Abstract
Neuroblastoma is a common childhood cancer typically treated by inducing differentiation with retinoic acid (RA). Peroxisome proliferator-activated receptor-β/δ, (PPARβ/δ) is known to promote terminal differentiation of many cell types. In the present study, PPARβ/δ was overexpressed in three human neuroblastoma cell lines, NGP, SK-N-BE(2), and IMR-32, that exhibit high, medium, and low sensitivity, respectively, to retinoic acid-induced differentiation to determine if PPARβ/δ and retinoic acid receptors (RARs) could be jointly targeted to increase the efficacy of treatment. All-trans-RA (atRA) decreased expression of SRY (sex determining region Y)-box 2 (SOX2), a stem cell regulator and marker of de-differentiation, in NGP and SK-N-BE(2) cells with inactive or mutant tumor suppressor p53, respectively. However, atRA did not suppress SOX2 expression in IMR-32 cells carrying wild-type p53. Over-expression and/or ligand activation of PPARβ/δ reduced the average volume and weight of ectopic tumor xenografts from NGP, SK-N-BE(2), or IMR-32 cells compared to controls. Compared with that found with atRA, PPARβ/δ suppressed SOX2 expression in NGP and SK-N-BE(2) cells and ectopic xenografts, and was also effective in suppressing SOX2 expression in IMR-32 cells that exhibit higher p53 expression compared to the former cell lines. Combined, these observations demonstrate that activating or over-expressing PPARβ/δ induces cell differentiation through p53- and SOX2-dependent signaling pathways in neuroblastoma cells and tumors. This suggests that combinatorial activation of both RARα and PPARβ/δ may be suitable as an alternative therapeutic approach for RA-resistant neuroblastoma patients.
Keywords: neuroblastoma, p53, peroxisome proliferator-activated receptor-β/δ, retinoid acid, SOX2
1 |. INTRODUCTION
Neuroblastoma accounts for 8–10% of all childhood cancers with an annual incidence of ~1 in 10,000 children less than 15 years of age in the United States.1,2 Over 60% of neuroblastoma tumors are highly metastatic and exhibit a high rate of relapse.3 This type of neuroblastoma exhibits poor prognosis with cytogenetic features, including chromosomal instability and amplification of the N-MYC gene.1,3 The differentiation stage of neuroblastoma is the most common measure of tumor aggressiveness.4 Thus, the current standard of care typically utilizes differentiation-inducing agents, such as retinoids, immunotherapy, or small molecule inhibitors of the cell cycle.5 The sensitivity to therapeutic interventions is dependent on several key factors including amplification of N-MYC and TP53 status.5 Interestingly, among resistant neuroblastomas, differences in the sensitivity to atRA can also be significant, and the mechanism underlying this variability is unknown. Thus, there remains a need to elucidate the mechanisms that mediate the differences observed in the inhibition of neuroblastoma tumorigenicity by selective agents in order to develop new therapeutic approaches for this disease.
Peroxisome proliferator-activated receptor-β/δ (PPARβ/δ) dynamically heterodimerizes with retinoid X receptor (RXR) by ligand activation, and regulates expression of target genes that modulate cell proliferation, differentiation, inflammation, and lipid and glucose homeostasis.6 The role of PPARβ/δ in carcinogenesis remains controversial as some studies suggest that activating PPARβ/δ promotes tumorigenesis, while others suggest the opposite.6,7 Recently, several studies have revealed that over-expression of PPARβ/δ inhibits tumorigenesis in different types of human cancer types including breast, colon, and testis.8–10 These studies are similar to results showing that knockdown of PPARβ/δ promotes tumorigenesis in human colon cancer models, an effect that is mediated in part by inhibition of induction of terminal differentiation.11–13 This is of interest to note because it is firmly established that PPARβ/δ promotes terminal differentiation in multiple cell types.14,15
Induction of terminal differentiation is one approach used for cancer therapy,16,17 including neuroblastoma patients.18 This is particularly true for high-risk neuroblastoma patients and can be accomplished by treatment with retinoic acid to induce terminal differentiation of neuroblastoma cells.19–24 It was recently hypothesized that atRA may promote tumorigenesis by alternatively activating PPARβ/δ, rather than retinoic acid receptors, based on the ratio of cellular retinoic acid binding protein-II and fatty acid binding protein-5.25 However, this hypothetical mechanism is not consistently observed26–28 and should be carefully re-evaluated.6,7,29 Since previous studies revealed that PPARβ/δ-induced differentiation inhibits tumorigenesis,6,7,14 the hypothesis that over-expressing and/or activating PPARβ/δ modulates tumorigenesis of human N-MYC-amplified neuroblastoma cells was examined in this study. Further, the effect of relative expression of PPARβ/δ on atRA-induced differentiation of retinoic acid-sensitive and -insensitive human neuroblastoma cells lines was examined.
2 |. MATERIALS AND METHODS
2.1 |. Cell culture
The human neuroblastoma cell lines, NGP, SK-N-BE(2), and IMR-32 (American Type Culture Collection) were cultured in Dulbecco’s modified Eagle’s media (DMEM, Cellgro, Manassas, VA) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin (Invitrogen, Grand Island, NY) at 37°C with 5% carbon dioxide.
2.2 |. Stable human neuroblastoma cells over-expressing hPPARβ/δ
Stable NGP, SK-N-BE(2), and IMR-32 cell lines were produced that express the enhanced green fluorescent protein (eGFP) or eGFP and the human PPARβ/δ (hPPARβ/δ).9 Expression of eGFP and hPPARβ/δ was verified as previously described.8,9 Cells were treated with or without 0.01–1.0 μM GW0742 (a specific PPARβ/δ agonist30), 1 μM DG172 (a selective repressive PPARβ/δ ligand31) or 10 μM atRA; an RAR agonist, Sigma-Aldrich, St. Louis, MO) as described below.
2.3 |. Western blot analysis
Quantitative western blot analysis was performed with radioactive detection as previously described.9 Primary antibodies used in the present study included p53, RARα, SP1, YY1, ACTIN (Santa Cruz Biotechnology, Santa Cruz, CA), PPARβ/δ (Abcam, Cambridge, MA), AP-2γ, TBP, SOX2 (Cell Signaling Technology, Danvers, MA), and lactate dehydrogenase (LDH; Rockland, Gilbertsville, PA). The expression level of total protein was normalized to LDH or ACTIN. The expression level of nuclear proteins was normalized to TBP, and the expression level of cytosolic protein was normalized to LDH.
2.4 |. Quantitative real-time polymerase chain reaction (qPCR)
Gene expression was measured by qPCR analysis as previously described.32 Relative mRNA levels were normalized to the mRNA level of glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
2.5 |. Cell proliferation assay
The xCELLigence system (ACEA Biosciences, Inc. San Diego, CA) was used for quantifying changes in real-time cell proliferation in NGP and SK-N-BE(2) cells as previously described.8,9 IMR-32 cells exhibited clumping when cultured, thus, proliferation of IMR-32 cells was determined by counting cell number daily using a Z1 Coulter Counter (Beckman Coulter, Indianapolis, IN) as previously described.26
2.6 |. Ectopic xenograft tumor assay
Ectopic xenografts were produced and assessed using NGP-MigR1 and NGP-hPPARβ/δ cells (5 × 106 cells/injection), SK-N-BE(2)-MigR1, and SK-N-BE(2)-hPPARβ/δ cells (1 × 107 cells/injection) or IMR-32MigR1 and IMR-32-hPPARβ/δ cells (1 × 107 cells/injection) in 6-weekold female immunodeficient athymic nude (nu/nu) mice (Frederick National Laboratory for Cancer Research, Frederick, MD) as previously described.9,10 Groups of mice (n = 5) were treated daily with either vehicle control (0.02% dimethylsulfoxide) or GW0742 (2.5 mg/kg/ day). After 2–3 weeks, mice were euthanized and tumors were excised and weighed. A tumor section was fixed in 10% phosphate buffered formalin overnight and then switched to 75% ethanol. The remaining tumors were snap frozen in liquid nitrogen for protein or mRNA analysis. Fixed tumor sections were processed for hematoxylin-eosin (H&E) staining and examined by a pathologist as previously described.9,10
2.7 |. Immunohistochemistry
Expression and localization of SOX2 in ectopic xenograft tumors was determined by immunohistochemistry using 3,3′-diaminobenzidine (DAB) as a substrate and counterstained by hematoxylin as previously described.9 Twenty fields per sections and two sections per tumor sample were analyzed. Relative expression was determined by normalizing the intensity of DAB to hematoxylin signals using ImageJ software (Version 1.47c).
