Abstract
Aim
Angina pectoris in the absence of obstructive coronary artery disease (CAD) is common and is associated with poor quality of life (QOL). Coronary microvascular endothelial dysfunction is associated with myocardial ischaemia and is a common cause of angina. We hypothesise that evaluation of coronary endothelial function, its diagnosis and treatment will favourably impact QOL in patients with angina symptoms and non-obstructive CAD.
Methods and results
Follow-up was done on 457 patients with chest pain and non-obstructive coronary arteries who had undergone coronary vascular reactivity evaluation by administration of intracoronary acetylcholine at the time of diagnostic study. After a mean follow-up of 8.4±4.7 years, QOL was assessed by administration of the SF-36 QOL survey. Patients diagnosed and treated for microvascular endothelial dysfunction had a higher (better) overall mental composite score (44.8 vs 40.9, p=0.036) and mental health score (44.2 vs 40.7, p=0.047), and a trend towards higher vitality scores (39.1 vs 35.9, p=0.053) and role emotional scores (43.6 vs 40.4, p=0.073), compared with patients with normal endothelial function.
Conclusion
Among patients with chest pain and normal coronaries, diagnosis and treatment of coronary microvascular endothelial dysfunction in those with angina pectoris and non-obstructive CAD are associated with better QOL compared with patients with normal endothelial function.
Keywords: microvascular endothelial function, quality of life, angina
Key questions.
What is already known about this subject?
Very little is known on the subject of quality of life (QOL) and coronary endothelial function.
No studies to our knowledge have specifically addressed the effects of diagnosis and treatment of coronary endothelial dysfunction on long-term QOL.
What does this study add?
In this manuscript we report the results of the impact of diagnosis and treatment of patients with coronary microvascular endothelial dysfunction and non-obstructive coronary artery disease (CAD) on long-term QOL.
These results demonstrate that among patients with chest pain and normal coronaries, the diagnosis and treatment of coronary microvascular endothelial dysfunction in those with angina pectoris and non-obstructive CAD are associated with better QOL compared with patients with normal endothelial function.
How might this impact on clinical practice?
The findings bring novel information to support the use of coronary endothelial function testing by skilled operators in the diagnosis of microvascular disease in patients with atypical chest pain that is not due to obstructive disease.
The results of the study suggest that the diagnosis and subsequent treatment of patients found to have microvascular disease may lead to an improvement in QOL.
Introduction
Angina pectoris with normal coronaries is a common phenomenon occurring in over 60% of patients undergoing coronary angiography in the USA.1 Patients with persistent chest pain and normal or non-obstructive coronary angiograms have poor quality of life (QOL) and consume large amounts of healthcare resources due to repeat evaluations for diagnostic and therapeutic uncertainty.2 3 Coronary microvascular endothelial dysfunction is associated with myocardial ischaemia4 and cardiovascular events,5–7 and can be an important mechanism for persistent chest pain in these patients.8
The identification and treatment of coronary microvascular endothelial dysfunction as a cause of angina in these patients may potentially modify their disease. Furthermore, recent studies have shown that reversal of endothelial dysfunction is associated with an improvement in cardiovascular prognosis.9 10 The effect of diagnosis and treatment of microvascular endothelial dysfunction on long-term QOL in patients with angina and normal angiograms has however not been studied.
Therefore, the aim of this study was to evaluate the impact of diagnosis and treatment of patients with coronary microvascular endothelial dysfunction and non-obstructive coronary artery disease (CAD) on long-term QOL.
Methods
Study design
This study is a prospective cohort study in a single centre.
Study population
The study group consisted of 457 patients with chest pain who were referred to the cardiac catheterisation laboratory for evaluation of CAD, found to have non-obstructive disease, and had comprehensive coronary physiology study including assessment of endothelial function and non-endothelium-independent coronary flow reserve.11–14
Exclusion criteria included significant coronary artery stenosis (>40%), ejection fraction <45%, unstable angina, previous myocardial infarction, use of radiographic contrast agents within 12 hours, significant systemic disease and pregnancy. Patients who did not return a follow-up questionnaire were excluded.
Study protocol
Medications that may affect cardiovascular haemodynamics were discontinued for at least 48 hours before the study. At baseline, diagnostic coronary angiography and determination of endothelium-dependent changes in Coronary Blood Flow (CBF) and endothelium-independent coronary flow reserve were performed as previously described.4 15 A Doppler guidewire (0.014-inch diameter, FloWire, Volcano) within a 2.2 F coronary infusion catheter (Ultrafuse, SciMed Life System) was advanced and positioned in the middle portion of the left anterior descending coronary artery (LAD). Intracoronary bolus injections of incremental doses (18–72 µg) of adenosine (Fujisawa), an endothelium-independent vasodilator (primarily of the microcirculation),16 were administered into the guiding catheter until maximal hyperaemia was achieved.
