In this prospective cohort study, we examine cognitive and academic outcomes in children with and without a history of PPB.
Abstract
Video Abstract
BACKGROUND:
Studies have revealed an association between positional plagiocephaly and/or brachycephaly (PPB) and development, although little is known about long-term outcomes. We examined cognition and academic achievement in children with and without PPB, testing the hypothesis that children who had PPB as infants would score lower than controls.
METHODS:
We enrolled 187 school-aged children with a history of PPB and 149 controls. Exposures were the presence or absence and severity of infancy PPB (mild, moderate to severe). Cognitive and academic outcomes were assessed by using the Differential Ability Scales, Second Edition and Wechsler Individual Achievement Test, Third Edition, respectively.
RESULTS:
Children with PPB scored lower than controls on most scales of the Differential Ability Scales, Second Edition (standardized effect sizes [ESs] = −0.38 to −0.20) and the Wechsler Individual Achievement Test, Third Edition (ESs = −0.22 to −0.17). Analyses by PPB severity revealed meaningful differences among children with moderate to severe PPB (ESs = −0.47 to −0.23 for 8 of 9 outcomes), but few differences in children with mild PPB (ESs = −0.28 to 0.14).
CONCLUSIONS:
School-aged children with moderate to severe PPB scored lower than controls on cognitive and academic measures; associations were negligible among children with mild PPB. The findings do not necessarily imply that these associations are causal; rather, PPB may serve as a marker of developmental risk. Our findings suggest a role for assessing PPB severity in clinical practice: providing developmental assessment and intervention for infants with more severe deformation and reassurance and anticipatory guidance for patients with mild deformation.
What’s Known on This Subject:
Infants and toddlers with positional plagiocephaly and/or brachycephaly (PPB) exhibit mild developmental delays relative to unaffected children. It is not known whether these developmental differences persist in school-aged children.
What This Study Adds:
School-aged children who had moderate to severe PPB as infants scored lower on measures of cognition and academic achievement than unaffected children. Differences between children who had mild PPB versus unaffected controls were negligible.
Positional skull deformation in infancy is common in the United States and other countries where supine sleep positioning is recommended to prevent sudden infant death syndrome (SIDS). Although these efforts reduced the incidence of SIDS, an unanticipated consequence was an increase in the rate of positional plagiocephaly and/or brachycephaly (PPB).1,2 Prevalence studies are limited, although the best estimates suggest that 20% to 30% of infants have noticeable skull deformation, and PPB has become a common reason for referral to craniofacial and neurosurgery clinics.3–6
Although PPB is considered a benign cosmetic condition, associations between PPB and development have been described. Miller and Clarren7 first reported on PPB and neurodevelopmental outcomes in a retrospective study of 63 school-aged children with a history of PPB and 91 unaffected siblings. Children with PPB were much more likely to have received developmental and educational interventions than siblings. Several others have since reported developmental deficits in children with PPB relative to test norms or controls. In a recent review, most of the studies reviewed revealed an association between PPB and developmental outcomes.8 Aside from the Miller and Clarren7 study, nearly all of this research involved infants and toddlers.
We have previously reported on the development of 235 children with PPB and 237 children without PPB in a cohort recruited in infancy and reevaluated at ages 18 and 36 months.6,9,10 At each assessment, children with PPB scored lower than controls on the Bayley Scales of Infant and Toddler Development, Third Edition. The nature of group differences changed over time: in infancy, PPB was most strongly associated with motor development. By age 3, motor differences were reduced and differences in cognition and language were the most prominent.10
The goals of this study were to evaluate development in school-aged children with and without a history of PPB, testing the hypothesis that children with PPB would score lower than controls and examining differences as a function of PPB severity.
Methods
Study Design
We used a prospective cohort design to assess development in children with and without a history of PPB. In phase 1 of this research, infants with PPB (cases) were recruited at the time of their diagnosis in the Seattle Children’s Craniofacial Center.6 The first 8 controls were recruited through area pediatricians, and remaining controls were recruited through an infant participant registry. To confirm the presence or absence of skull deformation, we used a 4-pod 3dMD digital camera to collect three-dimensional (3D) surface images of participants' head shapes in infancy.6 Images were deidentified, randomly sorted, and rated by 2 pediatricians from the Seattle Children’s Craniofacial Center who were unaware of the infants’ PPB diagnosis. Ratings of deformation were made on a scale of 0 = none, 1 = mild, 2 = moderate, and 3 = severe by using a scale based on a measure described by Argenta et al.1,11 Interrater agreement was excellent for the presence or absence of deformation (κ = 0.80) and good for severity rating (weighted κ = 0.72). We used the average of the 2 ratings to categorize infants as having PPB (diagnosed PPB, severity rating ≥0.5), unaffected controls (no diagnosed PPB, severity rating = 0), and affected controls (no diagnosed PPB, severity rating ≥0.5). The resulting samples included 233 children with confirmed PPB, 167 unaffected controls, and 70 affected controls.