2.8 |. Transient over-expression and activation of RARα in neuroblastoma cells
Cells were transiently transfected with 10 μg of a pSG5-hRARα plasmid using Lipofectamine LTX reagent (Invitrogen, Grand Island, NY) following the manufacturer’s recommended procedures. Transfected cells were treated with or without 10 μM atRA for 24 h. Over-expression of RARα was confirmed by quantitative Western blot analysis, and the expression of SOX2 and the RAR target gene CYP26A1 was determined by qPCR and/or Western blot analysis.
2.9 |. Luciferase assays
To identify the cis-regulatory element responsible for PPARβ/δ-dependent SOX2 expression in human neuroblastoma cells, three luciferase reporter gene constructs driven by the human SOX2 promoter were generated. Three deletion fragments of SOX2 promoter were amplified by PCR. Primers were designed to contain overhanging sites for restriction enzymes MluI and BglII, which allowed for directional cloning into the multiple cloning site of pGL3-basic luciferase reporter vector (Promega Corp., Madison, WI). The composition of each construct was confirmed by restriction endonuclease digestion and DNA sequencing.
Cells were transiently co-transfected with 1 μg of a β-galactosidase construct (pCMV-β) (Promega Corp., Madison, WI) and 10 μg of either SOX2 promoter-luciferase reporter constructs or the control plasmid (pGL3-basic vector) using Lipofectamine LTX reagent following the manufacturer’s recommended procedures. Cell lysate were prepared in passive lysis buffer. Luciferase and β-galactosidase activities were assessed using Luciferase and Beta-Glo assay system (Promega Corp., Madison, WI), respectively, following the manufacturer’s recommended procedures. Relative luciferase activity was normalized to β-galactosidase activity.
2.10 |. Chromatin immunoprecipitation (ChIP)-qPCR
Putative trans-acting factors in each region of SOX2 promoter were identified using the ConSite program.33 To confirm occupancy of trans-acting factors on the SOX2 promoter, ChIP-qPCR was performed to quantify relative promoter occupancy in response to overexpression of PPARβ/δ as previously described.9 Antibodies against SP1, p65, YY1 (Santa Cruz Biotechnology, Santa Cruz, CA), MYC, and AP-2γ (Cell Signaling Technology, Danvers, MA) were individually added to histone-DNA complexes for immunoprecipitation of protein bound chromatin. The relative level of occupied SOX2 promoter in each group was normalized to IgG input control.
2.11 |. Statistical analyses
All experimental groups were performed in triplicate and repeated using three independent biological replicates. The data were subjected to Student’s t-test or a parametric one-way analysis of variance (ANOVA) followed by Tukey test for post hoc comparisons. Statistical significance was considered when P ≤ 0.05.
3 |. RESULTS
3.1 |. PPARβ/δ suppressed SOX2 expression and selectively modulated p53 level in retinoic acid-sensitive and -resistant neuroblastoma cells
The human neuroblastoma cell lines NGP, SK-N-BE(2) and IMR-32 were treated with or without atRA for up to 14 days. Seven days after treatment with atRA, outgrowths of neurites were observed in NGP cells and S-N-BE(2) cells, whereas this was not found in IMR-32 cells (Fig. S1). Notably, the lengths of neurites were increased in NGP and SK-N-BE(2) cells at the end of treatment, while no outgrowth of neurites was observed in IMR-32 cells. These observations confirmed that NGP cells are highly sensitive to atRA-induced cell differentiation, SK-N-BE(2) cells exhibit medium sensitivity to atRA-induced cell differentiation, while IMR-32 cells are resistant to atRA-induced cell differentiation.34,35
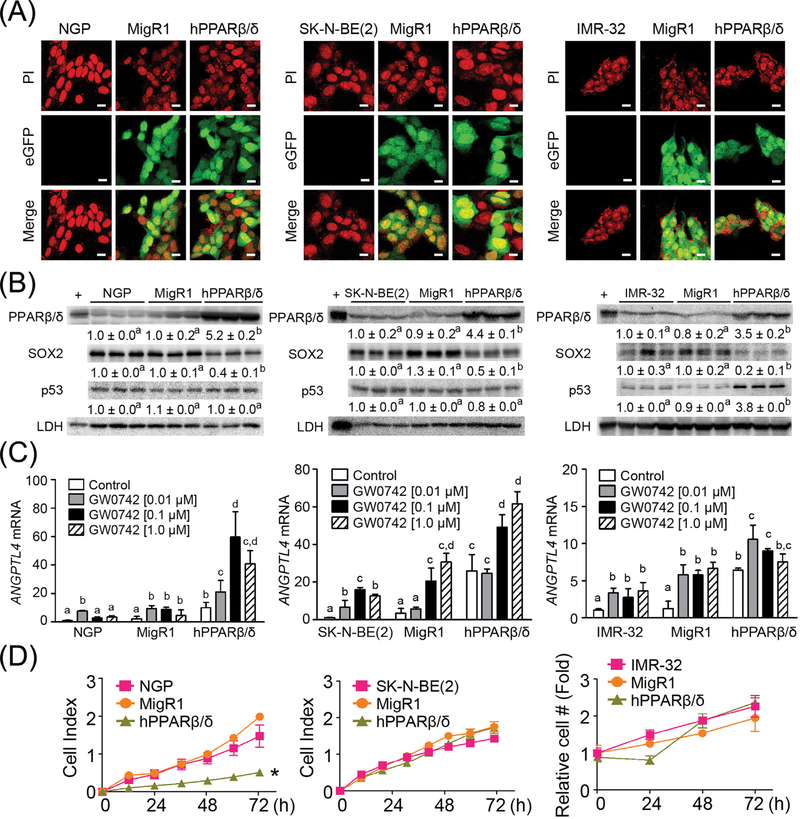
To determine whether PPARβ/δ can modulate the differentiation and tumorigenesis of neuroblastoma cells, NGP, SK-N-BE(2), and IMR-32 cells were engineered to stably over-express PPARβ/δ (NGP-hPPARβ/δ, SK-N-BE(2)-hPPARβ/δ, and IMR-32-hPPARβ/δ cells, respectively).8–10 Vector control cells lines (NGP-MigR1, SK-N-BE(2)-MigR1, and IMR-32-MigR1) that express eGFP, and the control parent NGP, SK-N-BE(2), and IMR-32 cells were also examined. The three neuroblastoma cell lines expressing PPARβ/δ and/or eGFP exhibited fluorescence whereas the control parent cell lines did not (Fig. 1A). Over-expression of PPARβ/δ in NGP, SK-N-BE(2), and IMR-32 cells was further confirmed by quantitative Western blot analysis (Figs. 1B and S2). Interestingly, over-expression of PPARβ/δ was consistently associated with the decreased expression of SOX2, a known stem cell and de-differentiation regulator, in all three neuroblastoma cell lines (Figs. 1B and S2), while increased expression of p53 was only observed in IMR-32-hPPARβ/δ cells compared to controls (Figs. 1B and S2). Over-expression and/or ligand activation of PPARβ/δ increased expression of angiopoietin-like protein 4 (ANGPTL4) mRNA, a known PPARβ/δ target gene, in NGP, SK-N-BE(2), and IMR-32 cells (Fig. 1C). Cells with PPARβ/δ had increased ligand-independent activation of ANGPTL4 mRNA suggesting the existence of endogenous PPARβ/δ agonists. Importantly, overexpression of PPARβ/δ suppressed proliferation in NGP cells; however, this effect was not observed in SK-N-BE(2) or IMR-32 cells compared to control (Fig. 1D).
FIGURE 1.
