NBS has improved the detection, diagnosis, treatment, and basic understanding of SCID; infants with TCL are also detected, facilitating management.
Abstract
OBJECTIVES:
Newborn screening for severe combined immunodeficiency (SCID) was instituted in California in 2010. In the ensuing 6.5 years, 3 252 156 infants in the state had DNA from dried blood spots assayed for T-cell receptor excision circles (TRECs). Abnormal TREC results were followed-up with liquid blood testing for T-cell abnormalities. We report the performance of the SCID screening program and the outcomes of infants who were identified.
METHODS:
Data that were reviewed and analyzed included demographics, nursery summaries, TREC and lymphocyte flow-cytometry values, and available follow-up, including clinical and genetic diagnoses, treatments, and outcomes.
RESULTS:
Infants with clinically significant T-cell lymphopenia (TCL) were successfully identified at a rate of 1 in 15 300 births. Of these, 50 cases of SCID, or 1 in 65 000 births (95% confidence interval 1 in 51 000–1 in 90 000) were found. Prompt treatment led to 94% survival. Infants with non-SCID TCL were also identified, diagnosed and managed, including 4 with complete DiGeorge syndrome who received thymus transplants. Although no cases of typical SCID are known to have been missed, 2 infants with delayed-onset leaky SCID had normal neonatal TREC screens but came to clinical attention at 7 and 23 months of age.
CONCLUSIONS:
Population-based TREC testing, although unable to detect immune defects in which T cells are present at birth, is effective for identifying SCID and clinically important TCL with high sensitivity and specificity. The experience in California supports the rapid, widespread adoption of SCID newborn screening.
What’s Known on This Subject:
Earlier results showed that newborn screening can be highly effective for the early detection of severe combined immunodeficiency (SCID).
What This Study Adds:
Screening 3.25 million California infants of diverse ethnicity with the T-cell receptor excision circle test substantiates the value of newborn screening for the diagnosis of SCID and non-SCID T-cell lymphopenia. Both are more common and genetically heterogeneous than previously thought.
Severe combined immunodeficiency (SCID) encompasses ∼20 known (and additional unknown), rare genetic disorders of adaptive immunity. Individuals who are affected lack T lymphocytes and have absent or nonfunctional B lymphocytes, rendering them susceptible to life-threatening bacterial, fungal, and viral infections with both ordinary and opportunistic pathogens.1,2 Because <20% of case patients with SCID have a recognized positive family history and because infants who are affected appear healthy at birth, population-based newborn screening (NBS) is the only strategy to identify all infants with SCID sufficiently early to provide treatment before the onset of infectious complications.3–7
Treatment of SCID most often requires an allogeneic hematopoietic cell transplant (HCT) from a suitably matched healthy donor; in addition, enzyme replacement therapy (ERT) is available for adenosine deaminase (ADA)–deficient SCID, and autologous hematopoietic cell correction by gene therapy (GT) has been successful for ADA-deficient and X-linked SCID.1,2,7–10 Best outcomes are achieved if infants are treated early to avoid infectious complications, as documented in single-center reports and by multicenter studies from the Primary Immune Deficiency Treatment Consortium (PIDTC).2,4,7,11
The PIDTC has established laboratory-based definitions for SCID independent of complications, such as opportunistic infections or failure to thrive.12 Infants with typical SCID have <300 autologous T cells per μL, absent naïve T cells, and proliferative responses to the mitogen phytohemagglutinin that are <10% of control values. They often have spontaneous engraftment of maternal T cells, and the diagnosis is further confirmed by documenting pathogenic mutations in known SCID genes. In addition, some infants have hypomorphic mutations in SCID genes that allow residual function and produce a leaky phenotype. These infants usually have <1500 autologous T cells per μL unless there has been oligoclonal proliferation of memory phenotype T cells, as occurs in Omenn syndrome; infants with leaky SCID generally have sufficient T-cell function to prevent maternal engraftment, but immunity is insufficient to fight infections.
The timely presymptomatic diagnosis of SCID is the primary goal of SCID NBS. California instituted population-wide screening for SCID in August 2010, following pilot programs in Wisconsin, Massachusetts, and the Navajo Reservation and on the recommendation by the Advisory Committee on Heritable Disorders in Newborns and Children (endorsed by the Secretary of the Department of Health and Human Services) that screening for SCID be added to the Recommended Uniform Screening Panel.13–16 The SCID NBS test is based on detection of T-cell receptor excision circles (TRECs), DNA biomarkers of normal T lymphopoiesis that can be measured by a polymerase chain reaction test by using dried blood spots (DBSs) routinely obtained from newborns by heel stick.3 DBSs from newborns with SCID have undetectable or low numbers of TRECs. Moreover, a low TREC copy number in a DBS is used to detect not only the primary screening target, SCID, but also several non-SCID conditions characterized by T-cell lymphopenia (TCL) that may also benefit from early diagnosis and intervention to prevent infections.
In 2014, researchers of an analysis of early pooled data from 11 screening programs endorsed the effectiveness of TREC screening.17 Although TREC-based SCID screening was highly effective for the diagnosis of SCID in all participating programs, the lack of uniformity in assay methodology between the programs precluded determination of rates of recall for further testing. As of December 10, 2018, all 50 states in the United States, plus the District of Columbia and Puerto Rico, as well as an increasing number of countries, including Israel, New Zealand, and Norway, have adopted universal NBS for SCID. Additional regional or pilot programs are under way in many other countries. Here we report the experience and outcomes of 6.5 years of screening >3.25 million newborns for SCID in California, the state with the largest number of births and high ethnic diversity.
Methods
Details of methods, including the statistical analysis, are in the Supplemental Information. Briefly, DBS specimens collected from essentially all infants born in California since mid-2010 had TREC testing for SCID, initially with a laboratory-developed assay at the Genetic Disease Laboratory (GDL) in Richmond, California, as published.18 The EnLite neonatal TREC kit (PerkinElmer, Inc, Waltham, MA) was adopted in June 2015, after which testing moved to regional NBS laboratories. Reported TREC and β-actin copy numbers differed between these methods, requiring adjustment of thresholds. The categories of test results are summarized in Table 1. After 1 positive or 2 incomplete TREC test results, infants had liquid blood analyzed for lymphocyte subsets and naïve and memory T-cell markers at a single laboratory (Quest Diagnostics, San Juan Capistrano, CA).
TABLE 1.
TREC Test Results, Interpretation, and Actions Required
| TREC Copy No., per μL | β-Actin Copy No., per μL | TREC Test Interpretation | Action Required |
|---|---|---|---|
| >18a | N/Ab | Normal | No further action |
| Undetectable or <4c | >35d | Urgent positive | Immediate callback for liquid blood for lymphocyte subsets |
| 4–18, infant not in NICU | >35 | Positive | Callback for liquid blood for lymphocyte subsets |
| 4–18, infant in NICU | N/A | Incomplete | Second DBS test in 2 wk or at discharge from nursery; after 2 incomplete test results, liquid blood for lymphocyte subsets |
N/A, not applicable.
TREC copies per μL of peripheral blood equivalent for the EnLite kit; >25 copies per μL for the original laboratory-developed test; values for both methods after adjustments based on initial experience.
β-actin was not reported if the initial TREC value was normal.
Fewer than 6 copies per μL for the laboratory-developed test.
For the EnLite kit, the cutoff for the laboratory-developed test was >5000 copies per μL.
