Abstract
A strong association between elevated plasma low-density-lipoprotein (LDL) and the development of cardiovascular diseases (CVD) has been established. Oxidation of LDL (Ox-LDL) promotes vascular dysfunction, enhances the production and release of inflammatory mediators such as reactive oxygen species and contribute to the initiation and progression of atherosclerosis. In addition, Ox-LDL enhances the production and release of tumor necrosis factor (TNF-α), interleukin (IL)-6, arachidonic acid metabolites and nitric oxide (NO) that are responsible for various human pathologies including cancer. Organosulfur compounds (OSC) from alliaceae modulate the glutathione (GSH) redox cycle and inhibits NFk-B activation in human T cells. Furthermore, OSC bioactivities include antioxidant, antibacterial, anticarcinogenic, antiatherogenic, immunostimulatory, and liver protection potential.
Keywords: Allium sativum, Allyl sulfur compounds, Helicopter pylori, Atherosclerosis, Cancer
1. The antioxidant potential of organosulfur compounds
Oxidative modification of DNA, proteins and lipids by free radicals and non-radical oxidants plays a role in wide range of diseases including cardiovascular [1], neurodegenerative and inflammatory diseases [2] and cancer (Table 1) [3,4]. Free radicals include superoxide, nitric oxide (NO) and hydroxyl radical are the most toxic of the reactive oxygen species (ROS). The non-radical oxidants include hydrogen peroxide (H2O2), singlet oxygen (1O2) and ozone (O3) which form free radicals in some tissues through various chemical reactions (Table 2). Most of the ROS produced by cells are from:
normal aerobic respiration in mitochondria, which generates superoxide radical (O2·−) and the ensuing toxic products, and the highly reactive hydroxyl radical (OH·),
stimulated macrophages and polymorphonuclear leukocytes, which release superoxide and the nitric oxide radical (NO·), that can interact to form the non-radical destructive peroxynitrite,
peroxisomes, cell organelles that produce H2O2 as a by-product of degrading fatty acid and other molecules, and
oxidant by-products that occur during the induction of cytochrome P450 enzymes.
Table 1.
Mechanisms involved in the etiology of oxidant-mediated disorders
| Disease | Pro-oxidative mechanism |
| Cancer | Oxidative damage to DNA Inactivation of DNA repair enzymes |
| Cardiovascular disease | Oxidative modification of LDL Adhesion of phagocytes to vascular endothe-lium |
| Pulmonary emphysema Tissue damage in autoimmune diseases (rheumatoid arthritis) | Potentiation of the proteolytic activity of phagocytes-derived proteases |
| Acquired immunosuppression | Oxidative inactivation of the protective activities of B- and T-lymphocytes and NK-cells |
Table 2.
Reactive oxygen species (ROS). Quenching ability of specific antioxidants
| Reactive species | Antioxidants | |
|---|---|---|
| 1O2 | Singlet oxygen | Vitamins A, C, E, (β-carotene and other carotenoids |
| O2.− | Superoxide free radical | Superoxide dismutase, vitamins C, E, β-carotene |
| ROO. | Peroxyl-free radical | Vitamins C, E |
| H2O2 | Hydrogen peroxide | Catalase, glutathione peroxidase |
| LOOH | Lipid peroxides | Glutathione peroxidase |
Endogenous levels of ROS increase during chronic infection and inflammation, strenuous physical exercise, hyper-metabolic states, trauma and sepsis, and during exposure to exogenous sources. The most common exogenous sources of ROS are derived from tobacco smoke, (that generates free radicals in exposed tissues notably the highly reactive OH· radical), UV light (which produces singlet oxygen (1O2) and OH·, ozone (O3), polluted air (which produces oxides of nitrogen), industrial toxins (such as carbon-tetrachloride), drugs (such as phenobarbital, a known tumor promoter in liver), and charcoal-broiled foods (which form a variety of carcinogens, notably benzo(a)pyrene). To protect molecules against toxic free radicals and other ROS, cells have developed antioxidant defences that include small molecules including glutathione, and the enzymes superoxide dismutase (SOD), which dismutates superoxide; catalase and glutathione peroxidase, which destroy toxic peroxides. External sources of antioxidant nutrients include antioxidant vitamins C and E, vitamin A/provitamin A and the mineral selenium, a component of selenium-dependent glutathione peroxidase (Table 2). Hence, dietary supplementation of these antioxidants may decrease the incidence of tissue damage that leads to a wide variety of diseases. One such source is phytochemicals.
Phytochemicals from plant-rich diets, including organo-sulfur compounds (OSC) from Allium sativum (garlic) such as alliin, diallylsulfides and allicin [5], provide most of its potent biological activity in the protection against oxidant damage (Fig. 1) [6]. Lipid-soluble allyl sulfur compounds are formed from the parent sulfur compound alliin by the action of alliinase, an enzyme released by crushing or chopping of garlic. The most commonly used lipid-soluble allyl sulfur compounds are ajoene, diallyl sulfide (DAS), diallyl disulfide (DADS) and diallyl trisulfide (DATS). Water soluble compounds can also occur in garlic especially after alcoholic fermentation. The parent compound to alliin, γ-glutamyl-S-allylcysteine is converted to S-allylcysteine (SAC), S-allylmercaptocysteine (SAMC) and others (Fig. 2) [7].
Fig. 1.
Hydro- and lipo-soluble compounds from Allium sativum.
Fig. 2.
Thiosulfonate in age garlic extract (AGE).
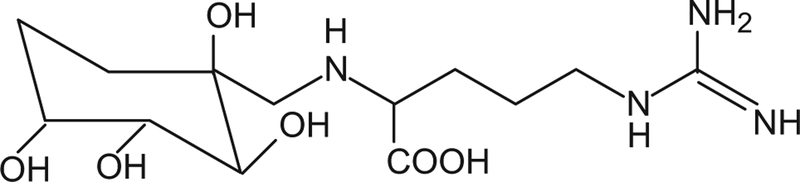
Water-soluble allyl sulfur compounds include SAC and SAMC and the lipid-soluble sulfur compounds, DADS, DATS and diallylpolysulfides that are obtained from aged garlic extract (AGE, Kyolic), a naturally modified form of raw garlic produced by a unique aging process. These compounds are characterized by their high antioxidant content and health protective potential [8–11]. Other antioxidants in AGE include phenolic compounds, allixin, [12], selenium, and N-(1-deoxy-D-fructos-1-yl)-L-arginine (Fru-Arg) and N-fructosyl glutamate that could not be detected in raw nor heated garlic (Fig. 3) [13]. These data suggest that supple mentation with aged garlic extracts are therapeutically beneficial.
Fig. 3.
Chemical structure of Fru-Arg.
The oxidant injury caused by ROS is linked to various pathologies including the development of cardiovascular disease (Table 1). The potential of AGE to protect endothelial cells is by modifying cellular scavenging enzymes. [10,19]. Endothelial exposure to the oxidants hypoxanthine and xanthine oxidase or H2O2, in the presence of AGE generated increased levels of SOD, catalase, and glutathione peroxidase, and in a dose- and time-related fashion suppressed the production of O2·− and H2O2 [10]. In the blood circulatory system, AGE components improve peripheral circulation, [27], protect vascular endothelial cells from oxidant injury [9–11,19], reduce plasma lipids [28] and alter platelet function [29].
Decreased tissue-glutathione levels are associated with cell damage, depressed immunity, progression of aging, and increased risk of cancer. Reduced GSH, as a substrate for the antioxidant enzyme glutathione peroxidase, protects cellular constituents from the damaging effects of peroxides formed in metabolism and through ROS reactions. AGE increases cellular GSH in a variety of cells, including those in normal liver and mammary tissue [16]. AGE increases also glutathione peroxidase and other ROS scavenging enzymes [10] that are important in radioprotection and UV suppression of certain forms of immunity [17]. In addition, AGE supplementation has been shown to reduce or prevent the range of ROS-induced DNA, lipid and protein damage implicated in aging processes [17,18] as well as chemically induced cancers (Fig. 4) [4]. It has been shown also to act as immuno-modulator [30–33], antiallergic [34], liver protective agent [35] and has an antiaging effect [36].
Fig. 4.
Oxidative damage in carcinogenesis.
Pharmacologic studies of SAC and SAMC, were proposed to be responsible in part for scavenging of active oxygen species [20,21], inhibition of lipid peroxidation [22] and cancer prevention [23–26]. AGE and SAC protect vascular endothelial cells (EC) from H2O2-induced injury [11], inhibit Cu2+-induced LDL oxidation [14], modulates the glutathione (GSH) redox cycle, inhibits NFk-B activation in human T cells [15] and prevent oxidant-induced dense-body formation in sickle red blood cells [37].
