Over an 11-year period after HPV vaccine introduction, we found in this community-based surveillance study evidence of vaccine effectiveness and herd protection among young women.
Abstract
BACKGROUND:
Clinical trials of the 4-valent human papillomavirus (HPV) vaccine demonstrate high efficacy, but surveillance studies are essential to examine the long-term impact of vaccine introduction on HPV prevalence in community settings. The aims of this study were to determine during the 11 years after vaccine introduction the prevalence of (1) vaccine-type HPV in adolescent and young adult women who were vaccinated (to assess vaccine effectiveness) and (2) vaccine-type HPV in women who were unvaccinated (to assess herd protection).
METHODS:
Young women 13 to 26 years of age were recruited from hospital-based and community health clinics for 4 surveillance studies from 2006 to 2017. We determined the proportion of vaccinated and unvaccinated women who were positive for vaccine-type HPV across the studies, and the odds of positivity for vaccine-type HPV using logistic regression; all analyses were propensity score–adjusted to control for between-wave differences in participant characteristics.
RESULTS:
Vaccination rates increased from 0% to 84.3% (97% of study participants received the 4-valent vaccine). Among women who were vaccinated, 4-valent vaccine–type HPV detection decreased from 35% to 6.7% (80.9% decline; odds ratio 0.13, 95% confidence interval 0.08 to 0.22). Among women who were unvaccinated, 4-valent vaccine–type HPV detection decreased from 32.4% to 19.4% (40% decline; odds ratio 0.50, 95% confidence interval 0.26 to 0.97). Estimated vaccine effectiveness was 90.6% in wave 3 and 80.1% in wave 4.
CONCLUSIONS:
In this study in which trends in HPV in a US community >10 years after 4-valent HPV vaccine introduction and after 9-valent vaccine introduction were examined, we found evidence of vaccine effectiveness and herd protection. Further research is needed to examine trends in 9-valent vaccine–type HPV after higher rates of vaccination are achieved.
What’s Known on This Subject:
Researchers in clinical trials of the 4-valent human papillomavirus (HPV) vaccine demonstrate high efficacy, but surveillance studies are essential to examine the long-term impact of vaccine introduction on HPV prevalence in community settings.
What This Study Adds:
In this study in which trends in HPV in a US community >10 years after 4-valent HPV vaccine introduction and after 9-valent vaccine introduction are examined, we found evidence of vaccine effectiveness and herd protection.
Infection with human papillomavirus (HPV) may cause genital warts and cancers. In women, HPV infection may cause cervical, vaginal, vulvar, anal, and oropharyngeal cancers, whereas in men, infection may cause anal, penile, and oropharyngeal cancers.1–3 The first prophylactic HPV vaccine, a 4-valent vaccine that prevents HPV-6, -11, -16, and -18, was licensed in 2006 in the United States.4 A 2-valent vaccine that prevents HPV-16 and -18 was licensed in 2009,5 and a 9-valent vaccine that prevents HPV-6, -11, -16, -18, -31, -33, -45, -52, and -58 was licensed in 2014.6 The 9-valent vaccine, the only vaccine available in the United States as of the end of 2016, prevents 5 additional oncogenic HPV types (-31, -33, -45, -52, and -58) and could prevent ∼90% of cervical cancers.7–10
Through evidence from clinical trials, researchers have demonstrated that all 3 HPV vaccines have high efficacy in preventing infection and disease caused by the specific HPV types targeted by the vaccines.7,11–14 However, vaccine effectiveness in community settings may be lower; women in the community may have been infected with vaccine-type HPV before vaccination and may have lower compliance with the vaccination series or be less healthy than those in the clinical trials. Researchers in studies have demonstrated that after introduction of the 2-valent and 4-valent vaccines, there is a substantial reduction in the prevalence of vaccine-type HPV as well as precancers in real-world settings among women who are vaccinated, indicating vaccine effectiveness.15–19 Studies are also emerging in which researchers demonstrate a reduction in vaccine-type HPV among women who are unvaccinated, suggesting herd protection,15,16,18 but findings are not consistent.17,20,21 Longer-term surveillance is essential to establish trends in vaccine-type HPV prevalence after vaccine introduction among women who are vaccinated and women who are unvaccinated, to examine HPV prevalence among younger women who are in the target age group for vaccination, and to examine the impact of 9-valent vaccine introduction on these trends. Assessments of vaccine effectiveness and herd protection are essential to guide public health messaging, clinical counseling, vaccination recommendations, and cervical cancer screening recommendations.
To evaluate effectiveness and herd protection after 4-valent and 9-valent HPV vaccine introduction in a community over the 11 years after vaccine introduction, we designed a study to extend our previous findings15,22 with the following primary specific aims: (1) to determine trends in vaccine-type HPV (types targeted by the 4-valent and 9-valent HPV vaccines) among young women who are vaccinated to examine vaccine effectiveness and (2) to determine trends in vaccine-type HPV among young women who are unvaccinated to assess for evidence of herd protection. We hypothesized that (1) the prevalence of 4-valent vaccine–type HPV would decrease significantly from 2006 to 2017 among women who were vaccinated, which would indicate vaccine effectiveness, and that (2) the prevalence of 4-valent vaccine–type HPV would decrease significantly in women who were unvaccinated, which would suggest herd protection. As an exploratory specific aim, we examined trends in the prevalence of the 5 additional types in the 9-valent vaccine (HPV-31, -33, -45, -52, and -58). Decreases in the prevalence of the 5 additional types in the 9-valent vaccine may be driven either by direct protection after vaccination with the 9-valent vaccine or crossprotection after vaccination with the 4-valent vaccine, given that these 5 types are genetically related to HPV-16 and HPV-18.
