng.521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaerer-Brodbeck C. and Riezman H. (2003). Genetic and biochemical interactions between the Arp2/3 complex, Cmd1p, casein kinase II, and Tub4p in yeast. FEMS Yeast Res. 4, 37-49. 10.1016/S1567-1356(03)00110-7 [DOI] [PubMed] [Google Scholar]
- Schmidt E.-M., Schmid E., Münzer P., Hermann A., Eyrich A.-K., Russo A., Walker B., Gu S., vom Hagen J. M., Faggio C. et al. (2013). Chorein sensitivity of cytoskeletal organization and degranulation of platelets. FASEB J. 27, 2799-2806. 10.1096/fj.13-229286 [DOI] [PubMed] [Google Scholar]
- Sekiya-Kawasaki M., Botstein D. and Ohya Y. (1998). Identification of functional connections between calmodulin and the yeast actin cytoskeleton. Genetics 150, 43-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski R. S. and Hieter P. (1989). A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122, 19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smaczynska-de Rooij I. I., Costa R. and Ayscough K. R. (2008). Yeast Arf3p modulates plasma membrane PtdIns(4,5)P2 levels to facilitate endocytosis. Traffic 9, 559-573. 10.1111/j.1600-0854.2008.00708.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smaczynska-de Rooij I. I., Allwood E. G., Mishra R., Booth W. I., Aghamohammadzadeh S., Goldberg M. W. and Ayscough K. R. (2012). Yeast dynamin Vps1 and amphiphysin Rvs167 function together during endocytosis. Traffic 13, 317-328. 10.1111/j.1600-0854.2011.01311.x [DOI] [PubMed] [Google Scholar]
- Stathopoulos A. M. and Cyert M. S. (1997). Calcineurin acts through the CRZ1/TCN1-encoded transcription factor to regulate gene expression in yeast. Genes Dev. 11, 3432-3444. 10.1101/gad.11.24.3432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stathopoulos-Gerontides A., Guo J. J. and Cyert M. S. (1999). Yeast calcineurin regulates nuclear localization of the Crz1p transcription factor through dephosphorylation. Genes Dev. 13, 798-803. 10.1101/gad.13.7.798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Martin A. C. and Drubin D. G. (2006). Endocytic internalization in budding yeast requires coordinated actin nucleation and myosin motor activity. Dev. Cell 11, 33-46. 10.1016/j.devcel.2006.05.008 [DOI] [PubMed] [Google Scholar]
- Ueno S.-I., Maruki Y., Nakamura M., Tomemori Y., Kamae K., Tanabe H., Yamashita Y., Matsuda S., Kaneko S. and Sano A. (2001). The gene encoding a newly discovered protein, chorein, is mutated in chorea-acanthocytosis. Nat. Genet. 28, 121-122. 10.1038/88825 [DOI] [PubMed] [Google Scholar]
- Varelas X., Stuart D., Ellison M. J. and Ptak C. (2006). The Cdc34/SCF ubiquitination complex mediates Saccharomyces cerevisiae cell wall integrity. Genetics 174, 1825-1839. 10.1534/genetics.106.059154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velayos-Baeza A., Vettori A., Copley R. R., Dobson-Stone C. and Monaco A. P. (2004). Analysis of the human VPS13 gene family. Genomics 84, 536-549. 10.1016/j.ygeno.2004.04.012 [DOI] [PubMed] [Google Scholar]
- Yaffe M. P. and Schatz G. (1984). Two nuclear mutations that block mitochondrial protein import in yeast. Proc. Natl. Acad. Sci. USA 81, 4819-4823. 10.1073/pnas.81.15.4819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap K. L., Ames J. B., Swindells M. B. and Ikura M. (1999). Diversity of conformational states and changes within the EF-hand protein superfamily. Proteins Struct. Funct. Genet. 37, 499-507. [DOI] [PubMed] [Google Scholar]
- Zhang M., Liu Z., Yu Q., Mao J., Zhang B., Xing L. and Li M. (2015). Deletion of genes encoding fatty acid desaturases leads to alterations in stress sensitivity in Pichia pastoris. FEMS Yeast Res. 15, fov020 10.1093/femsyr/fov020 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.