Abstract
Ecological and human health impairments related to excess nitrogen (N) in streams and rivers remain widespread in the United States (U.S.) despite recent efforts to reduce N pollution. Many studies have quantified the relationship between N loads to streams in terms of N mass and N inputs to watersheds; however, N concentrations, rather than loads, are more closely related to impacts on human health and aquatic life. Additionally, concentrations, rather than loads, trigger regulatory responses. In this study, we examined how N concentrations are related to N inputs to watersheds (atmospheric deposition, synthetic fertilizer, manure applied to agricultural land, cultivated biological N fixation, and point sources), land cover characteristics, and stream network characteristics, including stream size and the extent of lakes and reservoirs. N concentration data were collected across the conterminous U.S. during the U.S. Environmental Protection Agency’s 2008-09 National Rivers and Streams Assessment (n=1966). Median watershed N inputs were 15.7 kg N ha−1 yr−1. Atmospheric deposition accounted for over half the N inputs in 49% of watersheds, but watersheds with the highest N input rates were dominated by agricultural-related sources. Total N input to watersheds explained 42% and 38% of the variability in total N and dissolved inorganic N concentrations, respectively. Land cover characteristics were also important predictors, with wetland cover muting the effect of agricultural N inputs on N concentrations and riparian disturbance exacerbating it. In contrast, stream variables showed little correlation with N concentrations. This suggests that terrestrial factors that can be managed, such as agricultural N use practices and wetland or riparian areas, control the spatial variability in stream N concentrations across the conterminous U.S.
Keywords: Inorganic nitrogen, organic nitrogen, watershed, fertilizer, atmospheric deposition, land-use/land-cover
Graphical Abstract
INTRODUCTION
Human actions have more than doubled the global annual creation of reactive nitrogen (N) through production of synthetic N fertilizers, increased cultivation of N-fixing crops, and fossil fuel combustion (Fowler et al., 2013). Some of this N moves from where it is was originally produced or applied into aquatic ecosystems, where elevated N concentrations can lead to undesirable shifts in community structure, harmful algal blooms, low oxygen, and contaminated drinking water (Camargo and Alonso, 2006). Despite concerted national, state, and local efforts to reduce nutrient inputs to air, land, and water (Melillo and Cowling, 2002; Mississippi River/Gulf of Mexico Watershd Nutrient Task Force, 2008, 2001; United States Congress, 1990), U.S. rivers have seen little improvement in stream nitrate concentrations in recent decades (Sprague et al., 2011; Stets et al., 2015). A recent survey of nearly 2,000 rivers and streams across the U.S. indicates that 41% of stream and river miles are in poor condition with respect to total N (TN) concentrations (the sum of concentrations of all forms of suspended and dissolved reactive N), with TN higher than regional reference conditions (United States Environmental Protection Agency, 2016a). Numerous modeling studies have examined the drivers of N load to aquatic ecosystems (Harrison et al., 2005a; Preston et al., 2011; White et al., 2014); however, N concentrations more directly influence flora and fauna of stream ecosystems (Dodds and Welch, 2000; Dodds et al., 1997), and management and policy decisions, including the identification and listing of impaired waters under the Clean Water Act, often hinge upon N concentrations and water quality impairments directly related to N concentrations. Therefore, we need to improve our understanding of the drivers of N concentrations in aquatic ecosystems to guide management and restoration of critical water resources.
N makes its way into streams via point sources and non-point sources from watersheds (Dumont et al., 2005). Many studies have linked N inputs to watersheds with N export loads (kg N basin−1 y−1) (Alexander et al., 2002; Howarth et al., 1996; Robertson et al., 2014; Wise and Johnson, 2011). Fewer studies have linked watershed N inputs with stream N concentrations in the U.S., and these have been regional studies, focused on relatively undisturbed systems and/or on specific forms of N rather than TN concentrations (Aber et al., 2003; Brookshire et al., 2007; Lerch et al., 2015; Olson and Hawkins, 2013; Pellerin et al., 2006; Sobota et al., 2009). While dissolved inorganic N (DIN) – nitrate and ammonium – is biologically labile (Ensign and Doyle, 2006), TN concentration may indicate N availability in aquatic systems more accurately (Dodds, 2003). Because of the specific nature of previous work relating N inputs to N concentrations, predicting the extent to which management changes can address N-related impairments in streams and rivers nationally remains difficult. While studies have correlated land cover type with stream N concentrations (e.g. Greathouse et al., 2014; Omernik, 1977; Stanley and Maxted, 2008), N input rates can vary dramatically across the same land cover categories (Puckett, 1995; Rao et al., 2009), limiting the applicability of these studies to nutrient management efforts. Recent advances in high resolution inventories of N inputs across the U.S. (Preston et al., 2011; Sobota et al., 2013) allow for more refined, spatially explicit estimates of watershed N inputs to compare with stream N concentrations.
The relationship between N inputs and stream N concentrations may depend on the specific combination of N sinks and transport pathways within the landscape. For example, forests can retain N during periods of high biological demand (Arheimer et al., 1996; Mitchell et al., 1996), and vegetated riparian buffers can effectively retain N if hydrologic transport rates do not greatly exceed biological uptake rates in the buffer (Ocampo et al., 2006). Wetlands can physically slow the movement of N to streams, and their biogeochemical conditions can promote denitrification and remove N from local ecosystem cycling (Jordan et al., 2011; Saunders and Kalff, 2001). In contrast, impervious cover and drainage systems in urban and agricultural landscapes promote rapid movement of N to streams, bypassing biogeochemical processes that limit the movement of N to streams (Hale et al., 2015; McIsaac and Hu, 2004).
Once in the stream network, N can be processed and removed, with implications for N export (Alexander et al., 2009; Preston et al., 2011). Small streams have been found to efficiently remove N via denitrification in the benthos and biotic uptake, reducing N transport downstream (Mulholland et al., 2008). Lakes and reservoirs in stream networks also can be important N sinks via autochthonous uptake, denitrification, and burial (Harrison et al., 2009). Because headwater and low order streams comprise the majority of the drained landscape, these systems can receive and retain the majority of N inputs (Alexander et al., 2009; Mulholland et al., 2008; Wollheim et al., 2006). However, as N inputs to the stream network increase and N retention mechanisms in small streams become saturated, more N is transported downstream where N removal can continue until these stream reaches, in turn, become saturated (Mulholland et al., 2008; Wollheim et al., 2006). Consequently, N inputs from the landscape could have a more direct effect on stream N concentrations in smaller streams than in larger streams and rivers, which reflect cumulative N retention and removal throughout the upstream network.
The importance of in-stream processes relative to terrestrial drivers for controlling N concentrations has been debated (Bernhardt et al., 2005; Brookshire et al., 2009; Scanlon et al., 2010). While gross N uptake can be rapid, particularly in small streams, the capacity for net N storage in streams and rivers is typically small, and the timescale over which N uptake is balanced with release can range from instantaneous to seasonal or longer (Bernal et al., 2012; Brookshire et al., 2009; von Schiller et al., 2015). Clarifying the relative roles of terrestrial and aquatic drivers would help in identify and implement management approaches to control stream N concentrations.
The U.S. Environmental Protection Agency’s (EPA) National Rivers and Streams Assessment (NRSA) conducted in 2008-2009 included nearly 2,000 perennial stream and river sampling sites with a wide range of watershed characteristics in terms of size, land use and land cover, and climate. These data provide a unique opportunity to explore terrestrial and aquatic controls on N concentrations in streams and rivers across the nation under summer low flow conditions. In this study, we use NRSA stream N concentration, spatially explicit estimates of anthropogenic N inputs to the landscape, and stream and watershed attributes, to address the following questions: 1) What is the range of N inputs and stream concentrations across a statistical representation of U.S. watersheds? 2) How much does variation in N inputs and sources influence stream N concentrations and forms? And 3) How do landscape characteristics relative to stream network characteristics and climate variables influence spatial patterns of N concentrations across the conterminous U.S.?
METHODS
Sampling sites
The EPA sampled 2,123 stream and river sites across the conterminous U.S. as part of the 2008-2009 NRSA. Of these, we included 1,966 sites with complete data availability. Sampling occurred during summer low flow conditions from June 2008 through November 2009, with 71% of samples taken during June through August of those years (United States Environmental Protection Agency, 2016a). Sites were selected using a spatially-balanced, probability-based sampling design to provide statistically representative assessments of the physical, chemical, and biological conditions of U.S. streams and rivers (United States Environmental Protection Agency, 2016b). Based on this sampling approach, sites have associated weights corresponding to the length of stream they represent, so that weighted summary statistics accurately represent the conditions of streams across the conterminous U.S. that are represented by the sampled sites (e.g. perennial, accessible) (United States Environmental Protection Agency, 2016b). We used these weights to summarize N inputs and N concentrations. Watersheds for sampling sites range in size from less than 1 km2 to over 3 million km2 near the mouth of the Mississippi River (median watershed size 577 km2), and their drainage areas cover the majority of the conterminous U.S.
Stream sampling and chemical analysis
Stream reaches were established at each site to include a variety of habitat types (riffles, pools, bends, etc.), with reach lengths approximately 40 times as long as stream widths (United States Environmental Protection Agency, 2016a). Stream water was sampled from the mid-point of the stream reach and analyzed for TN, nitrate, and ammonium according to EPA protocols (United States Environmental Protection Agency, 2010). Assuming that the difference between TN and DIN was largely organic N, we subtracted dissolved inorganic N (DIN) - the sum of nitrate () and ammonium () - from TN to calculate total organic N (TON). We recognize that many studies report dissolved organic N (DON), but the methods used in NRSA prevented us from calculating DON concentrations, which limits our ability to directly compare our data to other studies that report DON. Samples with measured concentration values below detection limits, were assigned values of half the detection limit (analytical detection limits were 0.03 mg N L−1 for TN, 0.01 mg N L−1 for , and 0.005 mg N L−1 for ). Sites with measured DIN greater than TN, which indicates an analytical problem, were excluded from analyses (n=66, or 3% of sites). A subset of sites (8.8%) included in this analysis were re-visited within the same year to assess variation within a site during the sampling period as an indicator of noise in our analysis. (see Uncertainty and Independence section below). However, for modeling, we only used data collected during the first visits. No effect of sampling month was found for concentration across all data (results not shown), suggesting the use of first or second visit should not affect results.
