Abstract
Purpose: People with multiple sclerosis (PwMS) and their family caregivers often react to the impact of the disease as an interdependent dyad. The aim of this exploratory study was to examine interdependence in the physical activity (PA) patterns of dyads affected by moderate to severe MS disability. Method: A total of 15 pairs of PwMS and their family caregivers wore accelerometers for 7 days. By collecting data simultaneously from both partners, we tested interdependence using the dyad as the unit of analysis. Results: PwMS and caregivers averaged 4,091.3 (SD 2,726.3) and 6,160.2 (SD 1,653.0) steps per day, respectively. The mean number of minutes per day of sedentary, light, and moderate to vigorous activity for PwMS was 566.3 (SD 97.7), 167.4 (SD 94.0), and 7.6 (SD 12.4), respectively, and 551.9 (SD 92.4), 199.6 (SD 63.4), and 21.4 (SD 18.2), respectively, for caregivers. Interdependence between dyads for sedentary, light, moderate to vigorous activity, and step count was low and non-significant (rs=0.20, 0.26, 0.13, and –0.27, respectively; p>0.05). Conclusions: Although our findings do not support the interdependence of PA between caregivers and care recipients with MS, they do show that both partners are not engaging in sufficient PA to achieve important health benefits. These findings are important because they indicate that the dyads are likely to benefit from interventions for changing PA behavior.
Key Words: accelerometry, caregivers, multiple sclerosis, exercise
Abstract
Objectif : les personnes atteintes de sclérose en plaques (PaSP) et leur proche aidant de la famille réagissent souvent aux effets de la maladie comme une dyade interdépendante. La présente étude exploratoire visait à examiner les modes d'interdépendance des dyades touchées par une incapacité modérée à grave liée à la SP lorsqu'elles faisaient de l'activité physique (AP). Méthodologie : au total, 15 paires de PaSP et leur proche aidant familial ont porté des accéléromètres pendant sept jours. En amassant des données simultanément auprès des deux partenaires, les chercheurs ont examiné l'interdépendance en faisant de la dyade l'unité d'analyse. Résultats : les PaSP et les proches aidants faisaient une moyenne de 4091,3 (ÉT 2726,3) et 6160,2 (ÉT 1653,0) pas par jour, respectivement. Les PaSP faisaient un nombre moyen de 566,3 (ÉT 97,7), 167,4 (ÉT 94,0) et 7,6 (ÉT 12,4) minutes d'activité sédentaire, légère et modérée à vigoureuse par jour, respectivement, et les proches aidants, 551,9 (ÉT 92,4), 199,6 (ÉT 63,4), et 21,4 (ÉT 18,2) minutes par jour, respectivement. L'interdépendance entre les dyades pour ce qui est de l'activité sédentaire, légère et modérée à vigoureuse et le compte de pas était faible et non significative (r=0,20, 0,26, 0,13 et –0,27, respectivement; p >0,05). Conclusions : même si nos observations n'appuient pas l'interdépendance de l'AP dans la dyade aidant-aidé de SP, elles révèlent toutefois que les deux partenaires ne font pas assez d'AP pour en tirer des avantages importants pour leur santé. Ces observations sont importantes, car elles indiquent que les dyades sont susceptibles de profiter d'interventions pour modifier leur comportement en matière d'AP.
Mots clés : accélérométrie, exercice, proches aidants, sclérose en plaques
Multiple sclerosis (MS) is the leading cause of non-traumatic neurological disability among young adults in Canada:1 Approximately 100,000 people are currently living with the disease,2 and this number is expected to exceed 130,000 by 2031.3 Common features of MS, such as a decline in mobility and cognitive function, present challenges for managing associated life roles, and they have a negative impact on quality of life (QOL).4 Indeed, approximately 30% of people with MS (PwMS) need assistance from family members, usually spouses, to carry out their activities of daily living.5 Although medical advances have improved life expectancy for PwMS,3 the rising prevalence of the disease means that an increasing number of caregivers are providing assistance to PwMS living at home.6
The resulting demand for assistance may affect caregivers' own health. For example, caregivers of PwMS are more likely than the general population to report poor health-related QOL.7 Gupta and colleagues8 further demonstrated that, in comparison with caregivers of people with other chronic neurological conditions such as Alzheimer's disease, caregivers of PwMS experience more limitations on their activities, more emergency department visits, and more hospitalizations. Collectively, these issues can reflect caregiver burden, which has been described as the impact of the caregiving role on the well-being of the caregiver.9 Caregiver burden relates to the severity of symptoms and level of disability of the PwMS as well as the caregiver's coping strategies and the quality of the relationship between the caregiver and care recipient.10 Together, this research has suggested that there is an interdependent, or dyadic, component to the caregiving role, and it points to the need to consider the health of both the PwMS and the caregiver as an interdependent unit rather than the health of each in isolation.