Characterization of human neuroblastoma cell lines over-expressing PPARβ/δ. (A) Representative photomicrographs of NGP, NGP-MigR1 (MigR1, vector control) and NGP-hPPARβ/δ (hPPARβ/δ); SK-N-BE(2), SK-N-BE(2)-MigR1 (MigR1), and SK-N-BE(2)-hPPARβ/δ (hPPARβ/δ); or IMR-32, IMR-32-MigR1 (MigR1) and IMR-32-hPPARβ/δ (hPPARβ/δ) cells showing positive eGFP signals in MigR1 and hPPARβ/δ cells. PI: propidium iodide (stain for nucleus). Magnification = 600×. Bar = 10 μm. (B) Quantitative Western blot analysis of PPARβ/ δ, SOX2, and p53 expression. +: the positive control (cell lysate from COS1 cells transfected with human PPARβ/δ expression vector). (C) Relative ANGPTL4 mRNA expression in cells treated with or without the PPARβ/δ agonist GW0742 compared to NGP, SK-N-BE(2) or IMR-32 parent cells. (D) Real-time proliferation of NGP, NGP-MigR1, and NGP-hPPARβ/δ or SK-N-BE(2), SK-N-BE(2)-MigR1, and SK-N-BE(2)-hPPARβ/δ cells were examined using the xCELLigence system. Cell growth curve of IMR-32 cells was determined by counting cell numbers every 24 h. Relative cell number over time was normalized to the initial cell number and represents the fold change. Values represent the mean ± S.E.M. Values with different superscript letters are significantly different at P ≤ 0.05. *Significantly different than controls, P ≤ 0.05
3.2 |. SOX2 expression is decreased by PPARβ/δ independent of RARα-mediated signaling
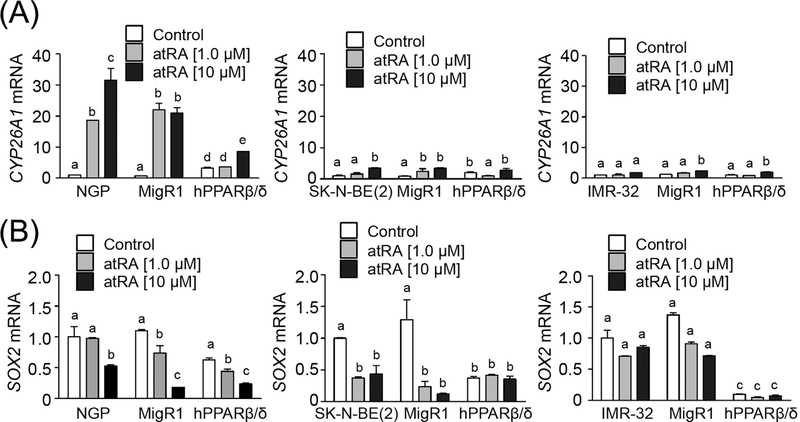
Expression of CYP26A1 mRNA, a known RARα target gene, was increased by atRA in NGP and SK-N-BE(2) cells (Fig. 2A). The induction of CYP26A1 mRNA in SK-N-BE(2) cells was not as high as that observed in NGP cells (Fig. 2A). By contrast, activating RARα with atRA decreased expression of SOX2 mRNA, in NGP and SK-N-BE(2) cells but not in IMR-32 cells (Fig. 2B), consistent with the morphological changes in the differentiation observed in NGP and SK-N-BE(2) cells but not IMR-32 cells (Fig. S1).
FIGURE 2.
Exposure to atRA decreases SOX2 mRNA expression in NGP and SK-N-BE(2) cells, but not in IMR-32 cells. (A) CYP26A and (B) SOX2 mRNA expression in cells treated with or without atRA. MigR1: cells with integrated MigR1 control vector; hPPARβ/δ: cells overexpressing PPARβ/δ. Values represent the mean ± S.E.M. Values with different superscript letters are significantly different at P ≤ 0.05
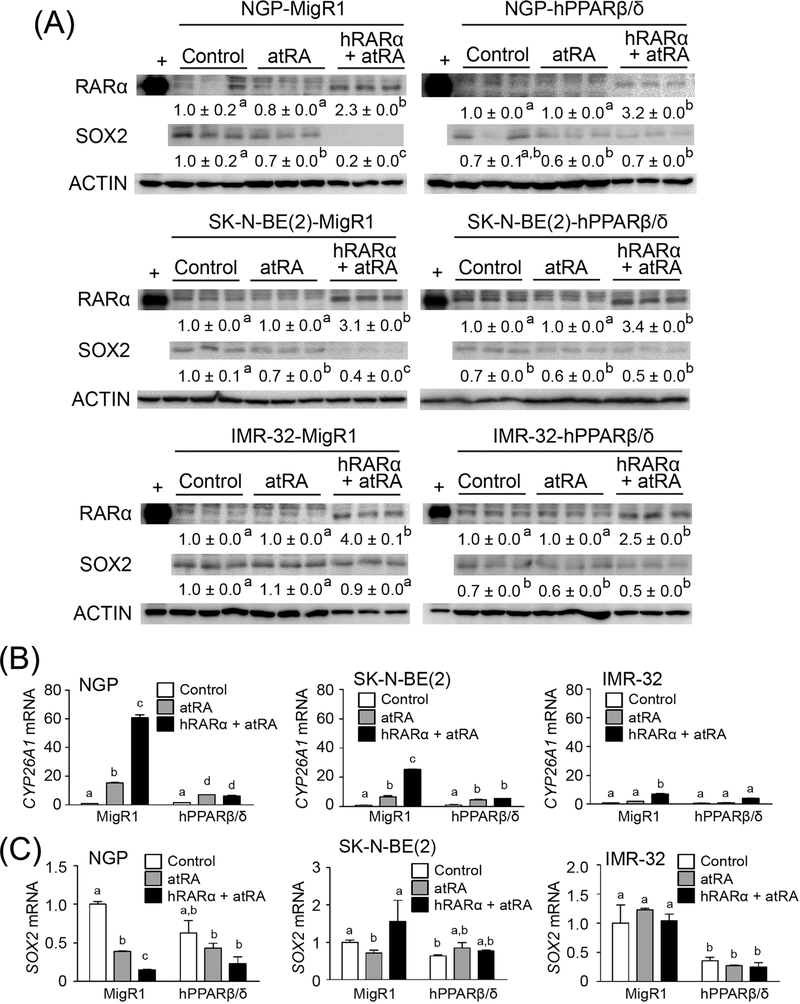
Over-expression of PPARβ/δ decreased the level of SOX2 mRNA in SK-N-BE(2) and IMR-32 cells, but not in NGP cells compared to controls (Fig. 2B). In neuroblastoma cell lines over-expressing PPARβ/ δ, activation of RARα with atRA did not further influence suppression of SOX2 mRNA expression in either SK-N-BE(2)-hPPARβ/δ or IMR-32-hPPARβ/δ cells (Fig. 2B). However, activation of RARα with atRA did decrease SOX2 mRNA expression in NGP-hPPARβ/δ cells (Fig. 2B). To more definitively examine any potential interaction between RARα and PPARβ/δ, RARα was over-expressed in NGP-MigR1 or NGP-hPPARβ/δ, SK-N-BE(2)-MigR1, or SK-N-BE(2)-hPPARβ/δ, and IMR-32-MigR1 or IMR-32-hPPARβ/δ, cells (Figs. 3A and S2). Expression of the RARα target gene CYP26A1 was increased in atRA-treated NGP-MigR1 and SK-N-BE(2)-MigR1 cells compared to controls, and this induction was greater in cells over-expressing RARα (Fig. 3B). By contrast, activation of RARα with atRA had no effect on the expression of CYP26A1 mRNA in IMR-32-MigR1 cells compared to controls, and exhibited limited effect in IMR-32-MigR1 cells over-expressing RARα (Fig. 3B). Interestingly, no significant difference in CYP26A1 mRNA expression was detected between NGP-hPPARβ/δ cells in response to atRA treatment regardless of RARα status (Fig. 3B). Similar results were observed in SK-N-BE(2)-hPPARβ/δ and IMR-32-hPPARβ/δ cells (Fig. 3B). Activation of RARα with atRA suppressed SOX2 protein and mRNA expression in NGP-MigR1 and NGP-hPPARβ/δ cells compared to controls (Fig. 3A and C). This reduction in SOX2 level was somewhat enhanced by over-expressing RARα in NGP-MigR1 cells, but not in NGP-hPPARβ/δ cells (Fig. 3A and C). Similar results were found in SK-N-BE(2)-MigR1 and SK-N-BE(2)-hPPARβ/δ cells (Fig. 3A and C). No change in SOX2 expression in IMR-32-MigR1 or IMR-32-hPPARβ/ δ cells following activation of RARα with atRA with or without overexpression of RARα (Fig. 3A and C). Thus, it appears that PPARβ/δ acts independently from RARα on inhibiting SOX2 expression.
