Abstract
Background:
Oxidative signaling to modulate redox-sensitive cell functions is a heretofore unexploited approach to developing new drugs for poorly treated oncology indications, where current therapies are often only palliative and accompanied by severe toxicities.
Objective:
Clinical and non-clinical findings with NOV-002 (a mimetic of glutathione disulfide that represents such an approach) are reviewed and evaluated.
Methods:
Published data on NOV-002 along with unpublished information from the drug’s sponsor were reviewed. Literature analysis also focused on protein S-glutathionylation as a regulatory mechanism, particularly in relation to cell signaling, proliferation and cytoskeletal architecture.
Results/conclusion:
NOV-002 is a mechanistically novel agent with potential for ameliorating hematologic toxicity and enhancing efficacy when used in combination with standard chemotherapy to treat cancer patients.
Keywords: biomarkers, glutathione, lung cancer, redox, S-glutathionylation, thiols
1. Introduction
It is an unusual circumstance when the availability of clinical data precedes full preclinical evaluation of a new cancer therapeutic. Such is the case for NOV-002, where significant clinical trial results have been generated from earlier Russian investigations and indicate a unique clinical profile in oncology indications, combining improved tolerance of standard chemotherapeutic drugs with increased efficacy [1]. While the Russian results were uniformly positive, the US Food and Drug Administration (FDA) requires confirmation and extension of such clinical data in order to gain approval in the US. Thus a number of US clinical trials are ongoing in several oncology indications and are discussed in the pertinent sections. Moreover, there is growing evidence that redox-sensitive targets are important in cancer drug discovery and development. NOV-002 is a mimetic of oxidized glutathione (GSSG) in a complex with cisplatin and has impact on cellular redox balance. This balance is critical to the maintenance of cell viability. In particular, thiol homeostasis is one important part of redox homeostasis and, as we shall see, is subject to pharmacological manipulation.
Modulation of glutathione (GSH)/GSSG levels and of glutathione S-transferase (GST) has been attempted as a means to improve response to cancer drugs. Use of buthionine sulfoximine (BSO) and ethacrynic acid, while effective preclinically [2], were not successful enough in the clinic to merit continued development [3,4]. However, one consequence of these studies was the design of a peptidomimetic inhibitor of GSTπ. TLK199 or γ-glutamyl-S-(benzyl)cysteinyl-(R)-(–)-phenylglycine diethyl ester. Preclinical and mechanism-of-action studies revealed an unanticipated myeloproliferative activity in rodents [5,6]. The discovery of a protein-protein interaction between GSTn and c-jun N-terminal kinase (JNK; [7,8]) provided a model for how TLK199 could produce proliferation in marrow progenitor cells [6]. Since N0V-002 affects bone marrow in both preclinical and clinical studies, there is reason to conclude that thiol manipulation may be a common factor in influencing myeloproliferation. Indeed, there are examples where glutathione is directly implicated in regulation of immune response. Manipulation of blood GSH/GSSG levels by administration of N-acetylcysteine has been shown to influence survival and quality of life in HIV-infected patients [9,10]. With NOV-002 it is possible to prescribe general pleiotropic mechanisms linking GSH/GSSG with myeloid proliferation and immune regulation.
2. Overview of the market
While NOV-002 could, theoretically, be used in a wide range of solid-tumor indications (in combination with standard chemotherapy), the main focus of its development is non-small-cell lung cancer (NSCLC), a highly prevalent, poorly treated and fatal disease. Lung cancer is now the leading cause of cancer death in both men and women, and accounts for 29% of cancer deaths overall. Non-small-cell lung cancer accounts for approximately 80% of all lung cancers and most patients present with inoperable advanced Stage III (IIIb) or Stage IV disease. One-year survival in advanced NSCLC is ~ 35% and median survival is ~ 8.5 months. Compounding these poor survival rates is the marked degree of morbidity associated with lung cancer, including pain associated with the primary tumor and with metastatic disease (especially metastases to bone) and neurological symptoms associated with metastases to the central nervous system [11].
Currently available therapy for NSCLC is suboptimal with respect to both efficacy and toxicity [12,13]. In the US, the combination of carboplatin and paclitaxel is most frequently used as first-line therapy, with the recent addition of bevacizumab (an angiogenesis inhibitor) for a subset of patients. A number of other ‘targeted’ therapies are in clinical development for NSCLC (e.g., cituximab, an anti-EGF antibody; bortezomib, a proteasome inhibitor; ASA404, a vascular disrupting agent). Overall, current therapies are palliative, providing marginal efficacy as measured by survival. In addition, such chemotherapy is accompanied by severe, sometimes life-threatening toxicities, which often limit its application. Thus, there is a clear need for new, more effective and safer therapies for advanced NSCLC.