Methods
The study population comprised young women recruited from a university-affiliated, hospital-based primary care clinic (Cincinnati Children’s Hospital Teen Health Center) and the Cincinnati Health Department (obstetrics and gynecology clinic and a sexually transmitted disease clinic). The study was approved by the hospital’s and health department’s institutional review boards, and participants provided written informed consent. Parental consent was waived for participants <18 years of age to protect patient confidentiality because history of sexual contact was an inclusion criterion. To participate, individuals had to be 13 to 26 years of age and sexually experienced, which was defined as having had sexual contact (oral-genital or genital-genital, with a male or female partner).22 Individuals were not eligible if they had participated in previous surveillance studies. Participants were enrolled by using a sequential recruitment strategy; 95% to 98% of those approached in each study agreed to participate. We collected the following 4 waves of data: wave 1 (2006–2007, N = 371), wave 2 (2009–2010, N = 409), wave 3 (2013–2014, N = 400), and wave 4 (2016–2017, N = 400). A survey instrument was used to collect data on sociodemographic and behavioral variables that may be risk factors for type-specific HPV infection; details about survey development and validity are described in previous articles.15,22 Cervicovaginal swabs were collected by self-swab or clinician swab from each female participant, and samples were genotyped for HPV by using a Roche Linear Array test, a polymerase chain reaction amplification technique that uses an L1 consensus primer system and a reverse line blot detection strip to identify 36 HPV genotypes.23 In previous studies, researchers have demonstrated that the results of self- and clinician testing for vaginal HPV in women are highly concordant.24–26 Vaccination status was defined as having received at least 1 HPV vaccine dose before enrollment, and it was assessed by using the Ohio statewide immunization registry and the electronic health record, both systems with high reliability.27 Documentation in at least 1 of these systems was available for 98% of the participants who were vaccinated. Vaccination status was determined by a positive record in either or both databases and, in the small number of cases in which no information was available, by self-report.
We first examined participant characteristics and behaviors using descriptive statistical methods. We then determined if there were differences by study wave in participant characteristics using univariable methods (eg, χ2 test, Fisher’s exact test, Kruskal-Wallis test, or analysis of variance). Because there were between-wave differences for some variables, we conducted a propensity score analysis adjusted by inverse probability of treatment weighting. This adjusts for selection bias, ensuring that any differences noted in HPV prevalence across the study waves were due to HPV vaccine introduction instead of differences in measured participant factors, which may have been confounders. These factors included sociodemographic characteristics, gynecologic history, sexual history, and enrollment site (see Table 1 for all factors included). We then determined HPV prevalence for all women in the study, women who were vaccinated, and women who were unvaccinated across the 4 study waves; these results were unadjusted and adjusted for the propensity score. Methodologic details are described in previous articles.15,22 Total vaccine effectiveness (the relative infection risk in individuals who were vaccinated versus the infection risk in individuals who were unvaccinated before the introduction of a vaccine) was assessed by comparing vaccine-type HPV prevalence in women who were vaccinated in waves 2, 3, and 4 with women in wave 1, all of whom were unvaccinated.28
TABLE 1.
Comparison of Participant Characteristics Across All 4 Study Waves, Unadjusted and Adjusted for Propensity Score
| Characteristic | Wave 1, N = 371 | Wave 2, N = 409 | Wave 3, N = 400 | Wave 4, N = 400 | P,a Unadjusted | P,a Propensity Score Adjusted | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| N (%) | Mean (SD) | N (%) | Mean (SD) | N (%) | Mean (SD) | N (%) | Mean (SD) | |||
| Enrollment site | ||||||||||
| Teen Health Center | 239 (64.4) | — | 268 (65.5) | — | 250 (62.5) | — | 307 (76.8) | — | <.0001 | .55 |
| Health department | 132 (35.6) | — | 141 (34.5) | — | 150 (37.5) | — | 93 (23.3) | — | — | — |
| Demographic characteristics and medical history | ||||||||||
| Age, y | — | 18.7 (3.0) | — | 18.8 (2.9) | — | 19.1 (2.8) | — | 19.1 (2.6) | .18 | .5 |
| Race and/or ethnicity | ||||||||||
| White or Asian American | 110 (30.1) | — | 114 (27.9) | — | 108 (27.0) | — | 91 (22.8) | — | .13 | .78 |
| African American or multiracial | 255 (69.9) | — | 295 (72.1) | — | 292 (73.0) | — | 309 (77.3) | — | — | — |
| Appalachian descent | 24 (6.7) | — | 16 (3.9) | — | 9 (2.3) | — | 6 (1.5) | — | .0006 | .22 |
| Hispanic ethnicity | 25 (6.9) | — | 24 (5.9) | — | 28 (7.0) | — | 21 (5.3) | — | .69 | .53 |
| Health insurance plan | ||||||||||
| Private | 32 (8.6) | — | 63 (15.4) | — | 35 (8.8) | — | 52 (13) | — | ˂.0001 | .69 |
| Medicaid | 196 (52.8) | — | 217 (53.1) | — | 269 (67.3) | — | 284 (71) | — | — | — |
| None or not sure | 143 (38.5) | — | 129 (31.5) | — | 96 (24.0) | — | 64 (16) | — | — | — |
| History of any STI | 170 (46.5) | — | 212 (52.0) | — | 202 (50.5) | — | 215 (53.8) | — | .22 | .29 |
| Behaviors | ||||||||||
| Age of first sexual intercourse ≤13 y of age | 76 (21.5) | — | 85 (20.8) | — | 63 (15.8) | — | 44 (11.1) | — | .0014 | .07 |
| No. male sexual partners in lifetime ≥2 | 282 (80.6) | — | 354 (87.8) | — | 327 (82.2) | — | 311 (78.7) | — | .02 | .29 |
| No. male sexual partners in the past 3 mo ≥2 | 71 (20.1) | — | 86 (21.0) | — | 77 (19.3) | — | 68 (17.4) | — | .48 | .15 |
| Main sexual partner male | 319 (89.1) | — | 380 (92.9) | — | 361 (90.3) | — | 314 (79.3) | — | ˂.0001 | .97 |
| Ever had anal sex with a male partner | 89 (25.3) | — | 93 (22.7) | — | 81 (20.3) | — | 83 (21.3) | — | .39 | .50 |
| Condom use with main partner in the past 3 mo | ||||||||||
| Less than every time | 298 (80.3) | — | 333 (81.4) | — | 342 (85.5) | — | 353 (88.3) | — | .01 | .45 |
| Every time | 73 (19.7) | — | 76 (18.6) | — | 58 (14.5) | — | 47 (11.8) | — | — | — |
| Condom use at last sexual intercourse | 121 (37.5) | — | 146 (38.5) | — | 139 (34.8) | — | 105 (26.3) | — | .02 | .42 |
| Smoked at least 100 cigarettes in lifetime | 114 (31.8) | — | 117 (29.1) | — | 86 (21.9) | — | 67 (16.9) | — | ˂.0001 | .58 |
—, not applicable.
