In a combined analysis from 5 studies, LAIV4 was less effective than IIV against influenza A/H1N1pdm09 in all pediatric age groups.
Abstract
BACKGROUND:
Researchers in observational studies of vaccine effectiveness (VE) in which they compared quadrivalent live attenuated vaccine (LAIV4) and inactivated influenza vaccine (IIV) among children and adolescents have shown inconsistent results, and the studies have been limited by small samples.
METHODS:
We combined data from 5 US studies from 2013–2014 through 2015–2016 to compare the VE of LAIV4 and IIV against medically attended, laboratory-confirmed influenza among patients aged 2 to 17 years by influenza season, subtype, age group, and prior vaccination status. The VE of IIV or LAIV4 was calculated as 100% × (1 − odds ratio), comparing the odds of vaccination among patients who were influenza-positive to patients who were influenza-negative from adjusted logistic regression models. Relative effectiveness was defined as the odds of influenza comparingLAIV4 and IIV recipients.
RESULTS:
Of 17 173 patients aged 2 to 17 years, 4579 received IIV, 1979 received LAIV4, and 10 615 were unvaccinated. Against influenza A/H1N1pdm09, VE was 67% (95% confidence interval [CI]: 62% to 72%) for IIV and 20% (95% CI: −6% to 39%) for LAIV4. Results were similar when stratified by vaccination in the previous season. LAIV4 recipients had significantly higher odds of influenza A/H1N1pdm09 compared with IIV recipients (odds ratio 2.66; 95% CI: 2.06 to 3.44). LAIV4 and IIV had similar effectiveness against influenza A/H3N2 and B. Our overall findings were consistent when stratified by influenza season and age group.
CONCLUSIONS:
From this pooled individual patient–level data analysis, we found reduced effectiveness of LAIV4 against influenza A/H1N1pdm09 compared with IIV, which is consistent with published results from the individual studies included.
What’s Known on This Subject:
Researchers in individual studies in the United States have reported low effectiveness of quadrivalent live attenuated vaccine (LAIV4) against influenza A/H1N1pdm09 relative to inactivated influenza vaccine (IIV). Estimations by age and prior vaccination status have been limited by small sample sizes.
What This Study Adds:
In a combined analysis from 5 studies, LAIV4 was less effective than IIV against influenza A/H1N1pdm09 in all pediatric age groups. Differences in prior vaccination did not explain this finding. LAIV4 and IIV had similar effectiveness against A/H3N2 and B.
Concerns about the effectiveness of the live attenuated influenza vaccine (LAIV) among young children in the 2013–2014 and 2015–2016 influenza seasons prompted the US Advisory Committee on Immunization Practices (ACIP) and the American Academy of Pediatrics to recommend against the use of LAIV in the United States during the 2016–2017 and 2017–2018 influenza seasons.1,2 Trivalent live attenuated influenza vaccine (LAIV3) was first licensed for use in the United States in 2003; quadrivalent live attenuated vaccine (LAIV4) followed in 2013. In clinical trials conducted before the 2009 influenza pandemic, researchers demonstrated immune responses and efficacy with all LAIV3 components and, in some studies, superiority to trivalent inactivated influenza vaccines (IIVs) among children and adolescents but not among adults.3–6 Postlicensure estimates of vaccine effectiveness (VE) of LAIV4 have been obtained from observational studies and may be subject to biases inherent in observational designs. Studies conducted in the United States have heterogeneous findings with LAIV VE estimates against influenza A/H1N1pdm09 ranging from −19% to 50%, although in no study were researchers able to detect statistically significant protection against influenza A/H1N1pdm09 since the 2013–2014 influenza season.7–14 In addition, differences between LAIV4 VE determined in United States versus European studies have raised questions about whether prior season vaccination (more common in US studies) may have contributed to the lower VE detected in the United States.15
Interpreting differences in VE estimates from individual studies is challenging because of small samples when patients are stratified by vaccine type, age group, and/or influenza subtype. In few studies of VE by vaccine type have researchers examined the effect of prior season vaccination status on current season VE. In this study, we analyzed pooled individual patient-level data from 5 studies that included children and adolescents aged 2 to 17 years in the United States during the 2013–2014 through 2015–2016 influenza seasons, during which all LAIV in the United States were quadrivalent.16 Our primary aims were to describe the VE of IIV (trivalent or quadrivalent) and LAIV4 in each season by influenza subtype for children aged 2 to 4, 5 to 8, and 9 to 17 years and to examine the association of prior season vaccination on current season VE.
