By following a birth cohort through 2 years of age, Bifidobacterium abundance in early infancy was seen to predict better vaccine responses later in life.
Abstract
BACKGROUND:
The intestinal microbiome in early infancy affects immunologic development and thus may affect vaccine memory, though few prospective studies have examined such associations. We examined the association of Bifidobacterium levels in early infancy with memory responses to early vaccination measured at 2 years of age.
METHODS:
In this prospective observational study, we examined the association of Bifidobacterium abundance in the stool of healthy infants at 6 to 15 weeks of age, near the time of vaccination, with T-cell and antibody responses measured at 6 weeks, 15 weeks, and 2 years of age. Infants were vaccinated with Bacillus Calmette-Guérin (BCG) (at birth), oral polio virus (at birth and at 6, 10, and 14 weeks), tetanus toxoid (TT) (at 6, 10, and 14 weeks), and hepatitis B virus (at 6, 10, and 14 weeks). Fecal Bifidobacterium was measured at 6, 11, and 15 weeks. Bifidobacterium species and subspecies were measured at 6 weeks.
RESULTS:
Mean Bifidobacterium abundance in early infancy was positively associated with the CD4 T-cell responses to BCG, TT, and hepatitis B virus at 15 weeks, with CD4 responses to BCG and TT at 2 years, and with plasma TT-specific immunoglobulin G and stool polio-specific immunoglobulin A at 2 years. Similar associations were seen for the predominant subspecies, Bifidobacterium longum subspecies infantis.
CONCLUSIONS:
Bifidobacterium abundance in early infancy may increase protective efficacy of vaccines by enhancing immunologic memory. This hypothesis could be tested in clinical trials of interventions to optimize Bifidobacterium abundance in appropriate populations.
What’s Known on This Subject:
The composition of the gut microbiome affects many aspects of immune function and is known to affect response to vaccination over the short-term.
What This Study Adds:
We demonstrate that a high abundance of Bifidobacterium in early infancy when infants receive several vaccines is associated with better vaccine memory, as indicated by higher responses to Bacillus Calmette-Guérin, polio, and tetanus vaccines measured at 2 years of age.
Vaccines are estimated to prevent 3 million childhood deaths annually.1 Vaccine memory involves the development of antibody-producing plasma cells and effector T cells, whereas maintenance of memory involves the persistence of memory B and T cells.2,3 Vaccine-elicited T-cell and antibody responses as well as protective efficacy vary substantially among individuals for reasons that are not completely understood.4–6 Recent evidence suggests that part of this variability is due to interactions between the intestinal microbiome and the developing infant immune system.7
The importance of intestinal microbes for the development of the mammalian immune system is well documented. The absence of bacteria in germ-free mice affects both local8–11 and systemic lymphoid compartments,9,10 whereas colonization with specific commensal bacteria12–14 affects both innate and adaptive immunity.15 Disruption of normal development of the intestinal microbiome may have adverse immunologic consequences. Quantitative or qualitative “deficiencies” in microbial exposure during infancy increases subsequent risk of atopic diseases including asthma,16 whereas the early administration of probiotic bacteria is associated with a decreased risk of atopic eczema.17 Researchers have examined in only a few human studies the relationship of naturally occurring gut bacteria with immune function, although members of the Bifidobacterium genus have been associated with higher levels of immunoglobulin A (IgA)-secreting plasma cells,18 memory B-cells,19 and salivary IgA,20 suggesting a beneficial relationship for the infant.
Establishment of the intestinal microbiome begins early in infancy and follows a typical pattern.21 Facultative anaerobic bacteria such as Staphylococcus, Streptococcus, Lactobacillus, and Escherichia initially colonize the colon, followed by strict anaerobes that come to predominate during the first few months of life. In breastfed infants Bifidobacterium longum subspecies infantis often dominates the microbiome because it is specifically adapted to both use human milk oligosaccharides as a carbon source and to restrict human milk oligosaccharides availability to other bacteria.22 Bacteroides and clostridia are also important commensal anaerobes in the infant gut.21 The abundance of Bifidobacterium species (relative to total bacteria) can exceed 60% in breastfed infants in countries like Bangladesh where breastfeeding is widespread.23,24 The abundance of B longum subspecies infantis is lower in the United States and other Western countries and appears to have decreased over recent decades,25 concurrent with the rise in autoimmune and allergic diseases.
In the current study, we evaluate the hypothesis that greater exposure to Bifidobacterium (and to B longum subspecies infantis in particular) early in infancy when vaccines are administered will result in better memory responses to these vaccines. We previously reported that a high abundance of Bifidobacterium, and of B longum subspecies infantis, at 15 weeks was associated with higher vaccine responses measured at the same time.24 In that study, we examined a subset (n = 48) of infants in a birth cohort of 306 infants. In the current study, we measure the vaccine responses of all available infants at 2 years of age to test the hypothesis that Bifidobacterium abundance at 6, 11, and 15 weeks of age is predictive of vaccine responses measured at 2 years. We examine responses to 4 vaccines: tetanus toxoid (TT) and hepatitis B virus (HBV) given at 6, 10, and 14 weeks; Bacillus Calmette-Guérin (BCG), given within 48 hours of birth; and oral polio virus (OPV), given within 48 hours of birth and at 6, 10, and 14 weeks. We also examine vaccine responses measured at 6 and 15 weeks to increase the sample size of our previous report.24
Methods
Study Design
In this study, we re-recruited all available participants from a randomized controlled trial of vitamin A supplementation at birth (www.clinicaltrials.gov identifier: NCT01583972) and measured additional vaccine responses at 2 years in the same infants (NCT02027610) (Supplemental Table 4). The study was approved by the Research Review Committee and the Ethical Review Committee of the International Centre for Diarrhoeal Disease Research, Bangladesh. The inclusion-exclusion criteria, study design, laboratory methodology, clinical procedures, and baseline characteristics of the participants have been described.26
Vaccine Responses Assay
Vaccine-specific CD4 T-cell stimulation index (SI), antibody in lymphocyte supernatant (ALS) responses and the purified protein derivative delayed-type hypersensitivity (DTH) skin test response were measured as described in our earlier report.26,27 All of the vaccines are T-cell–dependent in that a robust T-cell response is needed for protection. Thus, we view the SI as a proxy for the magnitude of memory response (ie, the number of memory T cells). Stool extracts were prepared as described28 to measure polio-specific IgA by using an in-house enzyme-linked immunosorbent assay (ELISA) described in the Supplemental Information. Antibody avidity index (AI) of plasma polio specific immunoglobulin G (IgG) and IgA, HBV-specific IgG, and TT-specific IgG at 2 years was determined by ELISA by using a chaotropic agent as described in the Supplemental Information.
