Abstract
Introduction:
Metabolic syndrome (MetS) is a world-wide epidemic disease with an increased risk of morbidity and mortality. Treatment strategies of MetS include pharmacologic and non-pharmacologic interventions and in this respect a relevant role has been shown for nutraceutical compounds (NCs). The aim of this study was to investigate the efficacy and safety of NCs incorporated with diet and lifestyle management versus diet alone, in lowering blood pressure (BP) values and improving lipid and glucose profile, in a group of hypertensives and hyper-cholesterolemic patients with MetS.
Methods:
104 subjects with MetS (mean age 57.4 ± 8.8 years, 51% males) without history of cardio-vascular (CV) diseases were enrolled in the study. 52 subjects were treated with a once-daily oral formulation of a NCs containing red yeast rice and coenzyme Q10 added to their diet for 2 months and were compared with the 52 patients following a diet program. Differences in BP, serum total cholesterol (TC), low- and high-density-lipo-protein cholesterol (LDLC and HDLC), triglycerides (TG) and glucose values were compared by analysis of variance.
Results:
A significant reduction of BP, TC, TG, LDLC and glucose levels was observed in both treatment groups. However, a greater reduction of systolic BP (−5.2 vs. −3.0 mmHg), diastolic BP (−4.9 vs. 2.9 mmHg), total cholesterol (−17.2%), LDLC (−21.8%), TG (−16.0%) and serum glucose (−3.4%) was observed in the treatment group relative to the control (p < 0.001 for all); HDLC remained unchanged (p = N.S.). Gender difference was not found in either group (p = N.S.).
Conclusions:
In patients with MetS, NC supplementation was safe, well tolerated and effective in improving clinic BP, lipid and glucose profile.
Keywords: Cholesterol, Lipids, Metabolic syndrome, Blood pressure, Diet, Monacolin K
1. Introduction
Metabolic syndrome (MetS), is a cluster of conditions that synergistically increase the risk of blood pressure (BP) and cardiovascular disease (CV) [1]. Its prevalence worldwide varies from 8% to 43% in men and from 7% to 56% in women (especially in the post-menopausal status) [1]. In the pathophysiology of MetS, visceral fat plays an important role in the development of dyslipidemia, that consists mainly in increased triglyceride (TG) and low-density lipoproteins (LDL) and decreased high density lipoproteins (HDL) levels [2]. Treatment strategies of MetS include non-pharmacologic and pharmacologic management. The first line treatment is weight loss, through increased exercise and caloric reduction with a diet containing low contents of saturated fats and cholesterol. Indeed, a relevant role in MetS management has been demonstrated through supplementation with nutraceutical compounds (NCs), when diet and lifestyle changes alone fail to correct the metabolic alterations.
Monacolins are statin-like molecules, contained in red yeast rice (RYR) that has been shown to inhibit the 3-hydroxy-3 methyl-glutarylcoenzyme A (HMG-CoA) reductases. In fact, the European Safety Authority has recently stated that there is a relationship between the consumption of monacolin K from RYR and maintenance of normal blood LDL-cholesterol concentrations [3] that has been observed in patients with MetS [4], in dyslipidemic [5], intolerant to statin [6], overweight [7], and also in elderly patients [8]. In addition, some NCs contains Coenzyme Q10 (CoQ10), a co-factor in the Krebs cycle that in adequate amount is essential for oxidative reduction processes, and is able to prevent LDL peroxidation [9]. In hyper-cholesterolemic subjects [10], low plasma levels of CoQ10 are associated with increased arterial stiffness, which is improved by dietary supplement with CoQ10 containing NCs [11]. Supplementation with CoQ10 resulted in a decrease in BP levels, and in a significant improvement of the glycemic control [12,13].
The aim of this study was to investigate the efficacy and safety of NCs containing Monacolin K and CoQ10 in lowering BP values and improving lipid and glucose profile, added to diet and lifestyle management versus diet alone in a group of hypertensives (HTs) and hyper-cholesterolemic subjects (HChs) with MetS.
