We discuss implementation of a resuscitation bundle aimed at improving Fm-PPV to decrease the DRI rate of preterm infants.
Abstract
BACKGROUND AND OBJECTIVES:
Avoidance of delivery room intubation (DRI) reduces death or bronchopulmonary dysplasia (BPD) in preterm neonates. Our objective with this quality improvement project was to decrease DRI rates by improving face mask positive pressure ventilation (Fm-PPV) among infants born ≤29 weeks’ gestation.
METHODS:
Key drivers of change were identified from a retrospective review of resuscitation records. A resuscitation bundle to optimize Fm-PPV including the use of a small round mask and end-tidal CO2 detectors, increasing peak inspiratory pressure when indicated, and debriefing after each intubation were implemented in consecutive plan-do-study-act cycles. The DRI rate was tracked by using a control chart. Resuscitation practice and outcomes of pre–quality improvement cohort (QIC) (January 2014–September 2015) were compared with post-QIC (October 2015–December 2016).
RESULTS:
Of the 314 infants who were resuscitated, 180 belonged to the pre-QIC and 134 to the post-QIC. The antenatal steroid administration rate was higher in the post-QIC (54% vs 88%). More infants in the post-QIC had resolution of bradycardia after Fm-PPV (56% vs 77%, P = .02). Infants in the post-QIC had lower DRI rates (58% vs 37%, P < .01), lower need for mechanical ventilation (85% vs 70%, P < .01), lower rates of BPD (26% vs 13%, P < .01), and severe retinopathy of prematurity (14% vs 5%, P = .01). Rates of DRI, BPD, and severe retinopathy of prematurity remained lower even after controlling for the potential confounders.
CONCLUSIONS:
Implementation of a resuscitation bundle decreased the DRI rate and improved outcomes of preterm infants.
Avoiding delivery room intubation (DRI) and stabilization of preterm infants on continuous positive airway pressure (CPAP) reduces death and bronchopulmonary dysplasia (BPD).1–3 The Neonatal Resuscitation Program (NRP) suggests that spontaneously breathing preterm infants with respiratory distress may be supported with CPAP.4 However, the majority of extremely low gestational age (GA) neonates receive face mask positive pressure ventilation (Fm-PPV) in the delivery room (DR).5–7 Inadequate Fm-PPV during these crucial initial minutes after birth may result in persistent hypoxia and bradycardia, necessitating emergent intubation.
Airway obstruction8–10 and mask leak9,10 are common during Fm-PPV. The NRP recommends certain steps (mask seal, repositioning head, suction, open mouth, and increase pressure [MRSOP]) to optimize Fm-PPV before resorting to intubation.11,12 In addition, the use of colorimetric end-tidal carbon dioxide (ETCO2) detectors and appropriate-size round masks help mitigate airway obstruction8 and mask leak,13 respectively. Inadequate positive inspiratory pressure (PIP) and positive end-expiratory pressure (PEEP) during Fm-PPV is a common occurrence.14
As previously reported, the DRI rate among extremely low GA neonates decreased in our hospital during and after participation in Surfactant, Positive Pressure, and Oxygenation Randomized trial.15 However, the DRI rate was higher at our center than what was reported in the trial (61% vs 34%).6,15 The DRI rate continued to be higher in the successive years despite efforts to optimize CPAP in the DR. We hypothesized that DRI can be decreased by implementing a resuscitation bundle. The specific aim of the quality improvement (QI) project was to decrease DRI rates of preterm infants born ≤29 weeks’ gestation by 10% within 12 months.
Methods
Setting
Parkland Hospital and Health System (PHHS) is a large public hospital with >12 000 deliveries annually. At PHHS, all deliveries of infants born ≤29 weeks’ gestation are attended by a dedicated resuscitation team, which includes a resuscitation nurse, a respiratory therapist (RT), a resident physician and/or nurse practitioner, an obstetric nurse, and a neonatal fellow and/or neonatal attending. Resuscitation is performed in the actual DR. The roles of team members are well defined, and validation of skills and knowledge is regularly assessed by using simulation and debriefing. Resuscitation details are verbalized by the team and entered on a paper form in real time by a trained obstetric nurse. The umbilical cord is clamped immediately after birth. All infants born ≤32 weeks’ gestation are started immediately on CPAP by using a T-piece resuscitator (Neopuff; Fisher & Paykel, Auckland, New Zealand). Fm-PPV is provided per the NRP guidelines. Resuscitation is started at 21% oxygen and titrated to meet the NRP saturation goals. PIP is usually set at 25 and PEEP at 5 cm H2O. The RT is primarily responsible for providing Fm-PPV but receives input from the team leader. No predetermined GA criteria is used for intubation. Infants who do not need intubation in the DR are maintained on CPAP through bi-nasal prongs (Hudson Prongs; Teleflex, Wayne, PA) connected to a PEEP valve, running 8 to 10 L/min flow before transport to the NICU. Infants are intubated in the NICU for surfactant therapy if they require 0.4 to 0.5 fraction of inspired oxygen at CPAP level of 6 to 7 cm H2O.
