Abstract
The purpose of this study was to analyze the rest-activity rhythm of type 2 diabetics mellitus (T2DM) and compare it to healthy controls using the nonparametric analytic approach. Twenty-one diabetics and 21 healthy subjects matched for gender and age were recruited to participate in the study. Data were analyzed using the Independent t-test, Mann-Whitney U test, and Spearmans correlation. T2DM subjects demonstrate lower interdaily stability (IS) (p=.03), higher intradaily variability (p=.046) and lower rhythm amplitude (p=.02) when compared to healthy controls. Also, there was a positive correlation between IS and most active 10 hr (M10) in the average of 24 hours pattern (r =.44; p=.046) in the diabetes group and a negative correlation between IV and M10 in the healthy group (r =-.57; p=.007). These data together suggest that T2DM exhibits a dysfunction in the rest-activity rhythm due to alterations in the circadian function as well as in the homeostatic capacity to maintain sleep; mainly characterized by less consistency across days of the daily circadian signal, higher rhythm fragmentation and lower rhythm amplitude. Future approaches may be developed considering the influence of circadian glucose variations throughout the day on the coupling of the rest-activity rhythm to zeitgeber and rhythm fragmentation.
Keywords: Sleep Wake Disorders; Diabetes Mellitus, Type 2; Circadian Rhythm
INTRODUCTION
There are approximately 30.3 million individuals with diabetes in the United States, of whom 90% to 95% are classified as type 2 diabetes mellitus (T2DM)1,2. The Centers for Disease Control and Prevention (CDC) have reported that sleep complaints have a high incidence rate in the U.S. diabetic population, and individuals with diabetes are more likely to have sleep impairments when compared to controls2,3.
According to Wild et al.4, the number of people diagnosed with T2DM will increase to 366 million by 2030, and countries such as China, India, the Middle East and sub-Saharan Africa will probably have the highest increase. It has estimated that the global prevalence of diabetes among adults has risen in recent years to about 422 million in 2014. High glucose levels were responsible for 1.5 million deaths in upper-middle income countries and 0.3 million in low-income countries5.
The main connection between type 2 diabetes and poor sleep has been attributed to impairments in metabolism of glucose and circadian variations of hormone levels6-8. Furthermore, glucose tolerance and insulin secretion are adjusted according to the sleep-wake cycle9. The normal glucose metabolic process is directly perturbed by inadequate sleep or a disruption of circadian rhythms10,11.
The circadian system functions to temporally organize the physiological processes of the body through specialized neural structures such as input pathways, which transmit signals such as light to the circadian master clock, that is the suprachiasmatic nucleus (SCN) of hypothalamus12. This master clock regulates the circadian rhythms in glucose, corticosteroids, leptin, and cardiovascular systems through the autonomic nervous system and humoral signs to the pancreas, liver, adrenal glands, adipose tissues, and heart13.
These interactions allow for synchronization between the central and peripheral tissue clocks. Moreover, various clock genes also participate in the regulation of metabolic homeostasis14. For example, it has been shown that a disorder in the master clock may disrupt glucose metabolism14. Also, feeding time can entrain the intrinsic oscillation of clocks in liver cells, similar to what occurs in central oscillators in the brain when entrained by light15. These previous findings support the understanding that circadian and metabolic systems are reciprocally regulated14,16-18.
The risk of microvascular complications in patients with T2DM may be reduced by approaches that focus on maintaining good glycemic control19,20. The American Diabetes Association (ADA) recommends maintaining HbA1c levels of less than 7%, since studies have shown that this approach reduces microvascular complications due to diabetes21-23. Therefore, measures that aim to improve glycemic control, such as improvement of sleep, are of great importance and studies that address the rest-activity rhythm may help to identify what parameters of this rhythm is potentially influencing sleep fluctuations in diabetic patients, while also helping to recognize which approaches can be administered for sleep improvement in this population.
The rest-activity rhythm is commonly studied using actigraphy. The adjustment of a cosine function to actigraphic data provides parameters that have been used in circadian rhythmicity studies24. The parameters that describe rhythm characteristics include: amplitude, mesor, acrophase and period. However, as the rest-activity rhythm does not behave exactly as a cosine function, other variables have been studied and new methodologies have been developed25. Since these variables are not associated with parameters of a known function, they are called nonparametric.
This is the first study that aimed to analyze the rest-activity rhythm in subjects with T2DM and compare it to healthy individuals using the nonparametric approach.
METHODS
Sample
A convenience sample of 25 diabetic subjects were recruited for the study. All subjects were ambulatory patients from Loma Linda University Medical Center. The healthy comparison group consisted of 21 healthy individuals matched for gender and age with the diabetes group. The study inclusion criteria consisted of subjects who had been diagnosed with diabetes for a year or longer. Exclusion criteria were: diagnosis of obstructive sleep apnea; neurological comorbidities such as Parkinson's and Alzheimer's disease; use of antidepressants or any other drug that may affect alertness or drowsiness (e.g., melatonin, antipsychotics, antidepressants, benzodiazepines, muscle relaxants); depression (a score of 17 and over was considered an indicator of the presence of the clinically significant depressive symptoms assessed by Beck's Depression Inventory); improper use of the actimeter device; incomplete sleep diaries kept by participants, and important changes in social routine during the week of data collection, including shift work. A total of four diabetic subjects were excluded from the study due to sleep apnea, severe depression as measured by Beck's Depression Inventory, and incorrect use of the actimeter device.