2. Material and methods
2.1. Patient recruitment
104 subjects with MetS (51% males, and mean age 57.4 ± 8.8 years) referred to the Hypertension Centre at Santa Maria della Misericordia Hospital, were enrolled in a multicentre, randomized, open-label, post-marketing clinical trial. MetS was defined, according to the NCEP ATP III definition [14], if three or more of the following five criteria were met: waist circumference (WC) over 102 cm (men) or 82 cm (women), BP over 130/85 mmHg, fasting triglyceride (TG) level over 150 mg/dl, fasting HDL cholesterol level less than 40 mg/dl (men) or 50 mg/dl (women) and fasting serum glucose over 100 mg/dl. Exclusion criteria were severe hypertension (clinic systolic BP ≥ 180 mmHg or diastolic BP ≥ 110 mmHg), secondary hypertension, diabetes mellitus, presence of neoplastic or hepatic disease, chronic heart or renal failure, positive history or clinical signs of ischemic heart disease, disabling diseases such as dementia or inability to cooperate, pregnancy or breast-feeding, anti-hypertensive and/or lipid lowering drugs treatment and organ damage (left ventricular hyper-trophy diagnosed by electrocardiogram, carotid plaque or albuminuria) due to HT.
The study was conducted in accordance with The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use Harmonized Tripartite Guidelines for Good Clinical Practice (ICH-GCP) and according to the set of ethical principles of the Declaration of Helsinki. Patients were enrolled after signing the informed consent for the study participation and were free to withdraw from the study at any time.
2.2. Data collection
At baseline, clinic BP (diastolic Korotkoff phase 5) was taken in triplicate in lying position using a mercury sphygmomanometer at 10-min intervals, taking special care to avoid any terminal digit preference. The average of the last two measurements was averaged to minimize white-coat effects. Heart rate was also taken at the same time. HT was defined as systolic blood pressure (SBP) ≥140 mmHg or diastolic blood pressure (DBP) ≥ 90 mmHg. Pulse pressure (PP) was the difference between SBP and DBP values.
Body mass index (BMI) was calculated as the ratio of weight (in kilograms) to squared height (in meters). Subjects were classified into non-smokers and current smokers (≥1 cigarette daily).
Fasting total cholesterol (TC), TG, HDLC, serum glycemia, serum creatinine (SCr), creatinkinase (CK) and transaminases levels were analysed by the enzymatic method. Low-density lipoproteins cholesterol (LDLC) was estimated by the Friedewald’s formula. As markers of biohumoral adverse effects, serum glycemia, SCr, CK and transaminases were measured. Albuminuria was also measured using the turbidimetric method (Cobas Mira Plus, Roche, Montclair, NJ) in a specimen of urine collected for 24 h. All subjects had normal SCr levels (< 1.2 mg/dl in men and < 0.9 mg/dl) in women and were normoalbuminuric (< 30 mg/24 h).
2.3. Study design
During the 2-week run-in period, all patients received a written prescription of a specific dietary regimen based on the standardized Mediterranean diet, including a high intake of fish, fruits, vegetables, legumes, olive oil, unrefined whole grains and a moderate intake of lean meats and alcohol. Subjects in the treatment group were supplemented with Liposcudil Plus (one tablet once-daily at evening before sleep for 2 months). Liposcudil Plus contains equivalent of 10 mg of Monacolin K and 30 mg of Coenzyme Q10. Clinic BP and lipid values were evaluated at baseline and at the end of the follow-up.
2.4. Statistical analysis
Continuous variables were averaged, expressed as mean and standard deviation, and compared with analysis of covariance (ANOVA) and the Bonferroni’s post hoc test. Comparison between categorical variables was performed using the χ2 test. Analysis of variance for repeated-measure was used to compare mean changes of BP components and serum lipids at baseline and at the follow-up visits. Delta-differences (in percentage) between continuous variables were evaluated in the two groups with the Tukey’s post hoc test after adjustment for age and difference of BMI, BP and serum lipids at baseline and at the follow-up. All statistical analyses were performed using the SPSS package version 17.0 for Windows (SPSS, Chicago, IL, USA). The null hypothesis was always rejected for values of p < 0.05.