Of note, PHHS relocated to a new larger facility in August 2015 where the DR is 1 floor below the NICU. The antenatal steroid (ANS) administration policy changed in September 2015 to include all mothers with an impending preterm delivery from the previous practice of selective administration excluding women with pregnancy-induced hypertension and diabetes mellitus.16
Planning the Intervention
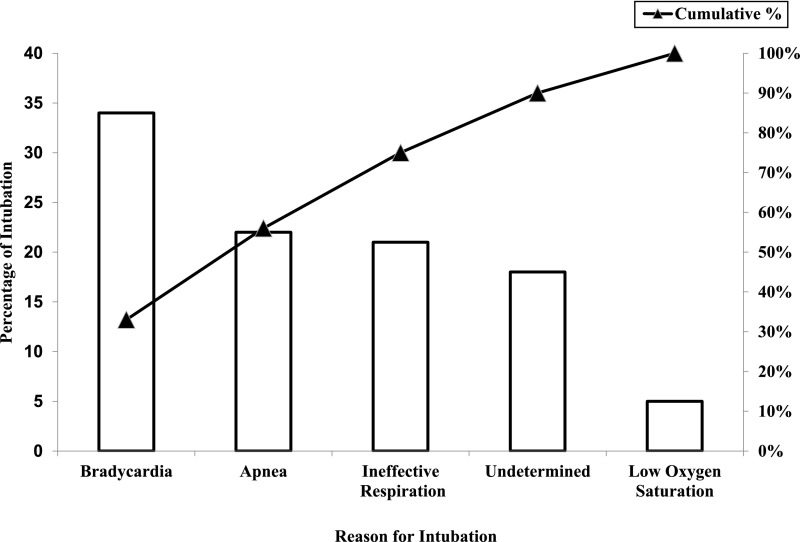
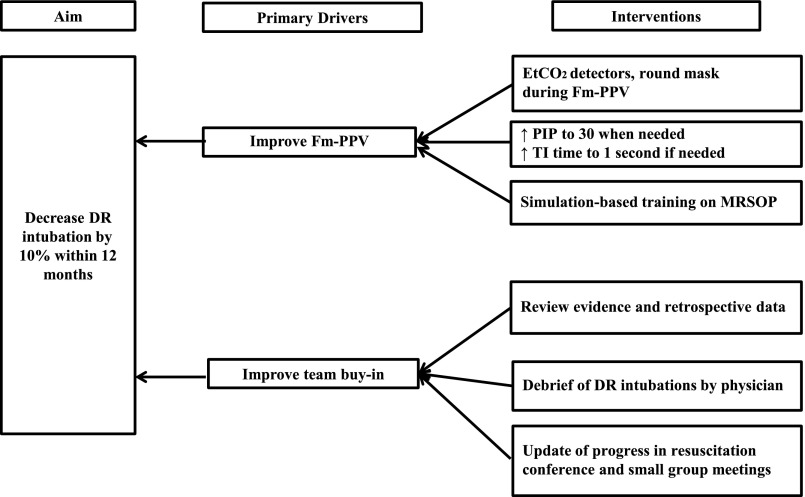
A multidisciplinary “Golden Hour” project team was formed in January 2014 to streamline the admission process of neonates born ≤29 weeks’ gestation. Data on DRI were prospectively collected among other relevant measures. The team identified the opportunity to decrease DRI. A retrospective analysis of resuscitation practices was conducted on infants born between January 2014 and September 2015 to determine key drivers of change. Analysis of indications for intubation (Fig 1) revealed that unresolved bradycardia (heart rate <100 beats per minute) after Fm-PPV was the most common reason for DRI, accounting for 34% of all intubations. Indeed, 18% of DRIs had no documented NRP-specified indications. Among intubated infants, only 45% had documentation of MRSOP steps and 27% received PIP >25 cm H2O before intubation. Based on these findings, improved Fm-PPV and team buy-in for noninvasive support were identified as key drivers of change (Fig 2). To meet these 2 objectives, a resuscitation bundle was designed with 9 key elements (Table 1). Briefly, bundle components included the use of round masks in combination with ETCO2 detectors (Pedi-Cap; Medtronic PLC, Minneapolis, MN) and strict adherence to MRSOP steps, particularly increasing pressure to 30 cm H2O and increasing inspiratory time (TI) to 1 second consecutively for unresolved bradycardia before resorting to intubation. Bundle also emphasized accurate documentation of MRSOP steps and debriefing after each DRI.
FIGURE 1.
Pareto chart of reasons for intubation.
FIGURE 2.
Key drivers of change.
TABLE 1.
DR Resuscitation QI Bundle
| Objective | Intervention |
|---|---|
| Improve Fm-PPV | Initiate PPV with PIP 25 and PEEP 5 if infant has bradycardia, apnea, or ineffective respiration; increase PIP to 30 as part of MRSOP steps before intubation attempt |
| Increase TI to 1 s if infant not responding to MRSOP steps | |
| Simulation-based training focused on MRSOP. Change to appropriate size round masks using size guide at the resuscitation cart. Use of ETCO2 detectors with Fm-PPV | |
| Document MRSOP | |
| Improve documentation | Document indication for intubation. RT to double check the accuracy of documentation with the resuscitation nurse |
| Debriefing | Debrief after each intubation |
Planning the Study of the Intervention
Infants born between January 2014 and September 2015 constituted the pre–quality improvement cohort (QIC) and infants born between October 2015 and December 2016 were included as the post-QIC. Three team members prospectively collected details of each resuscitation from the electronic medical records. In addition, data were obtained from the existing NICU database.
Description of Measures
The primary outcome of interest was the rate of DRI. Process measures included maximum PIP, duration of Fm-PPV, and documentation of reason for intubation. Balancing measures were duration of bradycardia, time to NICU admission, proportion of infants developing hypoglycemia (<40 mg/dL) and hypothermia (<36°C) on admission, and pneumothorax during hospital stay.
Statistical Analyses
Analysis was performed by using SAS version 9.2 (SAS Institute, Inc, Cary, NC). Categorical variables were analyzed by Pearson χ2 or Fisher’s exact test as applicable. Continuous variables were analyzed by Student’s t test or Mann–Whitney U test. A 2-sided 0.05 level of significance was used for all analyses. The rate of DRI was tracked by using statistical process control charts (QI Macros; KnowWare International, Inc, Denver, CO) with quarterly intervals. A stepwise forward multiple logistic regression was performed for DRI, BPD, death or BPD, and severe retinopathy of prematurity (sROP). In addition to those prespecified variables known to be associated with the outcomes of interest, all variables with P values <.1 were included in the logistic regression.