3. Introduction to NOV-002
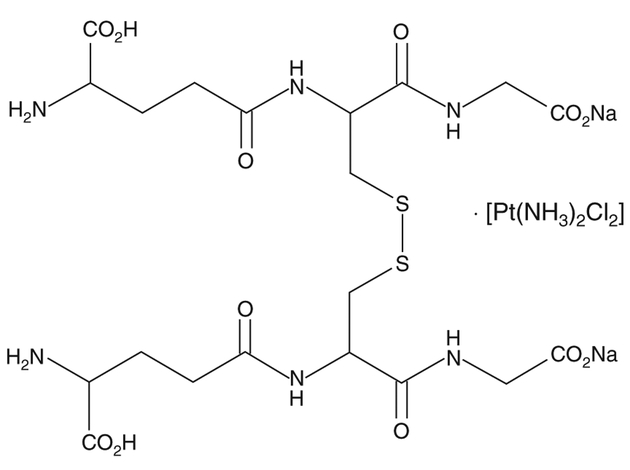
The structure of N0V-002 is shown in Figure 1. It is oxidized glutathione (GSSG) with cisplatin at a molar ratio of ~ 1000:1. The cisplatin may serve to stabilize the GSSG. It can be calculated that a typical therapeutic dose (60 mg) administered daily over 6 months would result in a cumulative total dose of cisplatin that is equivalent to < 2% of the single dose of cisplatin typically used in treatment of cancer patients. As such, it seems unlikely that the platinum component contributes substantially to the pharmacology of the compound and, indeed, the preclinical data confirm that GSSG is the active component of N0V-002 [14].
Figure 1.
Structure of NOV-002, the disodium salt of oxidized glutathione complexed with cisplatin.
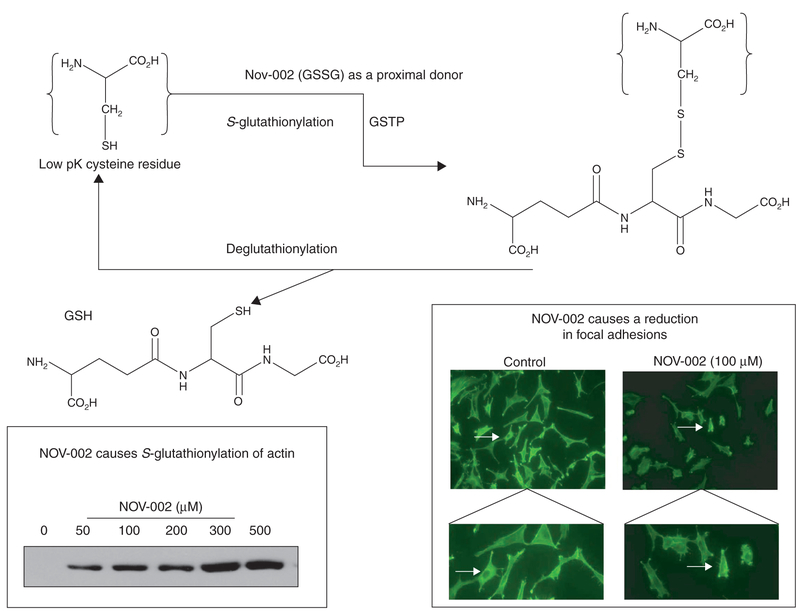
Sulfur has the essential chemical property of existing in a biologically reduced sulfhydryl state, where the pKa of the thiol group is ~ 9.65, accounting for the nucleophilicity of reduced glutathione. GSH homeostasis is maintained in cells by a complex series of balanced pathways. De novo synthesis can occur through the γ-glutamyl cycle [15], where the three constituent amino acids (Glu-Cys-Gly) are combined with rate-limiting catalysis through glutamate-cysteine ligase (GCS) and glutathione synthetase. Salvage of GSH can occur through the cleavage activity of the membrane-associated γ-glutamyl transpeptidase (GGT), which can recycle constituents. While intracellular concentrations of GSH may vary considerably, concentrations of 0.1 – 10 mM are commonly found in mammalian cells (10 – 30 μm in plasma). Glutathione can occur in reduced (GSH), oxidized (GSSG) or mixed disulfide forms and its ubiquitous abundance is testament to its biological importance. More recently, S-glutathionylation of proteins has been recognized as an important post-translational modification (Figure 2). S-glutathionylation can influence conformation of various structural proteins, including actin [16] and other clusters of proteins, which can be associated with energy metabolism/glycolysis, cell signaling, calcium homeostasis, protein folding or redox homeostasis (for review see [17]). Since GSSG can be a proximal donor in the S-glutathionylation reaction, the implications are that N0V-002 may also provide donor substrate. As a consequence of this and other effects on cellular redox balance, NOV-002 influences multiple cell processes and functions, including critical proliferation pathways.
Figure 2. Scheme for S-glutathionylation of proteins.