Microbiota Assay
Overall stool microbial composition was determined by using 16s V4 sequencing method24 modified as described in the Supplemental Information. Abundance at the genus level refers to the abundance of Bifidobacterium genomes relative to all bacterial genomes. A terminal restriction-fragment length-polymorphism assay was used to identify bifidobacteria at the species level by comparing fragment lengths to published data.29 Details are provided in the Supplemental Information and previous publications.24,30
Statistical Analysis
The associations between bifidobacteria and vaccine-specific immune responses were determined by multiple regression analysis by using the mean Bifidobacterium abundance during early infancy (6, 11, and 15 weeks) and at 6 weeks only, and the abundance of Bifidobacterium species and subspecies measured at 6 weeks, as independent variables. The analysis was adjusted for possible covariates, including sex, vitamin A or placebo treatment, birth weight (above or below median), and type of delivery (cesarean delivery or vaginal). Breastfeeding status (exclusive or nonexclusive) during early infancy affects Bifidobacterium abundance and could independently affect vaccine responses and was thus evaluated as a covariate in our model. It was statistically significant in only 2 cases and was thus not included in the final model. However, examination of stool IgA responses to OPV at 2 years could be affected if breastmilk contained OPV-specific IgA. Thus, we included breastfeeding status (yes or no) in the regression analysis at 2 years. A P value of <.05 was considered statistically significant for all analyses. Data are presented as means ± SD unless otherwise indicated. Statistical analysis was performed by using R version 3.4.2.31
Results
Characteristics of Study Infants
Microbiota data were available from 291 infants at 6, 11, and 15 weeks, and from 249 of these same infants at 2 years. Half of the participants were boys, and half received a high-dose vitamin A supplement within 48 hours of birth as prescribed by the parent study (Table 1). The majority of infants were born by elective cesarean delivery, as is typical for hospital deliveries in Bangladesh.26 At 6 weeks, all infants were exclusively or predominantly breastfed, and at 2 years, 55% received supplemental breastfeeding. Infants were healthy on the days of sample collection but a small percentage had elevated C-reactive protein (CRP), indicating an ongoing acute phase response (Table 1).
TABLE 1.
Characteristics of the Participants
| Characteristics | 6 wk (n = 280) | 2 ya (n = 249) |
|---|---|---|
| Sex, boy | 137 (48.9%) | 122 (48.8%) |
| Vitamin A supplement | 139 (49.6%) | 121 (48.8%) |
| Mode of delivery, cesarean delivery | 170 (60.7%) | 156 (62.7%) |
| Gestational age, wk | 39.1 ± 1.62 | 39.2 ± 1.61 |
| Preterm birth, <37 wk | 20 (7.19%) | 17 (6.91%) |
| Birth wt, g | 2735 ± 375 | 2762 ± 392 |
| Low birth wt, <2500 g | 77 (27.5%) | 63 (25.2%) |
| Wasting, WHZ <−2 | 13 (4.66%) | 24 (10.2%) |
| Stunting, HAZ <−2 | 48 (17.2%) | 81 (34.2%) |
| Breastfeeding status | ||
| Exclusive | 196 (70.0%) | 0 (0%) |
| Nonexclusive | 84 (30.0%) | 133 (55.0%) |
| No breastfeeding | 0 (0%) | 109 (45.0%) |
| CRP ≥5 mg/Lb | 1 (0.390%) | 16 (6.58%) |
Data presented as mean ± SD or number (frequency). HAZ, height-for-age z score; WHZ, weight-for-height z score.
Mean age = 27.7 ± 3.23 m, median age (25th, 75th percentile) = 28.0 (24.8, 30.1) m, range = 18.4 m.
CRP value was not available for 24 subjects at 6 wk and 6 subjects at 2 y.
Bifidobacteria Were the Most Abundant Taxa
All infants had detectable Bifidobacterium at 6 weeks and the mean abundance for 6, 11, and 15 weeks was 0.637 with a range 0.020 to 0.921 (Supplemental Table 5). Species and subspecies of Bifidobacterium were measured at 6 weeks, and B longum was by far the most abundant species, at 0.581, followed distantly by Bifidobacterium breve and Bifidobacterium bifidum at 0.032 and 0.014, respectively. We further analyzed the 2 subspecies of B longum and found that B longum subspecies infantis (abundance, 0.568) was predominant, being ∼10-fold more abundant than B longum subspecies longum (abundance, 0.058), as expected.