One of the rehabilitation strategies used to manage some of the impact of MS on the health of each partner and on the caregiver–care recipient dyad is increasing participation in physical activity (PA). In the caregiving literature, studies have shown that regular participation in PA can help reduce caregiver burden and improve the quality of sleep and QOL of caregivers of people with chronic health conditions such as Alzheimer's disease and cancer.11,12 Beyond these benefits, caregivers who engage in regular PA are better able to deal with the physical and mental challenges associated with caregiving and thereby delay the institutionalization of their care recipients.13 In the literature specific to PwMS, evidence has demonstrated that the benefits of PA extend to managing the symptoms, slowing the progression of the disease, and improving the QOL of people with the disease.14,15 However, the majority of these studies have restricted the inclusion criteria to PwMS who are independently mobile, excluding individuals with higher disability levels (e.g., with significant walking limitations that require support for gait—Patient Determined Disease Step (PDDS) score of 3–7 or Expanded Disability Status Scale score≥6).16,17 Furthermore, no studies have reported on the potential benefits of including both PwMS and their caregivers in the same PA intervention.
Although the research about dyadic PA behavior in the specific context of MS is very limited, examples of PA-related dyadic research are available for other populations.18–20 These studies have indicated that dyads exhibit similar health-seeking or risky behaviors, and, in particular, the characteristics of the dyad interact to affect PA behavior. For example, in a cross-sectional study, Lopes and colleagues21 reported a positive correlation between best friend dyads for both sedentary behavior and moderate to vigorous PA (MVPA). Similar findings were reported in another study, by Pettee and colleagues,22 involving healthy, older adult, spousal dyads. This evidence was extended by recent longitudinal studies showing that changes in both the absolute level and the trajectories of PA in middle-aged and older adult dyads are concordant over time.23
Current research is lacking, however, on the relationship between the PA behaviors of dyads affected by moderate to severe MS disability. An assessment of patterns of and interactions between dyads' PA would be an important first step in determining the potential utility of dyadic PA interventions in MS. Therefore, the objective of this study was to examine the interdependence between dyadic PA patterns—for example, time spent in sedentary behavior, light-intensity PA (LPA), and MVPA—in dyads affected by moderate to severe MS disability.
Methods
Design
We used an exploratory, descriptive, observational study design.
Participants
We recruited participants from three communities located in a single Canadian province as part of a larger study investigating the development of a dyadic PA intervention in MS. The larger study involved a series of focus groups exploring the shared perspectives on PA of people with moderate to severe MS disability and their family caregivers. We asked dyads who participated in the focus groups to indicate on their consent forms whether they would be willing to participate in the current study. We also recruited dyads outside the groups by advertising in local MS clinics.
The first author (AF) or a trained research assistant screened potential participants for their eligibility to participate in the study. The eligibility criteria are described in Table 1. The study was reviewed and approved by the research ethics board at Queen's University. All eligible participants provided written, informed consent.
Table 1.
Eligibility Criteria
| Inclusion criteria | Exclusion criteria |
| 1. Aged≥18 y* | 1. Severe cognitive deficits (weighted score of <12 on the short version of the Blessed Orientation–Memory–Concentration test)* |
| 2. Self-reported diagnosis of MS† | 2. Other medical conditions that might impair a participant's ability to engage in physical activity* |
| 3. Score between 3 (moderate disability) and 6 (bilateral support required) on the PDDS† | |
| 4. Providing at least 45 min/d of assistance to a PwMS who has a PDDS score of 3–6‡ |
Applies to both PwMS and caregiver.
Applies only to PwMS.
Applies only to family caregivers.
MS=multiple sclerosis; PDDS=Patient Determined Disease Steps; PwMS=person with MS.
Measures and procedures
The Actical accelerometer (Philips Respironics, Bend, OR) measures PA by registering the vibrations that occur during acceleration. The Actical produces a signal that is proportional to the magnitude and duration of the sensed acceleration. This signal is digitally converted into activity counts, which are then summed over a specified time interval (epoch). The device contains an internal processor that provides step count data. The step count function detects vertical movement events, which are then translated into steps accumulated per minute. The Actical accelerometer has established reliability and validity in both the general population and among PwMS.24,25
We measured participants' height (in cm) and weight (in kg) using standard techniques at an in-person orientation meeting. All the participants were then asked to complete a self-report questionnaire, after which time they received an accelerometer and a PA logbook. The questionnaire for PwMS captured background information on each PwMS demographic (age, sex, marital status, education, current employment status) and clinical (type of MS, years since diagnosis, perceived health status) characteristics. The family caregiver questionnaire captured background information on caregivers' demographic characteristics (age, sex, marital status, relationship to the PwMS, education, current employment status), general caregiving (type of support provided, years of support provided), and perceived health status.
We gave the participants verbal, written, and graphical instructions to wear the accelerometer on an elastic band over the non-dominant hip during all waking hours over a 7-day monitoring period. We defined waking hours as the moment participants got out of bed in the morning until the moment they got into bed in the evening. We instructed participants to remove the accelerometer only for sleeping or bathing and to maintain their routine daily activities during the 7-day period. Simultaneously, participants completed the logbook by recording the wear time and the type and duration of activities they performed. Either the first author or the research assistant called the participants every other day to remind them to wear the accelerometer and to troubleshoot any issues that arose with the device. Participants were given a prepaid envelope in which to return the study materials after the 7-day period. Data were collected between April and October 2015.