Assessment of the endothelium-dependent changes in CBF was performed by selective infusion of acetylcholine (ACh) into the LAD. ACh (Iolab Pharmaceuticals) 10−6, 10−5 and 10−4 mol/L were infused at 1 mL/min for 3 min.4 17 Haemodynamic data (heart rate and mean arterial pressure), Doppler measurements and coronary angiography were obtained after each infusion. Endothelium-independent epicardial vasodilation was assessed with an intracoronary bolus injection of nitroglycerin (200 µg, Abbott Laboratories).18
Microvascular endothelial function
Doppler flow velocity spectra were analysed online to determine the time-averaged peak velocity. Volumetric CBF was determined from the following relation: CBF=cross-sectional area × average peak velocity × 0.5.19 Endothelium-dependent microvascular function was calculated as % ∆ CBF in response to ACh as previously described.20 As previously reported microvascular endothelial dysfunction was defined as ≤50% increase in CBF in response to the maximal dose of ACh compared with baseline CBF.6 Patients were divided into two groups based on the presence of microvascular endothelial dysfunction.
Epicardial vascular function testing
Coronary artery diameter was analysed by quantitative coronary angiograms from digital images using a modified, previously described technique from this institution.4 13 The LAD was divided into proximal, middle and distal segments. For each segment, the measurements were performed in the region where the greatest change had occurred during the ACh infusion. An angiographically smooth segment of the proximal, middle and distal LAD, free of any overlapping branch vessels, was identified in each patient and served as the reference diameter for the calculation of diameter stenosis. End-diastolic cine frames that best showed the segment were selected and calibration of the video and cine images was done, identifying the diameter of the guide catheter. Quantitative measurements of the coronary arteries were obtained using a computer-based image analysis system. Segment diameters were determined at baseline and after both ACh and nitroglycerin administration. The proximal segment was not exposed to ACh and thus served a control segment.
Epicardial coronary endothelial dysfunction was defined as a decrease or no increase in coronary artery diameter in response to the maximal dose of ACh compared with baseline.21
Follow-up
After a mean follow-up of 8.4±4.7 years, QOL was assessed by administration of Short Form (36) Health (SF-36) survey.22 Patients were contacted by mail and the SF-36 survey sent to them.
Survey instrument
The SF-36 survey is a well-validated, multipurpose, short-form health survey with 36 questions. It is an 8-scale profile of functional health, well-being and mental health that forms a generic measure of participants’ perception of their health status and their mental and physical well-being (figure 1).22–25
Figure 1.
Summary of the measures and scales of the quality of life SF-36 survey.
Statistical analysis
Data are displayed as mean±SD or count and percentage as appropriate. Variables with heavily skewed distribution are reported as medians with first and third quartiles in parenthesis. Analysis to compare different demographic and baseline clinical data between the groups was performed using analysis of variance for continuous data and the Pearson’s χ2 test for categorical data. Baseline data for the CBF, CFR and coronary artery diameter were compared using a rank-sum test. Statistical analyses were performed using JMP V.14 (SAS Institute, Cary, NC, 1989–2018).
Quality of life
The responses to the SF-36 items for the 8-scale SF-36 scale were scored using the publisher’s normative methods, which in turn generated the summary physical and mental components scores. All of these scores are standardised such that in a normal age-matched and sex-matched reference population the mean score is equal to 50 and the SD of the scores is equal to 10. Patients who have SF-36 scores greater than 50 report better health-related QOL than the normal population, and scores less than 50 indicate poorer QOL. Differences between the groups in the QOL analysis were compared using Student’s t-test. Multiple linear regression was used to estimate the difference in QOL adjusted for other covariates. The model was adjusted for covariates which included the baseline characteristics (reported in table 1) as well as the medication use at baseline (reported in table 2). All statistical tests were two-sided, and a p<0.05 was considered to be statistically significant.
Table 1.
Baseline patient characteristics, by microvascular function
| Variable | Normal microvascular endothelial function (n=199) |
Microvascular endothelial dysfunction (n=258) |
P value |
| Age, years | 51.9±12.3 | 54.4±11.6 | 0.03 |
| Men, n (%) | 66 (33) | 78 (30) | 0.50 |
| Vascular disease, n (%) | 16 (8) | 22 (9) | 5 |
| Hypertension, n (%) | 76 (38) | 109 (43) | 0.34 |
| Diabetes, n (%) | 15 (8) | 24 (9) | 0.50 |
| Hyperlipidaemia, n (%) | 116 (59) | 148 (58) | 0.78 |
| Depression, n (%) | 60 (31) | 61 (24) | 0.13 |
| Smoking status, n (%) | 0.03 | ||
| Never smoked | 98 (49) | 150 (58) | |
| Former smoker | 77 (39) | 93 (36) | |
| Current smoker | 23 (12) | 15 (6) | |
| Height, cm | 168.6±10.0 | 167.6±9.2 | 0.29 |
| Weight, kg | 79.8±19.1 | 80.6±20.3 | 0.68 |
| Body mass index | 28.0±6.1 | 28.5±6.3 | 0.38 |
| Haemoglobin, g/L | 135±13 | 135±13 | 0.60 |
| Glucose, mg/dL, median (Q1, Q3) | 95.0 (88.0, 105.0) | 96.0 (89.0, 104.0) | 0.49 |
| Creatinine, mg/dL, median (Q1, Q3) | 1.0 (0.9, 1.1) | 1.0 (0.9, 1.1) | 0.58 |
| Total cholesterol, mg/dL | 194.5±47.1 | 191.4±43.4 | 0.49 |
| LDL-C, mg/dL | 111.2±39.4 | 110.1±38.1 | 0.77 |
| HDL-C, mg/dL | 54.4±16.8 | 54.4±16.1 | 1.00 |
| Triglycerides, mg/dL, median (Q1, Q3) | 121.0 (85.0, 176.5) | 113.5 (81.0, 171.0) | 0.32 |
| hsCRP, mg/L, median (Q1, Q3) | 0.5 (0.2, 1.6) | 0.6 (0.2, 1.9) | 0.65 |
HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; hsCRP, high-sensitivity C reactive protein.