Lymphocyte phenotypes were interpreted by California Department of Public Health (CDPH) immunology associates (M.J.B., J.A.C., S.A.M., and J.M.P.). Infants with <300 CD3 T cells per μL of blood or with <2% of their helper T cells bearing the naïve T-cell marker CD45RA were referred to pediatric hospitals with experience in treating SCID (Children’s Hospital Los Angeles; Stanford Lucille Packard Children’s Hospital; University of California, Los Angeles Mattel Children’s Hospital; or University of California, San Francisco Benioff Children’s Hospital). Additionally, infants with up to 1500 circulating T cells per μL with naïve T cells present were referred to pediatric immunology clinics for further management. Long-term follow-up was requested from transplant centers and immunologists according to the standard, comprehensive CDPH program to monitor outcomes of all California infants with positive NBS results.19 Children with SCID or TCL detected by NBS were managed until resolution or for at least 1 year and up to 7.5 years. Diagnoses were established by standard clinical testing, including gene sequencing, except as noted below.
Results
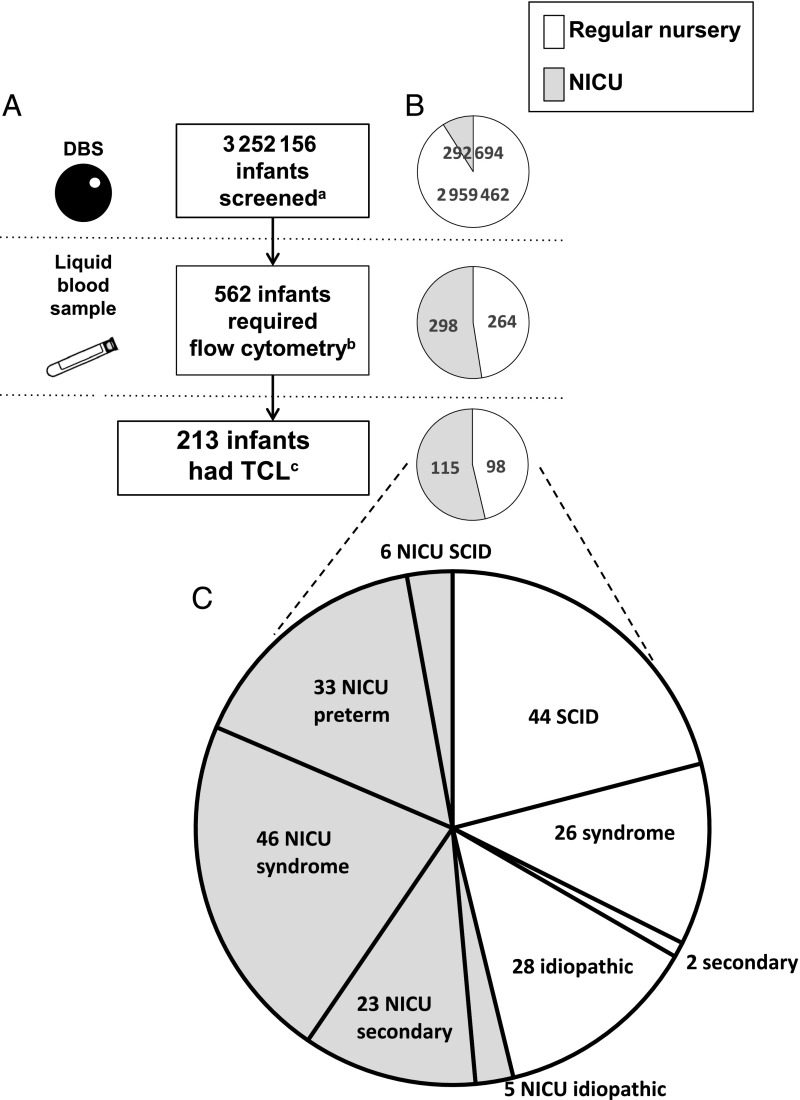
Between August 15, 2010, and March 31, 2017, a total of 3 252 156 newborns had SCID screening. Of these, 562 (1 of 5800 total births) had nonnormal TREC results followed by liquid blood lymphocyte phenotyping by flow cytometry (Fig 1A). Although only 9% of all infants were in NICUs, this population had over half of the TREC-positive screening results (53%; n = 298; Fig 1B). Cases were tracked to establish diagnoses of both the primary target of screening, SCID, and non-SCID TCL of <1500 T cells per μL, a clinically important secondary target. This T-cell cutoff was chosen to be highly selective but to avoid exposing infants with significant immune compromise to the otherwise-mandated live, attenuated rotavirus vaccine, which can cause serious diarrhea in immunodeficiency.20 TCL was documented in 38% (n = 213) of the cohort of infants requiring follow-up flow-cytometry tests or in 1 per 15 300 total newborns (95% confidence interval [CI] 1 per 13 500–1 per 17 700). An urgent positive TREC result (Table 1) was a specific predictor of both SCID and non-SCID TCL; of the 82 newborns with urgent positive results, 38 (46%) had SCID and 21 (26%) had non-SCID TCL. Positive or incomplete TREC test results and documented TCL in those infants who had abnormal TREC test results were more common in infants born very prematurely, as assessed by gestational age (GA) at birth or by birth weight (Tables 2 and 3; see Supplemental Information for further details). Ethnicity and sex were not significant risk factors for having a positive or incomplete TREC result or TCL (data not shown). TREC screening identified 50 cases of SCID (1 per 65 000 births [95% CI 1 per 51 000–1 per 90 000]), 44 cases from regular nurseries and 6 cases from NICUs. The proportions of case patients with true SCID in NICUs was the same as in regular nurseries (Fig 1 B and C). The 162 cases of non-SCID TCL were categorized as congenital syndromes (72 cases), including multisystem congenital abnormalities with TCL and non-SCID single-gene primary immunodeficiencies; as TCL resulting from another condition and resolving once that condition was corrected (25 cases); as preterm birth alone, in which TCL was self-limited if the infant survived (33 cases); or as idiopathic TCL (33 cases).
FIGURE 1.
Summary of NBS for SCID in California. A, Number of infants at each stage of screening. B, Numbers and proportions of infants in regular versus intensive care nurseries at each stage of the screening process. C, Final diagnosis category for infants with TCL in each nursery type. a Total infants screened included 91% of infants who were cared for in a regular well-infant nursery or born at home, and 9% who were cared for in a NICU. b Infants (47% from regular nurseries and 53% from NICUs) with a liquid blood sample tested after 1 positive or 2 incomplete TREC test results made up 0.17% of all births, excluding 20 newborns with TREC-positive test results who either died or were lost to follow-up before a liquid blood sample could be obtained and 2 infants born in another state who had positive TREC screen results and relocated to California for follow-up. c Infants with <1500 T cells per μL or <2% naïve CD3 CD4 CD45RA T helper cells (46% in the regular nursery and 54% in the NICU) made up 0.007% of the population (1 per 15 000 births).
TABLE 2.
Preterm Birth and TCL in Patients With Abnormal TREC Test Results by GA
| GA Group, wk | Not TCL, n | TCL, n |
|---|---|---|
| 22–31 | 114 | 47 |
| 32–41 | 225 | 171 |
χ2 (degrees of freedom = 1; N = 557) = 9.40; P < .05.
TABLE 3.