Watershed N inputs
For each sample site, we estimated N loading to the upstream watershed, which was delineated using the approach of the StreamCat Dataset (Hill et al., 2016) (see Supplemental Information S1). This approach summarized N inputs for each upstream watershed for each sample site. N inputs consisted of conterminous U.S.-wide geospatial estimates of synthetic fertilizer, cultivated biological N fixation, manure, atmospheric N deposition, and point sources (Maupin and Ivahnenko, 2011; United States Environmental Protection Agency, 2006a, 2006b, 2006c, 2006d). Atmospheric deposition of oxidized N, synthetic fertilizer, and cultivated biological N fixation represent newly created anthropogenic N, while manure application, atmospheric deposition of reduced N (i.e., ammonia), and point sources represent a re-location and concentration of previously produced N (Sobota et al., 2013). County-level estimates of synthetic fertilizer purchases, recoverable manure from concentrated animal feeding operations, and cultivated biological N fixation were uniformly applied to agricultural land area within each county [2006 National Land Cover Database (NLCD) row crop, hay, and pasture land cover classes; (Fry et al., 2011)]. Atmospheric deposition of reactive N, which includes agricultural and industrial sources, was total N deposition (wet + dry, organic + inorganic) estimated for the nation by the Community Multiscale Air Quality Model at a 12 by 12 km grid resolution for 2006 (United States Environmental Protection Agency, 2006d). We obtained point source N inputs from the EPA’s National Pollutant Discharge Elimination System (NPDES) (Maupin and Ivahnenko, 2011); we used N loads estimated for the latest year available (1992, 1997 or 2002), and only included those sources classified as “major” (discharging more than 1 million gallons per day) in the analysis because of uneven reporting associated with “minor” sources across states. We calculated areal input rates to the entire watershed upstream of the sampling point by dividing point source loads by watershed area.
Land cover, climate, and stream characteristics
We characterized land cover for the whole watershed and for a 100-m riparian buffer upstream of each sample site based on 2006 NLCD data. Land cover categories for the whole watershed included percent forest (total of coniferous, deciduous, and mixed), percent wetland (total of herbaceous and woody), and percent impervious man-made surfaces. Land cover categories for the riparian buffer included the former, as well as percent riparian disturbance, which we defined as the sum of percent row crop and high, medium, and low intensity urban development. To better delineate the separation between stream and riparian land, and aid in riparian land cover estimates, we combined NLCD water categories conterminous to the National Hydrography Dataset Plus version 2 (NHDPlusV2). Because agricultural land cover was highly correlated with total N inputs (r2 = 0.81), and because N inputs are mechanistically more directly linked to stream N concentrations, agricultural land cover was not also included as a predictor variable.
Additionally, we characterized climate, stream network, and reach-related variables. Climate variables included long-term (1981-2010) mean annual temperature and precipitation for each watershed (PRISM Climate Group). Long-term climate variables were included to reflect potentially important soil or vegetation characteristics that may affect N concentrations, not as proxies for more recent weather (e.g. drought) that can affect concentrations over the short-term. Stream network and reach-related variables included percent open water in the watershed (based on NLCD 2006 as above), annual mean discharge at the sampling site from the NHDPlusV2 (McKay et al., 2012) as a proxy for stream size, and geometric mean substrate size measured in the sampling reach at the time of sampling (United States Environmental Protection Agency, 2016b).
Relationships between stream N concentration and watershed characteristics
We used simple linear regression to examine the relationships between total anthropogenic N inputs and TN, DIN, and TON. To assess the additional effects of land cover, climate, and stream network and reach characteristics, we used multiple linear regression. Previous studies have found that transfer efficiency varies depending on N source [e.g. distributed atmospheric deposition versus direct point source inputs; (Preston et al., 2011)], so N inputs were separated into three predictor variables: agricultural N (sum of synthetic fertilizer, manure, and cultivated biological N fixation), atmospheric N deposition, and point source N. All land cover variables, climate variables, and stream network and reach variables that were described above were included in multiple regression models. Because the effect of N inputs on stream N concentration may vary depending on land cover in the watershed (Sudduth et al., 2013), we included the following interaction terms: agricultural N * percent wetland area, agricultural N * percent riparian disturbance, atmospheric N deposition * impervious cover, and atmospheric N deposition * forest cover. We log-transformed variables where necessary to meet assumptions of linearity and normality (Table 1), and standardized variables (mean = 0, standard deviation = 1) prior to analysis so that coefficients could be interpreted as relative importance. A stepwise approach (forward and backward) was used to select the most parsimonious model based on AIC (all models with AIC values within 2 of the lowest value are included in the Supplemental Information Tables S1-3). Partial r2 values reflect the average increase in model r2 with the addition of that variable, across all variable order permutations (Lindeman et al., 1980). We performed all statistical analyses in R (R Core Team, 2016).
Table 1.
Summary statistics for watershed variables associated with sampling sites used in multiple regression models. Any transformations made for regression analyses are noted. Site weights were used to estimate summary statistics for TN and total N inputs for the conterminous U.S.
| 25th | 75th | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Units | Transformation | Min | percentile | Median | Mean | percentile | max |
| TN | mg N L−1 | ln | 0.015 | 0.29 | 0.59 | 1.19 | 1.21 | 34.45 |
| DIN | mg N L−1 | ln | 0.01 | 0.03 | 0.13 | 0.68 | 0.50 | 34.32 |
| TON | mg N L−1 | ln | 0.00 | 0.16 | 0.32 | 0.51 | 0.59 | 19.23 |
| Total N inputs | kg ha−1 y− | ln | 1.0 | 6.2 | 15.7 | 28.7 | 36.9 | 182.4 |
| Agricultural Na | kg ha−1 y−1 | ln | 0.0 | 0.8 | 6.4 | 20.9 | 27.4 | 171.4 |
| Atmospheric N deposition | kg ha−1 y−1 | ln | 0.0 | 3.9 | 8.0 | 7.5 | 10.3 | 24.6 |
| Point Source N | kg ha−1 y−1 | ln | 0.0 | 0.0 | 0.0 | 0.3 | 0.03 | 80.7 |
| % Forest | % | -- | 0.0 | 10.7 | 38.4 | 40.0 | 65.9 | 100.0 |
| % Wetland | % | ln | 0.0 | 0.1 | 1.0 | 4.5 | 4.0 | 95.2 |
| % Riparian disturbance | % | ln | 0.0 | 0.3 | 3.2 | 13.0 | 16.5 | 100.0 |
| % Impervious cover | % | ln | 0.0 | 0.2 | 0.4 | 1.2 | 1.1 | 52.1 |
| % Open water | % | ln | 0.0 | 0.02 | 0.3 | 1.0 | 1.0 | 21. 5 |
| Mean annual discharge | cfs | ln | 0.0 | 0.25 | 1.9 | 251 | 25 | 42700 |
| Mean substrate size | mm | log(10) | 0.008 | 0.21 | 0.89 | 67 | 15 | 5657 |
| Mean annual precipitation | mm | -- | 222 | 610 | 961 | 938 | 1194 | 4376 |
| Mean annual air | °C | -- | -1.1 | 6.4 | 9.4 | 10.0 | 13.4 | 22.7 |
| temperature | ||||||||
| Weighted summary statistics |
||||||||
| TN | mg N L−1 | 0.015 | 0.24 | 0.52 | 1.37 | 1.27 | 34.45 | |
| Total N inputs | kg ha−1 y− | 1.0 | 9.0 | 18.0 | 36.5 | 46.5 | 182.4 | |
Agricultural N is the sum of fertilizer, cultivated biological N fixation, and manure N inputs.
Riparian land cover has been found to better predict stream chemistry than whole-watershed land cover in some cases (Floyd et al., 2009; Gove et al., 2001) but not in others (Compton et al., 2003), so we also modeled TN, DIN, and TON using land cover values (percent forest, wetland, and impervious surface) estimated for the 100-m riparian buffer and N inputs to the riparian buffer in place of whole watershed values. N input rates to the buffer were assumed to be the same for atmospheric deposition and point source inputs, while agricultural N inputs were adjusted by percent row crop in the 100-m riparian buffer relative to the total watershed.
Uncertainty and independence
N concentrations can be highly variable over daily to seasonal timescales within streams (Pellerin et al., 2012), and we expect that some variability in N concentrations across the nation cannot be explained by static predictor variables alone. Data from sites with multiple visits provide insight into the amount of variability that might be due to temporal variability during the sampling period, and the amount of variability that may be attributed to spatial factors. We find that the variability across sites relative to the variability within sites – the signal-to-noise ratio (Olson and Hawkins, 2013) – is 8.8 for TN, 7.9 for DIN, and 4.9 for TON. Based on this signal-to-noise ratio, the maximum r2 values that we can expect from regression analyses are 0.90, 0.89, and 0.83 (Olson and Hawkins, 2013) (see Supplemental Information S2 for calculation). Thus, variability in N concentrations measured in this study likely largely reflect variability in spatial drivers rather than temporal drivers.
Additionally, stream nutrient concentration measurements from sites nested within watersheds are not truly independent because the sampling sites share drainage areas and stream flow. However, if the model sufficiently accounts for spatial factors that control the variability in nutrient concentrations, the model residuals will not be spatially autocorrelated and coefficients should not be biased (Kuhn and Dormann, 2012). We assessed whether model residuals were spatially autocorrelated based on flow distance and degree of watershed overlap. We found some residual spatial autocorrelation (Fig. S1), but a subsequent comparison between results of the multiple regression models based on the entire data set and models based on boot-strapped samples of truly independent watersheds, indicated that the coefficients were not strongly affected by the lack of independence (Fig. S2-4). Therefore, we report results of models based on all available sites. A more detailed description of these analyses and results can be found in the Supplemental Information (S3-S5).