We reapproached children with confirmed PPB and unaffected controls from the infant cohort when they were age ≥7 years. Families received $200 for their participation as well as a written summary of their child’s evaluation results. The study was approved by the Seattle Children’s Hospital Institutional Review Board.
Children With PPB
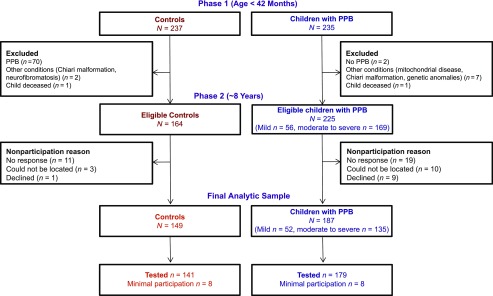
Phase 1 inclusion criteria for children with PPB were diagnosis of PPB by a clinician in the Seattle Children’s Craniofacial Center, confirmed by using independent ratings of 3D images by 2 pediatricians who were unaware of the children’s PPB status; age 4 to 11 months at the time of diagnosis; English reported as the primary language spoken in the home; and the child’s biologic mother was available for participation. Exclusions were prematurity (<35 weeks’ gestation); diagnosed neurodevelopmental conditions (eg, Down syndrome); and severe sensory impairments (eg, deafness or blindness). We enrolled 235 infants with PPB, representing 52% of those approached.6 No discernible deformation was observed on 3D imaging for 2 children with clinical PPB diagnoses, and they were not reapproached for phase 2 follow-up. Significant neurodevelopmental conditions were diagnosed after enrollment for 7 participants (eg, 22q11 deletion, Chiari malformation), who were not approached for follow-up, and 1 child with PPB died. This left a total of 225 potentially eligible participants with PPB (see Fig 1). Ten families could not be located, 9 declined participation, and 19 were unresponsive to outreach efforts. A total of 187 families consented to participate between August 2014 and March 2018, representing 83% of those eligible.
FIGURE 1.
Participants with and without PPB in the infant cohort (phase 1) and school-age follow-up (phase 2).
Unaffected Controls
In addition to the exclusions listed for children with PPB, controls were excluded if they had evidence of deformation on the 3D imaging completed in phase I. Specifically, although they had not been diagnosed with PPB, we excluded the 70 “affected control” children found to have some skull deformation in infancy (63 mild, 7 moderate). One child in the control sample died before phase 2. Out of 164 unaffected controls eligible for follow-up, 3 families could not be located, 1 family declined participation, and 11 families were unresponsive. In total, 149 unaffected control families consented to participate between December 2014 and February 2018, representing 91% of those eligible.
Study Measures
Participants completed an individually-administered neuropsychological assessment battery including the Differential Ability Scales, Second Edition (DAS-2),12 a measure of cognitive ability, and the Wechsler Individual Achievement Test, Third Edition (WIAT-3),13 a measure of academic achievement. Outcomes from the DAS-2 were the composite scales for global cognitive ability (GCA), verbal ability, nonverbal reasoning, spatial ability, working memory, and processing speed. From the WIAT-3, outcomes included composite total reading, written expression, and mathematics scores. Standardized, norm-referenced scores are generated for all DAS-2 and WIAT-3 indices, with an average standard score = 100, SD = 15. Tests were administered by trained psychometrists and video recorded for coding of inter-examiner reliability. Approximately 30% of the tests administered were reviewed by one of the study psychologists (B.R.C., M.L.S.). Average item-level agreement ranged from 95% to 100% for the DAS-2 and 94% to 100% for the WIAT-3.
We used clinician ratings of 3D head shape images described earlier to quantify PPB severity. On the basis of their infant data, children with PPB were categorized as having mild PPB (average severity rating = 0.5–1.0) or moderate to severe PPB (average severity rating = 1.5–3.0).
