A lifestyle exercise and dietary intervention, incorporating group-mediated behavioral counseling, resulted in meaningful improvements in clinically-relevant outcomes among prostate cancer patients undergoing androgen deprivation therapy.
Keywords: Androgen deprivation, Hormone therapy, Functional limitations, Prostate cancer, Exercise, Diet
Abstract
Background
Although androgen-deprivation therapy (ADT) is the foundation of treatment for prostate cancer, the physiological impacts of ADT result in functional decline and enhanced risk of chronic disease and metabolic syndrome.
Purpose
The Individualized Diet and Exercise Adherence Pilot Trial (IDEA-P) is a single-blind, randomized, pilot trial comparing the effects of a group-mediated, cognitive-behavioral (GMCB) exercise and dietary intervention (EX+D) with those of a standard-of-care (SC) control during the treatment of prostate cancer patients undergoing ADT.
Methods
A total of 32 prostate cancer patients (M age = 66.28, SD = 7.79) undergoing ADT were randomly assigned to the 12-week EX+D intervention (n = 16) or control (n = 16). The primary outcome in IDEA-P was change in mobility performance with secondary outcomes including body composition and muscular strength. Blinded assessment of outcomes were obtained at baseline and at 2- and 3-month follow-ups.
Results
Favorable adherence and retention rates were observed, and no serious intervention-related adverse events were documented. Intent-to-treat ANCOVA controlling for baseline value and ADT duration demonstrated that EX+D resulted in significantly greater improvements in mobility performance (p < .02), muscular strength (p < .01), body fat percentage (p < .05), and fat mass (p < .03) at 3-month follow-up, relative to control.
Conclusion
Findings from the IDEA-P trial suggest that a GMCB-based EX+D intervention resulted in significant, clinically meaningful improvements in mobility performance, muscular strength, and body composition, relative to controls. Collectively, these results suggest that the EX+D was a safe and well-tolerated intervention for prostate cancer patients on ADT. The utility of implementing this approach in the treatment of prostate cancer patients on ADT should be evaluated in future large-scale efficacy trials.
Clinical Trial information
Introduction
Despite the well-established therapeutic efficacy of androgen-deprivation therapy in the treatment of prostate cancer [1], the catabolic effects of androgen-deprivation therapy result in significant adverse effects, including loss of lean muscle mass, increased fat mass, reduced muscle strength, and lower bone mineral density, which place men at greater risk for functional decline and frailty [2–9]. Emerging evidence also suggests that androgen-deprivation therapy increases risk for metabolic syndrome and its consequences, including cardiovascular disease [9]. As prolonged administration of androgen-deprivation therapy becomes increasingly common, particularly with the growing array of effective antiandrogens and multimodality treatment strategies in the adjuvant and neoadjuvant settings, many men will cope with lasting treatment-related physiological effects that meaningfully compromise their physical function and quality of life and heighten the risk of comorbid chronic disease. Consequently, defining the feasibility and efficacy of supportive care interventions that preserve functional abilities and attenuate risk for chronic disease are primary clinical considerations for prostate cancer patients on androgen-deprivation therapy [2, 6, 10–17].
In this regard, there is now considerable evidence demonstrating that exercise interventions result in significant, clinically meaningful improvements in muscular strength, physical function, and quality of life in prostate cancer patients undergoing androgen-deprivation therapy [18–22]. Findings from these studies provide a compelling foundation to build upon in progressing toward integrating personalized lifestyle interventions in the supportive care of prostate cancer patients undergoing androgen-deprivation therapy. Nevertheless, despite the benefits accompanying exercise, it is also well-established within the weight management literature that modifying both energy expenditure via increased physical activity and energy intake through changes in dietary behavior is integral to successful behavioral weight management interventions [23–26]. A common adverse effect of androgen-deprivation therapy is an increase in weight and body fat in parallel with a decrease in muscle mass and strength, which in turn place prostate cancer patients at increased risk for functional decline, metabolic syndrome, and their respective complications. In addition to management of energy balance, diet composition is a critical risk factor for these pathologic processes, and optimization of dietary patterns to those defined by Dietary Guidelines for America [27] and the American Institute of Cancer Research [28] is desired. Thus, the additive or synergistic benefits of concomitant change in both exercise and dietary behavior could represent an optimal lifestyle intervention approach for offsetting the adverse effects experienced by prostate cancer patients during androgen-deprivation therapy.
Consistent with this position, recent findings from Bourke and colleagues revealed that lifestyle interventions combining primarily supervised exercise and dietary advice yielded significant improvements in select fitness, quality of life, and body weight-related outcomes, relative to a standard-of-care (SC) in prostate cancer patients undergoing prolonged androgen-deprivation therapy [29, 30]. Findings from these pilot and small-scale trials emphasize the potential value of implementing combined exercise and dietary interventions in the treatment of prostate cancer patients. However, attrition rates and erosion of select post-intervention treatment effects observed in both exercise and diet intervention studies [28, 29] as well as systematic review findings indicating exercise intervention adherence rates as low as 37% [31] also underscore the persisting challenges associated with successfully promoting adoption and maintenance to exercise and dietary behavior changes that are integral to determining treatment efficacy [28, 29].
There is growing recognition that implementing behavioral theory to guide the design, delivery, and evaluation of lifestyle interventions may enhance the efficacy of these approaches for promoting behavior change and concomitant improvements in key outcomes of interest [32–34]. However, relatively few lifestyle interventions targeting cancer patients have been based on established theories of behavior [35], and many can be characterized as theory-informed rather than theory-based [32–34]. The implications of implementing theory-based behavior change interventions for treatment efficacy are particularly important, given that the deterioration of benefits accompanying lifestyle interventions can be attributable to poor posttreatment, self-directed adherence to the desired exercise, and dietary behavior changes [36–38]. Collectively, these findings underscore the pressing need to explore novel, theory-based approaches to promoting maintenance of independent exercise and dietary behavior in efforts to delineate the utility of integrating lifestyle interventions in the treatment of prostate cancer patients. One theory-driven approach, a group-mediated cognitive-behavioral (GMCB) lifestyle intervention based on social cognitive theory [34] and the group dynamics literature [39, 40], has recently produced superior adherence to exercise and dietary behavior change and also yielded significant improvements in a variety of clinically relevant outcomes for prostate cancer patients in randomized trials targeting chronic disease patients [38, 41]. The GMCB intervention couples exercise and dietary behavior change with group-based self-regulatory skills counseling to promote independent maintenance of lifestyle behavior change and to sustain intervention-induced improvements in relevant outcomes. Although these findings suggest that an integrated lifestyle intervention holds promise for improving the utility of lifestyle exercise and dietary interventions targeting prostate cancer patients, the feasibility and efficacy of implementing this theory-driven, group-based intervention approach in the treatment of prostate patients undergoing androgen-deprivation therapy has not been investigated.
Therefore, the purpose of this single-blind, randomized, pilot trial is to examine the feasibility and preliminary efficacy of implementing a combined exercise and dietary intervention (EX+D), implementing a GMCB approach, relative to SC control in the treatment of prostate cancer patients undergoing androgen-deprivation therapy. A key component of our EX+D intervention is the use of the GMCB approach to promote the development and practice of the key behavioral self-regulatory skills, harness the social dynamics of small groups to support motivation for behavior change, and personalize the EX+D prescription to each patient’s individual capacity and needs to improve adoption, adherence, and intervention efficacy. The primary hypotheses of the IDEA-P trial are as follows: (a) the GMCB EX+D intervention will be a safe, well-tolerated intervention that yields acceptable rates for recruitment, adherence, and retention with few adverse events; (b) the GMCB EX+D intervention will result in superior improvements in mobility performance, muscular strength, body composition, and select patient-reported outcomes, relative to the control intervention; and (c) the GMCB EX+D intervention will successfully promote adoption and short-term maintenance of independent, self-regulated exercise and dietary behavior change at 3-month follow-up. If successful, a personalized EX+D intervention provides a foundation for future large-scale, longer-term multi-institutional studies.
Method
Participants
A total of 32 prostate cancer patients on androgen-deprivation therapy were recruited to participate in the IDEA-P trial at The Ohio State University James Cancer Hospital and Comprehensive Cancer Center (Columbus, OH). Eligibility criteria for the IDEA-P trial included: (a) histologically defined diagnosis of prostate cancer based on pathology reports and staging studies; (b) currently undergoing androgen-deprivation therapy with a planned course of at least 3 months of continuous therapy; (c) sedentary activity pattern with less than 60 min of structured exercise participation per week during the past 6 months, consistent with recent lifestyle intervention trial’s classification of inactivity [38, 42]; (d) free of poorly controlled medical conditions that precluded safe participation in an exercise program, such as uncontrolled coronary artery disease, hypertension, peripheral vascular disease, cerebral ischemia, congestive heart failure, chronic obstructive pulmonary disease, insulin-dependent diabetes, psychiatric disease, renal failure, liver failure, other active cancers, or anemia; (e) consent to participate from the treating oncologist and primary care physician; and (f) willingness to accept randomization and undergo the testing and intervention procedures. The trial was approved by The Ohio State University Comprehensive Cancer Center Institutional Review Board, and all participants completed informed consent prior to participation in the trial.