Data processing and analysis
We downloaded raw data from the accelerometer using the manufacturer's software. The downloaded data were then imported into the Personal Activity Location Measurement System (PALMS; Center for Wireless and Population Health Systems, University of California, San Diego, La Jolla) for data processing. PALMS is a Web-based software application that allows researchers to study the activity patterns of participants in free-living studies. Data were considered spurious if the accelerometer registered 20,000 or more counts per minute or 253 or more steps per minute (maximum number possible according to the manufacturer's specifications). Accelerometer data were visually checked for compliance by comparing the accelerometer wear time against the participant-recorded wear time from the logbook. Non–wear time was defined as a period registering a zero count for at least 60 consecutive minutes. We computed wear time by subtracting non–wear time from 24 hours. A day was considered valid when the data presented with 10 or more hours of wear time with no periods of consecutive zeros exceeding 60 minutes.26
Dyads with at least 3 days of valid data were included in the analysis.26 On the basis of previous research,27,28 an activity count cutoff points of less than 100 counts per minute was classified as sedentary, 100–1,534 counts per minute was classified as LPA, 1,535–3,959 counts per minute was classified as moderate-intensity PA, and 3,960 or more counts per minute was classified as vigorous-intensity PA. It is important to note that activity counts generated by the accelerometer have no real value until they are assigned a level of intensity.29 We calculated step counts and minutes as well as the percentage of wear time per day spent in sedentary behavior, LPA, and MVPA (calculated to adjust for individual wear time).
By collecting data from both partners in the dyad, we used the dyad as the unit of analysis and examined dyadic PA interaction in the context of the actor–partner interdependence model (APIM) developed by Kenny.30 The APIM is a framework for collecting and analyzing dyadic data. It is based on the idea that, in an interacting relationship, an individual's behavior is affected not only by his or her own characteristics (actor effects) but also by the other person's characteristics (partner effects) and the individual's perceptions of that other person. The APIM also provides appropriate statistical techniques for measuring and testing these effects (e.g., hierarchical linear modeling).30
Data analysis was performed using IBM SPSS Statistics for Windows, version 24.0 (IBM Corporation, Armonk, NY). Data were first checked for normality by visually inspecting histograms and normal quantile–quantile plots, then confirmed using the Shapiro–Wilk test. Descriptive statistics (e.g., frequencies, means, and SDs) were obtained for the demographic and clinical variables. Before using the APIM, we tested its assumption of interdependence between distinguishable dyads using Pearson correlations; these correlations provide insight into the degree to which dyad partners' scores on the accelerometer variables are significantly similar (or interdependent). Because our data violated this assumption, it was not advisable to continue using the APIM. Therefore, we had to treat our data as independent samples, using an independent sample t-test for normally distributed data and a Mann–Whitney U-test for data that did not follow a normal distribution. Statistical significance was set at p≤0.05. We calculated effect sizes (ESs) to provide information about the magnitude of the difference between groups. There are various ways to calculate ES;31 because of our study design, we calculated it using Cohen's d analysis (i.e., the difference between the mean scores for two groups divided by the pooled standard deviation). An ES greater than 0.8 is considered large; 0.5–0.8, moderate; and 0.3–0.5, small.32
Results
Participants' characteristics
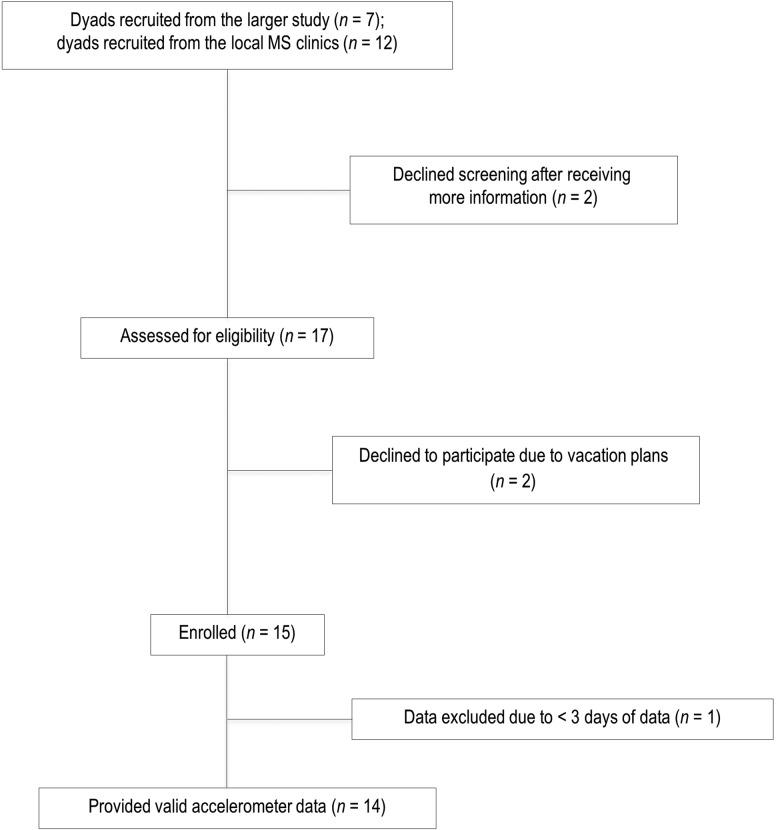
Of the 15 dyads enrolled in the study, 1 was excluded for having less than 3 days of data. Therefore, 14 dyads were retained for the analysis (see the flow diagram presented in Figure 1). Participants' characteristics are presented in Table 2. On average, PwMS were middle-aged women, with a mean age of 52.0 (SD 11.7) years, and with relapsing-remitting MS (42.9%). Their median PDDS score was 5.0 (interquartile range [IQR]=1.0), indicating disability severe enough to require the use of a cane or bilateral support for ambulation. The mean duration of the disease was 13.2 (SD 8.2) years. Caregivers were middle-aged and slightly older, with a mean age of 54.1 (SD 13.5) years and were primarily male spouses (71.4%) who had been providing assistance to a PwMS for a mean of 10.8 (SD 6.8) years.