2. Effect of organosulfur compounds in cardiovascular diseases
Cardiovascular diseases (CVD) are the major cause of death among people living a Western life style. The risk of developing CVD is greater for men than for premenopausal women. Moreover, as a person ages, there is a greater risk of CVD.A multitude of factors contribute to the development of CVD. Specifically, hypertension, diabetes and obesity are three clinical conditions that contribute to the increased incidence of CVD. Lifestyle, smoking, inactivity and stress are also important CVD risk factors [38–40]. Atherosclerosis is the principal contributor to the pathogenesis of myocardial and cerebral infarction. It is a complex disease that is associated with an excessive inflammatory, fibro-fatty, proliferative response to damage of the artery wall involving several cell types such as smooth muscle cells, monocyte-derived macrophages, T-lymphocytes and platelets [41].
The presence of a thrombus in a stenosed coronary artery can lead to acute syndromes such as myocardial infarction and angina [42]. Platelet aggregation plays a central role in coronary thrombosis and is related to a cascade of events which includes expression of adhesion molecules on the surface of the endothelium, the oxidation of lipoproteins, monocyte invasion of the vessel wall, foam cell formation, smooth muscle phenotypic change and proliferation and platelet deposition [43]. These events could be induced from dysfunction of the endothelial lining that occurs in hyperlipidemia, hypertension or cigarette smoke causing imbalance of angiotensin II and NO production in the artery wall [44,45].
Elevated plasma cholesterol particularly the LDL cholesterol but not the high-density-lipoprotein cholesterol (HDL cholesterol) and triglycerides are associated with an increased risk of CVD [46]. Oxidation of LDL cholesterol has been recognized as playing an important role in the initiation and progression of atherosclerosis [47–49]. LDL has been shown to be oxidized by cultured cells such as macrophages, endothelial and smooth muscle cells with transition metals [50–54]. The effect of Ox-LDL on vascular endothelial cells (EC), is evaluated by:
lactate dehydrogenase (LDH) release, used as index of membrane damage. LDH is an intracellular enzyme that leaks into the culture medium when cell membranes are damaged. Thus, as compared to untreated cells, exposure to Ox-LDL caused increase in LDH release;
cell viability, which is determined by the methylthiazol tetrazolium (MTT) ring cleaved by mitochondrial dehydrogenase, a reaction that occurs only in living cells;
lipid peroxidation evaluated by thiobarbituric acid reactive substances (TBARS). Products of lipid peroxidation increased greatly when cells were incubated with OX-LDL for 24 h.
A primary histologic feature of incipient atherosclerosis [55] is the presence of cholesterol-loaded foam cells and the fatty streak which occur when Ox-LDL is taken up by macrophage. The contribution of Ox-LDL to the initiation and progression of the atherosclerotic process includes events such as chemotaxis for monocytes, inhibition of macrophage motility, formation of foam cells, up-regulation of endothelial adhesion molecules, stimulation of growth factors and chemokines, and proliferation of smooth muscle cells [56]. Ox-LDL also appears to exert cytotoxicity and to initiate vascular dysfunction by altering the composition and permeability of the endothelial barrier [57–59]. Ox-LDL enhances also the production and release of inflammatory mediators such as ROS, tumor necrosis factor (TNF-alpha), interleukin (IL)-6, arachidonic acid metabolites and NO [60–62]. Acting as second messengers, these mediators stimulate cells to activate transcription factors regulated by the intracellular redox state and promote the development of inflammation leading to injury of surrounding cells and tissues. Among these factors, is nuclear factor NFk-B, a well-known transcription factor activated by oxidative stress. NFj-B is a heterodimeric transcription factor complex composed of two DNA-binding subunits, p50 and p65, and it is associated with the regulation of numerous genes encoding proteins in immune function, inflammation and cellular growth control [63]. Under stressed conditions in EC, activation of NFk-B leads to the expression of cell adhesion factors such as vascular cell adhesion molecule-1 (VCAM-1) and inter-cellular adhesion molecule-1 (ICAM-1) [64,65]. These events further accelerate the formation of atherogenic lesions and cell death. However, antioxidants can inhibit oxidant-induced NFk-B activation [66–68], protect EC [69,70] and normalize vascular functioning in hypercholesterolemia and atherosclerosis [71–73].
Relaxation mediated by endothelium derived NO is impaired in arteries from hypercholesterolemic and atherosclerotic animals [74]. The mechanisms suggested for the effect on vascular relaxation include increased diffusional barrier for NO due to the intima thickening [75], L-arginine depletion [76,77] altered endothelial cell receptor coupling mechanism and inactivation of NO by oxygen free radicals [78,79]. In addition, although blood lipids are in the normal or lower range, elevated blood homocysteine has also been associated with an increased incidence of cardiovascular disease [80–85].
2.1. The antiatheroslerotic activity of organosulfur compounds
OSC derived from garlic possess antiatherosclerotic properties by reducing serum cholesterol levels in humans [86,87], inhibit cholesterol biosynthesis [28], suppress LDL oxidation, lower plasma fibrinogen and increase fibrinolytic activity [88,89]. It has been also reported that in cell free homogenate and in vivo, garlic activates the NO synthase [90]. Amino acid analysis of garlic powder demonstrated that it is a rich source of arginine the precursor of NO. Since the hypertension induced by NO synthase inhibitor, L-NAME, and the decrease in the urinary levels of NO2−/NO3− induced by L-NAME in rats were prevented by treatment with garlic, it was suggested that garlic increases NO synthase activity in vivo.
2.2. Inhibition of platelet aggregation
The major arachidonic acid metabolite formed by platelets is TXA2, a potent vasoconstrictor and stimulator of aggregation. In contrast, PGI2, the major arachidonic acid metabolite formed by the vascular endothelial cells is a potent vasodilatator and inhibitor of platelet aggregation. It is the balance between these eicosanoids that is important in regulating hemostasis and platelet aggregation. These eicosanoids are extremely short-lived in plasma and are invariably measured as their stable metabolites, 6-keto-PGF1 and TXB2.
The production of NO by endothelial cells is another important regulator of platelet activity [91]. Aggregation of platelets is a consequence of exposure of fibrinogen receptors on the surface of the cells. These receptors bind fibrinogen in the presence of extracellular Ca2+ and cross-link the platelets to form aggregates. The fibrinogen receptor is a heterodimer of the membrane glycoproteins (GP)IIb and IIIa. Although unstimulated platelets express the GPIIb–IIIa complex at their surface, this complex is unable to bind fibrinogen until platelets are activated [92].
Although the mechanism of action of organosulfur is not clear, it was suggested that it may be due to a sulfhydryl group-mediated effect [100]. Studies in humans and animals have shown that certain constituents of fresh garlic or its extract have inhibitory activity on platelet aggregation [93–98]. The GPIIb–IIIa receptor has a high content of -SH groups, and binding of fibrinogen is inhibited by the organo-sulfur compound ajoene [99].
The inhibition of fibrinogen mediated platelet adhesion is due to an organosulfur compound in dried AGE reducing the functional competence of some GPIIb–IIIa receptors, whereas sufficient receptors remain to sustain full ADP-induced aggregation. In contrast, ADP-induced platelet aggregation was inhibited by the AGE supplementation. Thus, the most likely mechanism involves the ADP receptor. Platelet ADP receptors belong to the P2T subtype of purinoreceptors whose activation leads to a rise in intracellular Ca2+. Reduction of adhesion to collagen- and fibrinogen-coated surfaces and specificity of inhibition of platelet adhesion and individual receptors for these adhesive proteins may be affected differently by AGE [102]. Moreover, AGE contains a number of organosulfur components [101] and a large number of other substances including carbohydrates, proteins and saponins. Specific receptors, (epinephrine and collagen) could be also the mechanism by which AGE induced reduction of platelet aggregation rather than an inhibition of mediators of platelet aggregation such as observed with nonsteroidal antiinflammatory agents.
2.3. Cholesterol-lowering effect of organosulfur compounds
Elevated total and LDL cholesterol levels and blood pressure are the main cardiovascular risk factors [103].
Organosulfur compounds were shown to lower cholesterol level from cultured hepatocytes [104,105], blood cholesterol from animals [106–108] and humans [109–112]. Compounds such as S-allylcysteine (SAC), diallylsulfide (DADS) or allicin and its derivative (ajoene) were among compounds that contribute to reduce cholesterol level [113–115]. It has been suggested that the mechanism underlying the inhibitory action is related to the inhibition of cholesterol synthesis pathway and a decreased activity of several cholesterolgenic enzymes, including 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase and acetyl-CoA synthetase [114]. Several of the water soluble compounds present in AGE such as SAC, S-ethyl cysteine, and S-propyl cysteine have been shown to inhibit cholesterogenesis in hepatocytes [28]. Lipid-soluble organosulfurs such as diallylsulfide, diallyldisulfides and diallyltrisulfides as well as dipropylsulfide, dipropyldisulfides and methylallylsulfide, also decrease cholesterol synthesis of hepatocytes but to a less extent and by damaging the cells as evidenced by release of cellular lactate dehydrogenase. However, allicin one of the most effective organosulfurs of fresh garlic in vitro shows rapid reduction in the blood and appears to interact with the iron in hemoglobin, oxidizing it to the trivalent form to produce methemoglobin [116].
