Objectively-measured social engagement is associated with a combined measure of inflammation and response to latent viruses in a sample of recently divorced adults
Keywords: Divorce, Marital separation, Social integration, Social support, Immunological risk
Abstract
Background
Close relationships play an integral role in human development, and robust evidence links marital separation and divorce to poor health outcomes. Social integration may play a key role in this association. In many ways, the study of marital separation and divorce provides an ideal model system for a more complete understanding of the association between life stress and physical health.
Purpose
The current study investigated associations among objectively measured social integration, psychological distress, and biomarkers of immune health in recently separated adults (N = 49).
Methods
We collected four measures of immune functioning—interleukin-6, C-reactive protein, and antibody titers to latent cytomegalovirus and Epstein–Barr virus—that were combined to yield a viral-Immune Risk Profile. To assess how variability in social integration is associated with immunological correlates following the end of a marriage, we incorporated observational ecological momentary assessment data using a novel methodology (the Electronically Activated Recorder).
Results
We found that objectively measured social behaviors are associated with concurrent viral-Immune Risk Profile scores over and above the effects of psychological distress and that psychological distress may be linked to biomarkers of immune health through social integration.
Conclusions
This research expands current knowledge of biomarkers of immune health after divorce and separation and includes a new methodology for objective measures of social engagement.
Introduction
The link between stress and immune health is well established [1, 2]. Psychological stress activates a coordinated biological response, involving the endocrine, autonomic, and immune systems, to respond to environmental threats and challenges [3]. Initially, this response is adaptive; however, over time, exposure to chronic stress taxes the biological systems designed to respond to these demands and can ultimately impair health [1, 4, 5]. Marital separation and divorce are stressful life events that, although generally considered acute [6], engender a host of challenges that can become chronic. Indeed, divorce is consistently considered one of the life’s most stressful events [7], and given its association with psychological distress, disrupted health behaviors, and poor distal health outcomes, the dissolution of marriage represents an ideal “model system” for developing a deeper understanding of the mechanisms underlying stress–health associations in general [8, 9].
High-quality close relationships are associated with increased life satisfaction and physical well-being [10, 11], and the dissolution of these relationships is reliably associated with increased morbidity and mortality across the life span [12]. The epidemiological link between marital separation/divorce and mortality is robust. For example, in a meta-analysis of 104 studies published across more than 50 years and involving more than 600 million people, the mean hazard ratio for mortality in separated/divorced adults was 1.30 (95% confidence interval [CI]: 1.23–1.37) [13]. The magnitude of this effect is comparable to many recognized public health risks [14], although questions about the direction of causality in the divorce-health association remain [15]. In the current cross-sectional study, we explore the psychosocial correlates of immune health following the end of marriage; in doing so, we focus on a largely unexplored construct in the divorce–health literature: objectively measured daily social behavior and its correlation with immune biomarkers.
Stress and Immune Health
There are a variety of pathways through which the stress associated with marital separation can ultimately affect health [16]. Within the immune system, inflammatory processes release proinflammatory chemical messengers, including the cytokine interleukin-6 (IL-6) and C-reactive protein (CRP). IL-6 initiates the proliferation and differentiation of cells as part of the immune response to injury or infection [17]. CRP is produced by the liver in response to IL-6 and functions to recruit other cells as part of the innate immune response [18]. If these signals become dysregulated, the duration and intensity of inflammation within the body remains elevated and the proinflammatory response can run unchecked [19]. Over time, this process increases risk for inflammatory diseases including type II diabetes [20], autoimmune diseases [21], cancer [22], and cardiovascular diseases [5, 23, 24]
In a meta-analysis of stress and circulating inflammatory biomarkers, a wide range of psychosocial stressors was related to elevated levels of IL-6 and CRP [25]. Studies indicate spouses caregiving (for patients with dementia and Alzheimer’s disease) evidence increases in inflammatory biomarkers compared with noncaregivers [26]. Furthermore, the Trier Social Stress Test is associated with linear increases in IL-6 following the task [27], and levels of general perceived stress are positively associated with CRP levels [28].
The study of viral reactivation provides another window into the integrity of the immune system. Antibodies, produced following exposure to an antigen such as a virus, coordinate the body’s adaptive response to pathogens. For example, circulating levels of antibody titers to human cytomegalovirus (CMV) and Epstein–Barr virus (EBV) reflect prior viral activity, with greater antibody levels in part corresponding to initial response to infection as well as maintenance of viral latency [29]. Studies show that elevated CMV titers are found in the presence of psychosocial stressors including caregiving for dementia patients [30] and in response to depression, anxiety, and low socioeconomic status [31]. Stress related to academic examinations is associated with increased EBV titers [32] and CMV titers [33]. Recently, Fagundes et al. [34] demonstrated that anxious attachment in interpersonal relationships is associated with increased EBV titers. In addition to increased antibody titers in response to stress, there is evidence to suggest that the bodily resources associated with maintaining immunity to persistent latent viruses (i.e., immunosurveillance and alterations to important immune cell populations) may contribute to earlier mortality [35].
In sum, the literature suggests that inflammation and elevated antibody titers to latent viruses are important markers of immune function and are associated with psychological stress. Furthermore, as broadly predictive measures, they are used across a variety of fields, making them both accessible and applicable for studying links between the stress of marital separation and health.
Marital Disruption and Immunological Outcomes
Three decades ago, work by Kiecolt-Glaser et al. [36] established the link between divorce and immunological response. Compared with their married counterparts, separated/divorced women exhibited higher antibody titers to latent EBV [36], whereas separated men evidence elevated antibody titers to two forms of the herpes virus [37]. In the decades since this seminal study, our understanding of the broad-based health correlates of marital separation has expanded enormously (see [9]); however, no other investigations have extended this early work on the psychoneuroimmunology of divorce. A key goal of the current study is to provide a needed update to the research in this area by combining the assessment of immune functioning with modern ambulatory techniques for objectively assessing daily social functioning (see [38]).
Divorce, Social Integration, and Daily Behaviors
Although research establishes a clear link between the psychosocial stress of marital separation and immune function, questions about the role of social context remain. Social support and social integration are associated with a range of positive health-relevant outcomes [39, 40], and a lack of support constitutes a clear health risk [41]. When marriages come to an end, many adults experience disruptions in their social networks [42, 43]. Disruptions in social integration have consequences for adjustment following marital dissolution [36, 44, 45], and the availability of social support is thought to buffer against the negative consequences of divorce [46, 47].
In addition to the perceived availability of social resources, loneliness (i.e., low levels of perceived social integration) is highly associated with adjustment to marital separation. Divorced adults report higher levels of loneliness than their married counterparts [37, 48], and chronic loneliness is associated with a range of health-relevant biomarkers [49, 50] and increased risk for early death [51]. Importantly, lonely adults are not by definition socially isolated. Social isolation is an objective measure of how much time people spend by themselves, marked by living alone, having few relationships, and interacting rarely with social network ties [51], and isolation, independent of loneliness, appears important to health as well. In an 18-year follow-up study, data indicate that self-reported social isolation predicted mortality through levels of inflammation [52]. A recent meta-analysis of social isolation indicates that both subjective and objective measures predict increased mortality risk [51, 53].
One limitation of the loneliness–social isolation literature is that the previously employed “objective” measures of social isolation are not strictly objective. Typically, these measures are derived indirectly from participants’ first-person, subjective reports of “countable” aspects of their social life. These reports often include some combination of marital status, number of persons in the household, number of social activities, and frequency of contact with others (cf. [54, 55]), rather than being derived directly from a “third-person” measure that is fully independent of participants’ perceptions. In contrast, the Electronically Activated Recorder (EAR) [56, 57] provides a reliable measure of objective daily social behavior. Using a smartphone-based audio recorder, the EAR samples ambient sounds in the participants’ immediate social environment. The resulting collection of sound bites constitutes an acoustic log of a person’s day that then can be coded for target behaviors that reflect objective social isolation, including the amount of time a person spent socializing with others and alone (i.e., not engaged in any social interaction).
As a naturalistic observation method, the EAR provides a powerful, nonreactive characterization of daily behavior outside the laboratory while bypassing information processing limitations (e.g., forgetting or not noticing) and biases (e.g., demand characteristics; social desirability) that affect traditional self-report [58]. EAR-assessed constructs have been linked with immune parameters [44, 59], and in the current study, the EAR serves as an observational ecological momentary assessment tool for tracking objective social behaviors following the end of marriage. The EAR allows us to (a) examine if a group of objectively assessed daily behaviors representing social integration is associated with immune risk and (b) advance the understanding of how self-reported loneliness and perceived social support may differentially predict immune outcomes relative to an objective measure of these behaviors.
The Current Study
In this study, we used marital separation and divorce as a model system to examine the associations among social behavior, psychological distress, and measures of immune health. Specifically, we collected self-report, naturalistic observational data (using the EAR), and blood samples from 52 separated adults. Immune measures included circulating levels of IL-6, CRP, and antibody titers to CMV and EBV. These measures were then combined to yield a viral-Immune Risk Profile (vIRP) variable (described in greater detail below).