FIGURE 3.
PPARβ/δ decreases SOX2 expression independently of RARα-mediated pathway in human neuroblastoma cell lines. (A) Quantitative western blot analysis of RARα and SOX2 expression in cells in response to atRA treatment, or transiently transfected with pSG5-RARα plasmid and activated with atRA. (B) CYP26A1 mRNA expression in MigR1 and hPPARβ/δ cells or cells transiently overexpressing RARα after atRA treatment. (C) SOX2 mRNA expression in MigR1 and hPPARβ/δ cells or cells transiently over-expressing RARα following atRA treatment. MigR1: cells with integrated MigR1 control vector; hPPARβ/δ: cells over-expressing PPARβ/δ. Values represent the mean ± S.E.M. Values with different superscript letters are significantly different at P ≤ 0.05
3.3 |. PPARβ/δ selectively modulates expression of p53 and PTEN in atRA-insensitive neuroblastoma cells
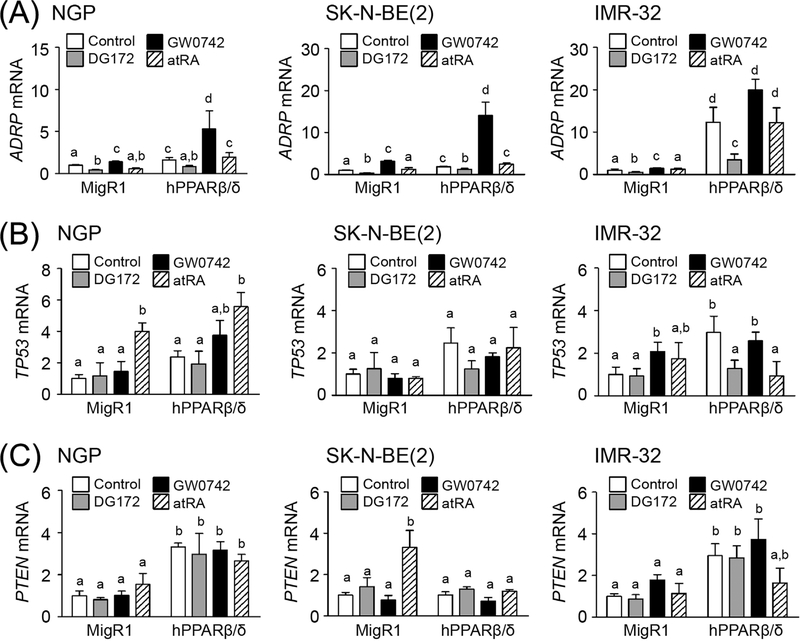
Since p53 status is different between the atRA sensitive cells (NGP; inactive p53 due to expression of MDM236,37) and atRA-insensitive cells (IMR-32; wild-type p53), the effect of PPARβ/δ on p53 expression was examined. Cells were treated with or without: 1) a PPARβ/δ ligand (GW0742), 2) a selective repressive PPARβ/δ ligand (DG172), or 3) the RARα ligand atRA. Previous studies showed that activating RARα with atRA increases p53 expression in neuroblastoma cells.38 Over-expression and/or ligand activation of PPARβ/δ with GW0742 caused an increase in ADRP mRNA, a known PPARβ/δ target gene, in NGP, SK-N-BE(2) and IMR-32 cells (Fig. 4A). By contrast, DG172 treatment down-regulated ADRP mRNA expression in all three neuroblastoma cell lines (Fig. 4A). Activation of RARα with atRA had no effect on ADRP mRNA expression in all three neuroblastoma cell lines (Fig. 4A). Interestingly, over-expression of PPARβ/δ up-regulated TP53 mRNA expression in IMR-32 cells, but not in NGP or SK-N-BE(2) cells (Fig. 4B). Ligand activation of PPARβ/δ with GW0742 induced TP53 mRNA expression in IMR-32-MigR1 cells, but not in NGP or SK-N-BE(2) cells (Fig. 4B). DG172-decreased TP53 mRNA expression was also found in IMR-32-hPPARβ/δ cells, but not in NGP or SK-N-BE(2) cells (Fig. 4B). Activation of RARα with atRA induced TP53 mRNA expression in both NGP-MigR1 and NGP-hPPARβ/δ cells; however, no significant difference was detected between these two groups (Fig. 4B). By contrast, activation of RARα with atRA had no effect on TP53 mRNA expression in SK-N-BE(2) or IMR-32 cells (Fig. 4B). Since p53 is known to increase PTEN expression,39 it is important to note that over-expression of PPARβ/δ caused an increase in PTEN mRNA expression in NGP-hPPARβ/δ and IMR-32-hPPARβ/δ cells compared to controls, but not in SK-N-BE(2)-hPPARβ/δ cells (Fig. 4C). Neither ligand activation nor selective repression of PPARβ/δ influenced PTEN mRNA expression in all three neuroblastoma cell lines (Fig. 4C). Surprisingly, activation of RARα with atRA induced PTEN mRNA expression in SK-N-BE(2)-MigR1 cells, but not in NGP-MigR1 or IMR-32-MigR1 cell lines (Fig. 4C).
FIGURE 4.
PPARβ/δ regulates p53/PTEN expression in human neuroblastoma cell lines. Expression of (A) ADRP, (B) TP53, and (C) PTEN mRNA expression in cells in response to GW0742, DG172, or atRA treatment. MigR1: cells with integrated MigR1 control vector; hPPARβ/δ: cells over-expressing PPARβ/δ. Values represent the mean ± S.E.M. Values with different superscript letters are significantly different at P ≤ 0.05
3.4 |. PPARβ/δ inhibits ectopic tumor xenografts by induction of differentiation, necrosis, and infiltration of inflammatory cells
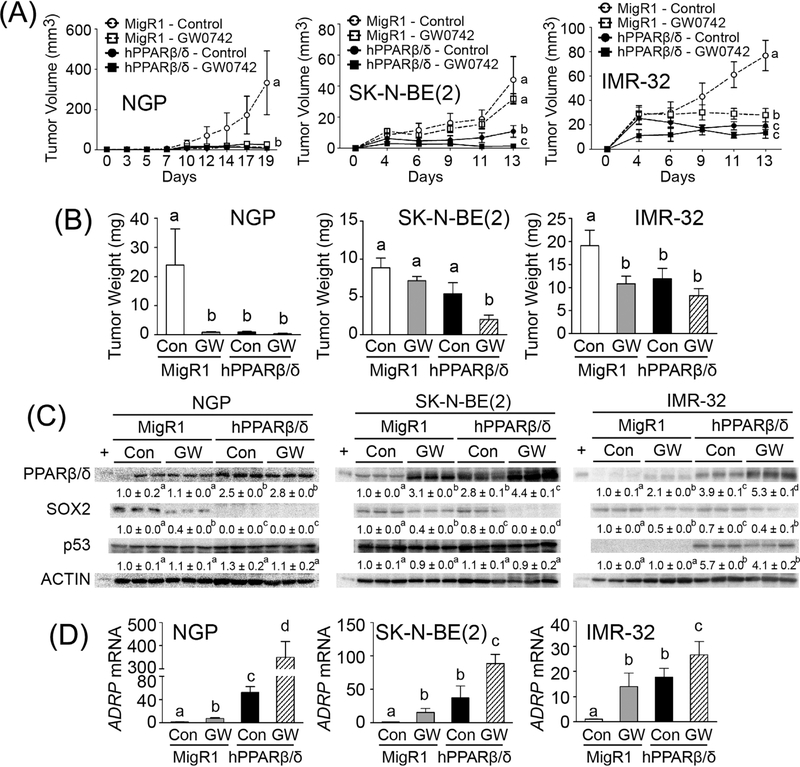
The average tumor volume and weight of ectopic xenografts from SK-N-BE(2)-MigR1 and IMR-32-MigR1 cells was comparatively smaller than those from NGP-MigR1 cells (Fig. 5A and B). This correlated with higher level of necrosis in xenografts from SK-N-BE(2)-MigR1 and IMR-32-MigR1 cells compared to those from NGP-MigR1 cells (Figs. S3 and S4). Additionally, tumor cells in nearby lymph nodes, consistent with metastasis, was also observed in control mice that developed ectopic xenografts from SK-N-BE(2)-MigR1 or IMR-32-MigR1 cells but not in NGP-MigR1 cells (data not shown). Tumor cells in xenografts from NGP-MigR1 cells appeared to be immature, pleomorphic and highly mitotic as assessed by histopathological analysis (Fig. S3).