In the example shown, NOV-002 can act as the proximal donor for S-glutathionylation of actin (immunoblot shown on lower left). At the cell level, this causes a decrease in the number of focal adhesions (lower right box: see arrows in top panels [magnification × 100] and bottom panels [magnification × 400]) that connect cells to the extracellular matrix and serve as a biochemical and biomechanical signaling hub, affecting numerous functions (e.g., cell migration, cell cycle control).
GSH: glutathione; GSSG: Glutathione disulfide; GSTP: glutathione S-transferase P.
4. Mechanism of action
N0V-002 is not cytotoxic alone even at high doses, and while N0V-002 causes protein S-glutathionylation, the platinum component does not [14]. Instead, findings collectively suggest that the capacity for modulation of redox conditions at the cell surface and/or intracellularly may underlie the pharmacological properties of NOV-002. NOV-002 has pleiotropic effects on preclinical model cell systems. Our published data [14] show that NOV-002 treatment of HL60 cells alters a number of cellular redox parameters both at the cell surface and intracellularly. HL60 cells were chosen as an example of a cell line of hematopoietic origin, thus providing focus for the clinical effects of NOV-002 on blood cell profiles. It is generally accepted that GSSG will not passively cross the cell membrane [18]. Thus, the effects of NOV-002 on cells may be mediated via direct effects on cell-surface targets. Alternatively, the interaction of NOV-002 with a membrane-associated enzyme such as γ-glutamyl transpeptidase (for which both GSSG and NOV-002 are substrates) could result in hydrolysis into constituent amino acids, whereupon extra availability of cysteine could stimulate GSH metabolism. In addition, there is evidence that GGT, through Fenton chemistry, can result in the liberation of hydrogen peroxide (H2O2), which is cell-permeable and capable of transmitting an oxidative signal into the cell [19]. The increased bioavailability of GSSG through NOV-002 administration could increase the flux of H2O2 as a consequence of stimulating the GGT activity. In addition, it has been shown that non-steroidal anti-inflammatory drugs and resveratrol can cause increases in the expression of glutamate-cysteine ligase mRNA and can stimulate glutathione metabolism. This same group has also demonstrated that GSSG can directly affect GSH levels through increasing H2O2 and stimulating glutamate-cysteine ligase [20].
Cell-surface protein thiols are believed to act as sensors of extracellular redox status, and their modification has been linked to regulation of cell signaling and other functions in a variety of cell types [21]. NOV-002 treatment of HL60 cells reduced cell-surface thiol content through oxidative modification of cell-surface proteins [14]. A growing body of evidence suggests that redox-based modulation of surface proteins is capable of regulating a variety of cell functions [22]. One potential target of such modification is cell-surface protein disulfide isomerase (PDI), which regulates, for example, viral entry into cells (e.g., HIV-1 entry as a consequence of redox modulation of CD4 on lymphocytes and the HIV-1 envelope glycoprotein gp120 [23]), cell-mediated adhesion by integrins [24] and tumor cell invasiveness [25]. Since N0V-002 also inhibits PDI activity, this enzyme may represent a cell-surface target for this drug, a possibility further supported by the finding that either N0V-002 or GSSG could act as a proximal donor in the S-glutathionylation of PDI (Townsend et al.. submitted), thus regulating its function.
Intracellularly, N0V-002 treatment of HL60 cells resulted in multiple changes indicative of alterations in redox balance against the backdrop of stimulating the rate of cell proliferation. Redox conditions can regulate a number of signaling pathways and directly control cell division and differentiation responses. In neuronal progenitor cells, a fine-tuning of the redox balance has led some investigators to suggest that as little as 15% increase or decrease in cellular redox can activate pathways that direct cells towards either proliferation and cell division or differentiation [26,27]. Our published results [14] showed that absolute levels of GSH and GSSG increased transiently following treatment. Moreover, N0V-002 does act as a substrate for glutathione reductase, and this will also serve to alter the potential of the cell to maintain redox homeostasis. The ratio of GSH to GSSG was decreased by approximately 36% after N0V-002 treatment, indicating the generation of a mild oxidative signal within the cell interior. In addition, HL60 cells treated with N0V-002 showed ~ 2.5-fold increase in H202 production compared with untreated cells. These results were temporally consistent with a time- and concentration-dependent increase in the phosphorylation of three kinases, which in bone marrow can play direct roles in cell proliferation (JNK, p38 and ERK), and in AKT, a kinase that acts in concert with JAK2 and STAT5 to regulate marrow proliferation. The JAK-STAT signaling pathway is intimately involved in governing the response of cells to cytokines and growth factors [28]. Activation of the pathway by N0V-002 indicates that the drug is affecting those pathways that lead to hematopoiesis/myeloproliferation. These results are consistent with earlier work with TLK199, another small-molecule myeloproliferative agent [6]. In each case, these stimulations were also coincident with the induction of S-glutathionylation of actin and could suggest a cause-and-effect relationship between signaling and cytoskeleton morphology. S-glutathionylation occurs to certain target proteins when a cell is exposed to oxidative or nitrosative stress [29]. Filomeni and colleagues have used a number of model systems to demonstrate that, under certain conditions, GSSG can act as a pro-oxidant activator of the p38 MAPK death pathway in some tumor cell lines [30,31]. While the fate of cells affected by these kinases is quite tissue-dependent, there is some general concurrence amongst the specific kinases targeted. Studies of specific kinases generally reflect the important role that GSSG has in mediating early-response pathways through redox changes that transduce to a kinase signaling cascade, affecting cell survival pathways. The concomitant changes in intracellular GSSG, actin S-glutathionylation, kinase phosphorylation and cell proliferation in HL60 cells treated with N0V-002 seem to be causally interrelated.
