Abstract
Circadian rhythms describe the behavioral and physiological changes that occur in living organisms in order to attune to a 24 hour cycle of day and night. The most striking aspect of circadian function is the sleep-wake cycle, however many other physiological processes are regulated in 24 hour oscillations, including blood pressure, body temperature, appetite, urine production, and the transcription and translation of thousands of circadian dependent genes. Circadian disruption and sleep disorders are strongly connected to neurodegenerative diseases including Parkinson’s disease, Alzheimer’s disease, and Huntington’s disease as well as others. Metal exposures have been implicated in neurodegenerative diseases, in some cases involving metals that are essential micronutrients but are toxic at high levels of exposure (such as manganese, copper, and zinc), and in other cases involving metals that have no biological role but are toxic to living systems (such as lead, mercury, and aluminum). In this review, we examine the evidence for circadian and sleep disorders with exposures to these metals and review the literature for possible mechanisms. We suggest that giving the aging population, the prevalence of environmental exposures to metals, and the increasing prevalence of neurodegenerative disease in the aged, more research into the mechanisms of circadian disruption subsequent to metal exposures is warranted.
Introduction
Circadian rhythms describe the roughly 24 hour oscillation of many physiological properties, behaviors, and gene transcription that has arisen through evolution to coordinate the activities of biological organisms with the periods of light and dark corresponding to day and night (Takahashi et al., 2008, Mohawk et al., 2012). Mechanisms to control circadian rhythms exist in many if not most organisms, from plants to human, with some mechanisms highly conserved and others perhaps evolving convergently. While much work is ongoing regarding the function of sleep in humans, it is clear that sleep is an essential physiological process, the lack of which causes or exacerbates a plethora of conditions ranging from causing a “bad day” to worsening neurodegenerative conditions such as Parkinson’s disease (PD) and Alzheimer’s disease (AD). In order to coordinate sleep-wake cycles, many biological functions need to be coordinated, including changes in alertness, appetite, body temperature, blood pressure, production of urine, heart rate and metabolism.
In humans, circadian control mechanisms exist at several levels. Centrally, the suprachiasmatic nucleus (SCN) in the brain receives signals from the retina and acts as a central clock (Welsh et al., 1995, Welsh et al., 2010). In total darkness, absent light cues to indicate daytime, the human circadian clock oscillates with a period slightly longer than 24 hours. In order to stay synchronized with the 24 hour day, specialized cells in the retina known as intrinsically photosensitive retinal ganglion cells (ipRGCs) sense light for the purpose of setting the circadian clock (Berson et al., 2002, Hattar et al., 2002). These cells do not participate in vision formation, but do express the photopigment melanopsin, which is particularly sensitive to blue light. These cells synapse with cells of the SCN in order to synchronize, or entrain, the central clock to the 24 hour day. Peripheral clocks also exist in organs such as the liver, lung and kidney (Yoo et al., 2004), and circadian oscillations are seen at the level of individual cells. Transcription of a large portion of the genome is controlled in a circadian manner.
The core genes involved in mammalian circadian control include Clock, Bmal1, Per1, Per2, Cry1, and Cry2 (Mohawk et al., 2012). Clock and Bmal1 dimerize and bind DNA resulting in the transcription of the Per and Cry genes as well as many others. Per and Cry, in turn, dimerize with each other and interact with the Clock-Bmal1 complex in an inhibitory fashion, repressing transcription (Takahashi et al., 2008). Thus, as the products of transcription accumulate they repress their own transcription, resulting in an oscillatory system (Figure 1).
Figure 1.