Procedures
The present study followed the World Medical Association Declaration of Helsinki for research involving human subjects26. Also, this study was approved by Loma Linda University's Institutional Review Board. All subjects were informed of the research procedures and signed an informed consent. The following demographic data were assessed: gender, age, weight and height, from which we calculated body mass index (BMI) (weight in kilograms divided by height in meters squared). The blood level of hemoglobin A1c (HbA1c) was measured using DCA VantageTM Analyzer (Siemens®, Malvern, PA, USA). HbA1c is the gold standard for monitoring glycemic control, and it reflects overall glucose levels over the past 2 to 3 months21,27.
For purposes of analysis, in order to assess whether different levels of glucose would influence circadian variables, subjects were classified in two subgroups according to HbA1c level. Subjects with HbA1c values less than 7% would be classified as “good glycemic control”; and subjects with HbA1c values equal or over 7% would be classified as “poor glycemic control”28,29. The length of time since the initial diabetes diagnosis and the type of T2DM treatment were also recorded. Due to finance constraints, A1c levels were not assessed on the healthy comparison group.
Depression was assessed using Beck's Depression Inventory (BDI), which consists of 21 questions which evaluate the severity of depressive symptoms. The total score is a sum of all questions and range from 0-63. It is classified in four categories: “These ups and downs are considered normal” (1-10 points); “Mild mood disturbances” (11-16 points); “Borderline clinical depression” (17-20 points); “Moderate depression” (21-30); “Severe depression” (31-40 points); and “Extreme depression” (over 40 points)30,31.
Subjects were screened for risk of obstructive sleep apnea (OSA) through the Apnea Risk Evaluation System (ARES) questionnaire, which assesses demographic characteristics, diseases that are associated with risk for OSA (i.e., high blood pressure, heart disease, diabetes, or stroke), Epworth sleepiness scale, and a five-scale response to the frequency rating for snoring, waking up choking, and breathing difficulties during sleep. Scoring between 4 or 5 is considered low risk for OSA, 6 to 10 is considered high risk, and a score of 11 or more is very high risk32.
Rest-activity rhythm was measured in subjects' own homes using the Actiwatch 2 (Philips Respironics®, Andover, MA, USA). Actimetry is a non-invasive method for the indirect evaluation of the sleep-wake cycle, recognized by the American Academy of Sleep Medicine as a helpful adjunct to clinically assess sleep disorders33. At the beginning of the data collection week, subjects were given the Actiwatch 2 device, instructions for its use, and a sleep log. It was advised to maintain their regular sleep-wake hours during the data collection week, avoiding caffeine, and alcohol to avoid masking of the rest-activity cycle.
Participants placed the device on their non-dominant wrist, and asked not to remove it during the 7-day data collection period. Subjects were asked to press a button on the Actiwatch 2 when they went to bed at night and when they got up in the morning; the device also has an ambient light monitor to assess the amount to Lux to which the subject has been exposed. Data on sleep and activity levels were primarily calculated using the Respironics Actiware 5.70.1 software (Philips Respironics®, Andover, MA, USA) and recordings per minute for six complete days and seven nights were used for the analysis.
The raw activity counts were extracted and analyzed by the nonparametric approach34. This method of assessing rest-activity circadian rhythm is composed of three main variables: interdaily stability (IS), which shows the relationship between the rest-activity rhythm and the zeitgeber (external time signaling stimuli); intradaily variability (IV), which represents the transitions between rest and activity; and amplitude (AMP), the difference between least active 5 h (L5) and most active 10 h (M10) patterns in an average 24 hour period, described previously by Van Someren et al.34. The calculations of each circadian variable were performed as follows:
Interdaily stability is described as the ratio between the variance of the average 24-hour pattern around the mean and the overall variance. The IS evaluates individual days to quantify the similarity of activity patterns. Higher values are indicative of rhythm stability, and have been reported to be in the 0.6 range in healthy adults35,36.
The formula for interdaily stability:
in which N is the total amount of data, p is the number of data per day; x¯h is the hourly means, x¯i represents the individual data points, and x¯ represents the mean of all days.
The interdaily variability ranges from 0 to 2; studies have shown that scores <1 are found in healthy adults. Rhythm fragmentation is characterized by higher values of IV. This variable is calculated as the ratio of the mean squares of the difference between successive hours and the mean squares around the grand mean, as shown below34:
Other variables were also analyzed from the Actiwatch 2: actual sleep time (the total number of epochs between the Start Time and the End Time of the given interval scored as SLEEP by Actiware software multiplied by the Epoch Length in minutes); % Sleep (Actual sleep time expressed as a percentage of the assumed sleep time); and wake after sleep onset (WASO, the total number of epochs between the Start Time and the End Time of the given Sleep Interval scored as WAKE by Actiware software multiplied by the Epoch Length in minutes).
Statistical analysis was performed using SPSS 21.0 software (Armonk, NY, USA). The Kolmogorov-Smirnov test was used to examine the distribution of the continuous variables. The independent t-test was used to compare groups in regards to mean of age, BMI, ARES score and BDI, as well as the differences in circadian variables, (which were followed by ANCOVA to control for risk of OSA), mean light exposure per minute, and average of all light in Lux. The Pearson's correlation test was applied to examine the intra-group correlation among circadian variables and HbA1c. The chi-square Fisher's exact test was used to compare proportions of males and females by group. Comparisons between subgroup of T2DM with HbA1c < 7% (good glycemic control) and HbA1c =7% (poor glycemic control) were assessed using the Mann-Whitney U test. Whereas the power of a study is defined as the ability that it has to demonstrate a statistically significant difference (or “Effect”), effect size was calculated using Cohen's d for comparisons between circadian variables among T2DM and healthy comparison group. The level of significance was set at p<.05.