3. The antiproliferative actions on human cancers
Evidence for the anticancer effect of OSC comes from both epidemiologic and laboratory investigations. In 1958, Weisberger and Pensky [117] demonstrated in vitro and in vivo that thiosulfinate extracts of garlic inhibited the growth of malignant cells and prevented growth of sarcoma 180 as-cites tumor.
Moreover, it was found thatA 549 lung and BJA-B Burkitt lymphoma cells [118], human prostate cancer cells (LNCaP) [119], human breast cancer cells (MCF-7) [120] and animal mammary tumor cells in culture [121] were more sensitive to the antiproliferative effects of DATS and ajoene than the non-neoplastic cells. However, the antiproliferative effects of allyl sulfides are reversible [122] and both ajoene and SAMC have been found to be inversely proportional to cell density [118,123].
The transformation of normal to neoplastic cells in vivo involves at least three distinctive phases, initiation, promotion and progression. Much of the work focused on OSC to suppress tumor incidence in breast [23,25,124–127]. In rats DMBA-induced mammary tumor, treatment with Se-garlic inhibited both the initiation and post-initiation phases of chemical carcinogenesis [128]. Organosulfur compounds suppressed tumor incidence in skin [129,130], uterine [131], oesophagus [132], gastric [133,134], human prostate [135] and colon [26,136] cancer models. It was also reported that diallyl disulfide is as effective as 5-fluorouracil (5-FU) in inhibiting tumor growth. Although, combining the diallyl disulfide and 5-FU did not increase the effect, concurrent diallyl disulfide did significantly reduce the depression of leukocytes counts and splenic weight associated with chemo-therapy administration [137].
3.1. Mechanisms of action
While the allyl group appears to be responsible for the growth depression, not all allyl sulfides are equal in their ability to reduce tumor proliferation. The magnitude of the increase in the G2/M phase of the cell cycle reflects the antiproliferative potential of allyl sulfur compounds. DADS and diallyl trisulfide (DATS) were more effective in the cell cycle alteration and growth of neoplasms inhibition than soluble allyl sulfur compounds such as SAC. Increased DADS, DAS or SAMC exposure led to a proportional but not permanent increase in the percentage of cells arrested in the G2/M phase of the cell cycle [122,138,139]. G2/M phase arrest induced by the allyl sulfur compounds coincide with a suppression in p34cdc2 kinase activity. The p34cdc2 kinase complex which governs the progression of cells from the G2 into the M phase of the cell cycle, is controlled by the association of the p34cdc2 catalytic unit with the cyclin B1 regulatory unit [140]. Activation of this complex is governed by both cyclin B1 protein synthesis and degradation and by the phosphorylation and dephosphorylation of threonine and tyrosine residues on the p34cdc2 subunit [140,141]. It promotes chromosomal condensation and cytoskeletal organization through the phosphorylation of multiple substrates, including histone H1 [141,142]. Factors that inhibit p34cdc2 kinase activity lead to a block in the G2/M phase.
The effects of allium derivatives is mediated by various mechanisms, including blockage of N-nitroso compound (NOC) formation. A reduction in nitrosamines may occur as a result of enhanced formation of nitrosothiols after ingestion of allium foods [143–146] and the bioactivation and carcinogenicity of several non-nitrosamines [147]. It suppresses also the bioactivation of several carcinogens [148–150]. Depressed carcinogen bioactivation may be related to cyclooxygenase and lipoxygenase activity reduction [150–152].
Ajoene a major component of garlic induces apoptosis in a human promyeloleukemic cell line (HL-60) as well as in peripheral blood cells of a chronic leukemic patients suffering from a myeloid blast crisis. In contrast ajoene does not induce apoptosis in proliferating as well as non-proliferating PMBC of healthy human donors. [153]. Induction of apoptosis and peroxide production by ajoene may be linked through activation of NF-kB which is known to be induced by oxidative stress. NF-kB has been shown to be involved in signaling of apoptotic processes [154] regulation of the cell cycle [155,156], enhanced DNA repair [157], modifying the signal transduction pathway, and regulating nuclear factors involved in immune function and inflammation [65]. In animal studies, AGE is reported to induce release of IL-2, TNFα and INFγ [158].
The loss of cancer progression with allyl sulfur compounds can be also related to several epigenetic changes such as DNA methylation and histone acetylation that can be modified by enhanced intake of allyl sulfur compounds. Thus, after treatment with N-nitrosomethylbenzylamine, DAS inhibited the formation of O6-methyldeoxyguanosine in lung by 78% [162] and DADS caused a marked increase in the acetylation of H4 and H3 histones in DS19 and K562 human leukemic cells [163].
3.2. Inhibition of protooncogenes
DADS can also inhibits the growth of H-ras oncogene-transformed tumors in nude mice. This inhibition correlate with the inhibition of p21H-ras membrane association in the tumor tissue [164]. The ras family of protooncogenes encode 21-kDa proteins (p21ras) which play an important role in the transduction of extracellular signals to the cell nucleus [165,166]. Plasma membrane association of mutated p21ras encoded by oncogenic ras, is essential for its cell transformation activity. A lipid post-translational modification (farnesylation) is critical for the plasma membrane association of p21ras and this reaction is catalyzed by a specific cytosolic enzyme, farnesyltransferase [167–169]. The inhibition of p21ras farnesylation can be achieved either by inhibiting farnesyltransferase activity or by lowering the farnesylpyro-phosphate pool through inhibition of HMG-CoA reductase activity. Naturally occurring organosulfides from garlic were shown to inhibit chemically induced cancers, including those in which tumorigenesis is associated with the activation of ras oncogenes. The organosulfides-mediated suppression of tumor growth correlates with the inhibition of p21H-ras membrane association in tumor tissues. It seems that this occurs via depletion of the farnesyl pyrophosphate pool through inhibition of hepatic as well as tumoral HMG-Co A reductase activity [26,164,170].
3.3. Antibacterial activity
While the exact cause of gastric cancer is not understood, there is strong evidence that adenocarcinomas of the distal stomach are largely due to environmental exposures early in life, including a diet rich in highly salted foods and infection by the bacterium Helicobacter pylori. Bacterial activation of procarcinogens may lead to the development of metaplasia dysplasia and ultimately carcinoma [159]. Garlic extract exhibited a selective potency against H. pylori. Thiosulfinates and particularly allicin account for all the antibiotic activity. Upon reduction of allicin to diallyl disulfide, the antibacterial activity is greatly reduced [134,160]. Allicin exhibits its antimicrobial activity by a rapid and total inhibition of RNA synthesis [161]. The lipid content of the membranes and their electrical charge will have an effect on the permeability of allicin [160].
4. Conclusion
A plurality of factors likely lead to ROS-induced tissue damage that contribute to a wide variety of diseases. However, reducing endogenously or exogenous can block the toxic effects. Thus, garlic-derived allylsulfides may exert also their anticarcinogenic effects in experimental animals and in cell culture systems by blocking P450 enzymes included in carcinogen activation and/or by enhancing P450 enzymes that catabolize carcinogens to less reactive intermediates [171–173]. Allium constituents inhibit the covalent binding of carcinogens to DNA [124] and inhibit the activities of demethylating and hydroxylating cytochromes P450 2E1, 2B1, 1A1 and 1A2 in both hormone-responsive and hormone unresponsive cells [174,175]. In addition allium derivatives stimulate the GSH synthesis and enhance glutathione peroxidase activity [176]. Allium has been shown to induce phase II conjugation systems which inactivate most carcinogens. Ingestion of allium derivatives by rats increases the activity of gutathione-S-transferase (GST) in both liver and mammary tissue [24,177–180]. However, not all GST isozymes are influenced equally and induction of GST pi may be particularly important in the anticarcinogenic properties associated with allyl sulfur components [181].
It was also reported that S-methylcysteine a water soluble organosulfur compound prevented elevation of ornithine decarboxylase and spermidine/spermine N1-acetyltransferase (SAT) both rate limiting enzymes of polyamine metabolism which are increased by chemically induced carcinogenesis [182]. Ornithine decarboxylase, contains nucleophilic thiol moieties at cysteines 360 and 70 that are highly accessible to oxidants and sulfhydril agents [183]. Depletion of reduced GSH by oxidative stress produces a concomitant induction of ornithine decarboxylase, whereas treatment of cells with compounds that increase reduced GSH formation inhibits ornithine decarboxylase induction [184]. The balance of polyamines and reduced glutathione may be critical to the regulation of cell proliferation and differentiation. S-Allylmercaptocysteine inhibits ornithine decarboxylase activity either by elevating intracellular reduced GSH, which in turn, inhibits induction of ornithine decarboxylase, or by reacting directly with ornithine decarboxylase at the nucleophilic thiol moieties of cysteines or both [119]. Thus, garlic derivatives markedly influence the steady state concentration of reduced GSH as well as the activities of enzymes that control its metabolism.