Methods
We included data from the following 5 studies conducted in outpatient settings in the United States from 2013–2014 through 2015–2016: the US Influenza Vaccine Effectiveness Network (USFLUVE, Centers for Disease Control and Prevention [CDC])7,10,11; a Louisiana State University Health Sciences Center (LSU) study13; the Influenza Clinical Investigation for Children (ICICLE, MedImmune)8,9,12; the Department of Defense Global, Laboratory-based, Influenza Surveillance Program (US Air Force School of Aerospace Medicine [USAFSAM])14; and the Influenza Incidence Surveillance Project (IISP, CDC).17 Study characteristics are compared in Table 1. In the USFLUVE, ICICLE, USAFSAM, and IISP studies, patients aged 2 to 17 years presenting to outpatient settings (including emergency departments) with acute respiratory infection with fever and/or cough were enrolled, and respiratory specimens were tested with real-time reverse transcription-polymerase chain reaction (RT-PCR) or viral culture. In the LSU study, patients who were clinically tested for influenza were enrolled; patients were first tested by a rapid antigen test and a subsequent polymerase chain reaction–based respiratory viral panel for patients who were rapid antigen test negative. Current season vaccination status was ascertained by using electronic immunization records (EIRs) including medical records and immunization information systems; in the USAFSAM study, vaccination status including vaccine type was also determined by parental or guardian report of the child’s vaccination. Prior season vaccination was ascertained from EIRs for all studies.
TABLE 1.
Characteristics of Included Studies
| Study | No. Patients Included | Study Inclusion | Testing | Current Season Vaccination Status Data Source(s) | Previous Season Vaccination Status Data Source(s) |
|---|---|---|---|---|---|
| USFLUVE, CDC | 6793 | ARI with cough ≤7 d duration | RT-PCR | Electronic medical record and immunization registries | Electronic medical record and immunization registries |
| LSU | 3822 | Clinical laboratory testing for influenza | Rapid antigen test and respiratory viral panel of rapid antigen test–negatives | Immunization registry | Immunization registry |
| ICICLE, MedImmune | 3521 | ARI with fever <5 d duration | RT-PCR | Electronic medical record and immunization registries | Electronic medical record and immunization registries |
| Department of Defense Global, Laboratory-based, Influenza Surveillance Program, USAFSAM | 1935 | ARI with fever and cough and/or sore throat <72 h duration | Viral culture and RT-PCR | Immunization registry and parent report | Immunization registry |
| IISP, CDC | 1102 | ARI with fever and cough and/or sore throat ≤7 d duration | RT-PCR | Electronic medical record and immunization registries | Immunization registry |
ARI, acute respiratory infection.
We included patients with conclusive testing results who were enrolled in the United States during the influenza season. Influenza seasons were defined as the period of consecutive weeks with regional or widespread influenza activity18 and the 3 weeks before and after that period for each state from which patients were enrolled. We excluded patients with unknown vaccination statuses or types, patients who received both IIV and LAIV4 within a season, patients who received a vaccine 0 to 13 days before illness onset, and patients who tested positive for ≥1 influenza type and/or subtype.
We used a test-negative, case-control design to calculate the VE of ≥1 dose of IIV or LAIV4 as 100% × (1 − odds ratio [OR]), comparing the odds of vaccination among case patients who were influenza-positive to controls who were influenza-negative. Relative effectiveness estimates are expressed as the odds of influenza among LAIV4 recipients compared with IIV recipients and 95% confidence intervals (CIs). Adjusted models included age group or age in years (for age-stratified models), the calendar time of illness onset (defined as prepeak, peak, or postpeak), and influenza season (for combined-season estimates). Peak period was defined as the week with the most case patients who were influenza-positive by season ±2 weeks. The study site was included as a random effect. VE estimates are not reported if the number of cases in a stratum was <50 or if the number of vaccinated cases was <5. Interaction terms were used to test for differences in VE by age group.
In a subgroup analysis, patients were stratified by prior season vaccination status (categorized as unvaccinated, received IIV, or received LAIV in the previous influenza season). In each stratum, current-season VE was calculated for each vaccine type by using patients who were unvaccinated in the current season as the reference group. Patients were excluded from this subgroup analysis if prior season vaccination records were unavailable, if prior season vaccine type was unknown, if both IIV and LAIV were received within the prior season, or if parent- or guardian-reported current-season vaccination could not be confirmed by EIRs (USAFSAM study only).
Several sensitivity analyses were conducted to examine factors incompletely collected across studies including illness severity (by using presentations with influenza-like illness [defined as fever plus cough and/or sore throat] and the time from illness onset to enrollment as proxies), high-risk status, and full versus partial vaccination status. Statistical analyses were conducted by using SAS software (SAS Institute, Inc, Cary, NC).
Results
Overall, 17 173 children and adolescents aged 2 to 17 years from 42 states were included in the analysis (Supplemental Table 3). Forty percent of the sample came from USFLUVE, 22% from LSU, 21% from ICICLE, 11% from USAFSAM, and 6% from IISP (Supplemental Table 4). Twenty-three percent of the sample enrolled in the 2013–2014 season, 47% in the 2014–2015 season, and 30% in the 2015–2016 season. The LSU study population was slightly younger (mean 6.4 years) than the average age of 7.4 years, and the IISP and USAFSAM study populations were slightly older (mean 8.0 years). Overall, one-fourth of patients (N = 4244) tested positive for influenza. Among them, 37% (N = 1582) were infected with influenza A/H3N2, 25% (N = 1082) influenza A/H1N1pdm09, 12% (N = 519) unsubtyped influenza A, and 25% (N = 1061) influenza B. Influenza B lineage was determined for 42% (N = 447) of influenza B–positive specimens; 234 (52%) and 213 (48%) were of Victoria and Yamagata lineage, respectively. One-third (38%, N = 6558) of patients were vaccinated in the current season, among whom 30% received LAIV4 (N = 1979). Among patients who tested negative for influenza, IIV recipients tended to be slightly younger with more high-risk conditions and asthma than LAIV4 recipients (Supplemental Table 5).
