Abstract
ATP-binding cassette (ABC) transporters are a family of proteins that translocate molecules across cellular membranes. Substrates can include lipids, cholesterol and drugs. Mutations in ABC transporter genes can cause human pathologies and drug resistance phenotypes in cancer cells. ABCA2, the second member the A sub-family to be identified, was found at high levels in ovarian carcinoma cells resistant to the anti-cancer agent, estramustine (EM). In vitro models with elevated levels of ABCA2 are resistant to a variety of compounds, including estradiol, mitoxantrone and a free radical initiator, 2,2′-azobis-(2-amidinopropane) (AAPH). ABCA2 is most abundant in the central nervous system (CNS), ovary and macrophages. Enhanced expression of ABCA2 and related proteins, including ABCA1, ABCA4 and ABCA7, is found in human macrophages upon bolus cholesterol treatment. ABCA2 also plays a role in the trafficking of low-density lipoprotein (LDL)-derived free cholesterol and is coordinately expressed with genes involved in cholesterol homeostasis. Additionally, ABCA2 expression has been linked with gene cluster patterns consistent with pathologies including Alzheimer’s disease (AD). A single-nucleotide polymorphism (SNP) in exon 14 of the ABCA2 gene was shown to be linked to early onset AD in humans, supporting the observation that ABCA2 expression influences levels of β-amyloid peptide (Aβ), the primary component of senile plaques. ABCA2 may play a role in cholesterol transport and affect a cellular phenotype conducive to the pathogenesis of a variety of human diseases including AD, atherosclerosis and cancer.
Keywords: ABC transporters, Drug resistance, Estramustine, Cholesterol, Alzheimer’s disease
1. Introduction
ATP-binding cassette (ABC) transporters are multi-domain membrane proteins that use energy from ATP hydrolysis to pump substrates directionally across cellular membranes [1]. The human ABC superfamily of proteins consists of at least seven sub-families: A (ABC1); B (MDR/TAP); C (CFTR/MRP); D (X-linked adrenoleukodystrophy, ALD); E (OABP); F (GCN20); G (WHITE). A wide diversity of tissue expression has been reported. Expression patterns are also influenced by genetic polymorphisms, some of which have been associated with human disease pathologies. Reports have shown at least 605 single-nucleotide polymorphisms (SNPs) among 13 ABC transporter genes [2]. ABC transporters have a generic structure composed of two transmembrane domains and two ABCs [3]. The ABC consensus sequence is the hallmark of the ABC protein superfamily and is comprised of highly conserved Walker A and B motifs separated by 90–120 amino acids, within which there is a characteristic “signature” motif [4]. Inhibition of hydrolysis of one ABC domain can abrogate the activity of the other and thereby function in an alternating fashion while recognizing diverse nucleotides as hydrolytic substrates. Topology analysis has shown that the number of trans-membrane helices can range from 6 to 11 [5], and these function in substrate recognition, binding, and channeling. ATP hydrolysis in the ABC domain provides the necessary energy for substrate transport. Interactions between the ABC and membrane domains are integral in executing energy dependent transport. Within the A sub-family (ABCA) there are seven members that function in tissue-specific fashions. Table 1 provides a summary of ABCA sub-family function and association with disease; however broader reviews of transporter structure/function are available in the literature [6–10].
Table 1.
Members of sub-family A of ABC transporter proteins
| Member | Chromosome | Expression | Function | Pathology |
|---|---|---|---|---|
| A1 | 9q22-q31 | Ubiquitous | Cellular lipid efflux | Tangier disease |
| A2 | 9q34 | Brain | Cholesterol homeostasis | Alzheimer’s disease |
| A3 | 16p13.3 | Lung alveolar type II cells | Lipid transport | Newborn surfactant deficiency |
| A4 | 1p21 | Retina | Flippase | Stargardt disease |
| A7 | 19 | Myelolymphatic tissue, spleen | Cellular lipid efflux | Sjorgren syndrome |
| A12 | 2q34 | Keratinocytes | Lipid traffic | Laellar ichthyosis type II |
| A13 | 7q12.3 | Kidney, skeletal muscle | Unknown | Unknown |
2. ABCA2
ABCA2 (270 kDa) is the largest of 49 identified members of the ABC transporter gene family (Fig. 1) [11]. This transporter was originally described along with ABCA1 in embryonic mouse brain [12,13]. Subsequent characterization of the full-length human ABCA2 cDNA and its detailed expression pattern showed that ABCA2 is most highly expressed in both fetal and adult brain, spinal cord, ovary and leukocytes [14]. Recently, ABCA2 protein was shown to localize in areas of the brain associated with adult neurogenesis and Alzheimer’s disease (AD) pathology (subventricular zone lining of the lateral ventricles and the dentate gyrus of the hippocampus), as well as in GABAergic and glutamatergic neurons [15]. Expression of ABCA2 has been detected in other tissues, including in descending order: lung, kidney, heart, liver, skeletal muscle, pancreas, testis, spleen and fetal liver. Cellular immunolocalization of ABCA2 revealed a distinct, punctate staining that vesicle-specific antibodies revealed to be a co-localization of ABCA2 with late endolysomes and trans-golgi organelles.