P value calculated by using χ2 test, Fisher’s exact test, Kruskal-Wallis test, or analysis of variance.
Finally, we conducted logistic regression analyses to determine the odds of HPV prevalence across the study waves, unadjusted and adjusted for the propensity score. The independent variables were study waves (eg, wave 4 versus wave 1), and the dependent variables were prevalence of at least 1 of the HPV types included in the 9-valent vaccine (HPV-6, -11, -16, -18, -31, -33, -45, -52, and -58), at least 1 of the HPV types included in the 4-valent vaccine (HPV-6, -11, -16, and -18), or at least 1 of the 5 HPV types in the 9-valent but not the 4-valent vaccine (-31, -33, -45, -52, and -58). Separate models were estimated for all women in the study, those who were unvaccinated, and those who were vaccinated. Data were analyzed by using SAS software version 9.4 (SAS Institute, Inc, Cary, NC).
Results
A total of 1580 participants were enrolled across the 4 studies. Participant characteristics are shown in Table 1. The range of mean ages across all waves was 18.7 to 19.1 years. Between 62.5% and 76.8% of participants were recruited from the Teen Health Center and 23.3% to 37.5% were recruited from the health department. The majority of participants (69.9%–77.3%) identified as African American or multiracial, 48.9% to 56.8% reported at least 2 male lifetime sexual partners, 26.3% to 38.5% used condoms during their last sexual intercourse, and 16.9% to 31.8% had smoked at least 100 cigarettes in their lifetime. The rate of HPV vaccination (defined as receipt of at least 1 dose) increased from 0% in wave 1 to 59.2% in wave 2, 71.5% in wave 3, and 84.3% in wave 4. Virtually all participants in waves 1 to 3 received the 4-valent vaccine; in wave 4, 88% received the 4-valent and 12% the 9-valent vaccine. Overall, 97% of participants received the 4-valent vaccine.
A propensity score analysis was performed to adjust for between-wave differences in participant characteristics (Table 1). The P values after propensity score adjustment were all >.05, and the standardized differences for most of the variables were <20%, indicating that variables were successfully balanced.
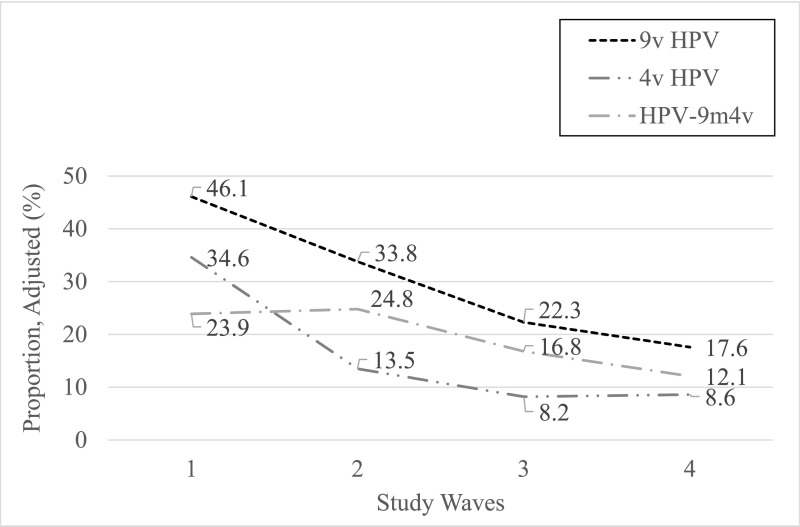
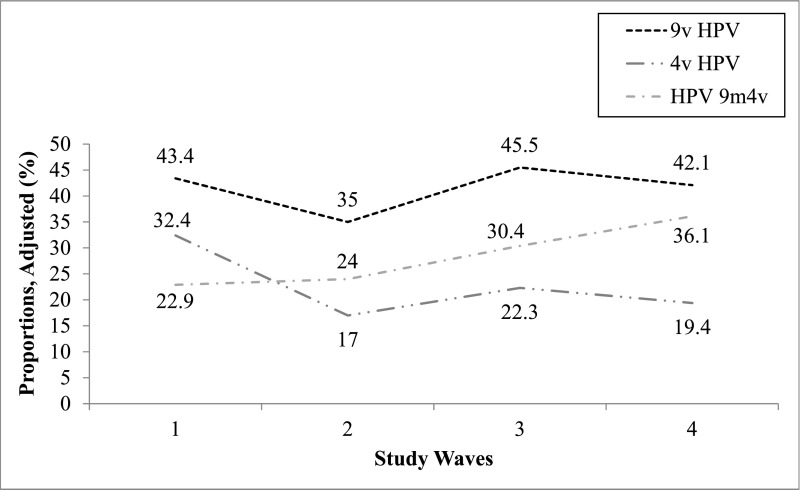
The proportions of women across the 4 study waves who were positive for 9-valent HPV types, 4-valent HPV types, and the 5 HPV types included in the 9-valent but not the 4-valent vaccine are shown adjusted for propensity score in Table 2 and in Figs 1–3. Among women who were vaccinated, the proportion infected with ≥1 9-valent vaccine–type HPV decreased from waves 1 to 4 from 46.6% to 13.5% (71% decline), the proportion infected with ≥1 4-valent vaccine–type HPV decreased from 35.0% to 6.7% (80.9% decline), and the proportion infected with ≥1 of the 5 types in the 9-valent but not the 4-valent vaccine decreased from 23.4% to 7.3% (68.8% decline); all were statistically significant. Among women who were unvaccinated, the proportion of women infected with ≥1 9-valent vaccine–type HPV decreased from 43.4% to 42.1% (3.0% decline; nonsignificant) from waves 1 to 4, the proportion infected with ≥1 4-valent vaccine–type HPV decreased from 32.4% to 19.4% (40.1% decline; significant), and the proportion infected with ≥1 of the 5 types in the 9-valent but not the 4-valent vaccine increased from 22.9% to 36.1% (57.6% increase; nonsignificant). Vaccine effectiveness was 71.7% in wave 2 versus wave 1, 90.6% in wave 3 versus wave 1, and 80.1% in wave 4 versus wave 1.