Table 2.
Medication use, by microvascular function
| Variable | Normal microvascular endothelial function (n=199) |
Microvascular endothelial dysfunction (n=258) |
P value |
| Aspirin use, n (%) | 85 (43) | 133 (52) | 0.06 |
| ACE inhibitor use, n (%) | 33 (17) | 40 (16) | 0.75 |
| Beta-blocker use, n (%) | 52 (26) | 81 (31) | 0.22 |
| Calcium channel blocker use, n (%) | 68 (34) | 88 (34) | 0.96 |
| Lipid-lowering drug use, n (%) | 66 (33) | 111 (43) | 0.03 |
| Antiarrhythmic use, n (%) | 14 (7) | 20 (8) | 0.77 |
| Anticoagulant use, n (%) | 18 (9) | 34 (13) | 0.18 |
| Antihypertensive use, n (%) | 41 (21) | 60 (23) | 0.50 |
| Nitrates use, n (%) | 67 (34) | 93 (36) | 0.60 |
| Diuretics use, n (%) | 33 (17) | 45 (17) | 0.81 |
| Oral hypoglycaemic use, n (%) | 7 (4) | 14 (5) | 0.33 |
| Insulin use, n (%) | 5 (3) | 8 (3) | 0.71 |
Results
Therefore 457 patients were included in the study, of whom 258 (56%) had microvascular endothelial dysfunction. There were 313 women (68%), and the mean age of the study population was 52 years (range, 17–78 years). Most patients (92%) had >1 risk factor for CAD. The mean duration of follow-up was 8.4±4.7 years.
Baseline characteristics
Patients with microvascular dysfunction were slightly older compared with patients with normal endothelial function (mean age 54 vs 51, p=0.028) and had a lower smoking rate. The groups were otherwise well matched at baseline with regard to gender, race, other cardiovascular risk factors and inflammatory marker (table 1).
Medications at baseline
Patients with microvascular dysfunction had a higher rate of lipid-lowering drugs use (43% vs 33%, p=0.032) and a lower rate of aspirin use (43% vs 52%, p=0.06) (did not reach statistical significance) compared with patients with normal microvascular function. The two groups matched well on the frequency of use of other cardiac medications including beta-blockers, ACE inhibitors use and calcium channel blockers (table 2).
Microvascular and epicardial endothelial function
Patients in the microvascular coronary dysfunction group had a lower mean per cent change in CAD after maximal dose of ACh from baseline (greater vasoconstriction) compared with patients with normal microvascular endothelial function (−25.7% vs −3.8%, p<0.001). There was also a higher percentage of patients with epicardial endothelial dysfunction (58% vs 16%, p<0.001) in the microvascular coronary dysfunction group compared with the group with normal microvascular endothelial function.
Quality of life
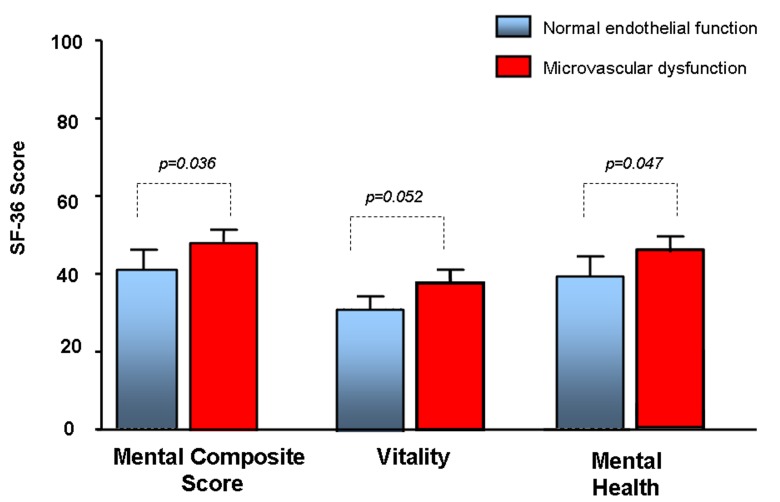
At the time they were surveyed, patients who were diagnosed and treated for microvascular endothelial dysfunction experienced higher overall mental composite score (44.8 vs 40.9, p=0.036) and mental health (44.2 vs 40.7, p=0.047), and a trend towards higher vitality (39.1 vs 35.9, p=0.053) and role emotional (43.6 vs 40.4, p=0.073), compared with patients with normal endothelial function (table 3 and figure 2).