In 2013–2014, 19% of specimens tested positive for influenza. Among those, 65% were subtyped as influenza A/H1N1pdm09 virus, and 19% were unsubtyped influenza A (Supplemental Table 6). VE against any influenza among those aged 2 to 17 years was 63% (95% CI: 55% to 70%) for IIV and 15% (95% CI: −1% to 28%) for LAIV4 (Supplemental Table 7). The VE of IIV was similar by age group (P = .15). LAIV4 VE estimates increased by age group, but the difference was not statistically significant (P = .65). VE against influenza B among those aged 2 to 17 years was 56% (95% CI: 33% to 71%) for IIV and 64% (95% CI: 8% to 86%) for LAIV4, although few influenza B cases were detected in this season.
In the 2014–2015 season, 27% of specimens tested positive for influenza. A majority (68%) were influenza A/H3N2 with an additional 12% unsubtyped influenza A and 20% influenza B (Supplemental Table 6). VE against any influenza among those aged 2 to 17 years was 37% (95% CI: 28% to 44%) for IIV and 25% (95% CI: 10% to 37%) for LAIV4 (Supplemental Table 7). Against influenza A/H3N2, VE was significantly protective only among IIV recipients aged 2 to 4 years. None of the LAIV4 VE estimates against influenza A/H3N2 reached statistical significance. The VE point estimate against influenza B among those aged 2 to 17 years was 49% (95% CI: 32% to 62%) for IIV and 76% (95% CI: 53% to 88%) for LAIV4.
In the 2015–2016 season, 26% of specimens tested positive for influenza, with 44% influenza A/H1N1pdm09, 8% unsubtyped influenza A, and 43% influenza B (Supplemental Table 6). Few influenza A/H3N2 cases were detected (N = 69). VE against any influenza among those aged 2 to 17 years was 60% (95% CI: 52% to 67%) for IIV and 39% (95% CI: 16% to 56%) for LAIV4 (Supplemental Table 7). Estimates were similar when stratified by age group. Against influenza A/H1N1pdm09, VE among those aged 2 to 17 years was 66% (95% CI: 59% to 71%) for IIV and 18% (95% CI: −31% to 49%) for LAIV4. Estimates were similar across age groups; none of the LAIV4 VE estimates against influenza A/H1N1pdm09 reached statistical significance. VE against influenza B was similar for IIV and LAIV4; VE of IIV among those aged 2 to 17 years was 55% (95% CI: 39% to 66%), and it was 54% (95% CI: 35% to 68%) for LAIV4.
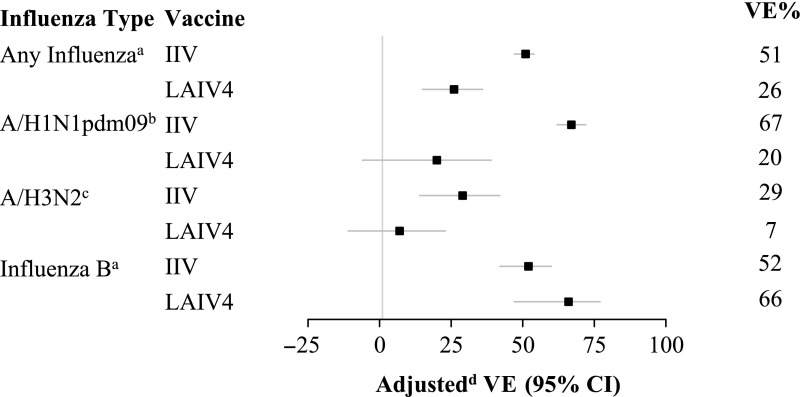
Combining data from all seasons, VE against any influenza among those aged 2 to 17 years was 51% (95% CI: 47% to 54%) for IIV and 26% (95% CI: 15% to 36%) for LAIV4 (Fig 1, Supplemental Table 8). VE estimates for LAIV4 increased by age group, but the difference was not statistically significant (P for interaction = .90). Against influenza A/H1N1pdm09, VE was 67% (95% CI: 62% to 72%) for IIV and 20% (95% CI: −6% to 39%) for LAIV4. VE estimates for LAIV4 increased by age group, but the difference was not statistically significant (P = .71). VE against influenza B viruses among those aged 2 to 17 years was 52% (95% CI: 42% to 60%) for IIV and 66% (95% CI: 47% to 77%) for LAIV4. There was little heterogeneity in VE estimates by age group.
FIGURE 1.