Ethical Considerations
The Institutional Review Board of University of Texas Southwestern Medical Center considered the project exempt from review because QI PHHS Office of Research Administration approved the project.
QI Activities
Plan-Do-Study-Act 1 (October 2015–December 2015)
In October 2015, findings of the retrospective analysis of DRI practices were presented to faculty, fellows, nurse practitioners, and the resuscitation team. A consensus resuscitation bundle was adopted.
Plan-Do-Study-Act 2 (January–May 2016)
The RTs led peer-to-peer simulation-based training of providing Fm-PPV using a round mask and ETCO2 detectors. Training was focused on mask holds and mask position to avoid mask leak and obstruction and to achieve chest rise. Extra small (35 mm diameter) and small (42 mm diameter) round masks (Fisher and Paykel Healthcare, Auckland, New Zealand) were deployed in the DR after the majority of staff completed training. ETCO2 detectors during Fm-PPV were set up before the delivery of the infant. During Fm-PPV, absence of color change prompted change in mask position and/or mask hold and assessment for other causes of airway obstruction. The goal was to achieve chest rise during PPV. The PIP was increased to 30 cm H2O and TI increased to 1 second sequentially if the heart rate continued to be <100 beats per minute and not rising. Data for the DRI rate were presented during a biweekly resuscitation conference and the reason for each intubation reviewed. MRSOP steps were emphasized.
Plan-Do-Study-Act 3 (June–December 2016)
A debriefing check list was created for infants needing DRI and were audited on a regular basis. Control charts for DRI were reviewed every month.
Results
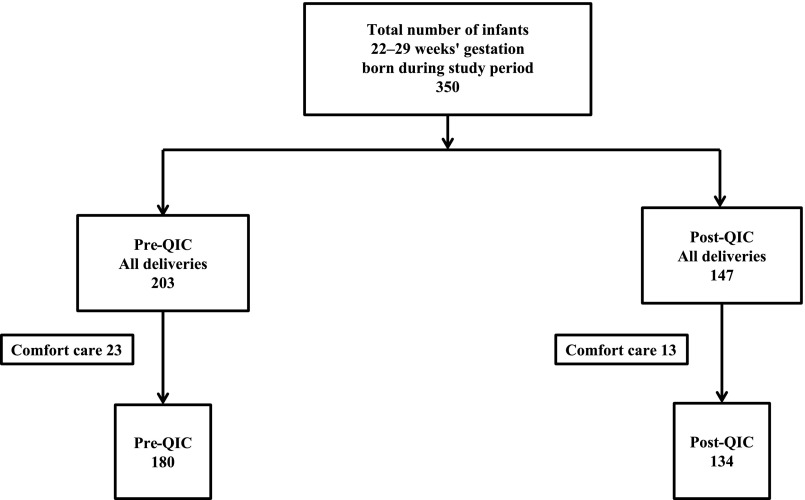
There were 350 infants between 22 and 29 weeks’ gestation born during the study period (Fig 3). After excluding neonates who received comfort care, 314 neonates were included. Of these, 180 were born in the pre-QIC and 134 in the post-QIC. There were no differences in maternal and neonatal characteristics between groups (Table 2), except for the higher ANS rate and less base deficit in the post-QIC.
FIGURE 3.
Flow diagram of study population.
TABLE 2.
Comparison of Maternal and Infant Characteristics
| Characteristic | Pre-QIC (n = 180) | Post-QIC (n = 134) | P |
|---|---|---|---|
| Maternal age, y, mean ± SD | 28.7 ± 7 | 28.6 ± 8 | NS |
| Hispanic, n (%) | 124 (69) | 90 (67) | NS |
| Preeclampsia, n (%) | 59 (33) | 41 (31) | NS |
| Diabetes, n (%) | 21 (12) | 15 (11) | NS |
| PROM (≥18 h), n (%) | 37 (20) | 22 (17) | NS |
| ANSs, n (%) | 98 (54) | 119 (89) | <.01 |
| Antenatal magnesium, n (%) | 112 (62) | 88 (66) | NS |
| Oligohydramnios, n (%) | 9 (5) | 3 (2) | NS |
| Multiple birth, n (%) | 29 (16) | 28 (21) | NS |
| Chorioamnionitis n (%) | 10 (6) | 7 (5) | NS |
| Cesarean delivery, n (%) | 118 (66) | 87 (65) | NS |
| General anesthesia, n (%) | 41 (23) | 24 (18) | NS |
| Male sex, n (%) | 90 (50) | 59 (44) | NS |
| GA, wk, mean ± SD | 26.8 ± 1.7 | 26.9 ± 1.9 | NS |
| Birth wt, g, mean ± SD | 1016 ± 0.09 | 1023 ± 305 | NS |
| Cord blood gas pH, mean ± SD | 7.26 ± 0.09 | 7.26 ± 0.08 | NS |
| Cord blood gas base deficit, mean ± SD | −6.2 ± 3.7 | −5.05 ± 3.54 | .02 |
NS, not significant; PROM, premature rupture of membranes.
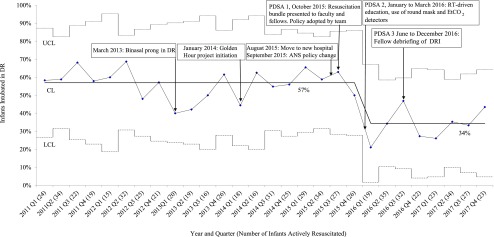
The control chart for DRI rate (Fig 4) revealed a special cause variation of high DRI rate up to the fourth quarter of 2015. Central line shifted down per standard rules of special cause variation in the first quarter of 2016. DRI remained low for 2016–2017 without further special cause variation demonstrating a stable process.