FIGURE 5.
PPARβ/δ attenuates tumor growth in neuroblastoma xenografts. (A) Average tumor volumes over time. (B) Average tumor weight at the end of the study. (C) Quantitative Western blot analysis of PPARβ/δ, SOX2, and p53 expression in xenograft tumors. +, positive control (cell lysate from COS1 cells transfected with human PPARβ/δ expression vector). Relative expression of protein was normalized to ACTIN. (D) ADRP mRNA expression in xenograft tumors was determined by qPCR. Control = (Con); GW0742-treated (2.5 mg/kg/day) = (GW). Values represent the mean ± S.E.M. Values with different superscript letters are significantly different at P ≤ 0.05
The average tumor volume and weight of ectopic xenografts from NGP, SK-N-BE(2), or IMR-32 cells over-expressing PPARβ/δ was significantly smaller compared to controls (Fig. 5A and B). PPARβ/δ expression was markedly higher in xenografts from NGP, SK-N-BE(2), or IMR-32 cells over-expressing PPARβ/δ compared to controls (Figs. 5C and S5). Over-expression and/or ligand activation of PPARβ/δ also caused an increase in expression of the PPARβ/δ target gene ADRP in xenografts compared to controls (Fig. 5D). Over-expression and/or ligand activation of PPARβ/δ increased the percentage of necrotic cells in xenografts derived from either NGP-MigR1 or NGP-hPPARβ/δ groups (Fig. S3C). The percentage of necrotic cells in xenografts derived from either SK-N-BE(2)-MigR1 or SK-N-BE(2)-hPPARβ/δ groups was not different (Fig. S4B). By contrast, over-expression and/or ligand activation of PPARβ/δ caused severe necrosis in xenografts from mice with IMR-32-MigR1 or IMR-32-hPPARβ/δ cells (Fig. S4D and E). Tumor cells in nearby lymph nodes, consistent with metastasis, was not found in xenografts following ligand activation of PPARβ/δ in any of the neuroblastoma cell lines over-expressing PPARβ/δ (data not shown). Interestingly, moderate to severe infiltration of inflammatory cells, mostly polymorphonuclear leukocytes, was also observed in xenografts from NGP-MigR1 or NGP-hPPARβ/δ cells in response to ligand activation of PPARβ/δ (Fig. S3A and C). Similarly, infiltration of inflammatory cells in xenografts from SK-N-BE(2) or IMR-32 cells by over-expression and/or ligand activation of PPARβ/ δ was also observed (Fig. S4C and F).
Higher expression of PPARβ/δ in the human neuroblastoma cell lines was associated with a decrease in SOX2 expression in xenografts derived from NGP-hPPARβ/δ, SK-N-BE(2)-PPARβ/δ, or IMR-32-hPPARβ/δ cells compared to controls (Figs. 5C and S5). Expression of p53 was markedly induced in xenografts from IMR-32-hPPARβ/δ cells, while this increase in p53 expression was not observed in xenografts from either NGP-hPPARβ/δ or SK-N-BE(2)-hPPARβ/δ cells (Figs. 5C and S5).
3.5 |. PPARβ/δ-dependent down-regulation of SOX2 is mediated by altered YY1, SP1 and AP-2γ activities in neuroblastoma cells
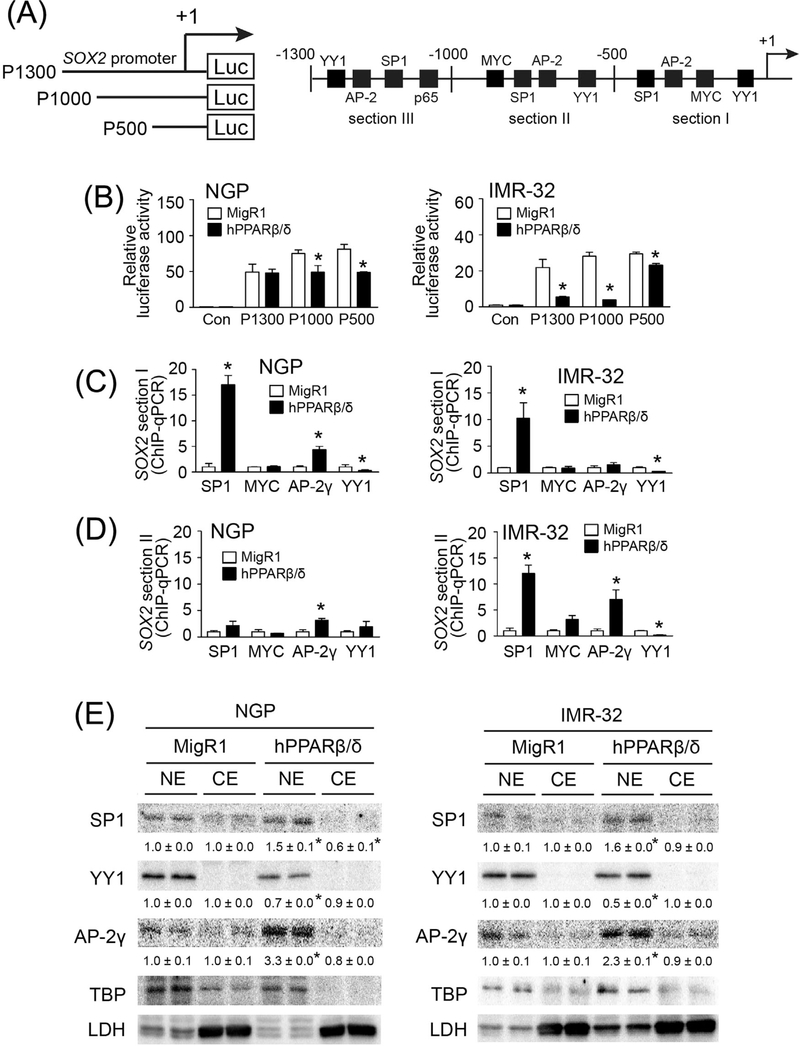
To identify the regulatory region in the SOX2 promoter required for mediating PPARβ/δ-dependent down-regulation of SOX2 expression, three luciferase reporter constructs (P1300, P1000, and P500) of the human SOX2 promoter (−1 to −1300, −1 to −1000, and −1 to −500, respectively) were generated (Fig. 6A), and transfected into control and PPARβ/δ over-expressing NGP and IMR-32 cells because they represent atRA sensitive (NGP) and atRA insensitive (IMR-32) cell lines. The data from these analyses indicate that the region from −1 to −1000 is critical for PPARβ/δ-dependent repression of SOX2 promoter activity in both of these cell lines, but this effect was more pronounced in the IMR-32 cell line (Fig. 6B). Putative cis-regulatory elements in this region of the SOX2 promoter included binding sites for YY1, AP-2γ, SP1, and MYC (Fig. 6A).
FIGURE 6.