TABLE 2.
Prevalence of Vaccine-Type HPV and Between-Wave Changes by Vaccination Status Adjusted for Propensity Score
| Vaccination Status | Prevalence of HPV Types Across Waves, Propensity Score Adjusted, % | Between-Wave Changes in HPV Prevalence, Propensity Score Adjusted, % (95% CI) [% Decline or Increase] | ||||||
|---|---|---|---|---|---|---|---|---|
| Wave 1, N = 371 | Wave 2, N = 409 | Wave 3, N = 400 | Wave 4, N = 400 | Wave 1–2 | Wave 2–3 | Wave 3–4 | Wave 1–4 | |
| Types in the 9-valent vaccinea | ||||||||
| All | 46.1 | 33.8 | 22.3 | 17.6 | −12.3 (−19.2 to −5.5) [−26.7]b | −11.5 (−17.6 to −5.4) [−34]b | −4.7 (−10.2 to 0.8) [−21.1] | −28.5 (−34.8 to −22.2) [−61.8]b |
| Vaccinated | 46.6 | 32.6 | 16.7 | 13.5 | −14 (−21.8 to −6.2) [−30]b | −15.9 (−23.2 to −8.5) [−48.8]b | −3.2 (−8.9 to 2.5) [−19.2] | −33.1 (−39.4 to −26.8) [−71]b |
| Unvaccinated | 43.4 | 35.0 | 45.5 | 42.1 | −8.3 (−17.3 to 0.6) [−19.4] | +10.5 (−1.3 to 22.2) [30] | −3.4 (−18.5 to 11.7) [−7.5] | −1.3 (−14.3 to 11.8) [−3.0] |
| Types in the 4-valent vaccinec | ||||||||
| All | 34.6 | 13.5 | 8.2 | 8.6 | −21.1 (−27.0 to −15.3) [−61]b | −5.3 (−9.6 to −1.0) [−39.3]b | +0.5 (−3.4 to 4.3) [4.9] | −26.0 (−31.6 to −20.4) [−75.1]b |
| Vaccinated | 35.0 | 9.9 | 3.3 | 6.7 | −25.1 (−31.2 to −18.9) [−71.7]b | −6.6 (−10.9 to −2.3) [−66.7]b | +3.5 (+0.05 to 6.9) [103]b | −28.2 (−33.8 to −22.7) [−80.9]b |
| Unvaccinated | 32.4 | 17 | 22.3 | 19.4 | −15.4 (−22.9 to −7.9) [−47.5]b | +5.3 (−4.3 to 14.9) [31.2] | −2.9 (−15.2 to 9.4) [−13] | −13 (−23.8 to −2.2) [−40.1]b |
| Types in the 9-valent but not 4-valent vaccined | ||||||||
| All | 23.9 | 24.8 | 16.8 | 12.1 | +1.0 (−5.1 to +7.0) [3.8] | −8.1 (−13.6 to −2.5) [−32.3]b | −4.7 (−9.6 to 0.2) [−28] | −11.8 (−17.2 to −6.4) [−48.1]b |
| Vaccinated | 23.4 | 26.6 | 14.1 | 7.3 | +3.1 (−3.9 to +10.2) [13.7] | −12.4 (−19.3 to −5.5) [−47]b | −6.8 (−11.8 to −1.9) [−48.2]b | −16.1 (−21.3 to −11.0) [−68.8]b |
| Unvaccinated | 22.9 | 24 | 30.4 | 36.1 | +1.1 (−6.7 to +9.0) [4.8] | +6.4 (−4.4 to 17.1) [26.7] | +5.8 (−8.7 to 20.2) [18.8] | +13.2 (−0.8 to +25.7) [57.6] |
CI, confidence interval.
HPV-6, -11, -16, -18, -31, -33, -45, -52, and -58.
P < .05.
HPV-6, -11, -16, and -18.
HPV-31, -33, -45, -52, and -58.
FIGURE 1.
Proportions of women who were vaccinated across the 4 study waves infected with any vaccine-type HPV, adjusted for propensity scores. HPV 9m4v, 5 additional HPV types only in 9-valent vaccine (HPV-31, -33, -45, -52, and -58); 4v HPV, 4-valent vaccine–type human papillomavirus; 9v HPV, 9-valent vaccine–type human papillomavirus.
FIGURE 3.
Proportions of all women across the 4 study waves infected with any vaccine-type HPV, adjusted for propensity scores. HPV 9m4v, 5 additional HPV types only in 9-valent vaccine (HPV-31, -33, -45, -52, and -58); 4v HPV, 4-valent vaccine–type human papillomavirus; 9v HPV, 9-valent vaccine–type human papillomavirus.
FIGURE 2.