Table 3.
Endothelial function and Short Form (36) Health (SF-36) survey results, by microvascular function
| Variable | Normal microvascular endothelial function (n=199) |
Microvascular endothelial dysfunction (n=258) |
P value |
| % change CBF (ACh) | 132.9±80.0 | −12.0±35.7 | <0.001 |
| % change CAD (ACh) | −3.8±15.2 | −25.7±20.5 | <0.001 |
| % change CAD ≤−20%, n (%) | 32 (16) | 149 (58) | <0.001 |
| Coronary flow reserve | 2.93±0.74 | 2.76±0.67 | 0.01 |
| Months from study to survey, mean±Standard Deviation | 85.5±55.4 | 82.7±48.2 | 0.27 |
| SF-36 Std. Physical component score, mean±SD | 37.0±19.5 | 38.7±17.7 | 0.33 |
| SF-36 Std. Mental component score, mean±SD | 40.1±20.1 | 43.9±18.1 | 0.04 |
| Physical functioning, age-adjusted and sex-adjusted, mean±SD | 39.5±20.4 | 41.9±18.3 | 0.19 |
| Role physical, age-adjusted and sex-adjusted, mean±SD | 37.0±19.4 | 39.2±17.6 | 0.20 |
| Body pain, age-adjusted and sex-adjusted, mean±SD | 37.6±18.2 | 39.3±16.7 | 0.28 |
| General health, age-adjusted and sex-adjusted, mean±SD | 37.8±19.6 | 40.2±17.7 | 0.16 |
| Vitality, age-adjusted and sex-adjusted, mean±SD | 36.0±17.9 | 39.1±17.0 | 0.05 |
| Social function, age-adjusted and sex-adjusted, mean±SD | 39.3±20 | 42.2±18.3 | 0.11 |
| Role emotional, age-adjusted and sex-adjusted, mean±SD | 40.4±20.0 | 43.6±18.5 | 0.07 |
| Mental health, age-adjusted and sex-adjusted, mean±SD | 40.7±20.0 | 44.2±17.7 | 0.04 |
ACh, acetylcholine; CAD, coronary artery disease.
Figure 2.
Short Form (36) Health (SF-36) survey mental composite score, vitality and mental health in patients with microvascular endothelial dysfunction compared with patients with normal microvascular functions.
Subgroup analysis of women
On subgroup analysis, women with microvascular endothelial dysfunction had similar baseline characteristics, frequency of cardiovascular risk factors, inflammatory markers and use of cardiovascular medication compared with women with normal microvascular endothelial function.
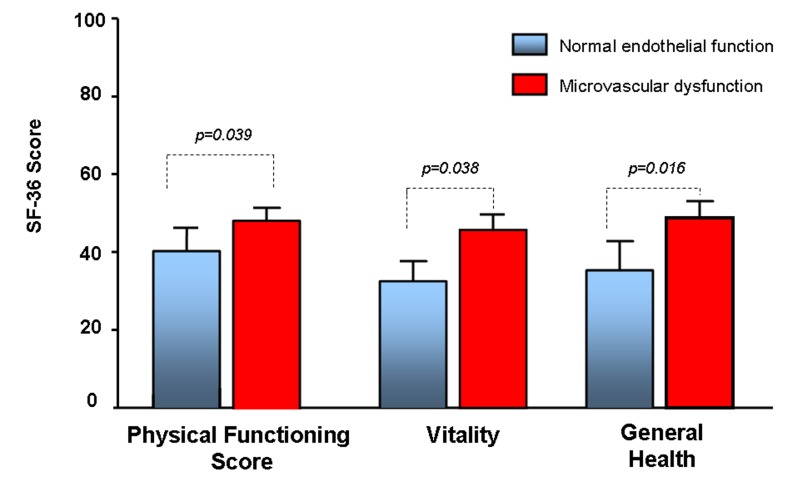
On QOL analysis women diagnosed and treated for microvascular endothelial dysfunction had a higher physical function (44.5 vs 40.4, p=0.039), general health (43.4 vs 38.7, p=0.016) and vitality (42.1 vs 37.3, p=0.008), and a trend towards higher overall mental composite score (46.8 vs 43.4, p=0.08), overall physical composite score (41.1 vs 37.3, p=0.05), role emotional (46.5 vs 43.2, p=0.09), social function (44.8 vs 41.4, p=0.08) and role physical (42.1 vs 38.9, p=0.09), compared with women with normal endothelial function (table 4 and figure 3).
Table 4.