Preterm Birth and TCL in Patients With Abnormal TREC Test Results by Birth Weight
| Birth wt Group, g | Not TCL, n | TCL, n |
|---|---|---|
| 0–1500 | 108 | 42 |
| >1501 | 231 | 176 |
χ2 (degrees of freedom = 1; N = 557) = 10.69; P < .05.
Cases of SCID
Of the 50 infants with an immunophenotype of SCID, 49 were immediately hospitalized at an immunodeficiency center for treatment with allogeneic HCT, autologous corrected cell GT, or ERT (Table 4). One infant with SCID born to a foreign visitor left the United States before receiving any further immune evaluation or treatment. All infants with SCID were healthy at diagnosis except for 2 who had already been brought to medical attention with infection or rashes and 1 who was born prematurely and had feeding intolerance, bronchiolitis, and leukopenia.21 Only 2 were known by providers at birth hospitals to be at risk for SCID because of a positive family history, whereas 7 others who were identified by SCID NBS had older relatives who were affected and diagnosed with SCID, and 1 had a sibling who had died of infantile pneumonia consistent with SCID. The median age at flow cytometry for infants lacking a recognized family history of SCID was 21 days (range 6–40 days).
TABLE 4.
Characteristics, Treatments, and Outcomes of 49 Infants With SCID Diagnosed by Using NBS
| SCID Genotype (No. Cases) | SCID Phenotypea | Nursery | TREC Result | Outcome After Treatmentb | |||
|---|---|---|---|---|---|---|---|
| Urgent Positivec | Positive | Died (Posttransplant d, Cause) | Full T and B Cell Recovery and Off IgG, Treatmentd | T but Not B Cells Reconstituted, Still on IgG, Treatment | |||
| IL2RG (14) | Typical | Regular | 12 | 0 | 1 (218 d, SOSe, CMV) | 2 cond MUD, 1 GT, 1 cond MUD after GT | 6 MMRD, 1 cond MUDf, 1 GT, 1 GT after MMRD |
| NICU | 2 | 0 | |||||
| ADA (9) | Typical | Regular | 5 | 2 | 0 | 8 GT, 1 MSD | 0 |
| NICU | 1 | 0 | |||||
| Leaky | Regular | 0 | 1 | ||||
| RAG1 (8) | Typical | Regular | 2 | 0 | 1 (28 d, SOS) | 1 cond MSD | 0 |
| Leaky | Regular | 2 leaky, 1 Omenn syndrome | 1 leaky, 2 Omenn syndrome | 0 | 4 cond MMRD | 2 cond MMRD (1 of whom planning to stop IgG) | |
| IL7R (6) | Typical | Regular | 5 | 0 | 0 | 4 MMRD, 1 MUD, 1 cond MSD after MSD | 0 |
| Typical | NICU | 0 | 1 | ||||
| JAK3 (3) | Typical | Regular | 3 | 0 | 0 | 1 cond MSD, 1 cond MUD | 1 MMRD |
| RAG2 (3) | Typical | Regular | 2 | 0 | 0 | 1 MSD, 2 cond MUD | 0 |
| Leaky | Regular | 0 | 1 | ||||
| BCL11B (1) | Leaky | Regular | 1 | 0 | 0 | 1 cond MUD | 0 |
| RMRP (1) | Leaky | Regular | 0 | 1 | 0 | 1 cond MUD | 0 |
| Unknown (4) | Typical | Regular | 1 | 1 | 1 (69 d, CMV) | 0 | 1 cond MUD planning to stop IgG |
| NICU | 1 | 0 | 0 | 0 | 1 Cond MUD | ||
| Leaky | Regular | 1 | 0 | 0 | 0 | 1 cond MUD planning to stop IgG | |
Cond, conditioning with busulfan chemotherapy and, in some instances, fludarabine or other agents; MMRD, mismatched related donor; MSD, matched sibling donor; MUD, matched adult or cord blood unrelated donor.
Criteria from the PIDTC for typical SCID and leaky SCID; Omenn syndrome, a subset of leaky SCID, includes erythroderma rash, adenopathy, oligoclonal T-cell expansion, eosinophilia, and elevated immunoglobin E levels.
One infant not included in this table had urgent-positive TRECs and the T−B−NK+ SCID lymphocyte profile but left the United States before HCT; no genotype or follow-up is available.
Three or fewer TREC copies with a normal control polymerase chain reaction copy number by the EnLite kit assay or equivalent with a previous in-laboratory assay.
Treatments included GT with low-dose busulfan followed by autologous CD34+ bone marrow cells transduced with a correct copy of the mutated gene (IL2RG or ADA), MMRD (usually a parent who is haploidentical), MSD, MUD. Cond, conditioning with busulfan chemotherapy and (in some instances) fludarabine or other agents (other infants may have had serotherapy with rabbit antithymocyte globulin or anti-CD52).
Posttransplant hepatic sinusoidal obstruction syndrome after busulfan chemotherapy.
One patient left the United States after conditioned MUD HCT and reconstitution; it is not known if the patient is still on IgG.
Of the 49 patients who were evaluable and treated (Table 4), 46 survived (94%). Of the 3 deaths, 1 infant of unknown genotype died of cytomegalovirus (CMV) infection with encephalitis. One with recombinase-activating gene 1 (RAG1)–deficient SCID and 1 with X-linked SCID, each of whom had received pretransplant conditioning with high-dose busulfan chemotherapy, developed fatal hepatic sinusoidal obstruction syndrome post-HCT; 1 of these also had a concomitant CMV infection that had been present before HCT.
Table 4 indicates genotype, nursery type, TREC result, and whether the SCID was typical or leaky. Whereas ∼90% of infants with typical SCID had urgent positive TREC results, half of the infants with leaky SCID had TREC numbers in the positive, but not urgent, range. See Supplemental Table 11 for patient mutations. As reported previously in the literature, the X-linked interleukin-2 receptor γ chain (IL2RG) gene was mutated most frequently.1,2,7,17,18 The 9 infants with ADA-deficient SCID had, in addition to lymphopenia of T, B, and natural killer (NK) cells, fewer neutrophils (mean 986 cells per μL) and monocytes (mean 372 cells per μL) than the other infants with SCID, who had means of 4700 neutrophils per μL and 1600 monocytes per μL, or healthy infants. Interestingly, the birth weights of infants with ADA-deficient SCID (median 2608 g) also tended to be lower than those of other infants with SCID (median 3288 g), perhaps reflecting that ADA deficiency is a systemic disorder of purine salvage. Despite clinical sequencing of known SCID genes, 5 infants (10%) remained without a proven genotype. Whole-exome sequencing failed to solve 4 of these, whereas confirmatory experiments revealed 1 infant to have a defect in a novel SCID gene, B cell lymphoma 11B (BCL11B).22
In the right columns of Table 4 outcomes after therapy for patients with SCID are summarized. GT, consisting of an autologous transplant of bone marrow–derived hematopoietic stem cells transduced with a vector encoding the correct complementary DNA, was used to treat 4 infants with X-linked SCID and 8 infants with ADA-deficient SCID, with uniform survival at 2.5 to 6.5 years and notable success. All infants receiving GT for ADA-deficient SCID have had multilineage immune reconstitution. Three recipients of GT for X-linked SCID have recovered T-cell immunity; 1 recipient had failed his initial haploidentical T-cell-depleted allogeneic HCT, and 1 recipient no longer required immunoglobulin G (IgG) infusions. However, 1 recipient of GT for X-linked SCID failed this initial treatment but recovered T- and B-cell immunity after an allogeneic transplant from a matched, unrelated donor.
