Abstract
Four bacterial strains identified as members of the Acidovorax genus were isolated from two geographically distinct but similarly contaminated soils in North Carolina, USA, characterized, and their genomes sequenced. Their 16S rRNA genes were highly similar to those previously recovered during stable-isotope probing (SIP) of one of the soils with the polycyclic aromatic hydrocarbon (PAH) phenanthrene. Heterotrophic growth of all strains occurred with a number of organic acids, as well as phenanthrene, but no other tested PAHs. Optimal growth occurred aerobically under mesophilic temperature, neutral pH, and low salinity conditions. Predominant fatty acids were C16:1ω7c/C16:1ω6c, C16:0, and C18:1ω7c, and were consistent with the genus. Genomic G+C contents ranged from 63.6 to 64.2%. A combination of whole genome comparisons and physiological analyses indicated that these four strains likely represent a single species within the Acidovorax genus. Chromosomal genes for phenanthrene degradation to phthalate were nearly identical to highly conserved regions in phenanthrene-degrading Delftia, Burkholderia, Alcaligenes, and Massilia species in regions flanked by transposable or extrachromosomal elements. The lower degradation pathway for phenanthrene metabolism was inferred by comparisons to described genes and proteins. The novel species Acidovorax carolinensis sp. nov. is proposed, comprising the four strains described in this study with strain NA3T as the type strain (=LMG 30136, =DSM 105008).
Keywords: Acidovorax, phenanthrene, polycyclic aromatic hydrocarbons
Introduction
Bacteria within the Acidovorax genus are found in a diverse range of habitats and have varied functions within those environments. Strains now classified as Acidovorax have been associated with soils [11, 45], wastewater treatment plants [23, 46], plants [19, 20, 35, 63], and clinical samples [59]. Mirroring the diversity inherent in this widespread distribution, isolates within this diverse genus have been linked to a variety of phenotypes ranging from plant pathogenicity, to denitrification, to the biodegradation of contaminants.
Environmentally derived 16S rRNA gene sequences from the Acidovorax genus have frequently been associated with samples impacted by petroleum contamination which typically contain high concentrations of polycyclic aromatic hydrocarbons (PAHs) [18, 26, 41, 58]. Of particular interest for PAH bioremediation, the isolates Acidovorax delafieldii strains P4–1 [44] and TNA921 [47], Acidovorax temperans strain GTI-19 [ 6], and several uncharacterized Acidovorax strains [40] have been demonstrated to grow on the three-ring, low-molecular-weight (LMW) PAH phenanthrene. In a prior study of weathered PAH-contaminated soils treated via biostimulation in ex situ, lab-scale, slurry-phase bioreactors, we directly linked the degradation of phenanthrene to uncultivated members of the Acidovorax genus through stable-isotope probing (SIP) [25, 49]. Additional SIP experiments of bacterial PAH degraders in a similarly contaminated soil from France also directly linked Acidovorax organisms to the disappearance of phenanthrene in those samples [38]. We subsequently isolated several strains of phenanthrene-degrading bacteria, including one (designated Acidovorax strain NA3) with high 16S rRNA gene sequence identity to SIP-detected Acidovorax sequences that could grow on the PAH phenanthrene [50].
A number of Acidovorax strains have publicly available draft or finished genomes. While some of these represent Acidovorax strains capable of the metabolism of aromatic compounds (e.g., 2-nitrotoluene by Acidovorax sp. JS42 [22] or biphenyl and polychlorinated biphenyls by Acidovorax sp. KKS102 [27]), no public Acidovorax genomes are available from organisms capable of degrading any PAH. In fact, there is little genetic information available for the strains previously shown to grown on phenanthrene, with only a partial 16S rRNA gene sequence for A. delafieldii P4–1 available (GenBank accession number DQ282184). The only published work on the genetics of PAH metabolism within the genus was performed on Acidovorax strain NA3T using partial sequences recovered from a fosmid library [50]. In that work, the genes likely to be associated with the first three steps of the “upper pathway” of PAH degradation in NA3T (comprising a ring-hydroxylating dioxygenase [RHD], dihydrodiol dehydrogenase, and ring- cleavage dioxygenase) were shown to be highly similar to homologous genes in phenanthrene- degrading members of the Alcaligenes, Delftia, and Burkholderia genera within the Betaproteobacterial order Bulkholderiales. Although the genes in strain NA3T were not directly linked to the transformation of phenanthrene, RNA transcripts derived from those genes were induced by the presence of either of the LMW PAHs phenanthrene or naphthalene added to the cell culture. A recent publication analyzing the genome of Delftia strain Cs1–4, a strain with putative phenanthrene-degradation genes nearly identical to those in Acidovorax NA3T, revealed a genomic “phn island,” a 232 kb region of the chromosome flanked by transposable elements containing the phn genes thought to be responsible for phenanthrene degradation [24]. It was suggested in that work that the genomes of organisms harboring highly similar genes, such as phenanthrene-degrading Acidovorax spp., may demonstrate a similar gene organization.
In this manuscript, we describe four Acidovorax strains isolated from two geographically distinct, weathered PAH-contaminated soils from former manufactured gas plant (MGP) sites in North Carolina, USA. Each isolate was characterized using a variety of physiological tests and the complete genome of each strain was determined. The 16S rRNA genes from all of the strains were highly similar to sequences derived from phenanthrene SIP [25, 49], and all were capable of growth on phenanthrene as a sole source of carbon and energy. The genome of each isolate was examined for genes and genomic islands potentially associated with phenanthrene metabolism. Based on genotypic and physiological differences to characterized Acidovorax species, Acidovorax carolinensis sp. nov. is proposed to encompass all four strains described herein, with strain NA3 T as the designated type strain.
Methods and Materials
Strain isolation.
Acidovorax sp. strains P3 and P4 were isolated from crystalline phenanthrene- enriched, PAH-contaminated soil collected from the site of a former MGP site in Salisbury, NC, USA that had previously undergone treatment in a lab-scale, slurry-phase aerobic bioreactor [51]. Pure cultures of strains P3 and P4 were obtained by serial dilution of phenanthrene-enriched bioreactor slurry on nutrient agar (NA) plates (Difco, Becton, Dickinson and Company, Franklin Lakes, NJ, USA). Well-separated colonies were screened by PCR using Acidovorax-selective primers and conditions as previously described [48]. Strains P3 and P4 specifically were selected for analysis based on genomic differences among 10 PCR-verified Acidovorax isolates (see below). Strains NA2 and NA3T were previously isolated in a similar manner, except that the source soil for treatment in the bioreactor was obtained from a former MGP site in Charlotte, NC, USA, and treated under slightly different conditions [50].
Determination of optimal growth conditions.
Strains were generally maintained on either NA or R2A plates (Difco), or in nutrient broth (NB; Difco). For determining optimal growth conditions for each strain, cultures in triplicate 5-mL tubes of NB were tested at 25°C, 27.5°C, 30°C, 32.5°C, and 35°C with shaking at 250 rpm. Optimal exponential growth rates were determined by measuring turbidity at OD600 using a DR/3000 Spectrophotometer (Hach, Loveland, CO, USA). Growth at 4°C, 15°C, 37°C, and 40°C was tested by examining NA plates for growth after 2 weeks. Growth under varying pH was determined by buffering triplicate 5-mL NB tubes with 50 mM of (2-(N-morpholino)ethanesulfonic acid) monohydrate (St. Louis, MO; pH 5.5, 6.0, and 6.5), (N-[2- hydroxyethyl]piperazine-N’-[2-ethanesulfonic acid]) (Acros Organics, NJ; pH 7.0, 7.5 and 8.0), or Tris·HCl (pH 8.9 and 9.0), and incubating at 32.5°C and 250 rpm for 7 hours. Growth in NB (pH 7.0, 32.5°C) amended with 0%, 1%, 2%, 3%, 4%, and 5% NaCl was also tested. Production of indigo was examined by amending NA plates with 1 mM indole (Aldrich Chemical Co., Milwaukee, WI, USA). Growth under low oxygen conditions was tested by incubating NA plates in a sealed vessel in the presence of a GasPak™ EZ anaerobe sachet (BD, Franklin Lakes, NJ) for two weeks at room temperature (~23°C). Unless otherwise stated, strains were routinely grown aerobically in NB buffered to pH 7.5 with no additional salt, and incubated 32.5°C.
Cellular morphology.
The morphology of overnight cultures was determined by scanning electron microscopy. Weak pellets were obtained by centrifugation for 2 minutes at ~1000 x g and the pellets washed twice with phosphate buffered saline (PBS).Specimens were observed and images acquired as previously described [13].. Motility of the strains was further examined using tubes of nutrient broth containing 0.3% agar (motility agar). Stabs of separate single colonies grown on NA plates were inoculated into triplicate tubes and incubated statically at 32.5°C. Cell suspensions in PBS derived from motility agar tube cultures were stained with Remel™ Flagella Stain (Thermo Scientific, Waltham, MA) according to manufacturer’s directions and examined under oil-immersion light microscopy.
Chemotaxonomic and physiological characteristics.
Catalase activity was tested by adding 3% hydrogen peroxide (v/v) solution to cells freshly scraped from the surface of an NA plate. Oxidase activity was determined by adding a few drops of freshly-prepared 1% N’,N’,N’,N’-tetramethyl- p-phenylenediamine dihydrochloride (Acros Organics, NJ) to cells scraped from a plate onto filter paper [30]. DNase activity of the strains was tested by streaking each isolate on DNase agar plates with toluidine blue (Remel, Lenaxa, KA, USA). Starch hydrolysis was examined using starch agar plates stained with Gram’s iodine solution after 48 hours of growth [32]. Gelatin hydrolysis was tested with a nutrient gelatin medium and examining for liquefaction of the media [15]. Protease activity was analyzed by streaking each of the strains on nutrient agar plates amended with 0.1% casein, as well as skim milk agar plates [2]. Urease activity was examined using slants of Christensen’s Urea Agar [12]. Nitrate reduction was determined by growing cells in triplicate tubes of nitrate broth (0.5% peptone, 0.3% beef extract, 0.1% KNO3), before adding several drops each of nitrate test reagents A and B (Gibson Bioscience, Lexington, KY, USA). Any negative results were confirmed by the addition of a small quantity of elemental zinc dust. Cellulolytic activity was tested on three types of media: NA, R2A, or MSB [53], each amended with 1% cellulose. Lipase activity was tested using plates containing 1% tributyrin.
Growth on polycyclic aromatic hydrocarbons (PAHs) and metabolites of PAH degradation were tested by adding salicylate, phthalate, naphthalene, acenaphthene, anthracene, phenanthrene, fluorene, pyrene, or fluoranthene (2 g·L−1 final concentration) to triplicate 5-mL tubes of either MM2 medium [28] or MSB medium. Tubes were incubated at 32.5°C with shaking at 225 rpm for up to 7 days and examined for turbidity. Uninoculated tubes containing growth substrates were tested as negative controls. Growth on benzene, toluene, ethylbenzene, or a mixture of o-, m-, and p-xylenes (BTEX compounds) was tested by adding each compound(s) to a final concentration of 100 mg·L−1 in triplicate, glass, screw-top tubes of 5-mL MM2 medium containing each strain and incubating for 5 days at 30°C with shaking. Growth was measured spectrophotometrically as described above. Inoculated MM2 plates without a carbon source were also incubated at room temperature in tightly sealed metal cannisters containing atmospheres with individual BTEX compounds (0.5 mL on a piece of filter paper). Growth was determined by visual examination after three weeks.