Study Design and Procedures
The Individualized Diet and Exercise Adherence Pilot Trial (IDEA-P) was a single-blind, randomized, pilot trial. Support for this study is provided by National Cancer Institute Grant no. R03 CA16296901 (Trial Registration: NCT02050906 Registered; January 24, 2014). The primary objectives of IDEA-P were (a) to determine the feasibility of delivering this specific EX+D intervention approach to prostate cancer patients on androgen-deprivation therapy; (b) to explore the preliminary efficacy of EX+D for improving clinically relevant functional, fitness, and patient-reported outcomes compared with SC treatment to set parameters for a future definitive trial; and (c) to examine the short-term adoption and maintenance of independent, self-regulated exercise and dietary behavior change for men undergoing androgen suppression therapy. A total of 32 prostate cancer patients on androgen-deprivation therapy were randomly assigned to either the EX+D (n = 16) or SC control (n = 16) arms.
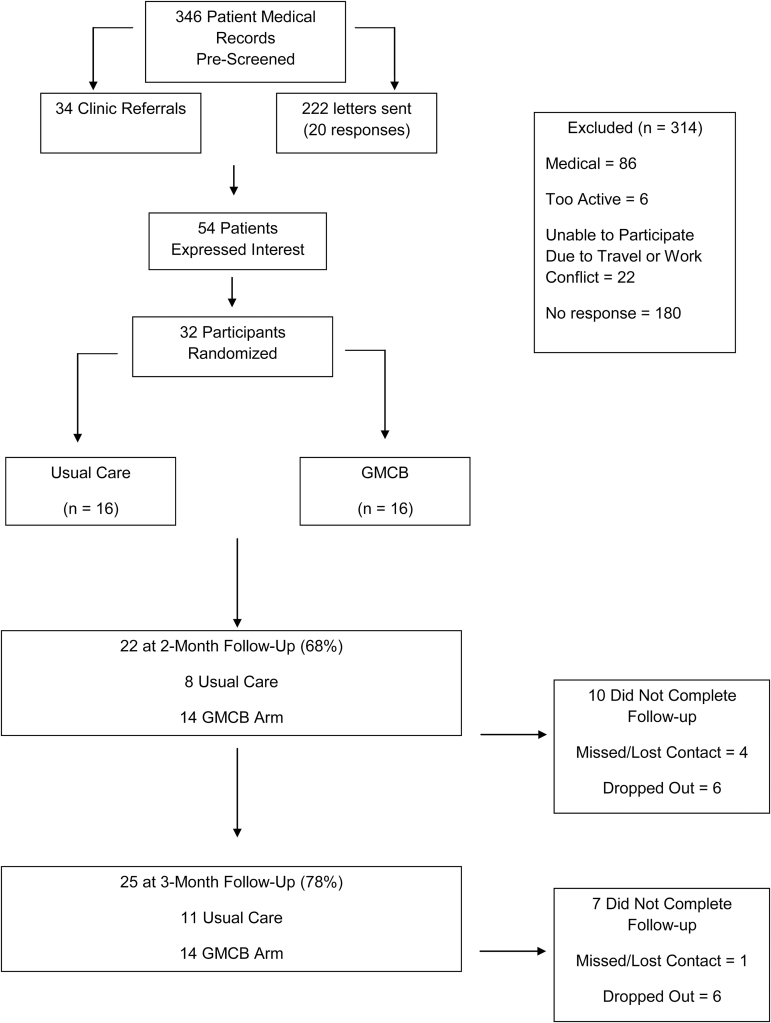
Men were referred to study investigators from physicians at the Genitourinary Oncology Clinics of the James Cancer Hospital at The Ohio State University. Volunteers interested in participating in the study completed a telephone or in-clinic screening to verify eligibility. Participants determined to be eligible were then scheduled for the baseline assessment visit. Assessments of the primary and secondary outcomes were obtained by study staff who were blinded to treatment arm assignment at baseline, 2-month, and 3-month follow-up visits. The Consolidated Standards of Reporting Trials (CONSORT) diagram illustrating the recruitment and retention of patients through the trial is summarized in Fig. 1.
Fig. 1.
CONSORT diagram of participant flow through the IDEA-P trial.
Procedures
At the beginning of the baseline screening visit, inclusion criteria were verified, and informed consent and Health Insurance Portability and Accountability Act forms were completed. Assessments of functional performance, body composition, muscular strength, and patient-reported questionnaire outcomes were obtained by study staff. After completion of the questionnaires, participants were instructed to wear an accelerometer at the right hip, from waking until they went to bed, for the next 7 consecutive days. The monitors were returned to trial staff via US postal service. Upon completion of the baseline screening visit, participants were randomly assigned into one of the two treatment arms (EX+D or control). At 2- and 3-month follow-up visits, assessments of all outcomes were obtained using the same procedures by study staff blinded to participants’ treatment group assignment.
Interventions
GMCB exercise and dietary intervention (EX+D)
The EX+D intervention involved a 12-week, multicomponent approach designed to facilitate exercise and dietary behavior change and promote adherence, independent of study staff, to these changes in lifestyle behavior. The exercise component of the EX+D intervention integrated a combination of supervised resistance and aerobic exercise performed twice per week. All supervised exercise sessions lasted 1 hr in duration. The exercise prescription was tailored to each individual’s baseline functional abilities and exercise tolerance/capacity. Consequently, resistance exercise load, volume, and volume-load and aerobic exercise duration and intensity were guided by each participant’s exercise tolerance and gradually increased across the intervention to progress toward optimal, targeted prescription ranges. Additionally, given that a primary goal of the intervention was to offset androgen deprivation-induced declines in muscle mass and strength, resistance exercise was the focal aspect of center-based training sessions. The progressive resistance exercise stimulus involved performing three sets at each individual’s 8–12 repetition maximum (8RM–12RM) and a rating of perceived exertion (1–10) ranging from 3 (Moderately Hard) to 6 (Hard) for nine different exercises (leg extension, leg curl, chest press, lateral pull-down, overhead press, triceps extension, bicep curl, calf raises, and abdominal crunch). To implement the resistance training principles of progression and overload, when participants were able to successfully complete two additional repetitions on two consecutive sets of an exercise, the resistance was increased by 5% for upper body exercises and 10% for lower body exercises. A 1–2 min rest interval was maintained between each set, and all sets were performed in a symptom-limited manner. The aerobic exercise stimulus consists of 10–20 min of exercise performed at a rating of perceived exertion (1–10) ranging from 3 (fairly light) to 4 (moderately hard) on the participant’s choice of a treadmill, stationary cycle, or elliptical trainer. Participants were also encouraged to gradually increase independent, home-based exercise participation and purposeful activity and decrease sedentary time to progress toward accruing a volume of physical activity consistent with national guidelines for health (i.e., 150 min of physical activity per week and 10,000 steps per day).
A GMCB counseling component, based on Social Cognitive Theory [43] and the group dynamics literature [39, 40], was also integrated with the intervention to promote adoption and adherence to independent, self-regulated exercise and dietary behavior and participant retention. The GMCB intervention is designed to create a supportive group learning environment in which patients use the group to facilitate the learning, development, and practice of self-regulatory skills, not only from the intervention facilitator, but from the group members themselves. The GMCB approach to harnessing group dynamics to actively promote self-regulatory skill development is unique from traditional group interventions in which patients passively receive education and counseling from the intervention leader. The promotion of group identity, to provide a common motivational base, and exchange between group members facilitated by the intervention leader are structured to develop a consistent group focus upon learning self-regulatory skills. Ultimately, this approach is structured to create a progressive, systematic group counseling effect that supports self-regulatory skill development for lifestyle behavior change. Counseling was delivered via six small group sessions (20–30 min in duration) conducted once per week immediately following a center-based exercise session during months 1–2. Participants also received four brief (20-min) individualized activity counseling sessions conducted via phone calls in months 1–3. Small group-mediated counseling sessions focussed upon development of group identity and social norms for activity, group-problem solving, sharing of peer-initiated barrier solutions, and fostering social support. Additionally, consistent with the self-efficacy and agency aspect of social cognitive theory, counseling addresses a systematic group focus on the learning, development, and practice of self-regulatory skills, including self-monitoring, building self-efficacy, goal setting, and anticipating and overcoming barriers to exercise behavior, and reducing sedentary time were focal aspects of the GMCB approach (see Table 1 for a summary of the GMCB intervention components) [44, 45]. Taken collectively, the purpose of the GMCB counseling component of the EX+D arm was to facilitate the development, practice, and mastery of self-regulatory skills necessary to adopt and maintain change in exercise and dietary behavior, and, through the use of the group as an agent of behavioral change, to increase motivation to develop and implement these behavioral skills to successfully plan and engage increasing frequency of independent exercise and healthier eating habits. A basic principle underlying these contacts and their sequencing is one of the gradually weaning participants from the dependency on staff and the group program toward independent self-regulation of exercise and diet. This process is one of a phased increase in the ratio of personal responsibility in conjunction with a phased decrease in staff, group, and clinic dependency. Thus, in contrast to most approaches in traditional exercise interventions, the present approach placed an emphasis on the self-regulation of behavior and social problem-solving barriers to promote independent exercise participation and dietary behavior.
Table 1.