References
- [1].Witztum JL. The role of oxidized low density lipoproteins in athero-genesis. Br Heart J 1993;69:12–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Richardson SJ. Free radicals in the genesis of Alzheimer’s disease. Ann NY Acad Sci 1993;695:73–6. [DOI] [PubMed] [Google Scholar]
- [3].Borek C Free radical processes in multistage carcinogenesis. Free Radic Res Commun 1991;12:745–50. [DOI] [PubMed] [Google Scholar]
- [4].Borek C Molecular mechanisms in cancer induction and prevention. Environ Health Perspect 1993;101:237–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kourounakis PN, Rekka EA. Effect on active oxygen species of alliin and Allium sativum (garlic) powder. Res Commun Chem Pathol Pharmacol 1991;74:249–52. [PubMed] [Google Scholar]
- [6].Borek C. Antioxidants and cancer. Sci Med 1997;4:51–62. [Google Scholar]
- [7].Lawson LD. Bioactive organosulfur compounds of garlic and garlic products: role in reducing blood lipids.ACS Symp Ser 1993;534:306–30. [Google Scholar]
- [8].Horie T, Awazu S, Itakura Y, Fuwa T. Identified diallyl polysulfides from an aged garlic extract which protects the membranes from lipid peroxidation. Planta Med 1992;58:468–9. [DOI] [PubMed] [Google Scholar]
- [9].Ide N, Lau BHS. Garlic compounds protect vascular endothelial cells from oxidized low density lipoprotein-induced injury. J Pharm Pharmacol 1997;49:908–11. [DOI] [PubMed] [Google Scholar]
- [10].Wei Z, Lau BHS. Garlic inhibits free radical generation and augments antioxidant enzyme activity in vascular endothelial cells. Nutr Res 1998;18:61–70. [Google Scholar]
- [11].Yamasaki T, Li L, Lau BHS. Garlic compounds protect vascular endothelial cells from hydrogen peroxide-induced oxidant injury. Phytother Res 1994;8:408–12. [Google Scholar]
- [12].Yamasaki T, Teel RW, Lau BHS. Effect of allixin, a phytoalexin produced by garlic, on mutagenesis, DNA-binding and metabolism of aflatoxin B1. Cancer Lett 1991;59:89–94. [DOI] [PubMed] [Google Scholar]
- [13].Ryu K, Ide N, Matsuura H, Itakura Y. N-(1-deoxy-D-fructos-1-yl)-L-arginine, an antioxidant compound identified in aged garlic extract. J Nutr 2001;131:972S–6S. [DOI] [PubMed] [Google Scholar]
- [14].Ide N, Nelson AB, Lau BHS. Aged garlic extract and its constituents inhibit Cu2+-induced oxidative modification of low density lipoprotein. Planta Med 1997;63:263–4. [DOI] [PubMed] [Google Scholar]
- [15].Geng Z, Lau BHS. Aged garlic extract modulates glutathione redox cycle and superoxide dismutase activity in vascular endothelial cells. Phytother Res 1997;11:54–6. [Google Scholar]
- [16].Liu JZ, Lin XY, Milner JA. Dietary garlic powder increases glutathione content and glutathione S-transferase activity in rat liver and mammary tissues. FASEB J 1992;6:A3230. [Google Scholar]
- [17].Reeve VE, Bosnic M, Rosinova E, Boehm-Wilcox C. A garlic extract protects from ultraviolet B (280–320 nm) radiation induced suppression of contact hypersensitivity. Photochem Photobiol 1993;58:813–7. [DOI] [PubMed] [Google Scholar]
- [18].Gutteridge JMC. Free radicals in disease processes: a compilation of cause and consequence. Free Radic Res Commun 1993;19:141–58. [DOI] [PubMed] [Google Scholar]
- [19].Efendy JL, Simmons DL, Campbell GR, Campbell JH. The effect of the aged garlic extract, “Kyolic” on the development of experimental atherosclerosis. Atherosclerosis 1997;132:37–42. [DOI] [PubMed] [Google Scholar]
- [20].Imai J, Ide N, Nagae S, Moriguchi T, Matsuura H, Itakura Y. Antioxidant and radical scavenging effects of aged garlic extract and its constituents. Planta Med 1994;60:417–20. [DOI] [PubMed] [Google Scholar]
- [21].Ide N, Matsuura H, Itakura Y. Scavenging effect of aged garlic extract and its constituent on active oxygen species. Phytother Res 1996;10: 340–1. [Google Scholar]
- [22].Ohnishi ST, Kojinra R. Antioxidant activities of aged garlic extracts and cancer chemotherapy In: Lachance PA, editor. Nutraceuticals designer foods III garlic, soy and licorice. Trumbull (CT): Food & Nutrition Press; 1997. p. 105–15. [Google Scholar]
- [23].Amagase H, Milner JA. Impact of various sources of garlic and their constituents on 7,12-dimethylbenz(a)anthracene binding to mammary cell DNA. Carcinogenesis 1993;14:1627–31. [DOI] [PubMed] [Google Scholar]
- [24].Hatono S, Jimenez A, Wargovich MJ. Chemopreventive effect of S-allylcysteine and its relationship to the detoxification enzyme glutathione S-transferase. Carcinogenesis 1996;17:1041–4. [DOI] [PubMed] [Google Scholar]
- [25].Nishino H, Iwashima A, Itakura Y, Matsuura H, Fuwa T. Antitumor-promoting activity of garlic extracts. Oncology 1989;46:277–80. [DOI] [PubMed] [Google Scholar]
- [26].Sumiyoshi H, Wargovich MJ. Chemoprevention of 1,2-dimethylhydrazine-induced colon cancer in mice by naturally occurring organosulfur compounds. Cancer Res 1990;50:5084–7. [PubMed] [Google Scholar]
- [27].Okuhara T Clinical study of aged garlic extract on peripheral circulation. Jpn Pharmacol Ther 1994;22:3695–701. [Google Scholar]
- [28].Yeh YY, Yeh SM. Garlic reduces plasma lipids by inhibiting hepatic cholesterol and triaglycerol synthesis. Lipids 1994;29:189–93. [DOI] [PubMed] [Google Scholar]
- [29].Steiner M, Lin RS. Changes in platelet function and susceptibility of lipoproteins to oxidation associated with administration of aged garlic extract. J Cardiovasc Pharmacol 1998;31:904–8. [DOI] [PubMed] [Google Scholar]
- [30].Hirao Y, Sumioka S, Yamamoto M, Hatono S, Yoshida S, Fuwa T. Activation of immunoresponder cells by the protein fraction from aged garlic extract. Phytother Res 1987;1:161–4. [Google Scholar]
- [31].Abdullah TH, Kirkpatrick DV, Carter J. Enhancement of natural killer cell activity in AIDS with garlic. J Oncol 1989;21:52–3. [Google Scholar]
- [32].Lau BHS, Yamasaki T, Gridley DS. Garlic compounds modulate macrophage and T-lymphocyte functions. Mol Biother 1991;3:103–7. [PubMed] [Google Scholar]
- [33].Morioka N, Sze LL, Morton DL, Irie RFA. Protein fraction from aged garlic extract enhances cytotoxicity and proliferation of human lymphocyte mediated by interleukin-2 and concavalin A. Cancer Immunol Immunother 1993;37:316–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kyo E, Uda N, Kakimoto M, Yokoyama K, Ushijima M, Sumioka I. Antiallergic effects of aged garlic extract. Phytomedicine 1997;4: 335–40. [DOI] [PubMed] [Google Scholar]
- [35].Nakagawa S, Kasuga S, Matsuura H. Prevention of liver damage by aged garlic extract and its components in mice. Phytother Res 1986; 3:50–3. [Google Scholar]
- [36].Moriguchi T, Saito H, Nishiyama N. Aged garlic extract prolongs longevity and improves spatial memory deficit in senescence-accelerated mouse. Biol Pharm Bull 1996;19:305–7. [DOI] [PubMed] [Google Scholar]
- [37].Moriguchi T, Takasugi N, Itakura Y. The effects of aged garlic extract on lipid peroxidation and the deformability of erythrocytes. J Nutr 2001;131:1016S–9S. [DOI] [PubMed] [Google Scholar]
- [38].Steyn K, Steyn M, Swanepoel AS, Jordaan PC, Jooste PL, Fourie JM, et al. Twelve-year results of the coronary risk factor study (CORIS). Int J Epidemiol 1997;26:964–71. [DOI] [PubMed] [Google Scholar]
- [39].Howard G, Wagenknecht LE, Burke GL, Diez-Roux A, Evans GW, McGovern P, et al. Cigarette smoking and progression of atherosclerosis: the atherosclerosis risk in communities (ARIC) study. J Am Med Assoc 1998;279:119–24. [DOI] [PubMed] [Google Scholar]
- [40].Villeneuve PJ, Morrison HI, Craig CL, Schaubel DE. Physical activity, physical fitness, and risk of dying. Epidemiology 1998;9:626–31. [PubMed] [Google Scholar]