Adjusted VE by influenza type and subtype for children aged 2 to 17 years receiving IIV or LAIV4 during 2013 to 2014 through 2015 to 2016. Bars indicate 95% CIs. a Includes the 2013–2014 influenza season through the 2015–2016 season. b Restricted to the 2013–2014 and 2015–2016 influenza seasons. c Restricted to the 2014–2015 influenza season. d Models are adjusted for age (group or years for age-stratified models), season, calendar time, and site (as a random effect).
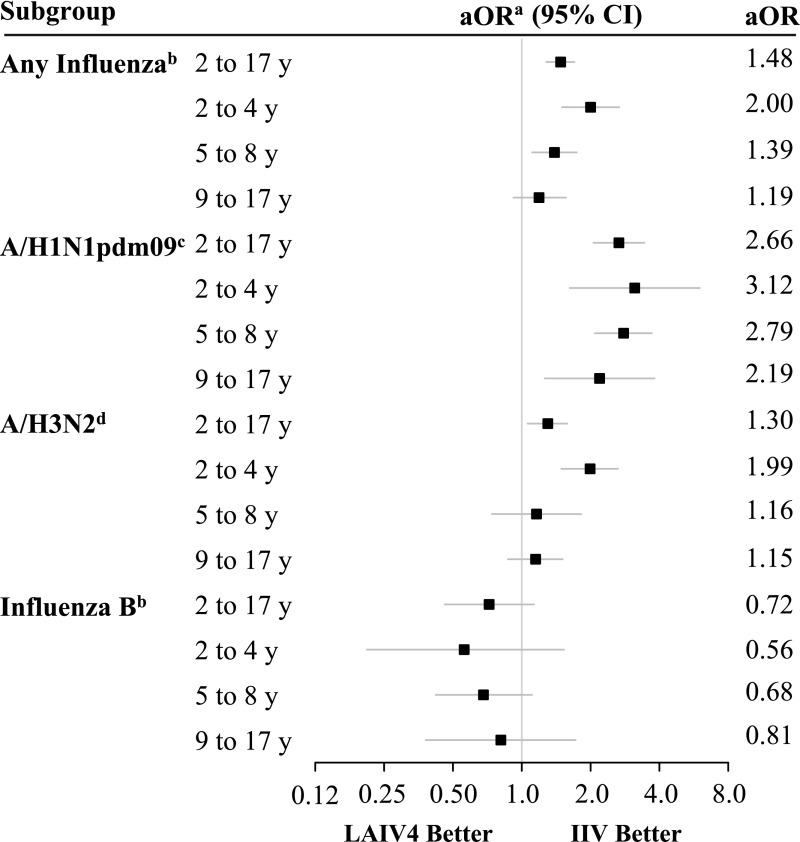
Overall, LAIV4 recipients were significantly more likely to have any influenza detected compared with IIV recipients (OR = 1.48, 95% CI: 1.28 to 1.70; Fig 2, Supplemental Table 9). A similar association was observed among those aged 2 to 4 and 5 to 8 years (Supplemental Table 9). There was no significant difference in the odds of influenza infection among those aged 9 to 17 years. LAIV4 recipients had significantly higher odds of influenza A/H1N1pdm09 infection compared with IIV recipients (OR = 2.66, 95% CI: 2.06 to 3.44), and this result was similar for all age groups. LAIV4 recipients aged 2 to 17 and 2 to 4 years had increased odds of influenza A/H3N2 infection compared with IIV recipients (OR = 1.30 [95% CI: 1.06 to 1.58] and OR = 1.99 [95% CI: 1.49 to 2.64], respectively). Estimates for the other age groups suggested higher odds of influenza A/H3N2 infection in LAIV4 recipients but were not statistically significant. The odds of influenza B infection were lower among LAIV4 recipients, but this finding was not statistically significant. No age-stratified relative effectiveness estimates reached statistical significance, but point estimates suggested higher odds of influenza B infection among IIV recipients.
FIGURE 2.
Adjusted relative effectiveness of LAIV4 versus IIV by influenza type, subtype, and age group. aOR, adjusted odds ratio. a Relative effectiveness estimates are expressed as the odds of influenza among LAIV4 recipients compared with IIV recipients and 95% CIs. An OR of <1 suggests lower odds of influenza in LAIV4 recipients compared with IIV recipients. Models are adjusted for age (group or years for age-stratified models), season, calendar time, and site (as a random effect). b Includes the 2013–2014 influenza season through the 2015–2016 season. c Restricted to the 2013–2014 and 2015–2016 influenza seasons. d Restricted to the 2014–2015 influenza season.