3. Results
In the study group, the mean age of individuals enrolled in the control and treatment arm was 57.4 ± 8.6 years. Age, BP and serum lipids levels were not different between genders (data not shown). The general characteristics of the two groups at baseline are summarized in Table 1.
Table 1.
General characteristics of the patients enrolled. MK 10: Monacolin K 10 mg. SBP: systolic blood pressure; DBP: diastolic blood pressure; HR: heart rate; TC: total cholesterol; HDL-C and LDL-C: high-density-lipoprotein and low-density-lipoprotein cholesterol; AST: aspartate aminotransferase, ALT: alanine amino-transferase; CK: creatinkinase.
| All subjects (n = 104) | Diet (n = 52) | MK 10 + Diet (n = 52) | p-value | |
|---|---|---|---|---|
| Age (years) | 57.4 ± 8.6 | 56.7 ± 6.1 | 58.1 ± 10.6 | NS |
| Men (%) | 51.0 | 42.3 | 59.6 | NS |
| Body mass index (kg/m2) | 27.8 ± 3.0 | 27.7 ± 3.5 | 28.0 ± 2.5 | NS |
| Waist circumference (cm) | 102.8 ± 8.1 | 101.4 ± 7.1 | 104.2 ± 8.8 | NS |
| Clinic BP values | ||||
| SBP (mmHg) | 145.2 ± 7.0 | 145.7 ± 5.1 | 144.8 ± 8.4 | NS |
| DBP (mmHg) | 91.2 ± 4.5 | 91.7 ± 4.6 | 90.7 ± 4.4 | NS |
| Heart rate (bpm) | 71.8 ± 8.3 | 70.9 ± 7.8 | 72.1 ± 8.6 | NS |
| TC (mg/dl) | 244.3 ± 19.7 | 247.5 ± 16.0 | 241.0 ± 22.5 | NS |
| LDL-C (mg/dl) | 165.9 ± 17.4 | 166.2 ± 15.6 | 165.7 ± 19.2 | NS |
| HDL-C (mg/dl) | 50.1 ± 8.1 | 49.4 ± 7.4 | 50.8 ± 8.7 | NS |
| Triglycerides (mg/dl) | 150.8 ± 50.5 | 159.3 ± 45.5 | 146.4 ± 53.1 | NS |
| Glucose (mg/dl) | 96.6 ± 9.1 | 97.3 ± 10.3 | 95.9 ± 7.8 | NS |
| Creatinine (mg/dl) | 0.95 ± 0.16 | 0.96 ± 0.16 | 0.94 ± 0.15 | NS |
| AST (U/L | 20.4 ± 7.9 | 21.7 ± 10.0 | 19.0 ± 4.6 | NS |
| ALT (U/L) | 28.3 ± 7.2 | 27.1 ± 7.7 | 29.7 ± 6.6 | NS |
| CK (U/L) | 94.2 ± 14.4 | 91.5 ± 15.1 | 96.9 ± 14.0 | NS |
| Smokers (%) | 28.0 | 32.7 | 23.1 | NS |
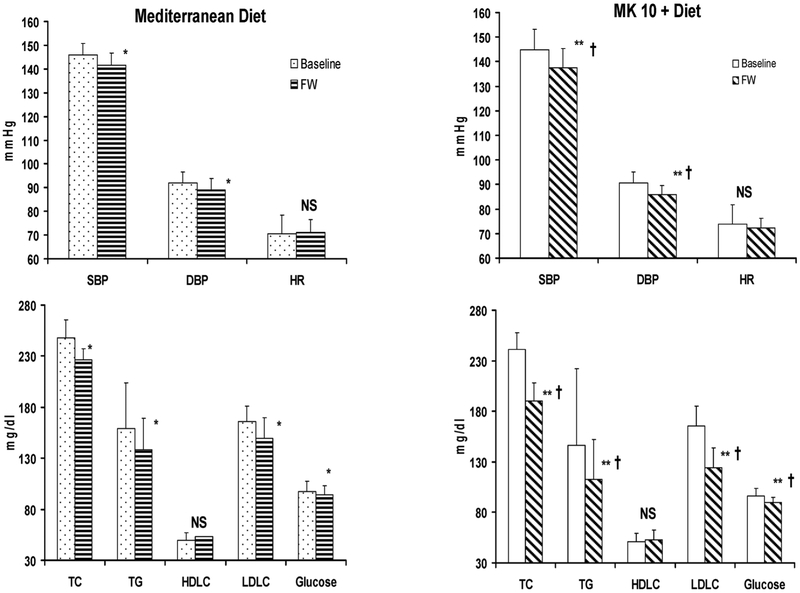
In the control group, significant changes were observed in serum TC, LDLC and for glucose and BP values from baseline compared to the follow-up (Fig. 1, Panel B), while HDLC remained unchanged. In the NC supplemented patients, clinic SBP and DBP values were significantly lower (p < 0.001) at the follow-up than baseline (Fig. 1, Panel B). A significant further reduction of SBP (−5.2 vs. −3.0 mmHg) and DBP (−4.9 vs. −2.9 mmHg), total cholesterol (−17.2%), LDLC (−21.8%), TG (−16.0%) and serum glucose (−3.4%) was observed in the treatment group compared to the control group (p < 0.001 for all); HDLC remained unchanged. No gender difference was observed.