PPARβ/δ-suppressed SOX2 expression is associated with altered activities of SP1, AP-2γ, and YY1 in neuroblastoma cells. (A) Left panel, three luciferase reporter constructs driven by the human SOX2 promoter with serial deletion; right panel, putative binding sites of trans-acting factors in the human SOX2 promoter. +1 represented the transcriptional start site. Section I, −1 to −500 bp region; section II, −500 to −1000 bp region; section III, −1000 to −1300 bp region of SOX2 promoter. (B) Relative luciferase activities of three luciferase reporter constructs in neuroblastoma cells with integrated MigR1 control vector (MigR1) and cells over-expressing PPARβ/δ (hPPARβ/δ). Control = Con. (C) ChIP-qPCR showing effect of PPARβ/δ on the occupancy of SP1, MYC, AP-2γ, and YY1 on the −1 to −500 bp region of SOX2 promoter in the indicated control cells or those over-expressing PPARβ/δ. (D) ChIP-qPCR showing effect of PPARβ/δ on the occupancy of SP1, MYC, AP-2γ, and YY1 on the −500 to −1000 bp region of SOX2 promoter in control cells or those over-expressing PPARβ/δ. (E) Differential expression of SP1, YY1, and AP-2γ in nuclear and cytosolic extracts of NGP-MigR1 and NGP-hPPARβ/δ cells or IMR-32-MigR1 and IMR-32-hPPARβ/δ cells were assessed by quantitative western blot analysis. NE, nuclear extract; CE, cytosolic extract. Values represent the mean ± S.E.M. *Significantly different than parent control group, P ≤ 0.05
ChIP assays revealed increased promoter occupancy of SP1 and reduced promoter occupancy of YY1 in the −1 to −500 region of the SOX2 promoter in NGP-hPPARβ/δ and IMR-32-hPPARβ/δ cells compared to controls (Fig. 6C). Greater promoter occupancy of AP-2γ in this region of the SOX2 promoter was also detected in NGP-hPPARβ/δ cells, but not in IMR-32-hPPARβ/δ cells compared to controls (Fig. 6C). Increased promoter occupancy of SP1 and AP-2γ, together with decreased promoter occupancy of YY1 in the −500 to −1000 region of the SOX2 promoter was observed in IMR-32 cells over-expressing PPARβ/δ (Fig. 6D); while NGP-hPPARβ/δ cells exhibited greater promoter occupancy of AP-2γ compared to controls (Fig. 6D). Quantitative western blot analysis further confirmed higher expression of SP1 and AP-2γ, and lower expression YY1 in nuclear fractions of NGP-hPPARβ/δ and IMR-32-hPPARβ/δ cells compared to controls (Fig. 6E). These observations suggest that PPARβ/δ-dependent activation of SP1 and/or AP-2γ together with PPARβ/δ-dependent inhibition of YY1 may co-operatively mediate down-regulation of SOX2 expression in neuroblastoma cells.
4 |. DISCUSSION
The average 5-year survival rate in children with high-risk neuroblastoma ranges from 20% to 50% due to poor response to chemotherapy, tumor recurrence or metastatic relapse.3 Developing new therapeutic approaches to enhance the sensitivity and/or efficacy of therapeutic interventions is strongly needed, in particular for neuroblastoma patients who exhibit resistance to therapies such as retinoic acid. The present study demonstrates for the first time that PPARβ/δ suppresses tumorigenesis in retinoic acid-resistant, N-MYC-amplified neuroblastoma cells through unique mechanisms. For example, the data from the present studies suggest that p53/PTEN and SOX2-mediated signaling co-operatively, yet differentially, regulate cell differentiation and survival in neuroblastoma cells. In neuroblastoma cells that respond to retinoids by inducing differentiation, PPARβ/δ is able to suppress SOX2 expression and increase PTEN expression, but does not influence expression of p53. This suggests that neuroblastoma cells that differentiate after retinoid treatment, are responsive to PPARβ/δ, and exhibit enhanced differentiation due in part to suppression of SOX2 expression and function as evidenced by reduced tumorigenicity. By contrast, in neuroblastoma cells that are relatively resistant to retinoid-induced differentiation, PPARβ/δ is able to suppress SOX2 expression, increase PTEN expression, and increase expression of p53 resulting in more effective attenuation of tumorigenicity. These studies are rigorous because they relied on several complementary in vitro and in vivo approaches, and included pharmacological activation of PPARβ/δ, selective repression of PPARβ/δ, and/or over-expression of PPARβ/δ and/or RARα coupled with mechanistic analyses.
The chemopreventive and chemotherapeutic effects induced by retinoids via activation of their cognate nuclear receptors (RAR/RXR) is mediated by regulating expression of genes involved in cell differentiation, cell cycle, apoptosis, and DNA damage.40,41 Relatively high expression of stem cell regulators, such as SOX2, NANOG and octamer-binding transcription factor 4 (OCT4), are critical in controlling tumor progression as these protein promote self-renewal of cancer stem cells, induce de-differentiation, facilitate cell invasion and migration, and enhance drug-resistance.42 The present studies demonstrate that atRA markedly represses SOX2 expression in NGP and SK-N-BE(2) cells, consistent with previous studies showing that retinoic acid inhibits the stemness of neuroblastoma cells43,44; while this effect is not observed in retinoic acid-resistant IMR-32 cells. It has been proposed that different responses to retinoic acid between retinoic acid-sensitive and retinoic acid-resistant neuroblastoma cells may be due to differential expression and activation of RAR in these two type of cells.45 Combinatorial treatment with synthetic ligands of RXR and RARα enhances atRA-inhibited cell growth in N-MYC-amplified neuroblastoma cells regardless of the sensitivity to atRA.46 However, the results from the present study revealed that transient over-expression of RARα in IMR-32 cells did not enhance sensitivity to atRA treatment. By contrast, over-expression of RARα caused even greater suppression of SOX2 expression in NGP and SK-N-BE(2) cells treated with atRA. These observations suggest that establishing drug-resistance in certain neuroblastoma cells is mediated by RAR-dependent and RAR-independent mechanisms. Interestingly, the PPARβ/δ-dependent decrease in SOX2 expression is observed in retinoic acid-resistant neuroblastoma cells, but not in retinoic-acid-sensitive neuroblastoma cells. As transient over-expression of RARα has no further effect on retinoic acid-suppressed SOX2 expression in all three neuroblastoma cells, this indicates that PPARβ/δ and RAR have no synergistic effect in modulating SOX2 expression in human neuroblastoma. In fact, a previous study demonstrated that PPARβ/δ interferes with RAR signaling by competing for the common binding partner, RXR, in testicular embryonal carcinoma.9 Thus, overexpressing PPARβ/δ may saturate the bioavailability of RXR to interact with RAR, resulting in no synergistic response to retinoic acid treatment in neuroblastoma cells.
Transcriptional regulation of SOX2 has been evaluated in human and mouse embryonic stem cells.47,48 The present study revealed novel regulation of SOX2 expression is mediated by modulating SP1, YY1, and AP-2γ activities in neuroblastoma cells through a mechanism that requires PPARβ/δ. SP1 acts as a transcription factor by interacting with GC-rich sequences of target gene promoters, especially for genes driven by a TATA-box-less promoter.49 Multiple putative SP1 binding sites were identified in the SOX2 promoter, a TATA-box-less promoter indeed, supporting the notion that SP1 co-operates with PPARβ/δ in regulating SOX2 expression. This is consistent with previous studies demonstrating that SP1 is critical in PPARβ/δ-dependent signaling,50 and negatively regulates the pluripotency in stem cells by targeting OCT4 and NANOG.51–53 This is consistent with previous studies showing that SP1 represses transcription in human cancer cells.54,55 Over-expression of PPARβ/δ caused nuclear accumulation of SP1 associated with a greater occupancy of SP1 in SOX2 promoter in neuroblastoma cells. This suggests that SP1 is likely required for PPARβ/δ-dependent suppression of SOX2 expression by directly binding to SOX2 promoter as a transcription repressor but this hypothesis requires further studies. Similar to SP1, AP-2γ also reveals a critical role in cell differentiation by suppressing stem cell marker expression.56 These observations are also collectively similar to previous studies showing that PPARβ/δ require co-recruitment of other transcription factors for optimal modulation of gene expression.57
The present study also showed that YY1 expression in the nuclear fraction was decreased in neuroblastoma cell over-expressing PPARβ/ δ, and these cells exhibited less YY1 occupancy on the SOX2 promoter. This suggests that PPARβ/δ may attenuate YY1-induced SOX2 expression in neuroblastoma cells. Additionally, YY1 is known to repress SP1-mediated transcriptional regulation.58 This suggests that PPARβ/δ-dependent activation of SP1 and PPARβ/δ-dependent inactivation of YY1 co-operatively inhibit SOX2 expression in neuroblastoma cells. It is worth noting that the alteration of SP1 and YY1 occupancy in −500 to −1000 region of SOX2 promoter was not observed in retinoic acid-sensitive neuroblastoma NGP cells overexpressing PPARβ/δ. The mechanism underlying this difference cannot be determined from these studies but could be due to differences in the expression patterns of other co-effector proteins that modify chromatin and allow PPARβ/δ to alter gene expression. Further studies are needed to examine this hypothesis.