Figure 1.
Participant flow through study. MS=multiple sclerosis.
Table 2.
Characteristics of the Participants
| No. (%) of participants* |
||
| Variable | PwMS (n=14) | Caregivers (n=14) |
| Mean (SD) age, y | 52.0 (11.7) | 54.1 (13.5) |
| Mean (SD) BMI, kg/m2 | 28.5 (8.5) | 27.6 (4.9) |
| Mean (SD) disease duration, y | 13.2 (8.2) | – |
| Mean (SD) caregiving duration, y | – | 10.8 (6.8) |
| Median PDDS (IQR) | 5.0 (1.0) | – |
| Sex | ||
| Male | 4.0 (28.6) | 10.0 (71.4) |
| Female | 10.0 (71.4) | 4.0 (28.6) |
| Education | ||
| High school or less | 5.0 (35.7) | 6.0 (42.8) |
| College or other | 7.0 (50.0) | 4.0 (28.6) |
| University | 2.0 (14.3) | 4.0 (28.6) |
| Employment | ||
| Employed | 0.0 (0.0) | 7.0 (50.0) |
| Unemployed or stay at home | 9.0 (64.3) | 2.0 (14.3) |
| Retired | 5.0 (35.7) | 5.0 (35.7) |
| Marital status | ||
| Married or common law | 12.0 (85.7) | 14.0 (100.0) |
| Single, divorced, or widowed | 2.0 (14.3) | 0.0 (0.0) |
| Type of MS† | ||
| Relapsing-remitting | 6.0 (42.9) | – |
| Primary progressive | 3.0 (21.4) | – |
| Secondary progressive | 2.0 (14.3) | – |
| Providing assistance with | ||
| Mobility | – | 8.0 (57.1) |
| Shower/bath | – | 2.0 (14.3) |
| Dressing | – | 4.0 (28.6) |
| Meal preparation | – | 12.0 (85.7) |
| Transportation | – | 13.0 (92.8) |
| Negotiating stairs | – | 10.0 (71.4) |
| Getting into or out of bed | – | 3.0 (21.4) |
Note: Dashes indicate not applicable.
Unless otherwise indicated.
Three PwMS did not report the type of MS.
PwMS=people with multiple sclerosis; PDDS=patient-determined disease steps; IQR=interquartile range.
Between-dyad correlations
The results of the Pearson correlations used to test the assumption of interdependence in each of the accelerometer variables indicate that the interdependence between dyads for sedentary behavior, LPA, MVPA, and step count was low and non-significant (rs=0.20, 0.26, 0.13, and –0.27, respectively; p>0.05). As explained previously, using the APIM any further was not advisable because none of the correlations were statistically significant (i.e., the assumption of interdependence between distinguishable dyads was not supported by our data).
Patterns of physical activity
Table 3 presents the means for step count and minutes per day of sedentary behavior, LPA, and MVPA. It also shows the proportion of wear time spent in the three categories of PA, along with the proportion of participants who met the Canadian PA guidelines. Across the 7-day period, caregivers accumulated significantly more steps per day than their care recipients (t26=–2.43; p=0.02), with a large, positive ES (d=0.95). However, no significant differences were found between the groups in the average number of minutes spent in sedentary behavior (t26=–0.40, p=0.69, d=0.16) or LPA (t26=–1.07, p=0.29, d=-0.42). Comparing the average time spent in MVPA showed significant differences between the groups (U=27.50, p=0.001), with a large, positive ES (d=0.92). Between the PwMS and their caregivers, there were no statistically significant associations between accelerometer-derived (i.e., step count, time spent in sedentary behavior, LPA, and MVPA) and sociodemographic variables (i.e., age, sex, marital status, education, and employment).
Table 3.
Step Count, Time Spent in Physical Activity, and Percentage Meeting Physical Activity Guidelines
| Mean (SD) |
|||||
| Category | PwMS | Caregivers | p-value | Effect size | 95% CI |
| Accelerometer variable | |||||
| Step count | 4,091.3 (2,726.3) | 6,160.2 (1,653.0) | 0.022 | −0.95 | −805.6, –803.7 |
| ST, min/d | 566.3 (97.7) | 551.9 (92.4) | 0.69 | 0.16 | −33.8, –34.1 |
| LPA, min/d | 167.4 (94.0) | 199.6 (63.4) | 0.29 | −0.42 | −28.2, –29.0 |
| MVPA, min/d | 7.6 (12.4) | 21.4 (18.2) | <0.001 | 0.92 | −6.8, –4.6 |
| % of day spent in PA | |||||
| ST | 76.4 (13.5) | 71.8 (8.3) | 0.28 | 0.43 | −3.6, –4.4 |
| LPA | 22.8 (12.6) | 25.4 (7.7) | 0.51 | −0.26 | −4.0, –3.5 |
| MVPA | 1.0 (1.6) | 2.8 (2.4) | <0.001 | 0.92 | −1.6, –0.2 |
| Meeting MVPA guidelines | Count (%) | ||||
| Yes | 0.0 (0.0) | 4 (28.6) | |||
| No | 14.0 (100.0) | 10 (71.4) | |||
PwMS=people with multiple sclerosis; ST=sedentary time; LPA=light physical activity; MVPA=moderate to vigorous physical activity; PA=physical activity; MVPA guidelines=≥150 min/wk MVPA.