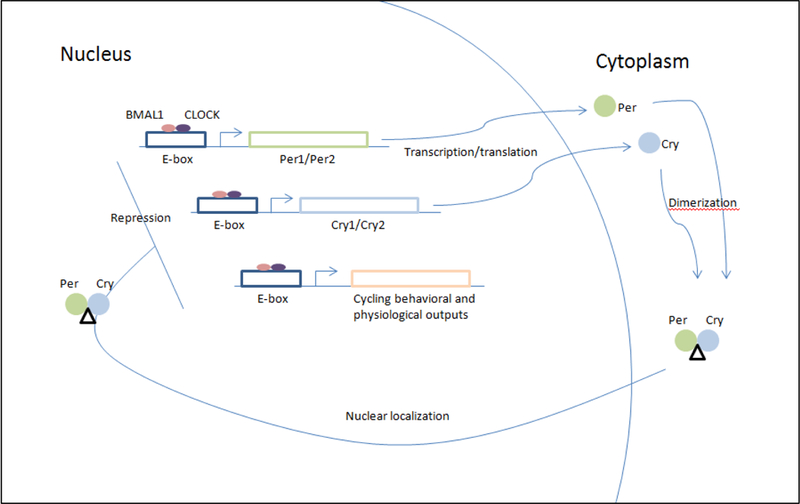
Circadian oscillations result from the transcription, translation, accumulation, and degradation of genes. This simplified schematic depicts the feedback mechanism of the canonical clock genes that results in circadian oscillation. The proteins BMAL1 and CLOCK dimerize and bind DNA at E-box binding sites, resulting in transcription of genes under the control of these binding elements. These genes include Per1 and Per2, Cry1 and Cry2, and other genes involved in circadian behavioral and physiological responses. The Per and Cry genes accumulate in the cytoplasm where they dimerize and are then transported back to the nucleus. The Per-Cry dimer inhibits binding of BMAL1 and CLOCK to DNA, repressing transcription of downstream genes. In this way, the accumulation of Per and Cry repress their own expression, establishing a system that oscillates based on the expression, accumulation, and subsequent repression of these genes.
Several lines of evidence indicate that various metals interact with components of the circadian clock mechanisms resulting in disruptions to circadian rhythms. The data that exists in the literature largely consists of observational correlations in humans, which does not address mechanisms which may be at work in such interactions. We propose that this is a rich area of interest that merits attention from the scientific community. Taking the example of manganese (Mn), a primary focus of our lab: there is a robust literature concerning high-level acute exposures, such as industrial exposures that occur in mining, welding, battery manufacture, and others (Rodier, 1955). There is less data on the effects of cumulative life-long exposures at lower levels that may be encountered in the day-to-day environment. We have written previously about the potential effects of such exposures on neurodegeneration and aging (Parmalee and Aschner, 2016). Notably, circadian disruption is observed in patients with PD, AD, and Huntington’s disease (HD), all neurodegenerative diseases that in addition to a genetic component are thought to be influenced by metal exposures (Videnovic et al., 2014). As lifespan increases and metals are present in the environment, these cumulative exposures to a variety of metals are likely to have implications on health. In this review we will present a subset of evidence for interactions between metals and circadian rhythms.
Manganese
Manganese (Mn) is a metal that occurs commonly in soil and water. It is one of the most abundant elements in the earth’s crust. It has been used since antiquity as a pigment in ceramics. It is used as an alloy with other metals, is used in battery manufacture, is present in pesticides, and in the gasoline anti-knock additive methylcyclopentadienyl manganese tricabonyl (MMT). Mn is an essential trace element, necessary as a cofactor for many enzymes. Humans obtain a sufficient amount of Mn through the diet and Mn deficiency is essentially unknown outside of the laboratory. Low-level exposures occur primarily via well water in regions with a naturally high level of Mn occurring in the soil, where it leaches into the water, and by exposure to contaminated soil in areas nearby to metal refineries. There is evidence for detrimental behavioral and cognitive effects in children exposed to relatively low levels of Mn (Bhang et al., 2013, Carvalho et al., 2014). At higher levels of exposure, especially unregulated industrial exposure, a condition known as manganism can develop that is well-characterized and has many features in common with Parkinson’s disease (Couper, 1837, Rodier, 1955, Mena et al., 1967, Huang et al., 1989). With excessive exposure, Mn tends to accumulate in the basal ganglia and putamen of the brain, whereas in Parkinson’s disease (PD) the substantia nigra pars compacta is primarily affected, with degeneration of dopaminergic neurons seen in this area. The similarities and differences between manganism and PD have been reviewed (Guilarte, 2010, Guilarte and Gonzales, 2015). Manganism and PD share the cardinal motor signs: bradykinesia, rigidity, postural instability and tremor. They also share many non-motor features, including depression, dementia, loss of sense of smell, and various sleep disorders, including difficulty falling and staying asleep, rapid eye movement (REM) behavior disorder (RBD), and circadian disruptions (Parkinson, 2002, Bowler et al., 2006, Bowler et al., 2007, Videnovic and Golombek, 2013). The mechanism of circadian disruption is unknown, but it is a prominent feature of both PD and manganism. Mn toxicity is thought to be mediated by dopamine so it is possible that Mn-dopamine interactions have an adverse effect on the sleep-wake cycle though undetermined mechanisms. Excess Mn is eliminated via bile and excreted in feces. Aside from industrial exposures, patients with liver disease are also known to be at risk for elevated Mn levels. Circadian disruptions have been reported in patients with chronic liver disease, cirrhosis of the liver, fatty liver disease, and hepatitis C (De Cruz et al., 2012), raising the possibility that elevated Mn levels could participate in sleep disruption in these patients.