RESULTS AND DISCUSSION
N concentration patterns
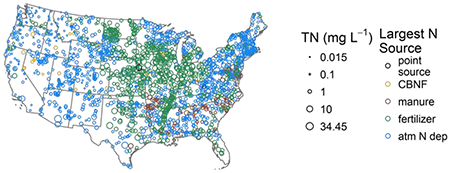
TN concentrations during summer low flow conditions ranged from below the detection limit (0.03) to 34.5 mg N L−1 (Table 1). Using the probabilistic weights, we estimated that 51% (974,121 km) of U.S. perennial U.S. stream length had TN greater than 0.5 mg N L−1 and 31% (592,113 km) had TN greater than 1.0 mg N L−1. Just 1% of stream length (19,100 km) was estimated to have concentrations above 10 mg N L−1, the EPA drinking water standard. However, undesirable ecological changes can occur at concentrations much lower than this human health standard; a recent review reported ecologically-relevant thresholds ranging from 0.27 to 1.72 mg N L−1 (Evans-White et al., 2013), values met or exceeded in an estimated 72% (1,375,230 km) and 18% (343,808 km) of streams, respectively. On average, DIN comprised 44% of TN, but ranged from less than 1% up to 100% of measured TN. The highest TN concentrations were observed in the Mississippi River valley and the lowest in the West (Fig. 1); however, considerable variability existed within ecoregions (Omernik et al., 2015).
Figure 1.
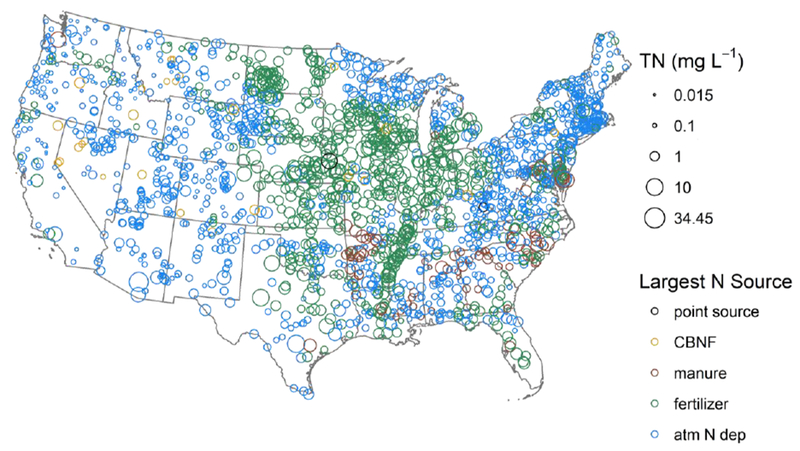
TN concentrations at NRSA sampling locations (n=1966). The color corresponds to the single largest N source to the watershed. CBNF is Cultivated biological N fixation.
N loading drives N concentrations
Across all watersheds associated with sampling sites, N inputs ranged from 1 to 182 kg N ha−1y−1 (Table 1, Fig. 2). The median total N input rate to these watersheds was 15.7 kg N ha−1y−1, higher than most empirically-derived critical loads for response of aquatic ecosystems to N deposition to watersheds within the U.S. (Baron et al., 2011; Pardo et al., 2011). For all sites with atmospheric deposition as the largest source of the five sources included in this analysis, the 25th percentile, median, and 75th percentile of inputs were just 4.0, 8.0, and 15 kg N ha−1 y−1, compared to 27, 46, and 80 kg N ha−1 y−1 for sites with synthetic fertilizer inputs as the single largest source. Although agriculture-related inputs dominated sites with the highest N input rates (Fig. 3), atmospheric deposition was the single largest N source in 58% of sampled sites, accounted for over half of inputs in 49% of watersheds, and was by far the dominant source of N to watersheds in the western U.S. (Fig. 2). Compared to agriculture-related inputs and atmospheric deposition, point sources represented a small contribution to total N inputs in most watersheds; only 28% of sampled watersheds had major recorded point sources; of these, the median areal input rate was only 0.28 kg N ha−1 y−1 (Table 1). Although point source input rates were low on an areal basis, they represent direct inputs to aquatic systems and can have important local and downstream effects on nutrient concentrations, whereas the other inputs can be attenuated before reaching aquatic systems. Additionally, potentially important episodic events, such as combined sewer overflows, are not well captured by the annual point source input rates used in this study. Of the sites included in our study, 42% were identified as in poor condition with respect to TN concentration in the 2008-2009 NRSA report (United States Environmental Protection Agency, 2016a); of the sites in poor condition, 51% of their watersheds were dominated by (single largest source was) fertilizer inputs, 40% by atmospheric deposition, 7% by manure, 2% by cultivated biological N fixation, and less than 1% by point sources.
Figure 2.
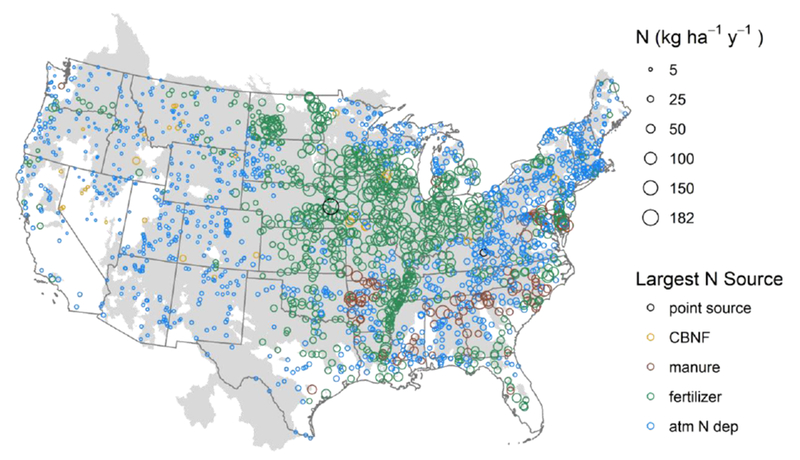
N inputs to study watersheds. Top: Bubbles are located at sampling sites and sizes indicate input rates to the watershed. Colors indicate which of the 5 sources was the largest contributor to each watershed (CBNF = cultivated biological N fixation). The gray shaded area indicates the watershed areas draining to sampling locations (For some small watersheds, the site bubble is larger than the watershed outline).
Figure 3.
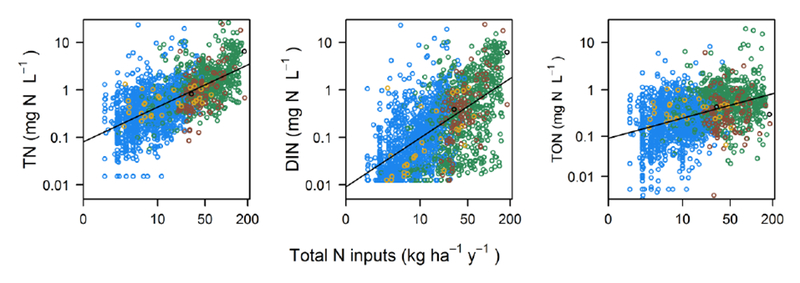
TN, DIN and TON concentration vs total watershed N input. Colors indicate the dominant (single largest) N source associated with each watershed: atmospheric deposition (blue), synthetic fertilizer (green), cultivated biological N fixation (gold), manure (brown), point source (black). Lines are linear regression fits (p < 0.001 for all three models, r2 = 0.42, 0.38, 0.17 for TN, DIN, and TON, respectively, n = 1966 sampling points). N input categories with fewer sites (cultivated biological N fixation, manure, point source) are plotted on top for better visibility
The terrestrial N saturation hypothesis predicts increasing soil inorganic N concentrations and export to surface water as N inputs exceed terrestrial biological N demand (Aber et al., 1998). Our study strongly supports this hypothesis as total N input to the watershed was a major driver of TN and DIN concentrations in stream water during summer low flow nationally (Fig. 3). Simple linear regression showed that total N input was positively and significantly correlated with stream N concentration for all forms of N, but TN had the strongest relationship with N inputs (r2 = 0.42), while total organic N (TON) had the weakest relationship (r2 = 0.17) (Fig. 3). However, the effect (slope) was largest for DIN, followed by TN and then TON (non-overlapping 95% confidence intervals around regression coefficients) (Fig. 3). Generally, sites with higher concentrations of all forms of N were dominated by agricultural N inputs, but sites dominated by atmospheric deposition also exhibited a wide range of N concentrations (0.015 to 23.2 mg N L−1) (Fig. 3). In the multiple regression models, N inputs collectively explained more variability in DIN than any other category of predictor variables, and N inputs explained nearly as much variability in TN as all land cover variables combined (Table 2, Fig. 4). In particular, agricultural N inputs had the largest partial r2 values of any of the predictor variables for TN and DIN (Table 2). Along with regional-scale studies that have identified the relationship between watershed N inputs and stream N concentrations (Aber et al., 2003; Lerch et al., 2015; Sobota et al., 2009), our study provides strong evidence that N inputs control stream DIN and TN concentrations during summer low flow conditions.
Table 2.
Variables and the direction of their effect, partial correlation and significance level in multiple regression models of TN, DIN, and TON. Variables are listed in order of decreasing standardized coefficient size (relative importance in terms of effect size on N concentration) for each model.
| TN model | DIN model | TON model | ||||||
|---|---|---|---|---|---|---|---|---|
| Variable | sign | partial r2 | Variable | sign | partial r2 | Variable | sign | partial r2 |
| Agricultural N | + | 0.16 *** | Impervious | + | 0.06 *** | Precipitation | − | 0.06 *** |
| Forest | − | 0.12 *** | Agricultural N | + | 0.14 *** | Forest | − | 0.13 *** |
| Impervious | + | 0.04 *** | Atmospheric N | + | 0.10 *** | Substrate size | − | 0.06 *** |
| Precipitation | − | 0.03 *** | Point N | + | 0.03 *** | Riparian disturbance | + | 0.06 *** |
| Atmospheric N | + | 0.07 *** | Atm N X Impervious | − | <0.01 *** | Wetland | + | 0.04 *** |
| Point N | + | 0.02 *** | Ag N X Riparian disturbance | + | 0.01 *** | Agricultural N | + | 0.08 *** |
| Ag N X Wetland | − | 0.01 *** | Wetland | − | <0.01 *** | Open Water | + | 0.01 *** |
| Forest | − | 0.02 * | Ag N X Wetland | − | 0.01 *** | |||
| Riparian disturbance | + | 0.11 ** | Ag N X Wetland | − | <0.01 *** | Ag N X Riparian disturbance | − | 0.01 *** |
| Atm N X Impervious | − | <0.01 ** | Open Water | − | <0.01 ** | Temperature | + | <0.01 ** |
| Substrate size | − | 0.03 *** | Temperature | − | 0.01 . | Atm N X Forest | + | <0.01 ** |
| Discharge | − | <0.01 ** | Precipitation | − | <0.01 | Atmospheric N | + | 0.02 . |
| Ag N X Riparian | ||||||||
| disturbance | + | <0.01 * | Riparian disturbance | − | 0.09 | Discharge | − | <0.01 . |
| Wetland | + | 0.01 . | Point N | + | <0.01 | |||
| Atm N X Forest | + | <0.01 . | ||||||
| Model adjusted r2 | 0.60 | 0.47 | 0.48 | |||||
| Model p | <2.2e-16 *** | <2.2e-16 *** | <2.2e-16 *** | |||||
p < 0.001
p < 0.01
p < 0.05
p < 0.10
Figure 4.