Caregivers completed a semistructured interview, which confirmed and updated family demographic information; categorized the child’s participation in developmental interventions (eg, occupational therapy, physical therapy, speech and/or language therapy); and documented any newly diagnosed medical conditions.
Statistical Analyses
Descriptive statistics (ie, means, SDs, frequencies) were used to summarize participant demographic and clinical characteristics separately for children with PPB and unaffected controls. We also used descriptive statistics to compare participants and nonparticipants on demographic information from phase 1. We used linear regression analyses with robust standard errors to compare children with PPB and unaffected controls on primary outcomes. Analyses were adjusted for demographics, including socioeconomic status (SES) based on the total Hollingshead14 score (continuous), age at assessment, sex, and race and/or ethnicity (white, non-Hispanic versus people of color or Hispanic). Because of the exploratory nature of these analyses, we did not adjust P values for multiple comparisons and did not consider P values alone to be dichotomous tests of significance. Instead, we evaluated the magnitude, precision, and consistency of effects across outcomes.15 To determine the magnitude of group differences, we calculated standardized mean difference effect sizes (ESs) using the adjusted group difference divided by the root mean square error. To evaluate for attrition bias, we repeated these analyses using inverse probability weighting (IPW).16 For these analyses, we determined propensity scores for phase 2 participation based on phase 1 characteristics (SES, sex, race and/or ethnicity, PPB severity, and Bayley Scales of Infant and Toddler Development, Third Edition scores at age 36 months). We then used these scores to generate propensity weights for linear regression analyses. In essence, these analyses give additional “weight” to participants who were retained in the study and were similar to participants who were lost to follow-up. We also evaluated the effects of developmental intervention on achievement and cognitive scores using censored normal regression.17 This approach assumes that the scores of children who received intervention are “left censored” and, although their true scores in the absence of intervention are unknown, they are presumed to be at least as low as the scores observed.
To evaluate case-control differences by severity of skull deformation, we repeated these analyses for children who had mild PPB in infancy versus controls and those who had moderate to severe PPB versus controls. We also ran sensitivity analyses for clinically relevant characteristics, including history of orthotic helmet treatment, torticollis, twin status, and prematurity (ie, delivery ≤38 weeks’ gestation).
Results
A total of 336 children (N = 187 children with PPB, N = 149 unaffected controls) were re-enrolled for participation. Participants and nonparticipants were similar in most respects, although nonparticipating families tended to be lower in SES, more likely to have moderate to severe PPB in infancy (90% versus 72% among participants), and to have had developmental delays as infants or toddlers (53% versus 38% among participants). Sixteen participants (n = 8 children with PPB, n = 8 controls) were unable to complete an in-person assessment (eg, because of family relocation out of the country) and completed only a caregiver interview. Neuropsychological assessment data were considered invalid for 1 control because of child refusal, resulting in available test data for 1 or more outcomes for 179 children with PPB and 140 unaffected controls.
Children in both groups were predominately boys (66% children with PPB, 57% unaffected controls), white and non-Hispanic (69% children with PPB, 60% unaffected controls), and from middle to upper SES families (ie, Hollingshead14 categories I–II; 80% children with PPB, 78% unaffected controls) (Table 1). Mean age at participation was 9.0 years (SD = 0.8) for children with PPB and 8.8 years (SD = 0.6) for unaffected controls. Children who had PPB in infancy were much more likely than controls to have received some form of developmental intervention (66% children with PPB, 21% unaffected controls). Among children with PPB, 52 (28%) had mild PPB as infants and 135 (72%) had moderate to severe PPB. Sixty-four (34%) children received orthotic helmet treatment of their skull deformation, and 84 (45%) had a history of torticollis.
TABLE 1.