Copper
Copper (Cu) is also an essential micronutrient which is found bound to many enzymes and unbound in serum. Wilson’s disease is a genetic disorder that results in dysregulated copper metabolism. In patients with Wilson’s disease copper accumulates in many organs, in the corneas, and in the basal ganglia and putamen of the brain, the same regions affected by Mn accumulation (Nevsimalova et al., 2011). Excess copper can cause parkinsonian movement symptoms, psychiatric disturbances, liver disease, and neurological degeneration. Amyloid precursor protein (APP), which is implicated in AD, is a copper binding protein (Nevsimalova et al., 2011). Patients with Wilson’s disease have been reported to have sleep disturbances including RBD, restless leg syndrome, daytime hypersomnolence, excessive nighttime wakefulness, and circadian disruption (Firneisz et al., 2000, Nevsimalova et al., 2011, Amann et al., 2015, Tribl et al., 2016).
Again, the mechanism by which copper overload may lead to circadian disruption is unknown, however, in addition to APP, suspect genes that could be involved include a night-specific ATPase PINA that is expressed in the pineal gland which participates in circadian biology. Variants in this gene are causative in Wilson’s disease, and the gene is expressed at 100 fold greater levels at night than during the day (Borjigin et al., 1999, Firneisz et al., 2000). Additionally, dopamine beta hydroxylase is a copper-binding enzyme that may be functionally affected by copper overload (Kitzberger et al., 2005, Nevsimalova et al., 2011). Copper has also been shown to interact with the vesicular H(+)ATPase, impacting dopamine metabolism (Wimalasena et al., 2007). The regulated cycling of dopamine is critical to maintaining circadian rhythms. In addition to dopamine, the neurotransmitter serotonin (5-hydroxytryptamine, 5-HT) has been shown to interact with unbound copper, resulting in conversion of 5-HT to an intermediate species (Jones et al., 2007). The psychiatric symptoms of Wilson’s disease include depression, anxiety and cognitive changes which could be attributable to changes in the serotonin system, which is also highly circadian. Studies have shown interaction between the prion protein, another copper-binding protein, and 5-HT, both of which are present in the synaptic cleft (Mouillet-Richard et al., 2005, Jones et al., 2007). Notably, symptoms of the human prion diseases Fatal Familial Insomnia and Cruetzfeldt-Jacob disease include severe sleep-wake disorders and behavioral disturbance (Wanschitz et al., 2000, Landolt et al., 2006, Jones et al., 2007).
Zinc
Zinc (Zn) is another essential metal, necessary for optimal biological functioning. Zinc and copper compete for absorption in the intestine, liver, and kidney. Both copper and zinc are also inhibitory against the NMDA receptor, which is involved in mood, cognitive functions, and sleep (Peters et al., 1987, Vlachova et al., 1996, Song et al., 2012). Song et al. found that duration of sleep correlated with the ratio of zinc to copper in adult women (Song et al., 2012), suggesting that a balance between these two metals is important for sleep-wake regulation. A similar finding was reported for men (Zhang et al., 2009). In mice, administration of zinc has been shown to decrease locomotion and induce non-REM sleep (Cherasse et al., 2015). Other metals are known to interact with each other, for instance iron and Mn compete for absorption, and iron deficiency is a risk factor for elevated levels of Mn. The balance between zinc and copper appears to be important in the sleep-wake cycle; however the mechanisms behind this interaction remain to be elucidated.