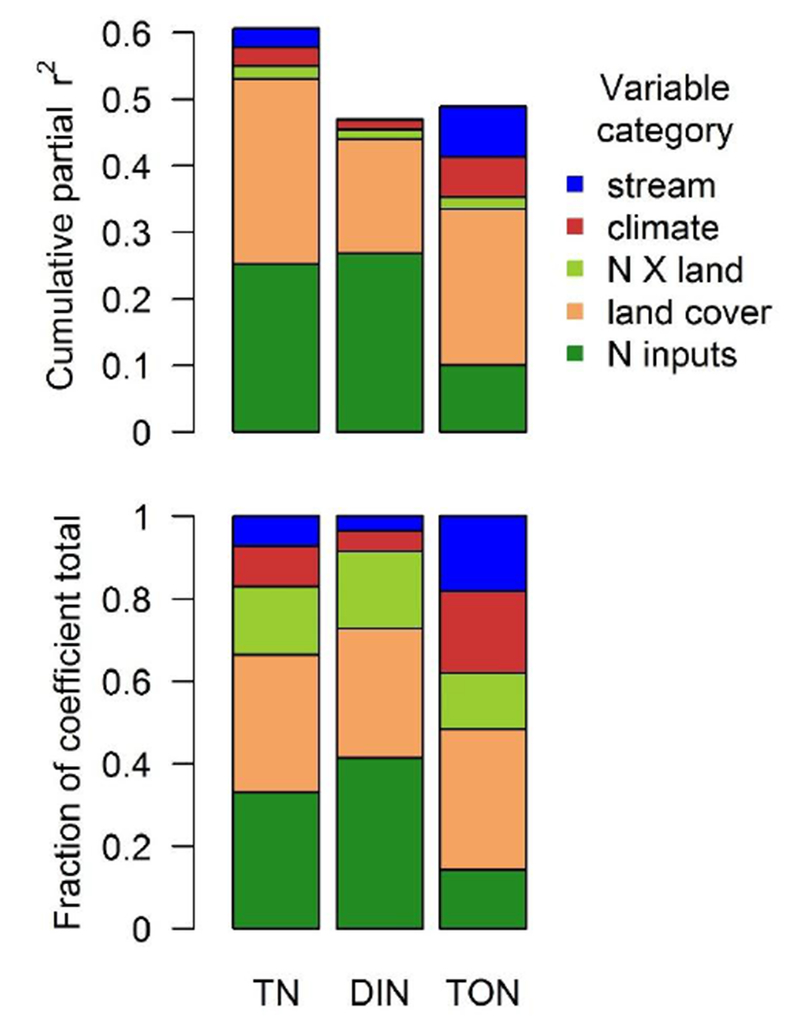
Top: Cumulative partial r2 values for each category of predictor variables. The total bar reflects the model r2. Bottom: Fraction of the total sum of standardized coefficients (absolute values) corresponding to each category of predictor variables. Standardized coefficients can be interpreted as relative effect size on the dependent variable. Variable categories are: stream (average annual discharge, % lake cover, mean substrate size), climate (mean annual temperature and precipitation), land cover (forest, wetland and impervious percent cover and riparian disturbance percent) N inputs (agricultural N, atmospheric deposition N, and point source N), and “N X land” corresponds to the effects of interactions between N inputs and land cover (agricultural N x wetland cover, agricultural N x riparian disturbance, atmospheric N x forest cover, and atmospheric N x impervious cover).
Compared with TN and DIN, TON responded weakly, although positively, to N inputs (Fig. 3). N inputs explained relatively little of the variability in TON, and point source N was not a significant predictor of TON (Fig. 4, Table 2). Some of dissolved organic N is thought to “leak” from terrestrial to aquatic ecosystems regardless of N saturation status (Perakis and Hedin, 2002), and most riverine DON originates from natural sources (Harrison et al., 2005b). Indeed, we found that TON was positively correlated with the proportion of wetlands in the watershed (Table 2). However, previous studies have found that, like DIN, dissolved organic N also increases with watershed N loading from atmospheric deposition (Aber et al., 2003; Brookshire et al., 2007; Pellerin et al., 2006), N fixation (Compton et al., 2003), and agricultural inputs (Sobota et al., 2009). Anthropogenic N inputs that contain organic N (e.g. manure, urea-based synthetic fertilizer, sewage waste water, organic N deposition) may contribute directly to elevated TON concentrations (Glibert et al., 2005; Heathwaite et al., 1998; Martí et al., 2004). Excess N inputs may also increase stream organic N concentrations via multiple indirect pathways, such as declining C:N of foliage, soil organic matter, and dissolved organic matter (Aber et al., 2003; Brookshire et al., 2007) and stimulating algal production (Sobota et al., 2012), but the relative importance of these mechanisms for controlling organic N concentrations is not well understood. Across the U.S., total organic N increases with N inputs (Table 2, Fig. 3), indicating that a component of organic N release is susceptible to increased loading.
Influence of landscape characteristics and climate on N sinks and transformations
Reactive N inputs to the terrestrial landscape can cycle through multiple pools and undergo many transformations, including denitrification, which removes N from local ecosystem cycling, before reaching surface water (Galloway et al., 2003). Landscape characteristics – such as vegetation cover and wetland soils – can promote N retention and removal (Groffman et al., 2004; Seitzinger et al., 2006). In particular, riparian vegetation and wetlands have been found to attenuate N loading to surface waters (Jordan et al., 2011; Mayer et al., 2007). Therefore, we expected these land cover characteristics would be associated with lower N concentrations, while other land cover characteristics that limit N processing and retention, particularly impervious cover, would be associated with higher N concentrations. We found that the expected effects of forest cover, wetland cover, and impervious cover on N concentrations were apparent in our national-scale analysis, and that land cover characteristics explained variability in N concentration that N inputs alone could not (Fig. 4, Table 2).
Forest cover was negatively related to concentrations of all forms of N. Forests could serve as a sink for N inputs in areas where deposition is the dominant N form. Forest composition, time since disturbance, and N saturation stage can affect the strength of forest land as an N sink, as well as the dominant form of N exported (Binkley et al., 2004; Lovett et al., 2000; Vitousek and Reiners, 1975). Because these factors were not differentiated in our models, the relationships observed in this study may simply reflect organic matter accumulation (and N retention) in many forested areas in the U.S. (Joyce et al., 2014). The observed relationship may also be due to limited erosion from forested land compared to disturbed land cover types (Corbett et al., 1997; Diaz-Ramirez et al., 2008), or forested watersheds having low rates of N export as compared to agricultural or urban land, the coverage of which are inversely related to forest cover. Regardless of the exact mechanism(s), higher forest cover in the U.S. was strongly associated with lower N concentrations during summer low flow conditions.
Wetland cover was negatively related to TN and DIN but positively related TON, consistent with empirical findings that wetlands can act as local N sinks, especially for inorganic N (Jordan et al., 2011), but can be sources of organic N at the landscape scale (Pellerin et al., 2004). Wetlands have the potential to retain N inputs to upslope agricultural and urban areas of the landscape if they are hydrologically connected. In fact, wetland cover interacted with agricultural N loading (Table 2), dampening the effect of N inputs on TN and DIN concentrations (Fig. 5). Modeled TN and DIN concentrations increased markedly less in response to agricultural N inputs assuming just a small increase wetland coverage (Fig. 5). We were not able to address within-watershed spatial relationships between agricultural N inputs and wetlands. It follows, then, that if strategically placed to intercept N-rich drainage water, wetlands may have an even stronger effect on DIN concentration than what was estimated in this model (Gardner et al., 2011; Johnson et al., 2013). These results indicate that wetlands are reducing TN and DIN to the nation’s waterways, and increasing wetland cover may be a useful approach to reduce stream DIN and TN concentrations nationally.
Figure 5.
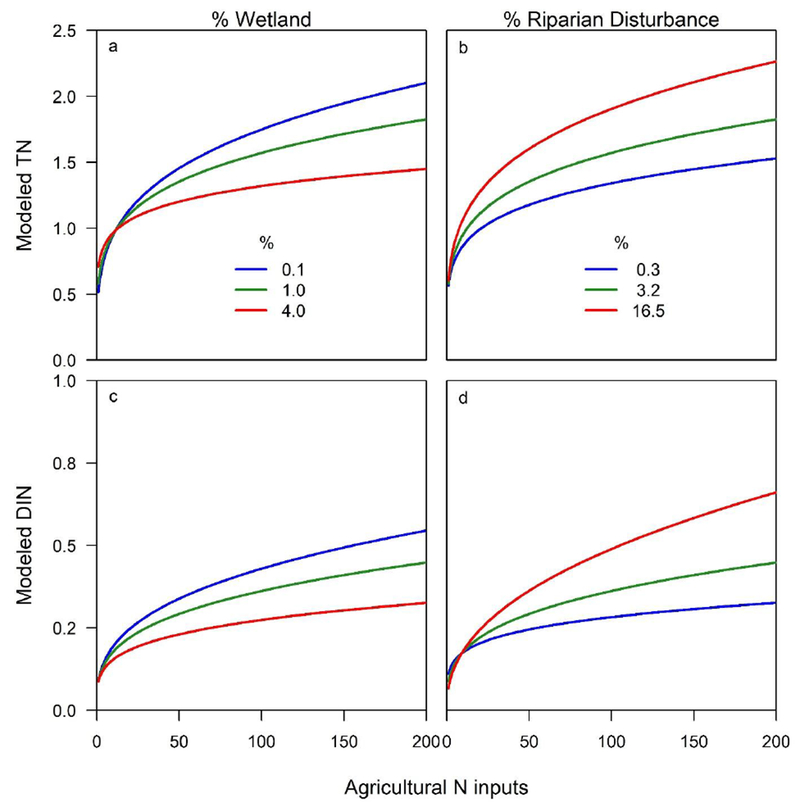
Estimated response of TN (a and b) and DIN (c and d) in mg N L−1 to increasing agricultural inputs (kg N ha−1 y−1), across 3 levels of wetland cover (a and c) and riparian disturbance (b and d) based on multiple regression model coefficients (Table 2). The low, medium, and high values correspond to the 1st, 2nd, and 3rd quartiles of wetland or riparian cover in the data set, while all other variables are set to their median values (Table 1). Agricultural N is the sum of fertilizer, cultivated biological N fixation, and manure N inputs.