Bifidobacteria Abundance Was Positively Associated With the CD4 T-Cell Response to the BCG Vaccine
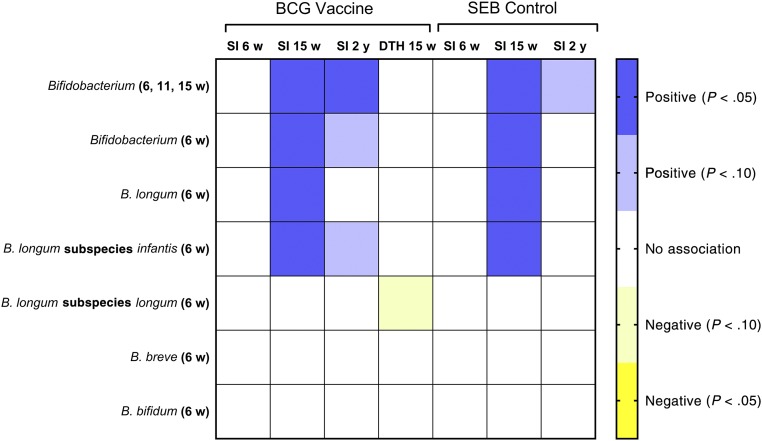
The BCG vaccine was given once within 48 hours of birth. Although intestinal bacterial communities were not established at that time, we hypothesized that such communities established between 6 and 15 weeks would affect maintenance of the BCG memory response. We tested this hypothesis using multivariate regression analysis to adjust for other factors that could also affect vaccine responses and found that mean Bifidobacterium abundance in early infancy (measured at 6, 11, and 15 weeks; Table 2) and Bifidobacterium abundance measured at 6 weeks only (the closest time to vaccination; Supplemental Table 6) were both positively associated with the CD4 memory T-cell responses (measured as the SI) at 15 weeks and 2 years but not at 6 weeks (Fig 1). For infants with high mean Bifidobacterium abundance (90th percentile of study population, abundance = 0.814), the estimated SI at 15 weeks was 85% higher than for infants with low Bifidobacterium abundance (10th percentile abundance = 0.396), whereas the difference at 2 years was 64% (Table 3). No significant associations were seen between Bifidobacterium abundance and the DTH skin-test response for BCG (Fig 1).
TABLE 2.
The Association Between Bifidobacterium Abundance During Early Infancy (Mean of 6, 11, and 15 Weeks of Age) and Vaccine-Specific CD4 T-Cell SI, DTH Skin Test Response, and Antibody Responses
| Regression Model | Average Bifidobacterium Abundance | |||
|---|---|---|---|---|
| R2 | P | β (SE) | P | |
| BCG | ||||
| 6 wk | ||||
| CD4+ T-cell SIa,b | 0.0859 | .019 | .144 (0.145) | .32 |
| 15 wk | ||||
| CD4+ T-cell SI | 0.0590 | .021 | .668 (0.214) | <.01 |
| Skin test area, cm2 | 0.0201 | .34 | .427 (0.362) | .24 |
| 2 y | ||||
| CD4+ T-cell SIa,c | 0.0883 | <.01 | .451 (0.221) | .043 |
| TT | ||||
| 15 wk | ||||
| CD4+ T-cell SIc | 0.0514 | .037 | .371 (0.131) | <.01 |
| ALS IgG, mIU/mL | 0.0166 | .49 | .408 (0.249) | .10 |
| 2 y | ||||
| CD4+ T-cell SI | 0.0453 | .17 | .465 (0.222) | .038 |
| Plasma IgG, IU/mL | 0.0402 | .076 | .378 (0.161) | .020 |
| Plasma IgG AI | 0.0117 | .72 | .00523 (0.0404) | .90 |
| HBV | ||||
| 15 wk | ||||
| CD4+ T-cell SIa | 0.0530 | .042 | 1.09 (0.429) | .012 |
| ALS IgG, mIU/mLc | 0.0471 | .19 | −.00162 (0.0119) | .89 |
| 2 y | ||||
| CD4+ T-cell SI | 0.0150 | .77 | .218 (0.202) | .28 |
| Plasma IgG, IU/mL | 0.0228 | .35 | −.398 (0.233) | .088 |
| Plasma IgG AId | 0.0285 | .22 | −.00723 (0.0599) | .90 |
| Polio | ||||
| 15 wk | ||||
| ALS polio IgG, mIU/mLc | 0.0334 | .11 | −4.03e−7 (2.89e−7) | .16 |
| 2 y | ||||
| Plasma polio1 IgG, IU/mL | 0.0138 | .64 | −.0117 (0.168) | .95 |
| Plasma polio2 IgG, IU/mL | 0.00802 | .85 | −.0163 (0.153) | .92 |
| Plasma polio3 IgG, IU/mL | 0.0156 | .57 | .155 (0.154) | .32 |
| Plasma polio1 IgA, IU/mL | 0.0211 | .39 | .144 (0.197) | .47 |
| Plasma polio2 IgA, IU/mL | 0.0150 | .60 | .111 (0.183) | .55 |
| Plasma polio3 IgA, IU/mLd | 0.0349 | .12 | .307 (0.212) | .15 |
| Stool polio1 IgA, IU/g protein | 0.0352 | .21 | .842 (0.326) | .011 |
| Stool polio2 IgA, IU/g protein | 0.0303 | .31 | .752 (0.311) | .016 |
| Stool polio3 IgA, IU/g protein | 0.0394 | .16 | .974 (0.356) | <.01 |
| Plasma polio IgG AI | 0.0317 | .17 | .123 (0.0595) | .039 |
| Plasma polio IgA AI | 0.00162 | .9955 | .0167 (0.0522) | .75 |
| SEB | ||||
| 6 wk | ||||
| CD4+ T-cell SIa,b | 0.112 | <.01 | .253 (0.163) | .12 |
| 15 wk | ||||
| CD4+ T-cell SIc | 0.0629 | .037 | .523 (0.230) | .024 |
| 2 y | ||||
| CD4+ T-cell SId | 0.0646 | .046 | .397 (0.231) | .088 |
Association between infant’s Bifibacterium abundance and 6 wk vaccine response was analyzed by using genus Bifibacterium abundance at 6 wk of age. Association between infant’s Bifibacterium and 15 wk and 2 y analysis were done on mean genus Bifibacterium abundance at 6 wk, 11 wk, and 15 wk of age. The regression models were adjusted with sex, treatment group, birth weight, and type of delivery. Stool antibody models were additionally adjusted for breastfeed status at 2 y of age.
Vaccine response was significantly different between boys and girls.
Vaccine response was significantly different between treatment groups.
Vaccine response was significantly different between normal and cesarean delivery.
Vaccine response was significantly different between below and above birth weight median.
FIGURE 1.