When stratified by prior season vaccination, VE estimates of LAIV4 against influenza A/H1N1pdm09 were similar with overlapping CIs that included 0 (Table 2). The current season VE of LAIV4 was 25% (95% CI: −9% to 49%) among patients who were unvaccinated in the prior season, −19% (95% CI: −126% to 38%) among patients who received IIV in the prior season, and 24% (95% CI: −16% to 50%) among patients who received LAIV in the prior season. The difference was not statistically significant (P for interaction = .21). The current season VE of IIV against influenza A/H1N1pdm09 was nonsignificantly (P = .05) reduced among those who received IIV in the prior season (46%, 95% CI: 15% to 66%) compared with patients who were unvaccinated in the prior season (77%, 95% CI: 64% to 85%) and patients who received LAIV in the prior season (73%, 95% CI: 55% to 84%). Against influenza A/H3N2, none of the current season LAIV4 VE estimates were statistically significant regardless of prior season vaccination. Among current season IIV recipients, VE against influenza A/H3N2 was similar among patients who were unvaccinated in the prior season (35%, 95% CI: 16% to 50%) and patients who received IIV in the prior season (36%, 95% CI: 10% to 54%), but VE was lower and not statistically significant among patients who received LAIV in the prior season (12%, 95% CI: −35% to 43%); however, significant interaction was not detected (P = .85). The number of case patients who were influenza-positive in each stratum of prior vaccination exposure was insufficient to examine this association for VE against influenza B (data not shown).
TABLE 2.
VE of Current Season Vaccine Against Influenza A Viruses Stratified by Vaccine Receipt in the Season Before Enrollment
| Vaccination | Total | No. Influenza-Positive (%)a | No. Influenza-Negative (%)a | Adjustedb VE, % | 95% CI |
|---|---|---|---|---|---|
| Influenza A/H1N1pdm09c | |||||
| Prior season unvaccinated | |||||
| Current season IIV | 567 | 28 (5) | 539 (95) | 77 | 64 to 85 |
| Current season LAIV | 213 | 32 (15) | 181 (85) | 25 | −9 to 49 |
| Current season unvaccinated | 3311 | 542 (16) | 2769 (84) | Ref | — |
| Prior season IIV | |||||
| Current season IIV | 1412 | 98 (7) | 1314 (93) | 46 | 15 to 66 |
| Current season LAIV | 220 | 29 (13) | 191 (87) | −19 | −126 to 38 |
| Current season unvaccinated | 859 | 97 (11) | 762 (89) | Ref | — |
| Prior season LAIV | |||||
| Current season IIV | 217 | 16 (7) | 201 (93) | 73 | 55 to 84 |
| Current season LAIV | 329 | 57 (17) | 272 (83) | 24 | −16 to 50 |
| Current season unvaccinated | 292 | 58 (20) | 234 (80) | Ref | — |
| Influenza A/H3N2d | |||||
| Prior season unvaccinated | |||||
| Current season IIV | 450 | 72 (16) | 378 (84) | 35 | 16 to 50 |
| Current season LAIV | 214 | 61 (29) | 153 (72) | −38 | −106 to 7 |
| Current season unvaccinated | 2963 | 605 (20) | 2358 (80) | Ref | — |
| Prior season IIV | |||||
| Current season IIV | 1226 | 196 (16) | 1030 (84) | 36 | 10 to 54 |
| Current season LAIV | 306 | 55 (18) | 251 (82) | 28 | −12 to 54 |
| Current season unvaccinated | 980 | 198 (20) | 782 (80) | Ref | — |
| Prior season LAIV | |||||
| Current season IIV | 144 | 29 (20) | 115 (80) | 12 | −35 to 43 |
| Current season LAIV | 402 | 90 (22) | 312 (78) | 13 | −30 to 42 |
| Current season unvaccinated | 285 | 70 (25) | 215 (75) | Ref | — |
Analyses exclude N = 62 patients for whom parent- or guardian-reported, current season vaccination could not be confirmed by EIRs (USAFSAM), N = 10 who received IIV and LAIV in the season before enrollment, N = 560 with unavailable records for the season before enrollment, and N = 53 with an unknown vaccine type in the season before enrollment. Ref, reference group; —, not applicable.
Percent of row total.
Adjusted for age (years), season, calendar time, and site (as a random effect).
Restricted to the 2013–2014 and 2015–2016 influenza seasons.
Restricted to the 2014–2015 influenza season.
Our findings were not sensitive to the results of individual studies; we removed studies individually and found similar estimates. Results were not sensitive to the definition of the influenza season; similar results were found with the season defined as July 1 to June 30. We also found similar results when analyses were (1) restricted to those with influenza-like illness, (2) restricted to those who presented within 3 days of illness onset, (3) restricted to those without high-risk conditions from USFLUVE and ICICLE, or (4) restricted to those who were fully vaccinated from USFLUVE and ICICLE (data not shown).
Discussion
The results of this pooled individual patient–level data analysis from 3 seasons are consistent with the previously published studies included in the analysis.7–14 In 2013–2014 and 2015–2016, low VE of LAIV4 was observed against influenza A/H1N1pdm09. Relative effectiveness estimates favored IIV for all age groups, suggesting a lower risk of influenza A/H1N1pdm09 infection among IIV recipients compared with LAIV4 recipients. Vaccination with either IIV or LAIV in the previous season did not explain the low VE detected for current season LAIV4. Against drifted influenza A/H3N2 virus in 2014–2015, effectiveness was poor for both vaccines regardless of previous season vaccination. These results are consistent with the low overall VE against drifted influenza A/H3N2 observed in the United States during that season.11,19 Results from a clinical trial of LAIV3 suggested a relative benefit of LAIV3 over trivalent IIV in seasons with drifted influenza A/H3N2 virus circulation in the 2004–2005 influenza season.4 Overall in our study, effectiveness against influenza B viruses was moderate for both vaccines.