Characteristics of Children With and Without Deformational Plagiocephaly at School Age
| Characteristic | Controls | Children With PPB | ||
|---|---|---|---|---|
| N or Mean | % or SD | N or Mean | % or SD | |
| Total | 149 | 100.0 | 187 | 100.0 |
| Sex | ||||
| Girls | 64 | 43.0 | 64 | 34.2 |
| Boys | 85 | 57.0 | 123 | 65.8 |
| Age, y | 8.8 | 0.6 | 9.0 | 0.8 |
| Race and/or ethnicity | ||||
| White, non-Hispanic | 89 | 59.7 | 130 | 69.5 |
| Asian American or Pacific Islander | 4 | 2.7 | 9 | 4.8 |
| African American | 5 | 3.4 | 0 | 0.0 |
| Hispanic or Latino | 20 | 13.4 | 22 | 11.8 |
| Mixed race or ethnicity | 31 | 20.8 | 26 | 13.9 |
| SES | ||||
| I, highest | 39 | 26.2 | 69 | 36.9 |
| II | 77 | 51.7 | 80 | 42.8 |
| III | 24 | 16.1 | 27 | 14.4 |
| IV | 8 | 5.4 | 10 | 5.3 |
| V, lowest | 1 | 0.7 | 1 | 0.5 |
| Child delivered ≤38 wk | ||||
| No | 139 | 93.3 | 146 | 78.1 |
| Yes | 10 | 6.7 | 41 | 21.9 |
| Developmental interventions, ever received | ||||
| Occupational or physical therapy | 15 | 10.1 | 106 | 56.7 |
| Speech or language therapy | 23 | 15.4 | 56 | 29.9 |
| Any intervention | 31 | 20.8 | 123 | 65.8 |
| Torticollis | ||||
| No | 146 | 98.0 | 103 | 55.1 |
| Yes | 3 | 2.0 | 84 | 44.9 |
| Helmet treatment | ||||
| No | — | — | 122 | 65.2 |
| Yes | — | — | 64 | 34.2 |
| Severity of plagiocephaly | ||||
| Mild | — | — | 52 | 27.8 |
| Moderate | — | — | 101 | 54.0 |
| Severe | — | — | 34 | 18.2 |
—, not applicable.
Mean cognitive and academic achievement scores for both children with PPB and controls were generally within the “average” range relative to test norms (Table 2). After adjustment for demographic characteristics, children with PPB scored lower than unaffected controls on most scales of the DAS-2 with ESs ranging from −0.38 to −0.20 (P = .001–.09). Processing speed was the only exception (ES = 0.04, P = .72). Children with PPB also scored lower than unaffected controls on the WIAT-3, although differences were smaller and statistically nonsignificant (ESs = −0.22 to −0.17, P = .06–.16). The magnitude of group differences increased when adjusting for attrition by using IPW analyses and when adjusting for intervention participation by using censored normal regression (Supplemental Table 3). For example, the ES for DAS-2 GCA scores increased from −0.38 to −0.42 in the primary and IPW-adjusted analyses, respectively, suggesting that the magnitude of group differences is slightly larger when accounting for differential attrition.
TABLE 2.
Comparison of Mean IQ and Achievement Scores in Children With and Without PPB
| Test and Scale | Controls | Children With PPB | Children With PPB Versus Controls | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusteda | |||||||||||||
| N | Mean (SD) | N | Mean (SD) | Mean Difference | 95% CI | ES | P | Mean Difference | 95% CI | ES | P | |||
| DAS-2 | ||||||||||||||
| GCA | 140 | 106.9 (11.9) | 179 | 103.5 (12.9) | −3.46 | −6.18 | −0.74 | −0.28 | .01 | −4.26 | −6.89 | −1.63 | −0.38 | .002 |
| Nonverbal reasoning | 140 | 104.4 (14.0) | 179 | 101.2 (13.6) | −3.23 | −6.28 | −0.19 | −0.24 | .04 | −4.00 | −7.03 | −0.97 | −0.31 | .01 |
| Spatial | 140 | 105.7 (10.9) | 179 | 104.2 (12.3) | −1.55 | −4.10 | 1.01 | −0.13 | .24 | −2.19 | −4.73 | 0.34 | −0.20 | .09 |
| Processing speed | 140 | 101.7 (12.5) | 177 | 102.4 (13.1) | 0.64 | −2.20 | 3.47 | 0.05 | .66 | 0.53 | −2.41 | 3.47 | 0.04 | .72 |
| Verbal | 140 | 107.4 (12.9) | 179 | 103.5 (13.1) | −3.94 | −6.82 | −1.06 | −0.30 | .01 | −4.66 | −7.51 | −1.81 | −0.38 | .001 |
| Working memory | 140 | 104.0 (11.1) | 179 | 101.2 (11.0) | −2.86 | −5.31 | −0.41 | −0.26 | .02 | −3.33 | −5.74 | −0.93 | −0.32 | .01 |
| WIAT-3 | ||||||||||||||
| Mathematics | 140 | 106.4 (15.5) | 176 | 104.9 (15.4) | −1.44 | −4.88 | 2.00 | −0.09 | .41 | −2.75 | −6.14 | 0.63 | −0.19 | .11 |
| Total reading | 137 | 107.4 (15.7) | 171 | 104.9 (13.1) | −2.51 | −5.97 | 0.96 | −0.16 | .16 | −3.19 | −6.47 | 0.10 | −0.22 | .06 |
| Written expression | 139 | 101.6 (14.8) | 169 | 99.6 (15.1) | −2.02 | −5.32 | 1.27 | −0.14 | .23 | −2.29 | −5.50 | 0.92 | −0.17 | .16 |
Adjusted for sex, SES (continuous), race (white or people of color), age in months (continuous).