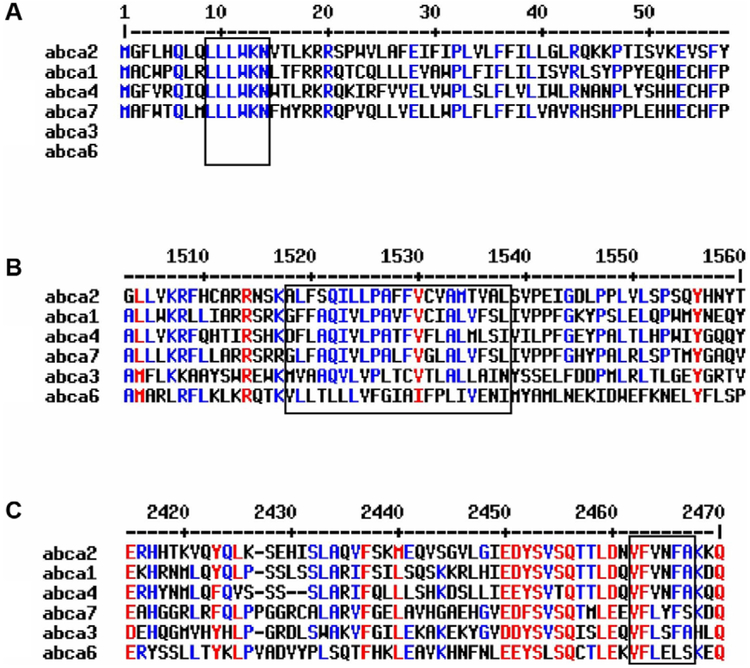
Fig. 1.
Alignment of ABCA transporters with homology to ABCA2; A: conserved motif at the beginning of the N-terminus with unknown function, B: high hydrophobic domain, C: highly conserved domain at the end of the C-terminus.
The ABCA2 gene is located at chromosome 9q34 within a genomic region of 21 kb [16]. The gene contains 48 exons with an open reading frame of 2436 amino acids [14,17]. The minimal promoter region has been mapped to 321 bp upstream of the translation start site [18]. Alternative splicing of the first exon to the second in ABCA2 results in two variants, 1A and 1B [19]. The first exon of 1B contains the coding sequence for 52 amino acids and is located 699 bp upstream of 1A, which contains coding sequence for 22 amino acids. Both splice variants co-localize with lysosome-associated membrane proteins-1 and −2 (LAMP-1 and −2) and share similar expression profiles [19]. The novel N-terminus of ABCA2 splice variants, while functionally redundant, may provide subtle gene regulatory differences to allow tissue-specific or temporal-specific protein expression during differentiation and/or development.
ABCA2 shares homology with other A sub-family proteins, including ABCA1 (50%) ABCA7 (44%), ABCA3 (43%), ABCA4 (40%) and ABCA6 (32%) [20]. Promoter elements identified in ABCA1 include an E box, AP-1, liver X receptor (LXR) element and SP1 motifs [21]. The proximal promoter of ABCA2 contains two GC-boxes and overlapping sites for the early growth response-1 (EGR-1) and Sp1 transcription factors [18]. While there are identical regions in ABCA1 and ABCA2 (e.g. two cytoplasmic ABCs, Walker domains, a conserved N-terminal sequence (LLLWKN) and a VFVNFA motif within the C-terminal domain [22]), their functional overlap may not be significant. The VFVNFA motif in ABCA1 is critical for apolipoprotein A-I binding and high density lipoproteins (HDL) cholesterol efflux at the plasma membrane [22]. Nevertheless, the ABCA2 sequence contains a lipocalin signature motif, implying a function in the transport of lipids, steroids and structurally similar molecules.