Our results are consistent with findings of reduced VE of LAIV4 against influenza A/H1N1pdm09 in the 2013–2014 influenza season, which prompted the change in the H1N1pdm09 LAIV4 vaccine virus from A/California/7/2009 to A/Bolivia/559/2013 for the 2015–2016 influenza season.7 Notably, we also observed a lower effectiveness of LAIV4 compared with IIV against influenza A/H1N1pdm09 virus in 2015–2016, suggesting the effectiveness remained low despite the change to the A/Bolivia/559/2013 vaccine formulation. Additional efforts have been taken by the manufacturer to identify the cause of the reduced effectiveness of influenza A/H1N1pdm09 LAIV strains. The LAIV H1N1 vaccine virus for the 2017–2018 and 2018–2019 influenza seasons, A/Slovenia/2309/2015, has demonstrated improved replicative fitness in the laboratory and increased shedding and serum antibody responses among children compared with A/Bolivia/559/2013.20,21 Preliminary estimates from the 2017–2018 influenza season in the United Kingdom suggest good effectiveness of the A/Slovenia/2309/2015 LAIV component against influenza A/H1N1pdm09, but additional data are needed.22
In contrast to findings of reduced LAIV4 effectiveness against influenza A/H1N1pdm09 viruses, our results suggest a possible but nonsignificant benefit of LAIV4 over IIV against influenza B viruses, which has been described previously.3,5 The difference in VE estimates was greatest in the 2013–2014 season, when most IIV used in the United States was trivalent.11 The relative benefit of LAIV4 over IIV may decrease as the proportion of all IIV use that is quadrivalent continues to increase.16 Because we were unable to distinguish between trivalent and quadrivalent IIV doses for the majority of patients in our study, LAIV4 was compared with a mixture of IIV products, among which VE may differ. In addition, B lineage testing was not available for most studies in this analysis. However, US surveillance data from these 3 seasons indicate circulation of both B lineages,23,24 and it is likely that our influenza B cases represent both influenza B lineages.
This analysis was subject to several limitations. Descriptive patient information beyond age, sex, and geographic region of enrollment was not available for all studies. As a result, the estimates from this pooled analysis may be subject to confounding by other, unmeasured factors. In an attempt to control for some of these factors, such as potential differences in disease severity and high-risk status, we conducted several sensitivity analyses on subsets of patients with information on these factors and found results similar to our overall findings. Another limitation of our study was that, for most patients, historical vaccination data were limited to the season immediately before enrollment rather than the complete vaccine priming history. This limited our ability to fully assess the effects of vaccine priming history on current season VE. In a sensitivity analysis restricted to patients with more complete vaccination histories, VE among fully vaccinated children was similar to results of the primary analysis, suggesting that receiving 1 dose of vaccine when 2 are recommended is not confounding our findings.
Conclusions
Combining data from multiple studies, we estimate VE in subgroups of interest, including narrower age groups and among children vaccinated in the previous season, which was not feasible in the individual studies because of small sample sizes. Our findings are consistent with that of a recently published meta-analysis that included data from outside the United States (including Canada, Finland, Germany, and the United Kingdom); researchers in this study reported suboptimal effectiveness of LAIV4 against influenza A/H1N1pdm09 compared with IIV and similar effectiveness against influenza A/H3N2 in 2014–2015 and influenza B viruses.25 Although LAIV4 containing the updated vaccine virus A/Slovenia/2309/2015 was used in Europe and Canada in 2017–2018, a limited circulation of influenza A/H1N1pdm09 hinders the ability to obtain precise effectiveness estimates.26,27 On the basis of evidence for immunogenicity and protection in animal models of the updated influenza A/H1N1pdm09 vaccine virus, ACIP reinstated the recommendation for use of LAIV4 in the United States as licensed for persons aged 2 to 49 years for the 2018–2019 influenza season as a vaccine option alongside age-appropriate IIVs and recombinant influenza vaccines.28
Acknowledgments
We thank the USAFSAM Epidemiology Laboratory; Alan Aspinall, GK Balasubramani, Rina Chabra, Heather Eng, Samantha Ford, Ed Garofolo, Richard Hoffmaster, Philip Iozzi, Krissy K. Moehling, Christopher Olbrich, Jonathan Raviotta, Evelyn C. Reis, Sandy Sauereisen, Sean Saul, Theresa Sax, Michael Susick, Joe Suyama, and Leonard Urbanski of the University of Pittsburgh; Michael Reis, Jessica Pruszynski, Archana Nangrani, Anne Robertson, Patricia Sleeth, Virginia Gandy, Teresa Ponder, Hope Gonzalez, Martha Zayed, and Deborah Furze of Baylor Scott & White Health; Pedro Piedra, W. Paul Glezen, and Vasanthi Avadhanula of Baylor College of Medicine; and all studies’ participants.