Climate factors were much less important than inputs and landscape sinks in explaining variations in N concentrations. The only important climate predictor was mean annual precipitation, which was significantly and negatively related to TON and TN concentrations. Regional studies report conflicting results with respect to the relationship between annual precipitation and TON and DON concentrations across watersheds [positive: (Perakis and Hedin, 2007; Vanderbilt et al., 2002); negative: (Sobota et al., 2009; Sponseller et al., 2014)]. Precipitation may directly affect concentrations through dilution, as suggested by Sponseller et al. (2014), or it may be a proxy variable for atmospheric N inputs, soil type or depth, vegetation, or other watershed characteristics that affect organic N production and/or delivery (Perakis and Hedin, 2007; Vanderbilt et al., 2002). Dodds et al. (2015) suggested that streams in regions with lower precipitation are likely to exhibit higher sediment transport and have more open riparian canopies, allowing for higher in-stream autochthonous production, both of which could lead to higher organic N concentrations. Precipitation was moderately and positively correlated with both percent open canopy at the sampling reach (r2 = 0.37, p < 0.001) and total suspended solids (r2 = 0.10, p < 0.001) in this data set, suggesting these factors may be more proximate causes of high TON concentration in low-precipitation watersheds. Although the mechanistic relationship between precipitation and TON concentration is not clear, this climate driver appears to regulate summer low flow TON concentrations across regions.
Riparian influence
The effects of near-stream N inputs and riparian land cover may be particularly important during summer low-flow conditions, which were the focus of this study. During low flow periods, streams have limited hydrologic connectivity with upland areas (Gardner and McGlynn, 2009; Jencso et al., 2009), and longer water residence times in riparian soils mean that plant uptake and denitrification can deplete N concentrations in water draining to streams (Gu et al., 2007; Ocampo et al., 2006). Despite these expectations, we found that using land cover characteristics of the riparian buffer – as opposed to the entire watershed – as predictor variables did not produce better model results. Notably, using whole-watershed predictor variables resulted in similar or slightly higher model r2 values compared to riparian buffer predictor variables, with r2 = 0.60, 0.47, and 0.48 for TN, DIN, and TON, respectively, for the whole-watershed model, and 0.59, 0.45, and 0.49 for TN, DIN, and TON, respectively, for the riparian buffer model (Tables S4-6). This similarity may be due to the high correlation between whole-watershed and 100-m riparian buffer land cover variables in study watersheds; correlations between the two for percent forest cover, percent wetland, percent impervious cover, and farm N inputs had r2 values of 0.88, 0.89, 0.79, and 0.96, respectively [variables were transformed as in the multiple regression models (Table 1)].
Although riparian land cover variables did not explain more variability in N concentrations than whole-watershed land cover variables, riparian disturbance, which was included as a predictor variable in the model with other whole-watershed land cover characteristics, was a significant predictor of N concentrations and exacerbated the effects of agricultural N inputs on TN and DIN (Fig. 5). These results are consistent with studies that have found that vegetated riparian buffers can limit the delivery of dissolved inorganic N to streams (Mayer et al., 2007; Weller et al., 2011). Previous research has found that the width of the riparian buffer that most influences water quality varies depending on season, riparian buffer characteristics, and the water quality parameter of interest (Floyd et al., 2009; Gove et al., 2001), and headwater riparian buffers may affect water quality more than downstream buffers (Christensen et al., 2013). This context-dependent effect of riparian buffers on water quality, as well as uncertainty about land cover within the riparian buffer, may explain why the relationship between stream N concentrations and characteristics of the 100-m riparian buffer was not stronger than for whole watershed characteristics. However, our results do suggest that N inputs and land management throughout the watershed – not just in riparian zones – regulates stream N concentrations.
In-stream effects
Terrestrial and riparian N dynamics control the delivery of N to surface water, but N retention and removal in the stream network itself can also affect aquatic N concentrations (Alexander et al., 2009; Bernal et al., 2012; Hensley et al., 2014). However, two variables that reflect characteristics of the stream and its network thought to be important for controlling N uptake – stream size and lakes and reservoirs in the network – had very weak or no relationships with N concentrations (Table 2). Modeled annual mean discharge from NHDPlusV2 was negatively, although weakly, related to TN concentration when N inputs and landscape drivers are taken into account, which we predicted, assuming most N uptake occurs in small streams (Table 2); however, it had no significant relationship with either DIN or TON concentration. Additionally, percent open water, which reflects the extent of lakes and reservoirs in watersheds, was only weakly related to DIN and TON, and had opposite effects on the different forms of N (Table 2), perhaps due to the conversion of inorganic N to organic N via autochthonous uptake. Together, these results suggest the effects of stream size and the extent of lakes and reservoirs in the watershed play a relatively small role in controlling the spatial variability of N concentrations nationally compared to N inputs and terrestrial characteristics (Table 2). This finding supports other reports that instream retention and removal is most efficient when concentrations are lowest, but has a muted effect on high concentrations. As a result, when terrestrial inputs vary widely, they largely control spatial variability in concentrations (Chiwa et al., 2015; Lin et al., 2015; Scanlon et al., 2010).
There was considerable variability in N concentrations not explained by N inputs and static landscape and stream network variables (40-53%; Table 2) that may be related to temporally variable in-stream dynamics or local in-stream factors. The signal-to-noise ratio (spatial variance relative to within-site variance over time) indicates that 10-17% of the overall variance in observed N concentrations could be attributed to temporal variability within the sampling period. Hydrologic conditions, disturbance history, and net heterotrophy or autotrophy, can all affect in-stream N uptake (Bernal et al., 2012; Brookshire et al., 2009; von Schiller et al., 2015) but are not reported as part of the NRSA. Additionally, we were not able to capture hyporheic exchange dynamics in this study, which recent research suggests may be more important than simply stream size for controlling in-stream N processing (Gomez-Velez and Harvey, 2014; Ye et al., 2012). Larger substrate size may promote hyporheic exchange and provide more stable substrate for the microbial community (Atkinson et al., 2008; Packman and Salehin, 2003), both of which can enhance local N uptake (Warren et al., 2013; Zarnetske et al., 2011). However, there was no detectable effect of local substrate size on DIN (Table 2). Instead, substrate size was negatively related to TON and TN (Table 2), perhaps because decreasing substrate size reflects erosion and higher concentrations of suspended solids. More detailed sampling would be required to assess the importance of these local, reach-level factors and temporal dynamics for controlling the spatial variability in N concentrations observed in this study.
CONCLUSIONS
We found that total anthropogenic N inputs to study watersheds spanned two orders of magnitude, from 1 to nearly 200 kg N ha−1 y−1. Agricultural sources were associated with the highest input rates, but atmospheric N deposition was the single largest source in nearly half of watersheds. We found that N input to the watershed was the best predictor of summer low flow N concentrations – particularly TN and DIN – in streams and rivers across the U.S., with agricultural sources being the best predictor of TN and DIN. Importantly, we found that the potential influences of N inputs were either exacerbated or dampened by land cover types that can be directly influenced by land management actions – riparian disturbance and cover of wetlands. Additionally, land cover and management in the whole watershed – not just the riparian zone – was strongly correlated with stream N concentrations. We found that neither stream size nor the extent of lakes and reservoirs in watersheds had a strong effect on the spatial patterns of N concentrations across the U.S. The apparently limited role of temperature and precipitation drivers and N processing in the stream network compared to terrestrial drivers in predicting observed spatial patterns of N concentrations, suggests the need for management throughout the watershed – not just in headwater stream catchments or in riparian and wetland areas – to improve water quality nationally.
Supplementary Material
HIGHLIGHTS.
Summer N concentrations were predicted from N inputs to the landscape and land cover
Atmospheric deposition, agriculture, and sewage contribute to aquatic N loading
Climate and stream characteristics explained little spatial variability in stream N
At a national scale, nitrogen inputs drive concentrations, but interact with land cover
Aquatic nitrogen impairment may be mitigated by managing inputs and land cover
ACKNOWLEDGEMENTS
We would like to thank everyone involved in the National Rivers and Streams Assessment who contributed to the collection and curation of data. We appreciate helpful comments from Tony Olsen, Alan Herlihy, John Stoddard, John Harrison, and Jim Omernik. We also appreciate the comments from four anonymous reviewers, which improved the content and clarity of the manuscript. This work was supported by the National Research Council [grant number 83557701] and is supported by EPA’s Safe and Sustainable Water Research Program. The views expressed in this article are those of the author(s) and do not necessarily represent the views or policies of the U.S. Environmental Protection Agency.
Footnotes
Conflict of interest
None of the authors have any conflicts of interest, personal or financial, that could inappropriately influence their work.