3. Cholesterol homeostasis and metabolism
Several of the ABC sub-family A members play a role in cholesterol homeostasis (Table 1). Specifically, ABCA1 and ABCA7, transporters that not only share the greatest homology with ABCA2, but are also sterol responsive genes functioning in cholesterol metabolism [20]. Cholesterol homeostasis is maintained by a feedback mechanism of de novo synthesis using acetyl CoA and by uptake of low-density lipoprotein (LDL)-cholesterol by receptor-mediated endocytosis through the LDL receptor (LDLR) [23]. Functional studies using exogenous, labeled LDL cholesterol provided evidence for ABCA2 function in sterol trafficking [24]. Forced ABCA2 overexpression was used as a model system in Chinese hamster ovary cells (CHOA2) to test the effect on transport of LDL-cholesterol. CHOA2 cells displayed a more intense staining of unesterified cholesterol using the fluorescent marker, filipin, in cytoplasmic and perinuclear vesicles, compared to the parental cell line (CHO). These vesicles were also positive for an endosome/lysosome acidic vesicle marker (Lysosensor green 189), with less intense filipin staining at the plasma membrane [24]. In addition to the sequestration of LDL-derived cholesterol into these vesicles, CHOA2 cells have also been shown to have elevated expression of the LDLR, sterol-response element binding protein-2 (SREBP2) and 3-hydroxy-3-methylglutaryl CoA synthase (HMGCoA S) and demonstrate reduced trafficking of LDL-derived cholesterol to the endoplasmic reticulum (ER) for esterification [24]. Since these cellular responses also occur when cells are grown under sterol-depleted conditions, these data suggest that ABCA2 up-regulation mimics sterol deprivation. LDLR (along with HMGCoA S and SREBP2) is regulated by the SREBP2 transcription factor [25]. The transcription of LDLR is reduced in CHOA2 cells with a mutant SREBP2 binding site within the LDLR promoter [18]. Taken together, these results show that ABCA2 over-expression causes a phenotype similar to cholesterol-depleted cells that sequester unesterified cholesterol into endolysosomal compartments.
Significant insight into the importance of cholesterol homeostasis has been gleaned from pre-clinical studies of ABCA1. For example, ABCA1 deficient mice have a ~70% decrease in serum cholesterol, phospholipids and lack HDL [26], while transgenic overexpressing mice show an increase in cholesterol efflux [27]. Cholesterol lowering agents, such as statins, impact ABCA1 expression, resulting in a reduction in intracellular cholesterol [28]. The significance of ABCA1 and dysregulation of cholesterol metabolism is noted in the mouse model for diabetes with associating cardiovascular disease. ABCA1 expression is decreased in diabetic mice with cardiovascular disease [29]. Although the generation of mouse models for other ABC A sub-family transporters began only recently [30–32], there will undoubtedly be increasing numbers of genetic models that should help to elucidate in vivo functions and genetic links with human disease.
3.1. ABCA2 and disease
ABCA2 expression is elevated in Niemann-Pick type C1 (NPC1) fibroblasts and in Familial Hypercholesterolemia (FHC) fibroblasts [24], as well as several cancer cell lines including an estramustine (EM)-resistant prostate cancer cell line, EM15. Although ABCA2 is expressed predominantly in normal and malignant central nervous system (CNS) tissues and cell lines, it is also abundant in several other cancer cell lines derived from non-CNS tissues. The expression of ABCA2 in oligodendrocytes is purported to be required for the generation of copious layers of phospholipid- and cholesterol-rich myelin sheath of white matter in the brain. The cell lines that express the highest levels are not derived from the CNS, suggesting a putative role for the deregulation of ABCA2 in tumorigenesis and/or cancer progression. However, the mounting evidence for a putative role of ABCA2 in the development of AD may lead to its use as a target for future therapeutic strategies.
AD is the most common neurological pathology associated with dementia and manifests itself by the accumulation of extracellular senile plaques in neurofibrillary tangles of the brain and cerebral blood vessels. These plaques are composed of both fibrillar and non-fibrillar forms of β-amyloid peptide (Aβ) and result in the loss of neuronal function in limbic and association cortices of the brain [33]. The Aβ peptide is derived from sequential cleavage reactions of amyloid precursor protein (APP) by a β-secretase, followed by cleavage by a γ-secretase within the transmembrane domain of APP. Approximately 40% of early-onset AD cases (in individuals younger than 60 years) are linked to mutation in the β-amyloid precursor protein (APP), presenilin1 (PSEN1), or presenilin2 (PSEN2) [34]. Late-onset AD occurs in patients older than 60 and accounts for nearly 95% of all AD cases. Several genes have shown a slight, but inconsistent association with this disease [35], but the most common of these is the ε4 isoform of APOE [33,36]. There is mounting epidemiological and genetic research that has drawn a close link between AD pathology and cholesterol [37–40]. Mid-life individuals with elevated serum cholesterol have an increased risk of developing AD [41] and treatment with statins, cholesterol lowering agents, is associated with a decreased risk of AD development [42] and a decrease in Aβ levels [43].
As a cholesterol-responsive gene expressed predominantly in the brain, ABCA2 became an excellent candidate for an association with AD when it was recently shown to impact the production of Aβ. Not only did ABCA2 co-localize with Aβ and APP [44], its overexpression also caused an up-regulation of a number of genes associated with resistance to oxidative stress. Using amplified differential gene expression (ADGE) microarray, several clusters of genes were shown to be differentially regulated upon ABCA2 over-expression in HEK293 cells, including 22 genes related to transport, membrane homeostasis, cell metabolism and substrate binding. Six of the genes from this study, APP, LDLR-related protein, calcineurin, seladin-1, vimentin and Slc23a1, are commonly associated with AD and response to oxidative stress. Overexpression was also shown to confer a slight resistance to oxidative stress rendered by the free radical initiator, 2,2′-azobis-(2-amidinopropane) (AAPH). ABCA2 levels were shown to be elevated in the temporal and frontal regions of the brain, areas frequently associated with AD pathology. Recently, a synonymous SNP in exon 14 of ABCA2 (rs908832) was determined to have a significant linkage with early-onset AD [45]. Taken together, these studies reflect the importance of determining a mechanistic role for ABCA2 in AD pathogenesis and/or progression. However, because cholesterol transport is critical to a number of important phenotypes, the ABCA2 protein may impact other diseases such as cancer.