Based on the literature reviewed above, we tested four hypotheses (H) concerning immune measures in this cross-sectional study; we expected that (H1) higher levels of self-reported psychological distress would be associated with higher levels of inflammation, higher antibody levels, and a higher vIRP score; (H2) higher levels of social integration, as measured objectively by the EAR, would be associated with lower levels of inflammation, a lower antibody response to latent viruses, and a lower vIRP score; (H3) self-reported loneliness would be associated with immune measures and vIRP after controlling for EAR-indexed time spent alone; and, similarly, (H4) self-reported perceived social support would predict immune measures and vIRP after accounting for objective, EAR-indexed positive social support received.
Finally, we conducted a set of two exploratory mediation analyses to investigate whether psychological distress might be related to immune parameters and vIRP through EAR-indexed social integration. We expected that (H5) high levels of psychological distress would negatively affect levels of social integration, which, in turn, would be associated with increased vIRP scores. We also considered the alternative possibility that social integration might be associated with immune risk through psychological distress.
Method
Participants
This study involved 49 adults (n = 15 men, mean age = 44.08, SD = 11.16) who reported a recent marital separation (mean months since separation = 3.67 months, SD = 2.61) and who took part in a larger NIH-funded study of psychosocial responses to marital separation (N = 128). This article focuses on this subsample of 49 participants unless otherwise noted. The average length of participants’ prior relationships was 14.63 years (SD = 8.91). Separation in this case was defined as the date of permanent physical separation from a spouse. Racial and ethnic composition of the sample is as follows: 61.2% Caucasian, 22.4% Hispanic, 4.1% African American, 2.0% Asian, 10.2% indicated their race was “Other.” Participants were required to have been married to their ex-partner for at least 3 years and have lived with them for at least 2 years, to be 18 years of age or older, and free of diagnoses for schizophrenia, bipolar disorder, suicidal ideation, or uncontrolled medical conditions. Subjects who met these initial criteria and who were interested in participating in the immune substudy (n = 71) were further screened for inclusion on the following: not currently pregnant, no history of psychotic disorders, no excessive caffeine intake (>400 mg of caffeine a day), no history of blood or needle phobias, and no recent history (prior 3 months) of active immunosuppressive treatments, surgeries, or autoimmune diseases. Of those interested and screened, eight declined to participate in the substudy, eight were not eligible based on screening criteria, and three eventually dropped out of the parent study. The remaining participants were asked to refrain from ingesting anti-inflammatory agents during the 24-hr period preceding the draw and to refrain from caffeine and tobacco for the 4-hr period preceding the draw. Following the draw, three participants had CRP levels above 10 mg/L, indicating possible acute infection, and were dropped from the sample. Thirty-one percent of the sample reported currently experiencing a controlled medical condition. The following issues were reported: allergies, high blood pressure, high cholesterol, migraines, pain, and thyroid conditions. The proportion of the sample who reported currently smoking was 12%. A comparison of the subsample (n = 49) to the full study sample (N = 128) revealed no significant differences in age, gender, length of marriage or time since separation, as well as no significant differences between the samples in measures of psychological adjustment, EAR-assessed social behaviors, or the composites of psychological adjustment or social integration (all R2 < .024).
An Institutional Review Board responsible for human subject research reviewed this project and found it to be acceptable, according to applicable state and federal regulations and University policies designed to protect the rights and welfare of participants in research.
Procedure
The current study examined associations between psychological distress, social integration, and immunological functioning following a recent marital separation. Subjects were recruited from a larger cohort study, the Divorce, Sleep, and EAR (DSE) study. Upon entry into the larger DSE study, participants were consented and completed questionnaires assessing their psychological response to the separation. Participants brought their completed questionnaire packets to the first study visit where waist-to-hip ratio (WHR) measurements were taken. Participants were given instructions regarding the EAR device and took the device home for data collection over the course of a weekend. Blood was drawn within 2 weeks of the initial study visit (M = 1.18 days, SD = 12.15 days). A final visit allowed participants to review their EAR data and delete sound files that they did not want to be included in the study.
Measures
Self-report measures
Demographic, health behaviors, and relationship history questionnaire.
(see [60]). This questionnaire consists of basic demographics, health behaviors (i.e., smoking, exercise, alcohol consumption, medical conditions, past hospitalizations/surgeries), WHR, and objective relationship history questions that may affect immune biomarkers [61]. We collected health history information to use as additional control variables when evaluating levels of inflammation.
Assessment of health status and health behaviors.
This questionnaire assessed compliance with study requests regarding consumption of alcohol, caffeine, and medications, as well as exercise and sleep quality before the blood draw.
Interpersonal support evaluation list.
Partici pants’ self-reported perception of social support resources was assessed using the Interpersonal Support Evaluation List—Short Form [62]. This 12-item measure includes items such as “There is someone I can turn to for advice about handling problems with my family,” which are indicated on a Likert scale (1 = “definitely false” to 4 = “definitely true”). The mean of participants’ responses was used (α = .89; M = 3.09, SD = 0.59, range = 1.75–4.00). Higher mean scores on the Interpersonal Support Evaluation List (ISEL) reflect higher levels of perceived social support.
Psychological composite.
A Psychological Composite score was created by taking the z-scored mean of each of the self-report measures detailed below and then averaging those z-scores. A factor analysis of these self-report measures revealed they all loaded on a single factor (which explained 59.7% of the variance, eigenvalue = 3.00). This was confirmed by visual inspection of the scree plot. The reliability of the Psychological Composite was acceptable (α = .82), with higher scores reflecting greater subjective emotional distress after the separation.
Revised inventory of complicated grief.
The original Inventory of Complicated Grief (ICG) is a factor-analytically derived measure of complicated grief, where individuals with high scores are associated with higher levels of depression and emotional distress [63]. We revised the ICG to relate to divorce, and the 15-item measure taps the participant’s maladaptive symptoms of loss, including grief, avoidance, and trouble accepting the end of the relationship. The revised ICG contains items such as “I think about my ex-partner so much that it is hard for me to do the things I need to do.” Responses ranged from 0 (never) to 4 (always; α = .95).
Center for epidemiological studies short depression scale.
The Center for Epidemiological Studies Short Depression (CES-D 10) scale is a 10-item version of the original CES-D scale [64] and serves as a self-report measure of depressive symptoms over the past week. Responses range from Rarely = 0 to All of the time = 3 to items such as “I felt depressed.” Higher scores indicate more depressive symptomatology, with a cutoff score of 10 and above considered depressed (α = .88).
Impact of events scale—revised.
The Impact of Events Scale—Revised (IES-R) [65] is a widely used measure of subjective responses to stressful events and assesses several dimensions of responding following a stressful event, including intrusive thoughts, hyper-arousal, emotional numbing, avoidance, and total subjective distress. Respondents report on the degree of distress of a given symptom over the last 7 days on a scale (from 0 = Not at all to 4 = Extremely). Sample items include statements such as “Any reminder brought back feelings about it” and “I thought about it when I didn’t mean to.” Higher scores reflect greater self-reported emotional distress following the separation (α = .95).
Loss of self—rediscovery of self.
The Loss of Self—Rediscovery of Self (LOSROS) scale [66] is a 12-item measure assessing the extent to which individuals feel they have “lost” or “rediscovered” their sense of self following their separation. Questions to evaluate the loss of self-concept include “I do not know who I am.” Questions that evaluate self-rediscovery include “I have done the things I once enjoyed that I could not do while I was in my relationship.” Participants respond on a 7-point Likert scale (from 1 = Not at all to 7 A great deal; α = .93).
Three-item loneliness scale.
The three-item version of the UCLA Loneliness Scale was used to assess subjective loneliness, the subjective experience of social isolation [67], with items such as “How often do you feel that you lack companionship?” Respondents indicate the frequency of these feelings on a scale (1 = Hardly ever to 3 = Often; α = .86).
Objective measures
Electronically activated recorder.
The EAR device is an observational, real-time, ecological data capture method composed of an audio sampling app installed on an iPod Touch device [56]. The app records ambient sounds for 30 s every 12 min or about five times an hour. Because the EAR samples only a fraction of the day (~5%), it protects participant privacy and makes large naturalistic observation studies viable. Over the course of the weekend, files were sampled beginning at approximately 6:00 pm on Friday evening with 6-hr black-out periods on Friday and Saturday nights during sleep (as indicated by self-report) and ending on Sunday at 11:59 pm. Each participant wore the EAR for a full weekend. Participants were to indicate in a diary when they did not wear the EAR and to report what percentage of the day they kept the EAR immediately on them (M response = 81.8%, SD = 17.7). Participants were able to listen to and delete any sound files they did not want in the study; four subsample participants excluded files, and of those four, they excluded an average of 5.75 sound files upon the final review (M number of waking files = 148.6; SD = 36.9).
EAR-observed daily social behavior.
Trained research assistants coded participants’ remaining EAR sound files for momentary social behaviors using a counting strategy based on the presence (“1”) or absence (“0”) of a target behavior based on a version of a standardized coding system (see [68]), which was adjusted for the purpose of the study. These codes capture acoustically detectable aspects of participants’ social environments and interactions in four broad categories (location, activity, interactions, affect).