The capability of each strain to metabolize a range of carbon sources was tested using Biolog GN2 microplates (Hayward, CA, USA) in duplicate using inocula from freshly grown R2A agar plates (Difco) suspended in GN/GP Inoculating Fluid as described by the manufacturer. Positive results were determined by visual examination and comparison to the water control after 24 and 48 hours of incubation at 30°C. Fatty acid profiles of the fours strains were provided by Microbial ID, Inc. (Newark, DE, USA) using the MIDI Sherlock® Microbial Identification System with cultures prepared on NA plates. Analysis of respiratory quinones and polar lipids was carried out by the Identification Service, DSMZ, Braunschweig, Germany [55–57].
Pulsed-field gel electrophoresis (PFGE).
Ten Acidovorax isolates from the Salisbury site (named P1 through P10) were screened for genomic variability by PFGE prior to selection and characterization. Embedded agarose plugs of whole cells grown in NB were prepared according to the CHEF-DR® III Pulsed Field Electrophoresis Systems Instruction Manual and Applications Guide, 2nd revision. Plugs containing DNA from each strain were digested with restriction enzyme SpeI overnight at 37°C (New England Biolabs, Ipswich, MA, USA) before being run on the CHEF- DR® III Pulsed Field Electrophoresis System under the following conditions: 1% agarose gel in 0.5×TBE at 14°C, 20 h run, 6 V/cm, switch times 4s-30s, and a 120° angle. The gel was stained post-run in ethidium bromide and the digest patterns examined for differences. One isolate from each of two observed banding patterns, designated P3 and P4, were selected for further analyses.
Embedded agarose plugs for determination of the presence of plasmids by PFGE were prepared and treated with S1 nuclease [3]. The running conditions of the gel were: 1% agarose gel in 0.5× TBE at 14°C, 18 h run, 6 V/cm, switch times 5s-25s, and a 120° angle. The gel was post- stained in ethidium bromide and plasmid sizes estimated based on comparison to λ-concatemer ladders.
Genomics.
High-quality DNA for genomic sequencing was obtained from overnight cultures grown in NB and extracted using a modification of the FastDNA Spin Kit for Soil protocol (MP Biomedicals, Santa Ana, CA, USA) in which cell suspensions were secured horizontally to a vortexer for 30 seconds at maximum speed for physical cell disruption. DNA quality and size were verified on a 1% agarose gel against size standards and quantified using a NanoDrop-3300 fluorospectrometer (Thermo Scientific, Waltham, MA, USA) with the Quant-iT Picogreen dsDNA Quantification Kit (Invitrogen, Grand Island, NY, USA). High-quality genome sequences of each of the four strains were obtained using the PacBio RSII system (Pacific Biosciences, Menlo Park, CA, USA) at the University of North Carolina High Throughput Sequencing Facility (UNC- HTSF). Data from two to four Single Molecule, Real-Time (SMRT) Cells were used for each strain to obtain the desired level of coverage. The PacBio reads were de novo assembled with SMRT Analysis (version 2.2) using the Hierarchical Genome Assembly Process 3 (HGAP3). In HGAP3, the assembly step is performed using a combination of the Celera Assembler and a custom PacBiounitig consensus caller. The resulting contigs were polished using the Quiver algorithm [10]. The assemblies were visualized using the software Gephi [4] and we observed from the resulting graphs that the longest contigs had circular assemblies for each of the four strains.
To circularize the longest contig from each strain, the contigs were broken into two pieces at the selected origin of replication. To find the origin of replication, the contigs were run through BLAST [1, 64]; the BLAST result with the highest score for all four strains was Alicycliphilus denitrificans K601. The region with highest similarity to the replication control/initiator dnaA gene for A. denitrificans K601 was selected to be the origin point for the four strains. BLAST was used to determine the location of best alignment of this gene with the contigs. Minimus2 [52] was used to perform the circularization and the two contig pieces were rejoined such that the start of the circularized contig was located close to the identified origin. Final polishing to reduce the number of insertion, deletion, and substitution errors was performed using Quiver. The resulting consensus sequences had an accuracy of at least 99.999%.
Gene calling and annotation of the genomes were independently performed using the Integrated Microbial Genomes and Microbiome Samples (IMG/MER) system of the U.S. Department of Energy Joint Genomics Institute (DOE/JGI) web portal [37] and the NCBI Prokaryotic Genome Automatic Annotation Pipeline (PGAAP) version 4.1. Alignments of select genes and genomes and graphical representations of those results were created using Geneious v.9.1.3 (Biomatters Ltd., Newark NJ, USA). Sequences for rRNA and tRNA genes were identified through RNammer [31] and through the JGI portal. A neighbor-joining phylogenetic tree of 16S rRNA genes was created using ClustalX v.2.1 [54], excluding positions with gaps, and bootstrapping 1000 replicates. The same alignment was used to create a maximum parsimony tree using the PAUP algorithm within Geneious [14]. Putative genes involved in aromatic compound metabolism were identified through analyses using AromaDeg [16] and any homologs between genomes were confirmed using the “Genome Gene Best Homologs” tool within IMG/MER . Whole-genome sequence similarities were determined using both formula 2 of Genome Blast Distance Phylogeny version 2.0 (GBDP) [39] and average nucleotide identity (ANI) (http://enveomics.ce.gatech.edu/g-matrix/) using ANI-Matrix estimates [21]. Publicly available Acidovorax genomes for comparison were downloaded from the NCBI GenBank archives using the most recent assemblies as of February 2017.
Complete genomes are available at the JGI-IMG/MER website as well as in GenBank under the Bioproject PRJNA285679. GenBank nucleotide accession numbers for strains NA2, 229 NA3T, P3, and P4 are CP021359–60, CP021361, CP021362–65, and CP021366–70, respectively.
Results and Discussion
Phylogeny.
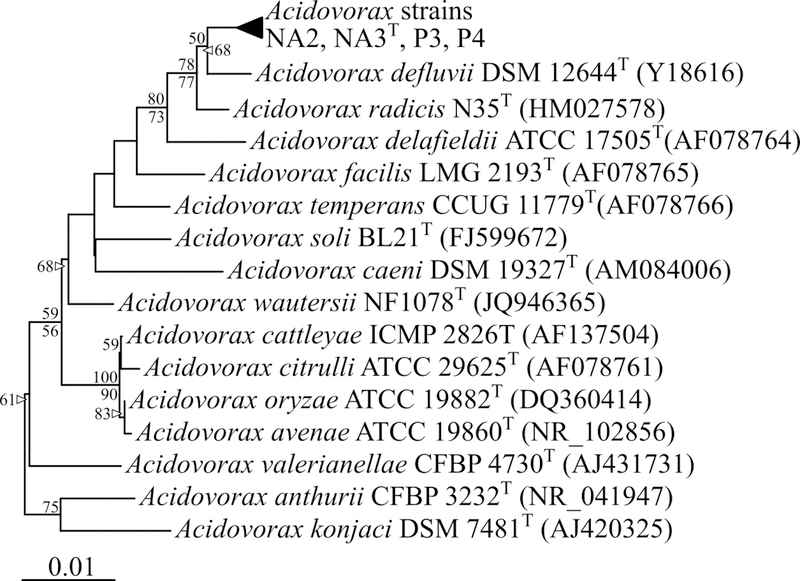
Each Acidovorax strain from this study contained four distinct rRNA operons. The 16S rRNA genes of the four strains (P3, P4, NA2, and NA3T) possessed between 99.6–100% similarity to one another. Strains P3 and P4 had identical 16S rRNA genes, which were distinct from the four identical copies found in strain NA3T. Interestingly, strain NA2 possessed four non- identical 16S rRNA genes with 99.6–99.9% identity to one another and variability observed at 6 of 1521 base positions (at positions 125, 126, 207, 208, 210, and 638; multiple signals were also observed in Sanger sequencing of PCR amplicons at those positions; data not shown). In strain NA2, one copy each of the 16S rRNA gene was identical to P3/P4 and NA3T, and the remaining two copies were 99.7% identical to one another, but distinct from all others.
Of the characterized Acidovorax species type strains, the most similar 16S rRNA gene sequences were from A. radicis sp. N35T (99.0–99.4% 16S rRNA gene similarity) and A. defluvii DSM 12644T (98.7–99.1%) (Figure 1). Similarly high 16S rRNA gene sequence similarity values were also observed to several uncharacterized environmental sequences (data not shown). High interspecies 16S rRNA gene identity (>97%) has previously been observed between described species of the Acidovorax genus [35]. The 16S rRNA gene sequence similarities of the four strains from this manuscript to a partial gene sequence from Acidovorax delafieldii P4–1 (Genbank accession DQ282184), the only other known phenanthrene-degrading Acidovorax isolate with a publicly available 16S rRNA gene sequence [44], ranged from 93.1–93.6% similarity over 828 bp. Efforts to obtain a culture of A. delafieldii P4–1 for further comparisons were unsuccessful. Comparisons of the RNA polymerase beta subunit (rpoB) gene additionally indicated that all four strains from this study comprised a cluster distinct from other characterized species with the closest relative being Acidovorax sp. JHL-3 (Appendix Figure A.1).
Figure 1.
Phylogenetic tree of 16S rRNA genes from Acidovorax species type strains compared to strains from this work. Labels at nodes indicate bootstrap support ≥ 50 (as a percentage of 1000 iterations) for neighbor-joining (above nodes) and maximum-parsimony (below nodes) algorithms. Genbank accession numbers are shown in parentheses. The tree was rooted with Variovorax paradoxus CBF3 (JN990697; not shown). The scale bar corresponds to 0.01 substitutions per nucleotide position.
Characterization.
Isolated Acidovorax strains grew well on common, complex growth media including NA and R2A with colonies generally appearing within 24–48 hours. Colonies on NA plates grown for 48 hours for each of the strains were pale yellow to yellow in color (cream to pale yellow on R2A plates), circular and convex with entire margins, and up to 1.0 – 1.5 mm in diameter. Addition of indole to plates resulted in purple colonies for each strain, indicating the probable production of indigo from an active aromatic ring-hydroxylating dioxygenase that catalyzes the initial step of aerobic bacterial polycyclic aromatic hydrocarbon degradation [17].
Growth was observed for all four stains at temperatures tested between 15°C and 35°C, with optimal growth at 30 – 32.5°C for all strains. Weak growth was observed after two weeks of incubation at 4°C, 37°C, and 40°C in regions of the plates with the densest inoculum for all strains but P4, which did not grow at 40ºC. All strains grew at pH 6.0 – 8.0, with optimal growth at either 7.0 or 7.5. In addition, strain NA3T grew weakly at pH 5.5 and strains P3 and P4 at pH 8.5. All strains grew optimally without amended salt but could tolerate 1% NaCl; no growth was observed after 24 hours in media supplemented with 2–5% NaCl. No growth was observed under anaerobic conditions on the media tested. All subsequent tests were generally conducted in media at a pH of 7.5, a temperature of 30 or 32.5°C, and with no amended salt.