Lead
While the metals previously discussed are all essential micronutrients that participate in enzymatic activity and are necessary to biological processes, lead (Pb) is not. Pb is an environmental pollutant well-characterized as having severe impacts on the central nervous system and cognitive function. Pb does not serve a biological role, but does interact with biological systems, therefore it is of interest to question what role Pb might play in circadian rhythms and sleep. It is worth noting that sleep studies invariably use leads, a homonym for the metal lead, confounding searches for the role of Pb in sleep. We suggest that researchers conducting studies related to Pb and sleep employ the elemental nomenclature Pb to allow these studies to be found in the literature.
As previously described, the human circadian system is photoentrained to the 24 hour day by sensing daylight via melanopsin expressing ipRGCs in the retina. Light signals are transmitted synaptically to the SCN, the central clock which coordinates and regulates peripheral and cellular clocks. While ipRGCs are not dopaminergic, they do form syncytial connections through gap junctions with amacrine cells which are dopaminergic. Gestational Pb exposure in mice has been shown to decrease dopamine content in the retina and decrease levels of dopamine byproducts 3,4-Dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA) (Fox et al., 2011). Many subtypes of amacrine cells exist. Dopaminergic amacrine cells were shown to be depleted, and their processes shortened, while non-dopaminergic amacrine cells were unaffected. The dopamine processing enzyme tyrosine hydroxylase was also depleted with gestational Pb exposure. This study did not address circadian rhythms but raised the possibility that this finding could have a circadian effect.
The early morning production of cortisol is an integral part of the circadian system, responsible for waking and alertness in the morning. In humans, cortisol production is regulated by the hypothalamus-pituitary-adrenal (HPA) axis. Cortisol levels rise dramatically first thing in the morning and decline throughout the day. High levels of stress can result in daytime cortisol production, disrupting this pattern. Braun et al. measured Pb levels and salivary cortisol levels in second trimester pregnant women from Mexico City and evaluated the relationship between Pb and cortisol levels (Braun et al., 2014). Cortisol is also critical for brain development and gestational Pb exposure is known to carry risk of neurological deficits, possibly resulting from HPA axis effects (LeWinn et al., 2009, Braun et al., 2014). This study found a weak relationship between Pb levels and cortisol awakening response in pregnant women. Diurnal slopes of cortisol production were flatter in those women with the highest Pb levels. Given the known effects of Pb on cognition and the central nervous system, more work remains to be done to understand the role of Pb and circadian rhythms.
Mercury
Mercury (Hg) is another metal that serves no biological purpose, but can accumulate in the body and interact with biological systems. Exposure to mercury can occur in industrial settings, such as gold mining operations and gilding. Mercury is an environmental contaminant that makes its way into waterways where it is taken up by fish and other aquatic animals which then frequently become seafood, resulting in exposure to humans. Because the body does not regulate mercury, exposure is cumulative and bioaccumulation occurs and smaller animals are eaten by larger animals, and eventually by humans. Coastal and island communities, especially indigenous people that traditionally rely on fish and shellfish as a food source, are especially vulnerable to high levels of mercury exposure. This was exemplified by the environmental disaster at Minamata Bay, a fishing village in Japan. In 1956 patients began appearing with what was later recognized as severe mercury poisoning. The cause was large scale disposal of industrial waste containing high levels of mercury into the bay. The surrounding community was dependent on fish from the region for food, resulting in dire health consequences. While industrial regulation has reduced dumping of mercury containing waste into waterways, exposure to mercury as a consequence of gold mining operations which use mercury in the extraction process remains an issue in the developing world.
The second oldest mercury mine in the world, the Idrija Mercury Mine, is located in what is now Slovenia and has been in operation since the 1400s. From the 1500s, writers have described the condition of miners at this location as sick, suffering from deformity, paralysis, asthma, and other conditions (Kobal and Grum, 2010). In 1754 Joannes Antonius Scopoli was assigned as physician to the mine and subsequently published the first description of mercury poisoning in Venice in 1761.In addition to tremor, respiratory difficulties, personality changes, difficulty eating and sleeping, and other conditions, Scopoli identified sleep disorders as a prominent sign of mercury toxicity (Kobal and Grum, 2010). Scopoli reported difficulty sleeping, restless sleep, dream disturbances, and what we would now term restless leg syndrome. Sleep disturbance subsequent to mercury exposure was also described by Freeman in 1860 in his description of mercury poisoning in hat makers, the caricature of which was thought to give rise to the character of the Mad Hatter in Lewis Carroll’s Through the Looking Glass.