- [41].Schwartz CJ, Valente AJ, Sprague EAA. Modern view of atherogenesis. Am J Cardiol 1993;71:9b–14b. [DOI] [PubMed] [Google Scholar]
- [42].Fuster VF, Badimon L, Badimon JJ, Chesebro JH. Mechanisms of disease: the pathogenesis of coronary artery disease and the acute coronary syndromes. N Engl J Med 1992;326:242–50. [DOI] [PubMed] [Google Scholar]
- [43].Ross R The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature (Lond) 1993;362:801–9. [DOI] [PubMed] [Google Scholar]
- [44].Dusting GJ, Fennessy P, Yin Z-L, Gurevih V. Nitric oxide in atherosclerosis: vascular protector or villain j. Clin Exp Pharmacol Physiol 1998;25:S34–41. [DOI] [PubMed] [Google Scholar]
- [45].Luscher TF, Noll G. The pathogenesis of cardiovascular disease: role of the endothelium as a target and mediator. Atheroslerosis 1995;118: S81–90. [PubMed] [Google Scholar]
- [46].Kannel WB, Castelli WP, Gordon T, McNamara PM. Serum cholesterol, lipoproteins, and the risk of coronary heart disease. Ann Intern Med 1971;74:1–12. [DOI] [PubMed] [Google Scholar]
- [47].Steinberg D, Parthasarathy S, Carew TE, Witztum JL. Beyond cholesterol: modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med 1989;320:915–24. [DOI] [PubMed] [Google Scholar]
- [48].Berliner JA, Heinecke JW. The role of oxidized lipoproteins in athero-genesis. Free Radic Biol Med 1996;20:707–27. [DOI] [PubMed] [Google Scholar]
- [49].Cox DA, Cohen ML. Effects of oxidized low-density lipoprotein on vascular contraction and relaxation: clinical and pharmacological implications in atherosclerosis. Pharmacol Rev 1996;48:3–19. [PubMed] [Google Scholar]
- [50].Henriksen T, Mahoney EM, Steinberg D. Enhanced macrophage degradation of low density lipoprotein previously incubated with cultured endothelial cells: recognition by receptors for acetylated low density lipoprotein. Proc Natl Acad Sci USA 1981;78:6449–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Henriksen T, Mahoney EM, Steinberg D. Enhanced macrophage degradation of biologically modified low density lipoprotein. Arteriosclerosis 1983;3:149–59. [DOI] [PubMed] [Google Scholar]
- [52].Cathcart MK, Morel DW, Chisolm GM. Monocytes and neutrophils oxidize low density lipoprotein making it cytotoxic. J Leukoc Biol 1985;38:341–50. [DOI] [PubMed] [Google Scholar]
- [53].Rosenfeld ME, Palinski W,Yla-Herttuala S, Carew TE. Macrophages, endothelial cells, and lipoprotein oxidation in the pathogenesis of atherosclerosis. Toxicol Pathol 1990;18:560–71. [PubMed] [Google Scholar]
- [54].Darley-Usmar VM, Hogg N, O’Leary VJ, Wilson MT, Moncada S. The simultaneous generation of superoxide and nitric oxide can initiate lipid peroxidation in human low density lipoprotein. Free Radic Res Commun 1992;17:9–20. [DOI] [PubMed] [Google Scholar]
- [55].Gerrity RG. The role of the monocyte in atherogenesis I: transition of blood borne monocytes into foam cells in fatty lesions. Am J Pathol 1981;103:181–90. [PMC free article] [PubMed] [Google Scholar]
- [56].Holvoet P, Collen D. Oxidized lipoproteins in atherosclerosis and thrombosis. FASEB J 1995;8:1279–84. [DOI] [PubMed] [Google Scholar]
- [57].Guretzki HJ, Gerbitz KD, Olgemoller B, Schleicher E. Atherogenic levels of low density lipoprotein alter the permeability and composition of the endothelial barrier. Atherosclerosis 1994;107:15–24. [DOI] [PubMed] [Google Scholar]
- [58].Quinn MT, Parthasarathy S, Fong LG, Steinberg D. Oxidatively modified low density lipoproteins: a potential role in recruitment and retention of monocyte/macrophages during atherogenesis. Proc Natl Acad Sci USA 1987;84:2995–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Kuzuya M, Naito M, Funaki C, Hayashi T, Asai K, Kuzuya F. Lipid peroxide and transition metals are required for the toxicity of oxidized low density lipoprotein to cultured endothelial cells. Biochim Biophys Acta 1991;1096:155–61. [DOI] [PubMed] [Google Scholar]
- [60].Marletta MA, Yoon PS, Iyengar R, Leaf CD, Wishnok JS. Macrophage oxidation of L-arginine to nitrite and nitrate: nitric oxide is an intermediate. Biochemistry 1988;27:8706–11. [DOI] [PubMed] [Google Scholar]
- [61].Durum SK, Oppenheim JJ. Macrophage-derived mediators: interleukin-1, tumor necrosis factor, interleukin-6, interferon and related cytokines 2nd ed In: Paul WE, editor. Fundamental immunology. Lancaster (CA): Raven Press; 1989. p. 639–61. [Google Scholar]
- [62].Fu J-Y, Masferrer JL, Seibert K, RazA. The induction and suppression of prostaglandin H2 synthase (cyclooxygenase) in human monocytes. J Biol Chem 1990;265:16737–40. [PubMed] [Google Scholar]
- [63].Grimm S, Baeuerle PA. The inducible transcription factor NF-B: structure–function relationship of its protein subunits. Biochem J 1993;290:297–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Sen CK, Packer L.Antioxidant and redox regulation of gene transcription. FASEB J 1996;10:709–20. [DOI] [PubMed] [Google Scholar]
- [65].Geng Z, Rong Y, Lau BHS. S-Allylcysteine inhibits activation of nuclear factor kappa B in human T cells. Free Radic Biol Med 1997;23:345–50. [DOI] [PubMed] [Google Scholar]
- [66].Meyer R, Caselmann WH, Schluter V, Schreck R, Hofschneider PH, Baeuerle PA. Hepatitis B virus transactivator MHBst: activation of NF-kappa B, selective inhibition by antioxidants and integral membrane location. EMBO J 1992;11:2992–3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Schreck R, Meier B, Maennel DN, Droge W, Baeuerle PA. Dithiocarbamates as potent inhibitors of nuclear factor B activation in intact cells. J Exp Med 1992;175:1181–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Sen CK, Traber K, Packer L. Inhibition of NF-B activation in human T-cell lines by anetholdithiolthione. Biochem Biophys Res Commun 1996;218:148–53. [DOI] [PubMed] [Google Scholar]
- [69].Kuzuya M, Naito M, Funaki C, Hayashi T, Asai K, Kuzuya F. Probucol prevents oxidative injury to endothelial cells. J Lipid Res 1991;32: 197–204. [PubMed] [Google Scholar]
- [70].Schmitt A, Salvayre R, Delchambre J, Negre-Salvayre A. Prevention by alpha-tocopherol and rutin of glutathione and ATP depletion induced by oxidized LDL in cultured endothelial cells. Br J Pharmacol 1995;116:1985–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Keaney JF Jr, Gaziano JM, Xu A, Frei B, Curran-Celentano J, Shwaery GT, et al. Low-dose alpha-tocopherol improves and high-dose alpha-tocopherol worsens endothelial vasodilator function in cholesterol-fed rabbits. J Clin Investig 1994;93:844–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Stewart-Lee AL, Forster LA, Nourooz-Zadeh J, Ferns GAA, Anggard EE. Vitamin E protects against impairment of endothelium-mediated relaxations in cholesterol-fed rabbits. Arterioscler Thromb 1994;14:494–9. [DOI] [PubMed] [Google Scholar]
- [73].Anderson TJ, Meredith IT,Yeung AC, Frei B, Selwyn AP, Ganz P. The effect of cholesterol-lowering and antioxidant therapy on endothelium-dependent coronary vasomotion. N Engl J Med 1995; 332:488–93. [DOI] [PubMed] [Google Scholar]
- [74].Verdeuren TJ, Jordaens FH, Zonnekeyn LL, Van Hove CE, Coene MC, Herman AG. Effect of hypercholesterolemia on vascular reactivity in the rabbit. Circ Res 1986;58:552–64. [DOI] [PubMed] [Google Scholar]
- [75].Lopez JA, Armstrong M, Harisson D, Piegors D, Heistad DD. Vascular responses to leukocytes products in atherosclerotic primates. Circ Res 1989;65:1078–86. [DOI] [PubMed] [Google Scholar]