A distinction of critical importance for this investigation is whether a participant appeared to be interacting with others or not in any given recorded sound file. “Alone” was coded when a participant was alone or when the participant was surrounded by other people but not engaged in any interaction. Theoretically, a participant was considered alone if, at a given time, they were not part of a social group (see [69]). For example, if a participant studies alone in a coffee shop and is not accompanied by anyone while working, they are a “party of one” and therefore “alone” despite the fact that other people might be heard in the background. However, if coders heard this participant interacting with someone, they would code this file as “not alone” or, more accurately, “with other.” Methodologically, the EAR is constrained to the identification of audible signal; if there is no sign of interaction for the entire sound file, coders score this file as “alone.” Conversations were further classified by content including substantive conversation, defined as an involved conversation of a substantive nature where thoughts, information, values, and/or ideas about a non-emotional topic are exchanged [70] and positive social support received. Positive support received is comprised of emotional support, practical support, and informational support and coded “globally” (i.e., not by each of the components). Finally, socializing/entertaining, defined as hanging out with others for fun, was included in the current analyses.
Each sound file was given a binary code (behavior present vs. absent) within each category. These raw codes were converted into a relative frequency variable indicating the number of waking EAR files in which a coding category applied (e.g., the percentage of time over the course of the weekend during which the participant was engaged in the target behavior). Two trained research assistants independently coded each of a participant’s recorded sound files, and the research assistants were regularly supervised for reliability (see [58]).
Social integration composite.
A social integration composite was constructed by taking the mean of the z-scored percentages (i.e., the percentage of sound files over the weekend where the behavior was present) of the following behaviors: time spent (a) with others, (b) socializing or entertaining, (c) in substantive conversation, and (d) receiving positive social support. We calculated the inter-rater reliability for each of the social behaviors separately in the subsample. For each participant, we averaged each coder’s ratings of the individual social behaviors across all of their EAR files, which provided two average ratings per participant (one from each coder) for each of the social behaviors assessed. Individually, the intercoder reliability for each is as follows: time spent (a) with others, intraclass correlation coefficient (ICC) (1,2) = .94, (b) socializing or entertaining, ICC (1,2) = .43, (c) in substantive conversation, ICC (1,2) = .86, and (d) time spent receiving positive social support, ICC (1,2) = .54. The social integration composite displayed acceptable reliability within the subsample (α = .76), and higher scores indicate a higher percentage of time spent engaging in the specified daily social behaviors.
Blood samples.
Subjects were directed to a local medical center for their blood draw. Here participants provided the additional informed consent specific to the immune parameters substudy and completed a short psychological assessment battery. All samples were collected between 8:00 am and 12:00 pm to control for known diurnal variations in markers of inflammation [25]. A certified phlebotomist drew approximately 8 mL of peripheral blood, which was collected and stored in vacutainer tubes and allowed to clot for 30 min. Serum was obtained after centrifugation and was stored at −80°C until testing (see IL-6 levels and CRP levels). Two milliliters of whole blood was collected for complete blood counts and held at room temperature for further processing. IL-6, CRP, and antibody titers were log transformed to correct for skewed distributions.
IL-6 levels.
Banked frozen serum was thawed and IL-6 levels were quantified in duplicate using a research grade Elisa kit (Quantikine HS; R&D Systems, Minneapolis, MN) according to manufacturer instructions. The intra-assay coefficients of variation were all less than 5%, which falls under the intra-assay variance of 20% as specified by the manufacturer. All standard curves correlated at r = .98 or greater. No values for IL-6 levels fell outside of the range of detection.
CRP levels.
Banked frozen serum was provided to a university medical center’s clinical chemistry lab for high-sensitivity CRP levels. Each sample was quantified (in duplicate). The high-sensitivity CRP level was determined with an FDA-approved clinical diagnostic assay (Abbott Laboratories) and analyzer (Architect C8000; Abbott Laboratories). Per manufacturer’s instruction assay controls were run and met assay validity criteria. Within-assay (intra-assay) coefficients of variation for each standard were below 5% and the limit of detection was below 20% coefficient of variation. No values for CRP fell outside of the range of detection. Because serum CRP > 10 mg/L can reflect acute infection, adults scoring above this cutoff (n = 3) were excluded from all analyses [71].
Antibody titers to CMV and EBV.
Serum samples with high immunofluorescence assay (IFA)-scored antibody titers (i.e., 2,560), obtained from prior studies, were used as the top standards for CMV and EBV. Seven twofold serial dilutions of the top standards (2,560, 1,280, 640, 320, 160, 80, 40, and 20) were made with phosphate-buffered saline in separate tubes. One hundred microliters of positive and negative controls, standards, and diluted patient samples (all dilutions were at 1:101 with phosphate-buffered saline) were pipetted in duplicate into individual microplate wells followed by a 30-min incubation (all steps were carried out at room temperature). The plates were then washed three times with 350-µL wash buffer using an Embla microplate washer (Molecular Devices, Menlo Park, CA). Next, 100 µL of enzyme conjugate (peroxidase-labeled anti-human IgG) was pipetted into the wells followed by another 30-min incubation period. The plates were then washed three times, and 100 µL of chromogen substrate (tetramethylbenzidine [TMB]/H2O2) was pipetted into the wells. The plates were then covered to protect from direct light and incubated for 15 min. One hundred microliters of 0.5 M sulfuric acid was added to each well to stop the reaction. Absorbance was then read at 450 nm (reference wavelength 620 nm) using a SpectraMax Plus 384 (Molecular Devices). The values of the unknown samples were assigned in relation to the standard curve. Consistent with other findings of seroprevalence, 51% of the sample had been exposed to CMV, while 98% were exposed to EBV.
Viral-immune risk profile.
Thus far, we have presented immune parameters as individual risk markers (e.g., levels of CRP). Another way to conceptualize how markers of inflammation and serologic response to latent viruses operate together is conceptually similar to allostatic load. Allostatic load represents the cumulative physiological wear-and-tear on the body as a result of maintaining homeostasis in response to environmental challenge [72]. The process of restoring homeostasis can be measured across multiple physiological systems (e.g., cardiovascular, endocrine, immune), and scores across systems may be combined to yield a summary measure of allostatic load (see [73]). It is likely that dysfunction across multiple parameters within a given physiological system operates in a manner similar to allostatic load; in this way, a summary index may better represent potential dysfunction in a biological system than any single measure in isolation [74–76]. In a study of inflammation and social networks, the authors created a “summary inflammation burden index” by summing across three dichotomous indicators of inflammation (CRP, fibrinogen, and serum albumin) to predict later mortality [52]. In a study focusing on allostatic load in older adults, selected immune variables (high CD8 and low CD4 counts, and poor proliferative response) were used to create an Immune Risk Phenotype [77, 78]. A follow-up study of the original Immune Risk Phenotype indicated that CMV seropositivity is an additional driver of mortality in the elderly adults [79, 80]. Borrowing conceptually from these previous approaches and given that measures of inflammation are predominant contributors to morbidity and mortality [76], and measures of antibody titer are broadly predictive of a variety of health-relevant outcomes [31], the current study combines the four immune parameters to yield a summary variable akin to the Immune Risk Phenotype. This allows us to study markers of inflammation and antibody titers as continuous variables in isolation in addition to an index of dysfunction across two major components of the immune system: inflammatory response and response to latent viruses. Consistent with the calculation of allostatic load, we calculated a quartile cutoff for each of the immune measures, CRP, IL-6, and antibody titers to CMV and EBV, and participants who fell within the top quartile of a category received a score of 1, while participants falling outside of the top quartile received a score of 0 (see [73]). These scores were combined to yield a four-item vIRP score, ranging from 0 to 4, with higher scores reflecting greater activity across inflammatory biomarkers and/or relative antibody titer to latent viruses (vIRP α = .52).
Missing Data
For the Psychological and Social Integration composite measures, missing data were handled by averaging across the available measures within a given domain (see above for specific details). We followed a procedure in which we computed the average score on the psychological composite if participants provided data for three or more of the constituent measures. Of the total sample, no participants were excluded due to missing more data than permitted with this approach. No participants were missing EAR data for the Social Integration Composite. Immune measures were missing for the following: one missing IL-6 value; no missing CRP values; one missing value for antibody titers to CMV; five titer levels missing for EBV as well as three values categorized as “missing” due to being above the detectable limits. In the case of missing individual immune measures, these participants were excluded from analyses requiring the value for the missing measure. To be conservative in our calculation of the vIRP composite, participants with a missing immune parameter for IL-6, CMV, or EBV received a 0 for that parameter, while participants whose EBV titers were above detectable limits (2,560 nm) received a 1 to indicate risk status.
Data Analysis
Data were inspected for outliers, and no outliers were identified or removed from the data. Hierarchical regression analysis was used to assess variability in concurrent immune response as a function of psychological distress and EAR-assessed social integration. Following the hypotheses outlined above, we tested each focal predictor in the first step of the model and then entered the covariates of interest in the second step. These covariates include age, WHR, race, and smoker status (coded as “1” if respondents report ever smoking, “0” if they have never smoked), all of which are known biobehavioral factors that may influence circulating levels of inflammation [61]. We tested this basic model using the individual, continuous immune parameters, as well as a final model predicting the summary vIRP. To examine the possibility that the EAR-assessed social integration variable operates indirectly to link self-reported psychological distress and immune function, we ran standard mediation analyses using the PROCESS macro [81] in SPSS 24.0; the macro provides the bootstrapped CIs for the indirect effect in a concurrent mediational model.