Fig. 1.
Clinic systolic blood pressure (SBP) and diastolic blood pressure (DBP), serum total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDLC), low-density lipoproteins cholesterol (LDLC) and glucose changes from baseline to the follow-up in both groups. *p < 0.05 vs. baseline. ** < 0.001 vs. baseline;**T < 0.001 vs. Mediterranean Diet; MK 10: Monacolin K 10 mg; NS: non significant.
3.1. Safety parameters
No adverse event was observed during the study and no subject withdrew from the study. Safety parameters are shown in Table 2.
Table 2.
Safety parameters monitoring values. MK 10: Monacolin K 10 mg. AST: aspartate aminotransferase ALT: alanine aminotransferase; CK: creatinkinase.
| Mediterranean Diet | MK10 | |||||
|---|---|---|---|---|---|---|
| Basal (N = 52) | Follow-up (N = 52) | p-value | Basal (N = 52) | Follow-up (N = 52) | p-value | |
| Creatinine (mg/dl) | 0.96 ± 0.16 | 0.97 ± 0.18 | NS | 0.94 ± 0.15 | 0.95 ± 0.19 | NS |
| AST (U/L) | 21.7 ± 10.0 | 23.8 ± 6.5 | NS | 19.0 ± 4.6 | 22.3 ± 4.5 | NS |
| ALT (U/L) | 27.1 ± 7.7 | 30.7 ± 8.0 | NS | 29.7 ± 6.6 | 31.6 ± 8.2 | NS |
| CK (mU/mL) | 91.5 ± 15.1 | 112.4 ± 29.4 | NS | 96.9 ± 14.0 | 125.2 ± 27.7 | NS |
4. Discussion
In this study, patients with Mets supplemented with Liposcudil Plus, a NC containing monacolin and CoQ10 once daily for two months, had a significant improvement in lipid profile, BP and glucose levels. These changes were higher than the improvement obtained with diet alone. In agreement with Gonelli et al, no significant increase of HDL-C was found after treatment with Liposcudil Plus [15]. This finding may be related to higher levels of HDL-C at baseline of our subjects, intrinsic characteristics of the subjects enrolled and to the duration of treatment.
The improvement of each clinical feature of MetS is essential to reduce the global CV risk factor associated. In this respect, the first approach consists of reducing the caloric intake specifically through the adoption of Mediterranean diet and regular physical activities [16]. It is well known that moderate weight loss increases insulin sensitivity, lowers triglycerides and BP values [17]. Unfortunately, many patients do not reach the criteria necessary and treatment with lipid lowering, oral antidiabetic and antihypertensive drugs are needed [18]. Until now, there is no single drug therapy for MetS and currently available pharmacotherapy of this condition necessitate prolonged use of multiple medications, which in the long term reduce compliance to treatment. The novelty of our study was to investigate the dual effects of RYR and Coenzyme Q10 administered in a single formulation (Liposcudil Plus) for modulating BP, serum glucose, and cholesterol.