Deficiency of the recombinase activating proteins RAG1 or RAG2, both required for T- and B-lymphocyte antigen receptor rearrangement, caused 4 cases of typical SCID and 7 cases of leaky SCID, of which 3 patients had Omenn syndrome, in which a limited pool of poorly regulated T cells expands, causing rashes, adenopathy, splenomegaly, eosinophilia, and elevated immunoglobulin E. An example was an infant with leaky RAG1-deficient SCID who had >4500 T cells per μL. Although this was in the normal range, only 46 of his 3680 CD4 helper T cells (1.2%) had the naïve CD45RA phenotype.
During the 6.5 years of screening, 2 cases of SCID were discovered to have been missed. Both had normal newborn DBS TREC numbers, each well above the designated cutoff for follow-up testing. One infant, who presented at age 7 months with Pneumocystis pneumonia, had the known, recurrent, hypomorphic X-linked IL2RG mutation, Arg222Cys.23 His delayed-onset leaky SCID was successfully treated with a conditioned HCT from a matched, unrelated donor. The other was a delayed-onset case of incomplete ADA deficiency, diagnosed at 23 months of age after repeated otitis and hospitalization for pneumonia.24 This child was successfully treated with GT.
Non-SCID TCL
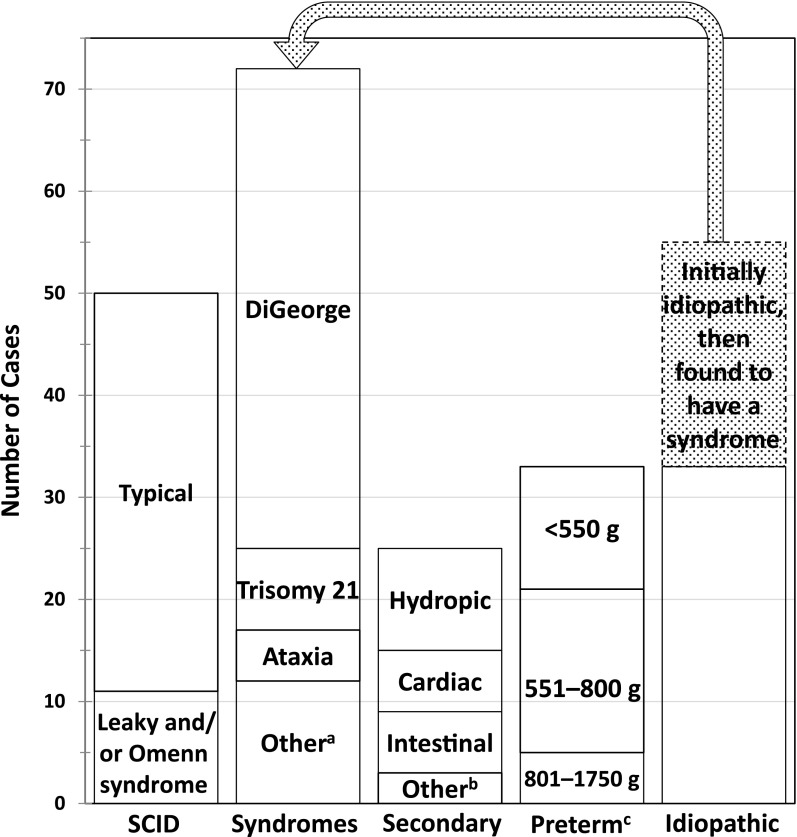
After ruling out SCID, infants with non-SCID TCL identified by SCID NBS were tracked by participating immunologists in the CDPH long-term follow-up program.19 Although many infants received a diagnosis in the neonatal period, 22 required further testing over the course of 3 to 18 months to establish a diagnosis (Fig 2), and 33 others remained idiopathic (Fig 2). The most frequent non-SCID TCL conditions were congenital syndromes (Fig 2, Table 5). Six infants with syndromes died before flow cytometry could be performed (Supplemental Table 12).
FIGURE 2.
Conditions diagnosed in infants with TCL identified by NBS, including 50 infants with typical or leaky SCID (including Omenn syndrome), 72 infants with a final diagnosis of a syndrome associated with T-cell impairment or of a non-SCID primary immunodeficiency disorder, 25 infants with congenital defects associated with secondary TCL, 33 infants with TCL and preterm birth alone, and 55 infants with an initial diagnosis of idiopathic TCL (in whom a syndrome was later diagnosed in 22, leaving 33 idiopathic cases with no underlying defect identified). a Includes 3 cases of CHARGE syndrome, 3 cases of diabetic embryopathy, and 1 each of CLOVES syndrome, EXTL3 deficiency (immune-skeletal dysplasia with neurodevelopmental abnormalities), Fryns syndrome (multiple anomalies with diaphragmatic defects), Nijmegen breakage syndrome (defect in the DNA repair protein nibrin, causing immune defects, microcephaly, short stature, and malignancy), Noonan syndrome (congenital heart disease, characteristic facial and physical features, and short stature), and RAC2 deficiency (a non-SCID primary immunodeficiency affecting neutrophils and lymphocytes). b Includes 2 cases of maternal immunosuppressive medication (azathioprine in 1, fingolimod in the other) and 1 case of neonatal teratoma of the thymus. c Infants with TCL associated with preterm birth alone were grouped by birth weight.
TABLE 5.
Diagnoses and Mortality in 72 Infants With TCL Found by Using SCID NBS Who Had Syndromes and Non-SCID Primary Immunodeficiency, Including Cases That Were Initially Classified as Idiopathic TCL
| Syndrome (No. Cases) | Typical Clinical Characteristics | Nursery Type | TREC Result | Outcome | |||
|---|---|---|---|---|---|---|---|
| Regular | NICU | Urgent Positive | Positive | Immune Therapy (If Known) | Died During Follow-up, Cause | ||
| DiGeorge syndrome (47) | Multiple congenital anomalies, including variable cardiac outflow tract defects, parathyroid hypoplasia, thymic hypoplasia, and immunodeficiency, and other congenital defects; most often due to interstitial deletion of chromosome 22q11.2 | 19 | 28 | 3 | 44 | 4 received thymus transplants | 1, post–thymus transplant medical complication |
| Trisomy 21 (8) | Wide range of neurodevelopmental and physical defects, including hypotonia, short stature, congenital heart disease, and defects in host defense against infection | 1 | 7 | 0 | 8 | — | 3 |
| AT (5) | Cerebellar ataxia with progressive neuromuscular deterioration, telangiectasias, immune defects, and predisposition to malignancy; DNA repair defect | 5 | 0 | 0 | 5 | 1 received IgG infusions | 0 |
| CHARGE syndrome (3) | Ocular coloboma, heart defect, atresia choanae, restricted growth and development, genital abnormality, and ear abnormality | 0 | 3 | 3 | 0 | 1 received IgG infusions | 1, respiratory failure |
| Diabetic embryopathy (3) | Multiple congenital malformations involving brain, cardiovascular, skeletal, and immune systems | 0 | 3 | 0 | 3 | TCL resolved in 1 | 1 |
| CLOVES syndrome (1) | Congenital lipomas, overgrowth, vascular malformations, epidermal nevi, and skeletal anomalies | 0 | 1 | 1 | 0 | IgG infusions | 1, respiratory failure |
| EXTL3 deficiency (1) | Immuno-skeletal dysplasia with neurodevelopmental abnormalities | 0 | 1 | 0 | 1 | IgG infusions | 0 |
| Fryns syndrome (1) | Multiple congenital anomalies with diaphragmatic defects and lung maldevelopment | 0 | 1 | 0 | 1 | — | 1, respiratory failure |
| Nijmegen syndrome (1) | Nibrin mutation causing DNA repair defect with immunodeficiency, microcephaly, short stature, and frequent malignancy | 0 | 1 | 0 | 1 | IgG infusions | 0 |
| Noonan syndrome (1) | Several known genotypes causing congenital heart disease, characteristic facial and physical features, and short stature | 0 | 1 | 0 | 1 | — | 1 |
| RAC2 deficiency (1) | Immunodeficiency affecting neutrophils and lymphocytes | 1 | 0 | 0 | 1 | — | 0 |
—, not applicable.