Hormone therapy of cancer patients has been shown to influence cholesterol metabolism [46,47]. Cholesterol also has critical functions in cell signaling, survival and differentiation [48] and may provide a novel target for cancer therapeutic agents [49] since cholesterol levels may be a limiting factor in membrane maintenance in rapidly dividing cancer cells. Although many cellular factors influence tumor cell proliferation, the role of the availability of growth-promoting metabolites is an important area of investigation. The potential role of ABCA2 in regulating availability of cholesterol as a critical metabolite for tumor cell proliferation may validate it as a target for pharmacological intervention. Overall, accumulating evidence has indicated that ABCA2 expression and subsequent endolysosomal compartmentalization of sterols is directly linked to cancer drug resistance [14,19,50] and may be an important regulator to maintain homeostatic levels of cholesterol for cellular function, growth and membrane integrity [14,51]. Recently, the specific properties of lysosomes within cancer cells were considered to provide a novel strategy for targeting of cancer chemotherapeutic agents [52]. Perhaps a plausible role for ABC transporters as functional members of dynamic protein complexes (rather than simple substrate transporters) in the processes of cell growth, differentiation and death has, thus far, been overlooked.
3.2. Cancer drug resistance
Adaptation of cancer cells to a single drug can render pleiotropic cellular effects and result in specific or general acquired resistance. The latter is appropriately termed multidrug resistance (MDR). The current strategy for cancer chemotherapy involves cocktails of pharmacological agents and MDR may be a cause of treatment failure. A number of ABC transporters have been implicated in cellular drug resistance [53]. In particular, members of the MDR and MRP transporter sub-families have been linked to simultaneous resistance to multiple cytotoxic drugs in cancer cells. MDR1 confers resistance to a variety of hydrophobic, amphipathic natural product drugs [11] whereas members of the MRP-sub-family are associated with resistance to anionic and neutral drugs frequently conjugated to acidic ligands [54]. Although several mechanisms can contribute to MDR, alterations in drug accumulation appears to be common both in cell culture and in model organisms [55]. The initial discovery linking membrane transporters with drug resistance came from an observation that MDR1 decreased the accumulation and toxic effects of various and structurally unrelated anti-neoplastic drugs in CHO cells [56,57]. Although the crystal structure of a human ABC transporter has yet to be resolved, the insights into putative structure and function of ABC transporters has been deduced from similarity with E. coli homologs, MsbA and BtuCD [58,59]. The function of MDR1 in drug efflux is generally ATP-dependent, similar to homologous bacterial ABC transporters. However, the mechanism(s) of ATP hydrolysis-coupled substrate transport remains theoretical [60].
Extending the drug resistance paradigm to the A sub-family, an ovarian carcinoma cell line (SKEM) with acquired resistance to the estradiol-based agent, EM, was found to have a gene amplification at 9q34 and contain an ABCA2 amplicon [51]. EM is a synthetic conjugate of nitrogen mustard and estradiol with an antimitotic activity [61]. A homogenously staining region, typical for gene amplification, was found in chromosome 9q34, and in situ hybridization with a specific probe indicated a approximately sixfold amplification of the ABCA2 gene. The approximately fivefold increase in gene expression in SKEM cells was accompanied by an increased rate of dansylated EM efflux and, hence, drug resistance. Cells were sensitized to EM upon treatment with antisense ABCA2 RNA [51]. Similarly, ABCA2 overexpressing HEK293 cells (HEK293-ABCA2) were also shown to be EM-resistant [14]. Somewhat lower levels of resistance (approximately threefold) were observed for the same cells upon exposure to a structurally similar compound, estradiol. Supporting the observation that ABCA2 contributes to EM and estradiol resistance was that cells transfected with a putative dominant-negative mutant of ABCA2 have virtually no differences in toxicity compared to mock-transfected cells [19]. This study also showed that HEK293-ABCA2 cells were not resistant to agents that are structurally dissimilar to EM (melphalan, mitoxantrone, cisplatinum, taxol, and vinblastine) with the exception of a slight doxorubicin resistance. Further studies showed that HEK293-ABCA2 cells are also resistant to a free radical initiator, AAPH [44]. Although AAPH is unrelated to other sterol-related substrates, free radical or reactive oxygen species (ROS) induced damage may damage sterols, lipids and lipoproteins where cell survival may be facilitated by sequesteration of damaged molecules into the endolysosomal compartment. Even though ABCA2 overexpression did not confer mitoxantrone resistance in HEK293 cells, this transporter was shown to be up-regulated in a mitoxantrone-resistant small cell lung cancer cell line, GLC4-MITO [50]. The same cell line was approximately twofold more resistant to EM compared to parental GLC4 cells. Exposure of GLC4-MITO cells to both EM and mitoxantrone increased cellular accumulation of the latter, indicating an ability of EM to block efflux of mitoxantrone. Taken together, these results indicate that molecules with steroid-like structures are putative substrates for transport by ABCA2 and may cause induced expression. It is not known, however, whether such compounds interact directly with ABCA2 for transport across intracellular membranes, or if they serve a signaling molecule to initiate some form of transport cascade.