Proportions of women who were unvaccinated across the 4 study waves infected with any vaccine-type HPV, adjusted for propensity scores. HPV 9m4v, 5 additional HPV types only in 9-valent vaccine (HPV-31, -33, -45, -52, and -58); 4v HPV, 4-valent vaccine–type human papillomavirus; 9v HPV, 9-valent vaccine–type human papillomavirus.
The logistic regression model results are shown in Table 3. In these models, we demonstrate the odds of infection (in waves 4 vs 1, 4 vs 2, and 4 vs 3) with 9-valent vaccine–type HPV, 4-valent vaccine–type HPV, and the 5 additional types in the 9-valent vaccine by vaccination status. Among women who were vaccinated, the odds of infection decreased significantly from wave 1 to wave 4 for 9-valent vaccine–type HPV (adjusted odds ratio [aOR] 0.18), 4-valent vaccine–type HPV (aOR 0.13), and the 5 additional HPV types included only in the 9-valent vaccine (aOR 0.26). Among women who were unvaccinated, the odds of infection did not change significantly from wave 1 to wave 4 for 9-valent vaccine–type HPV; it decreased significantly from wave 1 to wave 4 for 4-valent vaccine–type HPV (aOR 0.50) and increased significantly for the 5 additional HPV types only in the 9-valent vaccine (aOR 1.90).
TABLE 3.
Comparisons of the Proportions of Women Who Are Positive for 9-Valent HPV Types, 4-Valent HPV Types, and the 5 HPV Types Included in the 9-Valent But Not the 4-Valent Vaccine Across the 4 Study Waves by Vaccination Status: Results of Unadjusted and Adjusted Logistic Regression Analyses
| Vaccination Status | Wave 1–4 | Wave 2–4 | Wave 3–4 | |||
|---|---|---|---|---|---|---|
| Unadjusted OR (95% CI) | aOR (95% CI) | Unadjusted OR (95% CI) | aOR (95% CI) | Unadjusted OR (95% CI) | aOR (95% CI) | |
| 9-valent vaccine–type HPVa | ||||||
| All | 0.24 (0.17 to 0.34)b | 0.25 (0.18 to 0.35)b | 0.35 (0.25 to 0.49)b | 0.42 (0.30 to 0.58)b | 0.66 (0.46 to 0.94)b | 0.74 (0.52 to 1.05) |
| Vaccinated | 0.20 (0.14 to 0.29)b | 0.18 (0.12 to 0.26)b | 0.30 (0.20 to 0.46)b | 0.32 (0.21 to 0.49)b | 0.77 (0.50 to 1.19) | 0.77 (0.50 to 1.21) |
| Unvaccinated | 0.46 (0.26 to 0.84)b | 0.95 (0.56 to 1.62) | 0.66 (0.35 to 1.25) | 1.35 (0.75 to 2.43) | 0.68 (0.35 to 1.35) | 0.87 (0.47 to 1.61) |
| 4-valent vaccine–type HPVc | ||||||
| All | 0.12 (0.08 to 0.20)b | 0.18 (0.12 to 0.27)b | 0.36 (0.22 to 0.60)b | 0.61 (0.39 to 0.95)b | 0.66 (0.38 to 1.16) | 1.06 (0.64 to 1.75) |
| Vaccinated | 0.10 (0.06 to 0.17)b | 0.13 (0.08 to 0.22)b | 0.39 (0.20 to 0.75)b | 0.66 (0.36 to 1.20) | 1.15 (0.52 to 2.54) | 2.13 (0.97 to 4.69) |
| Unvaccinated | 0.26 (0.12 to 0.60)b | 0.50 (0.26 to 0.97)b | 0.55 (0.23 to 1.32) | 1.17 (0.56 to 2.46) | 0.55 (0.22 to 1.39) | 0.84 (0.39 to 1.79) |
| 5 HPV types in the 9-valent but not the 4-valent vaccined | ||||||
| All | 0.43 (0.29 to 0.63)b | 0.44 (0.30 to 0.64)b | 0.38 (0.26 to 0.55)b | 0.42 (0.29 to 0.60)b | 0.69 (0.46 to 1.04) | 0.68 (0.46 to 1.02) |
| Vaccinated | 0.37 (0.24 to 0.57)b | 0.26 (0.16 to 0.42)b | 0.30 (0.19 to 0.47)b | 0.22 (0.13 to 0.36)b | 0.68 (0.42 to 1.10) | 0.48 (0.28 to 0.82)b |
| Unvaccinated | 0.76 (0.39 to 1.49) | 1.90 (1.09 to 3.34)b | 0.77 (0.37 to 1.59) | 1.79 (0.96 to 3.33) | 0.93 (0.43 to 2.02) | 1.30 (0.68 to 2.48) |
CI, confidence interval; OR, odds ratio.
HPV-6, -11, -16, -18, -31, -33, -45, -52, and -58.
P < .05.
HPV-6, -11, -16, and -18.
HPV-31, -33, -45, -52, and -58.
Discussion
To our knowledge, this is the first study in which trends in 4-valent and 9-valent vaccine–type HPV among women who are vaccinated and women who are unvaccinated in the United States >10 years after HPV vaccine introduction are examined, and it is the first in which trends after 9-valent HPV vaccine introduction are examined. Consistent with our hypotheses, we found that from 2006 to 2017, the prevalence of 4-valent vaccine–type HPV and 9-valent vaccine–type HPV decreased significantly among women who were vaccinated, and the prevalence of 4-valent vaccine–type HPV decreased significantly among women who were unvaccinated. In exploratory analyses, we demonstrated a significant decrease in the 5 additional types targeted by the 9-valent vaccine among women who were vaccinated and, unexpectedly, a significant increase in the 5 additional types targeted by the 9-valent vaccine among women who were unvaccinated.