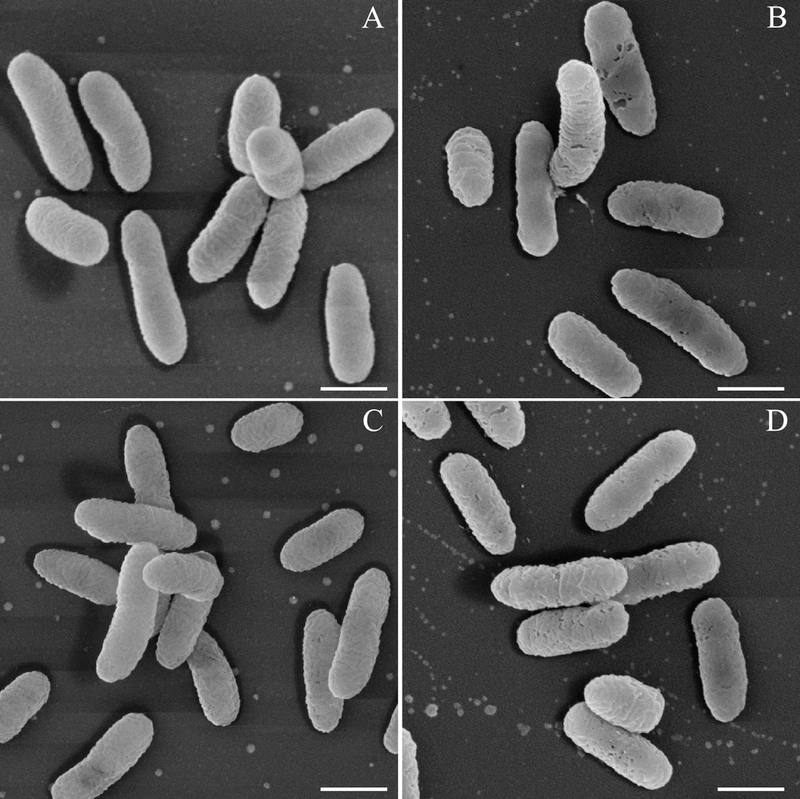
Under scanning electron microscopy, all four strains were observed to be slightly-curved or straight rods without distinguishing external features (Figure 2). The cell sizes ranged from 0.9 – 2.1 × 0.4 – 0.5 μm (P3), 0.8 – 1.6 × 0.4 – 0.6 μm (P4), 0.8 – 1.5 × 0.3 – 0.5 μm (NA2), and 0.9 – 1.9 × 0.4 – 0.5 μm (NA3T), with average cell sizes (n = 100) of 1.4 × 0.5 μm (P3), 1.1 × 0.4 μm (P4), 1.1 × 0.4 μm (NA2), and 1.2 × 0.4 μm (NA3T). No flagella were evident in scanning electron micrographs despite the fact that Acidovorax isolates typically exhibit one to three polar flagella [62] and the genomic sequences of these four strains indicated the presence of genes for flagellar synthesis. To examine this apparent discrepancy, each strain was inoculated into motility agar stabs and examined after several days of growth. A feathering of growth radiating from each stab indicated motility for all strains. Single, polar flagella were subsequently detected on cells extracted from those stabs (Appendix Figure A.2). The lack of flagella in electron micrographs was attributed to physical shearing during sample preparation and/or growth conditions within the preparative media.
Figure 2.
Scanning electron micrograph of the four Acidovorax strains from this work: (A) P3, (B) P4, (C) NA2, (D) NA3T. The scale bar for all panels is 0.5 μm.
The dominant fatty acids of the four strains were identified as summed feature 3 which 283 included C16:1ω7c and C16:1ω6c (47–48%), C16:0 (26–29%), and C18:1ω7c (12–16%) (Table 1). Based on the profiles of other Acidovorax strains the summed feature was likely to be predominantly C16:1ω7c. Minor fatty acids included C10:0 3-OH, C12:0, and C14:0. In addition to 3-hydroxydecanoic acid (C10:0 3-OH), cells from all strains also contained trace amounts of 3-hydroxyoctanoic acid (C8:0 3-OH), both of which are markers for the Acidovorax genus [62]. The fatty acid profiles of these strains were consistent with the genus. Major polar lipids in NA3T were phosphatidylethanolamine, diphosphatidylglycerol, phosphatidylglycerol, and two other lipids. The sole detected respiratory quinone in NA3T was Q8 (100%).
Table 1.
Characteristics of Acidovorax strains from this study.
| NA2 | NA3T | P3 | P4 | |
|---|---|---|---|---|
| Genome characteristicsa | ||||
| Total size (bp) | 4058023 | 4122625 | 4565632 | 4704737 |
| Chromosome size (bp) | 3986972 | 4122625 | 4077847 | 4077222 |
| Plasmid size(s) (bp) | 71051 | - | 258084, 156922, 72779 | 269150, 163888, 114839, 79638 |
| Mol%GC | 64.14 | 64.24 | 63.71 | 63.56 |
| No. of protein coding genes | 3803 | 3972 | 4472 | 4483 |
| No. of rRNA operons | 4 | 4 | 4 | 4 |
| No. of tRNA genes | 53 | 54 | 55 | 55 |
| No. of other RNA genes | 15 | 12 | 17 | 17 |
| No. of genes assigned to KEGG | 2108 | 2037 | 2111 | 2269 |
| No. of genes assigned to COGs | 2850 | 2763 | 2842 | 3055 |
| Biolog GN2 carbon utilizationb | ||||
| Tween 40 | ++ | ++ | ± | ++ |
| Tween 60 | ++ | ++ | ++ | ++ |
| Pyruvic acid methyl ester | ++ | ++ | ++ | ++ |
| Succinic acid mono-methyl ester | ++ | ++ | ++ | ++ |
| D-galacturonic acid | – | – | ++ | ++ |
| D-glucuronic acid | – | – | ++ | ++ |
| β-hydroxybutyric acid | ++ | ++ | ++ | ++ |
| α-ketobutryic acid | ++ | ++ | ++ | ++ |
| α-ketoglutaric acid | ++ | ++ | ++ | ++ |
| α-ketovaleric acid | ++ | ++ | ++ | ++ |
| DL-lactic acid | ++ | ++ | ++ | ++ |
| Succinamic acid | ++ | ++ | ++ | ++ |
| L-proline | ++ | – | ++ | ++ |
| 2,3-butanediol | ++ | ++ | ± | – |
| Glycerol | ++ | ++ | ++ | ++ |
| Fatty acids (%) | ||||
| C8:0 3-OH | 0.3 | 0.4 | 0.3 | 0.3 |
| C10:0 3-OH | 3.1 | 3.5 | 3.0 | 3.0 |
| C12:0 | 2.6 | 2.9 | 2.6 | 2.6 |
| C14:0 | 2.0 | 2.5 | 2.1 | 2.1 |
| iso-C15:0 | 0.2 | – | 0.1 | 0.1 |
| C15:1ω6c | – | 0.3 | – | 0.1 |
| C16:1ω7c/ω6c | 47.3 | 48.2 | 47.8 | 47.2 |
| C16:1ω6c | – | – | – | 0.1 |
| C16:0 | 27.2 | 28.8 | 26.3 | 26.8 |
| C17:1ω6c | – | 0.1 | – | – |
| iso-C17:1ω9c | 0.2 | – | 0.2 | 0.3 |
| iso-C17:0 | 0.1 | – | – | 0.2 |
| C17:0 cyclo | 0.3 | 0.2 | 0.5 | 0.7 |
| C17:0 | 0.3 | 0.6 | 0.3 | 0.3 |
| C18:1ω7c | 16.0 | 12.1 | 16.4 | 16.0 |
| C18:0 | 0.2 | – | 0.2 | 0.2 |
| C18:1ω7c 11-methyl | – | 0.2 | 0.1 | 0.1 |
| C19:0 cyclo ω8c | 0.2 | – | – | – |
Based on JGI/IMG annotations
Symbols: ++, positive results in both replicates; ±, positive in one of two replicates; –, negative in both replicates
Each of the four strains was catalase negative and oxidase positive. All strains were negative for starch and gelatin hydrolysis, as well as DNAse, urease, protease, and extracellular cellulase activity. Conversely, all four strains demonstrated lipase activity and could reduce nitrate to nitrite. All strains used the low-molecular weight polycyclic aromatic hydrocarbon (PAH) phenanthrene as a sole source of carbon and energy, but failed to grow on the PAHs acenaphthene, naphthalene, anthracene, pyrene, fluoranthene, or fluorene. No strain grew on the PAH metabolites salicylate or phthalate. Prior work with strain NA3T indicated that it could mineralize carbon from a variety of 14C-labelled aromatic substrates including naphthalene, chrysene, benz[a]anthracene, benzo[a]pyrene, and phthalate [50]. No growth was observed for any strain on benzene, toluene, ethylbenzene, o-xylene, p-xylene, or m-xylene when added to liquid medium culture or to plates incubated in the presence of those compounds in the vapor phase.
Metabolism of 95 carbon sources was tested using Biolog GN2 microplates (Table 1). All four stains metabolized Tween 40, Tween 80, pyruvic acid methyl ester, succinic acid mono- methyl ester, β-hydroxybutyric acid, α-ketobutyric acid, α-ketoglutaric acid, α-ketovaleric acid, DL-lactic acid, succinamic acid, and glycerol. Strains P3 and P4 could additionally metabolize D- galacturonic acid and D-glucuronic acid and strains NA2 and NA3T could utilize 2,3-butanediol. All strains but NA3T could metabolize L-proline. All other GN2 test substrates resulted in negative or inconsistent results.
Genomics.
The genomes of all four strains were determined and their general characteristics analyzed (Table 1). Circular chromosomes of the four strains averaged 4.07 ± 0.06 Mbp with chromosomal G+C contents of 64.1 ± 0.1%. These chromosomes were considerably smaller than the genome of the most closely related described Acidovorax species, A. radicis N35T (5.5 Mbp) [34]. Each of the four strains in this study displayed similar chromosomal arrangements with highly conserved blocks of genes presumably reflecting homology (Appendix Fig. A.3). Strains NA2 and NA3T each possessed an ~10 kbp region that was absent in P3/P4, containing a small number of hypothetical genes encoding proteins either associated with the inner membrane or of unknown function (Appendix Fig. A.3; Region “a”). In strain NA3T, this region was flanked by a putative plasmid stabilization protein-encoding gene, and in NA2 with putative transposases, suggesting the possibility of chromosomal integration of a plasmid or horizontal gene transfer. Strains P3/P4 additionally possessed a genomic region of ~50 kbp that was absent in NA2/NA3T (Fig. A.3; Region “b”). While several genes in this region were of unknown function, putative genes encoding for a type I restriction modification system, a lactate/2-hydroxyacid dehydrogenase, a phosphate/phosphite/phosphonate ABC transporter, and heavy metal sensor and response proteins were present. Two separate putative integrase genes within this block suggest a possible history of horizontal gene transfer for this region as well.