Behavior Change Techniques (BCTs) Used in the Lifestyle Intervention
| Week | Session content | BCTs implemented (Michie categories) |
|---|---|---|
| 1 | Introduction to EX+D intervention and GMCB sessions | 1, 2, 21 |
| Setting intervention and GMCB session expectations | 1, 2, 21 | |
| Discussion of gradual progression of change in EX+D behaviors and self-regulatory skill development, practice, and mastery | 9, 21 | |
| EX+D behavioral contract | 25 | |
| Facilitate social dynamics and social support for EX+D behavior | 28, 29 | |
| Provide behavioral self-monitoring logs | 16, 17 | |
| 2 | Review weekly EX+D behavior successes/challenges | 18, 19 |
| Introduce information on FITT principle, macronutrients, portion control approaches, and New American Plate guidelines | 1, 2, 21 | |
| Discuss IDEA-P self-monitoring approaches | 1, 2, 16, 17 | |
| Facilitate social dynamics/support/problem-solving for EX+D behavior | 28, 29 | |
| Prompt independent practice of self-regulatory skills for EX+D behavior | 26 | |
| Review and provide behavioral self-monitoring logs | 16, 17 | |
| 3 | Review weekly EX+D behavior successes/challenges | 18, 19 |
| Discuss IDEA-P group and individual goal setting approaches | 1, 2, 5, 10, 11, 18, 19 | |
| Discuss IDEA-P self-monitoring approaches | 1, 2, 16, 17 | |
| Discuss IDEA-P action planning approaches | 1, 2, 5 | |
| Discuss IDEA-P time management approaches | 1, 2, 38 | |
| Discuss approaches to increase fruit, vegetable, and whole grain intake | 1, 2, 21 | |
| Facilitate social dynamics/support/problem-solving for EX+D behavior | 28, 29 | |
| Prompt independent practice of self-regulatory skills for EX+D behavior | 26 | |
| Review and provide behavioral self-monitoring logs | 16, 17 | |
| 4 | Review weekly EX+D behavior successes/challenges | 18, 19 |
| Discuss approaches to shift toward a plant-based diet consistent with New American Plate guidelines | 1, 2, 21 | |
| Discuss IDEA-P group and individual barrier problem-solving approaches | 1, 2, 8 | |
| Discuss IDEA-P self-monitoring approaches | 1, 2, 16, 17, 18, 19 | |
| Discuss IDEA-P action planning approaches | 1, 2, 5, 18, 19 | |
| Discuss IDEA-P time management approaches | 1, 2, 18, 19, 38 | |
| Discuss IDEA-P group and individual goal setting approaches | 1, 2, 5, 10, 11, 18, 19 | |
| Facilitate social dynamics/support/problem-solving for EX+D behavior | 28, 29 | |
| Prompt independent practice of self-regulatory skills for EX+D behavior | 26 | |
| Review and provide behavioral self-monitoring logs | 16, 17 | |
| 5 | Review weekly EX+D behavior successes/challenges | 18, 19 |
| Discuss approaches to shift toward a plant-based diet consistent with New American Plate guidelines | 1, 2, 21 | |
| Discuss IDEA-P self-monitoring approaches | 1, 2, 16, 17, 18, 19 | |
| Discuss IDEA-P action planning approaches | 1, 2, 5, 18, 19 | |
| Discuss IDEA-P time management approaches | 1, 2, 18, 19, 38 | |
| Discuss IDEA-P group and individual goal setting approaches | 1, 2, 5, 10, 11, 18, 19 | |
| Facilitate social dynamics/support/problem-solving for EX+D behavior | 28, 29 | |
| Prompt independent practice of self-regulatory skills for EX+D behavior | 26 | |
| Review and provide behavioral self-monitoring logs | 16, 17 | |
| 6 | Review weekly EX+D behavior successes/challenges | 18, 19 |
| Discuss personal motivational value of lifestyle and outcomes changes observed during the lifestyle intervention | 16, 17, 18, 19 | |
| Discuss approaches to shift toward a plant-based diet consistent with New American Plate guidelines | 1, 2, 21 | |
| Discuss IDEA-P self-monitoring approaches | 1, 2, 16, 17, 18, 19 | |
| Discuss IDEA-P action planning approaches | 1, 2, 5, 18, 19 | |
| Discuss IDEA-P time management approaches | 1, 2, 18, 19, 38 | |
| Discuss IDEA-P group and individual goal setting approaches | 1, 2, 5, 10, 11, 18, 19 | |
| Facilitate social dynamics/support/problem-solving for EX+D behavior | 28, 29 | |
| Prompt independent practice of self-regulatory skills for EX+D behavior | 26 | |
| Review and provide behavioral self-monitoring logs | 16, 17 | |
| 7 | Review weekly EX+D behavior successes/challenges | 18, 19 |
| Discuss IDEA-P relapse prevention approaches | 16, 17, 18, 19 | |
| Discuss approaches to shift toward a plant-based diet consistent with New American Plate guidelines | 1, 2, 21 | |
| Discuss IDEA-P self-monitoring approaches | 1, 2, 16, 17, 18, 19 | |
| Discuss IDEA-P action planning approaches | 1, 2, 5, 18, 19 | |
| Discuss IDEA-P time management approaches | 1, 2, 18, 19, 38 | |
| Discuss IDEA-P group and individual goal setting approaches | 1, 2, 5, 10, 11, 18, 19 | |
| Facilitate social dynamics/support/problem-solving for EX+D behavior | 28, 29 | |
| Prompt independent practice of self-regulatory skills for EX+D behavior | 26 | |
| Review and provide behavioral self-monitoring logs | 16, 17 | |
| 8 | Review weekly EX+D behavior successes/challenges | 18, 19 |
| Discuss IDEA-P relapse prevention approaches | 16, 17, 18, 19 | |
| Discuss approaches to shift toward a plant-based diet consistent with New American Plate guidelines | 1, 2, 21 | |
| Discuss IDEA-P self-monitoring approaches | 1, 2, 16, 17, 18, 19 | |
| Discuss IDEA-P action planning approaches | 1, 2, 5, 18, 19 | |
| Discuss IDEA-P time management approaches | 1, 2, 18, 19, 38 | |
| Discuss IDEA-P group and individual goal setting approaches | 1, 2, 5, 10, 11, 18, 19 | |
| Facilitate social dynamics/support/problem-solving for EX+D behavior | 28, 29 | |
| Prompt independent practice of self-regulatory skills for EX+D behavior | 26 | |
| Review and provide behavioral self-monitoring logs | 16, 17 |
EX+D exercise and dietary intervention; GMCB group-mediated, cognitive-behavioral intervention; IDEA-P Individualized Diet and Exercise Adherence Pilot Trial.
To foster the practice/mastery of the newly acquired exercise and behavioral skills and to prevent participants from becoming dependent on the expertise of exercise staff to remain physically active, supervised center-based exercise decreased from two sessions per week in Weeks 1–6 to one supervised session/week in Weeks 7–8 of the intervention. During Weeks 7–8, participants had the goal of completing one center-based exercise session independent of study staff supervision during each week. During Weeks 9–12, participants had the goal of completing both center-based exercise sessions independent of study staff supervision. Participants were provided free access to the center-based exercise facility during its standard operating hours throughout the intervention. Access to the facility during times other than that used for Week 1–6’s supervised sessions was emphasized during Weeks 7–12 to aid patients in the transition from supervised to independent exercise participation. While the facility was supervised by trained fitness staff members during this time, the participants had no supervisory contact with the study staff during these independent exercise sessions. This approach of integrating counseling and the titration away from staff supervision is designed to help participants actively apply their developing lifestyle behavioral skills to exercise independently while also providing them access to the study’s exercise facility to support completion of the independent exercise sessions during Weeks 7–12. This approach additionally allowed for systematic evaluation of independent exercise adherence in Months 2–3.
The fully integrated dietary component of the EX+D intervention included 10 (30 min) nutritional counseling sessions with a registered dietitian. The first eight counseling sessions were conducted in a group setting of 4–8 patients, once per week for 1 hr immediately following a center-based exercise session during Months 1–2. The two remaining sessions were conducted via biweekly phone calls during Weeks 9–12. Our intervention aimed to provide basic nutrition education to all participants, address contemporary topics in nutrition and cancer, and provide individualized guidance toward adopting a plant-based diet. The nutrition intervention was designed consistent with the dietary objectives recommended by 2010–2015 Dietary Guidelines for Americans, the American Heart Association/American College of Cardiology [26, 46, 47], and the American Institute of Cancer Research [28] and the American Cancer Society [48]. The nutrition counseling also integrated group-mediated learning of key self-regulation strategies described previously to promote dietary behavior change through development of group identity and social normal for eating, group-problem solving and peer-initiated barrier solutions, and social support. Additionally, consistent with the self-efficacy and agency aspect of social cognitive theory, group counseling sessions targeted cognitive behavioral self-regulatory skills training such as self-monitoring, building self-efficacy, goal setting, and anticipating and overcoming barriers to dietary behavior change. Detailed descriptions of the GMCB approach have also been provided in multiple prior translational lifestyle intervention trials [38, 42, 49, 50].
SC control intervention
Men randomized to the SC control arm received usual prostate cancer treatment and standard disease management education, as well as additional educational literature describing the American Institute of Cancer Research dietary and physical activity guidelines and education. To equate contact between treatment arms to levels consistent with similar contemporary lifestyle intervention trials [50, 51], 20-min phone contacts delivered by study staff focusing on routine aspects of prostate cancer self-management were conducted biweekly with men in the control arm. As an incentive to promote retention across the trial, men randomized to the control intervention were offered two supervised exercise training sessions and dietary counseling sessions following the completion of the 3-month assessment. Patients randomized to control also completed assessments of all outcomes at baseline, 2-month, and 3-month screening visits.
Random assignment
Eligible participants were randomly assigned with equal probability to each of the two treatment arms using a 1:1 ratio following the completion of the baseline screening visit. The computer-generated randomization allocation sequence was sequentially numbered and sealed in opaque envelopes. The randomization allocation sequence was also concealed from study staff responsible for conducting the baseline assessments.