FIGURE 4.
DRI rate. CL, center line; LCL, lower control limit; Q1, quarter 1; Q2, quarter 2; Q3, quarter 3; Q4, quarter 4; UCL, upper control limit.
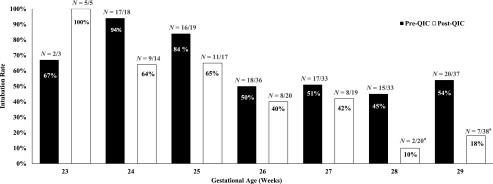
The rate of DRI, the primary outcome of the study, decreased in post-QIC (Table 3). Fm-PPV was more effective in resolving bradycardia in post-QIC. DRI decreased particularly in infants born at 28 to 29 weeks’ gestation (Fig 5). The rate of DRI remained lower in post-QIC even after adjusting for GA, sex, and ANS rate (adjusted odds ratio [aOR] 0.4 [confidence interval (CI) 0.3–0.7], P < .01). There was no difference in the reasons for intubation between groups. Of the balancing measures, there was no difference in the duration of bradycardia and incidence of pneumothorax, but the median time to NICU admission was longer and incidence of hypoglycemia was higher in the post-QIC.
TABLE 3.
Resuscitation and Admission Details
| Characteristics | Pre-QIC (n = 180) | Post-QIC (n = 134) | P |
|---|---|---|---|
| Received Fm-PPV, n (%) | 146 (81) | 113 (84) | NS |
| Bradycardia needing Fm-PPV, n (%) | 69 (38) | 57 (43) | NS |
| Successful resolution of bradycardia, n (%) | 39/69 (56) | 44/57 (77) | .02 |
| Duration of Fm-PPV, s, mean ± SD | 188 ± 138 | 189 ± 136 | NS |
| Duration of bradycardia, s | 120 (60 210) | 90 (60 180) | NSa |
| Maximum CPAP (cm H2O) | 5 (5, 5) | 5 (5, 5) | NSa |
| Maximum PIP (cm H2O) | 25 (25, 25) | 25 (25, 29) | <.01a |
| Maximum Fio2%, mean ± SD | 67 ± 25 | 71 ± 24 | NS |
| DR intubation, n (%) | 105 (58) | 50 (37) | <.01 |
| Minutes to first DR intubation | 3 (2, 5) | 4 (3, 7) | NSa |
| Apgar score at 1 min | 4 (2, 6) | 4 (2, 6) | NSa |
| Apgar score at 5 min | 7 (5, 8) | 7 (6, 8) | NSa |
| Time to admission, min | 21 (19, 25) | 24 (21, 27) | <.01a |
| Hypothermia on admission (≤36°C), n (%) | 9 (5) | 2 (2) | NS |
| Hyperthermia on admission (≥38°C), n (%) | 19 (11) | 23 (17) | NS |
| Hypoglycemia on admission (≤40 mg/dL), n (%) | 14 (8) | 20 (15) | .049 |
Fio2, fraction of inspired oxygen; NS, not significant.
Mann–Whitney U test; median (25th, 75th).
FIGURE 5.
Comparison of DRI rate at different GAs between pre- and post-QIC. a Fisher’s exact test P value < .05.
In the post-QIC, fewer infants needed surfactant therapy and mechanical ventilation (MV) during their NICU stay (Table 4). There was a lower incidence of BPD, composite outcome of death or BPD, and sROP compared with the pre-QIC. Death or BPD (aOR 0.53 [CI 0.30–0.96], P = .04), BPD (aOR 0.4 [CI 0.2–0.7], P < .01), and sROP (aOR 0.3 [CI 0.1–0.7], P < .01) rates were lower in the post-QIC even after adjusting for GA, sex, and ANS. There were no differences in DRI rate and neonatal outcomes between the plan-do-study-act (PDSA) cycles (Table 5).
TABLE 4.
Respiratory Care and Neonatal Outcome
| Characteristic | Pre-QIC (n = 180) | Post-QIC (n = 134) | P |
|---|---|---|---|
| Surfactant therapy, n (%) | 139 (77) | 81 (60) | <.01 |
| MV during NICU, n (%) | 153 (85) | 94 (70) | <.01 |
| Ventilator d | 5 (1, 21) | 4 (0, 13) | NSa |
| Pneumothorax, n (%) | 11 (6) | 13 (10) | NS |
| BPD, n (%)b | 47 (26) | 17 (13) | <.01 |
| Death or BPD, n (%) | 66 (37) | 33 (25) | .02 |
| Postnatal steroid, n (%) | 20 (11) | 8 (6) | NS |
| Necrotizing enterocolitis, n (%) | 16 (9) | 9 (7) | NS |
| sROP, n (%)c | 25 (14) | 7 (5) | .01 |
| Severe intraventricular hemorrhage, n (%)d | 21 (12) | 10 (8) | NS |
| Death, n (%) | 19 (11) | 16 (12) | NS |
NS, not significant.
Mann–Whitney U test; median (25th, 75th).
BPD: need for oxygen at 36 wk postmenstrual age.
sROP: stage ≥3.
Severe intraventricular hemorrhage: Papile stage ≥3.
TABLE 5.