In summary, N0V-002 exerts pleiotropic effects in HL60 cells consistent with the conclusion that its active pharmaceutical ingredient is GSSG and that its central mode of action involves modulation of redox balance, both intracellularly and at the cell surface. Redox modulation regulates downstream kinase events that may be responsible for its stimulation of proliferation in this premyeloid cell line [14], an effect that could be related to N0V-002’s clinical profile in oncology patients, which includes myeloproliferation and improved tolerance of cytotoxic chemotherapy.
5. Pharmacodynamics, pharmacokinetics and metabolism
Pharmacogenetic correlations are becoming more important in the establishment of new cancer therapeutics. Surrogate biomarkers can be potentially useful in establishing pharmacokinetic and pharmacodynamic properties. For N0V-002, there is the possibility that the S-glutathionylation pattern of plasma proteins may provide such information. Early analysis of murine serum data revealed that four protein bands are prominently S-glutathionylated. For three of these proteins, the intensities of labeling increase following treatment of the animals with a comparatively low dose of N0V-002 (15 mg/kg i.p.). Using matrix assisted laser desorption/ionization – time of flight (MALDI-T0FF)peptide sequencing, the proteins were identified as redox-sensitive serum proteases (serine protease inhibitor, contrapsin and α−1-antitrypsin 1 – 6 precursor) (Townsend et al., submitted). These biomarkers may prove to be valuable pharmacodynamic indicators in humans.
6. Clinical efficacy
N0V-002 has been the subject of multiple Phase II oncology studies in the Russian Federation and the US, primarily in non-small-cell lung cancer. In Russia, a multicenter, randomized, open-label, 12-month study was conducted to evaluate the safety and efficacy of N0V-002 in combination with chemotherapy in patients with advanced NSCLC (Novelos Therapeutics, Inc., unpublished information). During the first 2 months of the study, first-line chemotherapy was standardized to cisplatin (60 mg/m2 in a single i.v. infusion) and etoposide (three doses of 120 mg/m2 via i.v. infusions given every other day, starting with the day of cisplatin administration). Subsequent chemotherapy regimens included CAP (single i.v. doses of cisplatin 50 mg/m2, cyclophosphamide 500 mg/m2, and adriamycin 50 mg/m2), MACC (single i.v. doses of methotrexate 40 mg/m2, adriamycin 40 mg/m2, and cyclophosphamide 400 mg/m2), or lomustine (belustine or 1-(2-chloroethyl)-3-cyclohexyl-1-nitrosourea (CCNU) 30 mg orally).
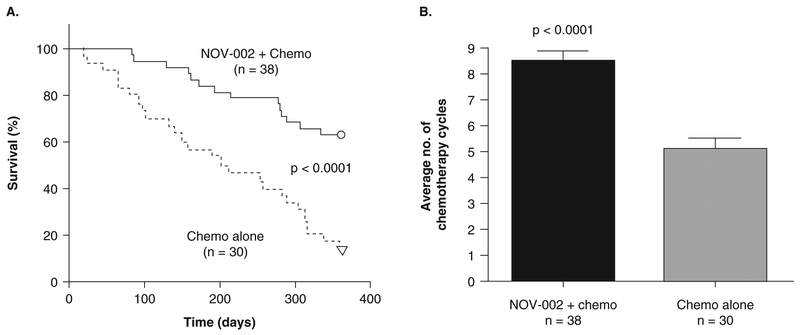
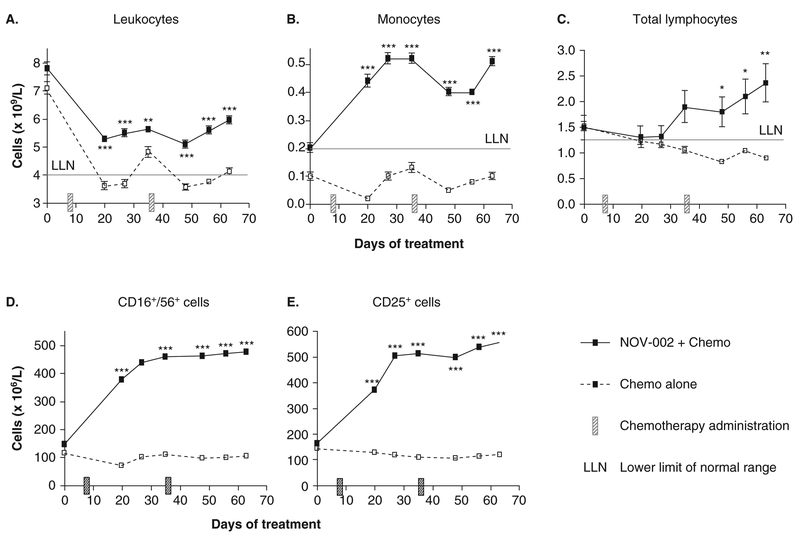
In the study, 68 chemotherapy-naive patients with Stage IIIb/IV NSCLC were randomized into two groups for 12 months of treatment. Group A patients (n = 38) received NOV-002 in combination with chemotherapy. For each nominal 28-day chemotherapy cycle, Group A patients received N0V-002 60 – 80 mg/day i.v. on days of chemotherapy administration and NOV-002 10 – 20 mg/day i.m. on non-chemotherapy days. Group B patients (n = 30) received chemotherapy alone. One-year survival was significantly improved from 17% in the chemotherapy-alone group (Group B) to 63% in the