Measures
Assessments of all study outcomes were obtained at baseline, 2-month, and 3-month follow-up visits by trained study personnel who were blinded to participants’ treatment assignment. The primary outcome of the IDEA-P trial was mobility performance and was assessed using the 400-m walk test (400MWT). A secondary mobility performance outcome, stair climb performance, was also assessed. These two mobility performance tests have established validity and reliability and have been incorporated as measures of functional performance in numerous prior lifestyle intervention trials [42, 50, 52]. The 400MWT was completed in a corridor with two cones spaced 20 m apart. Individuals were instructed to walk as quickly as they could, and the time to complete 10 laps around the cones was recorded as the performance measure. The stair climb task involved ascending a set of eight stairs, turning around on the top of the platform, and then descending. Participants were instructed to complete the task as quickly as they could, and performance was measured as the total time (in seconds) necessary to complete the task.
Muscular strength was assessed using standardized 1RM testing protocols for the chest press and leg extension exercises [53]. 1RM tests are the standard by which muscular strength is evaluated and have been established to be safe for both older adults and cancer patients. Participants were familiarized with the chest press and leg extension machines and received instruction on proper form. Participants began 1RM testing for each exercise by completing a warm-up set of four to six repetitions. Participants rated the difficulty of the set using a 10-point difficulty scale ranging from 1 (not at all difficult) to 10 (extremely difficult). The participant perceptions of difficulty rating were used to choose the first weight at which a 1RM test was attempted. Participants were subsequently asked to lift the weight once and to continue to perform single repetition lifts separated by at least a 2-min rest interval until a maximum weight was reached and recorded as the 1RM.
Body composition was assessed using the Bod Pod (Life Measurement Inc., Concord, CA). The Bod Pod system uses whole body densitometry to determine body composition (body fat and lean body mass). Whole body densitometry is based on the determination of body mass and body volume, since body density is equivalent to body weight divided by body volume. The Bod Pod has well-established validity and reliability as an assessment of body composition [54].
Self-reported resistance exercise was assessed using the Leisure-Time Exercise Questionnaire (LTEQ) [55]. The LTEQ has been shown to demonstrate adequate reliability and validity for assessing self-reported resistance exercise in previous research [56]. Moderate to vigorous physical activity (MVPA) and step counts were obtained via the LIFECORDER EX accelerometer (Suzuken Kenz Inc. Limited, Japan). Participants wore the LIFECORDER EX on their right hip, attached to either the waistband or the belt, during all waking hours, except when showering, bathing, or swimming, for 7 consecutive days following the completion of the baseline, 2-month, and 3-month screening visits. The LIFECORDER EX has well-established validity and reliability and has been used in prior lifestyle intervention trials targeting chronic disease patients [56].
Dietary intake was assessed using two methods. Longer term diet habits were assessed with a food frequency questionnaire (FFQ) (Dietary Health Questionnaire, DHQ II). The DHQ II is a validated, 134-item FFQ that queried participants about dietary intake over the previous 3 months [57]. This FFQ was completed at baseline and 3 months and was analyzed using Diet*Calc analysis [58, 59]. Additionally, we collected a 3-day diet record at baseline and 3 months. The baseline diet record was used to assist the team in providing personalized dietary pattern recommendations toward meeting dietary goals. We assessed changes in reported consumption of fruits and vegetables (servings per day), whole and refined grains, sugar sweetened beverages, processed meat and red meat consumption in the intervention group and compared both groups at all time points. Finally, assessments of select indicators of trial feasibility, including recruitment rates, intervention adherence, adverse events, and retention rates, were calculated prospectively throughout the trial.
Statistical Analysis
The effects of the EX+D and SC control interventions on changes in primary (mobility performance) and secondary outcomes (muscular strength, body composition, exercise and dietary behavior, and quality of life) were analyzed using separate 2 (Treatment: EX+D and Control) × 2 (Time: 2-month and 3-month) analysis of covariance (ANCOVA) models. In each ANCOVA, change from baseline in each measure was used as the outcome, and time on androgen-deprivation therapy (in months) and baseline value of each outcome were included in the models as covariates. ANCOVA analyses were conducted using the intent-to-treat principle to account for missing data with the last observation carried forward (LOCF) approach, used to impute change across time to be zero. All analyses were conducted using SPSS 22.0 (IBM SPSS Statistics for Windows, Version 22, Armonk, NY; IBBBM Corp.). Additionally, effect sizes (Cohen’s d) were calculated by taking the mean difference and dividing by the pooled standard deviation to determine the magnitude of differences observed for each outcome. Based on recent recommendations for estimating sample size in pilot, randomized trials [60], the IDEA-P sample size was adequate to obtain effect size estimates necessary to accurately set parameters for a subsequent, optimally powered randomized controlled lifestyle intervention trial.
Results
Patient Characteristics and Flow Through the Trial
We enrolled 32 prostate cancer patients undergoing androgen-deprivation therapy with data collection for the IDEA-P trial ending in May 2015. Select demographic characteristics of the patients at baseline are partitioned by treatment arm and summarized in Table 2 with no significant differences between treatment arms at baseline. As illustrated in the CONSORT diagram in Fig. 1, 346 patient medical records were screened to determine prostate cancer patients undergoing androgen-deprivation therapy being treated at the James Cancer Hospital who may be eligible for the trial. A total of 256 patients were directly contacted by study staff via mailing or clinic referral, and 54 (21%) of those patients expressed interest and lived within a convenient driving distance to facilitate trial participation. Distant travel and work schedules were identified as the primary obstacle to participation among interested patients. Taken collectively, the total accrual of 32 patients who were randomly assigned to EX+D (n = 16) or SC control (n = 16) arms yields relevant recruitment rates of 9.2% of the 346 initial medical records reviewed, 21% of the patients who were contacted by study staff, and 59% of the patients who expressed interest in the trial and completed the pre-screen for eligibility. In the IDEA-P trial, there was 69% retention at 2-month follow-up (EX+D = 88%; SC Control = 50%) and 78% retention at 3-month follow-up (EX+D = 88%; SC Control = 69%). Collectively, 25 of the 32 patients (78%) enrolled completed the baseline assessment and at least 1 of the follow-up assessments, with 68% (22 of 32) of patients completing the 2-month follow-up and 78% completing the 3-month follow-up assessment. Inspection of retention in each of the treatment arms demonstrates that 88% of patients randomized to EX+D completed baseline and at least 1 follow-up assessment, while 69% of patients randomized to control completed baseline and at least 1 follow-up. Of the seven patients who did not complete at least one follow-up assessment, two (6%) were from the EX+D arm and five were from the control arm (16%). It should be recognized that consistent with the objectives of an intention-to-treat approach, our retention strategies were successful in getting three control patients who did not complete 2-month testing to return for the final 3-month follow-up assessment. Additionally, it is relevant to note the two patients in the EX+D arm who did not complete either of the two follow-up assessments dropped out of the study prior to the start of the intervention. Thus, all patients who began the EX+D intervention completed at least one follow-up assessment.
Table 2.
Participant Characteristics
| Measure | Intervention arms, n (%) | |
|---|---|---|
| EX+D | SC | |
| Age, mean (SD) | 69.4 (9.0) | 64.5 (8.6) |
| Ethnicity | ||
| White | 12 (75) | 15 (93.8) |
| African American | 3 (18.8) | 0 (0) |
| Education | ||
| High school or less | 0 (0) | 0 (0) |
| More than high school | 14 (87.5) | 15 (93.8) |
| Income, USA | ||
| Less than $15K | 0(0) | 0 (0) |
| $15–35K | 1 (6.3) | 2 (12.5) |
| $35–50K | 3 (18.8) | 1 (6.3) |
| More than $50K | 11 (68.8) | 12 (75) |
| BMI, kg/m2, m (SD) | 28.5 (9.05) | 31.5 (6.23) |
| BMI classification | ||
| Underweight | 0 (0) | 0 (0) |
| Normal | 3 (18.8) | 0 (0) |
| Overweight/Obese | 12 (75) | 16 (100) |
| Gleason, m (SD) | 7.77 (1.0) | 7.64 (1.39) |
| Time on ADT (months), m (SD) | 32.18 (27.28) | 15.31 (19.39) |
EX+D exercise and dietary intervention; BMI body mass index; SC standard of care; SD standard deviation; ADT androgen-deprivation therapy.
Adverse Events
No serious adverse events attributable to the intervention (i.e., resulting in hospitalization or disability) were reported during the EX+D intervention. Two participants in the EX+D arm experienced nonserious adverse events during exercise. One patient reported dizziness during exercise. Subsequent investigation identified nonadherence to oral diabetes medication to be a contributing factor. After physician consultation, the patient completed all remaining exercise sessions without incident. One patient experienced musculoskeletal pain in the shoulder and back that required modification and/or cessation of select upper body exercises, and this pain resolved without additional treatment. During study participation, one patient in the control arm experienced cancer progression that necessitated discontinuing the study.