Heat map showing associations (and statistical significance) between early life bifidobacteria abundance and BCG vaccine responses measured at 6 weeks, 15 weeks and 2 years of age determined with multiple regression analysis as described in Methods. BCG vaccine responses include the CD4 T-cell SI and the DTH skin test response. Associations with the SI for the positive control for CD4 T-cell stimulation (SEB) are also shown. Bifidobacteria abundance measures were as follows: (1) mean abundance of the genus Bifidobacterium measured at 6, 11, and 15 weeks and single measures made at 6 weeks for (2) the genus Bifidobacterium; (3) the most abundant species, B longum; (4) the most abundant of 2 subspecies, B longum subspecies infantis; (5) the second subspecies, B longum subspecies longum; and 2 minor species, (6) B breve and (7) B bifidum.
TABLE 3.
Predicted Vaccine Response at Low (10th Percentile) and High (90th Percentile) Mean Bifidobacterium Abundance at 6, 11 and 15 Weeks
| Vaccine Response | 10th Percentilea | 90th Percentilea | Difference, % |
|---|---|---|---|
| BCG SI at 15 wk | 8.63 | 16.0 | 85 |
| TT SI at 15 wk | 7.15 | 12.60 | 76 |
| HBV SI 15 wk | 3.14 | 4.55 | 45 |
| BCG SI at 2 y | 10.0 | 16.4 | 64 |
| TT SI at 2 y | 4.60 | 7.23 | 57 |
| TT plasma IgG 2 y, IU/mL | 33.2 | 47.0 | 42 |
| Stool OPV IgA at 2 y (strain 2, mIU/g protein) | 0.0513 | 0.1060 | 107 |
Predicted from regression models described in Methods, holding covariates constant, and by using mean Bifidobacterium abundance at the 10th (abundance = 0.396) and 90th (abundance = 0.814) percentiles.
In addition to Bifidobacterium abundance at the genus level, Bifidobacterium species and subspecies were measured at 6 weeks, and we examined these associations as well, primarily to determine if the predominant species (B longum) and subspecies (B longum subspecies infantis) were also associated with these vaccine responses. In brief, we found that both B longum (Supplemental Table 7) and B longum subspecies infantis (Supplemental Table 8) were significantly associated with the SI response at 15 weeks but not 2 years (Fig 1). We also examined associations for the less abundant Bifidobacterium subspecies (B longum subspecies longum Supplemental Table 9) and 2 minor Bifidobacterium species (B breve and B bifidum; Supplemental Tables 10 and 11, respectively), and no significant associations were seen (Fig 1).
Because bifidobacteria might affect T-cell proliferation in general (which could affect development of vaccine memory responses), we examined the association of bifidobacteria with the SI for Staphylococcus enterotoxin B (SEB), a polyclonal stimulator of T-cell proliferation. As was seen for the BCG SI results, Bifidobacterium, B longum, and B longum subspecies infantis abundance were all significantly associated with the SI for SEB at 15 weeks (Fig 1).
Bifidobacteria Abundance Was Positively Associated With Responses to TT and HBV Vaccines
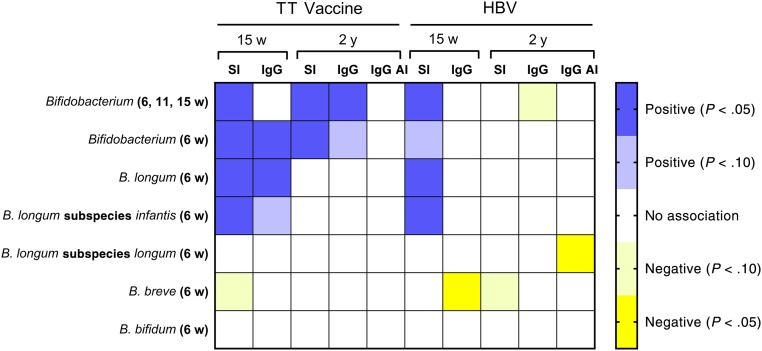
TT and HBV vaccines were administrated at 6, 10, and 14 weeks, within the period when bifidobacteria abundance was assessed, and we thus hypothesized that bifidobacterial abundance would be positively associated with the SI and IgG responses to both vaccines when they were measured at 15 weeks and 2 years. In partial confirmation of this hypothesis, Bifidobacterium, B longum, and B longum subspecies infantis abundance were all positively associated with the SI responses to both TT and HBV when they were measured at 15 weeks and with the TT SI response at 2 years, but they were not positively associated with the HBV response at 2 years (Fig 2). At 15 weeks, the predicted SI values for infants with high Bifidobacterium abundance were 76% and 45% higher than for those with low abundance for TT and HBV responses, respectively, whereas the corresponding difference at 2 years for TT was 57% (Table 3).
FIGURE 2.
Heat map showing associations (and statistical significance) between early life bifidobacteria abundance and TT and HBV responses measured at 15 weeks and 2 years of age determined with multiple regression analysis as described in Methods. Responses include the CD4 T-cell SI, the ALS assay for IgG at 15 weeks, plasma IgG at 2 years, and the IgG AI at 2 years. Bifidobacteria abundance measures were as follows: (1) mean abundance of the genus Bifidobacterium measured at 6, 11, and 15 weeks and single measures made at 6 weeks for (2) the genus Bifidobacterium; (3) the most abundant species, B longum; (4) the most abundant of 2 subspecies, B longum subspecies infantis; (5) the second subspecies, B longum subspecies longum; and 2 minor species, (6) B breve and (7) B bifidum.
Bifidobacterium abundance at the genus level was positively associated with the IgG responses to the TT vaccine when measured both at 15 weeks and at 2 years, whereas a similar association was seen at 15 weeks for B longum abundance (Fig 2). The predicted TT-specific IgG concentration at 2 years was 42% higher in infants with high versus low Bifidobacterium abundance (Table 3). Bifidobacterium at the genus level was not associated with the HBV IgG response at either 15 weeks or 2 years, although HBV-specific IgG at 15 weeks and plasma IgG maturation index at 2 years were negatively associated with B breve and B longum subspecies longum, respectively (Fig 2). All infants had protective IgG titres for both TT (≥0.1 IU/mL) and HBV (≥10 mIU/mL) at 2 years.