RESULTS
The subjects' characteristics are shown in Table 1. All diabetic subjects were on diabetic medication for disease control (61.9% and 38.1% for oral drug and insulin injection respectively). Among the oral medication included: metformin, sitagliptin, glyburide, glimepiride, glipizide and pioglitazone; administered once or twice daily. Injected medication included: regular insulin, insulin aspart, insulin glargine, insulin lispro and insulin determir. Diabetic subjects were under medication for diabetes control for 11.2 years on average. They have been diagnosed with T2DM for 11.7±9.6 years. HbA1c values were 7.7%±1.4 (38% for HbA1c < 7%; and 62% for HbA1c =7%). Significantly higher number of individuals with hypertension was found on the diabetic group compared to healthy comparison group (85.7% vs. 14.3%, p=.01).
Table 1.
Subjects' characteristics according to group.
| Variables | Diabetic Group | Healthy Group | p value |
|---|---|---|---|
| N | 21 | 21 | |
| Female | 61.9 | 57.1 | |
| Ageb (years) | 59.0±8.6 | 59.5±8.5 | .68 |
| Weightb (Kg) | 77.0±15.0 | 79.0±15.3 | .33 |
| Heightb (m) | 1.7±.1 | 1.7±.1 | .91 |
| BMIb (kg/m2) | 27.6±7.2 | 28.8±4.3 | .21 |
| High blood pressurea (%) | 85.7 | 14.3 | <.01* |
| Risk for OSAc | 5.8±2.7 | 3.4±2.7 | .01 |
n, number of subjects per group
Fisher's test comparisons
Independent t-test comparisons
Risk for OSA did not meet the modeling criterion for confounding when analyzing the differences between groups and the circadian variables
significant difference (p<.05)
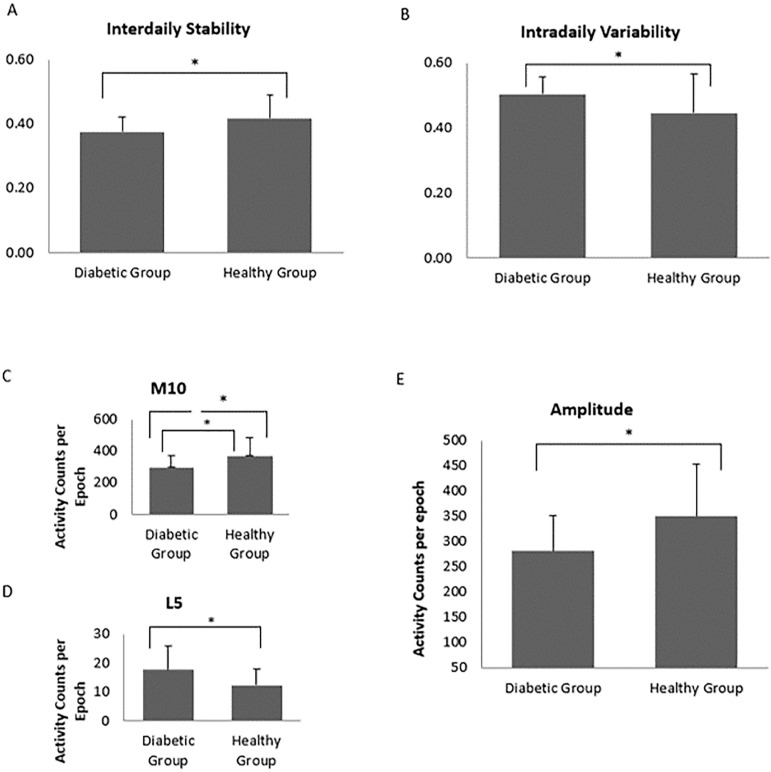
In the analysis of the circadian variable of rest-activity rhythm, the diabetic group showed a significant decreased IS (.37±.04 vs. .40±.07; p=.03) (Figure 1, A) and a significant increased IV (.50±.05 vs. .45±.12; p=.046) in related to healthy individuals (Figure 1, B). Additionally, diabetic subjects were significantly less active than the healthy comparison group, as shown by the result of M10 (298.3±72.2 vs. 370.1±114.0; p=.02) (Figure 1, C) and during the 5 hours of least activity, diabetic subjects had higher active counts when compared to healthy individuals (17.6±8.3 vs. 12.2±5.7; p=.02) (Figure 1, D). Calculations from M10 and L5 demonstrated a significant lower amplitude of the rest-activity rhythm for the diabetic group (281.0±70.4 vs. 349.3±104.6; p=.02) (Figure 1, E). Cohen's d calculation showed a medium effect for the circadian variables of IS and IV (d=.54 and d=.56 respectively); and a large effect for amplitude (d=.78).
Figure 1.
Distribution of PSQI by semester. A score of more than 5 in Pittsburgh Sleep Quality Index (PSQI) define bad sleepers.
We have found a positive correlation between IS and M10 (r=.44; p=.046) in the diabetic group, and a significant negative correlation between IV and M10 in the comparison group (r=-.57; p=.007). However, there was no significant correlation between IV and M10 in the diabetic group and IS and M10 in the comparison group (p>.05).
We have investigated the precision of actigraphy data. First, we estimated the end activity time and the start activity time recorded by the Actiwatch; second, the sleep time and wake up time recorded by the sleep log, and the results are shown in Table 2. Finally, we have studied the relation between the data from both instruments. We have found a significant correlation between the end activity time recorded by the Actiwatch and the sleep time recorded by the sleep log (r=.61; p<.001). Also, there was a strong significant correlation between the start activity time recorded by the Actiwacth and the wake-up time recorded by the sleep log (r=.96; p<.001).