Mobility Performance
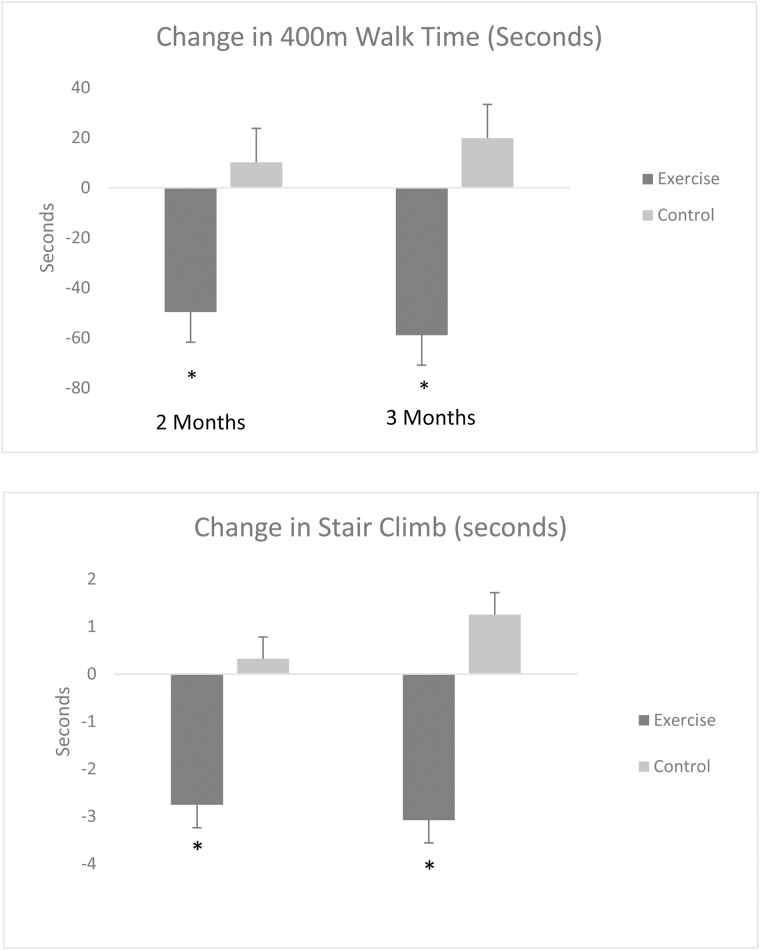
ANCOVA analysis of change in 400MWT yielded a significant treatment main effect (F = 7.76, p < .01). Inspection of the baseline-adjusted mean changes provided in Table 3 demonstrates that the EX+D intervention resulted in superior improvements in 400MWT at 2 months (d = 0.60) and 3 months (d = 0.72), relative to the control intervention. ANCOVA analysis of change in stair climb performance also resulted in a significant treatment main effect (F = 5.64, p < .02). The baseline-adjusted mean changes provided in Table 2 demonstrates that the EX+D intervention resulted in superior improvements in stair climb performance at 2-month (d = 0.47) and 3-month (d = 0.45) follow-up, relative to the control intervention. Results for change in mobility performance are also illustrated in Fig. 2.
Table 3.
Adjusted Values for the Changes in Mobility Performance (in Seconds)
| Measure | 2-month change | 3-month change | p | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Exercise | Control | Exercise | Control | ||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| 400mWalk | −49.64 | 137.98 | 10.25 | 29.35 | -58.85 | 137.49 | 19.82 | 48.21 | .009 |
| Stair climb | −2.76 | 9.20 | 0.32 | 1.89 | −3.08 | 9.91 | 1.25 | 1.86 | .03 |
SC standard of care; SD standard deviation; 400mWalk 400-meter walk test.
Fig. 2.
Adjusted means of the change in mobility performance (in seconds) from baseline by treatment arm.
Body Composition
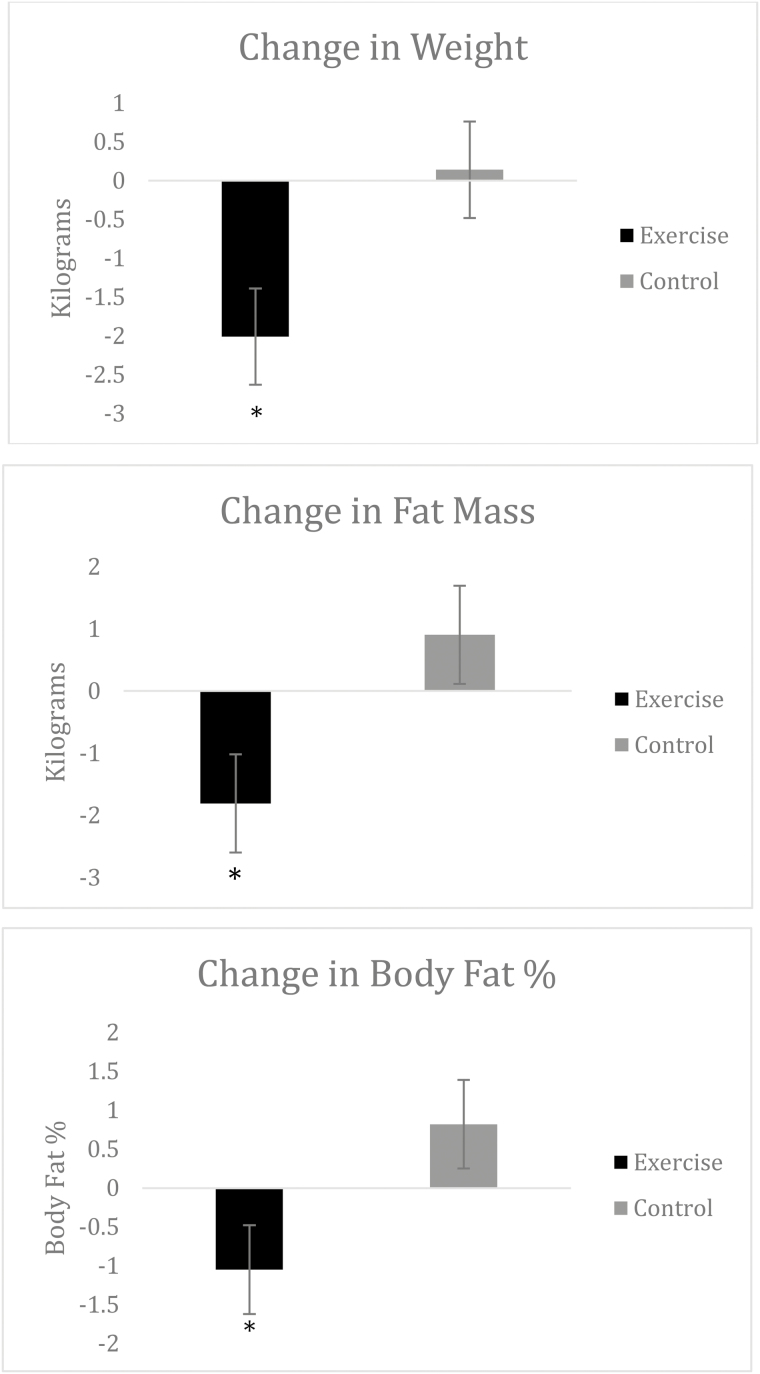
ANCOVA analysis of change in body composition yielded significant treatment main effects for total body weight (F = 5.35, p < .03), body fat percentage (F = 4.23, p < .05), and fat mass (F = 4.89, p < .04). The baseline-adjusted mean changes (Table 4) demonstrate that the EX+D intervention resulted in superior improvements in total body weight (d = 0.88), body fat % (d = 0.78), and fat mass (d = 0.82) at 3-month follow-up, relative to the control intervention. No significant differences in fat-free mass were observed between the two treatment arms at 3-month follow-up. Results for change in body composition are also illustrated in Fig. 3.
Table 4.
Adjusted Values for the Changes in Body Composition
| Measure | 3-month change | p | |||
|---|---|---|---|---|---|
| Exercise | Control | ||||
| Mean | SD | Mean | SD | ||
| Weight (kg) | −2.01 | 2.79 | 0.14 | 2.05 | .02 |
| Fat mass (kg) | −1.81 | 3.51 | 0.90 | 3.05 | .03 |
| Fat-free mass (kg) | −0.06 | 1.27 | −0.50 | 1.36 | .42 |
| Body fat (%) | −1.05 | 2.30 | 0.82 | 2.48 | .04 |
SD standard deviation.
Fig. 3.
Adjusted means of the change in body composition from baseline by treatment arm.
Muscular Strength
ANCOVA analysis of change in lower body strength (leg extension 1RM) yielded a significant treatment main effect (F = 6.98, p < .01). The baseline-adjusted mean changes (Table 5) demonstrate that the EX+D intervention resulted in superior improvements in lower body strength at 2-month (d = 0.93) and 3-month (d = 0.89) follow-up, relative to the controls. ANCOVA analysis of change in upper body strength (chest press 1RM) yielded a significant treatment × time interaction (F = 7.34, p < .01). The baseline-adjusted mean changes (Table 4) demonstrate that, although similar changes in 1RM strength were observed at 2-month follow-up (d = 0.28), the EX+D intervention resulted in superior improvements in upper body strength at 3-month (d = 0.86) follow-up, relative to the control intervention.
Table 5.
Adjusted Values for the Changes in Muscular Strength
| Measure | 2-month change | 3-month change | p | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Exercise | Control | Exercise | Control | ||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| Leg extension (lbs) | 14.87 | 30.51 | −8.75 | 18.92 | 19.56 | 35.47 | −5.00 | 16.32 | .01 |
| Chest press (lbs) | 23.13 | 26.31 | 7.50 | 23.52 | 23.43 | 26.31 | 2.81 | 21.67 | .01 |
SD standard deviation.
Exercise and Dietary Behavior
Adherence to participation in the supervised exercise sessions in the EX+D arm was 88% (range 43% to 100%; mean number of sessions attended = 12.5 [(SD = 1.95]). Based on the evaluation of exercise logs returned, patients in the EX+D arm completed a mean of 7.45 exercise sessions (SD = 2.58) during Month 3, the independent phase of the intervention. Adherence to the diet sessions in the EX+D arm was 84% (range 50% to 100%; mean number of sessions attended = 12 [SD = 2.10]). ANCOVA analysis of change in LTEQ yielded a significant treatment main effect (F = 19.87, p < .01). Inspection of the baseline-adjusted mean changes provided in Table 5 demonstrates that the EX+D intervention resulted in superior improvements in self-reported resistance exercise sessions at 2-month (d = 1.56) and 3-month (d = 1.83) follow-up, relative to the SC intervention. Complete data for each of the 7 days of accelerometer assessments were obtained in 13 participants in the EX+D arm and 15 participants in the SC Control arm. ANCOVA analysis of change in total weekly minutes of MVPA did not yield any significant treatment effects (all p > .26). ANCOVA analysis of change in average daily steps yielded a significant treatment × time interaction (F = 4.95, p = .035). These findings revealed that while the EX+D intervention resulted in more favorable improvements in daily steps at 2-month (d = 0.65) follow-up, relative to the control intervention, change in steps did not differ between treatment arms at 3-month follow-up (d = 0.01).