4. Conclusions
Several factors serve to limit progress in elucidating protein function for membrane-bound ABC transporters. These include structural biology studies that use traditional crystallization methods. Determination of protein–protein interactions of full-length, native proteins is hampered by their multiple domain structures and high molecular weight. Also, because of possible functional redundancy the high degree of homology among ABC transporters makes loss-of function studies difficult to interpret.
Although the structural and functional characterization of the ABCA2 transporter is in its infancy, there are several promising indications for future studies. The consequence of deregulated trafficking of cholesterol and sterol-related compounds at the cell and organism level, has effects on formation of arterial plaques and increases the risk for heart disease, AD and cancer. Links with the cellular response to ROS and elevated LDL cholesterol [62–65] make the fact that ABCA2 is abundantly expressed in macrophages [17], more pertinent to these pathologies. These connections and the identification of other cholesterol-responsive transporters from the same family (ABCA1 and ABCA7) may provide the platform for small molecule screening strategies for these novel targets. Impending studies could provide a wealth of information for the possibility of intercession in transport function as a conduit to pharmacological intervention in these diseases.
Abbreviations:
- AAPH
2,2′-azobis-(2-amidinopropane)
- Aβ
β-amyloid peptide
- ABC
ATP-binding cassette
- AD
Alzheimer’s disease
- ADGE
amplified differential gene expression
- ALD
X-linked adrenoleukodystrophy
- APP
β-amyloid precursor protein
- CF
cystic fibrosis
- CNS
central nervous system
- EGR-1
early growth response-1
- EM
estramustine
- ER
endoplasmic reticulum
- FHC
Familial Hypercholesterolemia
- HDL
high density lipoproteins
- HMGCoA S
3-hydroxy-3-methylglutaryl CoA synthase
- LA
linoleic acid
- LAMP
lysosome-associated membrane proteins
- LDL
low-density lipoprotein
- LDLR
LDL receptor
- LXR
liver X receptor
- MDR1/Pgp1
multidrug transporter/P-glycoprotein
- MRP1
multidrug resistance associated protein
- NPC1
Niemann-Pick type C1
- ROS
reactive oxygen species
- SNP
single-nucleotide polymorphisms
- SREBP2
sterol-response element binding protein-2
References
- [1].Higgins CF. ABC transporters: from microorganisms to man. Annu Rev Cell Biol 1992;8:67–113. [DOI] [PubMed] [Google Scholar]
- [2].Iida A, Saito S, Sekine A, Mishima C, Kitamura Y, Kondo K, et al. Catalog of 605 single-nucleotide polymorphisms (SNPs) among 13 genes encoding human ATP-binding cassette transporters: ABCA4, ABCA7, ABCA8, ABCD1, ABCD3, ABCD4, ABCE1, ABCF1, ABCG1, ABCG2, ABCG4, ABCG5, and ABCG8. J Hum Genet 2002;47(6): 285–310. [DOI] [PubMed] [Google Scholar]
- [3].Holland IB, Blight MA. ABC-ATPases, adaptable energy generators fuelling transmembrane movement of a variety of molecules in organisms from bacteria to humans. J Mol Biol 1999;293(2):381–99. [DOI] [PubMed] [Google Scholar]
- [4].Croop JM. Evolutionary relationships among ABC transporters. Methods Enzymol 1998;292:101–16. [DOI] [PubMed] [Google Scholar]
- [5].Tusnady GE, Bakos E, Varadi A, Sarkadi B. Membrane topology distinguishes a subfamily of the ATP-binding cassette (ABC) transporters. FEBS Lett 1997;402(1):1–3. [DOI] [PubMed] [Google Scholar]
- [6].Klein I, Sarkadi B, Varadi A. An inventory of the human ABC proteins. Biochim Biophys Acta 1999;1461(2):237–62. [DOI] [PubMed] [Google Scholar]
- [7].Lockhart AC, Tirona RG, Kim RB. Pharmacogenetics of ATP-binding cassette transporters in cancer and chemotherapy. Mol Cancer Ther 2003;2(7):685–98. [PubMed] [Google Scholar]
- [8].Sparreboom A, Danesi R, Ando Y, Chan J, Figg WD. Pharmacogenomics of ABC transporters and its role in cancer chemotherapy. Drug Resist Updat 2003;6(2):71–84. [DOI] [PubMed] [Google Scholar]
- [9].Broccardo C, Luciani M, Chimini G. The ABCA subclass of mammalian transporters. Biochim Biophys Acta 1999;1461(2):395–404. [DOI] [PubMed] [Google Scholar]