Overall, this sample of dyads spent approximately 9.3 hours per day (74.1% of wear time) in sedentary behavior, 2.6 hours per day (24.1% of wear time) in LPA, and 13.3 minutes (1.9% of wear time) in MVPA. Only four (28.6%) of the caregivers were meeting Canadian PA guidelines for MVPA (i.e., accumulating≥150 min per week of MVPA). No PwMS accumulated up to 150 minutes per week of MVPA. Although the family caregivers took significantly more steps per day than the PwMS, neither group was meeting the 10,000 steps-per-day recommendation.
Discussion
Our study extends the MS literature by exploring dyadic PA behavior, an important first step in determining the potential utility of incorporating MS caregiver–care recipient dyads into the same PA intervention. To our knowledge, no previous studies have reported the PA patterns of people with moderate to severe MS disability together with those of their family caregivers. Moreover, accelerometers were used to simultaneously measure PA in the dyads, which has never been done before.
Our findings provide new insights in this area by showing that the PA patterns of PwMS were not significantly correlated with those of their caregivers. This finding was unexpected, considering that previous studies have suggested that there is an interrelationship between dyadic PA patterns.20,33 For instance, Anderssen and Wold20 found a moderate correlation between adolescent male dyads (r=0.23) and adolescent female dyads (r=0.31) for leisure-time PA. Recently, Lopes and colleagues21 showed that best friend dyads (aged 13–18 years) were similar in moderate (intra-class correlation [ICC]=0.31) and vigorous (ICC=0.32) PA and sitting time (ICC=0.21). Other researchers have demonstrated a similarity in PA participation between older adult spousal dyads.33 For instance, in the study by Pettee and colleagues,22 an active husband was 2.97 (95% CI: 1.73, 5.10) times more likely to have an active wife. These authors also reported similar results when examining whether the wife's PA status was an important determinant of the husband's PA status (odds ratio=2.48, 95% CI: 1.40, 4.38).
We speculate that the difference in findings between previous studies and this one has at least two possible explanations. First, none of the previous studies included dyads in which one partner was affected by a chronic neurodegenerative condition such as MS. It is possible that disease-specific factors such as the progression of the disability, the unpredictability of symptoms, and a complementary decline in physical capacity34 may have a different effect on the pattern of engagement in PA in PwMS than on caregivers. For instance, caregivers may be more active because of their caregiving responsibilities; thus, it may be difficult for care recipients to engage in the same manner and level of PA as their caregivers. Nevertheless, one study reported insufficient PA in both partners in the stroke caregiver–care recipient dyad but did not measure the correlation in PA pattern.35
Another possible explanation is that previous studies typically measured PA using self-report questionnaires, which can introduce subjectivity bias and require participants to recall past events; both can result in inaccuracy in measurements. The use of accelerometers in this study eliminates the bias associated with self-reporting.
Our results show that PwMS spent about 76% of the wear time (566 min/d) in sedentary behavior and engaged in LPA and MVPA for approximately 23% (167 min/d) and 1% (5 min/d) of wear time, respectively. This finding is consistent with previous studies of PwMS across the disability spectrum.36,37 For instance, Ezeugwu and colleagues36 showed a similar trend in PwMS with mobility disability (i.e., PDDS≥3), who were sedentary for about 533 minutes per day (65% of the wear time) and engaged in LPA for approximately 280 minutes per day (34% of wear time), and MVPA made up only 10 minutes (1%) of their wear time. Recently, Klaren and colleagues37 showed that middle-aged adults (aged 40–59 y) with MS with mild disability (PDDS median=2; IQR=3) spent about 533 minutes per day in sedentary behavior and engaged in LPA for about 288 minutes per day, and MVPA made up only 19 minutes of their day.