WORKS CITED
- Aber J, McDowell W, Nadelhoffer K, Magill A, Berntson G, Kamakea M, McNulty S, Currie W, Rustad L, Fernandez I, 1998. Nitrogen saturation in temperate forest ecosystems. Bioscience 48, 921–934. [Google Scholar]
- Aber JD, Goodale CL, Ollinger SV, Smith M-L, Magill AH, Martin ME, Hallett RA, Stoddard JL, 2003. Is Nitrogen Deposition Altering the Nitrogen Status of Northeastern Forests? Bioscience 53, 375–389. 10.1641/0006-3568(2003)053[0357:NPITNU]2.0.CO;2 [DOI] [Google Scholar]
- Alexander RB, Johnes PJ, Boyer EW, Smith RA, 2002. A comparison of models for estimating the riverine export of nitrogen from large watersheds. Biogeochemistry 57-58, 295–339. 10.1023/A:1015752801818 [DOI] [Google Scholar]
- Alexander RB, Karl JB, Boyer EW, David MB, Harvey JW, Mulholland PJ, Seitzinger SP, Tobias CR, Tonitto C, Wollheim WM, 2009. Dynamic modeling of nitrogen losses in river networks unravels the coupled effects of hydrological and biogeochemical processes. Biogeochemistry 93, 91–116. 10.1007/s10533-008-9274-8 [DOI] [Google Scholar]
- Arheimer B, Andersson L, Lepisto A, 1996. Variation of nitrogen concentration in forest streams - influences of flow, seasonality and catchment characteristics. J. Hydrol. 179, 281–304. 10.1016/0022-1694(95)02831-5 [DOI] [Google Scholar]
- Atkinson BL, Grace MR, Hart BT, Vanderkruk KEN, 2008. Sediment instability affects the rate and location of primary production and respiration in a sand-bed stream. J. North Am. Benthol. Soc. 27, 581–592. 10.1899/07-143.1 [DOI] [Google Scholar]
- Baron JS, Driscoll CT, Stoddard JL, Richer EE, 2011. Empirical critical loads of atmospheric nitrogen deposition for nutrient enrichment and acidification of sensitive US lakes. Bioscience 61, 602–613. 10.1525/bio.2011.61.8.6 [DOI] [Google Scholar]
- Bernal S, von Schiller D, Martí E, Sabater F, 2012. In-stream net uptake regulates inorganic nitrogen export from catchments under base flow conditions. J. Geophys. Res 117, G00N05 10.1029/2012JG001985 [DOI] [Google Scholar]
- Bernhardt ES, Likens GE, Hall RO, Buso DC, Fisher SG, Burton TM, Meyer JL, McDowell WH, Mayer MS, Bowden WB, Findlay SEG, MacNeale KH, Stelzer RS, Lowe WH, 2005. Can’t See the Forest for the Stream? In-stream Processing and Terrestrial Nitrogen Exports. Bioscience 55, 219 10.1641/0006-3568(2005)055[0219:ACSTFF]2.0.CO;2 [DOI] [Google Scholar]
- Binkley D, Ice GG, Kaye J, Williams CA, 2004. Nitrogen and phosphorus concentrations in forest streams of the United States. J. Am. Water Resour. Assoc 40, 1277–1291. 10.1111/j.1752-1688.2004.tb01586.x [DOI] [Google Scholar]
- Brookshire ENJ, Valett HM, Gerber S, 2009. Maintenance of terrestrial nutrient loss signatures during in-stream transport. Ecology 90, 293–299. 10.1890/08-0949.1 [DOI] [PubMed] [Google Scholar]
- Brookshire ENJ, Valett HM, Thomas SA, Webster JR, 2007. Atmospheric N Deposition Increases Organic N Loss from Temperate Forests. Ecosystems 10, 252–262. 10.1007/s10021-007-9019-x [DOI] [Google Scholar]
- Camargo JA, Alonso Á, 2006. Ecological and toxicological effects of inorganic nitrogen pollution in aquatic ecosystems : A global assessment. Environ. Int 2, 8 1–849. 10.1016/j.envint.2006.05.002 [DOI] [PubMed] [Google Scholar]
- Chiwa M, Inoue S, Tashiro N, Ohgi D, Uehara Y, Shibata H, Kume A, 2015. Assessing the role of forests in mitigating eutrophication downstream of pasture during spring snowmelt. Hydrol. Process. 29, 615–623. 10.1002/hyp.10189 [DOI] [Google Scholar]
- Christensen JR, Nash MS, Neale A, 2013. Identifying Riparian Buffer Effects on Stream Nitrogen in Southeastern Coastal Plain Watersheds. Environ. Manage. 52, 1161–1176. 10.1007/s00267-013-0151-4 [DOI] [PubMed] [Google Scholar]
- Compton JE, Church MR, Larned ST, Hogsett WE, 2003. Nitrogen Export from Forested Watersheds in the Oregon Coast Range: The Role of N2-fixing Red Alder. Ecosystems 6, 773–785. 10.1007/s10021-002-0207-4 [DOI] [Google Scholar]
- Corbett CW, Wahl M, Porter DE, Edwards D, Moise C, 1997. Nonpoint source runoff modeling A comparison of a forested watershed and an urban watershed on the South Carolina coast. J. Exp. Mar. Bio. Ecol 213, 133–149. 10.1016/S0022-0981(97)00013-0 [DOI] [Google Scholar]
- Diaz-Ramirez JN, Alarcon VJ, Tagert ML, McAnally WH, Martin JL, O’Hara CG, 2008. Impacts of land use characterization in modeling hydrology and sediments for the Luxapilla Creek Watershed, Alabama and Mississippi. Trans. Am. Soc. Agric. Biol. Eng 51, 139–151. [Google Scholar]
- Dodds WK, 2003. Misuse of inorganic N and soluble reactive P concentrations to indicate nutrient status of surface waters. J. North Am. Benthol. Soc 22, 171–181. 10.2307/1467990 [DOI] [Google Scholar]
- Dodds WK, Gido K, Whiles MR, Daniels MD, Grudzinski BP, 2015. The stream biome gradient concept: factors controlling lotic systems across broad biogeographic scales. Freshw. Sci. 34, 1–19. 10.1086/679756 [DOI] [Google Scholar]
- Dodds WK, Smith VH, Zander B, 1997. Developing nutrient targets to control benthic chlorophyll levels in streams: A case study of the Clark Fork River. Water Res. 31, 1738–1750. 10.1016/S0043-1354(96)00389-2 [DOI] [Google Scholar]
- Dodds WK, Welch EB, 2000. Establishing nutrient criteria in streams. J. North Am. Benthol. Soc. 19, 186–196. 10.2307/1468291 [DOI] [Google Scholar]
- Dumont E, Harrison JA, Kroeze C, Bakker EJ, Seitzinger SP, 2005. Global distribution and sources of dissolved inorganic nitrogen export to the coastal zone: Results from a spatially explicit, global model. Global Biogeochem. Cycles 19, 1–14. 10.1029/2005GB002488 [DOI] [Google Scholar]
- Ensign S, Doyle M, 2006. Nutrient spiraling in streams and river networks. J. Geophys. Res … 111, G04009 10.1029/2005JG000114 [DOI] [Google Scholar]
- Evans-White MA, Haggard BE, Scott JT, 2013. A review of stream nutrient criteria development in the United States. J. Environ. Qual 42, 1002–1014. 10.2134/jeq2012.0491 [DOI] [PubMed] [Google Scholar]
- Floyd WC, Schoenholtz SH, Griffith S, Wigington PJJ, Steiner JJ, 2009. Nitrate-nitrogen, land use/land cover, and soil drainage associations at multiple spatial scales. J. Environ. Qual 38, 1473–1482. 10.2134/jeq2008.0099 [DOI] [PubMed] [Google Scholar]
- Fowler D, Coyle M, Skiba U, Sutton MA, Cape JN, Reis S, Sheppard LJ, Jenkins A, Grizzetti B, Galloway JN, Vitousek P, Leach A, Alexander F, Butterbach-bahl K, Dentener F, Stevenson D, Amann M, Galloway N, Bouwman AF, 2013. The global nitrogen cycle in the twenty-first century. Philos. Trans. R. Soc. Lond. B. Biol. Sci 368, 20130164 10.1098/rstb.2013.0164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry JA, Xian G, Jin S, Dewitz JA, Barnes CA, Herold ND, Wickham JD, 2011. Completion of the 2006 National Land Cover Database for the Conterminous United States. Photogramm. Eng. Remote Sensing 77, 858–864. [Google Scholar]
- Galloway JN, Aber JD, Erisman JW, Seitzinger SP, Howarth RW, Cowling EB, Cosby BJ, 2003. The Nitrogen Cascade. Bioscience 53, 341–356. 10.1641/0006-3568(2003)053[0341:TNC]2.0.CO;2 [DOI] [Google Scholar]
- Gardner KK, McGlynn BL, 2009. Seasonality in spatial variability and influence of land use/land cover and watershed characteristics on stream water nitrate concentrations in a developing watershed in the Rocky Mountain West. Water Resour. Res. 45, W08411 10.1029/2008WR007029 [DOI] [Google Scholar]
- Gardner KK, McGlynn BL, Marshall LA, 2011. Quantifying watershed sensitivity to spatially variable N loading and the relative importance of watershed N retention mechanisms. Water Resour. Res 47, W08524 10.1029/2010WR009738 [DOI] [Google Scholar]
- Glibert PM, Trice MT, Michael B, Lane L, 2005. Urea in the tributaries of the Chesapeake and coastal bays of Maryland. Water. Air. Soil Pollut 160, 229–243. 10.1007/s11270-005-2546-1 [DOI] [Google Scholar]
- Gomez-Velez JD, Harvey JW, 2014. A hydrogeomorphic river network model predicts where and why hyporheic exchange is important in large basins. Geophys. Res. Lett 41, 6403–6412. 10.1002/2014GL061099 [DOI] [Google Scholar]
- Gove NE, Edwards RT, Conquest LL, 2001. Effects of scale on land use and water quality relationships: A longitudinal basin-wide perspective. J. Am. Water Resour. Assoc 37, 1721–1734. 10.1111/j.1752-1688.2001.tb03672.x [DOI] [Google Scholar]
- Greathouse EA, Compton JE, Van Sickle J, 2014. Linking landscape characteristics and high stream nitrogen in the Oregon Coast Range: red alder complicates use of nutrient criteria. J. Am. Water Resour. Assoc 50, 1383–1400. 10.1111/jawr.12194 [DOI] [Google Scholar]