The significant decline (81%) in 4-valent vaccine–type HPV in women who were vaccinated and the high degree of vaccine effectiveness when comparing women who were vaccinated with women who were unvaccinated (90.6% in wave 3 versus wave 1 and 80.1% in wave 4 versus wave 1) provide evidence of direct protection by the 4-valent vaccine among the women who were vaccinated in this community, and it suggests high vaccine effectiveness in a real-world setting. This degree of effectiveness is remarkable given the fact that vaccination was defined as having received ≥1 dose (ie, was not defined as having completed the vaccination series) and that women in this study were likely at a substantially higher risk for preexisting HPV infection than those in the HPV vaccine clinical trials because of their reported sexual behaviors. The high efficacy of the licensed HPV vaccines and high rates of vaccination in this study sample likely contributed to this substantial decrease in HPV infection, even in young women at a high risk for sexually transmitted infections (STIs). Our findings of a decrease in vaccine-type HPV among women who were vaccinated extend the findings of those studies conducted over different time frames and in different populations and settings,16–19,21,29 and they support the real-world effectiveness of the 4-valent vaccine, especially in younger age groups and countries with high vaccination coverage.16,21 This decline in HPV prevalence is expected to result in a significant decrease in cervical precancers and cancers in the future. Of note, the findings should not be interpreted as suggesting that current recommendations for a 2- or 3-dose series and vaccinating before sexual initiation are not necessary. In additional exploratory analyses, we have demonstrated that receipt of 1 vs 3 doses was associated with 3.2 times the adjusted odds of vaccine-type HPV infection (P = .04) and that young women who did versus did not have sex before vaccination were more likely to be positive for vaccine-type HPV (8.8% vs 3.8%, P = .0021).
We also demonstrated a significant decline among women who were vaccinated in the prevalence of 9-valent vaccine–type HPV (71%), as hypothesized, and in the prevalence of the 5 additional HPV types included in the 9-valent vaccine (68.8%). All 5 additional types in the 9-valent vaccine are genetically related to HPV-16 (HPV-31, -33, -52, and -58) and HPV-18 (HPV-45). In clinical trials, researchers have demonstrated evidence of crossprotection against HPV types genetically related to HPV-16 and -18,14,30–32 and in studies conducted in clinical and community settings, researchers have also demonstrated evidence of crossprotection against HPV types genetically related to the 2-valent or 4-valent HPV vaccines33–35; however, it is not well established whether crossprotection occurs among younger women recruited from broader settings or after introduction of the 9-valent HPV vaccine. In this study, only 12% of women in wave 4 received at least 1 dose of the 9-valent vaccine; therefore, it is unlikely that these findings were due only to direct protection against the types in the 9-valent vaccine among women who were vaccinated. Instead, the decreases noted in 9-valent vaccine–type HPV and the 5 additional types in the 9-valent vaccine may represent evidence of crossprotection against genetically related types (-31, -33, -45, -52, and -58). In a separate analysis, we are examining trends in nonvaccine-type HPV in more depth by exploring the prevalence of nonvaccine-type HPV genetically related to HPV-16 and HPV-18 among women who received the 4-valent vaccine to examine for crossprotection and by exploring non–vaccine-type HPV genetically unrelated to vaccine-type HPV to examine for type replacement (C.S., L.D., D.B., et al, unpublished observations).
There was a significant decline of 40.1% in the prevalence of 4-valent vaccine–type HPV among women who were unvaccinated, suggesting herd protection. Evidence of herd protection was found in our previous study during the first 3 waves of data collection.15 In addition, evidence of herd protection was found in 2 studies conducted in Scotland among adult women attending cervical cancer screening,33,35 in studies in Australia among adult women attending cervical cancer screening19 and recruited through a social networking site,18 and in the United States before 9-valent vaccine introduction.29,36 As noted in a recent review,37 evidence about herd protection will be a key component of cost-effectiveness analysis evaluating cervical cancer screening strategies.
We unexpectedly found a significant increase from 2006 to 2017 in the prevalence of the 5 additional types in the 9-valent vaccine among women who were unvaccinated. A theoretical explanation could be type replacement, which would be an increase in non–4-valent vaccine–type HPV created by an ecological niche after the 4-valent vaccine was introduced. However, this phenomenon is thought to be unlikely given the genetic stability of HPV and that HPV types do not seem to compete for trophic epithelial niches.38 A more likely explanation is differences between women who are unvaccinated and women who are vaccinated. For example, if women who are unvaccinated versus women who are vaccinated are more likely to practice riskier behaviors that would increase their risk of acquiring HPV, they would be more likely to acquire non–vaccine-type HPV. In a previous analysis of the first 3 waves of data from this study, we found that women who were unvaccinated did differ significantly from women who were vaccinated in ways that could increase their risk for HPV acquisition, supporting this explanation.39 For example, women who were unvaccinated versus women who were vaccinated were more likely to lack health insurance and to have had at least 1 new sexual partner in the past 3 months. Continued community-level research is needed to determine if this trend reverses and if the prevalence of the 5 additional types included in the 9-valent vaccine begin to decline once a higher proportion of young women have received the 9-valent vaccine. In addition, the findings of differences between women who are unvaccinated and women who are vaccinated underscore the importance of understanding predictors of nonvaccination and designing clinical interventions to reach those youth who are unvaccinated and who may be at an elevated risk for HPV.
A limitation of this study was the small proportion of women who received the 9-valent vaccine in wave 4, which limited our ability to examine the trends in HPV prevalence due to the effects of the 9-valent vaccine. Also, the clinic-based recruitment strategy could limit generalizability to all young women in this age group in the United States. Instead, the sample could be viewed as generalizable to a group of adolescent and young adult women at a relatively high risk for HPV and other STIs and with a fairly high vaccination rate. Finally, risk behaviors were assessed by self-report, which may limit validity.
Conclusions
Eleven years after the introduction of the HPV vaccine, we noted significant decreases in 4-valent vaccine–type HPV, 9-valent vaccine–type HPV, and the 5 additional HPV types included only in 9-valent vaccine among women who were vaccinated, suggesting 4-valent vaccine effectiveness in a real-world setting and possible crossprotection against genetically related HPV types. The significant decrease in 4-valent HPV types among women who were unvaccinated suggests herd protection. Although these findings are important for clinical care and public health policy, continued surveillance will be important to assess for waning vaccine effectiveness, herd protection, and the impact of 9-valent vaccine introduction.