One, zero, three, and four circular plasmids were present in PFGE analyses of the strains NA2, NA3T, P3 and P4, respectively, and their sequences were determined from the genomes of the strains (Table 1; Appendix Fig. A.4). During assembly, manual inspection of the HGAP3 assemblies indicated missing plasmids from NA2 and P3 that were indicated by PFGE. The assembly for NA2 initially consisted of a single chromosome, however it was observed that two stretches matched segments of the largest P4 plasmid. Together these two pieces were roughly the same size as the missing plasmid for NA2 as determined by PFGE and thus were extracted from the NA2 chromosome and combined to form plasmid pACNA2.1. For strain P3, HGAP3 assembly indicated 2 plasmids. However, the largest P3 plasmid was considerably larger than expected based on PFGE. This large plasmid likely consisted of two separate plasmids that had incorrectly merged during the assembly process. The misassembled plasmid was split at the likely merge position, and the resulting two plasmids were consistent with PFGE results.
The largest plasmids from strains P3 and P4, designated pACP3.1 (258 kbp) and pACP4.1 (269 kbp), respectively, possessed >99% sequence similarity (100% query coverage), with only a putative transposase gene within the shared region accounting for the majority of the sequence variation. The second largest plasmids from strains P3 and P4, designated pACP3.2 (157 kbp) and pACP4.2 (164 kbp), were likewise nearly identical (99% query coverage, 99% identity) over their shared region and additionally possessed high similarity (99% identity) to sections of the Acidovorax sp. JS42 genome (Genbank accession CP000539; 27% query coverage) including a region encoding a heavy metal ATPase and a transposase in the Tn3 family. Additional plasmids from strains P3 and P4, pACP3.3 (73 kbp) and pACP4.4 (80 kbp), possessed 99% similarity to one another over the shared region (100% query coverage) and to plasmids from Acidovorax sp. JS42 (plasmid pAOVO01; 96% query coverage, 98% identity) and Alicycliphilus denitrificans K601 (plasmid pALIDE201; 92% query coverage, 99% identity). These plasmids contained putative genes encoding for (among other things) conjugal transfer proteins, a histidine kinase, as well as a DNA methyltransferase, polymerase, and primase. Plasmid pACP4.3 (115 kbp) was unique to strain P4 among these isolates and possessed only small regions of similarity to multiple elements in the Acidovorax citrulli AAC00–1 genome (Genbank accession CP000512; 2% query coverage, 73% identity). As the genomes of strains P3 and P4 were nearly identical, we presume that the fourth plasmid of P4 absent in P3 resulted in the differing PFGE-derived restriction nuclease patterns that distinguished the strains during screening of recovered isolates.
Plasmid pACNA2.1 of strain NA2 (71 kbp), despite being of a comparable size to plasmids pACP3.3 (73 kbp) and pACP4.4 (79 kbp), possessed 99.9% identity to a region spanning 26% of the larger plasmids pACP3.1 and pACP4.1. The largest region of similarity to known sequences in any of the plasmids pACP3.3, pACP4.4, or pACNA2.1 (representing 10% coverage of pACP4.1 and pACP3.1, 36% coverage of pACNA2.1) was to disparate sections of the Acidovorax avenae subsp. avenae ATCC 19860T genome (Genbank accession CP002521).
Differentiation of isolated strains from described Acidovorax species
As of the time of this work and not counting the strains described in this study, a total of 49 Acidovorax genomes representing much of the environmental and phenotypic diversity of the genus were publicly available. To distinguish strains NA2, NA3T, P3, and P4 from other species and strains in the genus, two different genome comparison methods with correlation to DNA-DNA hybridization (DDH) were performed: genome blast distance phylogeny (GBDP) and average nucleotide identity (ANI) (Table 2; Appendix Tables A.1 and A.2). The four strains described here possessed ≥ 97.0% ANI to one another, but no higher than 88.1% ANI to any other publicly available Acidovorax genome (an ANI value of >95% has been correlated with a DDH value >70% [21]). GBDP using the most conservative estimate predicted DDH values for the four strains from this study to be between 72.4 and 98.3% (with a corresponding 82–98% probability of these four strains comprising the same species); all were values above the 70% DDH threshold for establishing species similarity. Conversely, none of the four strains from this study possessed greater than 34.2% predicted DDH values to any other Acidovorax strain with a sequenced genome, including the species with the highest 16S rRNA gene similarity, A. radicis N35T (~26% DDH). Among an additional 30 Acidovorax strains with publicly available genomes but no species designation, none displayed a DDH value greater than 35.5%. These results indicate that when compared to Acidovorax species with sequenced genomes these four strains collectively comprise a new species.
Table 2.
Average nucleotide identity (ANI) between Acidovorax strains from this study and characterized Acidovorax strains with publicly available genomic data. Additional genomic comparisons to uncharacterized Acidovorax strains are available in Appendix A.
| Acidovorax sp. | strain | NA2 | NA3T | P3 | P4 |
|---|---|---|---|---|---|
| phenanthenivorans | NA2 | 100 | 96.9 | 97.1 | 97.1 |
| phenanthenivorans | NA3T | 96.9 | 100 | 97.0 | 97.1 |
| phenanthenivorans | P3 | 97.1 | 97.0 | 100 | 100 |
| phenanthenivorans | P4 | 97.1 | 97.1 | 100 | 100 |
| acenae | T10_61 | 80.6 | 80.8 | 80.7 | 80.7 |
| avenae | ATCC 19860T | 80.8 | 80.7 | 80.8 | 80.8 |
| acenae | RS-1 | 80.7 | 80.7 | 80.7 | 80.8 |
| caeni | R-24608T | 80.5 | 80.4 | 80.5 | 80.4 |
| cattleyae | DSM 17101 | 80.7 | 80.5 | 80.7 | 80.7 |
| citrulli | AAC00–1 | 80.9 | 80.9 | 80.9 | 80.9 |
| citrulli | DSM 17060 | 80.7 | 80.6 | 80.8 | 80.7 |
| citrulli | M6 | 80.8 | 80.8 | 80.9 | 80.8 |
| citrulli | pslb65 | 80.8 | 80.7 | 80.8 | 80.9 |
| citrulli | tw6 | 80.8 | 80.7 | 80.9 | 80.8 |
| citrulli | ZJU1106 | 81.7 | 81.7 | 81.8 | 81.8 |
| delafieldii | 2AN | 85.6 | 85.6 | 85.7 | 85.6 |
| delafieldii | CCH3-G3 | 83.9 | 83.9 | 84.2 | 84.2 |
| delafieldii | CCH4-A2 | n.d.a | n.d. | n.d. | n.d. |
| ebreus | TPSY | 81.1 | 81.2 | 81.0 | 81.1 |
| konjaci | DSM 7481T | 80.3 | 80.5 | 80.4 | 80.4 |
| oryzae | ATCC 19882T | 80.6 | 80.8 | 80.7 | 80.8 |
| radicis | N35T | 84.0 | 83.8 | 83.9 | 83.8 |
| radicis | N35v | 83.9 | 83.8 | 83.8 | 83.8 |
| soli | DSM 25157 | 87.4 | 87.3 | 87.6 | 87.6 |
| temperans | KY4 | 82.7 | 82.7 | 82.8 | 82.7 |
| valerianellae | DSM 16619 | 81.3 | 81.4 | 81.2 | 81.4 |
| wautersii | DSM 27981 | 81.6 | 81.7 | 81.7 | 81.8 |
Insufficient hits between genomes to estimate 2-way ANI.
Of described, non-phytopathogenic species of Acidovorax, only A. defluvii and A. facilis did not yet have a strain with a publicly available genome and thus could not be compared to these strains using ANI or GBDP methods. However, the type strains of those genera (DSM 12644T and DSM 649T, respectively) can be differentiated from strain NA3 phenotypically through differences in the utilization of various carbon sources using a standard Biolog GN2 microplate, as well as their catalase reactions (Table 3). Additionally, members of the A. defluvii species have been reported as possessing polyhydroxybutyrate granules [46], a phenotype not yet observed in these strains.
Table 3.
Differentiation of Acidovorax carolinensis from species without available genomic dataa. Strains: 1, A. carolinensis spp. NA2, NA3T, P3, and P4; 2, A. defluvii DSM 12644T; 3, A. facilis DSM 649T.
| 1 | 2 | 3 | |
|---|---|---|---|
| Biolog GN2 microplate carbon utilization | |||
| D-galactose | -b | + | + |
| D-mannitol | - | + | + |
| L-arabinose | - | + | + |
| L-serine | - | + | + |
| Monomethyl succinate | - | + | + |
| DL-carnitine | - | - | + |
| D-mannose | - | w | + |
| D-psicose | - | - | + |
| D-sorbitol | - | - | + |
| Glycyl L-aspartic acid | - | - | + |
| L-alaninamide | - | + | - |
| L-alanyl glycine | - | - | + |
| L-glutamic acid | - | + | - |
| L-ornithine | - | + | - |
| L-phenylalanine | - | - | + |
| N-acetyl-D-glucosamine | - | - | + |
| Succinamic acid | - | + | + |
| Succinate | + | - | w |
| Sucrose | - | + | - |
| Turanose | - | + | - |
| α-D-glucose | - | - | + |
| γ-aminobutyric acid | - | - | + |
| γ-hydroxybutyric acid | - | + | + |
| Other | |||
| Catalase reaction | - | + | + |
Genes putatively involved in aromatic and phenanthrene metabolism
The JGI-assembled and annotated genomes of each of the four strains were subjected to analysis using AromaDeg [15], a predictive algorithm for proteins involved in the metabolism of aromatic compounds. Between 8 and 11 putative oxygenases and dioxygenases were detected in each strain (Table 4). Based on high similarity to characterized genes and gene products in other organisms, several were predicted to encode proteins involved in PAH degradation.
Table 4.