Results
Table 1 displays descriptive statistics and a correlation matrix of the main variables in the study including values for individual immune parameters and the vIRP. As shown, none of the self-report psychological distress variables correlate with any of the individual immune parameters. The mean proportion of sound files where target social behaviors were coded as present is as follows: time spent alone (M = 59%), time spent with others (M = 41%), time spent socializing or entertaining (M = 13%), time spent receiving positive social support (M = 2%), and time spent in substantive conversation (M = 17%). The distribution of vIRP scores within the sample is as follows: 0 = 22 participants (45%), 1 = 17 (35%), 2 = 7 (14%), 3 = 1 (2%), 4 = 2 (4%). The only social behavior significantly associated with any individual immune outcome was positive support received with CRP levels, r = −.31, p = .031. All other correlations were nonsignificant.
Table 1.
Correlation matrix for variables of interest
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (1) | 1 | |||||||||||||||||||||
| WHR (2) | .24 | 1 | ||||||||||||||||||||
| Smoke (3) | .11 | .03 | 1 | |||||||||||||||||||
| Race (4) | −.03 | −.06 | .24 | 1 | ||||||||||||||||||
| Lone (5) | .10 | .08 | −.02 | .07 | 1 | |||||||||||||||||
| IES-R (6) | .04 | .20 | −.18 | .09 | .56** | 1 | ||||||||||||||||
| Grief (7) | −.09 | .01 | .01 | −.12 | .13 | .29* | 1 | |||||||||||||||
| CES-D (8) | .02 | .16 | .10 | .03 | .55** | .76** | .26 | 1 | ||||||||||||||
| Loss (9) | .01 | .16 | .08 | −.00 | .54** | .51** | .32* | .64** | 1 | |||||||||||||
| Psych (10) | .02 | .16 | −.01 | .02 | .74** | .84** | .52** | .86** | .80** | 1 | ||||||||||||
| ISEL (11) | .01 | −.25 | −.03 | −.17 | −.56** | −.41** | −.28 | −.37** | −.46** | −.55** | 1 | |||||||||||
| Subst (12) | −.31* | −.09 | −.12 | .04 | −.15 | −.09 | .04 | −.158 | −.10 | −.12 | .24 | 1 | ||||||||||
| Support (13) | −.08 | −.02 | −.15 | −.16 | −.33* | −.18 | −.24 | −.13 | −.27 | −.30* | .36* | .15 | 1 | |||||||||
| Soc/Ent (14) | −.17 | −.11 | −.22 | .07 | −.226 | −.06 | −.14 | −.17 | −.23 | −.22 | .32* | .54** | .51** | 1 | ||||||||
| Other (15) | −.54** | −.13 | −.09 | .20 | −.34* | −.07 | −.05 | −.04 | −.07 | −.15 | .20 | .70** | .22 | .53** | 1 | |||||||
| Social (16) | −.36* | −.12 | −.19 | .05 | −.34* | −.13 | −.13 | −.16 | −.22 | −.26 | .37* | .78** | .62** | .85** | .80** | 1 | ||||||
| Alone (17) | .54** | .13 | .10 | −.20 | .34* | .07 | .06 | .04 | .07 | .15 | −.20 | −.70** | −.22 | −.53** | −1.00** | −.80** | 1 | |||||
| IL-6 (18) | .25 | .02 | −.04 | −.019 | .23 | −.02 | .23 | .10 | .18 | .18 | −.41** | −.15 | −.13 | −.15 | −.16 | −.19 | .16 | 1 | ||||
| CRP (19) | −.06 | −.02 | −.16 | −.05 | .21 | −.03 | .07 | −.08 | −.16 | .00 | −.08 | −.13 | −.31* | −.17 | −.24 | −.28* | .25 | .15 | 1 | |||
| CMV (20) | .16 | −.19 | −.21 | −.04 | .08 | −.03 | .01 | .17 | −.02 | .06 | −.20 | −.16 | −.20 | −.20 | −.06 | −.23 | .06 | .60** | .18 | 1 | ||
| EBV (21) | .09 | −.17 | −.08 | −.12 | .06 | .13 | .28 | .07 | −.07 | .12 | −.26 | .04 | −.28 | −.11 | −.10 | −.15 | .10 | .09 | .07 | .73** | 1 | |
| vIRP (22) | .20 | −.15 | −.22 | −.02 | .21 | .11 | .20 | .12 | −.01 | .16 | −.34* | −.20 | −.35* | −.30* | −.27 | −.36* | .27 | .57** | .44** | .85** | .51** | 1 |
| Mean | 44.08 | 0.89 | – | – | 1.97 | 1.38 | 2.26 | 2.13 | 3.04 | 0.00 | 3.09 | .17 | 0.02 | 0.13 | 0.41 | 0.00 | 0.59 | 0.99 | 1.50 | 468 | 560 | 0.86 |
| SD | 11.16 | 0.14 | – | – | 0.67 | 0.88 | 0.96 | 0.68 | 1.38 | 0.75 | 0.60 | .12 | 0.02 | 0.13 | 0.23 | 0.76 | 0.22 | 0.92 | 1.88 | 712 | 668 | 1.02 |
Alone time spent alone or surrounded by others and not interacting; CES-D Center for Epidemiological Studies Short Depression scale; CMV antibody titers to cytomegalovirus; CRP C-reactive protein (mg/L); EBV antibody titers to Epstein–Barr virus; IES-R Impact of Events Scale—Revised; IL-6 interleukin-6 (pg/mL); ISEL Interpersonal Support Evaluation List; Lone loneliness; Loss loss of self; others time spent with others; Psych psychological distress composite; Smoke smoker status and is coded 0 = no, 1 = yes; Soc/Ent time spent socializing/entertaining; Social social integration composite; Subst time spent in substantive conversation; Support time spent receiving positive social support; vIRP viral-Immune Risk Profile; WHR waist-to-hip ratio.
*p < .05. **p < .01.
Given that the zero-order correlations between the self-reported psychological distress variables, the EAR-assessed social behaviors, and the individual immune parameters were largely nonsignificant, we investigated whether the psychological or EAR-assessed social integration composites were more reliably associated with the summary vIRP outcome. We hypothesized that (H1) higher scores on the psychological composite would be associated with higher scores on the vIRP index. The psychological distress composite was not significantly associated with vIRP (p > .05). In the next model, EAR-assessed social integration was a significant predictor of the vIRP outcome, B = −0.54, p = .007, 95% CI (−0.92, −0.15). These results, displayed as Model 1 in Table 2, provide partial support for the hypothesis (H2) that as social integration increases, concurrent immune risk scores decrease. The rightmost side of the table indicates that these associations remained significant after accounting for relevant covariates in each model. Model 2 provided a test of objective social integration relative to psychological distress. As shown, the social integration composite remained significantly related to vIRP scores when self-reported distress was entered into the model (Model 2, Table 2).
Table 2.
Unstandardized coefficients from regression models using psychological distress and social integration composites to predict vIRP
| B | 95% CI | B | 95% CI | |
|---|---|---|---|---|
| Model 1 | ||||
| Intercept | 0.86** | 0.58, 1.13 | 1.70 | −0.26, 3.66 |
| Social composite | −0.49** | −0.85, −0.12 | −0.54** | −0.92, −0.15 |
| Age | 0.01 | −0.01, 0.04 | ||
| Smoker status | 1.07* | −1.99, −0.15 | ||
| Race | .04 | −0.13, 0.22 | ||
| Waist-to-hip ratio | −1.58 | −3.58, 0.43 | ||
| Model 2 | ||||
| Intercept | 0.86** | 0.58, 1.14 | 1.76 | −0.21, 3.74 |
| Social composite | −0.46* | −0.84, −0.08 | −0.50* | −0.90, −0.10 |
| Psych composite | 0.10 | −0.29, 0.49 | 0.13 | −0.25, 0.51 |
| Age | 0.01 | −0.01, 0.04 | ||
| Smoker status | −1.05* | −1.98, −0.11 | ||
| Race | 0.04 | −0.14, 0.22 | ||
| Waist-to-hip ratio | −1.70 | −3.74, 0.35 |
CI confidence interval; psych composite psychological distress composite; smoker status coded as 0 = no, 1 = yes; social composite social integration composite; vIRP viral-Immune Risk Profile.
*p < .05. **p < .01.
Subjective and Objective Social Isolation
Under H3, we explored possible differential prediction of the immune parameters as a function of objective (EAR-indexed) time spent alone and loneliness. Consistent with the zero-order correlations presented in Table 1, neither time spent alone (abbreviated as “Alone”) nor self-reported loneliness (abbreviated as “Lone”) were significantly related to concurrent immune markers, including the vIRP. Thus, we find that even though the overall (EAR-indexed) social integration composite is negatively associated with vIRP scores (accounting for psychological distress), this association does not extend down to the more fine-grained assessment of time spent alone.