N0V-002-plus-chemotherapy group (Group A) (p < 0.0001), and patients receiving N0V-002 were able to receive more cycles of chemotherapy (Figure 3; p < 0.0001). Improved tolerance of chemotherapy in NOV-002-treated patients was also evidenced by significantly increased peripheral blood counts of leukocytes, monocytes, erythrocytes/hemoglobin and lymphocytes (including total lymphocytes, T-suppressor cells, IL-2 receptor-expressing T cells, and natural killer cells) compared to chemotherapy alone (Figure 4). In addition, performance status (using Karnofsky Score) was significantly improved in the N0V-002 plus chemotherapy group compared to the chemotherapy alone group (data not shown).
Figure 3. Survival over 1 year and average total number of chemotherapy cycles for advanced NSCLC patients in a Phase II clinical trial.
In this controlled trial, Patients were randomized to Group A (NOV-002 + chemotherapy) or Group B (chemotherapy alone). A. Kaplan-Meier plots of survival over time; O and ∇ symbols represent 24 and 5 censored patients in Groups A and B, respectively, who were alive at the end of 1 year; p<0.0001 using log rank χ2 test. B. Average total number of chemotherapy cycles administered to Group A and Group B patients. Values are mean ± standard error of mean; p < 0.0001 using unpaired, two-tailed t-test.
Figure 4. Peripheral blood counts of (A) leukocytes, (B) monocytes, (C) total lymphocytes, (D) CD25+ cells (IL-2 receptor-expressing lymphocytes), and (E) CD16+/CD56+ cells (NK lymphocytes) in advanced NSCLC patients in a Phase II clinical trial.
In this controlled trial, patients were randomized to Group A (Nov-002 + chemotherapy) or Group B (chemotherapy alone).
Values are mean ± standard error of mean (where error bars are not visible they lie within the data point). *** p < 0.001, ** p < 0.01, * p < 0.05 two-way ANOVA.
In a US Phase I/II trial [1], 44 chemotherapy-naive, Stage IIIb/IV NSCLC patients (Eastern Cooperative Oncology Group [ECOG] 0 – 2, stratified by disease stage) were randomized to one of three groups for 6 months of treatment. Groups A and B received NOV-002 in combination with carboplatin and paclitaxel (C+P). For each nominal 21-day chemotherapy cycle, these groups received 60 mg NOV-002 i.v. daily for the first 4 days, then 60 mg i.m. (A) or s.c. (B) on weekdays for the next 17 days. Group C received C+P alone. Initial doses of paclitaxel and carboplatin in all three groups were 225 mg/m2 and AUC 6, respectively. Initiation of repeat C+P cycles required an absence of hematologic and other toxicities in excess of predefined values. Primary study end points included tumor response (scans performed at baseline and then every 2 months) and safety. An intent-to-treat analysis of the best overall objective tumor response (WHO criteria) showed that 11 out of 16 (69%) Group B patients demonstrated greater than 50% tumor shrinkage versus 5 out of 15 (33%) in the control group (C) (p = 0.044, logistic regression stratified on disease stage). In Group A, 6 out of 13 (46%) patients demonstrated an objective response. In addition, 100% of NOV-002-treated patients in Group B and 85% in Group A were able to complete four cycles of C+P compared to 50% of control patients (Group C) (p = 0.004, χ2).