The role of nutraceuticals in the prevention of various diseases associated with MetS is not necessarily due to a single compound but to an additive and synergistic effect of several components present in the same product [6]. Thus, there is growing interest in the use of naturally occurring compounds in lowering the risk and progression of MetS that have proved to be effective and safe even in the long term [7]. As consequence, in the early stages of CV prevention, the use of NCs combined with dietary regimen, can be alternatives or added to pharmacological treatment of MetS.
The fermentation of red rice by the fungus Monascus purpureus, produces a number of by-products including Monacolin K. This compound is chemically related in structure to lovastatin, and competitively inhibits HMG-CoA reductase, a key enzyme within the mevalonate pathway. Monacolin K has an affinity of about 2000 times compared to the endogenous substrate and can modulate the lipid profile by reducing TC and LDLC [19]. A recent study carried out in patients intolerant to statins reported that RYR was as effective as pravastatin in inducing a dose-dependent decrease of LDLC levels, with a superior safety and tolerability profile [20]. In a large Chinese study of patients with a history of myocardial infarction, treatment with RYR significantly reduced not only LDLC, but also the risk of further major coronary events [21]. Impaired glucose metabolism and pre-diabetes status, conditions due to an excess body fat and insulin resistance, are considered an underlying aetiology of MetS [22]. Pre-diabetic patients compared with individuals with normal glucose tolerance, have been shown to have a deficiency in CoQ10 and a reduced total CoQ10/total cholesterol ratio [23]. The CoQ10 deficiency was attributed to increased oxidative stress in pre-diabetic patients, however supplementation of CoQ10 resulted in a significant improvement in long-term glycemic control [24].
Like hyperglycemia, high BP levels increase the global CV risk of subjects with Mets [25]. The antihypertensive effect of NCs has long been recognized and previous studies have evaluated its clinical value [26,27]. The HMG-CoA reductase inhibition by Monacolin K, like statins lower LDL cholesterol but may also impact the structural and functional components of the arteriosclerotic process [28]. Structural and functional effects of statins include reductions in vascular smooth muscle hypertrophy and proliferation, fibrin and collagen deposition [29], improving endothelial function, reducing inflammatory cytokines and reactive oxygen species, and the down-regulation of the angiotensin II and endothelin receptors [30]. We speculate a possible vasoactive action of Monacolin K leading to a mild BP lowering effect as well as observed with low doses of statins [31]. In addition, Monacolin K seems works better when paired with Mediterranean diet, thus opening the question if it shows the same benefit when added with other diets. Nutrition is a very complex research topic and it is not clear whether an individual component of the diet or a combination of nutrients and dietary habits may be responsible for any cardioprotective effects. Although appropriate diets, regular exercise, and weight control have potential for the prevention and treatment of MS, a high proportion of subjects with this condition continue to disattend these recommendations. This is true also for the Dietary Approaches to Stop Hypertension (DASH), a diet plan recommended by the US Department of Health and Human Service [32], due to its beneficial effects on some components of MS like hypertension [33]. The combined effects of DASH diet – a plan encouraging a high intake of whole grain, fruits, vegetables, and low-fat dairy products combined with a limited consumption of saturated fat, red meats, sweets and sugar-containing beverages and nutraceutical compounds were tested in randomized controlled trial [34], demonstrating a synergistic effect in some components of MS. However, several factors make it difficult in translating the findings of the original DASH trials to a broader population, firstly as the DASH trials was an efficacy study of short duration. In fact, the compliance to this dietary approach tends to be discontinued in the short term [35].
In addition, some randomized placebo-controlled clinical trials support the antihypertensive effect of the coenzyme Q10. In the meta-analysis by Ho et al., oral treatment with 100 mg/day of coenzyme Q10 decreased SBP by 11 mmHg and DBP by 7 mmHg in subjects with HT [36]. However, the dose of coenzyme Q10 tested is 30 mg and higher than other NCs [37], and in part this explains its possible BP-lowering effect. However, CoQ10 has shown to significantly lower both SBP and DBP also in patients receiving conventional antihypertensive drugs [13] and in patients with isolated systolic hypertension [38]. Finally, it is well known that participation in a clinical study enhances adherence, persistence and effectiveness of antihypertensive agents, particularly in patients with HT taking active treatment rather than those in the control group. This phenomenon is a possible explanation of the higher BP-lowering effect observed in the group treated with NCs compared to the control group.