- [76].Cooke JP, Anon NA, Girerd XJ, Hirsch AT, Creager MA. Arginine restores cholinergic relaxation of hypercholesterolemic rabbit thoracic aorta. Circulation 1991;83:1057–62. [DOI] [PubMed] [Google Scholar]
- [77].Shimokawa H, Vanhoutte PM. Impaired endothelium dependent relaxation to aggregating platelets and related vasoactive substances in porcine coronary arteries in hypercholesterolemia and atherosclerosis. Circ Res 1989;64:900–14. [DOI] [PubMed] [Google Scholar]
- [78].Gryglewski RJ, Palmer RM, Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived relating factor. Nature (Lond) 1986;320:454–6. [DOI] [PubMed] [Google Scholar]
- [79].Rubanyi GM, Vanhoutte PM. Oxygen-derived free radicals, endothelium, and responsiveness of vascular smooth muscle. Am J Physiol 1986;250:H815–21. [DOI] [PubMed] [Google Scholar]
- [80].Welch GN, Upchurch GR, Loscalzo J. Homocysteine, oxidative stress, and vascular disease. Hosp Pract 1997;32:81–92. [DOI] [PubMed] [Google Scholar]
- [81].Abby SL, Harris IM, Harris KM. Homocysteine and cardiovascular disease. J Am Board Fam Pract 1998;11:391–8. [DOI] [PubMed] [Google Scholar]
- [82].Jakubowski H Protein N-homocysteinylation: implications for atherosclerosis. Biomed Pharmacother 2001;55:443–7. [DOI] [PubMed] [Google Scholar]
- [83].Tapiero H, Tew KD, Gaté L, Machover D. Prevention of pathologies associated with oxidative stress and dietary intake deficiencies: folate deficiency and requirement. Biomed Pharmacother 2001;55:381–90. [DOI] [PubMed] [Google Scholar]
- [84].Ashfield-Watt PAL, Moat SJ, Doshi SN, McDowell IFW. Folate, homocysteine, endothelial function and cardiovascular disease. What is the linkj. Biomed Pharmacother 2001;55:425–33. [DOI] [PubMed] [Google Scholar]
- [85].Chern C-L, Huang R-FS, Chen Y-H, Cheng J-T, Liu T-Z. Folate deficiency-induced oxidative stress and apoptosis are mediated via homocysteine-dependent overproduction of hydrogen peroxide and enhanced activation of NF-kB in huma Hep G2 cells. Biomed Pharmacother 2001;55:434–42. [DOI] [PubMed] [Google Scholar]
- [86].Lau B, Lam F, Wang-Chen R. Effect of an odour-modified garlic preparation on blood lipids. Nutr Res 1987;7:139–49. [Google Scholar]
- [87].Phelps S, Harris W. Garlic supplementation and lipoprotein oxidation susceptibility. Lipids 1993;28:475–7. [DOI] [PubMed] [Google Scholar]
- [88].Bordia AK, Joshi HK, Sanadhya YK, Bhu N. Effect of essential oil of garlic on serum fibrinolytic activity in patients with coronary artery disease. Atherosclerosis 1977;28:155–9. [DOI] [PubMed] [Google Scholar]
- [89].Harenberg J, Giese C, Zimmermann R. Effect of dried garlic on blood coagulation, fibrinolysis, platelet aggregation and serum cholesterol levels in patients with hyperlipoprotienemia. Atherosclerosis 1988; 74:247–9. [DOI] [PubMed] [Google Scholar]
- [90].Das I, Khan NS, Sooranna SR. Potent activation of nitric oxide synthase by garlic: a basis for its therapeutic applications. Curr Med Opin 1995;13:257–63. [DOI] [PubMed] [Google Scholar]
- [91].Moncada S, Higgs EA. Molecular mechanisms and therapeutic strategies related to NO. FASEB J 1995;9:1319–30. [PubMed] [Google Scholar]
- [92].Hurani SMO, Hall DA. Receptors for ADP on human blood platelets. Trends Pharmacol Sci 1994;15:103–8. [DOI] [PubMed] [Google Scholar]
- [93].Bordia A Effect of garlic on human platelet aggregation in vitro. Atherosclerosis 1978;30:355–60. [DOI] [PubMed] [Google Scholar]
- [94].Boullin DJ. Garlic as a platelet inhibitor. Lancet 1981;1:776–7. [DOI] [PubMed] [Google Scholar]
- [95].Srivastava KC. Evidence for the mechanism by which garlic inhibits platelet aggregation. Prostaglandins Leukot Med 1986;22:313–21. [DOI] [PubMed] [Google Scholar]
- [96].Ali M, Mohamed SY. Selective suppression of platelet thromboxane formation by sparing of vascular prostacyclin synthesis by aqueous extract of garlic in rabbits. Prostaglandins Leukot Med 1986;25:139–46. [DOI] [PubMed] [Google Scholar]
- [97].Lawson LD, Ransom DK, Hughes BF. Inhibition of whole blood platelet aggregation by compounds in garlic clove extracts and commercial garlic products. Thromb Res 1992;65:141–56. [DOI] [PubMed] [Google Scholar]
- [98].Legnani C, Frascaro M, Guazzaloca G, Ludovici S, Cesarano G, Coccheri S. Effects of a dried garlic preparation on fibrinolysis and platelet aggregation in healthy subjects. Drug Res 1993;43:119–22. [PubMed] [Google Scholar]
- [99].Apitz-Castro R, Jain MK, Bartoli F, Ledezma E, Ruiz MC, Salas R. Evidence for direct coupling of primary agonist-receptor interaction to the exposure of functional IIb–IIIa complexes in human blood platelets: results from studies with the antiplatelet compound ajoene. Biochim Biophys Acta 1994;1094:269–80. [DOI] [PubMed] [Google Scholar]
- [100].Apitz-Castro R, Badimon JJ, Badimon LA. Garlic derivative ajoene, inhibits platelet deposition on severely damaged vessel wall in an in vivo porcine experimental model. Thromb Res 1994;75:243–9. [DOI] [PubMed] [Google Scholar]
- [101].Weinberg D, Manier ML, Richardson M, Haibach F. Identification and quantification of anticarcinogens in garlic extract and licorice root extract powder. J High Resolut Chromatogr 1992;15:641–54. [Google Scholar]
- [102].Steiner M, Li W. Aged garlic extract, modulator of cardiovascular risk factor: a dose-finding study on the effects of AGE on platelet functions. J Nutr 2001;131:980S–4S. [DOI] [PubMed] [Google Scholar]
- [103].Steiner M, Kahn AH, Holbert D, Lin RIA. Double blind crossover study in moderately hypercholesterolemic men that compared the effect of aged garlic extract and placebo administration on blood lipids. Am J Clin Nutr 1996;64:866–70. [DOI] [PubMed] [Google Scholar]
- [104].Gebhardt R Multiple inhibitory effects of garlic extracts on cholesterol biosynthesis in hepatocytes. Lipids 1993;28:613–9. [DOI] [PubMed] [Google Scholar]
- [105].Cho BHS, Xu S. Effects of allyl mercaptan and various Allium-derived compounds on cholesterol synthesis and secretion in Hep-G2 cells. Comp Biochem Physiol 2000;126:195–201. [DOI] [PubMed] [Google Scholar]
- [106].Aouadi R, Aouidet A, Elkadhi A, Ben Rayana MC, Jaafoura H, Tritar B, et al. Effect of fresh garlic (Allium sativum) on lipid metabolism in male rats. Nutr Res 2000;20:273–80. [Google Scholar]
- [107].Chi MS, Koh ET, Stewart TJ. Effect of garlic on lipid metabolism in rats fed cholesterol or lard. J Nutr 1982;112:241–8. [DOI] [PubMed] [Google Scholar]
- [108].Qureshi AA, Din ZZ, Abuirmeileh N, Burger WC, Ahmad Y, Elson CE. Suppression of avian hepatic metabolism by solvent extracts of garlic: impact of serum lipids. J Nutr 1983;113:1746–55. [DOI] [PubMed] [Google Scholar]
- [109].Stevinson C, Pittler MH, Ernst E. Garlic for treating hypercholesterolemia. Ann Intern Med 2000;133:420–9. [DOI] [PubMed] [Google Scholar]
- [110].Warshafsky S, Kamer RS, Sivak SL. Effect of garlic on total serum cholesterol: a meta-analysis. Ann Intern Med 1993;119:599–605. [DOI] [PubMed] [Google Scholar]
- [111].Bordia A, Verma SK, Srivastava KC. Effect of garlic (Allium sativum) on blood lipids, blood sugar, fibrinogen and fibrinolytic activity in patients with coronary artery disease. Prostaglandins Leukot Essent Fatty Acids 1998;58:257–63. [DOI] [PubMed] [Google Scholar]
- [112].Adler AJ, Holub BJ. Effect of garlic and fish-oil supplementation on serum lipid and lipoprotein concentrations in hypercholesterolemic men. Am J Nutr 1997;65:445–50. [DOI] [PubMed] [Google Scholar]
- [113].Liu L, Yeh YY. Inhibition of cholesterol biosynthesis by organosulfur compounds derived from garlic. Lipids 2000;35:197–203. [DOI] [PubMed] [Google Scholar]