NOV-002 is the subject of a pivotal ongoing Phase III trial in advanced NSCLC. This randomized, open-label trial is being conducted at nearly 100 clinical sites across 10 countries, and, like the above trials, compares NOV-002 and first-line chemotherapy (carboplatin and paclitaxel) with chemotherapy alone. Full patient accrual is complete at the time of writing, and results are expected in 2009. In addition, the drug is the subject of two smaller ongoing Phase II trials (again in combination with standard chemotherapy), one in platinum-resistant ovarian cancer and the other as neoadjuvant therapy in breast cancer.
7. Safety and tolerability
In cell cultures, NOV-002 was not cytotoxic at concentrations as high as 1 mM. In chronic (6-month) toxicology studies in both rats and dogs, NOV-002 demonstrated no measurable toxicities at 1000 and 100 mg/kg, respectively. In clinical studies conducted to date, NOV-002 has been well tolerated and without evidence of untoward side effects.
8. Regulatory affairs
The ongoing Phase III trial with NOV-002 in advanced NSCLC is being conducted under a Special Protocol Assessment from the FDA. NOV-002 has also received ‘fast-track’ designation from the FDA. As of the first quarter of 2008, the accrual goal of 840 patients had been reached. Analyses of results are anticipated in 2009. While other indications will be tested (including breast and ovarian cancer), initial review will focus on the NSCLC data.
9. Conclusions
N0V-002 is a well-tolerated therapeutic adjuvant to standard cancer drug therapies. It enhances myeloproliferation, primarily as a consequence of redox-induced changes that occur at both the cell surface and intracellularly. Clinical testing in Russia has already produced positive survival data in NSCLC patients. These trials require continued confirmation in the US and a critical Phase III trial is in progress in parallel with Phase II trials in other oncology indications.
10. Expert opinion
Overall, N0V-002 can be distinguished from other drugs for advanced NSCLC on the market or in development because, based on available data, it possesses the key attributes of safety, improved recovery from chemotherapy toxicity, potentiation of chemotherapy (increased survival rates and better antitumor effects) and low cost of manufacture. By comparison, new dosing regimens with existing cytotoxic drugs are likely to provide only incremental improvements in efficacy and/or safety. Similarly, emerging targeted biologic therapies, such as gefitinib, erlotinib, bevacizumab and cetuximab, may offer some benefit for certain patient subpopulations, but overall efficacy has remained low. Moreover, there are significant safety concerns and the costs of manufacture are very high. Thus, there remains a significant medical need for better drug therapy for advanced NSCLC patients, and NOV-002 offers promise in this regard.
In addition, NOV-002 is an interesting pharmaceutical case study. Cancer drug discovery/development in the US usually follows the standard route of target identification, small-molecule discovery, in vitro and in vivo testing, Phase I through Phase III clinical testing and eventual FDA registration. The existence of clinical results with N0V-002 generated outside the US prior to development in the US represents a more unusual path to drug approval. The earlier Russian data paint a fairly optimistic picture and US-based trials to date seem to be encouraging. If pending pivotal Phase III results are positive and the drug receives regulatory approval, N0V-002 has the potential for rather broad use in combination therapies for oncology indications, both for facilitating recovery from chemotherapy toxicities and increasing the efficacy of standard chemotherapy. With limited restrictions on which drugs are used with it, such off-label applications would add to the impact of N0V-002 on medical practice and augur well for its commercial success.
With respect to competitive drugs, given the apparently novel mechanism of action of NOV-002, historical parallels are not common. Amifostine is a cytoprotective agent working by detoxifying free-radical byproducts of drugs or radiation, with concomitant reduced toxicity to normal tissues. It has FDA approval for reducing kidney damage due to cisplatin chemotherapy in patients with ovarian cancer and for reducing moderate to severe dry mouth associated with radiation treatment after surgery for head and neck cancer. Any off-label uses of amifostine are related to its thiol detoxification functionality, and unlike NOV-002, there is no suggestion that it can improve the efficacy of concomitantly administered chemotherapy. Since the mechanism of action for N0V-002 relies more on its disulfide moiety, it does not seem likely that it would compete directly for the same indications as amifostine. TLK199 is in clinical trial in myelodysplastic syndrome patients, and data presented in abstract form at national meetings in the US are so far encouraging. However, it is too early to predict if there is likely to be clinical overlap with N0V-002, or whether the two drugs may have complementary effects.
Since the trace cisplatin content of N0V-002 does not appear to be a pharmacologically active component of the drug, one of the challenges lies in ascribing a definite mechanism of action for oxidized glutathione, which is essentially an endogenous molecule. Conceptualizing redox regulation of cellular function is complex, not least because of the volume of research characterizing both the positive and negative impact of oxidant effects on cells. For example, hydrogen peroxide is a toxin, but is also an endogenous messenger in a number of growth regulatory pathways. Critical signaling events governed by kinases or phosphatases are subject to control by S-glutathionylation of cysteine residues. This, in turn, is regulated by N0V-002 through a drug-induced alteration of thiol/disulfide balance. Generally, the maintenance of thiol homeostasis is subject to temporal fluxes that have regulatory effects upon cell growth, division and death, and it is now clear that pharmacological manipulation of thiols influences myeloproliferation. N0V-002 exerts an effect upon these pathways in a manner that is consistent with a general redox-based change in thiol and disulfide ratios and concomitant impact upon myeloproliferative status.