Spontaneous regression and induction of differentiation are the primary molecular mechanisms for developing therapeutic approaches for neuroblastoma patients.2 Interestingly, histopathological evaluation revealed that over-expression and/or ligand activation of PPARβ/δ caused an increased infiltration of inflammatory cells in xenograft tumors derived from human neuroblastoma cells. This suggests that PPARβ/δ may suppress tumor growth and/or inhibit metastasis by influencing the innate immunity.
Combined, results from these studies show that interactions among transcription factors can create complex networks to modulate cell differentiation. Results from these studies are highly inconsistent with the hypothesis that atRA promotes tumorigenesis by alternatively activating PPARβ/δ, rather than RAR/RXR as suggested by others.25 Although PPARβ/δ effectively inhibits tumor growth in both retinoic acid-sensitive and retinoic acid-resistant neuroblastoma cells, the underlying mechanism in these two types of cells appears to be different. In fact, over-expression of PPARβ/δ was clearly more effective at inhibiting tumorigenesis in neuroblastoma cells that exhibit relative resistance to retinoid-induced differentiation. This effect is likely due to the induction of differentiation that is at least in part mediated by down-regulation of SOX2 via interacting with SP1 and YY1 activities. Moreover, this provides preliminary data to support the development of an alternative combinatorial chemotherapeutic approach for neuroblastoma patients that are resistant to standard retinoic acid-induced differentiation.
Supplementary Material
ACKNOWLEDGMENT
The authors gratefully thank the Microscopy and Cytometry Facility at the Huck Institute of Life Sciences of The Pennsylvania State University for providing technique support with flow cytometer, histology equipment and confocal microscopy and Dr. Ugra Singh for providing the neuroblastoma cell lines. The authors also gratefully acknowledge Jyoti Singh, Megan E. Campbell and Jacqueline A. Krevitz for technical assistance with these studies. This work was supported by National Institutes of Health and National Cancer Institute R01-CA124533 (J. M. Peters), R01-CA141029 (J.M. Peters), 1ZIABC005561 (F.J. Gonzalez), 1ZIABC005562 (F.J. Gonzalez), and 1ZIABC005708 (F.J. Gonzalez) and by the German Cancer Research Foundation (grant Mu601/13-1).
Funding information
National Institutes of Health and National Cancer Institute, Grant numbers: R01-CA124533, R01-CA141029, 1ZIABC005561, 1ZIABC005562, 1ZIABC005708; German Cancer Research Foundation, Grant number: Mu601/13-1
Footnotes
SUPPORTING INFORMATION
Additional Supporting Information may be found online in the supporting information tab for this article.
REFERENCES
- 1.Cheung NK, Dyer MA. Neuroblastoma: developmental biology, cancer genomics and immunotherapy. Nat Rev Cancer. 2013;13:397–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brodeur GM, Bagatell R. Mechanisms of neuroblastoma regression.Nat Rev Clin Oncol. 2014;11:704–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Irwin MS, Park JR. Neuroblastoma: paradigm for precision medicine. Pediatr Clin North Am. 2015;62:225–256. [DOI] [PubMed] [Google Scholar]
- 4.Shimada H, Umehara S, Monobe Y, et al. International neuroblastoma pathology classification for prognostic evaluation of patients with peripheral neuroblastic tumors: a report from the Children’s Cancer Group. Cancer. 2001;92:2451–2461. [DOI] [PubMed] [Google Scholar]
- 5.Hara J Development of treatment strategies for advanced neuroblastoma. Int J Clin Oncol. 2012;17:196–203. [DOI] [PubMed] [Google Scholar]
- 6.Peters JM, Gonzalez FJ, Muller R. Establishing the role of PPARβ/δ in carcinogenesis. Trends Endocrinol Metab. 2015;26:595–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peters JM, Shah YM, Gonzalez FJ. The role of peroxisome proliferator activated receptors in carcinogenesis and chemoprevention. Nat Rev Cancer. 2012;12:181–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foreman JE, Chang WC, Palkar PS, et al. Functional characterization of peroxisome proliferator-activated receptor-β/δ expression in colon cancer. Mol Carcinog. 2011;50:884–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yao PL, Chen LP, Dobrzanski TP, et al. Inhibition of testicular embryonal carcinoma cell tumorigenicity by peroxisome proliferator activated receptor-β/δ- and retinoic acid receptor-dependent mechanisms. Oncotarget. 2015;6:36319–36337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yao PL, Morales JL, Zhu B, Kang BH, Gonzalez FJ, Peters JM. Activation of peroxisome proliferator-activated receptor-β/δ (PPAR-β/δ) inhibits human breast cancer cell line tumorigenicity. Mol Cancer Ther. 2014;13:1008–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang L, Olsson B, Pfeifer D, et al. Knockdown of peroxisome proliferator-activated receptor-β induces less differentiation and enhances cell-fibronectin adhesion of colon cancer cells. Oncogene. 2010;29:516–526. [DOI] [PubMed] [Google Scholar]
- 12.Yang L, Zhou J, Ma Q, et al. Knockdown of PPAR δ gene promotes the growth of colon cancer and reduces the sensitivity to bevacizumab in nude mice model. PLoS ONE. 2013;8:60715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang L, Zhou ZG, Zheng XL, et al. RNA interference against peroxisome proliferator-Activated receptor δ gene promotes proliferation of human colorectal cancer cells. Dis Colon Rectum. 2008;51:318–328. [DOI] [PubMed] [Google Scholar]
- 14.Peters JM, Gonzalez FJ. Sorting out the functional role(s) of peroxisome proliferator-activated receptor-β/δ (PPARβ/δ) in cell proliferation and cancer. Biochim Biophys Acta. 2009;1796:230–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peters JM, Yao PL, Gonzalez FJ. Targeting peroxisome proliferator activated receptor-β/δ (PPARβ/δ) for cancer chemoprevention. Curr Pharmacol Rep. 2015;1:121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buttitta LA, Edgar BA. Mechanisms controlling cell cycle exit upon terminal differentiation. Curr Opin Cell Biol. 2007;19:697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawamata H, Tachibana M, Fujimori T, Imai Y. Differentiation inducing therapy for solid tumors. Curr Pharm Des. 2006;12:379–385. [DOI] [PubMed] [Google Scholar]
- 18.Seeger RC, Siegel SE, Sidell N. Neuroblastoma: clinical perspectives, monoclonal antibodies, and retinoic acid. Ann Intern Med. 1982;97:873–884. [DOI] [PubMed] [Google Scholar]
- 19.Abemayor E The effects of retinoic acid on the in vitro and in vivo growth of neuroblastoma cells. Laryngoscope. 1992;102:1133–1149. [DOI] [PubMed] [Google Scholar]
- 20.Matthay KK, Villablanca JG, Seeger RC, et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children’s Cancer Group. N Engl J Med. 1999;341:1165–1173. [DOI] [PubMed] [Google Scholar]
- 21.Melino G, Thiele CJ, Knight RA, Piacentini M. Retinoids and the control of growth/death decisions in human neuroblastoma cell lines. J Neurooncol. 1997;31:65–83. [DOI] [PubMed] [Google Scholar]
- 22.Reynolds CP, Kane DJ, Einhorn PA, et al. Response of neuroblastoma to retinoic acid in vitro and in vivo. Prog Clin Biol Res. 1991;366:203–211. [PubMed] [Google Scholar]
- 23.Reynolds CP, Schindler PF, Jones DM, Gentile JL, Proffitt RT, Einhorn PA. Comparison of 13-cis-retinoic acid to trans-retinoic acid using human neuroblastoma cell lines. Prog Clin Biol Res. 1994;385:237–244. [PubMed] [Google Scholar]