Little objective PA measurement in caregivers has been documented. However, older adult caregivers (mean age of 69 years) in the study by Marquez and colleagues13 spent about 260 minutes per day in LPA and 8 minutes per day in MVPA. The authors did not document the time spent in sedentary behavior. Recently, Schulz and colleagues38 measured PA across four time points after cardiac surgery in 28 patients (mean age of 70.7 years) and their caregivers (mean age of 69 years). Across the four time points, the authors reported that the caregivers spent 11–16 minutes per day in MVPA. We observed a similar pattern in our current study, although caregivers in our study spent 5–10 minutes per day in MVPA longer than the caregivers in the studies by Shulz and colleagues and Marquez and colleagues. The minimal difference between these studies may be due to age-related factors because our caregivers were relatively younger (mean age of 54 years). Previous researchers have shown that PA declines with age in the general adult population.39
Although the caregivers in our study engaged in more MVPA than the care recipients, both groups were still far below the Canadian PA guidelines of 150 minutes per week or more of MVPA for the general population. The significant proportion of the day spent in sedentary behavior points to a risk of comorbidity in the dyads. Studies have shown that, regardless of PA status, sedentary behavior is associated with a greater risk of diabetes, high blood pressure, increased blood lipids, and poorer long-term mortality outcomes.40 Therefore, PA interventions that target reducing sedentary behavior and increasing MVPA may be particularly important for dyads affected by moderate to severe MS disability; this finding has been supported by previous researchers.37
Similar to previous research involving Canadian adults,41 participants in this study did not regularly meet the recommendations of 10,000 steps per day. Using the step count classification system for the general population proposed by Tudor-Locke and Bassett,42 the PwMS would be classified as sedentary, and their caregivers would be classified as low active. The number of steps accumulated by the PwMS in this study is consistent with previous research reporting an average step count of about 5,000 steps per day, with higher disability resulting in fewer steps.43 Although few studies have documented the step count of caregivers of people with chronic disease, our findings are consistent with research by Zalewski and Dvorak,35 who reported that caregivers of people with stroke accumulate a mean of 6,378 (SD 2,149) steps per day.
The significant difference in step count between PwMS and their caregivers suggests that dyadic PA interventions may need to incorporate different strategies to enhance care recipient and caregiver PA within the time constraints of caregiving responsibilities. Other researchers have suggested that shorter, more frequent bouts of activity may be more feasible for caregivers to accomplish given their time constraints.13 Educating MS dyads about the health benefits and methods of reallocating sedentary time to alternative activities (e.g., LPA and MVPA) may be an additional strategy for increasing PA participation in these groups; this idea is supported by previous studies.44–47 For instance, Hamer and colleagues46 reported that replacing sedentary time with an equal amount of MVPA was associated with favorable effects on the risk factors for cardiovascular disease. Other researchers have shown that replacing sedentary time with equivalent amounts of LPA is associated with an improvement in physical health and well-being among healthy middle-aged and older adults as well as individuals with chronic health conditions.47–50
The health benefits of reallocating sedentary time to LPA suggests that targeting an increase in at least LPA in PwMS and their caregivers may be a practical and achievable way to induce change in PA behaviors rather than seeking to increase the levels of MVPA, which may be more challenging. This body of evidence, together with the current findings, underscores the need for PA interventions that are tailored to the population affected by moderate to severe MS disability.
This study has limitations that warrant consideration. First, we used the same cutoff points for both PwMS and caregivers. Evidence has suggested that PwMS expend more energy than healthy controls despite similar activity counts.51 Therefore, the published cutoff points for interpreting the Actical outputs for healthy individuals may not be appropriate for PwMS. Although MS-specific activity cutoff points have been established for other accelerometer types (e.g., ActiGraph), no cutoff points have been published for the Actical among PwMS, and this may be an interesting avenue for future research. It is also possible that the step count classification system proposed by Tudor-Locke and Bassett42 for the general population may not be appropriate for the population with MS because it may increase the risk of incorrectly classifying the PA level of PwMS.
Second, our participants were recruited from three small urban areas in a single Canadian province, and this design limits the generalizability of our findings. Moreover, it is possible that regional or geographical differences in the services available to support PA participation may influence PA patterns. Future researchers may want to explore the differences in PA between caregiver–care recipient dyads living in major metropolitan cities versus those who live in smaller areas.
A third limitation is that our sample consisted of middle-aged to older adults, and our results may not represent young people with moderate to severe MS disability. Finally, our small sample size made it difficult to test actor–partner influences on PA behavior using the APIM. Future studies with larger samples may be interested in exploring specific caregiver–care recipient characteristics that interact to affect dyadic PA behavior.
Conclusion
Despite these limitations, the current study is the first, to our knowledge, to examine PA patterns in dyads of care recipients with MS and their caregivers. Although our data do not support the interdependence of PA within the dyads, our findings do show that both partners are not engaging in sufficient PA to achieve important health benefits. These findings are important because they indicate that these dyads could benefit from interventions to change PA behaviors.
Key Messages
What is already known on this topic
Physical activity (PA) has emerged as an alternative behavioral approach for managing the consequences of multiple sclerosis (MS). However, the PA behavior of caregiver–care recipient dyads in which the care recipient has moderate to severe MS disability has not yet been investigated.
What this study adds
This study shows that the PA patterns of people with MS were not significantly correlated with those of their caregivers. Nevertheless, both care recipients and caregivers were far below the recommended level of PA needed to achieve important health benefits. Clinicians and researchers need to begin to adopt an integrative approach to focusing on caregiver–care recipient dyads affected by moderate to severe MS for promoting PA interventions. Interventions may need to incorporate different strategies to enhance care recipient and caregiver PA within the time constraints of caregiving responsibilities.