The main findings drawn from our study suggest that dietary supplementation of CoQ10 and monakolin K are promising strategies for the treatment of MetS with minimal side effects. Liposcudil Plus is a novel formulation that combines these two important NCs in a single formulation for enhanced efficacy by targeting two important pathways impacted in MetS.
4.1. Study limitations
These studies provide the platform and rationale to expand to larger randomized controlled trials with longer duration and extended follow-up. Specifically, while clinical benefit was demonstrated the sample size was relatively small, although adequate to reach the primary endpoint with statistical relevance. The follow-up was short, and it does not provide any information to the long-term impact or to a possible rebound action at the treatment discontinuation.
In conclusion, it well known that pharmacological therapies are effective in the treatment of MetS, but often their side effects are dose limiting. Furthermore, 10%–15% of patients are intolerant to statins, and after 1 year almost one-third of patients discontinued the treatment. NCs, on the contrary, are well tolerated and can be combined with a dietetic regimen to yield positive clinical results. In our study, the safety of the NC containing CoQ10 and Monakolin K favourably demonstrated with no side effects or alterations in laboratory values. Liposcudil Plus in combination with diet and exercise was more effective than diet alone to improve lipid profile, glycemic control and BP values in patients with MetS.
Acknowledgments
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Footnotes
Conflicts of interest
None.
References
- [1].Kaur J, A comprehensive review on metabolic syndrome, Cardiol. Res. Pract (2014) 21 943162. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [2].El-Atat FA, Stas SN, McFarlane SI, Sowers J, Obesity and hypertension, Endocrinol. Metab. Clin. N. Am 32 (2003) 823–854. [DOI] [PubMed] [Google Scholar]
- [3].European Food Safety Authority (EFSA), Scientific opinion on the substantiation of health claims related to monacolin K from red yeast rice and maintenance of normal blood LDL-cholesterol concentrations (ID 1648, 1700) pursuant to article 13(1) of regulation (EC) no 1924/2006. EFSA panel on dietetic products, nutrition and allergies (NDA), EFSA J. 9 (7) (2011) 2304. [Google Scholar]
- [4].Izzo R, de Simone G, Giudice R, Chinali M, Trimarco V, De Luca N, et al. , Effects of nutraceuticals on prevalence of metabolic syndrome and on calculated Framingham Risk Score in individuals with dyslipidemia, J. Hypertens 28 (2010) 1482–1487. [DOI] [PubMed] [Google Scholar]
- [5].Trimarco B, Benvenuti C, Rozza F, Cimmino CS, Giudice R, Crispo S, Clinical evidence of efficacy of red yeast rice and berberine in a large controlled study versus diet, Med. J. Nutr. Metab 4 (2011) 133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Cicero AFG, Derosa G, Bove M, Imola F, Borghi C, Gaddi AV, Long-term effectiveness and safety of a nutraceutical based approach to reduce cholesterolemia in statin intolerant subjects with and without metabolic syndrome, Curr. Top. Nutraceutical Res 7 (2009) 121–126. [Google Scholar]
- [7].Cicero AFG, De Sando V, Benedetto D, Cevenini M, Grandi E, Borghi C, Long-term efficacy and tolerability of a multicomponent lipid-lowering nutraceutical in overweight and normo- weight patients, Nutrafood 11 (2012) 55–61. [Google Scholar]
- [8].Marazzi G, Cacciotti L, Pelliccia F, Iaia L, Volterrani M, Caminiti G, et al. , Long-term effects of nutraceuticals (berberine, red yeast rice, policosanol) in elderly hypercholesterolemic patients, Adv. Ther 28 (2011) 1105–1113. [DOI] [PubMed] [Google Scholar]