Subjective and Objective Social Support
Under H4, we explored possible differential associations between immune parameters as a function of EAR-assessed time spent receiving social support and subjective reports of positive support (as assessed by the ISEL). Time spent receiving social support (i.e., the total percentage of EAR files that were rated for the presence of received positive support) was significantly associated with CRP, B = −0.18, p = .009, 95% CI (−0.31, −0.05), in the expected, negative direction, and remained significant after controlling for covariates and self-reported support received (see Table 3). Contrary to our hypothesis, this effect was entirely independent of self-reported perceived social support, which was not significantly related to CRP levels. When the same model was run with the vIRP as the outcome variable, neither positive support received nor perceived positive support was associated with the vIRP. After including covariates, however, the self-report positive support variable was significantly associated with vIRP, B = −0.35, p = .022, 95% CI (−0.64, −0.05). Because this association is only revealed by entering covariates, it is likely a regression artifact and may not be particularly meaningful. In an attempt to further explore this association, we tested an interaction using sex as a moderator of the relationship between the ISEL and the vIRP. The interaction between sex and self-reported social support is significant, B = 1.12, p = .003, 95% CI (0.40, 1.85). For women, higher levels of self-reported social support were associated with a decrease in vIRP scores of −0.69, p < .001, 95% CI (−0.98, −0.40). For men, perceived social support is not associated with vIRP levels, β = 0.43, p = .197, 95% CI (−0.23, 1.09).
Table 3.
Unstandardized coefficients from regression models using social support variables to predict immune measures
| B | 95% CI | B | 95% CI | |
|---|---|---|---|---|
| Outcome: CRP | ||||
| Intercept | −0.04 | −0.16, 0.08 | −0.34 | −1.23, 0.56 |
| ISEL | 0.02 | −0.11, 0.15 | 0.03 | −0.10, 0.17 |
| Positive support | −0.16* | −0.29, −0.03 | −0.18** | −0.31, −0.05 |
| Age | 0.00 | −0.01, 0.02 | ||
| Smoker status | −0.46* | −0.87, −0.05 | ||
| Race | 0.02 | −0.06, 0.10 | ||
| Waist-to-hip ratio | 0.22 | −0.79, 1.22 | ||
| Outcome: vIRP | ||||
| Intercept | 0.87** | 0.59, 1.15 | 1.89 | −0.05, 3.82 |
| ISEL | −0.25 | −0.55, 0.05 | −0.35* | −0.64, −0.05 |
| Positive support | −0.26 | −0.55, −0.04 | −0.27 | −0.55, 0.01 |
| Age | 0.03* | 0.00, 0.05 | ||
| Smoker status | −0.91* | −1.79, −0.03 | ||
| Race | −0.04 | −0.21, 0.13 | ||
| Waist-to-hip ratio | −2.30* | −4.46, −0.14 |
CI confidence interval; CRP log10 C-reactive protein; ISEL mean score from Interpersonal Support Evaluation List; positive support time spent receiving positive support; smoker status coded as 0 = no, 1 = yes; vIRP viral-Immune Risk Profile.
*p < .05.
Concurrent Mediation Analyses
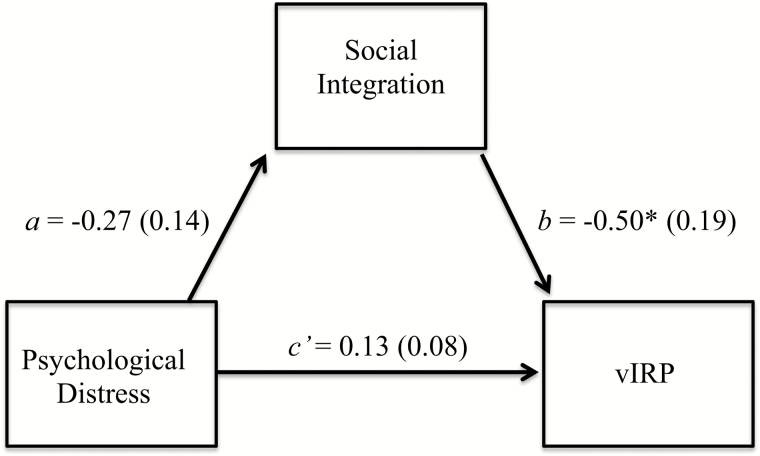
Finally, under H5 we assessed whether the EAR-assessed social integration composite would act indirectly to link the psychological distress composite and the vIRP after including our set of relevant covariates. This analysis revealed a nonsignificant a path, β = −0.27, p = .071, 95% CI (−0.55, 0.02), a significant b path, β = −0.50, p = .016, 95% CI (−0.90, −0.10), and a nonzero indirect effect, β = 0.13, 95% bootstrapped CI (0.01, 0.37); see Fig. 1. The EAR-assessed social integration composite statistically mediated the association between self-reported separation-related distress and vIRP score; higher levels of self-reported psychological distress are associated with less EAR-assessed social integration, which, in turn, is associated with increased scores on the vIRP composite index. We also assessed the alternative possibility that psychological distress mediates the relationship between social integration and immune risk scores; we found no support for the pathways in this model. A set of analyses using a three-item vIRP, which coded being in the top quartile for either titers to CMV or EBV as “1,” revealed similar results for every analysis described previously.
Fig. 1.
Concurrent mediation model predicting nonzero indirect effects (c’ path) of psychological distress on viral-Immune Risk Profile (vIRP) through social integration. The direct effect (c path) is 0.13, p = .482. Effects occur in the hypothesized direction where the (a path) psychological distress negatively influences social integration, which (b path) negatively influences the vIRP. SE values appear in parentheses and are unstandardized. *p < .05
Discussion
The primary purpose of this research was to explore associations among psychological adjustment, objective and subjective measures of social integration and behavior, and concurrent immunological measures in a sample of recently separated adults. Based on a large literature demonstrating that self-reported psychological distress and measures of social integration are associated with immunological functioning [82, 83], we hypothesized that psychological distress and objective measures of social integration would be associated with inflammatory biomarkers and elevated antibody titer to latent viruses. In addition to the individual immune response variables, we computed a vIRP composite, which is conceptually similar to a common index of multisystem biological risk (allostatic load).
We found mixed support for the study hypotheses. There was no evidence that self-reported psychological adjustment to marital separation was associated with any of the immune measures. We found partial support for our second hypothesis, as the EAR-assessed social integration variable (made up of time spent (a) with others, (b) in substantive conversation, (c) socializing or entertaining, and (d) receiving positive support) is significantly associated with concurrent immune activity. Although the social integration composite did not predict individual immune parameters, it was significantly associated with vIRP scores; lower levels of integration were associated with higher scores on the vIRP. Importantly, this effect persisted over and above the effects of the composite measure of psychological distress.
The association of social integration with the vIRP is consistent with a robust literature demonstrating the positive effects of social relationships on health. Social integration is related to beneficial effects across measures of cardiovascular, endocrine, and immune system health [10] and decreased risk for all-cause mortality [14]. Social support is further associated with positive health habits [84], buffers against the negative effects of stress [85], and increases feelings of personal control [86], all of which may operate indirectly to alter immunological responses. A notable contribution of this study is that our assessment of social behaviors and social integration was derived entirely from the EAR, a naturalistic behavioral observation tool that provides an objective account of daily behavior. To our knowledge, this work is the first to examine differential effects of subjective and objective measures of social support on the prediction of immune measures and it is among the first to link EAR-assessed social behaviors to immunological measures in general (see [85]).
An alternative explanation for the connection between social integration levels and the vIRP variable is cytokine-induced sickness behavior [87]. Elevated levels of inflammation coupled with the increased pathogen burden that accompanies poorly controlled latent viruses may have influenced social behaviors in this sample. The theory of sickness behavior contends that accumulated concentrations of proinflammatory cytokines within the brain promote a survival-oriented pattern of behavior [87], which includes withdrawal from social activities [88]. This is a plausible alternative explanation for our data.
With regard to specific associations of interest, we found no evidence that either self-reported loneliness (i.e., the perception of social isolation) or EAR-assessed time spent alone was associated with immune markers. This nonsignificant association is inconsistent with literature that generally indicates loneliness is linked to poorer control of latent viruses [33, 89] and inflammation [90]. Given previous findings, it is surprising that self-reported loneliness was not associated with any immune measures in the current study. It is possible that because loneliness is a normative feature of marital separation, corresponding associations with immune parameters may only emerge if loneliness becomes chronic in the aftermath of a separation. Another population-based study of 229 participants, the Chicago Health, Aging, and Social Relations (CHARS) study, used the same measure of loneliness and reports a similar mean total loneliness (6.1 vs. 6.0, respectively) [67].
We also focused more narrowly on self-reported perceptions of social support and expected (H4) this construct would be associated with lower levels of immune measures over and above the effect of EAR-indexed time spent receiving positive support. Contrary to our expectations, EAR-assessed positive social support received was a significant predictor of CRP beyond the effects of self-reported perceptions of support and relevant health covariates. This association was in the expected, negative direction such that higher levels of support were associated with lower levels of CRP. Interestingly, the ISEL was associated with the summary immune index over and above the effect of EAR-derived positive support received; however, this effect appears to be a regression artifact. In probing the link between the ISEL and the vIRP further, it was revealed that sex moderates the association between self-reported social support and the vIRP such that women reporting more social support demonstrated lower vIRPs. Given the size of this effect, reporting one standard deviation more perceived social support is close to eliminating the effects of falling into the risk category of one of the four immune indicators (e.g., taking a vIRP score of 3 down to 2). This might be reflective of the idea that women are more likely to recruit social support when needed [91, 92] or that women may be more likely to benefit from such support [93].