A subsample of trial participants provided complete assessments of dietary intake via 3-day diet records (EX+D: n = 9; SC: n = 7). Unfortunately, such a small sample set coupled with the imprecision of diet assessment tools makes it impossible to precisely quantify changes in dietary patterns. A major target of dietary intervention is increasing fruit and vegetable intake. ANCOVA analysis of change in fruit and vegetable intake yielded a significant treatment main effect (F = 4.69, p = .05). The EX+D intervention resulted in superior increase in fruit and vegetable intake (d = 1.04). The EX+D intervention also resulted in more favorable decreases in consumption of refined grains (d = 0.49) and sugar-sweetened beverages (d = 0.44).
Discussion
Findings from the present randomized pilot trial, IDEA-P, demonstrated that a lifestyle intervention integrating a personalized exercise and dietary prescription with group-mediated self-regulatory skills counseling to promote independent maintenance of lifestyle behavior change resulted in significant, clinically meaningful improvements in mobility performance, body composition, and muscular strength outcomes relative to SC control in prostate cancer patients undergoing androgen-deprivation therapy. The favorable retention and adherence rates, together with the absence of intervention-related adverse events, provide evidence that the GMCB EX+D intervention is safe and well tolerated by prostate cancer patients on androgen-deprivation therapy. In addition to the favorable outcomes observed in IDEA-P, the trial findings also suggest that the GMCB EX+D intervention was successful in promoting adoption and short-term maintenance of independent, self-regulated exercise and dietary behavior change. Integrating a theory-based GMCB approach in the delivery of a lifestyle intervention makes the IDEA-P trial unique from prior studies exploring the benefits of combined EX+D interventions in the treatment of prostate cancer patients undergoing androgen-deprivation therapy [29, 30]. Indeed, the IDEA-P is one of the first pilot trials demonstrating the feasibility and preliminary efficacy of a GMCB-based EX+D intervention in countering the well-established functional, musculoskeletal, and body composition changes associated with androgen-deprivation therapy. These findings suggest that the synergistic effects of a lifestyle intervention combining personalized exercise and dietary prescription with group-mediated self-regulatory skills counseling may make this approach a particularly beneficial adjuvant treatment for prostate cancer patients undergoing androgen-deprivation therapy.
It is well established that androgen-deprivation therapy is related to a loss of muscle strength and functional decline in prostate cancer patients [15, 16]. The present findings revealed that the GMCB EX+D intervention yielded significant improvements in mobility performance and muscular strength relative to SC control treatment. It is notable that the magnitude of improvement in 400MWT observed with the EX+D intervention was approximately three times greater than the established minimally clinical significant difference [42, 61]. The mobility performance benefits observed in IDEA-P are consistent with findings from recent randomized controlled trials implementing similar GMCB-based EX+D intervention in chronic disease patients at risk for mobility disability, and the present results extend these findings to prostate cancer patients [42, 50, 51]. Given that 400MWT and muscular strength are significant predictors of future disability, healthcare cost, and mortality, these promising findings underscore the potential utility of integrating lifestyle interventions combining group-mediated, self-regulatory skill counseling and personalized EX+D prescription in the treatment of prostate cancer patients on androgen-deprivation therapy.
In addition to a decline in strength and function, androgen-deprivation therapy consistently results in adverse effects upon body composition outcomes, thereby placing prostate cancer patients at increased risk for sarcopenic obesity, cardiovascular disease, and metabolic syndrome [3–6, 62]. In the present trial, the EX+D intervention resulted in significant reductions in body weight, body fat percentage, and fat mass relative to SC control. Thus, consistent with the findings observed for mobility performance, the EX+D intervention yielded meaningful improvements in clinically relevant body composition outcomes. EX+D yielded approximately a 3-kg net difference in fat mass and 2% difference in body fat percentage compared with SC control. Additionally, while fat-free mass remained stable in the EX+D arm, patients randomized to control experienced a ½ kg decline in fat-free mass at 3-month follow-up indicating that the GMCB EX+D intervention was successful in attenuating the decline in fat-free mass that has been consistently observed with ADT. Taken collectively, these findings provide initial evidence supporting the value of lifestyle interventions combining GMCB counseling with personalized exercise and diet prescription in offsetting androgen-deprivation-induced toxicities upon body composition.
Exercise interventions have consistently resulted in significant improvements in physical function and body composition in prostate cancer patients on androgen-deprivation therapy, and these beneficial effects for offsetting androgen deprivation-related toxicities have promoted growing recognition of the utility of implementing exercise in the adjuvant treatment of prostate cancer patients [20, 63–68]. The GMCB EX+D intervention in IDEA-P resulted in improvements in mobility performance and body composition that are comparable or superior to changes observed with exercise interventions in prior studies targeting prostate cancer patients on androgen-deprivation therapy [20, 63–68]. These findings provide initial evidence supporting the potential additive and/or synergistic benefit of combining personalized exercise and dietary prescription with group-mediated lifestyle behavioral counseling designed to promote independent adherence and goal achievement in offsetting and, for select outcomes, reversing the adverse effects of androgen-deprivation therapy upon decline in mobility performance and increases in body fat and fat mass among prostate cancer patients undergoing androgen-deprivation therapy.
Promoting independent adherence to exercise and dietary behavior change is integral to the efficacy of lifestyle interventions. Accordingly, a primary objective of the design and sequencing of the contacts in the GMCB approach to delivering the EX+D intervention was to gradually wean participants from dependency upon study staff in supervised exercise and diet sessions and to transition them toward independent self-regulation of these behaviors. Patients randomized to the GMCB EX+D arm reported a significant increase in participation in weekly resistance exercise sessions that was successfully sustained across the independent phase of the intervention at 3-month follow-up. It is notable that similar findings were observed for change in dietary behavior. Although only a subsample of patients completed comprehensive assessments of dietary intake, findings from the present trial demonstrate that patients in the EX+D intervention were successful in adopting and maintaining dietary modifications consistent with American Institute of Cancer Research and Dietary Guidelines for Americans recommendations, such as increased fruit and vegetable intake, at 3-month follow-up. In this regard, the emphasis upon group-mediated development, practice, and mastery of key self-regulatory skills such as self-monitoring, goal setting, and implementation of group and individualized barrier problem-solving integrated in the GMCB approach to delivering the EX+D intervention appears to have been particularly effective in promoting successful adoption and short-term maintenance of exercise and dietary behavior change. Given the promising short-term adherence observed in IDEA-P, determining the efficacy of a GMCB intervention for producing long-term maintenance of lifestyle behavior change in the treatment of prostate cancer patients undergoing androgen-deprivation therapy warrants further inquiry.
Although fat-free mass remained stable following the EX+D intervention, findings from prior trials have demonstrated that resistance exercise interventions have elicited significant, clinically meaningful increases in lean body mass [20, 21, 64, 67]. Thus, while the EX+D intervention appears to have attenuated androgen deprivation-induced loss of fat-free mass, exercise alone has reversed loss of lean body mass. It should be recognized that over 80% of patients randomized into the EX+D intervention in the present trial were classified as overweight or obese at baseline. Consequently, modest daily caloric restriction was a goal of the personalized intervention for the majority of patients in the EX+D intervention. Although the mechanisms underlying this effect cannot be delineated from the present pilot trial findings, it is reasonable to propose that the weight loss resulting from the combination of decreased caloric intake of the diet component and increased energy expenditure of the exercise component may have attenuated the effects of the resistance exercise component of the lifestyle intervention in successfully stimulating increases in fat-free mass. Collectively, findings of the benefits of the EX+D intervention from the present study and prior evidence of the efficacy of exercise both provide support for the value of integrating lifestyle interventions in the treatment of prostate cancer patients on ADT. Additional studies investigating the efficacy of combining personalized exercise and dietary interventions for improving physical function, body composition, and longer term metabolic and health outcomes in prostate cancer patients on ADT are warranted.
To offset the musculoskeletal toxicities of ADT, resistance exercise was the focal aspect of the center-based exercise sessions in this multicomponent lifestyle intervention. Results from IDEA-P revealed that prostate cancer patients successfully maintained independent resistance exercise participation in Month 3 of the trial. Conversely, the significant increase in daily steps observed following the supervised phase of EX+D was not maintained during the independent phase of the intervention at 3-month follow-up. In light of the emphasis upon resistance exercise during the supervised exercise sessions, the present approach of primarily promoting aerobic exercise and physical activity participation through unsupervised, home-based activity may have limited successful maintenance of this improvement in daily steps at 3-month follow-up. Providing additional emphasis of the GMCB group-mediated development and practice of self-regulatory skills upon use of the accelerometer/pedometer as a self-monitoring tool could be one promising strategy to aid in promoting improved maintenance of aerobic activity for prostate cancer patients.