Table 2.
End activity time and start activity time on average recorded by the Actiwatch 2 and mean seep time and mean wake up time recorded by the sleep log.
| Variables | Diabetic Group | Healthy Group |
|---|---|---|
| End activity time (Actiwatch 2) | 11:08 p.m. | 10:40 p.m. |
| Start activity time (Actiwatch 2) | 6:30 a.m | 5:33 a.m. |
| Sleep time (Sleep log) | 11:00 p.m. | 10:40 p.m. |
| Wake up time (Sleep log) | 6:43 a.m. | 5:30 a.m. |
When analyzing the actual sleep time on T2DM and healthy comparison group, it was found that it was shorter on the diabetic group compared to healthy individuals (320.9±66 vs. 361.82±56.9). The % Sleep was lower on the diabetic group (73.6% vs. 81.25%); and the WASO was higher in the T2DM group compared to healthy group (122.95±60.5 vs. 91.34±56.36). None of these variables were significantly different between group (p>.05).
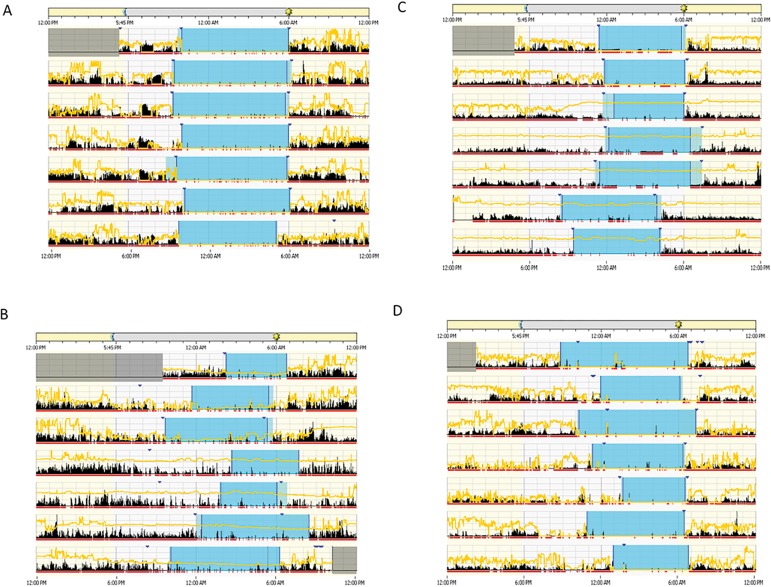
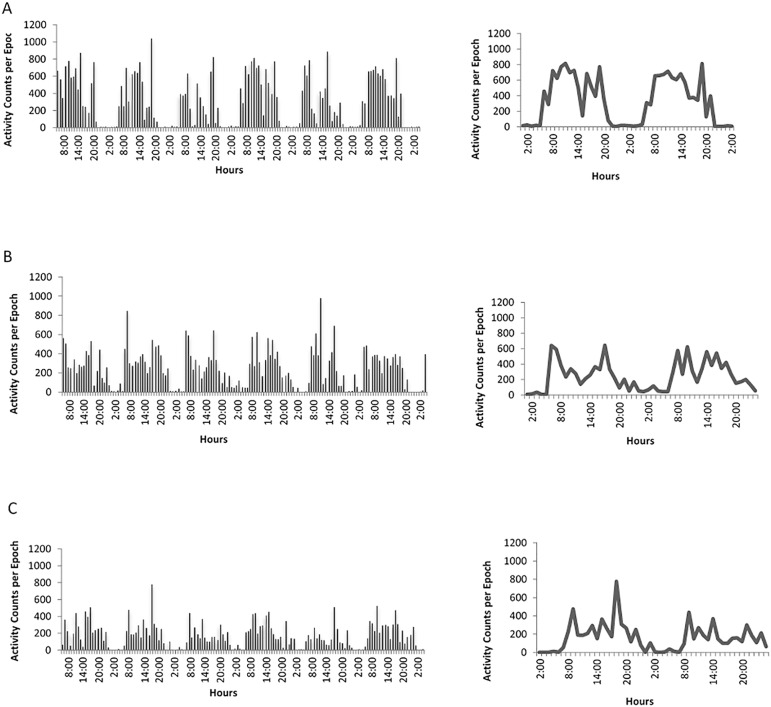
Actograms on Figure 2 visually illustrates differences of the rest-activity rhythm between healthy and diabetic subjects. Figure 2A illustrates the actogram of a healthy individual compared with the actogram of a diabetic subject. The actogram in Figure 2B shows high instability between days. The subject in Figure 2C shows low amplitude of the activity rhythm, and Figure 2D illustrates fragmentation in a subject with frequent records of periods of rest and activity. Figure 3, using raw actigraphy data, more closely illustrate differences between groups in regard to rhythm fragmentation and low rhythm amplitude. When analyzing HbA1c levels, there were no significant differences between subjects with HbA1c < 7% (good glycemic control) and subjects with HbA1c =7% (poor glycemic control) in the circadian variables (p>.05).
Figure 2.
Actograms showing activity data (vertical black bars) divided into light phase (daytime, from 6:00 a.m. to 5:45 p.m., beige horizontal bar on top) and dark phase (night time, from 5:45 p.m. to 6:00 a.m., gray horizontal bar on center top). Yellow lines represent the variation in light throughout the day (24h period). Horizontal red bar represents when individuals were awake, and blue area indicates sleep period, with the lighter blue area indicating rest, and dark blue arrow head representing when subjects pressed a button on Actiwatch 2 to mark when they went to bed and when they woke up in the morning. Graph (A) illustrates a healthy subject; (B) a diabetic subject showing high instability between days, (C) a diabetic subject showing low rhythm amplitude, and (D) a diabetic subject showing high fragmentation.