Studies consistently conclude that higher social support is associated with better immune functioning as measured by inflammatory cytokines [90, 94, 95], faster wound healing [96], and titers to latent viruses [37]. In the current study, the correlation between self-reported perceptions of support (as measured by the ISEL) and EAR-assessed social support was significant, r = .36, p = .010, suggesting the two are tapping similar constructs. However, these two measures evaluate different aspects of social support. The ISEL asks about hypothetical (perceived) support (e.g., “There is someone I can turn to for advice about handling problems with my family”) whereas the EAR measures literal receipt of support. These measures clearly represent the important difference between perceived (subjective) and actual (objective) social support. Compared with reports of four samples drawn from the Pittsburgh Mind-Body Center projects to which the ISEL-12 was administered, the current sample reports similar levels of perceived support (a total ISEL score of 37.1 vs. 41.2, 39.6, 41.0, and 42.3; accessed via the Common Cold Project website, www.commoncoldproject.com).
In the current study, the average percent of EAR files for a given participant that contained receipt of positive support was about 2% (ranging from 0% to 10% of files). Given the low percentage of positive support received in the sample, the strength of its association with CRP is telling—in this sense, a little received support may go a long way. Coupled with the nonsignificant association for time spent alone, these findings raise the possibility that it is the kind of social integration one experiences that matters for health, rather than the amount. This idea is consistent with the stress-support matching hypothesis, which asserts that social support can mitigate the influence of stress when the form of support is well matched to the demands of the stressor [62, 97]. In the case of a social loss like divorce, support that replaces the affiliation or belonging that has been lost in the separation may buffer against negative immunological health.
Beyond the finding that perceived social support is associated with CRP levels, we found no associations between predictor variables and individual immune parameters. Several studies have established the utility of looking at a summary immune measure [74–76], which is hypothesized to reflect shared variance in physiological health risk as opposed to individual measures. A summary index may represent a more accurate depiction of the state of the immune system as a whole; thus, individual associations with our variables of interest might be both less likely and potentially less meaningful. Determining what weight to give each measure in an immune variable may be an important route for future research.
Our study also explored the possibility that (H5) EAR-assessed social integration might operate indirectly to link psychological distress and immune functioning. We observed evidence for this indirect effect as hypothesized. Although no total effect existed between psychological distress and immune measures, the analysis yielded a nonzero indirect effect. Our confidence in the mediated effect is supported by its persistence across three disparate methodologies, which provides evidence that the association between our predictor and mediator cannot be explained by shared method variance. Research indicates that a lack of social support is linked with decreased immune health, and the indirect effect we reported here suggests that the extent to which divorce-related psychological distress is associated with immunological risk may be explained by differences in EAR-assessed social integration. We found no evidence this mediated effect acted in the opposite direction, largely because the zero-order associations between psychological stress and the individual variables were weak.
Limitations
Findings from the present study must be viewed in light of several limitations. The small sample size in this study limits the precision of our effect size estimates, and the results should therefore be considered preliminary rather than conclusive. These findings may overemphasize the experiences of women, as the sample included an unequal sex distribution. More generally, another limitation from the perspective of immune assessment is the relatively young age of the sample (M = 44 years, range = 25–64). Many studies indicate that age influences immune outcomes and that as people age their immune system undergoes a process of senescence, which renders them more vulnerable to negative life events [1]. In particular, the relatively young age of the sample may have contributed to the lack of association between psychological distress and the immune measures, which was particularly unexpected as research indicates marital dissolution is associated with increases in depression [36] and loneliness [48]. The mean level of depressive symptoms reported on the CES-D in this sample was 21.3, which is above the cutoff of 16 for diagnosing a major depressive episode [98]. Compared with a nationally representative study of adults in their midlife who reported a mean CES-D score of 7.8, the current sample appears to be relatively depressed [99], and these findings are consistent with other studies evaluating rates of mood disturbance in separated/divorced samples (M CES-D score = 22.5) [100]. This elevation, coupled with the findings that measures of inflammation are robustly associated with depression [101], suggests that the study was positioned to replicate these significant relationships.
Another limitation associated with this sample is the relatively healthy levels of individual biomarkers exhibited by the participants. The quartile cutoffs for each of the four biomarkers in this sample were as follows: IL-6 = 1.11 mg/pL; CRP = 1.70 mg/L; CMV = 351.70; EBV = 672.45. Other studies of samples with similar ages report higher mean levels of CRP [27, 74, 75] and higher means levels of IL-6 [74, 102]. Despite the comparative healthiness of the current sample, those participants who are “at risk” are more elevated on these measures than the rest of their cohort and may be on a path toward more clinically significant levels.
In addition, we did not have a baseline measure for immune parameters due to the nature of the study, so we cannot determine if the elevations in biomarkers existed prior to separation. Future studies are now ripe for design and modeling that incorporates such broad spectrum immunological baseline measures prior to stress exposure. This would also provide additional estimates of the reliability of the vIRP index, which, although relatively low by the standard applied to scales relying exclusively on self-reported items or measure, remains associated with predictor variables. Finally, given the large number of analyses conducted within the aims and exploratory investigation of this study, consideration of the effects of type 1 error is relevant. Multiple testing increases the chances of a “false positive.” Further study using a larger sample and examining preregistered hypotheses may provide increased precision and confidence in the current study’s results.
Conclusion
The primary findings of the current study add to the literature concerning stress, social integration, and physical health. The present analysis found that an objective composite of social integration, derived from a novel methodology assessing the frequency of naturalistic daily behaviors, was associated with a concurrent immune risk summary score in recently separated adults, and a similarly assessed objective measure of time spent receiving positive social support was associated with circulating levels of CRP. In addition, the EAR-assessed social behavior composite mediated the association between psychological distress and the immune summary score. These results provide an illustration of one distinct pathway through which the psychological stress associated with divorce may be associated with immune health and, if replicated, suggest several exciting lines of future inquiry, including whether targeting these social behaviors can improve health-relevant outcomes following marital separation.
Funding: The research reported in this article was supported by a grant from the National Institute of Child Health & Human Development (HD#069498).
Compliance with Ethical Standards Statements
Authors’ Statement of Conflict of Interest and Adherence to Ethical Standards: Authors Hasselmo, Mehl, Tackman, Carey, Wertheimer, Stowe, and Sbarra declare that they have no conflict of interest. All procedures, including the informed consent process, were conducted in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000.
Authors’ Contributions: All authors were involved in the preparation of this manuscript and read and approved the final version.
Informed Consent: Informed consent was obtained from all individual participants included in the study.