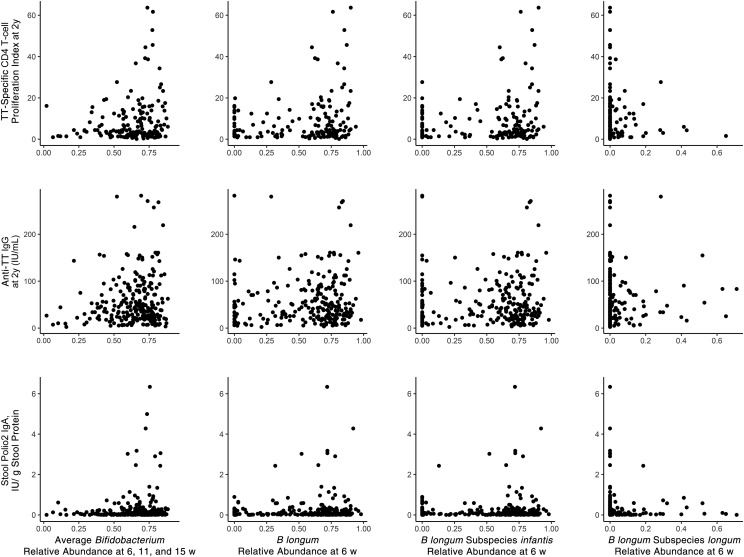
The generally positive associations of Bifidobacterium, B longum, and B longum subspecies infantis abundance with TT vaccine responses found in regression analysis can be seen graphically (without adjustment for covariates) in scatterplots of the raw data at 15 weeks (Supplemental Fig 5) and 2 years (Fig 3).
FIGURE 3.
Association of stool bifidobacteria in early infancy, at 6 to 15 weeks of age, with vaccine responses at 2 years of age. Top row, TT-specific CD4 T-cell SI (top row); middle row, TT-specific plasma IgG; bottom row, stool polio type 2–specific IgA. The asterisk (*) indicates a single, off-scale value (>7.5).
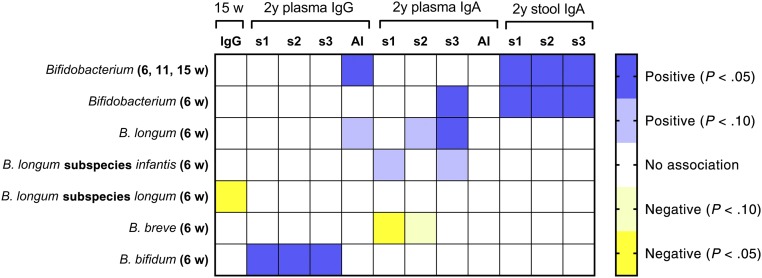
Bifidobacteria Abundance Was Positively Associated With IgG and IgA Responses to OPV Vaccine
OPV vaccine was administered within 48 hours of birth and again at 6, 10, and 14 weeks. We hypothesized that bifidobacterial abundance would be positively associated with OPV vaccine responses, which included the total IgG response at 15 weeks and strain-specific responses (3 strains are found in the OPV vaccine) at 2 years (plasma IgG and IgA, and stool IgA). In partial agreement with our hypothesis, the abundance of Bifidobacterium was positively associated with polio-specific stool IgA at 2 years (Fig 4), with the predicted response being 107% higher in infants with high versus low Bifidobacterium abundance (Table 3). This association can also be seen graphically with unadjusted data (Fig 3). Plasma IgA at 2 years also revealed some positive bifidobacterial associations (Fig 4), including positive associations of both Bifidobacterium and of B longum with the poliovirus strain 3 IgA response, whereas a negative association was seen between B breve and the strain 1 response (Fig 4). B bifidum abundance was positively associated with plasma IgG concentration at 2 years, whereas mean Bifidobacterium abundance was positively associated with the IgG AI (Fig 4). Two negative associations were also seen: B longum subspecies longum with 15 weeks IgG and B breve with 2 years plasma polio-specific IgA.
FIGURE 4.
Heat map showing associations (and statistical significance) between early life bifidobacteria abundance and OPV vaccine responses measured at 15 weeks and 2 years of age determined with multiple regression analyses as described in Methods. Responses include the CD4 T-cell SI, the ALS assay for IgG at 15 weeks, plasma IgG and IgA at 2 years for the 3 strains of virus included in the vaccine, the IgG AI and IgA AI for all 3 strains at 2 years. Bifidobacteria abundance measures were as follows: (1) mean abundance of the genus Bifidobacterium measured at 6, 11, and 15 weeks and single measures made at 6 weeks for (2) the genus Bifidobacterium; (3) the most abundant species, B longum; (4) the most abundant of 2 subspecies, B longum subspecies infantis; (5) the second subspecies, B longum subspecies longum; and 2 minor species, (6) B breve and (7) B bifidum. s1, strain 1; s2, strain 2; s3, strain 3.
Discussion
Higher bifidobacteria abundance in early infancy is associated with better memory responses to vaccines given at this time, as judged by the magnitude of the vaccine responses measured at 2 years. Mean Bifidobacterium abundance measured at 6, 11, and 15 weeks, and Bifidobacterium abundance measured at 6 weeks only, performed similarly in predicting later vaccine responses. These findings are novel and support current thinking about how gut microbiota may shape development of the infant immune system.7,32 For example, higher Th1 responses are associated with bifidobacterial abundance33 and a microbial state with low Bifidobacterium, Akkermansia, and Faecalibacterium in early infancy is associated with atopic CD4 T-cell responses at 2 years and asthma development at 4 years.34 In addition, vaccinia virus–specific interferon-γ production by CD8 T cells,35 and human serum albumin–specific and cholera toxin–specific interferon-γ and interleukin-5 production by splenocytes36 postvaccination are both influenced by gut microbial composition. These studies also suggest a cause-effect relationship between gut microbiota and vaccine responses. Our results suggest a general effect of bifidobacteria on T-cell proliferation or survival, as indicated by the association of bifidobacteria levels with both vaccine-specific and SEB-stimulated T-cell proliferation, whereas the lack of an association with SEB-stimulated proliferation at 2 years suggests an independent association with maintenance of vaccine memory.