Mercury accumulation has been shown in the pineal gland, which participates in circadian function through the secretion of melatonin and serotonin (Kosta et al., 1975, Falnoga et al., 2000). Methylmercury exposure was shown to result in circadian sleep-wake disruption in rats (Arito et al., 1983). One possible mechanism of sleep disruption is the effect of mercury on glutamate, resulting in increased extracellular glutamate and a possible excitotoxic effect (Aschner et al., 2007). Mercury exposure also results in changes in cytokine production. In children, mercury exposure was found to be associated with reduced levels of TNF-alpha and shorter sleep duration (Gump et al., 2014).
Insomnia and other sleep disorders have been reported subsequent to mercury exposure in worker in fluorescent light bulb plants (Rossini et al., 2000), among sport-fishers in Wisconsin (Knobeloch et al., 2006), among dental assistants exposed to mercury-containing amalgam used in fillings (Moen et al., 2008), among gold miners in Indonesia (Bose-O’Reilly et al., 2016), and among Iranian workers gilding a shrine (Vahabzadeh and Balali-Mood, 2016). Additional research is needed to address the mechanism underlying these sleep disruptions observed with mercury exposure.
Aluminium
Aluminum (Al) is yet another metal that plays no biological role in living systems but has toxic effects in vivo. Aluminum has been implicated as a neurotoxicant and a possible factor in neurodegenerative diseases such as amyotrophic lateral sclerosis (ALS), PD and AD, which as previously mentioned have strong circadian components (Rodella et al., 2008).While few studies of the effect of aluminum on human sleep and circadian rhythms have been reported, evidence from the fruit fly Drosophila melanogaster showed that in addition to shortening the lifespan of the flies, aluminum exposure influenced the level and rhythm of daily locomotion (Kijak et al., 2014). Given the connection to human neurodegenerative disease with circadian components, this finding in flies suggests that further observation into human aluminum exposure and further research into circadian mechanisms is warranted.
Conclusion
Many metals are essential for the optimal functional of many physiological processes, including countless enzymatic reactions that use metals as cofactors. Those metals that play a role in biological processes, including but not limited to manganese, copper, and zinc, are tightly regulated at the level of absorption, transport into and out of the cell, and excretion. Even with regulation, excessive levels of these metals can occur, in the case of high levels of environmental exposure, such as in industrial settings, or in the case of disease in which the regulation of the metal is impaired. Given that thousands of genes are under circadian control and involved in maintaining circadian rhythms, it would be surprising if the circadian system were not affected by metals.
Numerous neurological and neurodegenerative diseases have a circadian component, including mood disorders such as depression and bipolar disorder, neurodegenerative diseases such as PD, Alzheimer’s, and Huntingtons disease, and prion diseases such as Fatal Familial Insomnia and Cruetzfeldt-Jacob disease. There is evidence for the mood disorders and the neurodegenerative diseases that the disease process may initiate the sleep disorder, but dysregulated sleep-wake cycles may worsen the disease process, resulting in a spiraling pattern of dysfunction. Indeed, disturbed sleep patterns are a primary cause of institutionalization in patients suffering from dementia (Hatfield et al., 2004). Patients may benefit from pharmacological interventions to promote normative sleep-wake cycles, which may in turn slow the disease process. While epidemiological studies are useful in observing the correlation between circadian disruption and disease, more research is needed to understand the mechanisms behind these processes.
As the population ages and advances in medicine expand the human lifespan, healthy aging will be dependent on understanding factors related to neurodegenerative disease. Circadian rhythms are intricately entwined with neurodegenerative disease, as are metals. Understanding the interplay between these factors in health and disease will contribute to healthier aging.
Acknowledgements
This work is supported by NIEHS grant R03ES024849 and your grant.