Despite the promising findings of the IDEA-P trial, there are several study limitations that should be acknowledged. The relatively low recruitment percentages demonstrate the potential challenge of recruiting prostate cancer patients on androgen-deprivation therapy into lifestyle intervention trials and underscore the need to evaluate novel strategies to increase the access, reach, and scalability of exercise and dietary interventions in this population. Although the present pilot trial’s sample size was sufficient for setting parameters for a future definitive trial, the sample size clearly did not provide optimal power to detect differences in all potentially relevant outcomes of interest. Furthermore, the short duration of both the intervention and the follow-up assessment period may have limited the ability to observe meaningful changes in key outcomes. Future optimally powered, large-scale randomized lifestyle intervention trials incorporating longer duration interventions and follow-up periods are necessary to determine the efficacy of this EX+D intervention in the treatment of prostate cancer patients on androgen-deprivation therapy. The present sample’s ethnicity and socioeconomic homogeneity may also limit the generalizability of the findings, and interventions targeting more diverse samples of patients are needed to evaluate the extent to which the present findings can be appropriately generalized to the larger prostate cancer patient population. The pilot study lacked the resources required to obtain assessments of biomarkers of prostate cancer disease progression and the common metabolic diseases that contribute to comorbidity. Subsequent longer term and in-depth studies will aid in determining the extent to which the favorable changes observed with EX+D are linked with improvements in salient indicants of prostate cancer progression and other disease processes common in this population. Finally, since IDEA-P compared the GMCB EX+D intervention with a SC control treatment, the value of this approach relative to other active treatment and/or behavioral intervention strategies has yet to be delineated. Given the intensive nature of such interventions, determining both cost-effectiveness and comparative efficacy of such interventions in the treatment of prostate cancer patients on androgen-deprivation therapy is also warranted.
In summary, findings from the IDEA-P trial provide evidence of the feasibility, safety, and preliminary efficacy of implementing a GMBC-based EX+D intervention among prostate cancer patients undergoing androgen-deprivation therapy. The personalized EX+D intervention resulted in significant, clinically meaningful improvements in mobility performance, body composition, and muscular strength relative to a SC control treatment. Additionally, the GMCB approach, which targets the development and practice of key behavioral self-regulatory skills, yielded successful adoption and short-term maintenance of independent self-regulated exercise and dietary behavior change. Consequently, given that the EX+D intervention in the IDEA-P trial shows promise for countering and/or reversing the adverse effects of androgen-deprivation therapy, preserving mobility, and promoting short-term adherence to independent lifestyle behavior change, the utility of implementing this approach in the treatment of prostate cancer patients on androgen-deprivation therapy should be evaluated in future large-scale efficacy studies in diverse populations.
Acknowledgments:
This study is supported by National Cancer Institute (R03 CA16296901).
Compliance with Ethical Standards
Authors’ Statement of Conflict of Interest and Adherence to Ethical Standards: Authors Brian C. Focht, Alexander R. Lucas, Elizabeth Grainger, Christina Simpson, Ciaran M. Fairman, Jennifer M. Thomas-Ahner, Jackie Buell, J. Paul Monk, Amir Mortazavi, and Steven K. Clinton declare that they have no conflict of interest.
Author Contributions: BCF, SKC, EG, and JMTA conceived the study. BCF, SKC, and EG participated in the design of the study. SKC, PM, AM provided critical screening and recruitment of patients for the study. BCF, EG, ARL, CS, CMF, JB and SKC conducted the study. BCF, EG, ARL, CS, CMF, and SKC prepared the manuscript and all authors provided additional editing of the final manuscript.
Ethical Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- 1. Cooperberg MR, Moul JW, Carroll PR. The changing face of prostate cancer. J Clin Oncol. 2005;23(32):8146–8151. [DOI] [PubMed] [Google Scholar]
- 2. Basaria S, Lieb J 2nd, Tang AM et al. . Long-term effects of androgen deprivation therapy in prostate cancer patients. Clin Endocrinol (Oxf). 2002;56(6):779–786. [DOI] [PubMed] [Google Scholar]
- 3. Boxer RS, Kenny AM, Dowsett R, Taxel P. The effect of 6 months of androgen deprivation therapy on muscle and fat mass in older men with localized prostate cancer. Aging Male. 2005;8(3–4):207–212. [DOI] [PubMed] [Google Scholar]
- 4. Bylow K, Dale W, Mustian K et al. . Falls and physical performance deficits in older patients with prostate cancer undergoing androgen deprivation therapy. Urology. 2008;72(2):422–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bylow K, Mohile SG, Stadler WM, Dale W. Does androgen-deprivation therapy accelerate the development of frailty in older men with prostate cancer?: A conceptual review. Cancer. 2007;110(12):2604–2613. [DOI] [PubMed] [Google Scholar]
- 6. Chen Z, Maricic M, Nguyen P, Ahmann FR, Bruhn R, Dalkin BL. Low bone density and high percentage of body fat among men who were treated with androgen deprivation therapy for prostate carcinoma. Cancer. 2002;95(10):2136–2144. [DOI] [PubMed] [Google Scholar]
- 7. Dacal K, Sereika SM, Greenspan SL. Quality of life in prostate cancer patients taking androgen deprivation therapy. J Am Geriatr Soc. 2006;54(1):85–90. [DOI] [PubMed] [Google Scholar]
- 8. Diamond TH, Higano CS, Smith MR, Guise TA, Singer FR. Osteoporosis in men with prostate carcinoma receiving androgen-deprivation therapy: Recommendations for diagnosis and therapies. Cancer. 2004;100(5):892–899. [DOI] [PubMed] [Google Scholar]
- 9. Levine GN, D’Amico AV, Berger P et al. ; American Heart Association Council on Clinical Cardiology and Council on Epidemiology and Prevention, the American Cancer Society, and the American Urological Association Androgen-deprivation therapy in prostate cancer and cardiovascular risk: A science advisory from the American Heart Association, American Cancer Society, and American Urological Association: endorsed by the American Society for Radiation Oncology. CA Cancer J Clin. 2010;60(3):194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Courneya KS, Stevinson C, Vallance JKH. Exercise and psychosocial issues for cancer survivors. In: Handbook of Sport Psychology. 3rd ed Tenenbaum/Handbook. Hoboken, NJ: John Wiley & Sons, Inc; 2007:578–597. [Google Scholar]
- 11. Crawford ED. Epidemiology of prostate cancer. Urology. 2003;62(6 suppl 1):3–12. [DOI] [PubMed] [Google Scholar]
- 12. Ruchlin HS, Pellissier JM. An economic overview of prostate carcinoma. Cancer. 2001;92(11):2796–2810. [DOI] [PubMed] [Google Scholar]
- 13. American Cancer Society. Cancer Facts & Figures 2005. Atlanta; 2005. [Google Scholar]
- 14. American Cancer Society. Cancer Facts & Figures 2006. Atlanta; 2006. [Google Scholar]
- 15. Holzbeierlein JM, McLaughlin MD, Thrasher JB. Complications of androgen deprivation therapy for prostate cancer. Curr Opin Urol. 2004;14(3):177–183. [DOI] [PubMed] [Google Scholar]
- 16. Joly F, Alibhai SM, Galica J et al. . Impact of androgen deprivation therapy on physical and cognitive function, as well as quality of life of patients with nonmetastatic prostate cancer. J Urol. 2006;176(6 Pt 1):2443–2447. [DOI] [PubMed] [Google Scholar]
- 17. Kornblith AB, Herr HW, Ofman US, Scher HI, Holland JC. Quality of life of patients with prostate cancer and their spouses. The value of a data base in clinical care. Cancer. 1994;73(11):2791–2802. [DOI] [PubMed] [Google Scholar]
- 18. Carmack Taylor CL, Smith MA, de Moor C et al. . Quality of life intervention for prostate cancer patients: Design and baseline characteristics of the active for life after cancer trial. Control Clin Trials. 2004;25(3):265–285. [DOI] [PubMed] [Google Scholar]
- 19. Galvão DA, Taaffe DR, Spry N, Joseph D, Turner D, Newton RU. Reduced muscle strength and functional performance in men with prostate cancer undergoing androgen suppression: A comprehensive cross-sectional investigation. Prostate Cancer Prostatic Dis. 2009;12(2):198–203. [DOI] [PubMed] [Google Scholar]
- 20. Segal RJ, Reid RD, Courneya KS et al. . Resistance exercise in men receiving androgen deprivation therapy for prostate cancer. J Clin Oncol. 2003;21(9):1653–1659. [DOI] [PubMed] [Google Scholar]
- 21. Thorsen L, Courneya KS, Stevinson C, Fosså SD. A systematic review of physical activity in prostate cancer survivors: Outcomes, prevalence, and determinants. Support Care Cancer. 2008;16(9):987–997. [DOI] [PubMed] [Google Scholar]
- 22. Bourke L, Smith D, Steed L et al. . Exercise for men with prostate cancer: A systematic review and meta-analysis. Eur Urol. 2016;69(4):693–703. [DOI] [PubMed] [Google Scholar]
- 23. Goldberg JH, King AC. Physical activity and weight management across the lifespan. Annu Rev Public Health. 2007;28:145–170. [DOI] [PubMed] [Google Scholar]
- 24. Jakicic JM, Otto AD. Treatment and prevention of obesity: What is the role of exercise?Nutr Rev. 2006;64(2 Pt 2): S57–S61. [DOI] [PubMed] [Google Scholar]
- 25. Wing RR. Cross-cutting themes in maintenance of behavior change. Health Psychol. 2000;19(1S):84–88. [DOI] [PubMed] [Google Scholar]