See the Supplemental Information for details of patients with non-SCID TCL. The most common syndrome was DiGeorge syndrome, with 47 cases (Fig 2, Table 5). More than 90% had partial DiGeorge syndrome, in which T cells rose to age-adjusted reference ranges12 over time or became adequate as judged by the local immunologist. However, 4 infants with complete DiGeorge syndrome (having persistently <300 T cells per μL and/or absent naïve helper T cells) received thymus transplants at Duke University in Durham, North Carolina, with 3 surviving for at least 2 years.25–27 Trisomy 21 accounted for 8 (11%) infants with syndromes; TCL resolved in 3 infants, 3 infants died of nonimmune complications, and 2 were lost to follow-up. All 5 patients with ataxia telangiectasia (AT) were healthy, term newborns from regular nurseries with 800 to 1323 T cells per μL. Rising α-fetoprotein levels after age 6 months led to the identification of deleterious mutations in the AT mutated gene.28 One infant with AT developed infections requiring IgG infusions during his first year. Three infants with coloboma of the eye, heart defect, atresia choanae, restricted growth and development, genital abnormality, and ear abnormality (CHARGE) syndrome required NICU care for congenital malformations unrelated to the immune system. One was considered for a thymus transplant but died of respiratory failure. The 3 case patients with diabetic embryopathy with TCL were from NICUs and had additional congenital abnormalities. Single patients with rare conditions constituted the remainder of case patients with syndromes (Table 5; discussed in the Supplemental Information), including an infant with a novel short-limbed immuno-skeletal dysplasia in whom whole-exome sequencing revealed exostosinlike glycosyltransferase 3 (EXTL3) deficiency.29
Secondary TCL in 25 case patients (Table 6) resolved to normal once excess T-cell losses or suppression of T-cell maturation had been reversed. T-cell losses were associated with vascular leakage, whereas 2 infants had poor T-cell production because of in utero exposure to maternal immunosuppressive medications, fingolimod in 1 and azathioprine in the other. One infant had a thymic teratoma, with T cells normalizing after it was resected. TCL was also occasionally associated with preterm birth (Table 7, Supplemental Tables 9 and 10), affecting infants born at 32 weeks’ gestation or earlier (median GA at birth: 24 weeks), with a median birth weight of 610 g (range: 300–1720 g). TCL resolved or was improving in 26 of 33 preterm infants who were retested.
TABLE 6.
Causes of Secondary TCL Found by using SCID NBS With TRECs
| Primary Condition (No. Cases) | Nursery Type | TREC Result | Comments (Deaths Occurred During the NICU Stay) | ||
|---|---|---|---|---|---|
| Regular | NICU | Urgent Positive | Positive | ||
| Congenital heart disease (6) | 0 | 6 | 1 | 5 | All had urgent heart surgery; 1 died |
| Hydrops (6) | 0 | 6 | 1 | 5 | 1 died |
| Gastroschisis (4) | 0 | 4 | 0 | 4 | All had urgent surgical intervention |
| Chylothorax (2) | 0 | 2 | 0 | 2 | 1 died |
| Maternal immunosuppressive medication (2) | 2 | 0 | 1 | 1 | Azathioprine or fingolimod used by mothers during pregnancy; infants’ T-cell counts normalized spontaneously |
| Third-space fluid leakage (2) | 0 | 2 | 1 | 1 | 1 died |
| Intestinal atresia (1) | 0 | 1 | 1 | 0 | Urgent surgical intervention |
| Meconium ileus (1) | 0 | 1 | 1 | 0 | Surgical intervention (ileostomy) |
| Teratoma of the thymus (1) | 0 | 1 | 0 | 1 | Surgery; patient also had hydrops, ascites, and chylothorax |
TABLE 7.
TCL Associated With Preterm Birth and Low Birth Weight
| Birth wt, g | No. Patients | TREC Result | |
|---|---|---|---|
| Urgent Positive | Positive | ||
| 300–450 | 5 | 1 | 4 |
| 451–550 | 7 | 0 | 7 |
| 551–650 | 11 | 1 | 10 |
| 651–900 | 8 | 1 | 7 |
| 901–1720 | 2 | 0 | 2 |
Idiopathic TCL included cases for which an underlying condition could not be determined, even after immunologic evaluation and, in many instances, sequencing of gene panels or whole exomes. Of the 33 cases of idiopathic TCL (Table 8), 13 resolved or were improving, whereas 13 patients had persistent TCL, and 6 patients were lost to follow-up.
TABLE 8.
Idiopathic TCL Detected by TREC Screening
| Status at Last Follow-up (No. Cases) | Nursery Type | TREC Result | ||
|---|---|---|---|---|
| Regular | NICU | Urgent Positive | Positive | |
| TCL resolved (11) | 9 | 2 | 0 | 11 |
| TCL improving (2) | 2 | 0 | 2 | 0 |
| TCL persistent (13) | 12 | 1 | 3 | 10 |
| Infant died of respiratory failure, had a low CD4 T cell count (1) | 0 | 1 | 0 | 1 |
| Status unknown; lost to follow-up (6) | 5 | 1 | 1 | 5 |
Discussion
TREC screening for SCID has been used to establish the feasibility and utility of DNA-based, high-throughput screening, which, like tandem mass spectrometry, has already proven adaptable to additional conditions (including spinal muscular atrophy, as approved in July 2018) for addition to the Recommended Uniform Screening Panel on the basis of a review of evidence.30 TREC screening in California facilitated the diagnosis of SCID in 50 infants over a 6.5-year period, permitting timely referral to specialized treatment centers for immune-restoring therapy. Sensitivity appears complete in that no cases of typical or early-onset leaky SCID with low T cells are known to have been missed. Indeed, cases of SCID were readily identified by low TRECs, even when maternally engrafted T cells were present, or in Omenn syndrome, when up to several thousand T cells per micro liter had arisen from florid expansion of a small oligoclonal T-cell pool. TRECs are generated in newly differentiated T cells and are not replicated when T cells undergo mitosis. Thus, both maternally transferred cells and proliferating autologous cells have lost TRECs through dilution, and such cells also lack the naïve CD45RA marker and instead express the CD45RO memory maker, both part of the standard California SCID flow-cytometry panel. A positive family history of SCID might be expected to make NBS redundant in at least some case patients,5 but in our experience, obtaining such a history proved problematic. Two of the 50 infants with SCID were from known at-risk pregnancies and had a prenatal diagnosis. However, neonatal medical providers were unaware that 6 other infants had a previous sibling who had been treated for SCID, including 2 siblings who themselves had been identified by SCID NBS in California and received transplants. Although the rate of false-positive TREC screen results was higher in NICUs than in regular nurseries (Fig 1), 6 of the 50 case patients with true SCID (12%) were in NICUs, proportional to the total number of infants in NICUs (9% of all newborns).