Gut bacteria affect development of T cells, particularly Treg and Th17 cells.12,37 Bifidobacterial effects on T-cells could be direct or might involve effects on dendritic cells which then affect T cells, and these effects may be mediated by production of small-molecule bacterial metabolites including short chain fatty acids.38–40 Bacterial macromolecules also affect immunity. Both lipopolysaccharide41,42 and flagellin43 act as vaccine adjuvants, presumably via toll-like receptor 4 and toll-like receptor 5, respectively, suggesting that commensal bacteria may act as natural vaccine adjuvants.44 Indirect mechanisms may also be relevant. For example, Bifidobacterium protects against enteropathogenic infection45 and reduces the relative abundance of Enterobacteriaceae46 and thus may improve vaccine responses by reducing the risk of symptomatic infections or subclinical dysbiosis.
Many studies report associations of gut microbiota with vaccine-specific antibody responses.7 In the current study, we report positive associations of early life bifidobacteria with TT-specific IgG responses both in early infancy and at 2 years, and with polio-specific IgA at 2 years, suggesting a sustained effect on vaccine memory. A study examining rotavirus vaccine response among infants in Ghana47 found no associations with Actinobacteria (the phylum containing bifidobacteria), but reported that serum IgA was negatively associated with Bacteroidetes and positively associated with Streptococcus bovis. The same group performed a similar study in Pakistani infants and found no association with Bifidobacterium.48 The infants in the current study did not receive rotavirus vaccine. A previous study found that abundance of B breve and B longum49 were both positively correlated with polio-specific stool IgA measured shortly after vaccination. In the current study, we also report a nonsignificant (P = .088) positive association between Bifidobacterium and HBV-specific IgG at 2 years. A previous human study50 using B longum and Lactobacillus rhamnosus supplementation in infants also showed a trend toward an increased HBV-specific IgG response. In another study, the abundance of Bacteroides ovatus and Streptococcus geniculate in nasal microbiota were positively and negatively associated with IgA responses, respectively, to intranasal influenza vaccination.51 We also report that the B breve was negatively associated with polio-specific plasma IgA, a finding that is consistent with another study52 revealing that B breve supplementation lowers the serum IgA response to cholera vaccination. In our study, we also found a negative association between B longum subspecies longum and polio-specific ALS IgG at 15 weeks. Such results emphasize the need to identify the mechanisms by which specific bifidobacteria affect immunity.
Strengths of our study include the examination of short- and long-term memory responses, the relatively large sample size, the use of specific assays for bifidobacteria at the species and subspecies level, the use of multiple vaccines and of multiple vaccine end points, and the prospective study design. Our study is limited in that it is observational, thus we cannot infer causality from the associations described here, and participants were all breastfed, limiting the diversity in intestinal microbiota seen in study infants. Additionally, although BCG and the OPV are not used in developed countries, the similarity in associations seen in this study to that seen in a small study of inactivated polio virus,49 as well as the associations to the more widely administered HBV and TT vaccines, suggest that these results are relevant for many populations of infants around the world.
Conclusions
Colonization with Bifidobacterium at the time of vaccination is associated with sustainable systemic and mucosal vaccine-specific memory T-cell and antibody responses. Developing strategies to enhance immunologic memory is a high priority for vaccine research.53 A recent intervention trial demonstrated that administration of probiotic B longum subspecies infantis to healthy infants between 7 and 28 days significantly increased the abundance of this organism through at least 60 days,46 demonstrating prolonged colonization with this organism in breastfed infants. An adequately powered randomized controlled trial of a similar strategy to increase early colonization with B longum subspecies infantis to enhance responses to early vaccination is indicated by our findings.
Acknowledgments
We acknowledge all the study participants. The parent study was supported by the World Health Organization via grants from the Bill and Melinda Gates Foundation grant, the Thrasher Research Fund (CBS), and National Institutes of Health grants F32HD093185 (DHT) and R01AT008759 (DAM). We also acknowledge Janet M Peerson, Senior Statistician, Western Human Nutrition Research Center for her kind guiding on statistical analysis. We also give thanks to all the research staff, data entry staff, hospital nurses, and physicians at the collaborating clinic Maternal and Child Health Training Institute and International Centre for Diarrhoeal Disease Research, Bangladesh.
Glossary
- AI
avidity index
- ALS
antibody in lymphocyte supernatant
- BCG
Bacillus Calmette-Guérin
- CRP
C-reactive protein
- DTH
delayed-type hypersensitivity
- ELISA
enzyme-linked immunosorbent assay
- HBV
hepatitis B virus
- IgA
immunoglobulin A
- IgG
immunoglobulin G
- OPV
oral polio virus
- SEB
Staphylococcus enterotoxin B
- SI
stimulation index
- TT
tetanus toxoid
Footnotes
Dr Stephensen was principal investigator of the parent study, helped develop the study protocol, and mentored Dr Huda to perform laboratory analysis of the immunological assays, statistical analysis and manuscript writing; Dr Mills was a coinvestigator of this study and co-mentored Dr Huda to perform microbiota assay and bioinformatics analysis; Drs Underwood and Raqib were coinvestigators of this study and contributed to the development of the study protocol; Dr Ahmad was a coinvestigator of the parent study and directed research activities at the clinical site and in the laboratory in Bangladesh, contributed to the development of the study protocol, oversaw the overall study operations including providing feedback to the ethical committee, Data and Safety Monitoring Board, and report writing, and mentored Dr Huda to perform laboratory analysis; Dr Huda oversaw participant enrollment and follow-up, data collection, and performed laboratory analysis and performed statistical analysis and drafted the manuscript under the mentoring and supervision of Drs Stephensen and Mills. Mr Alam, Ms Khanam, Dr Kalanetra, and Dr Taft all participated in the data collection, laboratory analysis, and editing the manuscript; and all authors reviewed and approved the final manuscript as submitted.
This trial has been registered at www.clinicaltrials.org (identifiers NCT01583972 and NCT02027610).