- [10].Efferth T Adenosine triphosphate-binding cassette transporter genes in ageing and age-related diseases. Ageing Res Rev 2003;2(1):11–24. [DOI] [PubMed] [Google Scholar]
- [11].Ambudkar SV, Dey S, Hrycyna CA, Ramachandra M, Pastan I, Gottesman MM. Biochemical, cellular, and pharmacological aspects of the multidrug transporter. Annu Rev Pharmacol Toxicol 1999;39:361–98. [DOI] [PubMed] [Google Scholar]
- [12].Luciani MF, Denizot F, Savary S, Mattei MG, Chimini G. Cloning of two novel ABC transporters mapping on human chromosome 9. Genomics 1994;21(1):150–9. [DOI] [PubMed] [Google Scholar]
- [13].Kikuno R, Nagase T, Ishikawa K, Hirosawa M, Miyajima N, Tanaka A, et al. Prediction of the coding sequences of unidentified human genes.XIV. The complete sequences of 100 new cDNA clones from brain which code for large proteins in vitro. DNA Res 1999;6(3):197–205. [DOI] [PubMed] [Google Scholar]
- [14].Vulevic B, Chen Z, Boyd JT, Davis W Jr., Walsh ES, Belinsky MG, et al. Cloning and characterization of human adenosine 5′-triphosphate-binding cassette, sub-family A, transporter 2 (ABCA2). Cancer Res 2001;61(8):3339–47. [PubMed] [Google Scholar]
- [15].Broccardo C, Nieoullon V, Amin R, Masmejean F, Carta S, Tassi S, et al. ABCA2 is a marker of neural progenitors and neuronal subsets in the adult rodent brain. J Neurochem 2006;97(2):345–55. [DOI] [PubMed] [Google Scholar]
- [16].Zhao LX, Zhou CJ, Tanaka A, Nakata M, Hirabayashi T, Amachi T, et al. Cloning, characterization and tissue distribution of the rat ATP-binding cassette (ABC) transporter ABC2/ABCA2. Biochem J 2000;350 (Pt 3):865–72. [PMC free article] [PubMed] [Google Scholar]
- [17].Kaminski WE, Piehler A, Pullmann K, Porsch-Ozcurumez M, Duong C, Bared GM, et al. Complete coding sequence, promoter region, and genomic structure of the human ABCA2 gene and evidence for sterol-dependent regulation in macrophages. Biochem Biophys Res Commun 2001;281(1):249–58. [DOI] [PubMed] [Google Scholar]
- [18].Davis W Jr., Chen ZJ, Ile KE, Tew KD. Reciprocal regulation of expression of the human adenosine 5′-triphosphate binding cassette, sub-family A, transporter 2 (ABCA2) promoter by the early growth response-1 (EGR-1) and Sp-family transcription factors. Nucleic Acids Res 2003; 31(3):1097–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ile KE, Davis W Jr., Boyd JT, Soulika AM, Tew KD. Identification of a novel first exon of the human ABCA2 transporter gene encoding a unique N-terminus. Biochim Biophys Acta 2004;1678(1):22–32. [DOI] [PubMed] [Google Scholar]
- [20].Schmitz G, Kaminski WE. ABCA2: a candidate regulator of neural trans-membrane lipid transport. Cell Mol Life Sci 2002;59(8):1285–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Langmann T, Porsch-Ozcurumez M, Heimerl S, Probst M, Moehle C, Taher M, et al. Identification of sterol-independent regulatory elements in the human ATP-binding cassette transporter A1 promoter: role of Sp1/3, E-box binding factors, and an oncostatin M-responsive element. J Biol Chem 2002;277(17):14443–50. [DOI] [PubMed] [Google Scholar]
- [22].Fitzgerald ML, Okuhira KI, Short GF 3rd, Manning JJ, Bell SA, Freeman MW. ABCA1 contains a novel C-terminal VFVNFA motif that is required for its cholesterol efflux and apoA-I binding activities. J Biol Chem 2004. [DOI] [PubMed] [Google Scholar]
- [23].Brown MS, Goldstein JL. A receptor-mediated pathway for cholesterol homeostasis. Science 1986;232(4746):34–47. [DOI] [PubMed] [Google Scholar]
- [24].Davis W Jr., Boyd JT, Ile KE, Tew KD. Human ATP-binding cassette transporter-2 (ABCA2) positively regulates low-density lipoprotein receptor expression and negatively regulates cholesterol esterification in Chinese hamster ovary cells. Biochim Biophys Acta 2004;1683(1–3):89–100. [DOI] [PubMed] [Google Scholar]
- [25].Smith JR, Osborne TF, Goldstein JL, Brown MS. Identification of nucleotides responsible for enhancer activity of sterol regulatory element in low density lipoprotein receptor gene. J Biol Chem 1990;265(4):2306–10. [PubMed] [Google Scholar]