Survival of patients with SCID was 94% at 1 to 8 years of age. Furthermore, 31 of the 46 surviving children (67%) have fully reconstituted B- as well as T-cell immunity, no longer requiring IgG supplementation (Table 1); IgG production is expected to recover in additional patients over time, raising the proportion of children fully cured to 75%. The optimal treatment of SCID, HCT from a matched sibling, was available to only 3 infants. As previously published, the children with X-linked SCID who received HCT from a parent who was haploidentical without chemotherapy achieved T-cell reconstitution but continue to require IgG infusions.1,2,7,11 Chemotherapy conditioning, used in most cases of SCID in which B-cell recovery occurred, nonetheless did not ensure the development of normal antibody production and was associated with 2 deaths (see below). Moreover, B-cell recovery was achieved with unconditioned or conditioned HCT for all 6 cases of interleukin-7 receptor (IL7R)–deficient SCID, in which B cells are intrinsically functional once helper T cells are restored.31,32
Autologous GT after low-dose busulfan conditioning is available in clinical trials for patients with ADA-deficient and X-linked SCID who lack matched sibling donors.8–10 All 8 recipients of GT who were ADA-deficient have had full immune recovery, including independence from IgG infusions, as did the sole recipient of a matched-sibling HCT for ADA-SCID. ERT was used as a bridging therapy between diagnosis and GT for most ADA cases. Four of 14 case patients with X-linked SCID also received GT, although 1 required a subsequent allogeneic HCT for inadequate T-cell recovery. Importantly, and in contrast to previous unconditioned GT or unconditioned HCT with donors other than matched siblings, current GT for both ADA-deficient and X-linked SCID, when preceded by low-dose busulfan chemotherapy, restored both T- and B-cell immunity, allowing 10 of the 12 recipients of GT to discontinue IgG infusions.
The 3 deaths among infants with SCID present a challenge to further improve current therapy. One death followed hepatic sinusoidal obstruction from high-dose chemotherapy, 1 was due to CMV infection, and 1 was due to combined CMV infection and chemotherapy toxicity. Individual targeting of busulfan exposure to account for variable metabolism in young infants may prevent future chemotherapy-associated deaths.33 CMV is highly pathogenic in the absence of T-cell immunity. Infants with SCID are unlikely to have congenital CMV because maternal primary infection during pregnancy is uncommon; however, they may acquire CMV at or after birth from maternal virus shed into secretions or breast milk because most mothers have serologic evidence of previous CMV exposure.34 One infant with SCID of unknown genotype may have acquired CMV from breast milk from his mother who was CMV seropositive. On admission to the hospital at 21 days of age, this infant had CMV that was uncontrolled despite antiviral therapy and an accelerated haploidentical paternal HCT. California may have reduced CMV exposure time among infants with SCID by moving the TREC assay to regional laboratories with a faster turnaround. After regionalization, the median age at diagnosis of SCID by lymphocyte subsets for infants without a recognized family history was 8 days (n = 5), compared with 21 days (n = 40) before that time. Further study will be required to establish the sources and best preventive measures to combat CMV infection.
SCID did not occur more frequently in any ethnic group, nor did any California population subgroups demonstrate predominant founder mutations, as previously identified for Navajo American Indians and the Amish.35,36 However, the frequent occurrence of SCID because of homozygous autosomal recessive mutations reveals a pattern of shared parental ancestry. Of 30 cases of autosomal recessive SCID with mutations identified, 9 (30%) were homozygous (Supplemental Table 11).
A late diagnosis in 2 infants who had normal TREC NBS but who proved to have missense defects in the ADA and IL2RG genes, respectively, reveals that SCID has a broad spectrum of mutations and phenotypic presentations. Mutations that are sufficiently leaky to produce some T cells and TRECs may evade neonatal detection. Importantly, raising the TREC cutoff would not have helped to identify either of these case patients by NBS because both had TREC counts well above the designated level requiring follow-up.
In addition to diagnosing SCID, TREC NBS has identified non-SCID TCL, permitting intervention to avoid live rotavirus vaccination, which can cause severe diarrheal disease in infants with insufficient T-cell immunity.20 Secondary and premature TCL cases are expected to resolve, but until they do so, patients are immunocompromised. Although the TCL definition of 1500 CD3 cells per μL as the upper limit provides a medically prudent indication to intervene with immune supervision and avoidance of live viral vaccines, this cutoff does not ensure the identification of all cases of non-SCID, persistent, mild TCL.
Most of the non-SCID syndromes were multisystem disorders summarized in Table 5; of all individuals with these syndromes, those with low NBS TRECs and <1500 T cells per μL were only the ones with the most profound immunologic impairment. Thus, TREC testing is not a screen for DiGeorge syndrome, trisomy 21, or any other syndrome per se. Two-thirds of patients with syndromic TCL had DiGeorge syndrome, usually because of interstitial deletion of chromosome 22q11.2, but also associated with intragenic heterozygous mutations of T-box-containing transcription factor 1 (TBX1), a transcription factor located within the common 22q deletion region. Additionally, some patients with DiGeorge syndrome lacked a known genetic defect. In complete DiGeorge syndrome, some cases of CHARGE syndrome, and some infants of mothers with diabetes, profound TCL is due to the absence of a functional thymus. Urgent intervention to protect such infants from infections is required as in typical SCID, but curative treatment requires a thymus transplant, available only by experimental clinical trial.26,27 All of the 4 California infants found by NBS to have complete DiGeorge syndrome who were treated with a thymus transplant developed functional T cells, although 1 subsequently died of an unrelated complication. Infants with partial DiGeorge syndrome had stable or rising T cells over time, and those for whom data were available made protective responses to killed vaccines (data not shown).
Thirty-three infants with TCL from preterm birth alone (Table 7) had >2% of their CD4 helper T cells with the naïve CD45RA marker, and their TCL resolved to normal over time. However, longitudinal follow-up was essential to differentiate them from infants with SCID who were born prematurely.
Almost all infants with secondary TCL were cared for in NICUs because of recognized primary conditions, important exceptions being those whose mothers had taken immunosuppressive medication during pregnancy. Although self-limiting, the effects of these medications on infant T cells were profound. For example, despite being contraindicated in pregnancy, fingolimod was taken for multiple sclerosis by 1 woman who was pregnant. This drug is a sphingosine-1-phosphate receptor modulator that acts to sequester lymphocytes in the thymus and lymph nodes, preventing their release.37 The infant appeared healthy but at 8 days of age, had only 126 T cells, with undetectable naïve T cells, a profile typical of SCID that would require HCT. However, further workup revealed normal T-cell proliferation, and by the age of 2 months, the immune profile had completely resolved without treatment.