Figure 3.
Representation of the raw activity data (panels on the left) indicating the activity level (vertical black bars) throughout two consecutive days and double plots of the average 24-h activity level on solid line (panels on the right) of (A) a healthy subject; and (B and C) diabetic subjects both showing low rhythm amplitude, and high fragmentation.
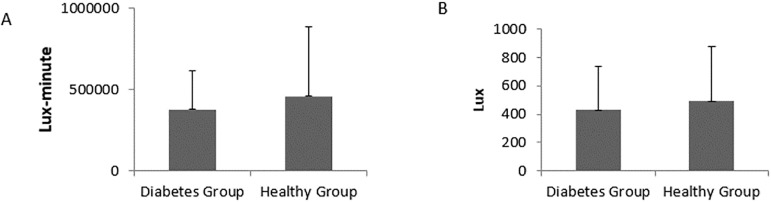
There were no significant differences in the sum of all light exposure (Lux multiplied by minute) and average of all valid light (Lux) from the start time to the end time of active interval between groups; however, the diabetic group was exposed to less light (424 654.1±263551.6 vs. 478 923.9±414 253.4; p=.61) (Figure 4). Also, the amount of all valid light for the diabetic group was less than the healthy comparison group (477.5±297.2 vs. 531.7±431.1; p=.64) (Figure 4).
Figure 4.
(A) mean±SD light exposure (Lux multiplied by minute); (B) mean±SD average of all valid light (Lux) from the start time to the end time of active interval according to group. There were no significant differences between groups.
DISCUSSION
This study suggests that T2DM individuals have less synchronization of rest-activity cycle with external zeitgebers and a more fragmentation of the rest phase. These were demonstrated by a lower IS, indicating less consistency across days of the daily circadian signal, a higher IV, showing greater variability within each 24-hour period; and also, by a lower amplitude in the T2DM group when compared to the healthy group. These data together suggest that T2DM exhibit a dysfunction in the rest-activity rhythm due to alterations in the circadian function, as well as in the homeostatic capacity to maintain sleep.
Previous studies also using similar non-parametric approach to assess the sleep-wake cycle were able to identify changes is circadian variables; also, associations between actigraphic variables and diabetic complications34,37. A study by Kadono et al.37 showed association between diurnal rest-activity rhythmicity and diabetes-related clinical status (including demographic, metabolic and neurovasculopathic factors). This study demonstrated that daytime and night-time activities were independently associated with the IV and IS. Differences in IS between T2DM and healthy comparison group was found in our study. The IS is indicative of the similarity of the activity pattern on different days. It can indicate the consistency of the daily circadian signal across days, also revealing the strength of its coupling master clock to stable zeitgebers. Typically, high values are indicative of a more stable rhythm, and were found to be around 0.6 for healthy adults36,38. Our finding is in agreement with this result, as we observed lower values in IS for the diabetic group (.37±.04) compared to healthy comparison group (.42±.07)38.
In regards to IV, the diabetic group showed significantly higher values when compared to healthy individuals, with higher IV values indicative of a more fragmented rhythm. Similar results were observed by Kadono et al. when assessing diabetic subjects across three stages of nephropathy, with higher values found in the proliferative diabetic retinopathy phase (.64±.05)37. Previous studies with T2DM individuals have shown that nocturia is a significant variable that is positively correlated with difficulty to maintain sleep39 and it might contribute to increased IV in the diabetic population. Previous studies have described associations between sleep fragmentation with higher fasting glucose levels, insulin levels and reduced insulin sensitivity in patients with T2DM40. A study by Trento et al. suggests that sleep fragmentation, together with increase activity during sleep time and decrease sleep efficiency are related to the degree of glycemic control (HbA1c levels)41.
Lower peak of physical activity and lower rhythm amplitude in T2DM were found in our study. Previous studies have suggested that the decreased activity level might be related to motor impairments, such as a study conducted by Whitehead et al.42 with Parkinson's patients and previous analysis of stroke patients43. It has been suggested that such changes in the amplitude negatively impact the quality of life of these individuals43. Also, motor alterations among stroke patients could be influencing the expression of this variable, considering that physical activity and exercise has been shown to influence synchronization of peripheral oscillators44. Lower levels of physical activity and low rhythm amplitude found in T2DM might be related to increased sedentary activity commonly found in these individuals.
Another finding suggesting alteration of the circadian rhythm in T2DM, is the association between IS and IV with daytime activity. It has been reported that this association does not result from the mathematical model, but from the organized pattern of the high levels of daytime activity which lead to an organized circadian rhythm45. Moreover, with activity level functioning as an output and as an input of the circadian timing system, Van Someren et al.45 suggested that daytime activity level is of great importance in circadian rest-active disturbances and might be one of the causes for rhythm disturbances in the Alzheimer's population. These findings may help to explain our results, in which IS showed positive association with daytime activity in the diabetic group. However, more studies are needed to determine if increasing the amplitude of the activity rhythm would result in better regulation of the sleep-wake cycle.
