Abstract
Purpose
Hypertensive disorders complicate 5–10% of all pregnancies and contribute greatly to maternal morbidity and mortality. There are various biomarkers for detection of preeclampsia. Several studies have reported that positive correlation exists between serum uric acid (UA) levels and adverse maternal and fetal outcome. Significant advances have been made toward validation of salivary biomarkers. We conducted this study to determine levels of salivary UA and its correlation with serum UA normal pregnancy and preeclampsia.
Methods
Present cross-sectional study was conducted in tertiary care teaching hospital in North India. One hundred and fifty participants were divided into control group (50 healthy non-pregnant females), study group I (50 normotensive pregnant females), study group II (50 pregnant females with preeclampsia), and both salivary and serum UA was estimated at the same time.
Results
Saliva UA of study group II (4.86 ± 2.37 mg/dl) was significantly higher (p < 0.001) than that of control group (2.09 ± 1.33 mg/dl) and study group I (3.32 ± 1.77 mg/dl). Serum UA of study group II (6.63 + 2.78 mg/dl) was significantly higher (p < 0.001) than that of control group (2.94 + 1.94 mg/dl) and also study group I (5.18 + 2.31 mg/dl) (p = 0.0006).
Conclusion
UA is present in the saliva of women with preeclampsia and has linear correlation with serum UA. Therefore, salivary UA can be used in place of invasive serum UA to monitor women with preeclampsia. Saliva collection is easy, noninvasive and cost-effective. Salivary UA testing may be useful for monitoring preeclampsia at home-based and hospital setting.
Keywords: Saliva, Uric acid, Preeclampsia
Introduction
Hypertensive disorders of pregnancy and specially preeclampsia are the leading cause of maternal and perinatal morbidity and mortality in developing and developed countries [1]. Hypertensive disorders complicate 5–10% of all pregnancies, and together they are one of the members of the deadly triad—along with hemorrhage and infection—that contributes greatly to maternal morbidity and mortality. Out of these, the preeclampsia syndrome, either alone or superimposed on chronic hypertension, is the most dangerous. Oxidative stress is thought to play an important role in all pathological consequences occurring in preeclampsia and associated with poor placental perfusion. Raised blood urea, a marker of oxidative stress, is associated with severity of preeclampsia and fetal outcome [2]. Hyperuricemia is a common finding in preeclamptic pregnancies. The elevation of uric acid in preeclamptic women often precedes hypertension and proteinuria [3]. Blood urate is also considered a predictor of preeclampsia [4]. There are several potential origins for uric acid in preeclampsia; abnormal renal function, increased tissue breakdown, acidosis, and increased activity of the enzyme xanthine oxidase/dehydrogenase, outside the pregnancy, high blood uric acid levels are also associated with a wide variety of diseases including hypertension, increased cardiovascular mortality, obesity and metabolic syndrome [5, 6].
Blood urate is a product of endogenous urate production, diet, renal excretion and gastrointestinal excretion. In the intestine, urate undergoes bacterial degradation (enteral uricolysis) [7]. Uric acid is 5% bound to plasma proteins in blood, but it is otherwise completely filtered by glomerulus, it is then reabsorbed by 90% in early proximal tubules, and then, 50% of the filtered urate is secreted via S2 segment of the proximal tubules. Finally, it undergoes post-secretory reabsorption in the last segment of proximal tubules [8].
There are several studies which report that positive correlation exists between serum uric acid (UA) levels and adverse maternal and fetal outcome [9–13]. In recent years, significant advances have been made toward validation of salivary biomarkers for disease detection. So we decided to conduct this study to determine levels of salivary uric acid and its correlation with serum uric acid in normal pregnancy and preeclampsia.
Materials and Methods
In this cross-sectional study, 150 participants were selected after taking informed consent and ethical clearance, over a period of 1 year. Women attending the antenatal O.P.D and admitted in the antenatal ward from 28th to 40th week of gestational age and also Gynaecology O.P.D of Department of Obstetrical and Gynaecology, Queen Mary Hospital, K.G.M.U, Lucknow, were selected.