- [26].McNeish J, Aiello RJ, Guyot D, Turi T, Gabel C, Aldinger C, et al. High density lipoprotein deficiency and foam cell accumulation in mice with targeted disruption of ATP-binding cassette transporter-1. Proc Natl Acad Sci USA 2000;97(8):4245–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Singaraja RR, Bocher V, James ER, Clee SM, Zhang LH, Leavitt BR, et al. Human ABCA1 BAC transgenic mice show increased high density lipoprotein cholesterol and ApoAI-dependent efflux stimulated by an internal promoter containing liver X receptor response elements in intron 1. J Biol Chem 2001;276(36):33969–79. [DOI] [PubMed] [Google Scholar]
- [28].Sone H, Shimano H, Shu M, Nakakuki M, Takahashi A, Sakai M, et al. Statins downregulate ATP-binding-cassette transporter A1 gene expression in macrophages. Biochem Biophys Res Commun 2004;316(3): 790–4. [DOI] [PubMed] [Google Scholar]
- [29].Uehara Y, Engel T, Li Z, Goepfert C, Rust S, Zhou X, et al. Polyunsaturated fatty acids and acetoacetate downregulate the expression of the ATP-binding cassette transporter A1. Diabetes 2002;51(10):2922–8. [DOI] [PubMed] [Google Scholar]
- [30].Radu RA, Mata NL, Bagla A, Travis GH. Light exposure stimulates formation of A2E oxiranes in a mouse model of Stargardt’s macular degeneration. Proc Natl Acad Sci USA 2004;101(16):5928–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kubo Y, Sekiya S, Ohigashi M, Takenaka C, Tamura K, Nada S, et al. ABCA5 resides in lysosomes, and ABCA5 knockout mice develop lysosomal disease-like symptoms. Mol Cell Biol 2005;25(10):4138–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kim WS, Fitzgerald ML, Kang K, Okuhira K, Bell SA, Manning JJ, et al. Abca7 null mice retain normal macrophage phosphatidylcholine and cholesterol efflux activity despite alterations in adipose mass and serum cholesterol levels. J Biol Chem 2005;280(5):3989–95. [DOI] [PubMed] [Google Scholar]
- [33].Tanzi RE, Bertram L. New frontiers in Alzheimer’s disease genetics. Neuron 2001;32(2):181–4. [DOI] [PubMed] [Google Scholar]
- [34].Thinakaran G The role of presenilins in Alzheimer’s disease. J Clin Invest 1999;104(10):1321–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Bertram L, Tanzi RE. Alzheimer’s disease: one disorder, too many genes? Hum Mol Genet 2004;1:R135–R141. [DOI] [PubMed] [Google Scholar]
- [36].Roses AD, Saunders AM. APOE is a major susceptibility gene for Alzheimer’s disease. Curr Opin Biotechnol 1994;5(6):663–7. [DOI] [PubMed] [Google Scholar]
- [37].Casserly I, Topol E. Convergence of atherosclerosis and Alzheimer’s disease: inflammation, cholesterol, and misfolded proteins. Lancet 2004;363 (9415):1139–46. [DOI] [PubMed] [Google Scholar]
- [38].Cholesterol Hartmann T., A beta and Alzheimer’s disease. Trends Neurosci 2001;24(11 Suppl):S45–8. [DOI] [PubMed] [Google Scholar]
- [39].Puglielli L, Tanzi RE, Kovacs DM. Alzheimer’s disease: the cholesterol connection. Nat Neurosci 2003;6(4):345–51. [DOI] [PubMed] [Google Scholar]
- [40].Simons M, Keller P, Dichgans J, Schulz JB. Cholesterol and Alzheimer’s disease: is there a link? Neurology 2001;57(6):1089–93. [DOI] [PubMed] [Google Scholar]
- [41].Kivipelto M, Helkala EL, Laakso MP, Hanninen T, Hallikainen M, Alhainen K, et al. Midlife vascular risk factors and Alzheimer’s disease in later life: longitudinal, population based study. BMJ 2001;322(7300): 1447–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Jick H, Zornberg GL, Jick SS, Seshadri S, Drachman DA. Statins and the risk of dementia. Lancet 2000;356(9242):1627–31. [DOI] [PubMed] [Google Scholar]
- [43].Fassbender K, Masters C, Beyreuther K. Alzheimer’s disease: molecular concepts and therapeutic targets. Naturwissenschaften 2001;88(6):261–7. [DOI] [PubMed] [Google Scholar]
- [44].Chen ZJ, Vulevic B, Ile KE, Soulika A Davis W Jr. Reiner PB et al. Association of ABCA2 expression with determinants of Alzheimer’s disease. FASEB J 2004;18(10):1129–31. [DOI] [PubMed] [Google Scholar]
- [45].Mace S, Cousin E, Ricard S, Genin E, Spanakis E, Lafargue-Soubigou C, et al. ABCA2 is a strong genetic risk factor for early-onset Alzheimer’s disease. Neurobiol Dis 2005;18(1):119–25. [DOI] [PubMed] [Google Scholar]