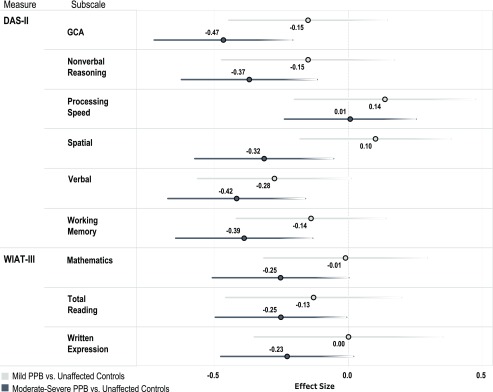
In analyses stratified by PPB severity, we found that case-control differences were consistently larger for children who had moderate to severe PPB than for those with a history of mild PPB (Fig 2). For example, on the DAS-2, case-control differences ranged from ESs = −0.47 to −0.32 (P < .001–.02) on most subscales when comparing children with moderate to severe PPB to unaffected controls. Processing speed was the only subtest on which moderate to severe cases did not score significantly lower than controls (ES = 0.01, P = .71). On the WIAT-3, case-control differences were ESs = −0.25 to −0.23 (P = .04–.07) for children who had moderate to severe PPB. When comparing children with mild PPB to controls, ESs ranged from −0.28 to 0.14 (P = .06–.48) on the DAS-2 and −0.13 to 0.00 (P = .44–.99) on the WIAT-3.
FIGURE 2.
Standardized mean difference ESs and confidence intervals for case-control comparisons, stratified by PPB severity: mild PPB versus unaffected controls and moderate to severe PPB versus unaffected controls.
In sensitivity analyses, we found that group differences were similar for children with and without a history of orthotic helmet treatment and in analyses excluding children with torticollis, those born ≤38 weeks’ gestation, and twins (Supplemental Tables 4 and 5).
Discussion
This is the first study to track developmental outcomes for school-aged children with and without a history of PPB. Strengths include a large, longitudinal cohort of children with and without PPB; the use of “blinded” clinician ratings of 3D images to document the presence and severity of PPB several years before the collection of the outcome data reported here; a low rate of attrition; and a broad, standardized neuropsychological test battery. Our findings are consistent with those observed in early childhood6,9,10 and suggest that children with PPB score lower than controls on measures of cognition and, to a lesser extent, academic achievement. There was a gradient in these differences as a function of the severity of PPB in infancy, with children who had mild PPB showing few if any differences relative to controls and those who had moderate to severe PPB exhibiting robust differences (eg, ESs = −0.47 to −0.32 on the DAS-2, representing ∼4 to 7 standard score points in IQ). Other factors that could modify group differences (eg, history of torticollis, orthotic helmet treatment) had little effect.
Some of the observed case-control differences were modest, and most children in both groups scored within the average range compared with test norms. However, as Bellinger18 has pointed out, even a modest effect can have significant cumulative public health consequences when an exposure or condition is highly prevalent. In addition, our findings were observed in a low risk sample with respect to both biological and social factors, making it unsurprising that both groups scored within the average range relative to test norms. Specifically, in the earlier phase of this research, we excluded children delivered at <35 weeks’ gestation and those with known neurodevelopmental conditions. Although overall retention was excellent, there was differential attrition among children from low SES households, those with more severe PPB in infancy, and children who exhibited developmental delays. Developmental risk was further attenuated by a high rate of intervention. Children with PPB were more likely than unaffected controls to receive developmental intervention (66% versus 21%), and their rate of intervention was higher than is typically observed in the general population.19 All of these factors could result in higher scores than might be observed in the general population, and, if anything, our results may be an underestimate of group differences.