Oxygenase and dioxygenase genes putatively involved in aromatic metabolism in A. carolinensis strains.
| IMG Gene ID; GenBank Protein Accession No. | Blastp results | ||||||
|---|---|---|---|---|---|---|---|
| Protein family (AromaDeg Cluster) | NA2 | NA3T | P3 | P4 | Top hit(s) (Accession No.) | Perc. Iden. (Simil.)a | Gene Design.a |
| Benzoate (II) | 2619698514; ART47637 | 2619702319; ART51195 | 2619707999; ART55684 | 2619710954; ART58452 | Cupriavidus necator (2Fe-2S)-binding protein (WP_051974707) | 77–78 (87) | |
| Biphenyl (XXIV) | 2619698485; ART50028 | 2619702290; ART53508 | 2619708030; ART56870 | 2619710924; ART60761 | Delftia sp. Cs1–4, aromatic ring-hydroxylating dioxygenase subunit alpha (WP_013801305); Burkholderia sp. Ch1–1 aromatic ring-hydroxylating dioxygenase subunit alpha (WP_007179244) | 98–99 (99) | phnAc |
| EXDO bicyclic substrates (XII, XIII) | 2619698494; ART47619 | 2619702299; ART51178 | 2619708021; ART55699 | 2619710933; ART58435 | Alcaligenes faecalis 3,4-dihydroxyphenanthrene dioxygenase (BAA76330) | 99–100 (100) | phnC |
| EXDO monocyclic substrates (XX) | 2619697639; ART46896 | 2619701445; ART50531 | 2619709018; ART56389 | 2619709989; ART57655 | Comamonas testosteroni catechol 2,3-dioxygenase (WP_034382469) | 97–98 (98–99) | |
| Gentisate (XIII) | 2619698491; ART50030 | 2619702296; ART53510 | 2619708024; ART56868 | 2619710930; ART60763 | Delftia sp. Cs1–4, cupin (WP_013801312); Alcaligenes faecalis 1-hydroxy-2-naphthoate dioxygenase (BAA76328) | 100 (100); 99 (99) | phnG |
| LigB protocatechuate (I) | 2619698471; n.a. | 2619702276; n.a. | 2619708046; n.a. | 2619710910; n.a. | Limnohabitans sp. 103DPR2 protocatechuate 3,4- dioxygenase (WP_055360450) | 86 (93) | pmdB |
| – | – | 2619709554; ART527279 | 2619713890; ART61320 | Acidovorax sp. CCH12-A10 protocatechuate 3,4- dioxyganse (WP_066789040) | 100 (100) | pmdBc | |
| LigB protocatechuate (XX) | 2619698512; ART47635 | 2619702317; ART51193 | 2619708001; ART55686 | 2619710952; ART58450 | Cupriavidus necator extradiol ring-cleavage dioxygenase (WP_042877061) | 80–81 (87) | |
| LigB protocatechuate (XXVI)b | – | 2619703300, 2619703301; n.a. | 2619707065; ART55085 | 2619711819; ART59161 | Noviherbaspirillum sp. Root189, 3-(2,3- dihydroxyphenyl)propionate dioxyganase (WP_057291057) | 72–74 (81–83) | |
| Phthalate (VI) | 2619698529; ART47651 | 2619702334; ART51209 | 2619707984; ART55670 | 2619710969; ART58466 | Hydrogenophaga sp. RAC07 MarR family transcriptional regulator (WP_069049060) | 85 (92) | ophA2 |
| Phthalate (VI) | – | – | 2619709578; ART57294 | 2619713912; ART61337 | Acidovorax sp. CCH12-A4 MarR family transcriptional regulator (WP_066786982) | 99 (100) | ophA2c |
Abbreviations: Perc. Iden. – amino acid identity (percentage); Simil. – amino acid similarity; Gene Design. – gene designation; n.a. – not annotated as protein using NCBI pipeline. Only gene designations for those potentially involved in phenanthrene metabolism are shown. Coverage for all Blastp comparisons between strains from this study was 98–100%.
Gene for putative dioxygenase in strain NA3T was split in two; Gene ID numbers for both genes are provided. Values for amino acid identity/similarity are for the in silico recombined whole gene.
Genes are plasmid-borne.
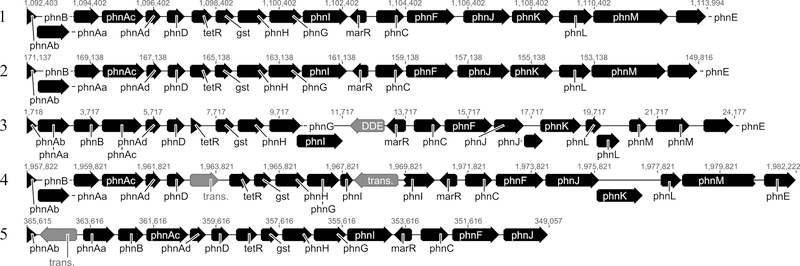
The genes likely responsible for the transformation of phenanthrene, including those encoding for the ring-hydroxylating dioxygenase (RHD) involved in the initial step in the aerobic bacterial metabolism of PAHs, were compared to genes from phenanthrene-degrading isolates from other genera within the Betaproteobacteria (Figure 3). The genes encoding enzymes for the transformation of phenanthrene to phthalate, including the four gene components of the initial RHD, were present in a single region with gene organization highly similar to homologous regions in the genomes of the phenanthrene-degrading bacteria Delftia sp. Cs1–4 and Burkholderia sp. Ch1–1, and to a GenBank sequence derived from the phenanthrene-degrading bacterium Alcaligenes faecalis strain AFK2. Only single copies of the four separate genes whose products comprise the subunits of the RHD holoenzyme (phnAabcd) were identified for each of the four genomes. For the four Acidovorax strains described in this study, the genes and organization of this cluster were nearly identical except for the putative phnK gene in strain P3, which was annotated as split into two adjacent genes (data not shown); with this caveat strain NA3T is presented as representative of all four strains from this study (Figure 3). The high similarity of the nucleotide sequence and gene organization of phenanthrene metabolic genes in NA3T to those in other phenanthrene-degrading strains was previously indicated by comparisons of partial Acidovorax sp. NA3 T gene sequences to the Alcaligenes faecalis AFK2 sequence [50] and more recently by comparisons of NA3T sequences to the complete genomes of Burkholderia sp. Ch1–1 and Delftia sp. Cs1–4 [24], and is confirmed here with this genomic sequencing effort. Interestingly, this gene cluster was also found in the recently sequenced genomes of Massilia sp. WG5 [36] and Massilia sp. WF1 [60]. While most representations of this gene cluster have been found on chromosomes, including in the Acidovorax strains presented here, the phn genes were present on a plasmid in both Massilia sp. WG5 (Genbank accession NZ_CP012641) and Alcaligenes faecalis AFK2 [29].
Figure 3.
Alignment of highly conserved genomic regions containing phn genes from (1) Acidovorax sp. NA3 [representing all strains from this work, except as noted in the text], (2) Massilia sp. WG5 [Genbank accession NZ_CP012641], also representing Massilia sp. WF1 [NZ_LELH00000000], (3) Alcaligenes faecalis AFK2 [AB024945], (4) Delftia sp. Cs1–4 695 [NC_015563], and (5) Burkholderia sp. Ch1–1 [NZ_ADNR00000000]. Previously unannotated genes for AFK2 were determined using Geneious (v8.1.7). Putative protein-encoding genes are shown in black and mobile genetic elements in grey. Numbers represent nucleotide positions in the associated GenBank or JGI entries. Gene designations were according to the naming convention established in the Alcaligenes faecalis AFK2 Genbank entry and Hickey et al. [24]. Abbreviations: tetR – TetR family transcriptional repressor; gst – glutathione S-transferase; marR – MarR family transcriptional regulator; DDE – DDE superfamily endonuclease; trans. – putative transposase. For more details on gene products and predicted pathway information refer to Hickey et al. [24].
The highly conserved phn gene cluster present in various members of the genera Acidovorax, Massilia, Delftia, Burkholderia, and Alcaligenes strains has been linked to phenanthrene metabolism through both transcription-based assays [50] and proteomic experiments [24], and has been referred to as the phn island or phnAFK2-type genes (after the Alcaligenes faecalis AFK2 Genbank entry first describing the genotype). As has been observed for other organisms possessing this cluster, the genomic region comprising the phn genes in these Acidovorax strains possessed a significantly lower G+C content than the chromosomal average of the organisms (50% for the phn cluster compared to 64% for the complete Acidovorax genomes). However, in contrast to Delftia sp. Cs1–4 in which the phn genes were contained within a large 232 kb genomic island, analyses of the Acidovorax strains presented here using the same methodology found no evidence for such a large chromosomal element encompassing the phn region. However, a much smaller potential genomic island (~10 kb) in strain NA3T comprising a region between a putative transposase upstream of the phnAb gene through the glutathione s-transferase (gst) gene in the phn operon was indicated (data not shown).
Unlike phn clusters of described Burkholderia, Delftia, and Alcaligenes strains, but similar to the Massilia gene clusters, the phn genes in these Acidovorax strains were uninterrupted by genes encoding for mobile elements (Figure 3). However, the presence of genes related to genetic mobility flanking the phn cluster in these and other organisms, in conjunction with the dissimilar G+C content compared to the rest of the genome, supports horizontal gene transfer as an explanation for the presence of extraordinarily highly-conserved degradation genes amongst these phenanthrene-degrading members of the Betaproteobacteria. Interestingly, this phn gene region from Acidovorax and other phenanthrene-degrading Betaproteobacterial strains does not appear to be present in the recently published genome of the phenanthrene-degrading Burkholderia sp. HB-1 [42] or in Burkholderia sp. RP007 [33], indicating multiple genetic determinants of phenanthrene metabolism within that genus.
The genes of the phenanthrene degradation pathway likely responsible for the transformation of o-phthalate to protocatechuate in Acidovorax strain NA3T were identified in a cluster approximately 46 kb downstream of the phn genes on the chromosome, and similar to the phn region were nearly identical in sequence and arrangement among the four Acidovorax strains (data not shown). Based on gene similarity to other organisms and predicted phenotypes of those genes, the transformation of o-phthalate likely proceeds through 4,5-dihydroxyphthalate using a two-component dioxygenase enzyme similar to that found in Burkholderia cepacia [5, 8]. The predicted phthalate 4,5-dioxygenase gene product (ophA2) was most similar in protein sequence to predicted products in the Comamonadaceae bacteria Variovorax sp. WDL1 (Genbank accession KWT97458; 75% identity) and Hydrogenophaga sp. Root209 (accession WP_056272285; 74% identity). Similar to Variovorax strain WDL1, the genes in these four Acidovorax strains encoding the putative phthalate dioxygense reductase (ophA1), phthalate 4,5-dioxygenase (ophA2), 4,5- dihydro-4,5-dihydroxyphthalate dehydrogenase (ophB), and 4,5-dihydroxyphthalate decarboxylase (ophC) were present in a single cluster with a MarR-type transcriptional regulator separating the divergently transcribed reductase/dehydrogenase and decarboxylase/dioxygenase genes. This gene arrangement is distinct from that described for Delftia sp. Cs1–4 and Comamonas testosteroni KF-1 [24]. No obvious homologs to a Burkholderia-type phthalate permease (ophD) [9] or an ABC-type phthalate transporter (ophFGH) [7] were found in the genomes any of the Acidovorax strains. The lack of a phthalate transporter may explain why none of these described Acidovorax strains were capable of growth on that compound as a sole source of carbon and energy despite presumably being able to transform it as part of phenanthrene metabolism. Strains P3 and P4 additionally contained a second, genetically distinct copy of a putative phthalate 4,5- dioxygenase (ophA2) located on plasmids pACP3.2 and pACP4.2, respectively (100% identical to one another; ~75.5% identical to the chromosomal ophA2 genes). However, no other genes predicted to be associated with phthalate metabolism were found in genetic proximity to those isolated copies, and their relevance to the transformation of phthalate or role in phenanthrene metabolism is unknown.