References
- Amann VC, Maru NK, Jain V (2015) Hypersomnolence in Wilson Disease. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine 11:1341–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arito H, Hara N, Torii S (1983) Effect of methylmercury chloride on sleep-waking rhythms in rats. Toxicology 28:335–345. [DOI] [PubMed] [Google Scholar]
- Aschner M, Syversen T, Souza DO, Rocha JB, Farina M (2007) Involvement of glutamate and reactive oxygen species in methylmercury neurotoxicity. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas 40:285–291. [DOI] [PubMed] [Google Scholar]
- Berson DM, Dunn FA, Takao M (2002) Phototransduction by retinal ganglion cells that set the circadian clock. Science 295:1070–1073. [DOI] [PubMed] [Google Scholar]
- Bhang SY, Cho SC, Kim JW, Hong YC, Shin MS, Yoo HJ, Cho IH, Kim Y, Kim BN (2013) Relationship between blood manganese levels and children’s attention, cognition, behavior, and academic performance--a nationwide cross-sectional study. Environmental research 126:9–16. [DOI] [PubMed] [Google Scholar]
- Borjigin J, Payne AS, Deng J, Li X, Wang MM, Ovodenko B, Gitlin JD, Snyder SH (1999) A novel pineal night-specific ATPase encoded by the Wilson disease gene. The Journal of neuroscience : the official journal of the Society for Neuroscience 19:1018–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose-O’Reilly S, Schierl R, Nowak D, Siebert U, William JF, Owi FT, Ir YI (2016) A preliminary study on health effects in villagers exposed to mercury in a small-scale artisanal gold mining area in Indonesia. Environmental research 149:274–281. [DOI] [PubMed] [Google Scholar]
- Bowler RM, Gysens S, Diamond E, Nakagawa S, Drezgic M, Roels HA (2006) Manganese exposure: neuropsychological and neurological symptoms and effects in welders. Neurotoxicology 27:315–326. [DOI] [PubMed] [Google Scholar]
- Bowler RM, Nakagawa S, Drezgic M, Roels HA, Park RM, Diamond E, Mergler D, Bouchard M, Bowler RP, Koller W (2007) Sequelae of fume exposure in confined space welding: a neurological and neuropsychological case series. Neurotoxicology 28:298–311. [DOI] [PubMed] [Google Scholar]
- Braun JM, Wright RJ, Just AC, Power MC, Tamayo YOM, Schnaas L, Hu H, Wright RO, Tellez-Rojo MM (2014) Relationships between lead biomarkers and diurnal salivary cortisol indices in pregnant women from Mexico City: a cross-sectional study. Environ Health 13:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho CF, Menezes-Filho JA, de Matos VP, Bessa JR, Coelho-Santos J, Viana GF, Argollo N, Abreu N (2014) Elevated airborne manganese and low executive function in school-aged children in Brazil. Neurotoxicology 45:301–308. [DOI] [PubMed] [Google Scholar]
- Cherasse Y, Saito H, Nagata N, Aritake K, Lazarus M, Urade Y (2015) Zinc-containing yeast extract promotes nonrapid eye movement sleep in mice. Molecular nutrition & food research 59:2087–2093. [DOI] [PubMed] [Google Scholar]
- Couper J (1837) On the effects of black oxide manganese when inhaled into the lungs. British annals of medicine, pharmacy, vital statistics and general science 1: 41–42. [Google Scholar]
- De Cruz S, Espiritu JR, Zeidler M, Wang TS (2012) Sleep disorders in chronic liver disease. Seminars in respiratory and critical care medicine 33:26–35. [DOI] [PubMed] [Google Scholar]
- Falnoga I, Tusek-Znidaric M, Horvat M, Stegnar P (2000) Mercury, selenium, and cadmium in human autopsy samples from Idrija residents and mercury mine workers. Environmental research 84:211–218. [DOI] [PubMed] [Google Scholar]
- Firneisz G, Szalay F, Halasz P, Komoly S (2000) Hypersomnia in Wilson’s disease: an unusual symptom in an unusual case. Acta neurologica Scandinavica 101:286–288. [DOI] [PubMed] [Google Scholar]