The decreased rhythm synchronization and increased rhythm fragmentation found in the T2DM group might be related the decreased light exposition in T2DM. Studies on the influence of light in circadian rest-activity rhythm regulation have shown that sessions of bright light during the day can increase IS and decrease IV34. The association between IS and daytime light exposure has also been reported (45). Although our study did not find differences between groups in light exposure (Lux multiplied by minute) and averages of all valid light (Lux), the diabetic group was exposed to less light, and had lower values for all valid light during the active phase. It has been previously reported that ophthalmic diseases such as cataracts, diabetes retinopathy, and glaucoma might affect photic input to the circadian system46. Therefore, it is possible that the presence of unknown retinopathy or other ophthalmic disease may have influenced the strength of the coupling of the rhythm to this environmental zeitgeber; these finding may also be related to the routine of study participants. We recommend that futures studies perform a clinical assessment for ophthalmic disease on T2DM in order to better assess the influence of light on these circadian variables.
The association between type 2 diabetes and hypertension has been well described in the literature47-49. Studies have shown that approximately 75% of people with T2DM will develop hypertension47. Moreover, similar pathophysiology that is responsible for the development of hypertension is also present in T2DM, such as obesity, inflammation, oxidative stress, and insulin resistance; which also characterizes the metabolic syndrome48,49). Our study found that 85.7% of the diabetes group was also diagnosed with hypertension against 14.3% of healthy comparison group (p=.01). Moreover, association between hypertension and sleep disorders such as apnea, insomnia, restless leg syndrome and decreased quality of sleep has been shown50-52.
Sleep duration is another factor previously described as associated with metabolic syndrome and T2DM53,54. Results from our study found that the start and end time (Table 2) was longer in the diabetic group, but the actual sleep time was shorter when compared to healthy individuals (320.9±66 vs. 361.82±56.9). Moreover, the WASO was higher and the % Sleep was lower (73.6% vs. 81.25%; 122.95±60.5 vs. 91.34±56.36, respectively). Although no significant difference was found between groups, these results together may further indicate the presence of sleep deprivation, fragmentation and decrease sleep efficient in T2DM group. A study by Ayas et al.53 indicates a relationship between both short and long self-reported sleep durations and increased risk for developing diabetes; also, a study by Choi et al.54 found that sleep duration is also associated with metabolic syndrome.
Several limitations should be considered in this study. First, this is an observational study and no causality can be assumed. Second, it should be considered that rest-activity rhythm recorded by the actigraphic method may be subject to masking effects24. Additionally, the observed associations between circadian variations and diabetes may be underestimated in this study due to the possible presence of metabolic syndrome on T2DM subjects. There are also limitations on data collection of light exposure using wrist actigraphy, as this method may not accurately reflect real corneal exposure and can be easily obstructed by clothing.
CONCLUSION
In summary, our results suggest that the rest-activity rhythm is disturbed in T2DM when compared to healthy individuals, and is mainly characterized by less consistency across days of the daily circadian signal, higher rhythm fragmentation, and lower rhythm amplitude. Future approaches may be developed to better investigate which mechanisms are involved in the rest-activity rhythm deregulation in T2DM, also considering the influence of meal times and circadian glucose variations throughout the day on the coupling of the rest-activity rhythm to zeitgeber and rhythm fragmentation.
Acknowledgments: We acknowledge the financial support for this research from the Clinical Research Molecular Laboratory and the Physical Therapy Department, School of Allied Health Professions, Loma Linda University.
REFERENCES
- 1.Department of Health and Human Services. Centers for Disease Control and Prevention . National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. Atlanta: Department of Health and Human; 2011. [Google Scholar]
- 2.Centers for Disease Control and Prevention . National Diabetes Statistics Report, 2017: Estimates of Diabetes and Its Burden in the United States. Atlanta: Centers for Disease Control and Prevention; 2017. [Google Scholar]
- 3.Plantinga L, Rao MN, Schillinger D. Prevalence of self-reported sleep problems among people with diabetes in the United States, 2005-2008. Prev Chronic Dis. 2012;9:E76. [PMC free article] [PubMed] [Google Scholar]
- 4.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(5):1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization . Methods and data sources for country-level causes of death 2000-2015. Geneva: World Health Organization; 2017. [Google Scholar]
- 6.Spiegel K, Tasali E, Leproult R, Van Cauter E. Effects of poor and short sleep on glucose metabolism and obesity risk. Nat Rev Endocrinol. 2009;5(5):253–261. doi: 10.1038/nrendo.2009.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Cauter E, Holmback U, Knutson K, Leproult R, Miller A, Nedeltcheva A, et al. Impact of sleep and sleep loss on neuroendocrine and metabolic function. Horm Res. 2007;67(Suppl 1):2–9. doi: 10.1159/000097543. [DOI] [PubMed] [Google Scholar]