- [114].Focke M, Feld A, Lichtenthaler K. Allicin, a naturally occurring antibiotic from garlic, specifically inhibits acetyl-CoA synthetase. FEBS Lett 1990;261:106–8. [DOI] [PubMed] [Google Scholar]
- [115].Sendl A, Schliack M, Loser R, Stanislaus F, Wagner H. Inhibition of cholesterol synthesis in vitro by extracts and isolated compounds prepared from garlic and wild garlic. Atherosclerosis 1992;94:79–85. [DOI] [PubMed] [Google Scholar]
- [116].Freeman F, Kodera Y. Garlic chemistry: stability of S-(2-propenyl)-2-propene-1-sulfothiate (allicin) in blood, solvents, and simulated physiological fluids. J Agric Food Chem 1995;43:2332–8. [Google Scholar]
- [117].Weisberger AS, Pensky J. Tumor inhibition by a sulfhydryl-blocking agent related to an active principle of garlic (Allium sativum). Cancer Res 1958;18:1301–8. [PubMed] [Google Scholar]
- [118].Scharfenberg K, Wagner R, Wagner KG. The cytotoxic effect of ajoene a natural product from garlic investigated with different cell lines. Cancer Lett 1990;53:103–8. [DOI] [PubMed] [Google Scholar]
- [119].Pinto JT, Qiao CH, Xing J, Rivlin RS, Protomastro ML, Weissler ML, et al. Effects of garlic thioallyl derivatives on growth, glutathione concentration, and polyamine formation of human prostate carcinoma cells in culture. Am J Clin Nutr 1997;66:398–405. [DOI] [PubMed] [Google Scholar]
- [120].Li G, Qiao CH, Lin RI, Pinto JT, Osborne MP, Tiwari RK. Antiproliferative effects of garlic constituents on culture human breast cancer cells. Oncol Rep 1995;2:787–91. [DOI] [PubMed] [Google Scholar]
- [121].Sundaram SG, Milner JA. Impact of organosulfur compounds in garlic on canine mammary tumor cells in culture. Cancer Lett 1993; 74:85–90. [DOI] [PubMed] [Google Scholar]
- [122].Knowles LM, Milner JA. Depressed p34cdc2 kinase activity and G2/M phase arrest induced by diallyl disulfide in HCT-15 cells. Nutr Cancer 1998;30:169–74. [DOI] [PubMed] [Google Scholar]
- [123].Sigounas G, Hooker J, Anagnostou A, Steiner M. S-allylmercaptocysteine inhibits cell proliferation and reduces the viability of erythroleukemia, breast and prostate cancer cell lines. Nutr Cancer 1997;27:186–91. [DOI] [PubMed] [Google Scholar]
- [124].Milner JA. Garlic: its anticarcinogenic and antitumorigenic properties. Nutr Rev 1996;54:S82–6. [DOI] [PubMed] [Google Scholar]
- [125].Ip C, Lisk DJ, Stoewsand GS. Mammary cancer prevention by regular garlic and selenium-enriched garlic. Nutr Cancer 1992;7:279–86. [DOI] [PubMed] [Google Scholar]
- [126].Liu JZ, Lin RI, Milner JA. Inhibition of 7,12-dimethylbenz(a)-anthracene induced mammary tumors and DNA adducts by garlic powder. Carcinogenesis 1992;13:1847–51. [DOI] [PubMed] [Google Scholar]
- [127].Fleishchauer AT, Arab L. Garlic and cancer: a critical review of the epidemiologic literature. J Nutr 2001;131:1032S–40S. [DOI] [PubMed] [Google Scholar]
- [128].Ip C, Lisk DJ. Efficacy of cancer prevention by high selenium-garlic is primarily dependent on the action of selenium. Carcinogenesis 1995; 16:2649–52. [DOI] [PubMed] [Google Scholar]
- [129].Shukla Y, Singh A, Srivastava B. Inhibition of carcinogen-induced activity of gamma-glutamyl transpeptidase by certain dietary constituents in mouse skin. Biomed Environ Sci 1999;12:110–5. [PubMed] [Google Scholar]
- [130].Takeyama H, Hoon DSB, Saxton RE, Morton DL, Irie RF. Growth inhibition and modulation of cell markers of melanoma by S-allylcysteine. Onology 1993;50:63–9. [DOI] [PubMed] [Google Scholar]
- [131].Hussain SP, Jannu LN, Rao AR. Chemopreventive action of garlic on methylcholanthrene-induced carcinogenesis in the uterine cervix of mice. Cancer Lett 1990;49:175–80. [DOI] [PubMed] [Google Scholar]
- [132].Wargovich MJ, Woods C, Eng VW, Stephens LC, Gray K. Chemoprevention of N-nitrosomethylbenzylamine-induced esophageal cancer in rats by the naturally occurring thioether, diallylsulfide. Cancer Res 1988;48:6872–5. [PubMed] [Google Scholar]
- [133].You WC, Blot WJ, Chang YS, Ershow A,Yang ZT, An Q, et al. Allium vegetables and reduced risk of stomach cancer. J Natl Cancer Inst 1989;81:162–4. [DOI] [PubMed] [Google Scholar]
- [134].Reuter HD, Koch HP, Lawson LD. Therapeutic effects and applications of garlic and its preparations In: Koch HP, Lawson LD, editors. Garlic: the science and therapeutic application of Allium sativum L and related species. Baltimore (MD): Williams & Wilkins; 1996. p. 162–72. [Google Scholar]
- [135].Pinto JT, Qiao CH, Xing J, Brian P, Suffoletto BP, Schubert KB, et al. Alterations of prostate biomarkers expression and testosterone utilization in human LNCaP prostatic carcinoma cells by garlic-derived S-allylmercaptocysteine. The Prostate 2000;45:304–14. [DOI] [PubMed] [Google Scholar]
- [136].Steinmetz KA, Kushi LH, Bostick RM, Folsom AR, Potter JD. Vegetables, fruit and colon cancer in the Iowa Woman’s study. J Epidemiol 1994;139:1–5. [DOI] [PubMed] [Google Scholar]
- [137].Sundaram SG, Milner JA. Diallyl disulfide suppresses the growth of human colon tumor cell xenografts in athymic nude mice. J Nutr 1996;126:1355–61. [DOI] [PubMed] [Google Scholar]
- [138].Sigounas G, Hooker J, Li W, Anagnostou A, Steiner M. S-allylmercaptocysteine a stable thioally compound, induces apoptosis in erythroleukemia cell line. Nutr Cancer 1997;28:153–9. [DOI] [PubMed] [Google Scholar]
- [139].Zheng S, Yang H, Zhang S, Wang X, Yu L, Lu J. Initial study on naturally occurring products from traditional Chinese herbs and vegetables for chemoprevention. J Cell Biochem 1997;27:106–12. [PubMed] [Google Scholar]
- [140].Morgan DO. Principles of CDK regulation. Nature (Lond) 1995;374: 131–4. [DOI] [PubMed] [Google Scholar]
- [141].Nurse P Universal control mechanism regulating onset of M-phase. Nature (Lond) 1990;344:503–8. [DOI] [PubMed] [Google Scholar]
- [142].Hartwell LH, Kastan MB. Cell cycle control and cancer. Science (Washington DC) 1994;266:1821–8. [DOI] [PubMed] [Google Scholar]
- [143].Atanasova-Goranova VK, Dimova PI, Pevicharova GT. Effect of food products on endogenous generation of N-nitrosmines in rats. Br J Nutr 1997;78:335–45. [DOI] [PubMed] [Google Scholar]
- [144].Dion ME, Agler M, Milner JA. S-allyl cysteine inhibits nitrosomorpholine formation and bioactivation. Nutr Cancer 1997;28:1–6. [DOI] [PubMed] [Google Scholar]
- [145].Shenoy NR, Choughuley AS. Inhibitory effect of diet related sulfhydryl compounds on the formation of carcinogenic nitrosamines. Cancer Lett 1992;65:227–32. [DOI] [PubMed] [Google Scholar]
- [146].Wattenberg LW, Sparnins VL, Barany G. Inhibition of N-nitrosodiethylamine carcinogenesis in mice by naturally occurring organosulfur compounds and monoterpenes. Cancer Res 1989;49: 2689–92. [PubMed] [Google Scholar]
- [147].Milner JA. A historical perspective on garlic and cancer. J Nutr 201;131:1027S–31S. [DOI] [PubMed] [Google Scholar]
- [148].Haber-Mignard D, Suschetet M, Berges R, Astorg P, Siess MH. Inhibition of aflatoxin B1- and N-nitrosodiethylamine-induced liver preneoplastic foci in rats fed naturally occurring allyl sulfides. Nutr Cancer 1996;25:61–70. [DOI] [PubMed] [Google Scholar]
- [149].Hong JY, Wang ZY, Smith TJ, Zhou S, Shi S, Pan J, et al. Inhibitory effects of diallyl sulfides on the metabolism and tumorigenicity of the tobacco-specific carcinogen 4-(methylnitrosoamino)-1-(3-pyridyl)-1-butanone (NKK) in A/J mouse lung. Carcinogenesis 1992;13:901–4. [DOI] [PubMed] [Google Scholar]
- [150].Liu Y, Levy GN, Weber WW. Activation of 2-aminofluorene by pros-taglandin endoperoxide H synthase-2. Biochem Biophys Res Commun 1995;215:346–54. [DOI] [PubMed] [Google Scholar]