Acknowledgments
We acknowledge the support provided by Charlene Morrow, RN, Susan Glynn, BA, Rachel Thomas, BS, and Lisa Higgins, RPh, as well as the support of the Cincinnati Children’s Hospital Teen Health Center and Cincinnati Health Department staff.
Glossary
- aOR
adjusted odds ratio
- HPV
human papillomavirus
- STI
sexually transmitted infection
Footnotes
Ms Spinner assisted with the design of the analyses, did the literature search, interpreted the results, and codrafted the initial manuscript; Dr Ding designed and conducted the statistical analyses, interpreted the results, and critically revised the manuscript for intellectual content; Drs Bernstein and Franco assisted with study conceptualization and design, interpreted the results, and critically revised the manuscript for intellectual content; Dr Brown assisted with study conceptualization and design, conducted the human papillomavirus DNA analyses, interpreted the results, and critically revised the manuscript for intellectual content; Ms Covert recruited participants for the study and interpreted the results; Dr Kahn conceptualized and designed the study, obtained funding for the study, interpreted the results, codrafted the initial manuscript, and revised the final manuscript; and all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Ms Spinner was an undergraduate at the University of Cincinnati when this research was conducted; she is currently an entering graduate student at the University of South Florida.
FINANCIAL DISCLOSURE: Dr Brown owns stock in shares of Merck & Co, Inc; the other authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by 2 grants from the National Institutes of Health National Institute of Allergy and Infectious Diseases (R01 AI073713 and R01 AI104709) and a grant from the National Institutes of Health National Center for Advancing Translational Sciences (UL1 TR001425). Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: Dr Brown has received an investigator initiated studies program award from Merck entitled “Cervical Cancer Prevention in Kenya” and owns stock in shares of Merck & Co, Inc. Dr Franco has occasionally served as consultant to companies involved with human papillomavirus (HPV) diagnostics (Roche, BD, and Abbott) and HPV vaccines (Merck and GSK). Dr Kahn served as cochair of a study of HPV vaccines in men with HIV. The study was funded by the National Institutes of Health, but Merck provided vaccine and serology testing; the other authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Bosch FX, de Sanjosé S. Chapter 1: human papillomavirus and cervical cancer–burden and assessment of causality. J Natl Cancer Inst Monogr. 2003;(31):3–13 [DOI] [PubMed] [Google Scholar]
- 2.Bosch FX, Lorincz A, Muñoz N, Meijer CJ, Shah KV. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol. 2002;55(4):244–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palefsky JM. HPV infection in men. Dis Markers. 2007;23(4):261–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER; Centers for Disease Control and Prevention; Advisory Committee on Immunization Practices . Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2007;56(RR-2):1–24 [PubMed] [Google Scholar]
- 5.Markowitz LE, Dunne EF, Saraiya M, et al. ; Centers for Disease Control and Prevention . Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP) [published correction appears in MMWR Recomm Rep. 2014;63(49):1182]. MMWR Recomm Rep. 2014;63(RR-05):1–30 [PubMed] [Google Scholar]
- 6.Petrosky E, Bocchini JA Jr, Hariri S, et al. ; Centers for Disease Control and Prevention (CDC) . Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2015;64(11):300–304 [PMC free article] [PubMed] [Google Scholar]
- 7.Joura EA, Giuliano AR, Iversen OE, et al. ; Broad Spectrum HPV Vaccine Study . A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372(8):711–723 [DOI] [PubMed] [Google Scholar]
- 8.Paz-Zulueta M, Álvarez-Paredes L, Rodríguez Díaz JC, et al. Prevalence of high-risk HPV genotypes, categorised by their quadrivalent and nine-valent HPV vaccination coverage, and the genotype association with high-grade lesions. BMC Cancer. 2018;18(1):112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luckett R, Feldman S. Impact of 2-, 4- and 9-valent HPV vaccines on morbidity and mortality from cervical cancer. Hum Vaccin Immunother. 2016;12(6):1332–1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brisson M, Laprise JF, Chesson HW, et al. Health and economic impact of switching from a 4-valent to a 9-valent HPV vaccination program in the United States. J Natl Cancer Inst. 2015;108(1):djv282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harper DM, Franco EL, Wheeler CM, et al. ; HPV Vaccine Study Group . Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet. 2006;367(9518):1247–1255 [DOI] [PubMed] [Google Scholar]
- 12.Joura EA, Kjaer SK, Wheeler CM, et al. HPV antibody levels and clinical efficacy following administration of a prophylactic quadrivalent HPV vaccine. Vaccine. 2008;26(52):6844–6851 [DOI] [PubMed] [Google Scholar]
- 13.Joura EA, Leodolter S, Hernandez-Avila M, et al. Efficacy of a quadrivalent prophylactic human papillomavirus (types 6, 11, 16, and 18) L1 virus-like-particle vaccine against high-grade vulval and vaginal lesions: a combined analysis of three randomised clinical trials. Lancet. 2007;369(9574):1693–1702 [DOI] [PubMed] [Google Scholar]
- 14.Paavonen J, Naud P, Salmerón J, et al. ; HPV PATRICIA Study Group . Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women [published correction appears in Lancet. 2010;376(9746):1054]. Lancet. 2009;374(9686):301–314 [DOI] [PubMed] [Google Scholar]