References
- 1. Segerstrom SC, Miller GE. Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry. Psychol Bull. 2004;130(4):601–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Herbert TB, Cohen S. Stress and immunity in humans: a meta-analytic review. Psychosom Med. 1993;55(4):364–379. [DOI] [PubMed] [Google Scholar]
- 3. McEwen BS. Stress, adaptation, and disease: allostasis and allostatic load. Ann NY Acad Sci. 1998;840:33–44. [DOI] [PubMed] [Google Scholar]
- 4. Gouin JP, Hantsoo L, Kiecolt-Glaser JK. Immune dysregulation and chronic stress among older adults: a review. Neuroimmunomodulation. 2008;15(4–6):251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cohen S, Janicki-Deverts D, Doyle WJ et al. . Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proc Natl Acad Sci USA. 2012;109(16):5995–5999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Amato PR. Research on divorce: continuing trends and new developments. J Marriage Fam. 2010;72(3):650–666. [Google Scholar]
- 7. Bloom BL, Asher SJ, White SW. Marital disruption as a stressor: a review and analysis. Psychol Bull. 1978;85(4):867–894. [PubMed] [Google Scholar]
- 8. Sbarra DA, Hasselmo K, Nojopranoto W. Divorce and death: a case study for health psychology. Soc Personal Psychol Compass. 2012;6(12):905–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sbarra DA. Divorce and health: current trends and future directions. Psychosom Med. 2015;77(3):227–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Uchino BN, Cacioppo JT, Kiecolt-Glaser JK. The relationship between social support and physiological processes: a review with emphasis on underlying mechanisms and implications for health. Psychol Bull. 1996;119(3):488–531. [DOI] [PubMed] [Google Scholar]
- 11. Kiecolt-Glaser JK, Gouin JP, Hantsoo L. Close relationships, inflammation, and health. Neurosci Biobehav Rev. 2010;35(1):33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sbarra DA, Law RW, Portley RM. Divorce and death: a meta-analysis and research agenda for clinical, social, and health psychology. Perspect Psychol Sci. 2011;6(5):454–474. [DOI] [PubMed] [Google Scholar]
- 13. Shor E, Roelfs DJ, Bugyi P, Schwartz JE. Meta-analysis of marital dissolution and mortality: reevaluating the intersection of gender and age. Soc Sci Med. 2012;75(1):46–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Holt-Lunstad J, Smith TB, Layton JB. Social relationships and mortality risk: a meta-analytic review. PLoS Med. 2010;7(7):e1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sbarra DA, Hasselmo K, Bourassa KJ. Divorce and health: beyond individual differences. Curr Dir Psychol Sci. 2015;24(2):109–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sbarra DA, Coan JA. Divorce and health: good data in need of better theory. Curr Opin Psychol. 2017;13:91–95. [DOI] [PubMed] [Google Scholar]
- 17. Ballou SP, Kushner I. C-reactive protein and the acute phase response. Adv Intern Med. 1992;37:313–336. [PubMed] [Google Scholar]
- 18. Thompson D, Pepys MB, Wood SP. The physiological structure of human C-reactive protein and its complex with phosphocholine. Structure. 1999;7(2):169–177. [DOI] [PubMed] [Google Scholar]
- 19. Miller GE, Cohen S, Ritchey AK. Chronic psychological stress and the regulation of pro-inflammatory cytokines: a glucocorticoid-resistance model. Health Psychol. 2002;21(6):531–541. [DOI] [PubMed] [Google Scholar]
- 20. Graves DT, Kayal RA. Diabetic complications and dysregulated innate immunity. Front Biosci. 2008;13:1227–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ishihara K, Hirano T. IL-6 in autoimmune disease and chronic inflammatory proliferative disease. Cytokine Growth Factor Rev. 2002;13(4–5):357–368. [DOI] [PubMed] [Google Scholar]
- 22. Lin WW, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest. 2007;117(5):1175–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kofler S, Nickel T, Weis M. Role of cytokines in cardiovascular diseases: a focus on endothelial responses to inflammation. Clin Sci. 2005;108(3):205–213. [DOI] [PubMed] [Google Scholar]
- 24. Elenkov IJ, Iezzoni DG, Daly A, Harris AG, Chrousos GP. Cytokine dysregulation, inflammation and well-being. Neuroimmunomodulation. 2005;12(5):255–269. [DOI] [PubMed] [Google Scholar]
- 25. Steptoe A, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain Behav Immun. 2007;21(7):901–912. [DOI] [PubMed] [Google Scholar]
- 26. Kiecolt-Glaser JK, Preacher KJ, MacCallum RC, Atkinson C, Malarkey WB, Glaser R. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proc Natl Acad Sci USA. 2003;100(15):9090–9095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. von Känel R, Kudielka BM, Preckel D, Hanebuth D, Fischer JE. Delayed response and lack of habituation in plasma interleukin-6 to acute mental stress in men. Brain Behav Immun. 2006;20(1):40–48. [DOI] [PubMed] [Google Scholar]
- 28. McDade TW, Hawkley LC, Cacioppo JT. Psychosocial and behavioral predictors of inflammation in middle-aged and older adults: the Chicago health, aging, and social relations study. Psychosom Med. 2006;68(3):376–381. [DOI] [PubMed] [Google Scholar]
- 29. Vedhara K, Wang ECY. Assessment of the immune system in human psychoneuroimmunology. In: Vedhara K, Wang ECY, eds. Human Psychoneuroimmunology. Oxford, UK: Oxford University Press; 2005:53–80. [Google Scholar]
- 30. Pariante CM, Carpiniello B, Orrù MG et al. . Chronic caregiving stress alters peripheral blood immune parameters: the role of age and severity of stress. Psychother Psychosom. 1997;66(4):199–207. [DOI] [PubMed] [Google Scholar]
- 31. Rector JL, Dowd JB, Loerbroks A et al. . Consistent associations between measures of psychological stress and CMV antibody levels in a large occupational sample. Brain Behav Immun. 2014;38:133–141. [DOI] [PubMed] [Google Scholar]
- 32. Sarid O, Anson O, Yaari A, Margalith M. Epstein–Barr virus specific salivary antibodies as related to stress caused by examinations. J Med Virol. 2001;64(2):149–156. [DOI] [PubMed] [Google Scholar]
- 33. Glaser R, Kiecolt-Glaser JK, Speicher CE, Holliday JE. Stress, loneliness, and changes in herpesvirus latency. J Behav Med. 1985;8(3):249–260. [DOI] [PubMed] [Google Scholar]
- 34. Fagundes CP, Jaremka LM, Glaser R et al. . Attachment anxiety is related to Epstein–Barr virus latency. Brain Behav Immun. 2014;41:232–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pawelec G, Akbar A, Caruso C, Effros R, Grubeck-Loebenstein B, Wikby A. Is immunosenescence infectious?Trends Immunol. 2004;25(8):406–410. [DOI] [PubMed] [Google Scholar]
- 36. Kiecolt-Glaser JK, Fisher LD, Ogrocki P, Stout JC, Speicher CE, Glaser R. Marital quality, marital disruption, and immune function. Psychosom Med. 1987;49(1):13–34. [DOI] [PubMed] [Google Scholar]
- 37. Kiecolt-Glaser JK, Kennedy S, Malkoff S, Fisher L, Speicher CE, Glaser R. Marital discord and immunity in males. Psychosom Med. 1988;50(3):213–229. [DOI] [PubMed] [Google Scholar]
- 38. Conner TS, Mehl MR. Ambulatory assessment – methods for studying everyday life. In: Scott R, Kosslyn S, Pinkerton N, eds. Emerging Trends in the Social and Behavioral Sciences. Hoboken, NJ: Wiley; 2015:1–15. [Google Scholar]
- 39. Cohen S, Wills TA. Stress, social support, and the buffering hypothesis. Psychol Bull. 1985;98(2):310–357. [PubMed] [Google Scholar]
- 40. Uchino BN. Understanding the links between social support and physical health: a life-span perspective with emphasis on the separability of perceived and received support. Perspect Psychol Sci. 2009;4(3):236–255. [DOI] [PubMed] [Google Scholar]
- 41. Uchino BN, Bowen K, Carlisle M, Birmingham W. Psychological pathways linking social support to health outcomes: a visit with the “ghosts” of research past, present, and future. Soc Sci Med. 2012;74(7):949–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hughes R Jr, Good ES, Candell K. A longitudinal study of the effects of social support on the psychological adjustment of divorced mothers. J Divorce Remarriage. 1993;19(1–2):37–56. [Google Scholar]
- 43. Terhell EL. Network dynamics in the long-term period after divorce. J Soc Pers Relat. 2004;21(6):719–738. [Google Scholar]
- 44. Slatcher RB, Robles TF. Preschoolers’ everyday conflict at home and diurnal cortisol patterns. Health Psychol. 2012;31(6):834–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Halford WK, Sweeper S. Trajectories of adjustment to couple relationship separation. Fam Process. 2013;52(2):228–243. [DOI] [PubMed] [Google Scholar]
- 46. Krumrei E, Coit C, Martin S, Fogo W, Mahoney A. Post-divorce adjustment and social relationships: a meta-analytic review. J Divorce Remarriage. 2007;46(3–4):145–166. [Google Scholar]
- 47. Gähler M. “To divorce is to die a bit…”: a longitudinal study of marital disruption and psychological distress among Swedish women and men. Fam J. 2006;14(4):372–382. [Google Scholar]
- 48. van Tilburg TG, Aartsen MJ, Pas S. Loneliness after divorce: a cohort comparison among Dutch older adults. Eur Sociol Rev. 2014;31(3):243–252. [Google Scholar]
- 49. Cacioppo JT, Cacioppo S, Capitanio JP, Cole SW. The neuroendocrinology of social isolation. Annu Rev Psychol. 2015;66:733–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cole SW, Capitanio JP, Chun K, Arevalo JM, Ma J, Cacioppo JT. Myeloid differentiation architecture of leukocyte transcriptome dynamics in perceived social isolation. Proc Natl Acad Sci USA. 2015;112(49):15142–15147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Holt-Lunstad J, Smith TB, Baker M, Harris T, Stephenson D. Loneliness and social isolation as risk factors for mortality: a meta-analytic review. Perspect Psychol Sci. 2015;10(2):227–237. [DOI] [PubMed] [Google Scholar]
- 52. Yang Claire Y, McClintock KM, Kozloski M, Li T. Social isolation and adult mortality: the role of chronic. J Health Soc Behav. 2013;54(2):183–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Steptoe A, Shankar A, Demakakos P, Wardle J. Social isolation, loneliness, and all-cause mortality in older men and women. Proc Natl Acad Sci USA. 2013;110(15):5797–5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Berkman LF, Syme SL. Social networks, host resistance, and mortality: a nine-year follow-up study of Alameda County residents. Am J Epidemiol. 1979;109(2):186–204. [DOI] [PubMed] [Google Scholar]