Participants were divided into two groups:
Control group Comprised of normal healthy normotensive non-pregnant women
Study group Further divided into two subgroups: Study group I (n 50) normotensive with singleton pregnancy and gestational age 28 weeks to 40 weeks. Study group II (n 50) singleton 28–40 weeks pregnancy with preeclampsia.
Study group II was further divided into two group:
Non-severe preeclampsia (n = 31)
Severe preeclampsia (n = 19)
Criteria for diagnosis of preeclampsia were taken as blood pressure ≥ 140/90 mmHg on two occasions 4 h apart with or without proteinuria ≥ 300 mg/24 h or persistent proteinuria 30 mg/dl (≥ 1+ dipstick) in random urine samples and also to differentiate severe and non-severe preeclampsia. Non-severe preeclampsia was taken as blood pressure systolic < 160 and diastolic < 110 mmHg, with or without proteinuria without any derangement of LFT & KFT. Severe preeclampsia was defined as blood pressure > 160/110 mmHg with without proteinuria and derangement of LFT or KFT [14].
Exclusion criteria for study groups I and II
Exclusion criteria were: multiple pregnancy, diabetes mellitus, chronic hypertension, any other cardiovascular disease, gout, connective tissue disorder, renal or liver disease, history of thromboembolism, history of any chronic illness, neural tube defect and other congenital malformation, patients with oral infection, poor oral hygiene and recent oral injuries.
Methodology
After informed consent and ethical clearance, all enrolled women were subjected to detailed history and examination, and relevant blood investigations were done.
After 10 min of rinsing of mouth, 5 ml of saliva was collected in a wide bore vial and was kept cool in normal domestic refrigerator at 4 °C until it was processed within 4 h in the laboratory of the Pathology department. 2 ml of blood sample from antecubital vein was also collected at the same time from the subject to measure serum uric acid levels; both serum and saliva samples were centrifuged for 5 min (3000 rpm). The supernatant was aspirated, and levels of serum and salivary uric acid were measured using Accurex Uric acid kit, with the help of an optical densitometer.
Red colored compound has maximum absorbance at 510 nm (500–530 nm). Its concentration is proportional to the amount of uric acid in specimen.
Statistical Tools Employed in the Study
The statistical analysis was done using SPSS (Statistical Package for Social Sciences) version 15.0 statistical analysis software. The values were represented in number (%) and mean ± SD. Time interval was analyzed with ANOVA test, and other data were analyzed with χ2 for qualitative and student’s t test for quantitative variables. P value of < 0.05 was taken as significant. Pearson correlation coefficient r < 0.3 was taken as weak or no correlation and > 0.9 was taken as very strong correlation.
Observation and Result
No statistically significant difference in age, parity, BMI and socioeconomic status of subjects in the above three groups was found (Table 1).
Table 1.
Demographic and obstetric characteristic of study and control groups
| Study group I | Study group II | Control group | Significance | |
|---|---|---|---|---|
| Maternal age in year | 25.62 ± 3.07 | 25.64 ± 3.60 | 27.44 ± 3.62 | None |
| Parity | 1.72 ± .08 | 1.85 ± 1.1 | 1.84 ± 0.9 | None |
| BMI | 24.63 ± 2.632 | 25.6 ± 2.51 | 24.28 ± 2.6 | None |
Proportion of subjects having educational status up to college level was higher in control group (36.00%) as compared to that in study group I (22.00%) and study group II (18.00%). Although the difference in educational status of above three groups was not found to be statistically significant.
Saliva uric acid in study group I was found to be 3.32 ± 0.1.77 mg/dl (range 0.25–8.60), that in study group II was 4.86 ± 2.37 mg/dl (range 1.70–14.60), and that in control group was 2.09 ± 1.33 mg/dl (range 0.20–8.40) (Table 2).
Table 2.