In these Acidovorax strains, the transformation of protocatechuate to central metabolism intermediates likely proceeds through a two-component protocatechuate 4,5-dioxygenase (genes pmdA and pmdB), similarly to that described in Delftia sp. Cs1–4 and Comamonas testosterone BR6020 [43]. In this pathway, conversion of the semialdehyde product to pyruvate and oxaloacetate occurs through the transformation products of a semialdehyde dehydrogenase (pmdC), dicarboxylic acid hydrolase (pmdD), hydratase (pmdE), and aldolase (pmdF). Putative genes encoding these enzymes were located on a single cluster in a region approximately 6 kb upstream of the phn gene cluster on the chromosome of Acidovorax strain NA3T and contained a divergently transcribed lysR-type regulator, as well as genes with similarities to an isomerase from the 2-methyl citrate pathway, a dehydrogenase with wide representation in bacterial genomes, and two hypothetical genes (data not shown). The entire pmd gene region was flanked by putative transposable elements; however, neither the oph nor pmd gene clusters were predicted to be part of a genomic island and the G+C% of the regions containing the relevant genes were 58.8% and 61.0%, respectively, significantly higher than the 50% of the phn gene region and closer to the 64% G+C of the Acidovorax chromosomes. Similar to the second copy of ophA2 on plasmids pACP3.2 and pACP4.2, strains P3 and P4 also harbored a second copy of the pmdA and pmdB genes on those plasmids with 100% identity to annotated protocatechuate 3,4-dioxygenase subunits from Acidovorax sp. CCH12-A4. In contrast to the plasmid-borne ophA2 genes in P3 and P4, which were not part of an obvious operon, these duplicate copies were also flanked by genes associated with a protocatechuate 4,5-cleavage pathway.
Based on phlyogenetic, phenotypic, and genomic characteristics, we propose that the four strains described in this manuscript constitute a new species within the Acidovorax genus. The formal proposal of the new species “Acidovorax carolinensis sp. nov.” is given in Table 5, which represents information taken from Digital Protologue Taxonumber TA00473.
Table 5.
Description of Acidovorax carolinensis sp. nov. according to Digital Protologue TA00473 assigned by the the www.imedea.uib.es/dprotologue website.
| TAXONUMBER | TA00473 |
| SPECIES NAME | Acidovorax carolinensis |
| GENUS NAME | Acidovorax |
| SPECIFIC EPITHET | carolinensis |
| SPECIES STATUS | sp. nov. |
| SPECIES ETYMOLOGY | ca.ro.li.nen’sis. N.L. masc. adj. carolinensis of or belonging to Carolina, refering to North Carolina, USA, where each of the strains were first isolated |
| SUBMITTER | David Singleton |
| E-MAIL OF THE SUBMITTER | drsingle@email.unc.edu |
| HAS THE TAXON BEEN SUBJECTED TO EMENDATION? | NO |
| DESIGNATION OF THE TYPE STRAIN | NA3 |
| STRAIN COLLECTION NUMBERS | =LMG 30136, =DSM 105008 |
| 16S rRNA GENE ACCESSION NUMBER | EU910093.1 |
| GENOME ACCESSION NUMBER [RefSeq] | NZ_CP021361.1 |
| GENOME STATUS | Complete |
| GENOME SIZE | 4122 kbp |
| GC mol % | 64.24 |
| COUNTRY OF ORIGIN | USA |
| REGION OF ORIGIN | Charlotte, North Carolina |
| DATE OF ISOLATION UNKNOWN (< yyyy) | 2005 |
| SOURCE OF ISOLATION | Contaminated soil |
| SAMPLING DATE | 2002–01-01 |
| GEOGRAPHIC LOCATION | Charlotte, NC former manufactured gas plant site |
| TEMPERATURE OF THE SAMPLE [In Celsius degrees] | 20 |
| pH OF THE SAMPLE | 7.5 |
| NUMBER OF STRAINS IN STUDY | 4 |
| SOURCE OF ISOLATION OF NON- TYPE STRAINS | Contaminated soil |
| GROWTH MEDIUM, INCUBATION CONDITIONS [Temperature, pH, and further information] USED FOR STANDARD CULTIVATION | Nutrient agar/R2A, 30–32.5°C, pH 7–7.5 |
| CONDITIONS OF PRESERVATION | 15% glycerol; −80°C |
| GRAM STAIN | Negative |
| CELL SHAPE | Rod |
| CELL SIZE (length or diameter) | 1.2 |
| MOTILITY | Motile |
| IF MOTILE | Flagellar |
| IF FLAGELLATED | Single, polar |
| SPORULATION (resting cells) | None |
| COLONY MORPHOLOGY | Cream color to pale yellow, circular, 1.0–1.5mm diameter. |
| TEMPERATURE RANGE | 4–40 |
| LOWEST TEMPERATURE FOR GROWTH | 4, nutrient agar plates |
| HIGHEST TEMPERATURE FOR GROWTH | 40, nutrient agar plates |
| TEMPERATURE OPTIMUM | 30–32.5 |
| LOWEST pH FOR GROWTH | 5.5, nutrient broth |
| HIGHEST pH FOR GROWTH | 8.0, nutrient broth |
| pH OPTIMUM | 7.0–7.5 |
| pH CATEGORY | Neutrophile |
| LOWEST NaCl CONCENTRATION FOR GROWTH | 0, nutrient broth |
| HIGHEST NaCI CONCENTRATION FOR GROWTH | 1%, nutrient broth |
| SALINITY CATEGORY | Nonhalophile (NaCl inhibitory at <1 % NaCl) |
| RELATIONSHIP TO O2 | Aerobe |
| O2 CONDITIONS FOR STRAIN TESTING | Aerobiosis; anaerobiosis |
| CARBON SOURCE USED [class of compounds] | Organic acids, aromatic compounds |
| CARBON SOURCE USED [specific compounds] | Phenanthrene |
| CARBON SOURCE NOT USED [specific compounds] | Acenaphthene, anthracene, benzene, ethylbenzene, fluoranthene, fluorene, naphthalene, phthalate, pyrene, salicylate, toluene, xylene |
| Positive tests with BIOLOG | Tween 40, Tween 80, Methyl pyruvate, succinic acid mono-methyl ester, β-hydroxybutyric acid, α-ketobutyric acid, α-ketoglutaric acid, α-ketovaleric acid, DL-lactic acid, succinamic acid, glycerol |
| Negative tests with BIOLOG | α-Cyclodextrin, Dextrin, Glycogen, N-Acetyl-D-galactosamine, N-Acetyl-D-glucosamine, Adonitol, L-Arabinose, D-Arabitol, D-Cellobiose, i-Erythritol, D-Fructose, L-Fucose, D- Galactose, Gentiobiose, D-Glucose, m-Inositol, α-D-Lactose, Lactulose, Maltose, D-Mannitol, D-Mannose, D-Melibiose, β-Methyl-D-Glucoside, D-Psicose, D-Raffinose, L-Rhamnose, D- Sorbitol, Sucrose, D-Trehalose, Turanose, Xylitol, Acetic Acid, Cis-Aconitic Acid, Citric Acid, Formic Acid, D-Galactonic Acid Lactone, D-Glucosaminic Acid, D-Glucuronic Acid, α- Hydroxy-Butyric Acid, γ-Hydroxy Butyric Acid, p-Hydroxy Phenylacetic Acid, Itaconic Acid, α-Keto Valeric Acid, Malonic Acid, Propionic Acid, Quinic Acid, D-Saccharic Acid, Sebacic Acid, Bromo-Succinic Acid, Glucuronamide, L-Alaninamide, D-Alanine, L-Alanine, L- Alanylglycine, L-Asparagine, L-Aspartic Acid, L-Glutamic Acid, Glycyl-L-Aspartic Acid, Glycyl-L-Glutamic Acid, L-Histidine, Hydroxy-L-Proline, L-Leucine, L-Ornithine, L- Phenylalanine, L-Pyroglutamic Acid, D-Serine, L-Serine, L-Threonine, DL-Carnitine, γ-Amino Butyric Acid, Urocanic Acid, Inosine, Uridine, Thymidine, Phenyethylamine, Putrescine, 2- Aminoethanol, D,L-α-Glycerol Phosphate, Gluose-1-Phosphate, Glucose-6-Phosphate |
| Variable tests with BIOLOG | D-galacturonic acid, D-glucuronic acid, 2,3-butanediol, L-proline |
| NITROGEN SOURCE | NH4 |
| TERMINAL ELECTRON ACCEPTOR | Oxygen |
| ENERGY METABOLISM | Chemoorganotroph |
| OXIDASE | Positive |
| CATALASE | Negative |
| POSITIVE TESTS | Lipase, nitrate reduction |
| NEGATIVE TESTS | Gelatin hydrolysis, cellulase, DNase, skim milk protease, starch hydrolysis, urease |
| QUINONE TYPE | R8 |
| MAJOR FATTY ACIDS | C16:1ω7c/C16:1ω6c, C16:0, C18:1ω7c |
| PHOSPOLIPID PATTERN OR DIAGNOSTIC PHOSPOLIPID | Phosphatidylethanolamine, diphosphatidylglycerol, phosphatidylglycerol |
| BIOSAFETY LEVEL | 1 |
| HABITAT | Terrestrial biome |
| BIOTIC RELATIONSHIP | Free-living |
| KNOWN PATHOGENICITY | None |
DIGITAL PROTOLOGUE
TAXONUMBER TA00473
SPECIES NAME Acidovorax phenanthrenivorans
GENUS NAME Acidovorax
SPECIFIC EPITHET phenanthrenivorans
SPECIES STATUS sp. nov.
SPECIES ETYMOLOGY Acidovorax phenanthrenivorans (phen.an′thre.ni.vo′rans. N.L. n. phenanthrenum phenanthrene; L. v. vorare to devour; L. part. adj. vorans devouring, digesting; N.L. part. adj. phenanthrenivorans digesting phenanthrene).
SUBMITTER
E-MAIL OF THE SUBMITTER david.r.singleton@duke.edu
HAS THE TAXON BEEN SUBJECTED TO EMENDATION? NO
DESIGNATION OF THE TYPE STRAIN NA3
STRAIN COLLECTION NUMBERS =LMG 30136, =DSM 105008
16S rRNA GENE ACCESSION NUMBER EU910093.1
GENOME ACCESSION NUMBER [RefSeq] NZ_CP021361.1
GENOME STATUS complete
GENOME SIZE 4122 kbp
GC mol % 64.24
COUNTRY OF ORIGIN USA
REGION OF ORIGIN Charlotte, North Carolina
DATE OF ISOLATION UNKNOWN (< yyyy) 2005
SOURCE OF ISOLATION Contaminated soil
SAMPLING DATE 2002–01-01
GEOGRAPHIC LOCATION Charlotte, NC former manufactured gas plant site
TEMPERATURE OF THE SAMPLE [In Celsius degrees] 20
pH OF THE SAMPLE 7.5
NUMBER OF STRAINS IN STUDY 4
SOURCE OF ISOLATION OF NON-TYPE STRAINS Contaminated soil
GROWTH MEDIUM, INCUBATION Nutrient agar/R2A
CONDITIONS [Temperature, pH, and further 30–32.5 degrees celcius
information] USED FOR STANDARD CULTIVATION pH 7–7.5
CONDITIONS OF PRESERVATION 15% glycerol; -80C
GRAM STAIN NEGATIVE
CELL SHAPE rod
CELL SIZE (length or diameter) 1.2
MOTILITY motile
IF MOTILE flagellar
IF FLAGELLATED single, polar
SPORULATION (resting cells) none
COLONY MORPHOLOGY Cream color to pale yellow,circular, 1.0–1.5mm diameter.