- [9].Kumar A, Kaur H, Devi P, Mohan V, Role of coenzyme Q10 (CoQ10) in cardiac disease, hypertension and Meniere-like syndrome, Pharmacol. Ther 124 (2009) 259–268. [DOI] [PubMed] [Google Scholar]
- [10].Young JM, Molyneux SL, Reinheimer AM, Florkowski CM, Frampton CM, Scott RS, George PM, Relationship between plasma coenzyme Q10, asymmetric dimethylarginine and arterial stiffness in patients with phenotypic or genotypic familial hypercholesterolemia on long term statin therapy, Atherosclerosis 218 (2011) 188–193. [DOI] [PubMed] [Google Scholar]
- [11].Larijani VN, Ahmadi N, Zeb I, Khan F, Flores F, Budoff M, Beneficial effects of aged garlic extract and coenzyme Q10 on vascular elasticity and endothelial function: the FAITH randomized clinical trial, Nutrition 29 (1) (2013) 71–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hodgson JM, Watts GF, Playford DA, Burke V, Croft KD, Coenzyme Q10 improves blood pressure and glycaemic control: a controlled trial in subjects with type 2 diabetes, Eur. J. Clin. Nutr 56 (2002) 1137–1142. [DOI] [PubMed] [Google Scholar]
- [13].Singh RB, Niaz MA, Rastogi SS, Shukla PK, Thakur AS, Effect of hydrosoluble coenzyme Q10 on blood pressures and insulin resistance in hypertensive patients with coronary artery disease, J. Hum. Hypertens 13 (1999) 203–208. [DOI] [PubMed] [Google Scholar]
- [14].National Institutes of Health: Third Report of the National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Executive Summary, National Institutes of Health, National Heart, Lung and Blood Institute, Bethesda, MD, 2001. (NIH publ. no. 01–3670). [Google Scholar]
- [15].Gonnelli S, Caffarelli C, Stolakis K, Cuda C, Giordano N, Nuti R, Efficacy and tolerability of a nutraceutical combination (red yeast rice, policosanols and berberine) in low-moderate risk hypercholesterolemic patients: a double-blind, placebo controlled study, Curr. Ther. Res 77 (2015) 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Velázquez-López L, Santiago-Díaz G, Nava-Hernández J, Muñoz-Torres AV, Medina-Bravo P, Torres-Tamayo M, Mediterranean-style diet reduces metabolic syndrome components in obese children and adolescents with obesity, BMC Pediatr. 14 (2014) 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Van Gaal LF, Wauters MA, De Leeuw IH, The beneficial effect of modest weight loss on cardiovascular risk factors, Int. J. Obes 21 (S1) (1997) S5–S9. [PubMed] [Google Scholar]
- [18].Mark AL, Weight reduction for treatment of obesity-associated hypertension: nuances and challenges, Curr. Hypertens. Rep 9 (2007) 368–372. [DOI] [PubMed] [Google Scholar]
- [19].Barrios V, Escobar C, Cicero AFG, Burke D, Fasching P, Banach M, et al. , A nutraceutical approach (Armolipid Plus) to reduce total and LDL cholesterol in individuals with mild to moderate dyslipidemia: review of the clinical evidence, Atheroscler. Suppl 24 (2017) 1–15. [DOI] [PubMed] [Google Scholar]
- [20].Halbert SC, French B, Gordon RY, Farrar JT, Schmitz K, Morris PB, et al. , Tolerability of red yeast rice (2,400 mg twice daily) versus pravastatin (20 mg twice daily) in patients with previous statin intolerance, Am J. Cardiol 105 (2010) 198–204. [DOI] [PubMed] [Google Scholar]
- [21].Lu Z, Kou W, Du B, Wu Y, Zhao S, Brusco OA, et al. , Effect of Xuezhikang, an extract from red yeast Chinese rice, on coronary events in a Chinese population with previous myocardial infarction, Am J. Cardiol 101 (2008) 1689–1693. [DOI] [PubMed] [Google Scholar]
- [22].Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes, Diabetes Care 32 (1) (2009) 193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lim SC, Tan HH, Goh SK, Subramaniam T, Sum CF, Tan IK, et al. , Oxidative burden in prediabetic and diabetic individuals: evidence from plasma coenzyme Q (10), Diabet. Med 23 (12) (2006) 1344–1349. [DOI] [PubMed] [Google Scholar]