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Funded by World Health Organization Project 2010168947, which was funded by the Bill and Melinda Gates Foundation, Thrasher Research Fund grant 11488 (Dr Stephensen), US Department of Agriculture-Agriculture Research Service project 2032-53000-001-00-D (Dr Stephensen), and National Institutes of Health grants F32HD093185 (Dr Taft) and R01AT008759 (Dr Mills). REDCap database usage was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant UL1TR001860. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: Dr Underwood’s institution (University of California, Davis) has received grant support from Evolve Biosystems to fund a clinical trial of a probiotic in term infants; he has consulted for Avexegen and received payment for travel and lectures from Abbott. Dr Mills is a cofounder of and consultant for Evolve Biosystems and has stock and stock options therein; he has received payment for lectures from Nestle and Abbott; the other authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Ehreth J. The global value of vaccination. Vaccine. 2003;21(7-8):596–600 [DOI] [PubMed] [Google Scholar]
- 2.Igietseme JU, Eko FO, He Q, Black CM. Antibody regulation of Tcell immunity: implications for vaccine strategies against intracellular pathogens. Expert Rev Vaccines. 2004;3(1):23–34 [DOI] [PubMed] [Google Scholar]
- 3.Siegrist C-A. Vaccine immunology In: Plotkin SA, Orenstein WA, Offit PA, eds. Vaccines. 6th ed. Philadelphia, PA: Saunders; 2013:14–32 [Google Scholar]
- 4.Querec TD, Akondy RS, Lee EK, et al. . Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nat Immunol. 2009;10(1):116–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakaya HI, Hagan T, Duraisingham SS, et al. . Systems analysis of immunity to influenza vaccination across multiple years and in diverse populations reveals shared molecular signatures. Immunity. 2015;43(6):1186–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finan C, Ota MO, Marchant A, Newport MJ. Natural variation in immune responses to neonatal Mycobacterium bovis Bacillus Calmette-Guerin (BCG) vaccination in a cohort of Gambian infants. PLoS One. 2008;3(10):e3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nguyen QN, Himes JE, Martinez DR, Permar SR. The impact of the gut microbiota on humoral immunity to pathogens and vaccination in early infancy. PLoS Pathog. 2016;12(12):e1005997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung H, Pamp SJ, Hill JA, et al. . Gut immune maturation depends on colonization with a host-specific microbiota. Cell. 2012;149(7):1578–1593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kamada N, Seo SU, Chen GY, Núñez G. Role of the gut microbiota in immunity and inflammatory disease. Nat Rev Immunol. 2013;13(5):321–335 [DOI] [PubMed] [Google Scholar]
- 10.Maynard CL, Elson CO, Hatton RD, Weaver CT. Reciprocal interactions of the intestinal microbiota and immune system. Nature. 2012;489(7415):231–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith K, McCoy KD, Macpherson AJ. Use of axenic animals in studying the adaptation of mammals to their commensal intestinal microbiota. Semin Immunol. 2007;19(2):59–69 [DOI] [PubMed] [Google Scholar]
- 12.Ivanov II, Atarashi K, Manel N, et al. . Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139(3):485–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Atarashi K, Tanoue T, Shima T, et al. . Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331(6015):337–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Savino W, Dardenne M, Velloso LA, Dayse Silva-Barbosa S. The thymus is a common target in malnutrition and infection. Br J Nutr. 2007;98(suppl 1):S11–S16 [DOI] [PubMed] [Google Scholar]
- 15.Rivière A, Selak M, Lantin D, Leroy F, De Vuyst L. Bifidobacteria and butyrate-producing colon bacteria: importance and strategies for their stimulation in the human gut. Front Microbiol. 2016;7:979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frei R, Lauener RP, Crameri R, O’Mahony L. Microbiota and dietary interactions: an update to the hygiene hypothesis? Allergy. 2012;67(4):451–461 [DOI] [PubMed] [Google Scholar]
- 17.Kuitunen M. Probiotics and prebiotics in preventing food allergy and eczema. Curr Opin Allergy Clin Immunol. 2013;13(3):280–286 [DOI] [PubMed] [Google Scholar]
- 18.Grönlund MM, Arvilommi H, Kero P, Lehtonen OP, Isolauri E. Importance of intestinal colonisation in the maturation of humoral immunity in early infancy: a prospective follow up study of healthy infants aged 0-6 months. Arch Dis Child Fetal Neonatal Ed. 2000;83(3):F186–F192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lundell AC, Björnsson V, Ljung A, et al. . Infant B cell memory differentiation and early gut bacterial colonization. J Immunol. 2012;188(9):4315–4322 [DOI] [PubMed] [Google Scholar]
- 20.Sjögren YM, Tomicic S, Lundberg A, et al. . Influence of early gut microbiota on the maturation of childhood mucosal and systemic immune responses. Clin Exp Allergy. 2009;39(12):1842–1851 [DOI] [PubMed] [Google Scholar]
- 21.Davis EC, Wang M, Donovan SM. The role of early life nutrition in the establishment of gastrointestinal microbial composition and function. Gut Microbes. 2017;8(2):143–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Underwood MA, German JB, Lebrilla CB, Mills DA. Bifidobacterium longum subspecies infantis: champion colonizer of the infant gut. Pediatr Res. 2015;77(1–2):229–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lewis ZT, Mills DA. Differential establishment of bifidobacteria in the breastfed infant gut. Nestle Nutr Inst Workshop Ser. 2017;88:149–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huda MN, Lewis Z, Kalanetra KM, et al. . Stool microbiota and vaccine responses of infants. Pediatrics. 2014;134(2). Available at: www.pediatrics.org/cgi/content/full/134/2/e362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henrick BM, Hutton AA, Palumbo MC, et al. . Elevated fecal pH indicates a profound change in the breastfed infant gut microbiome due to reduction of Bifidobacterium over the past century. MSphere. 2018;3(2):e00041-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahmad SM, Raqib R, Qadri F, Stephensen CB. The effect of newborn vitamin A supplementation on infant immune functions: trial design, interventions, and baseline data. Contemp Clin Trials. 2014;39(2):269–279 [DOI] [PubMed] [Google Scholar]