Infant Characteristics and Neonatal Outcomes by PDSA Cycles
| PDSA 1 (October 15–December 15), n = 26 | PDSA 2 (June 16–May 16), n = 44 | PDSA 3 (June 16–December 16), n = 64 | P | |
|---|---|---|---|---|
| ANSs, n (%) | 25 (96) | 38 (86) | 56 (88) | NS |
| GA, wk, mean ± SD | 27.4 ± 1.9 | 26.8 ± 1.9 | 26.6 ± 1.9 | NS |
| Birth wt, g, mean ± SD | 1081 ± 320 | 1023 ± 301 | 999 ± 304 | NS |
| DR intubation, n (%) | 13 (50) | 12 (27) | 25 (40) | NS |
| Surfactant therapy, n (%) | 18 (69) | 24 (55) | 39 (61) | NS |
| Intubated during NICU stay, n (%) | 20 (77) | 29 (66) | 45 (70) | NS |
| BPD, n (%) | 2 (8) | 6 (14) | 9 (14) | NS |
| sROP, n (%) | 2 (8) | 2 (5) | 3 (5) | NS |
| Death, n (%) | 4 (15) | 2 (5) | 10 (16) | NS |
| Death or BPD, n (%) | 6 (23) | 8 (18) | 19 (30) | NS |
Discussion
This single-center QI project shows that the rate of DRI can be decreased among preterm infants by implementing a resuscitation bundle aimed at improving the effectiveness of Fm-PPV. To the best of our knowledge, this is the first QI study in which the use of MRSOP ventilation corrective steps was systematically evaluated along with training in effective Fm-PPV aimed at reducing the rate of DRI among preterm infants. The rate of DRI dropped by 21% in the post-QIC. The decreased rate of DRI was associated with a lower need for surfactant therapy and MV during the NICU stay. The incidence of BPD, the composite outcome of death or BPD, and sROP also decreased in the post-QIC.
Mask leak and airway obstruction are common with Fm-PPV and often remain unrecognized.8–10,17–21 Correct positioning,22 appropriate mask size, prevention of mask leak, as well as simulation training and assistive devices to determine obstructed breaths are critical to avoid DRI.20 Palme et al13 reported less mask leak with round mask compared with triangular mask while providing PPV. However, a study from O’Donnell et al17 did not see differences between the masks in regards to leak. Out of the available masks at PHHS, the smallest one was found to be too large for infants aged 23 to 25 weeks’ gestation in the pre-QIC.23 Round preterm masks appropriate for GA were used in post-QIC. Mask hold and mask position are important in achieving a proper seal.18 The use of an ETCO2 detector during Fm-PPV can help identify obstructed breaths early. Persistent absence of color change on the ETCO2 detector along with absence of chest rise indicates the need for ventilation corrective measures (MRSOP).8 There is a paucity of data on the optimal PIP for preterm infants. A PIP of 20 to 30 cm H2O has been reported to be adequate.4,24,25 Higher PIPs may be necessary to compensate for mask leak and overcome airway obstruction, although one should be careful not to deliver excessive pressures to avoid lung injury and air leaks.8 In our algorithm, the PIP was increased to 30 cm H2O and TI to 1 second as part of the MRSOP intervention for unresolved bradycardia. Finally, achieving a team buy-in is important in implementing the desired interventions. Peer-to-peer simulation and debriefing of individual resuscitations were designed to place accountability on the team in achieving set goals and to create an environment of self-learning.
Several single-center studies have revealed decreased DRI after implementing strategies favoring early initiation of CPAP in spontaneously breathing newborns compared with the previous practice of intubation for prophylactic surfactant.26–33 However, there is limited evidence to support decreased rates of DRI beyond what can be achieved by the adoption of strategies to stabilize spontaneously breathing infants who are not bradycardic at birth. Recently, use of a respiratory function monitor (RFM) during resuscitation has shown to help better identification of mask leak, obstruction, and avoid excessive tidal volumes, decrease DRI and oxygen use.18,19,34 In addition, written instruction and simulation-based learning using an RFM decreases mask leak.18,21 RFMs are not routinely available and are used primarily in research settings. In our study, we used ETCO2 colorimetric detector and round masks, which are easily available and were associated with a decrease in DRI. Our rate of DRI is similar to the collaborative study that reported a decrease in DRI rate by promoting early CPAP, avoiding intubation and decreased hypothermia.35 The collaborative centers also reported decreased BPD and sROP.36 However, no specific strategy was described to reduce intubation rates. Recently, 2 other strategies have been studied in decreasing DRI rates. Sustained inflation using nasopharyngeal prongs with PIP of 20 to 30 H2O for 10- to 15-second inspiration time reduced the rate of DRI compared with intermittent Fm-PPV but was associated with increased pneumothorax.37 Large randomized controlled trials (RCTs) are currently underway in which sustained inflation is compared with intermittent Fm-PPV.38 The authors of a single-center study reported decreased DRI rate using modified nasal cannulae.39 Because both these strategies are still experimental, we did not include them as part of the resuscitation bundle.
Interestingly, the decreased rate of DRI in the post-QIC was associated with decreased need for surfactant and MV consistent with the findings of RCTs.6,40–42 The decrease in the incidence of BPD and composite death or BPD seen in our study is consistent with the findings of previous meta-analyses.1–3 The incidence of sROP was also lower in the post-QIC. However, RCTs have not shown any reduction in sROP with increased use of ANS or promoting stabilization on CPAP in DR.2,43 Pulse oximeter saturation target limits for supplemental oxygen therapy in the NICU was similar in both cohorts (88%–94%). Contributing factors for reduced sROP could be due to decreased exposure to MV or increase in rate of ANS. Although we used logistic regression to address potential confounders including ANS, secular trends, other QI initiatives, or unknown confounders could account for the observed differences.