Out of an initial total of 55 idiopathic TCL cases, 22 defined syndromes were eventually diagnosed, most commonly DiGeorge syndrome in 9 case patients who did not demonstrate the commonly observed neonatal cardiac disease, hypocalcemia, or dysmorphic features. AT was also found in 5 cases that, without NBS, would have most likely been diagnosed only after the appearance of neurologic manifestations, ocular telangiectasias, or frequent infections.28 The remaining syndromes initially identified as idiopathic TCL by NBS included 3 cases of diabetic embryopathy and 1 each of CHARGE syndrome; congenital, lipomatous, overgrowth, vascular malformations, epidermal nevi, and spinal and/or skeletal anomalies and/or scoliosis (CLOVES) syndrome; Nijmegen breakage syndrome38; EXTL3 deficiency29; and member 2 of Rac subfamily of ρ GTPases (RAC2) deficiency39 (A.P. Hsu, J.A. Church, et al, unpublished observations). The last 3 were diagnosed after whole-exome sequencing and analysis.
Conclusions
As an early adopter of TREC screening, the California NBS program has demonstrated not only detection of SCID and clinically important non-SCID TCL with near-complete sensitivity and outstanding specificity but also implementation of high-throughput, DNA-based methodology applicable to other conditions, such as spinal muscular atrophy. The potential harm of live attenuated rotavirus vaccination in infants who are immunodeficient has been averted, strengthening this otherwise beneficial public health measure. The incidence of SCID has proven higher than originally thought, and new disease genes have been discovered in infants identified by SCID NBS. Treatment of SCID detected by NBS has led to 94% survival while revealing areas for future further improvement in targeted chemotherapy and avoidance of CMV infection.
Acknowledgments
We thank Fred Lorey for his energy and vision in bringing about SCID screening in California. Important support and encouragement for us and SCID families came from Fred and Vicki Modell, the SCID Angels for Life, and the Immune Deficiency Foundation.
Glossary
- ADA
adenosine deaminase
- AT
ataxia telangiectasia
- BCL11B
B cell lymphoma 11B
- CDPH
California Department of Public Health
- CHARGE
coloboma of the eye, heart defect, atresia choanae, restricted growth and development, genital abnormality, and ear abnormality
- CI
confidence interval
- CLOVES
congenital lipomatous overgrowth, vascular malformations, epidermal nevi, and spinal and/or skeletal anomalies and/or scoliosis
- CMV
cytomegalovirus
- DBS
dried blood spot
- ERT
enzyme replacement therapy
- EXTL3
exostosinlike glycosyltransferase 3
- GA
gestational age
- GDL
Genetic Disease Laboratory
- GT
gene therapy
- HCT
hematopoietic cell transplant
- IgG
immunoglobulin G
- IL2RG
interleukin-2 receptor γ chain
- IL7R
interleukin-7 receptor
- JAK3
Janus kinase 3
- NBS
newborn screening
- NK
natural killer
- PIDTC
Primary Immune Deficiency Treatment Consortium
- RAC2
member 2 of Rac subfamily of ρ GTPases
- RAG1
recombinase-activating gene 1
- RAG2
recombinase-activating gene 2
- RMRP
RNA component of mitochondrial RNA processing endoribonuclease
- SCID
severe combined immunodeficiency
- TCL
T-cell lymphopenia
- TREC
T-cell receptor excision circle
Footnotes
Dr Puck conceptualized and designed the study, collected and analyzed data, and revised the manuscript; Mr Amatuni collected and analyzed data and wrote the initial manuscript; Dr Currier collaborated on the study design, conducted data analysis and interpretation, and critically reviewed and revised the manuscript; Drs Church, Cowan, Dorsey, Dvorak, Agarwal-Hashmi, Butte, Kapoor, Kohn, Markert, and Moore contributed essential clinical data and reviewed and revised the manuscript; Ms Bishop and Drs Aznar, Feuchtbaum, Sciortino, and Koupaei organized and led the California severe combined immunodeficiency newborn screening program, for which Dr Koupaei was the Genetic Disease Laboratory Director in charge of T-cell receptor excision circle screening; Ms Grimbacher and Dr Nguyen collected and analyzed data and reviewed the manuscript; Dr Naides provided the analysis of flow-cytometric data and reviewed and revised the manuscript; and all authors approved of the final manuscript as submitted and agree to be accountable for all aspects of it.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Dr Puck received support from grant R01 AI105776 and cooperative agreement U54 AI082973 for the Primary Immune Deficiency Treatment Consortium, a member of the Rare Diseases Clinical Research Network funded by the National Institute of Allergy and Infectious Diseases, the Office of Rare Diseases Research, the National Center for Advance Translational Sciences, and the National Institutes of Health; the Jeffrey Modell Foundation; the Lisa and Douglas Goldman Fund; and the Michelle Platt-Ross Foundation. Drs Butte and Church received support from the Jeffrey Modell Foundation. Dr Dvorak received support from grant U54 AI082973. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: Dr Markert developed technology for RVT-802, which has been licensed to Enzyvant Therapeutics GmbH. Dr Markert has received royalties from Enzyvant. Portions of Dr Markert and her research team’s salaries are being paid by the funding from Enzyvant. If the technology is commercially successful in the future, Dr Markert and Duke University may benefit financially. Dr Naides is employed by Quest Diagnostics. Donald Kohn is an inventor of a lentiviral vector for gene therapy of adenosine deaminase severe combined immunodeficiency, which Orchard Therapeutics Ltd has licensed from the University of California Regents; Dr Kohn is a consultant to Orchard Therapeutics Ltd and a member of its Scientific Advisory Board. Dr Cowan serves on the Scientific Advisory Boards for Homology Medicine, Inc, and Exogen, Inc, and on the Data and Safety Monitoring Board for bluebird bio, Inc. Dr Puck discloses spousal employment at a clinical DNA sequencing company, Invitae; the other authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Buckley RH, Schiff RI, Schiff SE, et al. Human severe combined immunodeficiency: genetic, phenotypic, and functional diversity in one hundred eight infants. J Pediatr. 1997;130(3):378–387 [DOI] [PubMed] [Google Scholar]
- 2.Dvorak CC, Cowan MJ, Logan BR, et al. The natural history of children with severe combined immunodeficiency: baseline features of the first fifty patients of the primary immune deficiency treatment consortium prospective study 6901. J Clin Immunol. 2013;33(7):1156–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan K, Puck JM. Development of population-based newborn screening for severe combined immunodeficiency. J Allergy Clin Immunol. 2005;115(2):391–398 [DOI] [PubMed] [Google Scholar]
- 4.Myers LA, Patel DD, Puck JM, Buckley RH. Hematopoietic stem cell transplantation for severe combined immunodeficiency in the neonatal period leads to superior thymic output and improved survival. Blood. 2002;99(3):872–878 [DOI] [PubMed] [Google Scholar]