- Fox DA, Hamilton WR, Johnson JE, Xiao W, Chaney S, Mukherjee S, Miller DB, O’Callaghan JP (2011) Gestational lead exposure selectively decreases retinal dopamine amacrine cells and dopamine content in adult mice. Toxicology and applied pharmacology 256:258–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilarte TR (2010) Manganese and Parkinson’s disease: a critical review and new findings. Environ Health Perspect 118:1071–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilarte TR, Gonzales KK (2015) Manganese-Induced Parkinsonism Is Not Idiopathic Parkinson’s Disease: Environmental and Genetic Evidence. Toxicological sciences : an official journal of the Society of Toxicology 146:204–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gump BB, Gabrikova E, Bendinskas K, Dumas AK, Palmer CD, Parsons PJ, MacKenzie JA (2014) Low-level mercury in children: associations with sleep duration and cytokines TNF-alpha and IL-6. Environmental research 134:228–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfield CF, Herbert J, van Someren EJ, Hodges JR, Hastings MH (2004) Disrupted daily activity/rest cycles in relation to daily cortisol rhythms of home-dwelling patients with early Alzheimer’s dementia. Brain : a journal of neurology 127:1061–1074. [DOI] [PubMed] [Google Scholar]
- Hattar S, Liao HW, Takao M, Berson DM, Yau KW (2002) Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science 295:1065–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CC, Chu NS, Lu CS, Wang JD, Tsai JL, Tzeng JL, Wolters EC, Calne DB (1989) Chronic manganese intoxication. Archives of neurology 46:1104–1106. [DOI] [PubMed] [Google Scholar]
- Jones CE, Underwood CK, Coulson EJ, Taylor PJ (2007) Copper induced oxidation of serotonin: analysis of products and toxicity. J Neurochem 102:1035–1043. [DOI] [PubMed] [Google Scholar]
- Kijak E, Rosato E, Knapczyk K, Pyza E (2014) Drosophila melanogaster as a model system of aluminum toxicity and aging. Insect science 21:189–202. [DOI] [PubMed] [Google Scholar]
- Kitzberger R, Madl C, Ferenci P (2005) Wilson disease. Metabolic brain disease 20:295–302. [DOI] [PubMed] [Google Scholar]
- Knobeloch L, Steenport D, Schrank C, Anderson H (2006) Methylmercury exposure in Wisconsin: A case study series. Environmental research 101:113–122. [DOI] [PubMed] [Google Scholar]
- Kobal AB, Grum DK (2010) Scopoli’s work in the field of mercurialism in light of today’s knowledge: past and present perspectives. American journal of industrial medicine 53:535–547. [DOI] [PubMed] [Google Scholar]
- Kosta L, Byrne AR, Zelenko V (1975) Correlation between selenium and mercury in man following exposure to inorganic mercury. Nature 254:238–239. [DOI] [PubMed] [Google Scholar]
- Landolt HP, Glatzel M, Blattler T, Achermann P, Roth C, Mathis J, Weis J, Tobler I, Aguzzi A, Bassetti CL (2006) Sleep-wake disturbances in sporadic Creutzfeldt-Jakob disease. Neurology 66:1418–1424. [DOI] [PubMed] [Google Scholar]
- LeWinn KZ, Stroud LR, Molnar BE, Ware JH, Koenen KC, Buka SL (2009) Elevated maternal cortisol levels during pregnancy are associated with reduced childhood IQ. International journal of epidemiology 38:1700–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mena I, Marin O, Fuenzalida S, Cotzias GC (1967) Chronic manganese poisoning. Clinical picture and manganese turnover. Neurology 17:128–136. [DOI] [PubMed] [Google Scholar]
- Moen B, Hollund B, Riise T (2008) Neurological symptoms among dental assistants: a cross-sectional study. Journal of occupational medicine and toxicology 3:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohawk JA, Green CB, Takahashi JS (2012) Central and peripheral circadian clocks in mammals. Annual review of neuroscience 35:445–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouillet-Richard S, Pietri M, Schneider B, Vidal C, Mutel V, Launay JM, Kellermann O (2005) Modulation of serotonergic receptor signaling and cross-talk by prion protein. The Journal of biological chemistry 280:4592–4601. [DOI] [PubMed] [Google Scholar]
- Nevsimalova S, Buskova J, Bruha R, Kemlink D, Sonka K, Vitek L, Marecek Z (2011) Sleep disorders in Wilson’s disease. Eur J Neurol 18:184–190. [DOI] [PubMed] [Google Scholar]