TEMPERATURE RANGE 4–40
LOWEST TEMPERATURE FOR GROWTH 4, nutrient agar plates HIGHEST TEMPERATURE FOR GROWTH 40, nutrient agar plates TEMPERATURE OPTIMUM 30–32.5
LOWEST pH FOR GROWTH 5.5, nutrient broth
HIGHEST pH FOR GROWTH 8.0, nutrient broth
pH OPTIMUM 7.0–7.5
pH CATEGORY neutrophile
LOWEST NaCl CONCENTRATION FOR GROWTH 0, nutrient broth
HIGHEST NaCI CONCENTRATION FOR GROWTH 1%, nutrient broth
SALINITY CATEGORY nonhalophile (NaCl inhibitory at <1 % NaCl)
RELATIONSHIP TO O2 aerobe
O2 CONDITIONS FOR STRAIN TESTING aerobiosis; anaerobiosis
CARBON SOURCE USED [class of compounds] organic acids, aromatic compounds
CARBON SOURCE USED [specific compounds] phenanthrene
CARBON SOURCE NOT USED [specific compounds] acenaphthene, anthracene, benzene, ethylbenzene, fluoranthene, fluorene, naphthalene, phthalate, pyrene, salicylate, toluene, xylene
Positive tests with BIOLOG Tween 40, Tween 80, Methyl pyruvate, succinic acid mono-methyl ester, β- hydroxybutyric acid, α-ketobutyric acid, α-ketoglutaric acid, α-ketovaleric acid, DL-lactic acid, succinamic acid, glycerol
Negative tests with BIOLOG α-Cyclodextrin, Dextrin, Glycogen, N-Acetyl-D-galactosamine, N-Acetyl-D- glucosamine, Adonitol, L-Arabinose, D-Arabitol, D-Cellobiose, i-Erythritol, D- Fructose, L-Fucose, D-Galactose, Gentiobiose, D-Glucose, m-Inositol, α-D- Lactose, Lactulose, Maltose, D-Mannitol, D-Mannose, D-Melibiose, β-MethylD- Glucoside, D-Psicose, D-Raffinose, L-Rhamnose, D-Sorbitol, Sucrose, D-Trehalose, Turanose, Xylitol, Acetic Acid, Cis-Aconitic Acid, Citric Acid, Formic Acid, D- Galactonic Acid Lactone, D-Glucosaminic Acid, D-Glucuronic Acid, α-Hydroxy- Butyric Acid, γ-Hydroxy Butyric Acid, p-Hydroxy Phenylacetic Acid, Itaconic Acid, α-Keto Valeric Acid, Malonic Acid, Propionic Acid, Quinic Acid, D-Saccharic Acid, Sebacic Acid, Bromo-Succinic Acid, Glucuronamide, L-Alaninamide, D-Alanine, L-Alanine, L- Alanylglycine, L-Asparagine, L-Aspartic Acid, L-Glutamic Acid, Glycyl-L-Aspartic Acid, Glycyl-L-Glutamic Acid, L-Histidine, Hydroxy-L-Proline, L-Leucine, L-Ornithine, L-Phenylalanine, L-Pyroglutamic Acid, D-Serine, L-Serine, L-Threonine, D,L-Carnitine, γ-Amino Butyric Acid, Urocanic Acid, Inosine, Uridine, Thymidine, Phenyethylamine, Putrescine, 2-Aminoethanol, D,L-α-Glycerol Phosphate, Gluose-1-Phosphate, Glucose-6-Phosphate
Variable tests with BIOLOG D-galacturonic acid, D-glucuronic acid, 2,3-butanediol, L-proline
NITROGEN SOURCE NH4
TERMINAL ELECTRON ACCEPTOR oxygen
ENERGY METABOLISM chemoorganotroph
OXIDASE positive
CATALASE negative
POSITIVE TESTS lipase, nitrate reduction
NEGATIVE TESTS gelatin hydrolysis, cellulase, DNase, skim milk protease, starch hydrolysis, urease
QUINONE TYPE R8
MAJOR FATTY ACIDS C16:1ω7c/C16:1ω6c, C16:0, C18:1ω7c
PHOSPOLIPID PATTERN OR DIAGNOSTIC PHOSPOLIPID phosphatidylethanolamine, diphosphatidylglycerol, phosphatidylglycerol
BIOSAFETY LEVEL 1
HABITAT terrestrial biome
BIOTIC RELATIONSHIP free-living
KNOWN PATHOGENICITY none
Supplementary Material
Acknowledgements
We wish to acknowledge the efforts of Victoria Madden of the Microscopy Services Laboratory at the University of North Carolina for assistance provided in electron microscopy.
Funding – This work was supported by the U.S. National Institute of Environmental Health Sciences (NIEHS) Superfund Research Program [5 P42ES005948].
References
- [1].Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ, Basic local alignment search tool, J. Mol. Biol, 215 (1990) 403–410. [DOI] [PubMed] [Google Scholar]
- [2].Atlas RM, Handbook of Microbiological Media, Third edition ed., CRC Press, Boca Raton FL, 2004. [Google Scholar]
- [3].Basta T, Keck A, Klein J, Stolz A, Detection and characterization of conjugative degradative plasmids in xenobiotic-degrading Sphingomonas strains, J. Bacteriol, 186 (2004) 3862–3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bastian M, Heymann S, Jacomy M, Gephi: An Open Source Software for Exploring and Manipulating Networks, 2009. [Google Scholar]
- [5].Batie C, LaHaie E, Ballou D, Purification and characterization of phthalate oxygenase and phthalate oxygenase reductase from Pseudomonas cepacia, J. Biol. Chem, 262 (1987) 1510–1518. [PubMed] [Google Scholar]
- [6].Bogan BW, Lahner LM, Sullivan WR, Paterek JR, Degradation of straight-chain aliphatic and high-molecular-weight polycyclic aromatic hydrocarbons by a strain of Mycobacterium austroafricanum, J. Appl. Microbiol, 94 (2003) 230–239. [DOI] [PubMed] [Google Scholar]
- [7].Chang HK, Dennis JJ, Zylstra GJ, Involvement of two transport systems and a specific porin in the uptake of phthalate by Burkholderia spp, J. Bacteriol, 191 (2009) 4671–4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chang HK, Zylstra GJ, Novel organization of the genes for phthalate degradation from Burkholderia cepacia DBO1, J. Bacteriol, 180 (1998) 6529–6537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chang HK, Zylstra GJ, Characterization of the phthalate permease OphD from Burkholderia cepacia ATCC 17616, J. Bacteriol, 181 (1999) 6197–6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chin C-S, Alexander DH, Marks P, Klammer AA, Drake J, Heiner C, Clum A, Copeland A, Huddleston J, Eichler EE, Turner SW, Korlach J, Nonhybrid, finished microbial genome assemblies from long-read SMRT sequencing data, Nat. Meth, 10 (2013) 563–569. [DOI] [PubMed] [Google Scholar]
- [11].Choi J-H, Kim M-S, Roh SW, Bae J-W, Acidovorax soli sp. nov., isolated from landfill soil, Int. J. Syst. Evol. Microbiol, 60 (2010) 2715–2718. [DOI] [PubMed] [Google Scholar]
- [12].Christensen WB, Urea decomposition as a means of differentiating Proteus and Paracolon cultures from each other and from Salmonella and Shigella types, J. Bacteriol, 52 (1946) 461–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Corteselli EM, Aitken MD, Singleton DR, Rugosibacter aromaticivorans gen. nov., sp. nov., a novel bacterium within the family Rhodocyclaceae isolated from contaminated soil, capable of degrading aromatic compounds, Int. J. Syst. Evol. Microbiol, 67 (2016) 311–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Cummings MP, Hancock JM, Zvelebil MJ, PAUP* (phylogenetic analysis using parsimony (and other methods)), in: Dictionary of Bioinformatics and Computational Biology, John Wiley & Sons, Ltd, 2004. [Google Scholar]
- [15].dela Cruz TE, Torres JMO, Gelatin hydrolysis test protocol, in, American Society for Microbiology, Washington, DC, 2012. [Google Scholar]
- [16].Duarte M, Jauregui R, Vilchez-Vargas R, Junca H, Pieper DH, AromaDeg, a novel database for phylogenomics of aerobic bacterial degradation of aromatics, Database (Oxford), 2014 (2014) bau118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Eaton RW, Chapman PJ, Formation of indigo and related compounds from indolecarboxylic acids by aromatic acid-degrading bacteria: chromogenic reactions for cloning genes encoding dioxygenases that act on aromatic acids, J. Bacteriol, 177 (1995) 6983–6988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Eriksson M, Sodersten E, Yu Z, Dalhammar G, Mohn WW, Degradation of polycyclic aromatic hydrocarbons at low temperature under aerobic and nitrate-reducing conditions in enrichment cultures from northern soils, Appl. Environ. Microbiol, 69 (2003) 275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gardan L, Dauga C, Prior P, Gillis M, Saddler GS, Acidovorax anthurii sp. nov., a new phytopathogenic bacterium which causes bacterial leaf-spot of anthurium, Int. J. Syst. Evol Microbiol, 50 (2000) 235–246. [DOI] [PubMed] [Google Scholar]
- [20].Gardan L, Stead DE, Dauga C, Gillis M, Acidovorax valerianellae sp. nov., a novel pathogen of lamb’s lettuce [Valerianella locusta (L.) Laterr.], Int. J. Syst. Evol. Microbiol, 53 (2003) 795–800. [DOI] [PubMed] [Google Scholar]
- [21].Goris J, Konstantinidis KT, Klappenbach JA, Coenye T, Vandamme P, Tiedje JM, DNA-DNA hybridization values and their relationship to whole-genome sequence similarities, Int. J. Syst. Evol. Microbiol, 57 (2007) 81–91. [DOI] [PubMed] [Google Scholar]
- [22].Haigler BE, Wallace WH, Spain JC, Biodegradation of 2-nitrotoluene by Pseudomonas sp. strain JS42, Appl. Environ. Microbiol, 60 (1994) 3466–3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Heylen K, Lebbe L, De Vos P, Acidovorax caeni sp. nov., a denitrifying species with genetically diverse isolates from activated sludge, Int. J. Syst. Evol. Microbiol, 58 (2008) 73–77. [DOI] [PubMed] [Google Scholar]
- [24].Hickey WJ, Chen S, Zhao J, The phn island: a new genomic island encoding catabolism of polynuclear aromatic hydrocarbons, Front. Microbiol, 3 (2012) 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Jones MD, Crandell DW, Singleton DR, Aitken MD, Stable-isotope probing of the polycyclic aromatic hydrocarbon-degrading bacterial guild in a contaminated soil, Environ. Microbiol, 13 (2011) 2623–2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Jurelevicius D, Alvarez VM, Peixoto R, Rosado AS, Seldin L, Bacterial polycyclic aromatic hydrocarbon ring-hydroxylating dioxygenases (PAH-RHD) encoding genes in different soils from King George Bay, Antarctic Peninsula, Appl. Soil Ecol, 55 (2012) 1–9. [Google Scholar]