Glossary
- ACIP
Advisory Committee on Immunization Practices
- CDC
Centers for Disease Control and Prevention
- CI
confidence interval
- EIR
electronic immunization record
- ICICLE
Influenza Clinical Investigation for Children
- IISP
Influenza Incidence Surveillance Project
- IIV
inactivated influenza vaccine
- LAIV
live attenuated influenza vaccine
- LAIV3
trivalent live attenuated influenza vaccine
- LAIV4
quadrivalent live attenuated vaccine
- LSU
Louisiana State University Health Sciences Center
- OR
odds ratio
- RT-PCR
real-time reverse transcription-polymerase chain reaction
- USAFSAM
US Air Force School of Aerospace Medicine
- USFLUVE
US Influenza Vaccine Effectiveness Network
- VE
vaccine effectiveness
Footnotes
A complete list of nonauthor contributors appears in the Supplemental Information.
Ms Chung conducted the analyses and drafted the initial manuscript; Drs Flannery and Fry conceptualized and designed the study, contributed to the analyses and interpretation of the results, and critically reviewed and revised the manuscript; Drs Ambrose, Bégué, and Caspard and Ms DeMarcus, Ms Fowlkes, Ms Kersellius, and Ms Steffens coordinated and supervised the study design and data collection for their respective studies and critically reviewed the manuscript; group authors recruited participants for studies, provided feedback for the data analysis methods, and critically reviewed and revised the manuscript; and all authors approved the final manuscript as submitted.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
FINANCIAL DISCLOSURE: The Influenza Clinical Investigation for Children was supported by MedImmune, a member of the AstraZeneca Group. Drs Ambrose and Caspard are employees of AstraZeneca; the other authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: The US Influenza Vaccine Effectiveness Network was supported by the Centers for Disease Control and Prevention through cooperative agreements with the University of Michigan (U01 IP000474), Kaiser Permanente Washington Health Research Institute (U01 IP000466), Marshfield Clinic Research Institute (U01 IP000471), University of Pittsburgh (U01 IP000467), and Baylor Scott & White Health (U01 IP000473). At the University of Pittsburgh, the project was also supported by the National Institutes of Health through grants UL1 RR024153 and UL1TR000005. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: Drs Ambrose and Caspard are employees of AstraZeneca; the other authors have indicated they have no potential conflicts of interest to disclose.
COMPANION PAPER: A companion to this article can be found online at www.pediatrics.org/cgi/doi/10.1542/peds.2018-3290.
References
- 1.Freeland AL, Vaughan GH Jr, Banerjee SN. Acute gastroenteritis on cruise ships - United States, 2008-2014. MMWR Morb Mortal Wkly Rep. 2016;65(1):1–5 [DOI] [PubMed] [Google Scholar]
- 2.Committee on Infectious Diseases Recommendations for prevention and control of influenza in children, 2016-2017. Pediatrics. 2016;138(4):e20162527. [DOI] [PubMed] [Google Scholar]
- 3.Ashkenazi S, Vertruyen A, Arístegui J, et al. ; CAIV-T Study Group . Superior relative efficacy of live attenuated influenza vaccine compared with inactivated influenza vaccine in young children with recurrent respiratory tract infections. Pediatr Infect Dis J. 2006;25(10):870–879 [DOI] [PubMed] [Google Scholar]
- 4.Belshe RB, Edwards KM, Vesikari T, et al. ; CAIV-T Comparative Efficacy Study Group . Live attenuated versus inactivated influenza vaccine in infants and young children [published correction appears in N Engl J Med. 2007;356(12):1283]. N Engl J Med. 2007;356(7):685–696 [DOI] [PubMed] [Google Scholar]
- 5.Fleming DM, Crovari P, Wahn U, et al. ; CAIV-T Asthma Study Group . Comparison of the efficacy and safety of live attenuated cold-adapted influenza vaccine, trivalent, with trivalent inactivated influenza virus vaccine in children and adolescents with asthma. Pediatr Infect Dis J. 2006;25(10):860–869 [DOI] [PubMed] [Google Scholar]
- 6.Neuzil KM, Dupont WD, Wright PF, Edwards KM. Efficacy of inactivated and cold-adapted vaccines against influenza A infection, 1985 to 1990: the pediatric experience. Pediatr Infect Dis J. 2001;20(8):733–740 [DOI] [PubMed] [Google Scholar]
- 7.Jackson ML, Chung JR, Jackson LA, et al. . Influenza vaccine effectiveness in the United States during the 2015-2016 season. N Engl J Med. 2017;377(6):534–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poehling KA, Caspard H, Peters TR, et al. . 2015-2016 vaccine effectiveness of live attenuated and inactivated influenza vaccines in children in the United States. Clin Infect Dis. 2018;66(5):665–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caspard H, Gaglani M, Clipper L, et al. . Effectiveness of live attenuated influenza vaccine and inactivated influenza vaccine in children 2-17 years of age in 2013-2014 in the United States. Vaccine. 2016;34(1):77–82 [DOI] [PubMed] [Google Scholar]