Levels of saliva uric acid (mg/dl) and serum uric acid (mg/dl) in different groups
| Saliva uric acid (mg/dl) | Serum uric acid (mg/dl) | |||||||
|---|---|---|---|---|---|---|---|---|
| Min | Max | Mean | SD | Min | Max | Mean | SD | |
| Study group I | 0.25 | 8.60 | 3.32 | 1.77 | 1.50 | 10.80 | 5.18 | 2.31 |
| Study group II | 1.70 | 14.60 | 4.86 | 2.37 | 2.50 | 17.40 | 6.62 | 2.78 |
| Control | 0.20 | 8.40 | 2.09 | 1.33 | 0.90 | 5.50 | 2.94 | 0.94 |
Serum uric acid in study group I was found to be 5.18 ± 2.31 mg/dl (range 1.50–10.80), that in study group II was 6.62 + 2.78 mg/dl (range 2.50–17.40), and that in control group was 2.94 ± 0.94 mg/dl (range 0.90–5.50) (Table 2).
In study group I, saliva uric acid ranged from 0.25 to 8.6 mg/dl and mean value was found to be 3.32 ± 1.77 mg/dl, while serum uric acid of study group I ranged from 1.5 to 10.8 mg/dl and mean value was found to be 5.18 ± 2.31 mg/dl.
A mild and statistically significant correlation (Pearson correlation coefficient r = 0.466, p = 0.001) between saliva uric acid and serum uric acid of subjects of group I was found (Table 3).
Table 3.
Correlation of saliva uric acid (mg/dl) and serum uric acid (mg/dl) in study group I
| No. of subjects | Min. (mg/dl) | Max. (mg/dl) | Mean (mg/dl) | SD | |
|---|---|---|---|---|---|
| Saliva uric acid (mg/dl) | 50 | 0.25 | 8.6 | 3.32 | 1.77 |
| Serum uric acid (mg/dl) | 50 | 1.5 | 10.8 | 5.18 | 2.31 |
Pearson’s correlation coefficient (r) = 0.466 (mild); p = 0.001
In study group II, saliva uric acid ranged from 1.7 to 14.6 mg/dl and mean value was found to be 4.86 ± 2.37 mg/dl, while serum uric acid of study group II ranged from 2.5 to 17.4 mg/dl and mean value was found to be 6.63 ± 2.78 mg/dl (Table 2).
A moderate and statistically significant correlation (Pearson correlation coefficient r = 0.690, p = 0.001) between saliva uric acid and serum uric acid of subjects of Group II was found (Fig. 1).
Fig. 1.
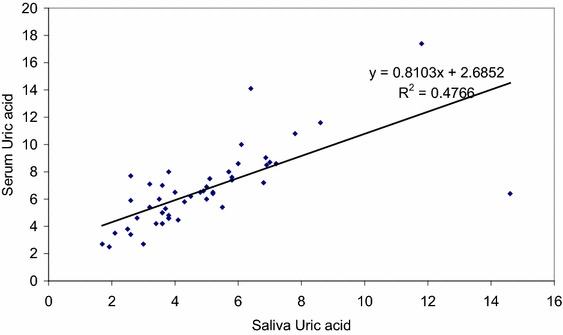
Pearson’s correlation coefficient of group II subjects, (r) = 0.690 (moderate)
Saliva uric acid of study group II (4.86 ± 2.37 mg/dl) was found to be significantly higher (p < 0.001) than that of control group (2.09 ± 1.33 mg/dl) and study group I (3.32 ± 1.77 mg/dl).
Serum uric acid of study group II (6.63 + 2.78 mg/dl) was found to be significantly higher (p < 0.001) than that of control group (2.94 + 1.94 mg/dl). Similarly, serum uric acid of study group II (6.63 + 2.78 mg/dl) was found to be significantly higher (p = 0.006) than that of study group I (5.18 + 2.31 mg/dl).
Difference in saliva uric acid of above three groups was found to be statistically significant (p < 0.001). Difference in serum uric acid of above three groups was found to be statistically significant (p < 0.001) (Table 4).
Table 4.