In addition to differential attrition, study limitations include clinic-based recruitment of children with PPB and recruitment of unaffected controls through a participant registry. Although our case and control groups were demographically similar, children referred to a specialty clinic may differ from nonreferred children. For example, referral may be associated with a higher level of concern and vigilance among parents regarding their child’s health, or better access to health care. Similarly, control families participating in a research registry may differ in ways that influence their child’s development (eg, parents’ curiosity or interest in research) but are inadequately captured by demographic measures. Ideally, our findings would be replicated in a study in which population-based sampling of children with and without PPB is used. Another limitation relates to our assessment of academic achievement at a young age, when measures may not be sensitive enough to detect differences.
As we have written previously,10 the association between PPB and neurodevelopment does not necessarily indicate a causal relationship. That is, we cannot say that PPB causes developmental disadvantage. Determining causality is always problematic in observational research. However, future prospective, population-based studies that track early development and head shape from birth would help to clarify whether motor deficits precede or follow skull deformation. We have hypothesized that PPB may, in fact, serve as a marker for developmental vulnerability. For example, infants with subtle neuromotor deficits may be less able to reposition themselves and more likely to develop PPB, particularly in the context of routine supine positioning. As children mature, these motor deficits may have a cascading effect on other areas of development.20,21 If this were true, treating the skull deformation associated with PPB may be less important than evaluating the developmental progress of affected infants and providing early intervention as needed. Sensitivity analyses adjusting for developmental treatments such as physical therapy and occupational therapy suggest their possible benefit for most outcomes.
Our findings reveal that the severity of skull deformation is important when considering developmental risk for infants with PPB, implying the need for reliable measures of PPB to guide pediatricians’ decisions to either refer a child for further evaluation or provide caregiver reassurance and anticipatory guidance. Such measures have been described in the literature11; however, they are not widely used in clinical practice. Rather, we suspect that the decision to refer a child for further evaluation of positional skull deformity or its developmental implications often depends on less data-driven factors such as the degree of expressed caregiver concern, clinicians’ perceptions of caregivers’ potential to benefit from specialty services, or clinicians’ beliefs about the importance of infant skull deformation.
Among infants with moderate to severe deformation, we recommend developmental assessment, monitoring, and intervention. For example, infants with more noticeable skull deformation might receive developmental assessment in primary care with a low threshold for referral to a developmental specialist (eg, physical therapist or occupational therapist). In addition to ruling out other etiologies for head shape anomalies (eg, craniosynostosis), an important function for specialty clinics within tertiary care centers would be to provide developmental assessment and parent coaching to optimize early motor development. In contrast, given that children with mild PPB as infants did not differ from controls (at least in the current study), caregiver reassurance and anticipatory guidance would seem to be most appropriate. Randomized clinical trials for children with varying degrees of skull deformation are needed to confirm these impressions.
Acknowledgments
We thank all of the families who participated in this research for their time and dedication to our project. We also thank Tristen Nash, project coordinator, and the psychometrists and research staff who assisted with data collection.
Glossary
- 3D
three-dimensional
- DAS-2
Differential Ability Scales, Second Edition
- ES
effect size
- GCA
global cognitive ability
- IPW
inverse probability weighting
- PPB
positional plagiocephaly and/or brachycephaly
- SES
socioeconomic status
- SIDS
sudden infant death syndrome
- WIAT-3
Wechsler Individual Achievement Test, Third Edition
Footnotes