What does the future hold for N0V-002? The results of the Phase III NSCLC trial should be available in 2009, with analysis available for registration application later that year. Premised on existing clinical data and the benign toxicity profile, there is reason to be optimistic, notwithstanding the vagaries of FDA review. If approval is received, the next 5 to 10 years should result in steady market growth in a large and poorly treated patient population (advanced NSCLC), with plausible expansion of use into other oncology indications in combination with chemotherapy. The general public is keen on therapies that are non-toxic, and the present emphasis on targeting of new drugs is shifting towards combinatorial agents, such as N0V-002.
Acknowledgements
The authors wish to acknowledge Georgy Moiseyvich Manichas of the St Petersburg City Oncological Dispensary and Yuri Nikolayevich Levashev and Sergey Vladimirovich Orlov of the State Research Center on Pulmonology of the Health Ministry of the Russian Federation, St Petersburg, for their pivotal roles in the conduct of the Phase II trial of NOV-002 in advanced non-small-cell lung cancer patients in Russia.
Footnotes
Declaration of interest
Christopher J Pazoles is an employee of Novelos.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Pazoles CJ, Gernstein H. N0V-002, a chemoprotectant/immunomodulator, added to first line carboplatin/paclitaxel in advanced non-small cell lung cancer (NSCLC): a randomized phase 1/2, open label, controlled study. J Clin Oncol, 2006 ASC0 Annual Meeting Proceedings Part I. 2006;24(18S Supplement):17021 [Google Scholar]
- 2.Tew KD, Bomber AM, Hoffman SJ. Ethacrynic acid and piriprost as enhancers of cytotoxicity in drug resistant and sensitive cell lines. Cancer Res 1988;48 (13):3622–5 [PubMed] [Google Scholar]
- 3.Bailey HH, Ripple G, Tutsch KD, et al. Phase I study of continuous-infusion L-S,R-buthionine sulfoximine with intravenous melphalan. J Natl Cancer Inst 1997;89(23):1789–96 [DOI] [PubMed] [Google Scholar]
- 4.O’dwyer PJ, Lacreta F, Nash S, et al. Phase I study of thiotepa in combination with the glutathione transferase inhibitor ethacrynic acid. Cancer Res 1991;51 (22): 6059–65 [PubMed] [Google Scholar]
- 5.Ruscoe JE, Rosario LA, Wang T, et al. Pharmacologic or genetic manipulation of glutathione S-transferase P1–1 (GSTpi) influences cell proliferation pathways. J Pharmacol Exp Ther 2001;298(1):339–45 [PubMed] [Google Scholar]
- 6.Gate L, Majumdar RS, Lunk A, et al. Increased myeloproliferation in glutathione S -transferase pi-deficient mice is associated with a deregulation of JNK and Janus kinase/STAT pathways. J Biol Chem 2004;279(10):8608–16 [DOI] [PubMed] [Google Scholar]
- 7.Adler V, Yin Z, Fuchs SY, et al. Regulation of JNK signaling by GSTp. EMBOJ 1999; 18 (5): 1321–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang T, Arifoglu P, Ronai Z, et al. Glutathione S-transferase P1–1 (GSTP1–1) inhibits c-Jun N-terminal kinase (JNK1) signaling through interaction with the C terminus. J Biol Chem 2001; 276 (24): 20999–1003 [DOI] [PubMed] [Google Scholar]
- 9.Peterson JD, Herzenberg LA, Vasquez K, et al. Glutathione levels in antigen-presenting cells modulate Th1 versus Th2 response patterns. Proc Natl Acad Sci USA 1998; 95 (6):3071–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herzenberg LA, De Rosa SC, Dubs JG, et al. Glutathione deficiency is associated with impaired survival in HIV disease. Proc Natl Acad Sci USA 1997; 94 (5): 1967–72• Reports on the value of glutathione in ameliorating symptoms in HIV patients. Provides a strong clinical correlate for glutathione pathways in immune regulation.
- 11.El Maalouf G, Rodier JM, Faivre S, et al. Could we expect to improve survival in small cell lung cancer? Lung Cancer 2007;57(Suppl 2):S30–4 [DOI] [PubMed] [Google Scholar]
- 12.Socinski MA, Bogart JA. Limited-stage small-cell lung cancer: the current status of combined-modality therapy. J Clin Oncol 2007;25(26):4137–45 [DOI] [PubMed] [Google Scholar]
- 13.Rigas JR, Kelly K. Current treatment paradigms for locally advanced non-small cell lung cancer. J Thorac Oncol 2007;2(Suppl 2):S77–85 [DOI] [PubMed] [Google Scholar]
- 14.Townsend DM, HE L, Hutchens S, et al. NOV-002, a glutathione disulfide mimetic, as a modulator of cellular redox balance. Cancer Res 2008;68:2870–7•• This is the first paper detailing NOV-002’s mechanism of action. It discusses how signaling pathways are affected and how drug-induced 5-glutathionylation of actin can affect cell structure.