- [46].Gylling H, Pyrhonen S, Mantyla E, Maenpaa H, Kangas L, Miettinen TA. Tamoxifen and toremifene lower serum cholesterol by inhibition of delta 8-cholesterol conversion to lathosterol in women with breast cancer. J Clin Oncol 1995;13(12):2900–5. [DOI] [PubMed] [Google Scholar]
- [47].Mastroianni A, Bellati C, Facchetti G, Oldani S, Franzini C, Berrino F. Increased plasma HDL-cholesterol and apo A-I in breast cancer patients undergoing adjuvant tamoxifen therapy. Clin Biochem 2000;33(6):513–6. [DOI] [PubMed] [Google Scholar]
- [48].Kim J, Adam RM, Solomon KR, Freeman MR. Involvement of cholesterol-rich lipid rafts in interleukin-6-induced neuroendocrine differentiation of LNCaP prostate cancer cells. Endocrinology 2004;145(2): 613–9. [DOI] [PubMed] [Google Scholar]
- [49].Brower V Of cancer and cholesterol: studies elucidate anticancer mechanisms of statins. J Natl Cancer Inst 2003;95(12):844–6. [DOI] [PubMed] [Google Scholar]
- [50].Boonstra R, Timmer-Bosscha H, van Echten-Arends J, van der Kolk DM, van den Berg A, de Jong B, et al. Mitoxantrone resistance in a small cell lung cancer cell line is associated with ABCA2 upregulation. Br J Cancer 2004;90(12):2411–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Laing NM, Belinsky MG, Kruh GD, Bell DW, Boyd JT, Barone L, et al. Amplification of the ATP-binding cassette 2 transporter gene is functionally linked with enhanced efflux of estramustine in ovarian carcinoma cells. Cancer Res 1998;58(7):1332–7. [PubMed] [Google Scholar]
- [52].Fehrenbacher N, Jaattela M. Lysosomes as targets for cancer therapy. Cancer Res 2005;65(8):2993–5. [DOI] [PubMed] [Google Scholar]
- [53].Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer 2005;5(4):275–84. [DOI] [PubMed] [Google Scholar]
- [54].Borst P, Evers R, Kool M, Wijnholds J. A family of drug transporters: the multidrug resistance-associated proteins. J Natl Cancer Inst 2000;92 (16):1295–302. [DOI] [PubMed] [Google Scholar]
- [55].Ambudkar SV, Kimchi-Sarfaty C, Sauna ZE, Gottesman MM. P-glycoprotein: from genomics to mechanism. Oncogene 2003;22(47): 7468–85. [DOI] [PubMed] [Google Scholar]
- [56].Juliano RL, Ling V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim Biophys Acta 1976; 455(1):152–62. [DOI] [PubMed] [Google Scholar]
- [57].Riordan JR, Ling V. Purification of P-glycoprotein from plasma membrane vesicles of Chinese hamster ovary cell mutants with reduced colchicine permeability. J Biol Chem 1979;254(24):12701–5. [PubMed] [Google Scholar]
- [58].Chang G, Roth CB. Structure of MsbA from E. coli: a homolog of the multidrug resistance ATP binding cassette (ABC) transporters. Science 2001;293(5536):1793–800. [DOI] [PubMed] [Google Scholar]
- [59].Locher KP, Lee AT, Rees D. The E. coli BtuCD structure: a framework for ABC transporter architecture and mechanism. Science 2002;296 (5570):1091–8. [DOI] [PubMed] [Google Scholar]
- [60].Liao JL, Beratan DN. How does protein architecture facilitate the transduction of ATP chemical-bond energy into mechanical work? The cases of nitrogenase and ATP binding-cassette proteins. Biophys J 2004;87(2): 1369–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Stearns ME, Jenkins DP, Tew KD. Dansylated estramustine, a fluorescent probe for studies of estramustine uptake and identification of intra-cellular targets. Proc Natl Acad Sci USA 1985;82(24):8483–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Witztum JL. The oxidation hypothesis of atherosclerosis. Lancet 1994; 344(8925):793–5. [DOI] [PubMed] [Google Scholar]
- [63].Griendling KK, FitzGerald GA. Oxidative stress and cardiovascular injury: Part I: basic mechanisms and in vivo monitoring of ROS. Circulation 2003;108(16):1912–6. [DOI] [PubMed] [Google Scholar]
- [64].Barnham KJ, Masters CL, Bush AI. Neurodegenerative diseases and oxidative stress. Nat Rev Drug Discov 2004;3(3):205–14. [DOI] [PubMed] [Google Scholar]
- [65].Ames BN, Gold LS, Willett WC. The causes and prevention of cancer. Proc Natl Acad Sci USA 1995;92(12):5258–65. [DOI] [PMC free article] [PubMed] [Google Scholar]