- 27.Huda MN, Ahmad SM, Alam MJ, et al. . Infant cortisol stress-response is associated with thymic function and vaccine response. Stress. 2018;1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qadri F, Jonson G, Begum YA, et al. . Immune response to the mannose-sensitive hemagglutinin in patients with cholera due to Vibrio cholerae O1 and O0139. Clin Diagn Lab Immunol. 1997;4(4):429–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lewis ZT, Bokulich NA, Kalanetra KM, Ruiz-Moyano S, Underwood MA, Mills DA. Use of bifidobacterial specific terminal restriction fragment length polymorphisms to complement next generation sequence profiling of infant gut communities. Anaerobe. 2013;19:62–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lewis ZT, Shani G, Masarweh CF, et al. . Validating bifidobacterial species and subspecies identity in commercial probiotic products. Pediatr Res. 2016;79(3):445–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.R Core Team A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2017 [Google Scholar]
- 32.Gensollen T, Iyer SS, Kasper DL, Blumberg RS. How colonization by microbiota in early life shapes the immune system. Science. 2016;352(6285):539–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu BB, Yang Y, Xu X, Wang WP. Effects of Bifidobacterium supplementation on intestinal microbiota composition and the immune response in healthy infants. World J Pediatr. 2016;12(2):177–182 [DOI] [PubMed] [Google Scholar]
- 34.Fujimura KE, Sitarik AR, Havstad S, et al. . Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat Med. 2016;22(10):1187–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gonzalez-Perez G, Hicks AL, Tekieli TM, Radens CM, Williams BL, Lamousé-Smith ES. Maternal antibiotic treatment impacts development of the neonatal intestinal microbiome and antiviral immunity. J Immunol. 2016;196(9):3768–3779 [DOI] [PubMed] [Google Scholar]
- 36.Kim D, Kim Y-G, Seo S-U, et al. . Nod2-mediated recognition of the microbiota is critical for mucosal adjuvant activity of cholera toxin [published correction appears in Nat Med. 2016;22(8):961]. Nat Med. 2016;22(5):524–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arpaia N, Campbell C, Fan X, et al. . Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504(7480):451–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iwabuchi N, Takahashi N, Xiao JZ, Miyaji K, Iwatsuki K. In vitro Th1 cytokine-independent Th2 suppressive effects of bifidobacteria. Microbiol Immunol. 2007;51(7):649–660 [DOI] [PubMed] [Google Scholar]
- 39.Honda K, Littman DR. The microbiota in adaptive immune homeostasis and disease. Nature. 2016;535(7610):75–84 [DOI] [PubMed] [Google Scholar]
- 40.Chen X, Su W, Wan T, et al. . Sodium butyrate regulates Th17/Treg cell balance to ameliorate uveitis via the Nrf2/HO-1 pathway. Biochem Pharmacol. 2017;142:111–119 [DOI] [PubMed] [Google Scholar]
- 41.Pulendran B, Kumar P, Cutler CW, Mohamadzadeh M, Van Dyke T, Banchereau J. Lipopolysaccharides from distinct pathogens induce different classes of immune responses in vivo. J Immunol. 2001;167(9):5067–5076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zeng MY, Cisalpino D, Varadarajan S, et al. . Gut microbiota-induced immunoglobulin G controls systemic infection by symbiotic bacteria and pathogens. Immunity. 2016;44(3):647–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oh JZ, Ravindran R, Chassaing B, et al. . TLR5-mediated sensing of gut microbiota is necessary for antibody responses to seasonal influenza vaccination. Immunity. 2014;41(3):478–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pabst O, Hornef M. Gut microbiota: a natural adjuvant for vaccination. Immunity. 2014;41(3):349–351 [DOI] [PubMed] [Google Scholar]
- 45.Fukuda S, Toh H, Hase K, et al. . Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature. 2011;469(7331):543–547 [DOI] [PubMed] [Google Scholar]
- 46.Frese SA, Hutton AA, Contreras LN, et al. . Persistence of supplemented Bifidobacterium longum subsp. infantis EVC001 in breastfed infants. MSphere. 2017;2(6):e00501-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harris VC, Armah G, Fuentes S, et al. . Significant correlation between the infant gut microbiome and rotavirus vaccine response in rural Ghana. J Infect Dis. 2017;215(1):34–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harris V, Ali A, Fuentes S, et al. . Rotavirus vaccine response correlates with the infant gut microbiota composition in Pakistan. Gut Microbes. 2018;9(2):93–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mullié C, Yazourh A, Thibault H, et al. . Increased poliovirus-specific intestinal antibody response coincides with promotion of Bifidobacterium longum-infantis and Bifidobacterium breve in infants: a randomized, double-blind, placebo-controlled trial. Pediatr Res. 2004;56(5):791–795 [DOI] [PubMed] [Google Scholar]
- 50.Soh SE, Ong DQ, Gerez I, et al. . Effect of probiotic supplementation in the first 6 months of life on specific antibody responses to infant Hepatitis B vaccination. Vaccine. 2010;28(14):2577–2579 [DOI] [PubMed] [Google Scholar]
- 51.Salk HM, Simon WL, Lambert ND, et al. . Taxa of the nasal microbiome are associated with influenza-specific IgA response to live attenuated influenza vaccine. PLoS One. 2016;11(9):e0162803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Matsuda F, Chowdhury MI, Saha A, et al. . Evaluation of a probiotics, Bifidobacterium breve BBG-01, for enhancement of immunogenicity of an oral inactivated cholera vaccine and safety: a randomized, double-blind, placebo-controlled trial in Bangladeshi children under 5 years of age [published correction appears in Vaccine. 2011;29(35)6068]. Vaccine. 2011;29(10):1855–1858 [DOI] [PubMed] [Google Scholar]
- 53.Sheerin D, Openshaw PJ, Pollard AJ. Issues in vaccinology: present challenges and future directions. Eur J Immunol. 2017;47(12):2017–2025 [DOI] [PMC free article] [PubMed] [Google Scholar]