Intergroup comparison of saliva uric acid (mg/dl) and serum uric acid (mg/dl)
| Saliva uric acid (mg/dl) | Serum uric acid (mg/dl) | |||||||
|---|---|---|---|---|---|---|---|---|
| Min | Max | Mean | SD | Min | Max | Mean | SD | |
| Study group I | 0.25 | 8.60 | 3.32 | 1.77 | 1.50 | 10.80 | 5.18 | 2.31 |
| Study group II | 1.70 | 14.60 | 4.86 | 2.37 | 2.50 | 17.40 | 6.62 | 2.78 |
| Control | 0.20 | 8.40 | 2.09 | 1.33 | 0.90 | 5.50 | 2.94 | 0.94 |
| Statistical significance | F = 27.494; p < 0.001 | F = 36.961; p < 0.001 | ||||||
Though serum uric acid of severe preeclampsia subjects of group II (7.40 ± 3.29 mg/dl) was found to be higher than that of non-severe preeclampsia subjects of group II (6.15 ± 2.35 mg/dl), but difference in serum uric acid of both the groups was not found to be statistically significant (p = 0.124) (Table 5).
Table 5.
Saliva uric acid and serum uric acid (mg/dl) in study group II according to severity of preeclampsia
| Non-severe preeclampsia (n = 31) | Severe preeclampsia (n = 19) | |||||||
|---|---|---|---|---|---|---|---|---|
| Min. | Max. | Mean | SD | Min. | Max. | Mean | SD | |
| Saliva uric acid (mg/dl) | 1.7 | 14.6 | 4.80 | 2.36 | 1.92 | 11.8 | 4.97 | 2.45 |
| Statistical significance | t = 0.241; p = 0.811 | |||||||
| Serum uric acid (mg/dl) | 2.70 | 14.1 | 6.15 | 2.35 | 2.50 | 17.40 | 7.40 | 3.29 |
| Statistical significance | t = 1.566; p = 0.124 | |||||||
Based on the direction of assessment, saliva uric acid and serum uric acid were evaluated for prediction of preeclampsia at a cutoff with a larger value indicating positive result. Areas under curve findings were 0.810 (indicating a projected accuracy of 81.0%) and 0.800, respectively, for saliva uric acid and serum uric acid. For Saliva uric acid, a cutoff value ≥ 3.350 was predicted to be 78.0% sensitive and 73% specific. For Serum uric acid, a cutoff value ≥ 4.655 was predicted to be 74.0% sensitive and 71 specific (Fig. 2, Table 6).
Fig. 2.
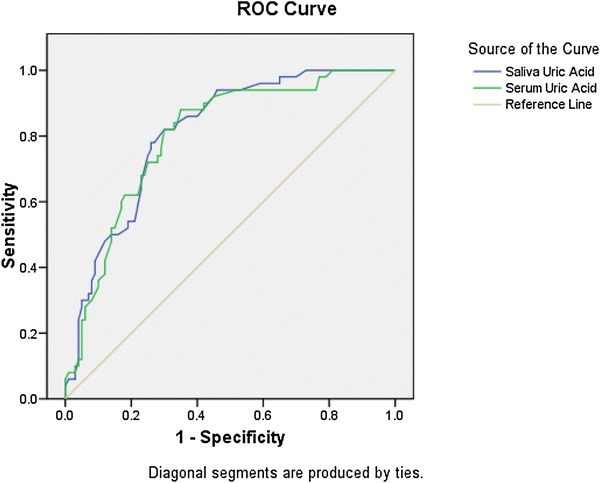
ROC curve depicting value of salivary uric acid level preeclampsia patients (group II)
Table 6.
ROC curve analysis
| Test result variable(s) | Area | Std. error(a) | Asymptotic Sig.(b) | Asymptotic 95% CI | |
|---|---|---|---|---|---|
| Lower bound | Upper bound | ||||
| Saliva uric acid | 0.810 | 0.035 | < 0.001 | 0.741 | 0.878 |
| Serum uric acid | 0.800 | 0.037 | < 0.001 | 0.727 | 0.872 |
All parameters of preeclampsia were measured to differentiate between severe and non-severe preeclampsia, but they were not compared as our aim of this study was to compare salivary and serum uric acid and its correlation with severity of preeclampsia.
Various statistical parameters were used to correlate the level of salivary and serum uric acid and severity of preeclampsia. We tried to make a cutoff value of uric acid to predict preeclampsia.