Women and SF-36 results by microvascular function
| Variable | Normal microvascular endothelial function (n=119) |
Microvascular endothelial dysfunction (n=194) |
P value |
| % chg CBF | 138.5±84.6 | −2.1±47.1 | <0.001 |
| % chg CBF ≤50%, n (%) | 0 (0) | 180 (93) | <0.001 |
| % chg CAD | 0.3±12.4 | −24.4±18.3 | <0.001 |
| %chg. CAD ≤ −20%, n (%) | 0 (0) | 112 (58) | <0.001 |
| Months from ACh study to survey, mean±SD | 84.3±56.9 | 82.7±48.3 | 0.580 |
| SF-36 Std. Physical component score, mean±SD | 37.4±18.4 | 41.1±15.8 | 0.05 |
| SF-36 Std. Mental component score, mean±SD | 43.4±18.9 | 46.8±15.4 | 0.08 |
| Physical functioning, age-adjusted and sex-adjusted, mean±SD | 40.4±18.8 | 44.5±16.0 | 0.05 |
| Role physical, age-adjusted and sex-adjusted, mean±SD | 38.9±18.2 | 42.1±15.5 | 0.09 |
| Body pain, age-adjusted and sex-adjusted, mean±SD | 38.8±17.3 | 41.3±14.8 | 0.16 |
| General health, age-adjusted and sex-adjusted, mean±SD | 38.7±18.6 | 43.4±15.5 | 0.02 |
| Vitality, age-adjusted and sex-adjusted, mean±SD | 37.3±16.8 | 42.1±14.8 | 0.01 |
| Social function, age-adjusted and sex-adjusted, mean±SD | 41.4±18.8 | 44.8±16.2 | 0.09 |
| Role emotional, age-adjusted and sex-adjusted, mean±SD | 43.2±18.5 | 46.5±15.6 | 0.09 |
| Mental health, age-adjusted and sex-adjusted, mean±SD | 43.9±18.8 | 47.0±15.1 | 0.11 |
ACh, acetylcholine; CAD, coronary artery disease.
Figure 3.
SF-36 physical functioning, vitality and general health in women with microvascular endothelial dysfunction compared with women with normal microvascular functions.
Discussion
The current study demonstrated that among patients with chest pain and non-obstructive CAD, QOL with respect to measures of mental well-being during long-term follow-up is better in those patients who were assessed, diagnosed and received treatment for microvascular endothelial dysfunction compared with those who are found to have normal endothelial function. The current study suggests that assessment and treatment of microvascular angina may improve their QOL.
Patients with chest pain and non-obstructive CAD have been shown to have high morbidity and mortality than previously thought.26 27 These patients constitute a large percentage (up to 50%) of patients who undergo coronary angiography studies for suspected myocardial ischaemia.28 These patients face diagnostic uncertainty and are high users of healthcare resources,26 29 30 due to the perceived need for frequent hospitalisation and multiple angiograms.31 They also have poor QOL, functional disability and limitations in activities of daily living.26 32 33
The aetiologies for chronic chest pain encompass a wide array of potential diagnoses, including cardiac and non-cardiac causes. Recent evidence suggests ischaemia due to coronary microvascular or macrovascular endothelial dysfunction34–37 may play a role, especially in women.28 37–40 Furthermore, the presence of microvascular dysfunction is associated with adverse cardiovascular events and its treatment may lead to improved prognosis.4 The need for proper diagnosis of chest pain in these patients is underscored by the recent guidelines and endorsement by the European Society of Cardiology. To our knowledge there are no studies in patients with chest pain showing the effect of diagnosis and treatment of microvascular endothelial dysfunction on QOL.
Our finding of higher QOL scores in patients with microvascular endothelial dysfunction who were on treatment compared with patients with normal endothelial function may seem paradoxical. Previous studies have however found similar results. In one study women with chest pain and myocardial ischaemia had a higher QOL compared with women without.41 There are several potential mechanisms for this observation. First, lack of diagnosis for chest pain is a source of frustration for both the physician and the patient. Conversely, establishment of a diagnosis may alleviate anxiety for the patients, which would lead to improvement in the measures within the mental domains of QOL. Similar observations have also been made in patients admitted to hospitals for evaluation of suspected myocardial infarction. Patients in whom acute myocardial infarction was not confirmed reported more cardiovascular symptoms of chest pain, dyspnoea and palpitations, as well as more psychosomatic and psychological parameters, at 1 year compared with patients diagnosed with acute myocardial infarctions.42 Furthermore patients with unstable angina pectoris are likely to experience poorer QOL following an acute hospitalisation than patients with other types of acute coronary syndrome.43 Second, the treatment of microvascular dysfunction may relieve their symptoms, which may improve QOL. Previous studies have shown that treatment of angina is associated with improvement in physical, mental and social domains of QOL.44 Third, the paradoxical finding of better QOL in women with ischaemia compared with those without ischaemia41 45 may also in part explain our finding. Patients able to exercise to a sufficient workload to elicit an ischaemic response may have better QOL because they are less functionally impaired.41 The mechanism is thought to be due to less functional impairment in women with myocardial ischaemia compared with women without myocardial ischaemia. We have previously shown that microvascular coronary disease is associated with myocardial ischaemia in patients with non-obstructive coronary disease.4 Although we did not specifically measure the exercise tolerance of our patients, it may be speculated that a similar mechanism may play a role in patients with chest pain and impaired microvascular function.