- 26. American College of Cardiology/American Heart Association Task Force on Practice Guidelines, Obesity Expert Panel, 2013. Executive summary: Guidelines (2013) for the management of overweight and obesity in adults: A report of the American College of Cardiology/American Heart Association task force on practice guidelines and the Obesity Society published by the Obesity Society and American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Based on a systematic review from the the Obesity Expert Panel, 2013. Obesity (Silver Spring). 2014;22 (suppl 2:S5–S39. [DOI] [PubMed] [Google Scholar]
- 27. The U.S. Department of Health and Human Services. 2015–2020 Dietary Guidelines for Americans. 2015. [Google Scholar]
- 28. Wiseman M. The second World Cancer Research Fund/American Institute for Cancer Research expert report. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Proc Nutr Soc. 2008;67(3):253–256. [DOI] [PubMed] [Google Scholar]
- 29. Bourke L, Gilbert S, Hooper R et al. . Lifestyle changes for improving disease-specific quality of life in sedentary men on long-term androgen-deprivation therapy for advanced prostate cancer: A randomised controlled trial. Eur Urol. 2014;65(5):865–872. [DOI] [PubMed] [Google Scholar]
- 30. Bourke L, Doll H, Crank H, Daley A, Rosario D, Saxton JM. Lifestyle intervention in men with advanced prostate cancer receiving androgen suppression therapy: A feasibility study. Cancer Epidemiol Biomarkers Prev. 2011;20(4):647–657. [DOI] [PubMed] [Google Scholar]
- 31. Buffart LM, Galvão DA, Brug J, Chinapaw MJ, Newton RU. Evidence-based physical activity guidelines for cancer survivors: Current guidelines, knowledge gaps and future research directions. Cancer Treat Rev. 2014;40(2):327–340. [DOI] [PubMed] [Google Scholar]
- 32. Bluethmann SM, Bartholomew LK, Murphy CC, Vernon SW. Use of theory in behavior change interventions. Health Educ Behav. 2017;44(2):245–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Abraham C, Michie S. A taxonomy of behavior change techniques used in interventions. Health Psychol. 2008;27(3):379–387. [DOI] [PubMed] [Google Scholar]
- 34. Michie S, Carey RN, Johnston M et al. . From theory-inspired to theory-based interventions: A protocol for developing and testing a methodology for linking behaviour change techniques to theoretical mechanisms of action. Ann Behav Med. 2016;369(10):954–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pinto BM, Ciccolo JT. Physical activity motivation and cancer survivorship. Recent Results Cancer Res. 2011;186:367–387. [DOI] [PubMed] [Google Scholar]
- 36. Ettinger WH Jr, Burns R, Messier SP et al. . A randomized trial comparing aerobic exercise and resistance exercise with a health education program in older adults with knee osteoarthritis. The Fitness Arthritis and Seniors Trial (FAST). JAMA. 1997;277(1):25–31. [PubMed] [Google Scholar]
- 37. Focht BC. Effectiveness of exercise interventions in reducing pain symptoms among older adults with knee osteoarthritis: A review. J Aging Phys Act. 2006;14(2):212–235. [DOI] [PubMed] [Google Scholar]
- 38. Rejeski WJ, Brawley LR, Ambrosius WT et al. . Older adults with chronic disease: Benefits of group-mediated counseling in the promotion of physically active lifestyles. Health Psychol. 2003;22(4):414–423. [DOI] [PubMed] [Google Scholar]
- 39. Cartwright D. Achieving change in people: Some applications of group dynamics theory. Hum Relat. 1951;4(4):381–392. [Google Scholar]
- 40. Zander A. Making Groups Effective. San Francisco, CA; 1982. [Google Scholar]
- 41. Focht BC, Garver MJ, Devor ST et al. . Improving maintenance of physical activity in older, knee osteoarthritis patients trial-pilot (IMPACT-P): design and methods. Contemp Clin Trials. 2012;33(5):976–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Focht BC, Garver MJ, Devor ST et al. . Group-mediated physical activity promotion and mobility in sedentary patients with knee osteoarthritis: Results from the IMPACT-pilot trial. J Rheumatol. 2014;41(10):2068–2077. [DOI] [PubMed] [Google Scholar]
- 43. Bandura A.In: Freeman WH, ed. Self-Efficacy. The Exercise of Control. New York; 1997. [Google Scholar]
- 44. Ussher M, Aveyard P, Manyonda I et al. . Physical activity as an aid to smoking cessation during pregnancy (LEAP) trial: Study protocol for a randomized controlled trial. Trials. 2012;13:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Michie S, Ashford S, Sniehotta FF, Dombrowski SU, Bishop A, French DP. A refined taxonomy of behaviour change techniques to help people change their physical activity and healthy eating behaviours: The CALO-RE taxonomy. Psychol Health. 2011;26(11):1479–1498. [DOI] [PubMed] [Google Scholar]
- 46. Eckel RH, Jakicic JM, Ard JD et al. ; American College of Cardiology/American Heart Association Task Force on Practice Guidelines 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 Pt B):2960–2984. [DOI] [PubMed] [Google Scholar]
- 47. Jensen MD, Ryan DH, Apovian CM et al. ; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol. 2014;63(25 Pt B):2985–3023. [DOI] [PubMed] [Google Scholar]
- 48. Rock CL, Doyle C, Demark-Wahnefried W et al. . Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012;62(4):243–274. [DOI] [PubMed] [Google Scholar]
- 49. Brawley L, Rejeski WJ, Gaukstern JE, Ambrosius WT. Social cognitive changes following weight loss and physical activity interventions in obese, older adults in poor cardiovascular health. Ann Behav Med. 2012;44(3):353–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rejeski WJ, Brubaker PH, Goff DC Jr et al. . Translating weight loss and physical activity programs into the community to preserve mobility in older, obese adults in poor cardiovascular health. Arch Intern Med. 2011;171(10):880–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rejeski WJ, Fielding RA, Blair SN et al. . The lifestyle interventions and independence for elders (LIFE) pilot study: Design and methods. Contemp Clin Trials. 2005;26(2):141–154. [DOI] [PubMed] [Google Scholar]
- 52. Rejeski WJ, Ettinger WH Jr, Schumaker S, James P, Burns R, Elam JT. Assessing performance-related disability in patients with knee osteoarthritis. Osteoarthritis Cartilage. 1995;3(3):157–167. [DOI] [PubMed] [Google Scholar]
- 53. Wilkins LW, ed. ACSM’s Guide to Exercise and Cancer Survivorship. Philadelphia, PA; 2010. [Google Scholar]
- 54. Heymsfield SB, Lichtman S, Baumgartner RN et al. . Body composition of humans: comparison of two improved four-compartment models that differ in expense, technical complexity, and radiation exposure. Am J Clin Nutr. 1990;52(1):52–58. [DOI] [PubMed] [Google Scholar]
- 55. Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Can J Appl Sport Sci. 1985;10(3):141–146. [PubMed] [Google Scholar]
- 56. Dean RN, Farrell JM, Kelley ML, Taylor MJ, Rhodes RE. Testing the efficacy of the theory of planned behavior to explain strength training in older adults. J Aging Phys Act. 2007;15(1):1–12. [DOI] [PubMed] [Google Scholar]
- 57. Subar AF, Thompson FE, Kipnis V et al. . Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires: The Eating at America’s Table Study. Am J Epidemiol. 2001;154(12):1089–1099. [DOI] [PubMed] [Google Scholar]
- 58. Diet*Calc Analysis Program Version 1.5.0. October 2012. [Google Scholar]
- 59. Diet History Questionnaire, Version 2.0. [Google Scholar]
- 60. Whitehead AL, Julious SA, Cooper CL, Campbell MJ. Estimating the sample size for a pilot randomised trial to minimise the overall trial sample size for the external pilot and main trial for a continuous outcome variable. Stat Methods Med Res. 2016;25(3):1057–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kwon S, Perera S, Pahor M et al. . What is a meaningful change in physical performance? Findings from a clinical trial in older adults (the LIFE-P study). J Nutr Health Aging. 2009;13(6):538–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Galvão DA, Newton RU, Taaffe DR, Spry N. Can exercise ameliorate the increased risk of cardiovascular disease and diabetes associated with ADT?Nat Clin Pract Urol. 2008;5(6):306–307. [DOI] [PubMed] [Google Scholar]
- 63. Carmack Taylor CL, Demoor C, Smith MA et al. . Active for life after cancer: A randomized trial examining a lifestyle physical activity program for prostate cancer patients. Psycho-Oncology. 2006;15(10):847–862. [DOI] [PubMed] [Google Scholar]
- 64. Galvão DA, Taaffe DR, Spry N, Joseph D, Newton RU. Combined resistance and aerobic exercise program reverses muscle loss in men undergoing androgen suppression therapy for prostate cancer without bone metastases: A randomized controlled trial. J Clin Oncol. 2010;28(2):340–347. [DOI] [PubMed] [Google Scholar]
- 65. Segal RJ, Reid RD, Courneya KS et al. . Randomized controlled trial of resistance or aerobic exercise in men receiving radiation therapy for prostate cancer. J Clin Oncol. 2009;27(3):344–351. [DOI] [PubMed] [Google Scholar]
- 66. Culos-Reed SN, Robinson JW, Lau H et al. . Physical activity for men receiving androgen deprivation therapy for prostate cancer: Benefits from a 16-week intervention. Support Care Cancer. 2010;18(5):591–599. [DOI] [PubMed] [Google Scholar]
- 67. Galvão DA, Nosaka K, Taaffe DR et al. . Resistance training and reduction of treatment side effects in prostate cancer patients. Med Sci Sports Exerc. 2006;38(12):2045–2052. [DOI] [PubMed] [Google Scholar]
- 68. Culos-Reed SN, Robinson JL, Lau H, O’Connor K, Keats MR. Benefits of a physical activity intervention for men with prostate cancer. J Sport Exerc Psychol. 2007;29(1):118–127. [DOI] [PubMed] [Google Scholar]