- Groffman PM, Law NL, Belt KT, Band LE, Fisher GT, 2004. Nitrogen Fluxes and Retention in Urban Watershed Ecosystems. Ecosystems 7, 393–403. 10.1007/s10021-003-0039-x [DOI] [Google Scholar]
- Gu C, Hornberger GM, Mills AL, Herman JS, Flewelling SA, 2007. Nitrate reduction in streambed sediments: Effects of flow and biogeochemical kinetics. Water Resour. Res 43, W12413 10.1029/2007WR006027 [DOI] [Google Scholar]
- Hale RL, Turnbull L, Earl SR, Childers DL, Grimm NB, 2015. Stormwater infrastructure controls runoff and dissolved material export from arid urban watersheds. Ecosystems 18, 62–75. 10.1007/s10021-014-9812-2 [DOI] [Google Scholar]
- Harrison JA, Caraco N, Seitzinger SP, 2005a. Global patterns and sources of dissolved organic matter export to the coastal zone: Results from a spatially explicit, global model. Global Biogeochem. Cycles 19, GB4S04 10.1029/2005GB002480 [DOI] [Google Scholar]
- Harrison JA, Caraco N, Seitzinger SP, 2005b. Global patterns and sources of dissolved organic matter export to the coastal zone: Results from a spatially explicit, global model. Global Biogeochem. Cycles 19, n/a.n/a. 10.1029/2005GB002480 [DOI] [Google Scholar]
- Harrison JA, Maranger RJ, Alexander RB, Giblin AE, Jacinthe P-A, Mayorga E, Seitzinger S, Sobota D, Wollheim WM, 2009. The regional and global significance of nitrogen removal in lakes and reservoirs. Biogeochemistry 93, 143–157. 10.1007/S10533-008-9272-X [DOI] [Google Scholar]
- Heathwaite AL, Griffiths P, Parkinson RJ, 1998. Nitrogen and phosphorus in runoff from grassland with buffer strips following application of fertilizers and manures. Soil Use Manag. 14, 142–148. 10.1111/j.1475-2743.1998.tb00140.x [DOI] [Google Scholar]
- Hensley RT, Cohen MJ, Korhnak LV, 2014. Inferring nitrogen removal in large rivers from high resolution longitudinal profiling. Limnol. Oceanogr. 59, 1152–1170. 10.4319/lo.2014.59.4.1152 [DOI] [Google Scholar]
- Hill RA, Weber MH, Leibowitz SG, Olsen AR, Thornbrugh DJ, 2016. The stream-catchment (StreamCat) dataset: A databased of watershed metrics for the conterminous United States. J. Am. Water Resour. Assoc. 52, 120–128. 10.1111/1752-1688.12372 [DOI] [Google Scholar]
- Howarth RW, Billen G, Swaney D, Townsend A, Jaworski N, Lajtha K, Downing JA, Elmgren R, Caraco N, Jordan T, Berendse F, Freney J, Kudeyarov V, Murdoch P, Zhao-Liang Z, 1996. Regional nitrogen budgets and riverine N & P fluxes for the drainages to the North Atlantic Ocean: Natural and human influences. Biogeochemistry 35, 75–139. 10.1007/BF02179825 [DOI] [Google Scholar]
- Jencso KG, McGlynn BL, Gooseff MN, Wondzell SM, Bencala KE, Marshall LA, 2009. ydrologic connectivity between landscapes and streams: Transferring reach-and plot-scale understanding to the catchment scale. Water Resour. Res 45, 1–16. 10.1029/2008WR007225 [DOI] [Google Scholar]
- Johnson SR, Burchell MRI, Evans RO, Osmond DL, Gilliam JW, 2013. Riparian buffer located in an upland landscape position does not enhance nitrate-nitrogen removal. Ecol. Eng 52, 252–261. 10.1016/j.ecoleng.2012.11.006 [DOI] [Google Scholar]
- Jordan SJ, Stoffer J, Nestlerode JA, 2011. Wetlands as Sinks for Reactive Nitrogen at Continental and Global Scales: A Meta-Analysis. Ecosystems 14, 144–155. 10.1007/s10021-010-9400-z [DOI] [Google Scholar]
- Joyce LA, Running SW, Breshears DD, Dale VH, Malmsheimer RW, Sampson RN, Sohngen B, Woodall CW, 2014. Ch. 7: Forests. Climate Change Impacts in the United States:, in: Melillo JM, Richmond TC, Yohe GW (Eds.), The Third National Climate Assessment. U.S. Global Change Research Program, pp. 175–194. 10.7930/J0Z60KZC.On [DOI] [Google Scholar]
- Kuhn I, Dormann CF, 2012. Less than eight (and a half) mis-conceptions of spatial analysis. J. Biogeogr 39, 995–998. 10.1111/j.1365-2699.2012.02707.x [DOI] [Google Scholar]
- Lerch RN, Baffaut C, Kitchen NR, Sadler EJ, 2015. Long-term agroecosystem research in the Central Mississippi River Basin: dissolved nitrogen and phosphorus transport in a high-runoff potential watershed. J. Environ. Qual 44, 44–57. 10.2134/jeq2014.02.0059 [DOI] [PubMed] [Google Scholar]
- Lin L, Webster JR, Hwang T, Band LE, 2015. Effects of lateral nitrate flux and instream processes on dissolved inorganic nitrogen export in a forested catchment: A model sensitivity analysis. Water Resour. Res. 51, 2680–2695. 10.1002/2014WR015962 [DOI] [Google Scholar]
- Lindeman RH, Merenda PF and Gold RZ, 1980. Introduction to Bivariate and Multivariate Analysis, Glenview IL: Scott, Foresman. [Google Scholar]
- Lovett GM, Weathers KC, Sobczak WV, 2000. Nitrogen saturation and retention in forested watersheds of the Catskill Mountains, New York. Ecol. Appl 10, 73–84. 10.1890/1051-0761(2000)010[0073:NSARIF]2.0.CO;2 [DOI] [Google Scholar]
- Martí E, Aumatell J, Lluis G, Poch M, Sabater F, 2004. Nutrient retention efficiency in streams receiving inputs from wastewater treatment plants. J. Environ. Qual 33, 285–293. 10.2134/jeq2004.2850 [DOI] [PubMed] [Google Scholar]
- Maupin MA, Ivahnenko T, 2011. Nutrient loadings 635 to streams of the continental United States from municipal and industrial effluent. J. Am. Water Resour. Assoc 47, 950–964. 10.1111/j.1752-1688.2011.00576.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer PM, Reynolds SKJ, McCutchen MD, Canfield TJ, 2007. Meta-analysis of nitrogen removal in riparian buffers. J. Environ. Qual 36, 1172–1180. 10.2134/jeq2006.0462 [DOI] [PubMed] [Google Scholar]
- McIsaac GF, Hu X, 2004. Net N input and riverine N export from Illinois agricultural watersheds with and without extensive tile drainage. Biogeochemistry 70, 251–271. 10.1023/B:BIOG.0000049342.08183.90 [DOI] [Google Scholar]
- McKay L, Bondelid T, Dewald T, Johnston J, Moore R, Reah A, 2012. NHDPlus Version 2 User Guide. [Google Scholar]
- Melillo JM, Cowling EB, 2002. Reactive nitrogen and public policies for environmental protection. Ambio 31, 150–158. [DOI] [PubMed] [Google Scholar]
- Mississippi River/Gulf of Mexico Watershd Nutrient Task Force, 2008. Gulf hypoxia action plan 2008 for reducing, mitigating, and controlling hypoxia inthe northern Gulf of Mexico and improving water quality in the Mississippi River Basin. Washington, DC. [Google Scholar]
- Mississippi River/Gulf of Mexico Watershd Nutrient Task Force, 2001. Action plan for reducing, mitigating, and controlling hypoxia in the northern Gulf of Mexico. Washington, DC. [Google Scholar]
- Mitchell MJ, Driscoll CT, Kahl JS, Likens GE, Murdoch PS, Pardo LH, 1996. Climatic control of nitrate lossfrom forested watersheds in the northeast United States. Environ. Sci. Technol 30, 2609–2612. 10.1021/es9600237 [DOI] [Google Scholar]
- Mulholland PJ, Helton AM, Poole GC, Hall RO, Hamilton SK, Peterson BJ, Tank JL, Ashkenas LR, Cooper LW, Dahm CN, Dodds WK, Findlay SEG, Gregory SV, Grimm NB, Johnson SL, McDowell WH, Meyer JL, Valett HM, Webster JR, Arango CP, Beaulieu JJ, Bernot MJ, Burgin AJ, Crenshaw CL, Johnson LT, iederlehner BR, ‘Brien JM, Potter JD, Sheibley RW, Sobota DJ, Thomas SM, 2008. Stream denitrification across biomes and its response to anthropogenic nitrate loading. Nature 452, 202–5. 10.1038/nature06686 [DOI] [PubMed] [Google Scholar]
- Ocampo CJ, Oldham CE, Sivapalan M, 2006. Nitrate attenuation in agricultural catchments: Shifting balances between transport and reaction. Water Resour. Res 42, W01408 10.1029/2004WR003773 [DOI] [Google Scholar]
- Olson JR, Hawkins CP, 2013. Developing site-specific nutrient criteria from empirical models. Freshw. Sci 32, 719–740. 10.1899/12-113.1 [DOI] [Google Scholar]
- Omernik JM, 1977. Nonpoint source-stream nutrient level relationships: A nationwide study. U.S. Environmental Protection Agency, Environmental Research Laboratory, Corvallis, OR, EPA-600/3-77-105, 151 p [Google Scholar]
- Omernik JM, Paulsen SG, Griffith GE , Weber MH, 2015. Regional patterns of total nitrogen concentration in the National Rivers and Streams Assessment. Soil and Water Conservation Society, 7, 167–181. doi: 10.2489/jswc.71.3.167. [DOI] [Google Scholar]
- Packman AI, Salehin M, 2003. Relative roles of stream flow and sedimentary conditions in controlling hyporheic exchange. Hydrobiologia 494, 291–297. 10.1023/A:1025403424063 [DOI] [Google Scholar]
- Pardo LH, Fenn ME, Goodale CL, Geiser LH, Driscoll CT, Allen EB, Baron JS, Bobbink R, Bowman WD, Clark CM, Emmett B, Gilliam FS, Greaver TL, Hall SJ, Lilleskov EA, Liu L, Lynch JA, Nadelhoffer KJ, Perakis SS, Robin-Abbott MJ, Stoddard JL, Weathers KC, Dennis RL, 2011. Effects of nitrogen deposition and empirical nitrogen critical loads for ecoregions of the United States. Ecol. Appl 21, 3049–3082. 10.1890/10-2341.1 [DOI] [Google Scholar]