Persistent chest pain in the absence of coronary disease is more prevalent in women.46 47 Indeed, we found in our study a higher percentage of women having microvascular endothelial dysfunction compared with men. In a subgroup analysis, the differences in QOL were more marked among women. We speculate that microvascular disease is thus more prevalent women, and its diagnosis and treatment may have greater benefit compared with men. The current study further supports the notion that coronary microvascular dysfunction is one of the main mechanisms of chest pain in women.
Limitations
There are several limitations to be mentioned. No baseline QOL scores were obtained. Although the characteristics of the two groups were similar at baseline, including cardiovascular risk factors and medication use, we do not have baseline QOL data for comparison. This precludes stronger inference about the cause and effect of any findings. In addition there was no standardised treatment protocol for patients with microvascular disease, and the attending clinician determined the treatment based on the patient’s risk factors. We thus cannot draw definite conclusion on the effect of a specific treatment on QOL. In addition, we were unable to ascertain the medication use at follow-up and verify it with medical records because many of the patients did not follow up in our institution.
Our study was not designed to measure healthcare utilisation and functional status or to do cost analysis of potential healthcare saving of diagnosis and treatment of microvascular disease in patients with chest pain. This would have provided more outcome benefits, which could potentially be linked to improved QOL.
Conclusions
Among patients with chest pain and non-obstructive CAD or normal coronary arteries, it is suggested that the diagnosis and subsequent treatment of patients found to have microvascular disease may lead to an improvement in QOL. These results support the use of coronary endothelial function testing by skilled operators in the diagnosis of microvascular disease in patients with atypical chest pain that is not due to obstructive CAD.
Footnotes
Contributors: The authors of the paper take full responsibility.
Funding: The study was supported by grants from the NIH (NIH K24 HL-69840, NIH R01 HL-63911, HL-77131, HL 92954, HL 085307, DK 73608) and the Mayo Foundation.
Competing interests: None declared.
Patient consent for publication: Obtained.
Ethics approval: The Mayo Clinic Institutional Review Board approved the study.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1. Patel MR, Peterson ED, Dai D, et al. Low diagnostic yield of elective coronary angiography. N Engl J Med 2010;362:886–95. 10.1056/NEJMoa0907272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bairey Merz CN, Shaw LJ. Stable angina in women: lessons from the national heart, lung and blood Institute-sponsored womenʼs ischemia syndrome evaluation. J Cardiovasc Med 2011;12:85–7. 10.2459/JCM.0b013e3283430969 [DOI] [PubMed] [Google Scholar]
- 3. Shaw LJ, Bairey Merz CN, Pepine CJ, et al. Insights from the NHLBI-Sponsored women's ischemia syndrome evaluation (wise) study: part I: gender differences in traditional and novel risk factors, symptom evaluation, and gender-optimized diagnostic strategies. J Am Coll Cardiol 2006;47 S4–20. 10.1016/j.jacc.2005.01.072 [DOI] [PubMed] [Google Scholar]
- 4. Hasdai D, Gibbons RJ, Holmes DR, et al. Coronary endothelial dysfunction in humans is associated with myocardial perfusion defects. Circulation 1997;96:3390–5. 10.1161/01.CIR.96.10.3390 [DOI] [PubMed] [Google Scholar]
- 5. Lerman A, Zeiher AM. Endothelial function: cardiac events. Circulation 2005;111:363–8. 10.1161/01.CIR.0000153339.27064.14 [DOI] [PubMed] [Google Scholar]
- 6. Suwaidi JA, Hamasaki S, Higano ST, et al. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation 2000;101:948–54. 10.1161/01.CIR.101.9.948 [DOI] [PubMed] [Google Scholar]
- 7. Jespersen L, Hvelplund A, Abildstrøm SZ, et al. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur Heart J 2012;33:734–44. 10.1093/eurheartj/ehr331 [DOI] [PubMed] [Google Scholar]
- 8. Johnson BD, Shaw LJ, Pepine CJ, et al. Persistent chest pain predicts cardiovascular events in women without obstructive coronary artery disease: results from the NIH-NHLBI-sponsored women's ischaemia syndrome evaluation (wise) study. Eur Heart J 2006;27:1408–15. 10.1093/eurheartj/ehl040 [DOI] [PubMed] [Google Scholar]