Drs Collett and Speltz conceptualized and designed the study, supervised data collection, and drafted the manuscript; Dr Wallace completed the data analyses and drafted the analyses and results sections of the manuscript; Dr Kartin assisted with the selection of outcome measures; Dr Cunningham assisted with the design of the study and helped to develop the methods for measuring positional plagiocephaly and/or brachycephaly severity in the earlier phase of this research; and all authors reviewed and revised the final manuscript.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Funded by a grant from the Eunice Kennedy Shriver National Institute of Child Health and Development (R01HD080462; Dr Collett, Principal Investigator). Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Argenta LC, David LR, Wilson JA, Bell WO. An increase in infant cranial deformity with supine sleeping position. J Craniofac Surg. 1996;7(1):5–11 [DOI] [PubMed] [Google Scholar]
- 2.Turk AE, McCarthy JG, Thorne CH, Wisoff JH. The “back to sleep campaign” and deformational plagiocephaly: is there cause for concern? J Craniofac Surg. 1996;7(1):12–18 [DOI] [PubMed] [Google Scholar]
- 3.Hutchison BL, Hutchison LA, Thompson JM, Mitchell EA. Plagiocephaly and brachycephaly in the first two years of life: a prospective cohort study. Pediatrics. 2004;114(4):970–980 [DOI] [PubMed] [Google Scholar]
- 4.Kane AA, Mitchell LE, Craven KP, Marsh JL. Observations on a recent increase in plagiocephaly without synostosis. Pediatrics. 1996;97(6 pt 1):877–885 [PubMed] [Google Scholar]
- 5.McKinney CM, Cunningham ML, Holt VL, Leroux B, Starr JR. Characteristics of 2733 cases diagnosed with deformational plagiocephaly and changes in risk factors over time. Cleft Palate Craniofac J. 2008;45(2):208–216 [DOI] [PubMed] [Google Scholar]
- 6.Speltz ML, Collett BR, Stott-Miller M, et al. . Case-control study of neurodevelopment in deformational plagiocephaly. Pediatrics. 2010;125(3). Available at: www.pediatrics.org/cgi/content/full/125/3/e537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller RI, Clarren SK. Long-term developmental outcomes in patients with deformational plagiocephaly. Pediatrics. 2000;105(2). Available at: www.pediatrics.org/cgi/content/full/105/2/e26 [DOI] [PubMed] [Google Scholar]
- 8.Martiniuk A, Jacob J, Faruqui N, Yu W. Positional plagiocephaly reduces parental adherence to SIDS guidelines and inundates the health system. Child Care Health Dev. 2016;42(6):941–950 [DOI] [PubMed] [Google Scholar]
- 9.Collett BR, Starr JR, Kartin D, et al. . Development in toddlers with and without deformational plagiocephaly. Arch Pediatr Adolesc Med. 2011;165(7):653–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collett BR, Gray KE, Starr JR, Heike CL, Cunningham ML, Speltz ML. Development at age 36 months in children with deformational plagiocephaly. Pediatrics. 2013;131(1). Available at: www.pediatrics.org/cgi/content/full/131/1/e109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spermon J, Spermon-Marijnen R, Scholten-Peeters W. Clinical classification of deformational plagiocephaly according to Argenta: a reliability study. J Craniofac Surg. 2008;19(3):664–668 [DOI] [PubMed] [Google Scholar]
- 12.Elliott CD. Differential Ability Scales. 2nd ed. San Antonio, TX: Psychological Corporation; 2007 [Google Scholar]
- 13.Wechsler D. Wechsler Individual Achievement Test. 3rd ed. San Antonio, TX: Psychological Corporation; 2009 [Google Scholar]
- 14.Hollingshead AB. Four Factor Index of Social Status. New Haven, CT: Yale University; 1975 [Google Scholar]
- 15.Rothman KJ. Six persistent research misconceptions. J Gen Intern Med. 2014;29(7):1060–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heyting A, Tolboom JTBM, Essers JGA. Statistical handling of drop-outs in longitudinal clinical trials. Stat Med. 1992;11(16):2043–2061 [DOI] [PubMed] [Google Scholar]
- 17.Tobin MD, Sheehan NA, Scurrah KJ, Burton PR. Adjusting for treatment effects in studies of quantitative traits: antihypertensive therapy and systolic blood pressure. Stat Med. 2005;24(19):2911–2935 [DOI] [PubMed] [Google Scholar]
- 18.Bellinger DC. A strategy for comparing the contributions of environmental chemicals and other risk factors to neurodevelopment of children. Environ Health Perspect. 2012;120(4):501–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rice CE, Naarden Braun KV, Kogan MD, et al. ; Centers for Disease Control and Prevention (CDC) . Screening for developmental delays among young children–National Survey of Children’s Health, United States, 2007. MMWR Suppl. 2014;63(2):27–35 [PubMed] [Google Scholar]
- 20.Bornstein MH, Hahn CS, Suwalsky JTD. Physically developed and exploratory young infants contribute to their own long-term academic achievement. Psychol Sci. 2013;24(10):1906–1917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Viholainen H, Ahonen T, Lyytinen P, Cantell M, Tolvanen A, Lyytinen H. Early motor development and later language and reading skills in children at risk of familial dyslexia. Dev Med Child Neurol. 2006;48(5):367–373 [DOI] [PubMed] [Google Scholar]