- 24.Sidell N Retinoic acid-induced growth inhibition and morphologic differentiation of human neuroblastoma cells in vitro. J Natl Cancer Inst. 1982;68:589–596. [PubMed] [Google Scholar]
- 25.Schug TT, Berry DC, Shaw NS, Travis SN, Noy N. Opposing effects of retinoic acid on cell growth result from alternate activation of two different nuclear receptors. Cell. 2007;129:723–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Borland MG, Foreman JE, Girroir EE, et al. Ligand activation of peroxisome proliferator-Activated receptor-β/δ (PPARβ/δ) inhibits cell proliferation in human HaCaT keratinocytes. Mol Pharmacol. 2008;74:1429–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borland MG, Khozoie C, Albrecht PP, et al. Stable over-expression of PPARβ/δ and PPARγ to examine receptor signaling in human HaCaT keratinocytes. Cell Signal. 2011;23:2039–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rieck M, Meissner W, Ries S, Muller-Brusselbach S, Muller R. Ligand mediated regulation of peroxisome proliferator-activated receptor (PPAR) β/δ: a comparative analysis of PPAR-selective agonists and all trans retinoic acid. Mol Pharmacol. 2008;74:1269–1277. [DOI] [PubMed] [Google Scholar]
- 29.Peters JM, Foreman JE, Gonzalez FJ. Dissecting the role of peroxisome proliferator-activated receptor-β/δ (PPARβ/δ) in colon, breast and lung carcinogenesis. Cancer Metastasis Rev. 2011;30:619–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sznaidman ML, Haffner CD, Maloney PR, et al. Novel selective small molecule agonists for peroxisome proliferator-activated receptor δ (PPARδ)-synthesis and biological activity. Bioorg Med Chem Lett.2003;13:1517–1521. [DOI] [PubMed] [Google Scholar]
- 31.Lieber S, Scheer F, Meissner W, et al. (Z)-2-(2-bromophenyl)-3-{[4-(1methyl-piperazine)amino]phenyl}acrylonitrile (DG172): an orally bioavailable PPARβ/δ-selective ligand with inverse agonistic properties.J Med Chem. 2012;55:2858–2868. [DOI] [PubMed] [Google Scholar]
- 32.Zhu B, Ferry CH, Blazanin N, et al. PPARβ/δ promotes HRAS-induced senescence and tumor suppression by potentiating p-ERK and repressing p-AKT signaling. Oncogene. 2014;33:5348–5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sandelin A, Wasserman WW, Lenhard B. ConSite: web-based prediction of regulatory elements using cross-species comparison. Nucleic Acids Res. 2004;32:W249–W252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joshi S, Guleria RS, Pan J, Dipette D, Singh US. Heterogeneity in retinoic acid signaling in neuroblastomas: role of matrix metalloproteinases in retinoic acid-induced differentiation. Biochim Biophys Acta. 2007;1772:1093–1102. [DOI] [PubMed] [Google Scholar]
- 35.Armstrong JL, Ruiz M, Boddy AV, Redfern CP, Pearson AD, Veal GJ. Increasing the intracellular availability of all-trans retinoic acid in neuroblastoma cells. Br J Cancer. 2005;92:696–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goldschneider D, Horvilleur E, Plassa LF, et al. Expression of C-terminal deleted p53 isoforms in neuroblastoma. Nucleic Acids Res. 2006;34:5603–5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tweddle DA, Malcolm AJ, Cole M, Pearson AD, Lunec J. P53 cellular localization and function in neuroblastoma: evidence for defective G(1) arrest despite WAF1 induction in MYCN-amplified cells. Am J Pathol. 2001;158:2067–2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nagai J, Yazawa T, Okudela K, Kigasawa H, Kitamura H, Osaka H.Retinoic acid induces neuroblastoma cell death by inhibiting proteasomal degradation of retinoic acid receptor α. Cancer Res. 2004;64:7910–7917. [DOI] [PubMed] [Google Scholar]
- 39.Mayo LD, Donner DB. The PTEN, Mdm2, p53 tumor suppressor-oncoprotein network. Trends Biochem Sci. 2002;27:462–467. [DOI] [PubMed] [Google Scholar]
- 40.Altucci L, Gronemeyer H. The promise of retinoids to fight against cancer. Nat Rev Cancer. 2001;1:181–193. [DOI] [PubMed] [Google Scholar]
- 41.Freemantle SJ, Spinella MJ, Dmitrovsky E. Retinoids in cancer therapy and chemoprevention: promise meets resistance. Oncogene. 2003;22:7305–7315. [DOI] [PubMed] [Google Scholar]
- 42.Weina K, Utikal J. SOX2 and cancer: current research and its implications in the clinic. Clin Transl Med. 2014;3:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hammerle B, Yanez Y, Palanca S, et al. Targeting neuroblastoma stem cells with retinoic acid and proteasome inhibitor. PLoS ONE. 2013;8: e76761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Das S, Bryan K, Buckley PG, et al. Modulation of neuroblastoma disease pathogenesis by an extensive network of epigenetically regulated microRNAs. Oncogene. 2013;32:2927–2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Joshi S, Guleria R, Pan J, DiPette D, Singh US. Retinoic acid receptors and tissue-transglutaminase mediate short-term effect of retinoic acid on migration and invasion of neuroblastoma SH-SY5Y cells. Oncogene. 2006;25:240–247. [DOI] [PubMed] [Google Scholar]
- 46.Nguyen T, Hocker JE, Thomas W, et al. Combined RAR α- and RXR-specific ligands overcome N-myc-associated retinoid resistance in neuroblastoma cells. Biochem Biophys Res Commun. 2003;302:462–468. [DOI] [PubMed] [Google Scholar]
- 47.Chew JL, Loh YH, Zhang W, et al. Reciprocal transcriptional regulation of Pou5f1 and Sox2 via the Oct4/Sox2 complex in embryonic stem cells. Mol Cell Biol. 2005;25:6031–6046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vilas JM, Ferreiros A, Carneiro C, et al. Transcriptional regulation of Sox2 by the retinoblastoma family of pocket proteins. Oncotarget. 2015;6:2992–3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pugh BF, Tjian R. Mechanism of transcriptional activation by Sp1: evidence for coactivators. Cell. 1990;61:1187–1197. [DOI] [PubMed] [Google Scholar]
- 50.Okazaki M, Iwasaki Y, Nishiyama M, et al. PPARβ/δ regulates the human SIRT1 gene transcription via Sp1. Endocr J. 2010;57:403–413. [DOI] [PubMed] [Google Scholar]
- 51.Zhang JP, Zhang H, Wang HB, et al. Down-regulation of Sp1 suppresses cell proliferation, clonogenicity and the expressions of stem cell markers in nasopharyngeal carcinoma. J Transl Med. 2014;12:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pesce M, Marin Gomez M, Philipsen S, Scholer HR. Binding of Sp1 and Sp3 transcription factors to the Oct-4 gene promoter. Cell Mol Biol (Noisy-le-grand). 1999;45:709–716. [PubMed] [Google Scholar]
- 53.Wu DY, Yao Z. Functional analysis of two Sp1/Sp3 binding sites in murine Nanog gene promoter. Cell Res. 2006;16:319–322. [DOI] [PubMed] [Google Scholar]
- 54.Law AY, Yeung BH, Ching LY, Wong CK. Sp1 is a transcription repressor to stanniocalcin-1 expression in TSA-treated human colon cancer cells, HT29. J Cell Biochem. 2011;112:2089–2096. [DOI] [PubMed] [Google Scholar]
- 55.Zaid A, Hodny Z, Li R, Nelson BD. Sp1 acts as a repressor of the human adenine nucleotide translocase-2 (ANT2) promoter. Eur J Biochem. 2001;268:5497–5503. [DOI] [PubMed] [Google Scholar]
- 56.Kuckenberg P, Buhl S, Woynecki T, et al. The transcription factorTCFAP2C/AP-2γ cooperates with CDX2 to maintain trophectoderm formation. Mol Cell Biol. 2010;30:3310–3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Khozoie C, Borland MG, Zhu B, et al. Analysis of the peroxisome proliferator-activated receptor-β/δ (PPARβ/δ) cistrome reveals novel co-regulatory role of ATF4. BMC Genomics. 2012;13:665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.He CQ, Ding NZ, Fan W. YY1 repressing peroxisome proliferator-activated receptor δ promoter. Mol Cell Biochem. 2008;308:247–252. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.