- 15.Kahn JA, Widdice LE, Ding L, et al. Substantial decline in vaccine-type human papillomavirus (HPV) among vaccinated young women during the first 8 years after HPV vaccine introduction in a community. Clin Infect Dis. 2016;63(10):1281–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drolet M, Bénard É, Boily MC, et al. Population-level impact and herd effects following human papillomavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis. 2015;15(5):565–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Markowitz LE, Liu G, Hariri S, Steinau M, Dunne EF, Unger ER. Prevalence of HPV after introduction of the vaccination program in the United States. Pediatrics. 2016;137(3):e20151968. [DOI] [PubMed] [Google Scholar]
- 18.Garland SM, Cornall AM, Brotherton JML, Wark JD, Malloy MJ, Tabrizi SN; VACCINE Study Group . Final analysis of a study assessing genital human papillomavirus genoprevalence in young Australian women, following eight years of a national vaccination program. Vaccine. 2018;36(23):3221–3230 [DOI] [PubMed] [Google Scholar]
- 19.Machalek DA, Garland SM, Brotherton JML, et al. Very low prevalence of vaccine human papillomavirus types among 18- to 35-year old Australian women 9 years following implementation of vaccination. J Infect Dis. 2018;217(10):1590–1600 [DOI] [PubMed] [Google Scholar]
- 20.Markowitz LE, Hariri S, Lin C, et al. Reduction in human papillomavirus (HPV) prevalence among young women following HPV vaccine introduction in the United States, National Health and Nutrition Examination Surveys, 2003-2010. J Infect Dis. 2013;208(3):385–393 [DOI] [PubMed] [Google Scholar]
- 21.Mesher D, Panwar K, Thomas SL, Beddows S, Soldan K. Continuing reductions in HPV 16/18 in a population with high coverage of bivalent HPV vaccination in England: an ongoing cross-sectional study. BMJ Open. 2016;6(2):e009915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kahn JA, Brown DR, Ding L, et al. Vaccine-type human papillomavirus and evidence of herd protection after vaccine introduction. Pediatrics. 2012;130(2). Available at: www.pediatrics.org/cgi/content/full/130/2/e249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gravitt PE, Peyton CL, Alessi TQ, et al. Improved amplification of genital human papillomaviruses. J Clin Microbiol. 2000;38(1):357–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kahn JA, Slap GB, Huang B, et al. Comparison of adolescent and young adult self-collected and clinician-collected samples for human papillomavirus. Obstet Gynecol. 2004;103(5, pt 1):952–959 [DOI] [PubMed] [Google Scholar]
- 25.Sellors JW, Lorincz AT, Mahony JB, et al. Comparison of self-collected vaginal, vulvar and urine samples with physician-collected cervical samples for human papillomavirus testing to detect high-grade squamous intraepithelial lesions. CMAJ. 2000;163(5):513–518 [PMC free article] [PubMed] [Google Scholar]
- 26.Gravitt PE, Lacey JV Jr, Brinton LA, et al. Evaluation of self-collected cervicovaginal cell samples for human papillomavirus testing by polymerase chain reaction. Cancer Epidemiol Biomarkers Prev. 2001;10(2):95–100 [PubMed] [Google Scholar]
- 27.Thomas R, Higgins L, Ding L, Widdice LE, Chandler E, Kahn JA. Factors associated with HPV vaccine initiation, vaccine completion, and accuracy of self-reported vaccination status among 13- to 26-year-old men. Am J Men Health. 2018;12(4):819–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shim E, Galvani AP. Distinguishing vaccine efficacy and effectiveness. Vaccine. 2012;30(47):6700–6705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oliver SE, Unger ER, Lewis R, et al. Prevalence of human papillomavirus among females after vaccine introduction-National Health and Nutrition Examination Survey, United States, 2003-2014. J Infect Dis. 2017;216(5):594–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown DR, Kjaer SK, Sigurdsson K, et al. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in generally HPV-naive women aged 16-26 years. J Infect Dis. 2009;199(7):926–935 [DOI] [PubMed] [Google Scholar]
- 31.Wheeler CM, Castellsagué X, Garland SM, et al. ; HPV PATRICIA Study Group . Cross-protective efficacy of HPV-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by non-vaccine oncogenic HPV types: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial [published correction appears in Lancet Oncol. 2012;13(1):e1]. Lancet Oncol. 2012;13(1):100–110 [DOI] [PubMed] [Google Scholar]
- 32.Malagón T, Drolet M, Boily MC, et al. Cross-protective efficacy of two human papillomavirus vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12(10):781–789 [DOI] [PubMed] [Google Scholar]
- 33.Kavanagh K, Pollock KG, Potts A, et al. Introduction and sustained high coverage of the HPV bivalent vaccine leads to a reduction in prevalence of HPV 16/18 and closely related HPV types. Br J Cancer. 2014;110(11):2804–2811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tabrizi SN, Brotherton JM, Kaldor JM, et al. Assessment of herd immunity and cross-protection after a human papillomavirus vaccination programme in Australia: a repeat cross-sectional study. Lancet Infect Dis. 2014;14(10):958–966 [DOI] [PubMed] [Google Scholar]
- 35.Cameron RL, Kavanagh K, Pan J, et al. Human papillomavirus prevalence and herd immunity after introduction of vaccination program, Scotland, 2009-2013. Emerg Infect Dis. 2016;22(1):56–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berenson AB, Hirth JM, Chang M. Change in human papillomavirus prevalence among U.S. women aged 18-59 years, 2009-2014. Obstet Gynecol. 2017;130(4):693–701 [DOI] [PubMed] [Google Scholar]
- 37.Malagón T, Laurie C, Franco EL. Human papillomavirus vaccination and the role of herd effects in future cancer control planning: a review. Expert Rev Vaccines. 2018;17(5):395–409 [DOI] [PubMed] [Google Scholar]
- 38.Tota JE, Ramanakumar AV, Jiang M, et al. Epidemiologic approaches to evaluating the potential for human papillomavirus type replacement postvaccination. Am J Epidemiol. 2013;178(4):625–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ding L, Widdice LE, Kahn JA. Differences between vaccinated and unvaccinated women explain increase in non-vaccine-type human papillomavirus in unvaccinated women after vaccine introduction. Vaccine. 2017;35(52):7217–7221 [DOI] [PMC free article] [PubMed] [Google Scholar]