- Pellerin BA, Kaushal SS, McDowell WH, 2006. Does Anthropogenic Nitrogen Enrichment Increase Organic Nitrogen Concentrations in Runoff from Forested and Human-dominated Watersheds? Ecosystems 9, 852–864. 10.1007/s10021-006-0076-3 [DOI] [Google Scholar]
- Pellerin BA, Saraceno JF, Shanley JB, Sebestyen SD, Aiken GR, Wollheim WM, Bergamaschi BA, 2012. Taking the pulse of snowmelt: in situ sensors reveal seasonal, event and diurnal patterns of nitrate and dissolved organic matter variability in an upland forest stream. Biogeochemistry 108, 183–198. 10.1007/s10533-011-9589-8 [DOI] [Google Scholar]
- Pellerin BA, Wollheim WM, Hopkinson CS, McDowell WH, Williams MR, Vörösmarty CJ, Daley ML, 2004. Role of wetlands and developed land use on dissolved organic nitrogen concentrations and DON/TDN in northeastern U.S. rivers and streams. Limnol. Oceanogr 49, 910–918. 10.4319/lo.2004.49.4.0910 [DOI] [Google Scholar]
- Perakis SS, Hedin LO, 2007. State factor relationships of dissolved organic carbon and nitrogen losses from unpolluted temperate forest watersheds. J. Geophys. Res 112, G02010 10.1029/2006JG000276 [DOI] [Google Scholar]
- Perakis SS, Hedin LO, 2002. Nitrogen loss from unpolluted South American forests mainly via dissolved organic compounds. Nature 415, 416–419. 10.1038/415416a [DOI] [PubMed] [Google Scholar]
- Preston SD, Alexander RB, Schwarz GE, Crawford CG, 2011. Factors affecting stream nutrient loads: a synthesis of regional SPARROW model results for the continental United States. J. Am. Water Resour. Assoc. 47, 891–915. 10.1111/j.1752-1688.2011.00577.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRISM Climate Group, n.d. Oregon State University. [Google Scholar]
- Puckett LJ, 1995. Identifying the major sources of nutrient water pollution. Environ. Sci. Technol. 29, 408A–414A. 10.1021/es00009a001 [DOI] [Google Scholar]
- R Core Team, 2016. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: ISBN 3-900051-07-0, http://www.R-project.org/. [Google Scholar]
- Rao LE, Parker DR, Bytnerowicz A, Allen EB, 2009. Nitrogen mineralization across an atmospheric nitrogen deposition gradient in Southern California deserts. J. Arid Environ 73, 920–930. 10.1016/j.jaridenv.2009.04.007 [DOI] [Google Scholar]
- Robertson DM, Saad DA, Schwarz GE, 2014. Spatial Variability in Nutrient Transport by HUC8, State, and Subbasin Based on Mississippi/Atchafalaya River Basin SPARROW Models. J. Am. Water Resour. Assoc 50, 988–1009. 10.1111/jawr.12153 [DOI] [Google Scholar]
- Saunders D, Kalff J, 2001. Nitrogen retention in wetlands, lakes and rivers. Hydrobiologia 443, 205–212. 10.1023/A:1017506914063 [DOI] [Google Scholar]
- Scanlon TM, Ingram SM, Riscassi AL, 2010. Terrestrial and in-stream influences on the spatial variability of nitrate in a forested headwater catchment. J. Geophys. Res 115, G02022 10.1029/2009JG001091 [DOI] [Google Scholar]
- Seitzinger S, Harrison J, Bohlke J, Bouwman A, Lowrance R, Peterson B, Tobias C, Van Drecht G, 2006. Denitrification across landscapes and waterscapes: a synthesis. Ecol. Appl. 16, 2064–2090. 10.1890/1051-0761(2006)016[2064:DALAWA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Sobota DJ, Compton JE, Harrison JA, 2013. Reactive nitrogen inputs to US lands and waterways: how certain are we about sources and fluxes? Front. Ecol. Environ 11, 82–90. 10.1890/110216 [DOI] [Google Scholar]
- Sobota DJ, Harrison JA, Dahlgren RA, 2009. Influences of climate, hydrology, and land use on input and export of nitrogen in California watersheds. Biogeochemistry 94, 43–62. 10.1007/s10533-009-9307-y [DOI] [Google Scholar]
- Sponseller RA, Temnerud J, Bishop K, Laudon H, 2014. Patterns and drivers of riverine nitrogen (N) across alpine, subarctice, and boreal Sweden. Biogeochemistry 120, 105–120. 10.1007/s10533-014-9984-z [DOI] [Google Scholar]
- Sprague LA, Hirsch RM, Aulenbach BT, 2011. Nitrate in the mississippi river and its tributaries, 1980 to 2008: Are we making progress? Environ. Sci. Technol. 45, 7209–7216. 10.1021/es201221s [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley EH, Maxted JT, 2008. Changes in the dissolved nitrogen pool across land cover gradients in Wisconsin streams. Ecol. Appl 18, 1579–1590. 10.1890/07-1379.1 [DOI] [PubMed] [Google Scholar]
- Stets EG, Kelly VJ, Crawford CG, 2015. Regional and temporal differences in nitrate trends discerned from long-term water quality monitoring data. J. Am. Water Resour. Assoc 1–14. 10.1111/1752-1688.12321 [DOI] [Google Scholar]
- Sudduth EB, Perakis SS, Bernhardt ES, 2013. Nitrate in watersheds: straight from soils to streams? J. Geophys. Res. Biogeosciences 118, 291–302. 10.1002/jgrg.20030 [DOI] [Google Scholar]
- United States Congress, 1990. Clean Air Act Amendments: Title IV-A - Acid deposition control. [Google Scholar]
- United States Environmental Protection Agency, 2016a. National Rivers and Streams Assessment 2008-2009: A collaborative survey (EPA/841/R-16/007). Washington, DC. [Google Scholar]
- United States Environmental Protection Agency, 2016b. National Rivers and Streams Assessment 2008-2008 Technical Report (EPA/841/R-16/008). Washington, DC. [Google Scholar]
- United States Environmental Protection Agency, 2010. National Rivers and Streams Assessment Quality Assurance Project Plan. Office of Wetlands, Oceans, and Watersheds, Washington, DC, EPA 841-B-07-007, 120 p. [Google Scholar]
- United States Environmental Protection Agency, 2006a. Synthetic N fertilizer application to agricultural lands by 12-digit HUC for the Conterminous United States, 2006. [Google Scholar]
- United States Environmental Protection Agency, 2006b. Cultivated biological nitrogen fixation in agricultural lands by 12-digit HUC for the Conterminous United States, 2006. [Google Scholar]
- United States Environmental Protection Agency, 2006c. Manure application to agricultural lands from confined animal feeding operations by 12-digit HUC for the Conterminous United States (2006). [Google Scholar]
- United States Environmental Protection Agency, 2006d. Atmospheric Nitrogen Deposition by 12-digit HUC for the Conterminous United States, 2006. [Google Scholar]
- Vanderbilt KL, Lajtha K, Frederick J, 2002. Biogeochemistry of unpolluted forested watersheds in the regon Cascades : temporal patterns of precipitation and stream nitrogen fluxes 87-118. [Google Scholar]
- Vitousek PM, Reiners WA, 1975. Ecosystem succession and nutrient retention: A hypothesis. Bioscience 25, 376–381. 10.2307/1297148 [DOI] [Google Scholar]
- von Schiller D, Bernal S, Sabater F, Martí E, 2015. A round-trip ticket: The importance of release processes for in-stream nutrient spiraling. Freshw. Sci 34, 20–30. 10.1086/679015 [DOI] [Google Scholar]
- Warren DR, Judd KE, Bade DL, Likens GE, Kraft CE, 2013. Effects of wood removal on stream habitat and nitrate uptake in two northeastern US headwater streams. Hydrobiologia 717, 119–131. 10.1007/s10750-013-1578-6 [DOI] [Google Scholar]
- Weller DE, Baker ME, Jordan TE, 2011. Effects of riparian buffers on nitrate concentrations in watershed discharges: new models and management implications. Ecol. Appl 21, 1679–1695. [DOI] [PubMed] [Google Scholar]
- White MJ, Santhi C, Kannan N, Arnold JG, Harmel D, Norfleet L, Allen P, DiLuzio M, Wang X, Atwood J, Haney E, Johnson MV, 2014. Nutrient delivery from the Mississippi River to the Gulf of Mexico and effects of cropland conservation. J. Soil Water Conserv. 69, 26–40. 10.2489/jswc.69.1.26 [DOI] [Google Scholar]
- Wise DR, Johnson HM, 2011. Surface-Water Nutrient Conditions and Sources in the United States Pacific Northwest. J. Am. Water Resour. Assoc. 47, 1110–1135. 10.1111/j.1752-1688.2011.00580.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollheim WM, Vörösmarty CJ, Peterson BJ, Seitzinger SP, Hopkinson CS, 2006. Relationship between river size and nutrient removal. Geophys. Res. Lett 33, L06410 10.1029/2006GL025845 [DOI] [Google Scholar]
- Ye S, Covino TP, Sivapalan M, Basu NB, Li H-Y, Wang S-W, 2012. Dissolved nutrient retention dynamics in river networks: A modeling investigation of transient flows and scale effects. Water Resour. Res 48, W00J17 10.1029/2011WR010508 [DOI] [Google Scholar]
- Zarnetske JP, Haggerty R, Wondzell SM, Baker MA, 2011. Dynamics of nitrate production and removal as a function of residence time in the hyporheic zone. J. Geophys. Res 116, G01025 10.1029/2010JG001356 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