- [151].McGrath BC, Milner JA. Diallyl disulfide, S-allylsulfide and conjugated linoleic acid retard 12/15-lipoxygenase-mediated bioactivation of 7,12-dimethylbenz(a)anthracene (DMBA) in vitro. FASEB J 1999; 13:A540. [Google Scholar]
- [152].Rioux N, Castonguay A. Inhibitors of lipoxygenase: a new class of cancer chemopreventive agents. Carcinogenesis 1998;19:1393–400. [DOI] [PubMed] [Google Scholar]
- [153].Dirsch VM, Gerbes AL, Vollmar AM. Ajoene, a compound of garlic, induces apoptosis in human promyeloleukemic cells accompanied by generation of reactive oxygen species and activation of nuclear factor. Br Molec Pharmacol 1998;53:402–7. [DOI] [PubMed] [Google Scholar]
- [154].Grimm S, Bauer MKA, Bauerle PA, Schulze-Osthoff K. Bcl-2down regulates the activity of transcription factor NF-kappa B induced upon apoptosis. J cell Biol 1996;134:13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Lea MA, Ayyala UM. Differentiating and growth inhibitory effects of diallyl disulfide on cancer cells. Int J Oncol 1997;11:181–5. [PubMed] [Google Scholar]
- [156].Lee ES, Steiner M, Lin R. Thioallyl compounds: potent inhibitors of cell proliferation. Biochim Biophys Acta 1994;1221:73–7. [DOI] [PubMed] [Google Scholar]
- [157].Lin X-Y, Liu JZ, Milner JA. Dietary garlic suppresses DNA adducts caused by N-nitroso compounds. Carcinogenesis 1994;15:349–52. [DOI] [PubMed] [Google Scholar]
- [158].Kyo E, Suzuki A, Kakimoto M, Ushijima M, Kasuga S, Itakura Y. Immunomodulation and antitumor activities of aged garlic extract. Phytomedicine 1998;5:259–67. [DOI] [PubMed] [Google Scholar]
- [159].Fuchs CS, Mayer RJ. Gastric carcinoma. N Engl J Med 1995;333:32–41. [DOI] [PubMed] [Google Scholar]
- [160].Sivam GP, Lampe JW, Ulness B, Swanzy SR, Potter JD. Helicobacter pylori: in vitro susceptibility to garlic (Allium sativum) extract. Nutr Cancer 1997;27:118–21. [DOI] [PubMed] [Google Scholar]
- [161].Feldberg RS, Chang SC, Kotik AN, Nadler M, Neuwirth Z, Sund-strom DC, et al. In vitro mechanism of inhibition of bacterial growth by allicin. Antimicrob Agents Chemother 1988;32:1763–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Ludeke BI, Domine F, Ohgaki H, Kleihues P. Modulation of N-nitrosomethylbenzylamine bioactivation by diallylulfide in vivo. Carcinogenesis 1992;13:2467–70. [DOI] [PubMed] [Google Scholar]
- [163].Lea MA, Randolph VM, Patel M. Increased acetylation of histones induced by allyl disulfide and structurally related molecules. Int J Oncol 1999;15:347–52. [DOI] [PubMed] [Google Scholar]
- [164].Singh SV, Mohan RR, Agarwal R, Benson PJ, Hu X, Rudy MA, et al. Novel anticarcinogenic activity of an organosulfide from garlic: inhibition of H-ras oncogene transformed tumor growth in vivo by dial-lylsulfide is associated with inhibition of p21H-ras processing. Biochem Biophys Res Commun 1996;225:660–5. [DOI] [PubMed] [Google Scholar]
- [165].Barbacid M Ras genes. Annu Rev Biochem 1987;56:779–828. [DOI] [PubMed] [Google Scholar]
- [166].McCormick F How receptors turn Ras onj. Nature (Lond) 1993;363: 15–6. [DOI] [PubMed] [Google Scholar]
- [167].Manne V, Roberts D, Tobin A, O’Rourke E, De Virgilio M, Meyers C, et al. Identification and preliminary characterization of protein-cysteine farnesyltransferase. Proc Natl Acad Sci USA 1990;87:7541–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Der CJ, Cox AD. Isoprenoid modification and plasma membrane association: critical factors for ras oncogenicity. Cancer Cells 1991;3: 331–40. [PubMed] [Google Scholar]
- [169].Kato K, Cox AD, Hisaka MM, Graham SM, Buss JE, Der CJ. Isoprenoid addition to ras protein is the critical modification for its membrane association and transforming activity. Proc Natl Acad Sci USA 1992;89:6403–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Reddy BS, Rao CV, Rivenson A, Kelloff G. Chemoprevention of colon carcinogenesis by organosulfur compounds. Cancer Res 1993; 53:3493–8. [PubMed] [Google Scholar]
- [171].Brady JF, Ishizaki H, Fukuto JM, Lin MC, Fadel A, Gapac JM, et al. Inhibition of cytochrome P450 IIE1 by diallyl sulfide and its metabolites. Chem Res Toxicol 1991;4:642–7. [DOI] [PubMed] [Google Scholar]
- [172].Chen L, Lee M, Hong JY, Huang W, Wang E, Yang CS. Relationship between cytochrome P450 2E1 and acetone catabolism in rats as studied with diallyl sulfide as an inhibitor. Biochem Pharmacol 1994; 48:2199–205. [DOI] [PubMed] [Google Scholar]
- [173].Yang CS. Mechanisms of inhibition of chemical toxicity and carino-genesis by diallylsulfide (DAS) and related compounds from garlic. J Nutr 2001;131:1041S–5S. [DOI] [PubMed] [Google Scholar]
- [174].Pan J, Hong JY, Ma BL, Ning SM, Paranawithana SR, Yang CS. Transcriptional activation of P450 2B1/2 genes in rat liver by diallyl sulfone compound derived from garlic. Arch Biochem Biophys 1993; 302:337–42. [DOI] [PubMed] [Google Scholar]
- [175].Siess MH, Le Bon AM, Canivenc-Lavier MC, Suschetet M. Modification of hepatic drug-metabolizing enzymes in rat treated with alkyl sulfides. Cancer Lett 1997;120:195–201. [DOI] [PubMed] [Google Scholar]
- [176].Perchellet JP, Perchellet EM, Abney NL, Zirnstein JA, Belman S. Effects of garlic and onion oils on glutathione peroxides activity, the ratio of reduced and oxidized glutathione and ornithine decarboxylase induction in isolated mouse epidermal cells treated with tumor promoters. Cancer Biochem Biophys 1986;8:299–312. [PubMed] [Google Scholar]
- [177].Hu X, Sigh SV. Glutathione S-transferases of female A/J mouse lung and their induction by anticarcinogenic organosulfides from garlic. Arch Biochem Biophys 1997;340:279–86. [DOI] [PubMed] [Google Scholar]
- [178].Manson MM, Ball HW, Barrett MC, Clark HL, Judah DJ, Williamson G, et al. Mechanism of action of dietary chemoprotective agents in rat liver: induction of phase I and II drug metabolizing enzymes and aflatoxin B1 metabolism. Carcinogenesis 1997;18:1729–38. [DOI] [PubMed] [Google Scholar]
- [179].Jin L, Baillie TA. Metabolism of the chemoprotective agent diallyl sulfide to glutathione conjugates in rats. Chem Res Toxicol 1997;10: 318–27. [DOI] [PubMed] [Google Scholar]
- [180].Sparnins VL, Barany G, Wattenberg LW. Effects of organosulfur compounds from garlic and onion on benzo[a]pyrene-induced neoplasia and glutathione-S-transferase activity in the mouse. Carcinogen-esis 1988;9:131–4. [DOI] [PubMed] [Google Scholar]
- [181].Hu X, Benson PJ, Srivastava SK, Xia H, Bleicher RJ, Zaren HA, et al. Induction of glutathione S-transferase pi as a bioassay for the evaluation of potency of inhibitors of benzo(a)pyrene-induced cancer in a murine model. Int J Cancer 1997;73:897–902. [DOI] [PubMed] [Google Scholar]
- [182].Matsui-Yuasa I, Otani S, Yano Y, Takada N, Shibata M, Fukushima S. Spermidine/spermine N′-acetyltransferase, a new biochemical marker for epithelial proliferation in rat bladder. Jpn J Cancer Res 1992;83: 1037–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Coleman CS, Stanley BA, Pegg AE. Effect of mutations at active site residues on the activity of ornithine decarboxylase and its inhibition by active site-directed irreversible inhibitors. J Biol Chem 1993;268: 24572–9. [PubMed] [Google Scholar]
- [184].Oguro T,Yoshida T, Numazawa S, KuroiwaY. Comparative studies of the effects of stilbene compounds on hepatic ornithine decarboxylase and S-adenosylmethionine decarboxylase induction in rats. Life Sci 1991;48:195–202. [DOI] [PubMed] [Google Scholar]