Prolonged apnea or ineffective respiration in the absence of bradycardia may require DRI to provide a stable airway for transport. In this situation, it is unclear when the resuscitation team should proceed to intubation in the DR. The average duration of Fm-PPV in both cohorts was 160 seconds. No studies have contained evaluations of the safety of longer durations of Fm-PPV. However, a longer time in the DR with continued Fm-PPV could increase the likelihood of other complications such as hypothermia and hypoglycemia. The higher incidence of hypoglycemia and longer time to NICU admission in the post-QIC could be related to the need to travel a longer distance between DR and NICU in the new hospital. The incidence of pneumothorax in the post-QIC was 10%, which is considerable but not statistically different from pre-QIC. None of the infants in the study had pneumothorax on admission to NICU. We speculate that the incidence of pneumothorax reported in this study likely reflects respiratory management after admission to NICU.
DRI rates decreased markedly among the infants born 28 to 29 weeks’ gestation. The difference was not statistically significant for infants of other GAs, likely because of the relatively smaller sample size.
Our study has several important strengths. First, such a systematic approach to improve effectiveness of Fm-PPV using rigorous QI methodology is new. Second, our study demonstrates how detailed documentation can help improve processes in the DR, even when video-recordings are not available or feasible. Video-recordings of DR resuscitation is a useful tool in advancing training and research,44,45 but its use has some ethical, economical, and legal constraints.45 Although centers that use video have reported inconsistencies between guidelines and actual practice,14,44,46,47 use of such information to decrease DRI rate has not been reported.
There are several limitations to our study. The post-QIC had a higher rate of ANS administration. In animal studies, ANS administration before delivery increases lung volume and dynamic compliance.48 The effect of ANS administration on the rate of DRI is not known. Meta-analyses of studies of ANS do not reveal a decrease in the incidence of BPD.43 The DRI rate remained lower in the post-QIC even after controlling for the potential confounding effect of ANS administration. However, it is still possible that the higher rate of ANS in the post-QIC may have had an impact on the neonatal response to DR resuscitation, need for surfactant, MV, and incidence of BPD more than what could be accounted for in the logistic regression. This study was done over a period of 3 years, which creates potential for confounding due to secular trends and changes in NICU practice. PHHS moved from a cramped unit in which multiple infants were cared for in 1 bay to a newer facility with single-patient rooms. Although no obvious changes in approach were noted, there could be undetermined differences in the care provided in such different settings. Considerable recruitment of new faculty occurred between the 2 periods. Although the nurses that attended DR resuscitations remained consistent during the 2 epochs, several new RTs joined the team. Although these dynamics could make the DR processes more unstable, it is possible that the ongoing training of the resuscitation bundle led to improved processes.
Focus of our future PDSA cycle steps include achieving further reduction in the rate of DRI among infants born 23 to 25 weeks’ gestation and implement delayed cord clamping in vigorous preterm infants.
Conclusions
In our study, we demonstrate the safety and effectiveness of a resuscitation bundle to improve Fm-PPV and decrease the DRI rate among infants born between 23 and 29 weeks’ gestation. Larger studies in different centers should be conducted to confirm the effectiveness of this bundle.
Acknowledgments
We thank Dr Rashmin Savani and Dr Myra Wyckoff for their input throughout this QI project and review of the article.
Glossary
- ANS
antenatal steroid
- aOR
adjusted odds ratio
- BPD
bronchopulmonary dysplasia
- CI
confidence interval
- CPAP
continuous positive airway pressure
- DR
delivery room
- DRI
delivery room intubation
- ETCO2
end-tidal CO2
- Fm-PPV
face mask positive pressure ventilation
- GA
gestational age
- MRSOP
mask seal, repositioning head, suction, open mouth, and increase pressure
- MV
mechanical ventilation
- NRP
Neonatal Resuscitation Program
- PDSA
plan-do-study-act
- PEEP
positive end-expiratory pressure
- PHHS
Parkland Hospital and Health System
- PIP
positive inspiratory pressure
- QI
quality improvement
- QIC
quality improvement cohort
- RCT
randomized controlled trial
- RFM
respiratory function monitor
- RT
respiratory therapist
- sROP
severe retinopathy of prematurity
- TI
inspiratory time
Footnotes
Dr Kakkilaya conceptualized and designed the study, wrote all drafts of the manuscript, and assisted with data analysis and interpretation; Dr Jubran participated in the designing of the study and reviewed the manuscript; Dr Mashruwala, Ms Simcik, and Ms Ramon prospectively collected data and reviewed the manuscript; Ms Marshall led the simulation-based training and reviewed the manuscript; Mr Brown conducted the analysis of data and reviewed and revised the manuscript; Dr Jaleel participated in the design of the study and reviewed and revised the manuscript; Dr Kapadia conceptualized and designed the study, assisted with data analysis, and reviewed and revised the manuscript; and all authors approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development and National Institutes of Health grant 1K23HD083511-01A1 (to Dr Kapadia). Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Rojas-Reyes MX, Morley CJ, Soll R. Prophylactic versus selective use of surfactant in preventing morbidity and mortality in preterm infants. Cochrane Database Syst Rev. 2012;(3):CD000510. [DOI] [PubMed] [Google Scholar]