- [24].Shen Q, Pierce JD, Supplementation of coenzyme Q10 among patients with type 2 diabetes mellitus, Healthcare (Basel) 3 (2) (2015) 296–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Staessen JA, Li Y, Thijs L, Wang JG, Blood pressure reduction and cardiovascular prevention; an update including the 2003–2004 secondary prevention trials, Hypertens. Res 28 (2005) 385–407. [DOI] [PubMed] [Google Scholar]
- [26].Trimarco V, Cimmino CS, Santoro M, Pagnano G, Manzi MV, Piglia A, et al. , Nutraceuticals for blood pressure control in patients with high-normal or grade 1 hypertension, High Blood Press. Cardiovasc. Prev 19 (2012) 117–122. [DOI] [PubMed] [Google Scholar]
- [27].Mazza A, Lenti S, Schiavon L, Zuin M, D’Avino M, Ramazzina E, et al. , Nutraceuticals for serum lipid and blood pressure control in hypertensive and hypercholesterolemic subjects at low cardiovascular risk, Adv. Ther 32 (7) (2015) 680–690. [DOI] [PubMed] [Google Scholar]
- [28].Mangat S, Agarwal S, Rosendorff C, Do statins lower blood pressure? J. Cardiovasc. Pharmacol. Ther 12 (2) (2007) 112–123. [DOI] [PubMed] [Google Scholar]
- [29].Strazzullo P, Kerry SM, Barbato A, Versiero M, D’Elia L, Cappuccio FP, Do statins reduce blood pressure?: A meta-analysis of randomized, controlled trials, Hypertension 49 (4) (2007) 792–798. [DOI] [PubMed] [Google Scholar]
- [30].Drapala A, Sikora M, Ufnal M, Statins, the renin-angiotensin-aldosterone system and hypertension–a tale of another beneficial effect of statins, J. Renin Angiotensin Aldosterone Syst 15 (3) (2014) 250–258. [DOI] [PubMed] [Google Scholar]
- [31].Kanaki AI, Sarafidis PA, Georgianos PI, Stafylas PC, Kanavos K, Tziolas IM, et al. , Low-dose atorvastatin reduces ambulatory blood pressure in patients with mild hypertension and hypercholesterolaemia: a double-blind, randomized, placebo-controlled study, J. Hum. Hypertens 26 (10) (2012) 577–584. [DOI] [PubMed] [Google Scholar]
- [32].US Department of Health and Human Services, Your Guide to Lowering Your Blood Pressure With DASH, National Heart, Lung, and Blood Institute, Bethesda, MD, 2006. (NIH Publication No. 06–4082). [Google Scholar]
- [33].Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MM, et al. , A clinical trial of the effects of dietary patterns on blood pressure, DASH Collaborative Research Group, N. Engl. J. Med 336 (1997) 1117–1124. [DOI] [PubMed] [Google Scholar]
- [34].Al-Solaiman Y, Jesri A, Mountford WK, Lackland DT, Zhao Y, Egan BM, DASH lowers blood pressure in obese hypertensives beyond potassium, magnesium and fibre. Randomized controlled trial, J. Hum. Hypertens 24 (2010) 237–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kwan MW, Wong MC, Wang HH, Liu KQ, Lee CL, Yan BP, Yu CM, Griffiths SM, Compliance with the Aietary Approaches to Stop Hypertension (DASH) diet: a systematic review, PLoS One 8 (2013) e78412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ho MJ, Bellusci A, Wright JM, Blood pressure lowering efficacy of coenzyme Q10 for primary hypertension, Cochrane Database Syst. Rev 4 (2009) CD007435. [DOI] [PubMed] [Google Scholar]
- [37].Gentile M, Calcaterra I, Strazzullo A, Pagano C, Pacioni D, Speranza E, et al. , Effects of Armolipid Plus on small dense LDL particles in a sample of patients affected by familial combined hyperlipidemia, Clin. Lipidol 10 (6) (2015) 475–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Burke BE, Neuenschwander R, Olson RD, Randomized, double-blind, placebo-controlled trial of coenzyme Q10 in isolated systolic hypertension, South. Med. J 94 (11) (2001) 1112–1117. [DOI] [PubMed] [Google Scholar]