- [27].Kimbara K, Hashimoto T, Fukuda M, Koana T, Takagi M, Oishi M, Yano K, Isolation and characterization of a mixed culture that degrades polychlorinated biphenyls Agric. Biol. Chem, 52 (1988) 2885–2891. [Google Scholar]
- [28].Kiyohara H, Nagao K, Yana K, Rapid screen for bacteria degrading water-insoluble, solid hydrocarbons on agar plates, Appl. Environ. Microbiol, 43 (1982) 454–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kiyohara H, Takizawa N, Date H, Torigoe S, Yano K, Characterization of a phenanthrene degradation plasmid from Alcaligenes faecalis AFK2, J. Ferment. Bioeng, 69 (1990) 54–56. [Google Scholar]
- [30].Kovacs N, Identification of Pseudomonas pyocyanea by the oxidase reaction, Nature, 178 (1956) 703–703. [DOI] [PubMed] [Google Scholar]
- [31].Lagesen K, Hallin P, Rødland EA, Stærfeldt H-H, Rognes T, Ussery DW, RNAmmer: consistent and rapid annotation of ribosomal RNA genes, Nucleic Acids Res, 35 (2007) 3100– 3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lal A, Cheeptham N, Starch agar protocol, in: Protocols, American Society for Microbiology, Washington, DC, 2012. [Google Scholar]
- [33].Laurie AD, Lloyd-Jones G, The phn genes of Burkholderia sp. strain RP007 constitute a divergent gene cluster for polycyclic aromatic hydrocarbon catabolism, J. Bacteriol, 181 (1999) 585 531–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Li D, Rothballer M, Engel M, Hoser J, Schmidt T, Kuttler C, Schmid M, Schloter M, Hartmann A, Phenotypic variation in Acidovorax radicis N35 influences plant growth promotion, FEMS Microbiol. Ecol, 79 (2012) 751–762. [DOI] [PubMed] [Google Scholar]
- [35].Li D, Rothballer M, Schmid M, Esperschütz J, Hartmann A, Acidovorax radicis sp. nov., a wheat-root-colonizing bacterium, Int. J. Syst. Evol. Microbiol, 61 (2011) 2589–2594. [DOI] [PubMed] [Google Scholar]
- [36].Lou J, Gu H, Wang H, An Q, Xu J, Complete genome sequence of Massilia sp. WG5, an efficient phenanthrene-degrading bacterium from soil, J. Biotechnol, 218 (2016) 49–50. [DOI] [PubMed] [Google Scholar]
- [37].Markowitz VM, Chen I-MA, Palaniappan K, Chu K, Szeto E, Pillay M, Ratner A, Huang J, Woyke T, Huntemann M, Anderson I, Billis K, Varghese N, Mavromatis K, Pati A, Ivanova NN, Kyrpides NC, IMG 4 version of the integrated microbial genomes comparative analysis system, Nucleic Acids Res, 42 (2014) D560–D567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Martin F, Torelli S, Le Paslier D, Barbance A, Martin-Laurent F, Bru D, Geremia R, Blake G, Jouanneau Y, Betaproteobacteria dominance and diversity shifts in the bacterial community of a PAH-contaminated soil exposed to phenanthrene, Environ. Pollut, 162 (2012) 600 345–353. [DOI] [PubMed] [Google Scholar]
- [39].Meier-Kolthoff JP, Auch AF, Klenk HP, Goker M, Genome sequence-based species delimitation with confidence intervals and improved distance functions, BMC Bioinformatics, 14 (2013) 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Meyer S, Moser R, Neef A, Stahl U, Kämpfer P, Differential detection of key enzymes of polyaromatic-hydrocarbon-degrading bacteria using PCR and gene probes, Microbiology, 145 ( Pt 606 7) (1999) 1731–1741. [DOI] [PubMed] [Google Scholar]
- [41].Mizzouri NS, Shaaban MG, Kinetic and hydrodynamic assessment of an aerobic purification system for petroleum refinery wastewater treatment in a continuous regime, Int. Biodeterior. Biodegrad, 83 (2013) 1–9. [Google Scholar]
- [42].Ohtsubo Y, Moriya A, Kato H, Ogawa N, Nagata Y, Tsuda M, Complete genome sequence of a phenanthrene degrader, Burkholderia sp. HB-1 (NBRC 110738), Genome Announcements, 3 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Providenti MA, Mampel J, MacSween S, Cook AM, Wyndham RC, Comamonas testosteroni BR6020 possesses a single genetic locus for extradiol cleavage of protocatechuate, Microbiology, 147 (2001) 2157–2167. [DOI] [PubMed] [Google Scholar]
- [44].Samanta SK, Chakraborti AK, Jain RK, Degradation of phenanthrene by different bacteria: evidence for novel transformation sequences involving the formation of 1-naphthol, Appl. Microbiol. Biotechnol, 53 (1999) 98–107. [DOI] [PubMed] [Google Scholar]
- [45].Schatz A, Bovell C, Growth and hydrogenase activity of a new bacterium, Hydrogenomonas facilis, J. Bacteriol, 63 (1952) 87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Schulze R, Spring S, Amann R, Huber I, Ludwig W, Schleifer K-H, Kämpfer P, Genotypic diversity of Acidovorax strains isolated from activated sludge and description of Acidovorax defluvii sp. nov, Syst. Appl. Microbiol, 22 (1999) 205–214. [DOI] [PubMed] [Google Scholar]
- [47].Shuttleworth KL, Cerniglia CE, Bacterial degradation of low concentrations of phenanthrene and inhibition by naphthalene, Microb. Ecol, 31 (1996) 305–317. [PubMed] [Google Scholar]
- [48].Singleton DR, Hunt M, Powell SN, Frontera-Suau R, Aitken MD, Stable-isotope probing with multiple growth substrates to determine substrate specificity of uncultivated bacteria, J. Microbiol. Methods, 69 (2007) 180–187. [DOI] [PubMed] [Google Scholar]
- [49].Singleton DR, Powell SN, Sangaiah R, Gold A, Ball LM, Aitken MD, Stable-isotope probing of bacteria capable of degrading salicylate, naphthalene, or phenanthrene in a bioreactor treating contaminated soil, Appl. Environ. Microbiol, 71 (2005) 1202–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Singleton DR, Ramirez LG, Aitken MD, Characterization of a polycyclic aromatic hydrocarbon degradation gene cluster in a phenanthrene-degrading Acidovorax strain, Appl. Environ. Microbiol, 75 (2009) 2613–2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Singleton DR, Richardson SD, Aitken MD, Pyrosequence analysis of bacterial communities in aerobic bioreactors treating polycyclic aromatic hydrocarbon-contamined soil Biodegradation, 22 (2011) 1061–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Sommer DD, Delcher AL, Salzberg SL, Pop M, Minimus: a fast, lightweight genome assembler, BMC Bioinformatics, 8 (2007) 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Stanier RY, Palleroni NJ, Doudoroff M, The aerobic Pseudomonads: a taxonomic study, Journal of General Microbiology, 43 (1966) 159–271. [DOI] [PubMed] [Google Scholar]
- [54].Thompson JD, Gibson TJ, Higgins DG, Multiple sequence alignment using ClustalW and ClustalX, Curr. Protoc. Bioinformatics, Chapter 2 (2002) Unit 2 3. [DOI] [PubMed] [Google Scholar]
- [55].Tindall BJ, A comparative study of the lipid composition of Halobacterium saccharovorum from various sources, Syst. Appl. Microbiol, 13 (1990) 128–130. [Google Scholar]
- [56].Tindall BJ, Lipid composition of Halobacterium lacusprofundi, FEMS Microbiol. Lett, 66 (1990) 199–202. [Google Scholar]
- [57].Tindall BJ, Sikorski J, Smibert RM, Kreig NR, Phenotypic characterization and the principles of comparative systematics., in: Reddy CA, Beveridge TJ, Breznak JA, Marzluf G, Schmidt TM, Snyder LR (Eds.) Methods for General and Molecular Microbiology, ASM Press, Washington DC, USA, 2007, pp. 330–393. [Google Scholar]
- [58].Tischer K, Kleinsteuber S, Schleinitz KM, Fetzer I, Spott O, Stange F, Lohse U, Franz J, Neumann F, Gerling S, Schmidt C, Hasselwander E, Harms H, Wendeberg A, Microbial communities along biogeochemical gradients in a hydrocarbon-contaminated aquifer, Environ. Microbiol, 15 (2013) 2603–2615. [DOI] [PubMed] [Google Scholar]
- [59].Vaneechoutte M, Janssens M, Avesani V, Delmée M, Deschaght P, Description of Acidovorax wautersii sp. nov. to accommodate clinical isolates and an environmental isolate, most closely related to Acidovorax avenae, Int. J. Syst. Evol. Microbiol, 63 (2013) 2203–2206. [DOI] [PubMed] [Google Scholar]
- [60].Wang H, Lou J, Gu H, Luo X, Yang L, Wu L, Liu Y, Wu J, Xu J, Efficient biodegradation of phenanthrene by a novel strain Massilia sp. WF1 isolated from a PAH-contaminated soil, Environ. Sci. Pollut. Res. Int, (2016). [DOI] [PubMed] [Google Scholar]
- [61].Willems A, Falsen E, Pot B, Jantzen E, Hoste B, Vandamme P, Gillis M, Kersters K, De Ley J, Acidovorax, a new genus for Pseudomonas facilis, Pseudomonas delafieldii, E. Falsen (EF) group 13, EF group 16, and several clinical isolates, with the species Acidovorax facilis comb. nov., Acidovorax delafieldii comb. nov., and Acidovorax temperans sp. nov, Int. J. Syst. Evol. Microbiol, 40 (1990) 384–398. [DOI] [PubMed] [Google Scholar]
- [62].Willems A, Gillis M, Genus II Acidovorax Willems, Falsen, Pot, Jantzen, Hoste, Vandamme, Gillis, Kersters and De Ley 1990, 394VP, in: Brenner DJ, Krieg NR, Staley JT, Garrity GM (Eds.) Bergey’s Manual of Systematic Bacteriology, Springer, New York, 2005, pp. 696–703. [Google Scholar]
- [63].Willems A, Goor M, Thielemans S, Gillis M, Kersters K, De Ley J, Transfer of several phytopathogenic Pseudomonas species to Acidovorax as Acidovorax avenae subsp. avenae subsp. nov., comb. nov., Acidovorax avenae subsp. citrulli, Acidovorax avenae subsp. cattleyae, and 674Acidovorax konjaci, Int. J. Syst. Bacteriol, 42 (1992) 107–119. [DOI] [PubMed] [Google Scholar]
- [64].Zhang Z, Schwartz S, Wagner L, Miller W, A greedy algorithm for aligning DNA sequences, J. Comput. Biol, 7 (2000) 203–214. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.