References
- 1. Spring J, Beauregard N, Vorobeychik G.. Multiple sclerosis: myths and realities. B C Med J. 2006;48(2):72–5 [Google Scholar]
- 2. Multiple Sclerosis International Foundation. Atlas of MS 2013: Mapping multiple sclerosis around the world. London: Multiple Sclerosis International Foundation; 2013. [Google Scholar]
- 3. Amankwah N, Marrie RA, Bancej C, et al. [Multiple sclerosis in Canada 2011 to 2031: results of a microsimulation modelling study of epidemiological and economic impacts.] Health Promot Chronic Dis Prev Can. 2017;37(2):37–48. 10.24095/hpcdp.37.2.02. Medline:28273039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yorkston KM, Johnson KL, Klasner ER.. Taking part in life: enhancing participation in multiple sclerosis. Phys Med Rehabil Clin N Am. 2005;16(2):583–94. 10.1016/j.pmr.2005.01.003. Medline:15893688 [DOI] [PubMed] [Google Scholar]
- 5. Holland NJ, Northrop DE.. Young adults with multiple sclerosis: management in the home. Home Health Care Manage Pract. 2006;18(3):186–95. 10.1177/1084822305281952 [DOI] [Google Scholar]
- 6. Hurwitz BJ. The diagnosis of multiple sclerosis and the clinical subtypes. Ann Indian Acad Neurol. 2009;12(4):226–30. 10.4103/0972-2327.58276. Medline:20182569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Patti F, Amato MP, Battaglia MA, et al. Caregiver quality of life in multiple sclerosis: a multicentre Italian study. Mult Scler. 2007;13(3):412–9. 10.1177/1352458506070707. Medline:17439911 [DOI] [PubMed] [Google Scholar]
- 8. Gupta S, Goren A, Phillips AL, et al. Self-reported burden among caregivers of patients with multiple sclerosis. Int J MS Care. 2012;14(4):179–87. 10.7224/1537-2073-14.4.179. Medline:24453750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Forbes A, While A, Mathes L.. Informal carer activities, carer burden and health status in multiple sclerosis. Clin Rehabil. 2007;21(6):563–75. 10.1177/0269215507075035. Medline:17613586 [DOI] [PubMed] [Google Scholar]
- 10. Pakenham KI. Relations between coping and positive and negative outcomes in carers of persons with multiple sclerosis (MS). J Clin Psychol Med Settings. 2005;12(1):25–38. 10.1007/s10880-005-0910-3 [DOI] [Google Scholar]
- 11. King AC, Baumann K, O'Sullivan P, et al. Effects of moderate-intensity exercise on physiological, behavioral, and emotional responses to family caregiving: a randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2002;57(1):M26–36. 10.1093/gerona/57.1.M26. Medline:11773209 [DOI] [PubMed] [Google Scholar]
- 12. Orgeta V, Miranda-Castillo C.. Does physical activity reduce burden in carers of people with dementia? A literature review. Int J Geriatr Psychiatry. 2014;29(8):771–83. 10.1002/gps.4060. Medline:25191688 [DOI] [PubMed] [Google Scholar]
- 13. Marquez DX, Bustamante EE, Kozey-Keadle S, et al. Physical activity and psychosocial and mental health of older caregivers and non-caregivers. Geriatr Nurs. 2012;33(5):358–65. 10.1016/j.gerinurse.2012.03.003. Medline:22595335 [DOI] [PubMed] [Google Scholar]
- 14. Dalgas U, Stenager E.. Exercise and disease progression in multiple sclerosis: can exercise slow down the progression of multiple sclerosis? Ther Adv Neurol Disorder. 2012;5(2):81–95. 10.1177/1756285611430719. Medline:22435073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Latimer-Cheung AE, Pilutti LA, Hicks AL, et al. Effects of exercise training on fitness, mobility, fatigue, and health-related quality of life among adults with multiple sclerosis: a systematic review to inform guideline development. Arch Phys Med Rehabil . 2013;94(9):1800–1828.e3. 10.1016/j.apmr.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 16. Toomey E, Coote SB.. Physical rehabilitation interventions in nonambulatory people with multiple sclerosis: a systematic review. Int J Rehabil Res. 2012;35(4):281–91. 10.1097/MRR.0b013e32835a241a. Medline:23060086 [DOI] [PubMed] [Google Scholar]
- 17. Motl RW, Learmonth YC, Pilutti LA, et al. Top 10 research questions related to physical activity and multiple sclerosis. Res Q Exerc Sport. 2015;86(2):117–29. 10.1080/02701367.2015.1023099. Medline:25874730 [DOI] [PubMed] [Google Scholar]
- 18. Prick A-E, de Lange J, Scherder E, et al. The effects of a multicomponent dyadic intervention with physical exercise on the cognitive functioning of people with dementia: a randomized controlled trial. J Aging Phys Act. 2017;25(4):539–52. 10.1123/japa.2016-0038. Medline:28120631 [DOI] [PubMed] [Google Scholar]