- 5.Chan A, Scalchunes C, Boyle M, Puck JM. Early vs. delayed diagnosis of severe combined immunodeficiency: a family perspective survey. Clin Immunol. 2011;138(1):3–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kwan A, Puck JM. History and current status of newborn screening for severe combined immunodeficiency. Semin Perinatol. 2015;39(3):194–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heimall J, Logan BR, Cowan MJ, et al. Immune reconstitution and survival of 100 SCID patients post-hematopoietic cell transplant: a PIDTC natural history study. Blood. 2017;130(25):2718–2727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaw KL, Garabedian E, Mishra S, et al. Clinical efficacy of gene-modified stem cells in adenosine deaminase-deficient immunodeficiency. J Clin Invest. 2017;127(5):1689–1699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Ravin SS, Wu X, Moir S, et al. Lentiviral hematopoietic stem cell gene therapy for X-linked severe combined immunodeficiency [published correction appears in Sci Transl Med. 2016;8(341):341er5. Sci Transl Med. 2016;8(335):335ra57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mamcarz E, Zhou S, Lockey T, et al. Interim results from a phase I/II clinical gene therapy study for newly diagnosed infants with X-linked severe combined immunodeficiency using a safety-modified lentiviral vector and targeted reduced exposure to busulfan [abstract]. Blood. 2017;130(suppl 1):523 [Google Scholar]
- 11.Pai SY, Logan BR, Griffith LM, et al. Transplantation outcomes for severe combined immunodeficiency, 2000-2009. N Engl J Med. 2014;371(5):434–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shearer WT, Dunn E, Notarangelo LD, et al. Establishing diagnostic criteria for severe combined immunodeficiency disease (SCID), leaky SCID, and Omenn syndrome: the Primary Immune Deficiency Treatment Consortium experience. J Allergy Clin Immunol. 2014;133(4):1092–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buckley RH. The long quest for neonatal screening for severe combined immunodeficiency. J Allergy Clin Immunol. 2012;129(3):597–604; quiz 605–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Puck JM. Laboratory technology for population-based screening for severe combined immunodeficiency in neonates: the winner is T-cell receptor excision circles. J Allergy Clin Immunol. 2012;129(3):607–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verbsky JW, Baker MW, Grossman WJ, et al. Newborn screening for severe combined immunodeficiency; the Wisconsin experience (2008-2011). J Clin Immunol. 2012;32(1):82–88 [DOI] [PubMed] [Google Scholar]
- 16.Maternal and Child Health Bureau; Health Resources and Services Administration Newborn screening for severe combined immunodeficiency: a summary of the evidence and advisory committee decision. 2009. Available at: https://www.hrsa.gov/sites/default/files/hrsa/advisory-committees/heritable-disorders/rusp/previous-nominations/scid-27-june-2018.pdf. Accessed October 19, 2018
- 17.Kwan A, Abraham RS, Currier R, et al. Newborn screening for severe combined immunodeficiency in 11 screening programs in the United States [published correction appears in JAMA. 2014;312(20):2169]. JAMA. 2014;312(7):729–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kwan A, Church JA, Cowan MJ, et al. Newborn screening for severe combined immunodeficiency and T-cell lymphopenia in California: results of the first 2 years. J Allergy Clin Immunol. 2013;132(1):140–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feuchtbaum L, Yang J, Currier R. Follow-up status during the first 5 years of life for metabolic disorders on the federal Recommended Uniform Screening Panel. Genet Med. 2018;20(8):831–839 [DOI] [PubMed] [Google Scholar]
- 20.Bakare N, Menschik D, Tiernan R, Hua W, Martin D. Severe combined immunodeficiency (SCID) and rotavirus vaccination: reports to the Vaccine Adverse Events Reporting System (VAERS). Vaccine. 2010;28(40):6609–6612 [DOI] [PubMed] [Google Scholar]
- 21.Buchbinder D, Puthenveetil G, Soni A, Hsieh L, Nugent D, Church JA. Newborn screening for severe combined immunodeficiency: an opportunity for intervention. J Perinatol. 2013;33(8):657–658 [DOI] [PubMed] [Google Scholar]
- 22.Punwani D, Zhang Y, Yu J, et al. Multisystem anomalies in severe combined immunodeficiency with mutant BCL11B. N Engl J Med. 2016;375(22):2165–2176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fuchs S, Rensing-Ehl A, Erlacher M, et al. Patients with T+/low NK+ IL-2 receptor γ chain deficiency have differentially-impaired cytokine signaling resulting in severe combined immunodeficiency. Eur J Immunol. 2014;44(10):3129–3140 [DOI] [PubMed] [Google Scholar]
- 24.la Marca G, Canessa C, Giocaliere E, et al. Tandem mass spectrometry, but not T-cell receptor excision circle analysis, identifies newborns with late-onset adenosine deaminase deficiency. J Allergy Clin Immunol. 2013;131(6):1604–1610 [DOI] [PubMed] [Google Scholar]
- 25.Markert ML, Devlin BH, McCarthy EA. Thymus transplantation. Clin Immunol. 2010;135(2):236–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee JH, Markert ML, Hornik CP, et al. Clinical course and outcome predictors of critically ill infants with complete DiGeorge anomaly following thymus transplantation. Pediatr Crit Care Med. 2014;15(7):e321–e326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davies EG, Cheung M, Gilmour K, et al. Thymus transplantation for complete DiGeorge syndrome: European experience. J Allergy Clin Immunol. 2017;140(6):1660–1670.e16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mallott J, Kwan A, Church J, et al. Newborn screening for SCID identifies patients with ataxia telangiectasia. J Clin Immunol. 2013;33(3):540–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Volpi S, Yamazaki Y, Brauer PM, et al. EXTL3 mutations cause skeletal dysplasia, immune deficiency, and developmental delay. J Exp Med. 2017;214(3):623–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kemper AR, Lam KK, Comeau AM, et al. ; Evidence-based Review Group Evidence-based review of newborn screening for spinal muscular atrophy (SMA): final report (v5.2). 2018. Available at: https://www.hrsa.gov/sites/default/files/hrsa/advisory-committees/heritable-disorders/reports-recommendations/sma-final-report.pdf. Accessed October 19, 2018
- 31.Haddad E, Leroy S, Buckley RH. B-cell reconstitution for SCID: should a conditioning regimen be used in SCID treatment? J Allergy Clin Immunol. 2013;131(4):994–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haddad E, Logan BR, Griffith LM, et al. SCID genotype and 6-month posttransplant CD4 count predict survival and immune recovery: a PIDTC retrospective study. Blood. 2018;132(17):1737–1749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Long-Boyle JR, Savic R, Yan S, et al. Population pharmacokinetics of busulfan in pediatric and young adult patients undergoing hematopoietic cell transplant: a model-based dosing algorithm for personalized therapy and implementation into routine clinical use. Ther Drug Monit. 2015;37(2):236–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hamprecht K, Maschmann J, Vochem M, Dietz K, Speer CP, Jahn G. Epidemiology of transmission of cytomegalovirus from mother to preterm infant by breastfeeding. Lancet. 2001;357(9255):513–518 [DOI] [PubMed] [Google Scholar]
- 35.Kwan A, Hu D, Song M, et al. Successful newborn screening for SCID in the Navajo Nation. Clin Immunol. 2015;158(1):29–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strauss KA, Puffenberger EG, Bunin N, et al. Clinical application of DNA microarrays: molecular diagnosis and HLA matching of an Amish child with severe combined immune deficiency. Clin Immunol. 2008;128(1):31–38 [DOI] [PubMed] [Google Scholar]
- 37.Massberg S, von Andrian UH. Fingolimod and sphingosine-1-phosphate–modifiers of lymphocyte migration. N Engl J Med. 2006;355(11):1088–1091 [DOI] [PubMed] [Google Scholar]
- 38.Patel JP, Puck JM, Srinivasan R, et al. Nijmegen breakage syndrome detected by newborn screening for T cell receptor excision circles (TRECs). J Clin Immunol. 2015;35(2):227–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Accetta D, Syverson G, Bonacci B, et al. Human phagocyte defect caused by a Rac2 mutation detected by means of neonatal screening for T-cell lymphopenia. J Allergy Clin Immunol. 2011;127(2):535–538.e1–e2 [DOI] [PubMed] [Google Scholar]