- 2.Schmölzer GM, Kumar M, Pichler G, Aziz K, O’Reilly M, Cheung PY. Non-invasive versus invasive respiratory support in preterm infants at birth: systematic review and meta-analysis [published correction appears in BMJ. 2014;348:g58]. BMJ. 2013;347:f5980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Subramaniam P, Ho JJ, Davis PG. Prophylactic nasal continuous positive airway pressure for preventing morbidity and mortality in very preterm infants. Cochrane Database Syst Rev. 2016;(6):CD001243. [DOI] [PubMed] [Google Scholar]
- 4.Wyckoff MH, Aziz K, Escobedo MB, et al. . Part 13: neonatal resuscitation: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care (reprint). Pediatrics. 2015;136(suppl 2):S196–S218 [DOI] [PubMed] [Google Scholar]
- 5.Finer NN, Carlo WA, Duara S, et al. ; National Institute of Child Health and Human Development Neonatal Research Network . Delivery room continuous positive airway pressure/positive end-expiratory pressure in extremely low birth weight infants: a feasibility trial. Pediatrics. 2004;114(3):651–657 [DOI] [PubMed] [Google Scholar]
- 6.Finer NN, Carlo WA, Walsh MC, et al. ; SUPPORT Study Group of the Eunice Kennedy Shriver NICHD Neonatal Research Network . Early CPAP versus surfactant in extremely preterm infants [published correction appears in N Engl J Med. 2010;362(23):2235]. N Engl J Med. 2010;362(21):1970–1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phillipos E, Solevåg AL, Aziz K, et al. . Oxygen saturation and heart rate ranges in very preterm infants requiring respiratory support at birth. J Pediatr. 2017;182:41–46.e2 [DOI] [PubMed] [Google Scholar]
- 8.Finer NN, Rich W, Wang C, Leone T. Airway obstruction during mask ventilation of very low birth weight infants during neonatal resuscitation. Pediatrics. 2009;123(3):865–869 [DOI] [PubMed] [Google Scholar]
- 9.Schilleman K, Witlox RS, Lopriore E, Morley CJ, Walther FJ, te Pas AB. Leak and obstruction with mask ventilation during simulated neonatal resuscitation. Arch Dis Child Fetal Neonatal Ed. 2010;95(6):F398–F402 [DOI] [PubMed] [Google Scholar]
- 10.Schmölzer GM, Dawson JA, Kamlin CO, O’Donnell CP, Morley CJ, Davis PG. Airway obstruction and gas leak during mask ventilation of preterm infants in the delivery room. Arch Dis Child Fetal Neonatal Ed. 2011;96(4):F254–F257 [DOI] [PubMed] [Google Scholar]
- 11.Wyckoff MH, Aziz K, Escobedo MB, et al. . Part 13: neonatal resuscitation: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132(18suppl 2):S543–S560 [DOI] [PubMed] [Google Scholar]
- 12.Weiner GM, Zaichkin J, eds. Textbook of Neonatal Resuscitation. 7th ed. Elk Grove Village, IL: American Academy of Pediatrics and American Heart Association; 2016 [Google Scholar]
- 13.Palme C, Nyström B, Tunell R. An evaluation of the efficiency of face masks in the resuscitation of newborn infants. Lancet. 1985;1(8422):207–210 [DOI] [PubMed] [Google Scholar]
- 14.Yamada NK, Yaeger KA, Halamek LP. Analysis and classification of errors made by teams during neonatal resuscitation. Resuscitation. 2015;96:109–113 [DOI] [PubMed] [Google Scholar]
- 15.LeVan JM, Wyckoff MH, Ahn C, et al. . Change in care among nonenrolled patients during and after a randomized trial. Pediatrics. 2013;132(4). Available at: www.pediatrics.org/cgi/content/full/132/4/e960 [DOI] [PubMed] [Google Scholar]
- 16.Bloom SL, Leveno KJ. Corticosteroid use in special circumstances: preterm ruptured membranes, hypertension, fetal growth restriction, multiple fetuses. Clin Obstet Gynecol. 2003;46(1):150–160 [DOI] [PubMed] [Google Scholar]
- 17.O’Donnell CP, Davis PG, Lau R, Dargaville PA, Doyle LW, Morley CJ. Neonatal resuscitation 2: an evaluation of manual ventilation devices and face masks. Arch Dis Child Fetal Neonatal Ed. 2005;90(5):F392–F396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wood FE, Morley CJ, Dawson JA, et al. . Improved techniques reduce face mask leak during simulated neonatal resuscitation: study 2. Arch Dis Child Fetal Neonatal Ed. 2008;93(3):F230–F234 [DOI] [PubMed] [Google Scholar]
- 19.Schmölzer GM, Morley CJ, Davis PG. Respiratory function monitoring to reduce mortality and morbidity in newborn infants receiving resuscitation. Cochrane Database Syst Rev. 2010;(9):CD008437. [DOI] [PubMed] [Google Scholar]
- 20.O’Donnell CP, Schmölzer GM. Resuscitation of preterm infants: delivery room interventions and their effect on outcomes. Clin Perinatol. 2012;39(4):857–869 [DOI] [PubMed] [Google Scholar]
- 21.Wood FE, Morley CJ. Face mask ventilation–the dos and don’ts. Semin Fetal Neonatal Med. 2013;18(6):344–351 [DOI] [PubMed] [Google Scholar]
- 22.Chua C, Schmölzer GM, Davis PG. Airway manoeuvres to achieve upper airway patency during mask ventilation in newborn infants - an historical perspective. Resuscitation. 2012;83(4):411–416 [DOI] [PubMed] [Google Scholar]
- 23.O’Shea JE, Thio M, Owen LS, Wong C, Dawson JA, Davis PG. Measurements from preterm infants to guide face mask size. Arch Dis Child Fetal Neonatal Ed. 2016;101(4):F294–F298 [DOI] [PubMed] [Google Scholar]