- 55. Cohen S, Doyle WJ, Skoner DP, Rabin BS, Gwaltney JM Jr. Social ties and susceptibility to the common cold. JAMA. 1997;277(24):1940–1944. [PubMed] [Google Scholar]
- 56. Mehl MR, Pennebaker JW, Crow DM, Dabbs J, Price JH. The Electronically Activated Recorder (EAR): a device for sampling naturalistic daily activities and conversations. Behav Res Methods Instrum Comput. 2001;33(4):517–523. [DOI] [PubMed] [Google Scholar]
- 57. Mehl MR, Pennebaker JW. The sounds of social life: a psychometric analysis of students’ daily social environments and natural conversations. J Pers Soc Psychol. 2003;84(4):857–870. [DOI] [PubMed] [Google Scholar]
- 58. Mehl MR, Robbins ML, Deters FG. Naturalistic observation of health-relevant social processes: the electronically activated recorder methodology in psychosomatics. Psychosom Med. 2012;74(4):410–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tobin ET, Kane HS, Saleh DJ et al. . Asthma-related immune responses in youth with asthma. Psychosom Med. 2015;77(8):892–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sbarra DA, Law RW, Lee LA, Mason AE. Marital dissolution and blood pressure reactivity: evidence for the specificity of emotional intrusion-hyperarousal and task-rated emotional difficulty. Psychosom Med. 2009;71(5):532–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. O’Connor MF, Bower JE, Cho HJ et al. . To assess, to control, to exclude: effects of biobehavioral factors on circulating inflammatory markers. Brain Behav Immun. 2009;23(7):887–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cohen S, Hoberman H. Positive events and social supports as buffers of life change stress. J Appl Soc Psychol. 1983;13(2):99–125. [Google Scholar]
- 63. Prigerson HG, Maciejewski PK, Reynolds CF 3rd et al. . Inventory of Complicated Grief: a scale to measure maladaptive symptoms of loss. Psychiatry Res. 1995;59(1–2):65–79. [DOI] [PubMed] [Google Scholar]
- 64. Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- 65. Creamer M, Bell R, Failla S. Psychometric properties of the Impact of Event Scale – Revised. Behav Res Ther. 2003;41(12):1489–1496. [DOI] [PubMed] [Google Scholar]
- 66. Lewandowski GW, Bizzoco NM. Addition through subtraction: growth following the dissolution of a low quality relationship. J Posit Psychol. 2007;2(1):40–54. [Google Scholar]
- 67. Hughes ME, Waite LJ, Hawkley LC, Cacioppo JT. A short scale for measuring loneliness in large surveys: results from two population-based studies. Res Aging. 2004;26(6):655–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Mehl MR, Gosling SD, Pennebaker JW. Personality in its natural habitat: manifestations and implicit folk theories of personality in daily life. J Pers Soc Psychol. 2006;90(5):862–877. [DOI] [PubMed] [Google Scholar]
- 69. Goffman E. Forms of Talk. Philadelphia, PA: University of Pennsylvania Press; 1981. [Google Scholar]
- 70. Mehl MR, Vazire S, Holleran SE, Clark CS. Eavesdropping on happiness: well-being is related to having less small talk and more substantive conversations. Psychol Sci. 2010;21(4):539–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Pearson TA, Mensah GA, Alexander RW et al. ; Centers for Disease Control and Prevention; American Heart Association Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107(3):499–511. [DOI] [PubMed] [Google Scholar]
- 72. McEwen BS, Stellar E. Stress and the individual: mechanisms leading to disease. Arch Intern Med. 1993;153(18):2093–2101. [PubMed] [Google Scholar]
- 73. Seeman TE, Singer BH, Rowe JW, Horwitz RI, McEwen BS. Price of adaptation – allostatic load and its health consequences. MacArthur studies of successful aging. Arch Intern Med. 1997;157(19):2259–2268. [PubMed] [Google Scholar]
- 74. McCaffery JM, Marsland AL, Strohacker K, Muldoon MF, Manuck SB. Factor structure underlying components of allostatic load. PLoS One. 2012;7(10):e47246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wiley JF, Gruenewald TL, Karlamangla AS, Seeman TE. Modeling multisystem physiological dysregulation. Psychosom Med. 2016;78(3):290–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Gruenewald TL, Seeman TE, Ryff CD, Karlamangla AS, Singer BH. Combinations of biomarkers predictive of later life mortality. Proc Natl Acad Sci USA. 2006;103(38):14158–14163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ferguson FG, Wikby A, Maxson P, Olsson J, Johansson B. Immune parameters in a longitudinal study of a very old population of Swedish people: a comparison between survivors and nonsurvivors. J Gerontol A Biol Sci Med Sci. 1995;50(6):B378–B382. [DOI] [PubMed] [Google Scholar]
- 78. Wikby A, Ferguson F, Forsey R et al. . An immune risk phenotype, cognitive impairment, and survival in very late life: impact of allostatic load in Swedish octogenarian and nonagenarian humans. J Gerontol A Biol Sci Med Sci. 2005;60(5):556–565. [DOI] [PubMed] [Google Scholar]
- 79. Wikby A, Johansson B, Olsson J, Löfgren S, Nilsson BO, Ferguson F. Expansions of peripheral blood CD8 T-lymphocyte subpopulations and an association with cytomegalovirus seropositivity in the elderly: the Swedish NONA immune study. Exp Gerontol. 2002;37(2–3):445–453. [DOI] [PubMed] [Google Scholar]
- 80. Olsson J, Wikby A, Johansson B, Löfgren S, Nilsson BO, Ferguson FG. Age-related change in peripheral blood T-lymphocyte subpopulations and cytomegalovirus infection in the very old: the Swedish longitudinal OCTO immune study. Mech Ageing Dev. 2000;121(1–3):187–201. [DOI] [PubMed] [Google Scholar]
- 81. Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Methods Instrum Comput. 2004;36(4):717–731. [DOI] [PubMed] [Google Scholar]
- 82. Seeman TE. Social ties and health: the benefits of social integration. Ann Epidemiol. 1996;6(5):442–451. [DOI] [PubMed] [Google Scholar]
- 83. Uchino BN. Social support and health: a review of physiological processes potentially underlying links to disease outcomes. J Behav Med. 2006;29(4):377–387. [DOI] [PubMed] [Google Scholar]
- 84. Uchino BN. Social Support and Physical Health: Understanding the Health Consequences of Relationships. New Haven, CT: Yale University Press; 2004. [Google Scholar]
- 85. Cohen S, Gotlieb BH, Underwood LG. Social relationships and health. In: Cohen S, Underwood LG, Gottlieb BH, eds. Measuring and Intervening in Social Support New York, NY: Oxford University Press; 2000; 3–25. [Google Scholar]
- 86. Berkman LF, Glass T, Brissette I, Seeman TE. From social integration to health: Durkheim in the new millennium. Soc Sci Med. 2000;51(6):843–857. [DOI] [PubMed] [Google Scholar]
- 87. Hart BL. Biological basis of the behavior of sick animals. Neurosci Biobehav Rev. 1988;12(2):123–137. [DOI] [PubMed] [Google Scholar]
- 88. Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain Behav Immun. 2007;21(2):153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Pressman SD, Cohen S, Miller GE, Barkin A, Rabin BS, Treanor JJ. Loneliness, social network size, and immune response to influenza vaccination in college freshmen. Health Psychol. 2005;24(3):297–306. [DOI] [PubMed] [Google Scholar]
- 90. Loucks EB, Sullivan LM, D’Agostino RB Sr, Larson MG, Berkman LF, Benjamin EJ. Social networks and inflammatory markers in the Framingham Heart Study. J Biosoc Sci. 2006;38(6):835–842. [DOI] [PubMed] [Google Scholar]
- 91. Butler T, Giordano S, Neren S. Gender and sex-role attributes as predictors of utilization of natural support systems during personal stress events. Sex Roles. 1985;13(9–10):515–524. [Google Scholar]
- 92. Belle D. Gender differences in the social moderators of stress. In: Monat A, Lazarus RS, eds. Stress and Coping: An Anthology. New York, NY: Columbia University Press; 1991: 258–274. [Google Scholar]
- 93. Antonucci TC, Akiyama H. An examination of sex differences in social support among older men and women. Sex Roles. 1987;17(11–12):737–749. [Google Scholar]
- 94. Whisman MA, Sbarra DA. Marital adjustment and interleukin-6 (IL-6). J Fam Psychol. 2012;26(2):290–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Costanzo ES, Lutgendorf SK, Sood AK, Anderson B, Sorosky J, Lubaroff DM. Psychosocial factors and interleukin-6 among women with advanced ovarian cancer. Cancer. 2005;104(2):305–313. [DOI] [PubMed] [Google Scholar]
- 96. Kiecolt-Glaser JK, Loving TJ, Stowell JR et al. . Hostile marital interactions, proinflammatory cytokine production, and wound healing. Arch Gen Psychiatry. 2005;62(12): 1377–1384. [DOI] [PubMed] [Google Scholar]
- 97. Cohen S, McKay G. Social support, stress, and the buffering hypothesis: a theoretical analysis. In: Baum A, Singer JE, Taylor SE, eds. Handbook of Psychology and Health Hillsdale, NJ: Erlbaum; 1984; 253–267. [Google Scholar]
- 98. Lewinsohn PM, Seeley JR, Roberts RE, Allen NB. Center for Epidemiologic Studies Depression Scale (CES-D) as a screening instrument for depression among community-residing older adults. Psychol Aging. 1997;12(2):277–287. [DOI] [PubMed] [Google Scholar]
- 99. Morozink JA, Friedman EM, Coe CL, Ryff CD. Socioeconomic and psychosocial predictors of interleukin-6 in the MIDUS national sample. Health Psychol. 2010;29(6):626–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Powell LH, Lovallo WR, Matthews KA et al. . Physiologic markers of chronic stress in premenopausal, middle-aged women. Psychosom Med. 2002;64(3):502–509. [DOI] [PubMed] [Google Scholar]
- 101. Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9(1):46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. von Känel R, Mills PJ, Mausbach BT et al. . Effect of Alzheimer caregiving on circulating levels of C-reactive protein and other biomarkers relevant to cardiovascular disease risk: a longitudinal study. Gerontology. 2012;58(4):354–365. [DOI] [PMC free article] [PubMed] [Google Scholar]