- 9. Kitta Y, Obata JE, Nakamura T, et al. Persistent impairment of endothelial vasomotor function has a negative impact on outcome in patients with coronary artery disease. J Am Coll Cardiol 2009;53:323–30. 10.1016/j.jacc.2008.08.074 [DOI] [PubMed] [Google Scholar]
- 10. Modena MG, Bonetti L, Coppi F, et al. Prognostic role of reversible endothelial dysfunction in hypertensive postmenopausal women. J Am Coll Cardiol 2002;40:505–10. 10.1016/S0735-1097(02)01976-9 [DOI] [PubMed] [Google Scholar]
- 11. Lavi S, Bae JH, Rihal CS, et al. Segmental coronary endothelial dysfunction in patients with minimal atherosclerosis is associated with necrotic core plaques. Heart 2009;95:1525–30. 10.1136/hrt.2009.166017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Raichlin E, Prasad A, Mathew V, et al. Efficacy and safety of atrasentan in patients with cardiovascular risk and early atherosclerosis. Hypertension 2008;52:522–8. 10.1161/HYPERTENSIONAHA.108.113068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Reriani M, Raichlin E, Prasad A, et al. Long-term administration of endothelin receptor antagonist improves coronary endothelial function in patients with early atherosclerosis. Circulation 2010;122:958–66. 10.1161/CIRCULATIONAHA.110.967406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rubinshtein R, Yang EH, Rihal CS, et al. Coronary microcirculatory vasodilator function in relation to risk factors among patients without obstructive coronary disease and low to intermediate Framingham score. Eur Heart J 2010;31:936–42. 10.1093/eurheartj/ehp459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Reriani MK, Lerman LO, Lerman A. Endothelial function as a functional expression of cardiovascular risk factors. Biomark Med 2010;4:351–60. 10.2217/bmm.10.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hori M, Kitakaze M, Adenosine KM. Adenosine, the heart, and coronary circulation. Hypertension 1991;18:565–74. 10.1161/01.HYP.18.5.565 [DOI] [PubMed] [Google Scholar]
- 17. Lerman A, Holmes DR, Bell MR, et al. Endothelin in coronary endothelial dysfunction and early atherosclerosis in humans. Circulation 1995;92:2426–31. 10.1161/01.CIR.92.9.2426 [DOI] [PubMed] [Google Scholar]
- 18. Harrison DG, Bates JN. The nitrovasodilators. new ideas about old drugs. Circulation 1993;87:1461–7. 10.1161/01.CIR.87.5.1461 [DOI] [PubMed] [Google Scholar]
- 19. Doucette JW, Corl PD, Payne HM, et al. Validation of a Doppler guide wire for intravascular measurement of coronary artery flow velocity. Circulation 1992;85:1899–911. 10.1161/01.CIR.85.5.1899 [DOI] [PubMed] [Google Scholar]
- 20. Ofili EO, Labovitz AJ, Kern MJ. Coronary flow velocity dynamics in normal and diseased arteries. Am J Cardiol 1993;71:D3–9. 10.1016/0002-9149(93)90128-Y [DOI] [PubMed] [Google Scholar]
- 21. Halcox JP, Schenke WH, Zalos G, et al. Prognostic value of coronary vascular endothelial dysfunction. Circulation 2002;106:653–8. 10.1161/01.CIR.0000025404.78001.D8 [DOI] [PubMed] [Google Scholar]
- 22. Ware JE, Sherbourne CD. The mos 36-item short-form health Survey (SF-36). I. conceptual framework and item selection. Med Care 1992;30:473–83. [PubMed] [Google Scholar]
- 23. Hays RD, Sherbourne CD, Mazel RM. The rand 36-item health survey 1.0. Health Econ 1993;2:217–27. 10.1002/hec.4730020305 [DOI] [PubMed] [Google Scholar]
- 24. McHorney CA, Ware JE, Lu JF, et al. The mos 36-item short-form health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care 1994;32:40–66. 10.1097/00005650-199401000-00004 [DOI] [PubMed] [Google Scholar]
- 25. McHorney CA, Ware JE, Raczek AE. The mos 36-Item short-form health survey (SF-36): II. psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care 1993;31:247–63. [DOI] [PubMed] [Google Scholar]
- 26. Kemp HG, Kronmal RA, Vlietstra RE, et al. Seven year survival of patients with normal or near normal coronary arteriograms: a CASS registry study. J Am Coll Cardiol 1986;7:479–83. 10.1016/S0735-1097(86)80456-9 [DOI] [PubMed] [Google Scholar]
- 27. Kaski JC, Rosano GM, Collins P, et al. Cardiac syndrome X: clinical characteristics and left ventricular function. Long-term follow-up study. J Am Coll Cardiol 1995;25:807–14. 10.1016/0735-1097(94)00507-M [DOI] [PubMed] [Google Scholar]
- 28. Reis SE, Holubkov R, Conrad Smith AJ, et al. Coronary microvascular dysfunction is highly prevalent in women with chest pain in the absence of coronary artery disease: results from the NHLBI wise study. Am Heart J 2001;141:735–41. 10.1067/mhj.2001.114198 [DOI] [PubMed] [Google Scholar]
- 29. Merz NB, Johnson BD, Kelsey PSF, et al. Diagnostic, prognostic, and cost assessment of coronary artery disease in women. Am J Manag Care 2001;7:959–65. [PubMed] [Google Scholar]