- 8.Gottlieb DJ, Punjabi NM, Newman AB, Resnick HE, Redline S, Baldwin CM, et al. Association of sleep time with diabetes mellitus and impaired glucose tolerance. Arch Intern Med. 2005;165(8):863–867. doi: 10.1001/archinte.165.8.863. [DOI] [PubMed] [Google Scholar]
- 9.Van Cauter E, Blackman JD, Roland D, Spire JP, Refetoff S, Polonsky KS. Modulation of glucose regulation and insulin secretion by circadian rhythmicity and sleep. J Clin Invest. 1991;88(3):934–942. doi: 10.1172/JCI115396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laposky AD, Bass J, Kohsaka A, Turek FW. Sleep and circadian rhythms: key components in the regulation of energy metabolism. FEBS Lett. 2008;582(1):142–151. doi: 10.1016/j.febslet.2007.06.079. [DOI] [PubMed] [Google Scholar]
- 11.Gangwisch JE. Epidemiological evidence for the links between sleep, circadian rhythms and metabolism. Obes Rev. 2009;10(Suppl 2):37–45. doi: 10.1111/j.1467-789X.2009.00663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dijk DJ, von Schantz M. Timing and consolidation of human sleep, wakefulness, and performance by a symphony of oscillators. J Biol Rhythms. 2005;20(4):279–290. doi: 10.1177/0748730405278292. [DOI] [PubMed] [Google Scholar]
- 13.Buijs RM, Scheer FA, Kreier F, Yi C, Bos N, Goncharuk VD, et al. Organization of circadian functions: interaction with the body. Prog Brain Res. 2006;153:341–360. doi: 10.1016/S0079-6123(06)53020-1. [DOI] [PubMed] [Google Scholar]
- 14.Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science. 2010;330(6009):1349–1354. doi: 10.1126/science.1195027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stokkan KA, Yamazaki S, Tei H, Sakaki Y, Menaker M. Entrainment of the circadian clock in the liver by feeding. Science. 2001;291(5503):490–493. doi: 10.1126/science.291.5503.490. [DOI] [PubMed] [Google Scholar]
- 16.Young ME, Bray MS. Potential role for peripheral circadian clock dyssynchrony in the pathogenesis of cardiovascular dysfunction. Sleep Med. 2007;8(6):656–667. doi: 10.1016/j.sleep.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carneiro BT, Araujo JF. The food-entrainable oscillator: a network of interconnected brain structures entrained by humoral signals? Chronobiol Int. 2009;26(7):1273–1289. doi: 10.3109/07420520903404480. [DOI] [PubMed] [Google Scholar]
- 18.Carneiro BTS, Fernandes DAC, Medeiros CFP, Diniz NL, Araujo JF. Daily anticipatory rhythms of behavior and body temperature in response to glucose availability in rats. Psychol Neurosci. 2012;5(2):191–197. [Google Scholar]
- 19.Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348(5):383–393. doi: 10.1056/NEJMoa021778. [DOI] [PubMed] [Google Scholar]
- 20.Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M, STOP-NIDDM Trial Research Group Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the STOP-NIDDM trial. JAMA. 2003;290(4):486–494. doi: 10.1001/jama.290.4.486. [DOI] [PubMed] [Google Scholar]
- 21.American Diabetes Association Standards of medical care in diabetes--2012. Diabetes Care. 2012;35(Suppl 1):S11–S63. doi: 10.2337/dc12-s011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34): UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):854–865. [PubMed] [Google Scholar]
- 23.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33): UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):837–853. [PubMed] [Google Scholar]
- 24.Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26(3):342–392. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- 25.Witting W, Kwa IH, Eikelenboom P, Mirmiran M, Swaab DF. Alterations in the circadian rest-activity rhythm in aging and Alzheimer's disease. Biol Psychiatry. 1990;27(6):563–572. doi: 10.1016/0006-3223(90)90523-5. [DOI] [PubMed] [Google Scholar]
- 26.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 27.International Expert Committee International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32(7):1327–1334. doi: 10.2337/dc09-9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hartemann-Heurtier A, Sultan S, Sachon C, Bosquet F, Grimaldi A. How type 1 diabetic patients with good or poor glycemic control cope with diabetes-related stress. Pt 1Diabetes Metab. 2001;27(5):553–559. [PubMed] [Google Scholar]
- 29.Juarez DT, Sentell T, Tokumaru S, Goo R, Davis JW, Mau MM. Factors associated with poor glycemic control or wide glycemic variability among diabetes patients in Hawaii, 2006-2009. Prev Chronic Dis. 2012;9:120065–120065. doi: 10.5888/pcd9.120065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Contreras S, Fernandez S, Malcarne VL, Ingram RE, Vaccarino VR. Reliability and Validity of the Beck Depression and Anxiety Inventories in Caucasian Americans and Latinos. Hisp J Behav Sci. 2004;26(4):446–462. [Google Scholar]
- 31.Richter P, Werner J, Heerlein A, Kraus A, Sauer H. On the validity of the Beck Depression Inventory: A review. Psychopathology. 1998;31(3):160–168. doi: 10.1159/000066239. [DOI] [PubMed] [Google Scholar]
- 32.Levendowski DJ, Olmstead R, Popovic D, Carper DL, Berka C, Westbrook PR. Assessment of Obstructive Sleep Apnea Risk and Severity in Truck Drivers: Validation of a Screening Questionnaire. Sleep Diagn Therapy. 2007;2(2):20–26. [Google Scholar]