Discussion
In our study, the salivary uric acid in study group I was 3.32 ± 1.77 mg/dl, that in study group II was 4.86 ± 2.37 mg/dl, and that in control group 2.09 ± 1.33 mg/dl. Serum uric acid in study group I was found to be 5.18 ± 2.31 mg/dl, that in study group II was 6.62 ± 2.78 mg/dl, and that in control group was 2.94 ± 0.94 mg/dl.
Several studies have demonstrated the presence of uric acid in saliva (Soukup et al. [15], Bahaa et al. [16]). They stressed that there is a linear relationship between serum and saliva uric acid levels. Hence, saliva may serve as a useful surrogate marker for blood testing. Soukup et al. [15], in their study “salivary uric acid as a noninvasive biomarker of metabolic syndrome,” found that salivary uric acid was significantly elevated in patients with metabolic syndrome (p = 0.002). Salivary uric acid concentration without metabolic disease was 184.9 ± 78.4 µM (3.08 ± 1.3 mg/dl), and with metabolic syndrome it was 278.1 ± 135.3 µM (4.6 ± 2.2 mg/dl) which was statistically significant. This is in agreement with our study where we have observed statistically significant difference in salivary uric acid levels between the control group (2.09 ± 1.33 mg/dl), study group II of preeclamptic patients (4.86 ± 2.37 mg/dl), and study group I (3.32 + 1.77 mg/dl) with p < 0.001.
Our findings are well correlated with the observation made by Bahaa et al. [16]. They studied oxidative stress biomarkers (uric acid and malondialdehyde) in saliva and plasma of patients with end stage renal failure. They observed increased serum and salivary uric acid levels (5.46 ± 0.66, 2.74 ± 0.86 mg/dl), respectively, in patients as compared to healthy controls (4.39 ± 1.27, 2.08 ± 0.64 mg/dl), respectively.
Blicharz et al. [17] in their study titled use of colorimetric test strips for monitoring the effect of hemodialysis on salivary nitrite and uric acid in patients with end stage renal disease: a proof of principle reported that changes in UA concentrations occurring during dialysis can be monitored in saliva and, therefore, salivary UA warrants further examination for its clinical utility. In a retrospective cohort study conducted by Hawkins et al. [18], it was shown that plasma uric acid remains a marker of poor outcome in hypertensive pregnancy. The percentage of women diagnosed with preeclampsia was significantly higher (56 vs. 29%; p < 0.001) if there was a 0.10 mmol/l (≥ 1.7 mg/dl) or more increase in plasma uric acid between the time of initial consultation and that closest to delivery (uric acid). Uric acid ≥ 0.10 mmol/l (≥ 1.7 mg/dl) (n = 481).
According to Sangeeta et al. [19], the cause of hyperuricemia in preeclampsia is attributed to either a decreased excretion or to an increased production of uric acid.
In this study, the serum uric acid levels in the cases were higher than the control which is similar to the values found by us in our study. The serum uric acid mean ± SD (mg %) for the cases was 8.82 ± 1.68, whereas in the control group (normal pregnant) it was 4.06 ± 0.96. This difference in the values was very highly significant (p ≤ 0.001).
In the present study, serum and salivary uric acid were found to be higher in women with severe preeclampsia as compared with non-severe preeclampsia, but the difference is statistically nonsignificant (p = 0.124). Our findings were in line with the several studies (Tejal et al. [20]; Bainbridge et al. [21]). These studies concluded that an elevation of mean values of serum uric acid correlates with degree of severity of toxemia and that the levels of serum uric acid appear to be a sensitive index of the severity of preeclampsia. In the current study, we observed that there exists a linear correlation between serum and salivary uric acid and salivary uric acid reflects changes in the serum. We therefore assume that salivary uric acid can also be used as an index of severity of preeclampsia.
In the present study, we also made an attempt to calculate the cutoff value of salivary uric acid for prediction of preeclampsia. On evaluating salivary uric acid, cutoff value > 3.350 mg/dl was predicted to be 78% sensitive and 73% specific.