- 30. Shaw LJ, Merz CN, Pepine CJ, et al. The economic burden of angina in women with suspected ischemic heart disease: results from the national institutes of health--national heart, lung, and blood institute–sponsored women's ischemia syndrome evaluation. Circulation 2006;114:894–904. 10.1161/CIRCULATIONAHA.105.609990 [DOI] [PubMed] [Google Scholar]
- 31. Cannon RO, Quyyumi AA, Mincemoyer R, et al. Imipramine in patients with chest pain despite normal coronary angiograms. N Engl J Med 1994;330:1411–7. 10.1056/NEJM199405193302003 [DOI] [PubMed] [Google Scholar]
- 32. Glagov S, Weisenberg E, Zarins CK, et al. Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med 1987;316:1371–5. 10.1056/NEJM198705283162204 [DOI] [PubMed] [Google Scholar]
- 33. Schoenhagen P, Nissen SE, Tuzcu EM. Coronary arterial remodeling: from bench to bedside. Curr Atheroscler Rep 2003;5:150–4. 10.1007/s11883-003-0088-9 [DOI] [PubMed] [Google Scholar]
- 34. Cannon RO, Camici PG, Epstein SE. Pathophysiological dilemma of syndrome X. Circulation 1992;85:883–92. 10.1161/01.CIR.85.3.883 [DOI] [PubMed] [Google Scholar]
- 35. Panting JR, Gatehouse PD, Yang GZ, et al. Abnormal subendocardial perfusion in cardiac syndrome X detected by cardiovascular magnetic resonance imaging. N Engl J Med 2002;346:1948–53. 10.1056/NEJMoa012369 [DOI] [PubMed] [Google Scholar]
- 36. Quyyumi AA, Cannon RO, Panza JA, et al. Endothelial dysfunction in patients with chest pain and normal coronary arteries. Circulation 1992;86:1864–71. 10.1161/01.CIR.86.6.1864 [DOI] [PubMed] [Google Scholar]
- 37. Bugiardini R, Bairey Merz CN. Angina with "normal" coronary arteries: a changing philosophy. JAMA 2005;293:477–84. 10.1001/jama.293.4.477 [DOI] [PubMed] [Google Scholar]
- 38. Johnson BD, Shaw LJ, Buchthal SD, et al. Prognosis in women with myocardial ischemia in the absence of obstructive coronary disease: results from the National Institutes of Health-National heart, lung, and blood Institute-Sponsored women's ischemia syndrome evaluation (wise). Circulation 2004;109:2993–9. 10.1161/01.CIR.0000130642.79868.B2 [DOI] [PubMed] [Google Scholar]
- 39. von Mering GO, Arant CB, Wessel TR, et al. Abnormal coronary vasomotion as a prognostic indicator of cardiovascular events in women: results from the National Heart, lung, and blood institute-sponsored women's ischemia syndrome evaluation (wise). Circulation 2004;109:722–5. 10.1161/01.CIR.0000115525.92645.16 [DOI] [PubMed] [Google Scholar]
- 40. Summers MR, Lerman A, Lennon RJ, et al. Myocardial ischaemia in patients with coronary endothelial dysfunction: insights from body surface ECG mapping and implications for invasive evaluation of chronic chest pain. Eur Heart J 2011;32:2758–65. 10.1093/eurheartj/ehr221 [DOI] [PubMed] [Google Scholar]
- 41. Olson MB, Kelsey SF, Matthews K, et al. Symptoms, myocardial ischaemia and quality of life in women: results from the NHLBI-sponsored wise study. Eur Heart J 2003;24:1506–14. 10.1016/S0195-668X(03)00279-3 [DOI] [PubMed] [Google Scholar]
- 42. Karlson BW, Wiklund I, Bengtson A, et al. Prognosis, severity of symptoms, and aspects of well-being among patients in whom myocardial infarction was ruled out. Clin Cardiol 1994;17:427–31. 10.1002/clc.4960170805 [DOI] [PubMed] [Google Scholar]
- 43. Perers E, From Attebring M, Caidahl K, et al. Low risk is associated with poorer quality of life than high risk following acute coronary syndrome. Coron Artery Dis 2006;17:501–10. 10.1097/00019501-200609000-00002 [DOI] [PubMed] [Google Scholar]
- 44. Buser MA, Buser PT, Kuster GM, et al. Improvements in physical and mental domains of quality of life by anti-ischaemic drug and revascularisation treatment in elderly men and women with chronic angina. Heart 2008;94:1413–8. 10.1136/hrt.2007.127589 [DOI] [PubMed] [Google Scholar]
- 45. Mattera JA, De Leon CM, Wackers FJ, et al. Association of patients' perception of health status and exercise electrocardiogram, myocardial perfusion imaging, and ventricular function measures. Am Heart J 2000;140:409–18. 10.1067/mhj.2000.108518 [DOI] [PubMed] [Google Scholar]
- 46. Kemp HG, Elliott WC, Gorlin R. The anginal syndrome with normal coronary arteriography. Trans Assoc Am Physicians 1967;80:59–70. [PubMed] [Google Scholar]
- 47. Likoff W, Segal BL, Kasparian H. Paradox of normal selective coronary arteriograms in patients considered to have unmistakable coronary heart disease. N Engl J Med 1967;276:1063–6. 10.1056/NEJM196705112761904 [DOI] [PubMed] [Google Scholar]