- 15.Meister A Glutathione metabolism and its selective modification. J Biol Chem 1988;263(33): 17205–8 [PubMed] [Google Scholar]
- 16.Wang J, Boja ES, Tan W, et al. Reversible glutathionylation regulates actin polymerization in A431 cells. J Biol Chem 2001;276(51):47763–6 [DOI] [PubMed] [Google Scholar]
- 17.Townsend DM. S -glutathionylation: indicator of cell stress and regulator of the unfolded protein response. Mol Interv 2007;7(6):313–24• A comprehensive review of protein S-glutathionylation, including those clusters of proteins susceptible to the post-translational modification.
- 18.Brennan JP, Miller JI, Fuller W, et al. The utility of N,N-biotinyl glutathione disulfide in the study of protein S-glutathiolation. Mol Cell Proteomics 2006;5(2):215–25 [DOI] [PubMed] [Google Scholar]
- 19.Pompella A, Corti A, Paolicchi A, et al. Gamma-glutamyltransferase, redox regulation and cancer drug resistance. Curr Opin Pharmacol 2007;7(4):360–6 [DOI] [PubMed] [Google Scholar]
- 20.Sekhar KR, Spitz DR, Harris S, et al. Redox-sensitive interaction between KIAA0132 and Nrf2 mediates indomethacin-induced expression of gamma-glutamylcysteine synthetase. Free Radic Biol Med 2002;32(7):650–62 [DOI] [PubMed] [Google Scholar]
- 21.Dominici S, et al. Redox modulation of cell surface protein thiols in U937 lymphoma cells: the role of gamma-glutamyl transpeptidase-dependent H2O2 production and S-thiolation. Free Radic Biol Med 1999;27:623–35• Provides a link between oxidized glutathione and GGT, with particular reference to membrane-signaling events transmitted through hydrogen peroxide.
- 22.Jordan PA, Gibbins JM. Extracellular disulfide exchange and the regulation of cellular function. Antioxid Redox Signal 2006;8(3–4):312–24 [DOI] [PubMed] [Google Scholar]
- 23.Matthias LJ, Hogg PJ. Redox control on the cell surface: implications for HIV-1 entry. Antioxid Redox Signal 2003; 5 (1): 133–8 [DOI] [PubMed] [Google Scholar]
- 24.Lahav J, Wijnen EM, Hess O, et al. Enzymatically catalyzed disulfide exchange is required for platelet adhesion to collagen via integrin alpha2beta1. Blood 2003;102(6):2085–92 [DOI] [PubMed] [Google Scholar]
- 25.Goplen D, Wang J, Enger PO, et al. Protein disulfide isomerase expression is related to the invasive properties of malignant glioma. Cancer Res 2006; 66 (20): 9895–902 [DOI] [PubMed] [Google Scholar]
- 26.Smith J, Ladi E, Mayer-Proschel M, et al. Redox state is a central modulator of the balance between self-renewal and differentiation in a dividing glial precursor cell. Proc Natl Acad Sci USA 2000;97(18): 10032–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Noble M, Mayer-Proschel M, Proschel C. Redox regulation of precursor cell function: insights and paradoxes. Antioxid Redox Signal 2005;7(11–12): 1456–67 [DOI] [PubMed] [Google Scholar]
- 28.O’shea JJ, Gadina M, Schreiber RD. Cytokine signaling in 2002: new surprises in the Jak/Stat pathway. Cell 2002;109(Suppl):S121–31 [DOI] [PubMed] [Google Scholar]
- 29.Tew KD. Redox in redux. emergent roles for glutathione S-transferase P (GSTP) in regulation of cell signaling and S-glutathionylation. Biochem Pharmacol 2007;73(9): 1257–69• Discusses general glutathione pathways and their interface with myeloproliferation. Emphasis is placed on the GST-mediated pathways of signaling/proliferation.
- 30.Filomeni G, Aquilano K, Civitareale P, et al. Activation of c-Jun-N-terminal kinase is required for apoptosis triggered by glutathione disulfide in neuroblastomacells. Free Radic Biol Med 2005;39(3):345–54 [DOI] [PubMed] [Google Scholar]
- 31.Filomeni G, Rotilio G, Ciriolo MR. Glutathione disulfide induces apoptosis in U937 cells by a redox-mediated p38 MAP kinase pathway. FASEB J 2003;171:64–6• Shows that oxidized glutathione causes redox-mediated changes in tissue culture cells. 1