Literature search did not reveal any study to compare our cutoff value of salivary uric acid for predication of preeclampsia.
Conclusion
We conclude that uric acid is present in the saliva of women with preeclampsia and has linear correlation with serum uric acid. Past studies have proved that serum uric acid has association with severity of preeclampsia and has also been used to predict maternal and fetal outcome in women with preeclampsia. Therefore, we propose that salivary uric acid can be used in place of invasive serum uric acid to monitor women with preeclampsia. Saliva collection is easy, noninvasive, cost-effective requiring simple instruction for collection. So salivary uric acid testing may be useful approach for monitoring preeclampsia at home-based and hospital setting.
Further studies with large sample size are required to study the levels of salivary uric acid in early pregnancy for prediction of preeclampsia.
Abbreviation
- UA
Uric acid
Urmila Singh
DGO, MD, FICMCH, FICOG, MAMS, is Professor and Unit Head in the Department of Obstetrics and Gynaecology Queen Mary Hospital, King George’s Medical University, Lucknow. Her special areas of interest include Gynae-oncology, Operative Endoscopy and Reproductive and Child health. She is an excellent teacher and has trained many undergraduates and postgraduates. She is Nodal Officer, PPTCT centre, Lucknow. She has received Doctor of Millennium award 2000, Dr. Manorama Diamond Jubilee award, and Vishishta Chikitsa Seva Bhushan Samman in 2016. She has more than 50 publications in national and international journals. She has been organizing secretary of UPCOG 2013, NARCHI 2014, ISCCP2015, and North Zone Yuva FOGSI 2017. She was the Joint Editor of Journal of U. P. Chapter of Obstetrics & Gynaecology 2011.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5).
Informed Consent
Informed consent was obtained from all patients for being included in the study.
Footnotes
Urmila Singh is DGO, MD, FICMCH, FICOG, MAMS, Professor and Unit Head in the Department of Obstetrics and Gynaecology, King George´s Medical University, Lucknow, Uttar Pradesh, India; Vandana Solanki is Assistant Professor at Department of Obstetrics and Gynaecology, King George´s Medical University, Lucknow, Uttar Pradesh, India; Seema Mehrotra is Professor at Department of Obstetrics and Gynaecology, King George´s Medical University, Lucknow, Uttar Pradesh, India; Ruchita Sharma is Resident at Department of Obstetrics and Gynaecology, King George´s Medical University, Lucknow, Uttar Pradesh, India.
References
- 1.Duley L. Maternal mortality associated with hypertensive disorder of pregnancy in Africa, Asia, Latin America and the Caribbean. Br J Obstet Gynaecol. 1992;99(7):547–553. doi: 10.1111/j.1471-0528.1992.tb13818.x. [DOI] [PubMed] [Google Scholar]
- 2.Many A, Hubel CA, Roberts JM. Hyperuricaemia and xanthine oxidase in preeclampsia, revisited. Am J Obstet Gynecol. 1996;174(1):288–291. doi: 10.1016/S0002-9378(96)70410-6. [DOI] [PubMed] [Google Scholar]
- 3.Powers RW, Bodnar LM, Ness RB, et al. Uric acid concentrations in early pregnancy among preeclamptic women with gestational hyperuricemia at delivery. Am J Obstet Gynecol. 2006;194(1):160. doi: 10.1016/j.ajog.2005.06.066. [DOI] [PubMed] [Google Scholar]
- 4.Lippi G, Montagnana M, Franchini M, et al. The paradoxical relationship between serum uric acid and cardiovascular disease. Clin Chim Acta. 2008;392(1–2):1–7. doi: 10.1016/j.cca.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 5.Johnson RJ, Kang DH, Feig D, et al. Is there a pathogenetic role for uric acid in hypertension and cardiovascular and renal disease? Hypertension. 2003;41:1183–1190. doi: 10.1161/01.HYP.0000069700.62727.C5. [DOI] [PubMed] [Google Scholar]