- Parkinson J (2002) An essay on the shaking palsy. 1817. The Journal of neuropsychiatry and clinical neurosciences 14:223–236; discussion 222. [DOI] [PubMed] [Google Scholar]
- Parmalee NL, Aschner M (2016) Manganese and aging. Neurotoxicology 56:262–268. [DOI] [PubMed] [Google Scholar]
- Peters S, Koh J, Choi DW (1987) Zinc selectively blocks the action of N-methyl-D-aspartate on cortical neurons. Science 236:589–593. [DOI] [PubMed] [Google Scholar]
- Rodella LF, Ricci F, Borsani E, Stacchiotti A, Foglio E, Favero G, Rezzani R, Mariani C, Bianchi R (2008) Aluminium exposure induces Alzheimer’s disease-like histopathological alterations in mouse brain. Histology and histopathology 23:433–439. [DOI] [PubMed] [Google Scholar]
- Rodier J (1955) Manganese poisoning in Moroccan miners. British journal of industrial medicine 12:21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini SR, Reimao R, Lefevre BH, Medrado-Faria MA (2000) Chronic insomnia in workers poisoned by inorganic mercury: psychological and adaptive aspects. Arquivos de neuro-psiquiatria 58:32–38. [DOI] [PubMed] [Google Scholar]
- Song CH, Kim YH, Jung KI (2012) Associations of zinc and copper levels in serum and hair with sleep duration in adult women. Biol Trace Elem Res 149:16–21. [DOI] [PubMed] [Google Scholar]
- Takahashi JS, Hong HK, Ko CH, McDearmon EL (2008) The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nature reviews Genetics 9:764–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribl GG, Trindade MC, Bittencourt T, Lorenzi-Filho G, Cardoso Alves R, Ciampi de Andrade D, Fonoff ET, Bor-Seng-Shu E, Machado AA, Schenck CH, Teixeira MJ, Barbosa ER (2016) Wilson’s disease with and without rapid eye movement sleep behavior disorder compared to healthy matched controls. Sleep medicine 17:179–185. [DOI] [PubMed] [Google Scholar]
- Vahabzadeh M, Balali-Mood M (2016) Occupational Metallic Mercury Poisoning in Gilders. The international journal of occupational and environmental medicine 7:116–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videnovic A, Golombek D (2013) Circadian and sleep disorders in Parkinson’s disease. Experimental neurology 243:45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videnovic A, Lazar AS, Barker RA, Overeem S (2014) ‘The clocks that time us’--circadian rhythms in neurodegenerative disorders. Nature reviews Neurology 10:683–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlachova V, Zemkova H, Vyklicky L Jr, (1996) Copper modulation of NMDA responses in mouse and rat cultured hippocampal neurons. The European journal of neuroscience 8:2257–2264. [DOI] [PubMed] [Google Scholar]
- Wanschitz J, Kloppel S, Jarius C, Birner P, Flicker H, Hainfellner JA, Gambetti P, Guentchev M, Budka H (2000) Alteration of the serotonergic nervous system in fatal familial insomnia. Ann Neurol 48:788–791. [PubMed] [Google Scholar]
- Welsh DK, Logothetis DE, Meister M, Reppert SM (1995) Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron 14:697–706. [DOI] [PubMed] [Google Scholar]
- Welsh DK, Takahashi JS, Kay SA (2010) Suprachiasmatic nucleus: cell autonomy and network properties. Annual review of physiology 72:551–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimalasena DS, Wiese TJ, Wimalasena K (2007) Copper ions disrupt dopamine metabolism via inhibition of V-H+-ATPase: a possible contributing factor to neurotoxicity. J Neurochem 101:313–326. [DOI] [PubMed] [Google Scholar]
- Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ, Menaker M, Takahashi JS (2004) PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proceedings of the National Academy of Sciences of the United States of America 101:5339–5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HQ, Li N, Zhang Z, Gao S, Yin HY, Guo DM, Gao X (2009) Serum zinc, copper, and zinc/copper in healthy residents of Jinan. Biol Trace Elem Res 131:25–32. [DOI] [PubMed] [Google Scholar]