- 10.Gaglani M, Pruszynski J, Murthy K, et al. . Influenza vaccine effectiveness against 2009 pandemic influenza A(H1N1) virus differed by vaccine type during 2013-2014 in the United States. J Infect Dis. 2016;213(10):1546–1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zimmerman RK, Nowalk MP, Chung J, et al. ; US Flu VE Investigators . 2014-2015 influenza vaccine effectiveness in the United States by vaccine type. Clin Infect Dis. 2016;63(12):1564–1573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McLean HQ, Caspard H, Griffin MR, et al. . Effectiveness of live attenuated influenza vaccine and inactivated influenza vaccine in children during the 2014-2015 season. Vaccine. 2017;35(20):2685–2693 [DOI] [PubMed] [Google Scholar]
- 13.Valdin HL, Bégué RE. Influenza vaccines effectiveness 2013-14 through 2015-16, a test-negative study in children. Vaccine. 2017;35(33):4088–4093 [DOI] [PubMed] [Google Scholar]
- 14.DeMarcus LS, Parms TA, Thervil JW. The DoD global, laboratory-based, influenza surveillance program: summary for the 2013-2014 influenza season. MSMR. 2016;23(3):2–5 [PubMed] [Google Scholar]
- 15.Pebody R, McMenamin J, Nohynek H. Live attenuated influenza vaccine (LAIV): recent effectiveness results from the USA and implications for LAIV programmes elsewhere. Arch Dis Child. 2018;103(1):101–105 [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention (CDC) Prevention and control of seasonal influenza with vaccines. Recommendations of the Advisory Committee on Immunization Practices–United States, 2013-2014 [published correction appears in MMWR Recomm Rep. 2013;62(45):906]. MMWR Recomm Rep. 2013;62(RR–07):1–43 [PubMed] [Google Scholar]
- 17.Fowlkes A, Steffens A, Temte J, et al. ; Influenza Incidence Surveillance Project Working Group . Incidence of medically attended influenza during pandemic and post-pandemic seasons through the Influenza Incidence Surveillance Project, 2009-13. Lancet Respir Med. 2015;3(9):709–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention Weekly influenza activity estimates reported by state and territorial epidemiologists. Available at: https://gis.cdc.gov/grasp/fluview/FluView8.html. Accessed June 1, 2017
- 19.Petrie JG, Malosh RE, Cheng CK, et al. . The household influenza vaccine effectiveness study: lack of antibody response and protection following receipt of 2014-2015 influenza vaccine. Clin Infect Dis. 2017;65(10):1644–1651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bright H, Mallory R. Update on status of investigation of reduced LAIV effectiveness. In: Advisory Committee on Immunization Practices; February 22, 2017; Atlanta, GA [Google Scholar]
- 21.Mallory RM. Results of randomized trial of a new H1N1 LAIV strain in US Children. In: Advisory Committee on Immunization Practices; February 21, 2018; Atlanta, GA [Google Scholar]
- 22.Public Health England Influenza vaccine effectiveness (VE) in adults and children in primary care in the United Kingdom (UK): provisional end-of-season results 2017-18. 2018. Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/726342/Influenza_vaccine_effectiveness_in_primary_care_2017_2018.pdf. Accessed August 15, 2018
- 23.Epperson S, Blanton L, Kniss K, et al. ; Influenza Division, National Center for Immunization and Respiratory Diseases, CDC . Influenza activity - United States, 2013-14 season and composition of the 2014-15 influenza vaccines. MMWR Morb Mortal Wkly Rep. 2014;63(22):483–490 [PMC free article] [PubMed] [Google Scholar]
- 24.Appiah GD, Blanton L, D’Mello T, et al. ; Centers for Disease Control and Prevention (CDC) . Influenza activity - United States, 2014-15 season and composition of the 2015-16 influenza vaccine. MMWR Morb Mortal Wkly Rep. 2015;64(21):583–590 [PMC free article] [PubMed] [Google Scholar]
- 25.Caspard H, Mallory RM, Yu J, Ambrose CS. Live-attenuated influenza vaccine effectiveness in children from 2009 to 2015-2016: a systematic review and meta-analysis. Open Forum Infect Dis. 2017;4(3):ofx111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skowronski DM, Chambers C, De Serres G, et al. . Early season co-circulation of influenza A(H3N2) and B(Yamagata): interim estimates of 2017/18 vaccine effectiveness, Canada, January 2018. Euro Surveill. 2018;23(5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rondy M, Kissling E, Emborg HD, et al. ; I-MOVE/I-MOVE+ Group . Interim 2017/18 influenza seasonal vaccine effectiveness: combined results from five European studies. Euro Surveill. 2018;23(9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grohskopf LA, Sokolow LZ, Broder KR, et al. . Prevention and control of seasonal influenza with vaccines: Recommendations of the Advisory Committee on Immunization Practices - United States, 2018-19 influenza season. MMWR Morb Mortal Wkly Rep. 2018;67(3):1–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention People at high risk of developing flu–related complications. 2018. Available at: https://www.cdc.gov/flu/about/disease/high_risk.htm. Accessed March 30, 2018