- 6.Soukup M, Biesiada I, Henderson A, et al. Salivary uric acid as a non invasive biomarker of metabolic syndrome. Diabetol Metab Syndr. 2012;4(1):14. doi: 10.1186/1758-5996-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sorensen LB. Role of the intestinal tract in the elimination of Uric acid. Arthritis And Rheum. 1965;8(5):694–706. doi: 10.1002/art.1780080429. [DOI] [PubMed] [Google Scholar]
- 8.Lam C, Lim K-H, Kang D-H, et al. Uric acid and preeclampsia. Semin Nephrol. 2005;25(1):56–60. doi: 10.1016/j.semnephrol.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 9.Liedholm H, Montan S, Aberg A. Risk grouping of 113 patients with hypertensive disorders during pregnancy, with respect to serum urate, proteinuria and time of onset of hypertension. Acta Obstet Gynecol Scand Suppl. 1984;62(Suppl 118):43–48. doi: 10.3109/00016348409157122. [DOI] [PubMed] [Google Scholar]
- 10.Redman CW, Beilin LJ, Bonnar J, et al. Plasma-urate measurements in predicting fetal death in hypertensive pregnancy. Lancet. 1976;26:1370–1373. doi: 10.1016/S0140-6736(76)93024-5. [DOI] [PubMed] [Google Scholar]
- 11.Johnson RJ, Kanbay M, Kang D-H, et al. Uric acid: a clinically useful marker to distinguish preeclampsia from gestational hypertension. Hypertension. 2011;58(4):548–549. doi: 10.1161/HYPERTENSIONAHA.111.178921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akahori Y, Masuyama H, Hiramatsu Y. Correlation of maternal serum uric acid concentration with small for date gestational fetus in normotensive pregnant women. Gynecol Obstet Investig. 2012;73:162–167. doi: 10.1159/000332391. [DOI] [PubMed] [Google Scholar]
- 13.Chappell LC, Seed PT, Briley A, et al. A longitudinal study of biochemical variables in women at risk of preeclampsia. Am J Obstet Gynaecol. 2002;186:127–136. doi: 10.1067/mob.2002.122969. [DOI] [PubMed] [Google Scholar]
- 14.Cunningham FG (2014). Chapter 40: hypertensive disorders. In: Leveno KJ, Bloom SL, Spong CY, Dashe JS, Hoffman BL, Casey BM, Sheffield JS, editors. Williams obstetrics, 24th ed. New York: McGraw-Hill Education. ISBN 9780071798938. OCLC 871619675.
- 15.Soukup M, Biesiada I, Henderson A, et al. Salivary uric acid as a noninvasive biomarker of metabolic syndrome. Diabetol Metab Syndr. 2012;4:14. doi: 10.1186/1758-5996-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hadi BA, Al-jubouri RH. Salivary and plasma analysis of oxidative stress biomarkers in end stage renal failure patients. J Bagh Coll Dent. 2011;23(2):46–50. [Google Scholar]
- 17.Blicharz TM, Rissin DM, Bowden M, et al. Use of colorimetric test strips for monitoring the effect of hemodialysis on salivary nitrite and uric acid in patients with end-stage renal disease: a proof of principle. Clin Chem. 2008;54(9):1473–1480. doi: 10.1373/clinchem.2008.105320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hawkins TL-A, Roberts JM, Mangos GJ, et al. Plasma uric acid remains a marker of poor outcome in hypertensive pregnancy: a retrospective cohort study. BJOG. 2012;119(4):484–492. doi: 10.1111/j.1471-0528.2011.03232.x. [DOI] [PubMed] [Google Scholar]
- 19.Sangeeta N, Shaini L, Basar G, et al. Serum uric acid and homocysteine as predictors of pre-eclampsia. J Diabetes Metab. 2013;4:259. [Google Scholar]
- 20.Tejal P, Astha D. Relationship of serum uric acid level to maternal and perinatal outcome in patients with hypertensive disorders of pregnancy. Gujarat Med J. 2014;69(2):45–47. [Google Scholar]
- 21.Bainbridge SA, Roberts JM. Uric acid as a pathogenic factor in preeclampsia. Placenta. 2008;29(Suppl A):S67–S72. doi: 10.1016/j.placenta.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]