- 24.Hoskyns EW, Milner AD, Hopkin IE. A simple method of face mask resuscitation at birth. Arch Dis Child. 1987;62(4):376–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hird MF, Greenough A, Gamsu HR. Inflating pressures for effective resuscitation of preterm infants. Early Hum Dev. 1991;26(1):69–72 [DOI] [PubMed] [Google Scholar]
- 26.Jacobsen T, Grønvall J, Petersen S, Andersen GE. “Minitouch” treatment of very low-birth-weight infants. Acta Paediatr. 1993;82(11):934–938 [DOI] [PubMed] [Google Scholar]
- 27.Lundstrom KE, Greisen G. Early treatment with nasal-CPAP. Acta Paediatr. 1993;82(10):856. [DOI] [PubMed] [Google Scholar]
- 28.Gittermann MK, Fusch C, Gittermann AR, Regazzoni BM, Moessinger AC. Early nasal continuous positive airway pressure treatment reduces the need for intubation in very low birth weight infants. Eur J Pediatr. 1997;156(5):384–388 [DOI] [PubMed] [Google Scholar]
- 29.Poets CF, Rau GA, Neuber K, Gappa M, Seidenberg J. Determinants of lung volume in spontaneously breathing preterm infants. Am J Respir Crit Care Med. 1997;155(2):649–653 [DOI] [PubMed] [Google Scholar]
- 30.Lindner W, Vossbeck S, Hummler H, Pohlandt F. Delivery room management of extremely low birth weight infants: spontaneous breathing or intubation? Pediatrics. 1999;103(5 pt 1):961–967 [DOI] [PubMed] [Google Scholar]
- 31.De Klerk AM, De Klerk RK. Nasal continuous positive airway pressure and outcomes of preterm infants. J Paediatr Child Health. 2001;37(2):161–167 [DOI] [PubMed] [Google Scholar]
- 32.Narendran V, Donovan EF, Hoath SB, Akinbi HT, Steichen JJ, Jobe AH. Early bubble CPAP and outcomes in ELBW preterm infants. J Perinatol. 2003;23(3):195–199 [DOI] [PubMed] [Google Scholar]
- 33.Aly H, Milner JD, Patel K, El-Mohandes AA. Does the experience with the use of nasal continuous positive airway pressure improve over time in extremely low birth weight infants? Pediatrics. 2004;114(3):697–702 [DOI] [PubMed] [Google Scholar]
- 34.Schmölzer GM, Morley CJ, Wong C, et al. . Respiratory function monitor guidance of mask ventilation in the delivery room: a feasibility study. J Pediatr. 2012;160(3):377–381.e2 [DOI] [PubMed] [Google Scholar]
- 35.Lee HC, Powers RJ, Bennett MV, et al. . Implementation methods for delivery room management: a quality improvement comparison study. Pediatrics. 2014;134(5). Available at: www.pediatrics.org/cgi/content/full/134/5/e1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lapcharoensap W, Bennett MV, Powers RJ, et al. . Effects of delivery room quality improvement on premature infant outcomes. J Perinatol. 2017;37(4):349–354 [DOI] [PubMed] [Google Scholar]
- 37.te Pas AB, Walther FJ. A randomized, controlled trial of delivery-room respiratory management in very preterm infants [published correction appears in Pediatrics. 2007;120(4):936]. Pediatrics. 2007;120(2):322–329 [DOI] [PubMed] [Google Scholar]
- 38.Foglia EE, Owen LS, Thio M, et al. . Sustained Aeration of Infant Lungs (SAIL) trial: study protocol for a randomized controlled trial. Trials. 2015;16:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Biniwale M, Wertheimer F. Decrease in delivery room intubation rates after use of nasal intermittent positive pressure ventilation in the delivery room for resuscitation of very low birth weight infants. Resuscitation. 2017;116:33–38 [DOI] [PubMed] [Google Scholar]
- 40.Morley CJ, Davis PG, Doyle LW, Brion LP, Hascoet JM, Carlin JB; COIN Trial Investigators . Nasal CPAP or intubation at birth for very preterm infants [published correction appears in N Engl J Med. 2008;358(14):1529]. N Engl J Med. 2008;358(7):700–708 [DOI] [PubMed] [Google Scholar]
- 41.Sandri F, Plavka R, Ancora G, et al. ; CURPAP Study Group . Prophylactic or early selective surfactant combined with nCPAP in very preterm infants. Pediatrics. 2010;125(6). Available at: www.pediatrics.org/cgi/content/full/125/6/e1402 [DOI] [PubMed] [Google Scholar]
- 42.Dunn MS, Kaempf J, de Klerk A, et al. ; Vermont Oxford Network DRM Study Group . Randomized trial comparing 3 approaches to the initial respiratory management of preterm neonates. Pediatrics. 2011;128(5). Available at: www.pediatrics.org/cgi/content/full/128/5/e1069 [DOI] [PubMed] [Google Scholar]
- 43.Roberts D, Brown J, Medley N, Dalziel SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2017;3:CD004454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carbine DN, Finer NN, Knodel E, Rich W. Video recording as a means of evaluating neonatal resuscitation performance. Pediatrics. 2000;106(4):654–658 [DOI] [PubMed] [Google Scholar]
- 45.O’Donnell CP, Kamlin CO, Davis PG, Morley CJ. Ethical and legal aspects of video recording neonatal resuscitation. Arch Dis Child Fetal Neonatal Ed. 2008;93(2):F82–F84 [DOI] [PubMed] [Google Scholar]
- 46.McCarthy LK, Morley CJ, Davis PG, Kamlin CO, O’Donnell CP. Timing of interventions in the delivery room: does reality compare with neonatal resuscitation guidelines? J Pediatr. 2013;163(6):1553–1557.e1 [DOI] [PubMed] [Google Scholar]
- 47.Schilleman K, Witlox RS, van Vonderen JJ, Roegholt E, Walther FJ, te Pas AB. Auditing documentation on delivery room management using video and physiological recordings. Arch Dis Child Fetal Neonatal Ed. 2014;99(6):F485–F490 [DOI] [PubMed] [Google Scholar]
- 48.Ikegami M, Polk DH, Jobe AH, et al. . Postnatal lung function in lambs after fetal hormone treatment. Effects of gestational age. Am J Respir Crit Care Med. 1995;152(4 pt 1):1256–1261 [DOI] [PubMed] [Google Scholar]