- 33.Practice parameters for the use of actigraphy in the clinical assessment of sleep disorders. American Sleep Disorders Association. Sleep. 1995;18(4):285–287. doi: 10.1093/sleep/18.4.285. [DOI] [PubMed] [Google Scholar]
- 34.Van Someren EJ, Kessler A, Mirmiran M, Swaab DF. Indirect bright light improves circadian rest-activity rhythm disturbances in demented patients. Biol Psychiatry. 1997;41(9):955–963. doi: 10.1016/S0006-3223(97)89928-3. [DOI] [PubMed] [Google Scholar]
- 35.Huang YL, Liu RY, Wang QS, Van Someren EJ, Xu H, Zhou JN. Age-associated difference in circadian sleep-wake and rest-activity rhythms. Physiol Behav. 2002;76(4-5):597–603. doi: 10.1016/s0031-9384(02)00733-3. [DOI] [PubMed] [Google Scholar]
- 36.Calogiuri G, Weydahl A, Carandente F. Methodological issues for studying the rest-activity cycle and sleep disturbances: a chronobiological approach using actigraphy data. Biol Res Nurs. 2013;15(1):5–12. doi: 10.1177/1099800411416224. [DOI] [PubMed] [Google Scholar]
- 37.Kadono M, Nakanishi N, Yamazaki M, Hasegawa G, Nakamura N, Fukui M. Various patterns of disrupted daily rest-activity rhythmicity associated with diabetes. J Sleep Res. 2016;25(4):426–437. doi: 10.1111/jsr.12385. [DOI] [PubMed] [Google Scholar]
- 38.Van Someren EJ, Lijzenga C, Mirmiran M, Swaab DF. Long-term fitness training improves the circadian rest-activity rhythm in healthy elderly males. J Biol Rhythms. 1997;12(2):146–156. doi: 10.1177/074873049701200206. [DOI] [PubMed] [Google Scholar]
- 39.Lamond N, Tiggemann M, Dawson D. Factors predicting sleep disruption in Type II diabetes. Sleep. 2000;23(3):415–416. [PubMed] [Google Scholar]
- 40.Knutson KL, Van Cauter E, Zee P, Liu K, Lauderdale DS. Cross-sectional associations between measures of sleep and markers of glucose metabolism among subjects with and without diabetes: the Coronary Artery Risk Development in Young Adults (CARDIA) Sleep Study. Diabetes Care. 2011;34(5):1171–1176. doi: 10.2337/dc10-1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trento M, Broglio F, Riganti F, Basile M, Borgo E, Kucich C, et al. Sleep abnormalities in type 2 diabetes may be associated with glycemic control. Acta Diabetol. 2008;45(4):225–229. doi: 10.1007/s00592-008-0047-6. [DOI] [PubMed] [Google Scholar]
- 42.Whitehead DL, Davies AD, Playfer JR, Turnbull CJ. Circadian rest-activity rhythm is altered in Parkinson's disease patients with hallucinations. Mov Disord. 2008;23(8):1137–1145. doi: 10.1002/mds.22057. [DOI] [PubMed] [Google Scholar]
- 43.Cavalcanti PR, Campos TF, Araujo JF. Circadian and homeostatic changes of sleep-wake and quality of life in stroke: implications for neurorehabilitation. NeuroRehabilitation. 2013;32(2):337–343. doi: 10.3233/NRE-130853. [DOI] [PubMed] [Google Scholar]
- 44.Cavalcanti P, Campos T, Araujo J. Actigraphic analysis of the sleep-wake cycle and physical activity level in patients with stroke: implications for clinical practice. Chronobiol Int. 2012;29(9):1267–1272. doi: 10.3109/07420528.2012.719960. [DOI] [PubMed] [Google Scholar]
- 45.van Someren EJ, Hagebeuk EE, Lijzenga C, Scheltens P, de Rooij SE, Jonker C, et al. Circadian rest-activity rhythm disturbances in Alzheimer's disease. Biol Psychiatry. 1996;40(4):259–270. doi: 10.1016/0006-3223(95)00370-3. [DOI] [PubMed] [Google Scholar]
- 46.Jean-Louis G, Zizi F, Lazzaro DR, Wolintz AH. Circadian rhythm dysfunction in glaucoma: A hypothesis. J Circadian Rhythms. 2008;6:1–1. doi: 10.1186/1740-3391-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Long AN, Dagogo-Jack S. Comorbidities of diabetes and hypertension: mechanisms and approach to target organ protection. J Clin Hypertens (Greenwich) 2011;13(4):244–251. doi: 10.1111/j.1751-7176.2011.00434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pouvreau C, Dayre A, Butkowski EG, de Jong B, Jelinek HF. Inflammation and oxidative stress markers in diabetes and hypertension. J Inflamm Res. 2018;11:61–68. doi: 10.2147/JIR.S148911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ndisang JF, Vannacci A, Rastogi S. Oxidative stress and inflammation in obesity, diabetes, hypertension, and related cardiometabolic complications. Oxid Med Cell Longev. 2014;2014:506948–506948. doi: 10.1155/2014/506948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dhillon S, Chung SA, Fargher T, Huterer N, Shapiro CM. Sleep apnea, hypertension, and the effects of continuous positive airway pressure. Pt 1Am J Hypertens. 2005;18(5):594–600. doi: 10.1016/j.amjhyper.2004.11.031. [DOI] [PubMed] [Google Scholar]
- 51.Vgontzas AN, Liao D, Bixler EO, Chrousos GP, Vela-Bueno A. Insomnia with objective short sleep duration is associated with a high risk for hypertension. Sleep. 2009;32(4):491–497. doi: 10.1093/sleep/32.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Winkelman JW, Shahar E, Sharief I, Gottlieb DJ. Association of restless legs syndrome and cardiovascular disease in the Sleep Heart Health Study. Neurology. 2008;70(1):35–42. doi: 10.1212/01.wnl.0000287072.93277.c9. [DOI] [PubMed] [Google Scholar]
- 53.Ayas NT, White DP, Al-Delaimy WK, Manson JE, Stampfer MJ, Speizer FE, et al. A prospective study of self-reported sleep duration and incident diabetes in women. Diabetes Care. 2003;26(2):380–384. doi: 10.2337/diacare.26.2.380. [DOI] [PubMed] [Google Scholar]
- 54.Choi KM, Lee JS, Park HS, Baik SH, Choi DS, Kim SM. Relationship between sleep duration and the metabolic syndrome: Korean National Health and Nutrition Survey 2001. Int J Obes (Lond) 2008;32(7):1091–1097. doi: 10.1038/ijo.2008.62. [DOI] [PubMed] [Google Scholar]