- 19. Ellis KR, Janevic MR, Kershaw T, et al. Engagement in health-promoting behaviors and patient-caregiver interdependence in dyads facing advanced cancer: an exploratory study. J Behav Med. 2017;40(3):506–19. 10.1007/s10865-016-9819-6. Medline:28078502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Anderssen N, Wold B.. Parental and peer influences on leisure-time physical activity in young adolescents. Res Q Exerc Sport. 1992;63(4):341–8. 10.1080/02701367.1992.10608754. Medline:1439157 [DOI] [PubMed] [Google Scholar]
- 21. Lopes VP, Gabbard C, Rodrigues LP.. Physical activity in adolescents: examining influence of the best friend dyad. J Adolesc Health. 2013;52(6):752–6. 10.1016/j.jadohealth.2012.12.004. Medline:23360898 [DOI] [PubMed] [Google Scholar]
- 22. Pettee KK, Brach JS, Kriska AM, et al. Influence of marital status on physical activity levels among older adults. Med Sci Sports Exerc. 2006;38(3):541–6. 10.1249/01.mss.0000191346.95244.f7. Medline:16540843 [DOI] [PubMed] [Google Scholar]
- 23. Li KK, Cardinal BJ, Acock AC.. Concordance of physical activity trajectories among middle-aged and older married couples: impact of diseases and functional difficulties. J Gerontol B Psychol Sci Soc Sci. 2013;68(5):794–806. 10.1093/geronb/gbt068. Medline:23873967 [DOI] [PubMed] [Google Scholar]
- 24. Crouter SE, Dellavalle DM, Horton M, et al. Validity of the Actical for estimating free-living physical activity. Eur J Appl Physiol. 2011;111(7):1381–9. 10.1007/s00421-010-1758-2. Medline:21153659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kayes NM, Schluter PJ, McPherson KM, et al. Exploring Actical accelerometers as an objective measure of physical activity in people with multiple sclerosis. Arch Phys Med Rehabil. 2009;90(4):594–601. 10.1016/j.apmr.2008.10.012. Medline:19345774 [DOI] [PubMed] [Google Scholar]
- 26. Colley R, Connor Gorber S, Tremblay MS.. Quality control and data reduction procedures for accelerometry-derived measures of physical activity. Health Rep. 2010;21(1):63–9. Medline:20426228 [PubMed] [Google Scholar]
- 27. Colley RC, Tremblay MS.. Moderate and vigorous physical activity intensity cut-points for the Actical accelerometer. J Sports Sci. 2011;29(8):783–9. 10.1080/02640414.2011.557744. Medline:21424979 [DOI] [PubMed] [Google Scholar]
- 28. Wong SL, Colley R, Connor Gorber S, et al. Actical accelerometer sedentary activity thresholds for adults. J Phys Act Health. 2011;8(4):587–91. 10.1123/jpah.8.4.587. Medline:21597132 [DOI] [PubMed] [Google Scholar]
- 29. Reilly JJ, Penpraze V, Hislop J, et al. Objective measurement of physical activity and sedentary behaviour: review with new data. Arch Dis Child. 2008;93(7):614–9. 10.1136/adc.2007.133272. Medline:18305072 [DOI] [PubMed] [Google Scholar]
- 30. Kenny DA. Models of non-independence in dyadic research. J Soc Pers Relat. 1996;13(2):279–94. 10.1177/0265407596132007 [DOI] [Google Scholar]
- 31. Durlak JA. How to select, calculate, and interpret effect sizes. J Pediatr Psychol. 2009;34(9):917–28. 10.1093/jpepsy/jsp004. Medline:19223279 [DOI] [PubMed] [Google Scholar]
- 32. Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed London: Routledge Academic; 2013. [Google Scholar]
- 33. Satariano WA, Haight TJ, Tager IB.. Living arrangements and participation in leisure-time physical activities in an older population. J Aging Health. 2002;14(4):427–51. 10.1177/089826402237177. Medline:12391994 [DOI] [PubMed] [Google Scholar]
- 34. Goldman MD, Marrie RA, Cohen JA.. Evaluation of the six-minute walk in multiple sclerosis subjects and healthy controls. Mult Scler. 2008;14(3):383–90. 10.1177/1352458507082607. Medline:17942508 [DOI] [PubMed] [Google Scholar]
- 35. Zalewski KR, Dvorak L.. Barriers to physical activity between adults with stroke and their care partners. Top Stroke Rehabil. 2011;18(Suppl 1):666–75. 10.1310/tsr18s01-666. Medline:22120035 [DOI] [PubMed] [Google Scholar]
- 36. Ezeugwu V, Klaren REA, Hubbard EA, et al. Mobility disability and the pattern of accelerometer-derived sedentary and physical activity behaviors in people with multiple sclerosis. Prev Med Rep. 2015;2:241–6. 10.1016/j.pmedr.2015.03.007. Medline:26844077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Klaren RE, Sebastiao E, Chiu C-Y, et al. Levels and rates of physical activity in older adults with multiple sclerosis. Aging Dis. 2016;7(3):278–84. Medline:27330842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schulz P, Zimmerman L, Johansson P, et al. Physical activity patterns in rural-residing spousal caregivers and cardiac surgery patients in the first 6 months post-surgery. Online J Rural Nurs Health Care. 2014;14(2):123–44. 10.14574/ojrnhc.v14i2.330 [DOI] [Google Scholar]
- 39. Ingram DK. Age-related decline in physical activity: generalization to nonhumans. Med Sci Sports Exerc. 2000;32(9):1623–9. 10.1097/00005768-200009000-00016. Medline:10994915 [DOI] [PubMed] [Google Scholar]
- 40. Katzmarzyk PT, Church TS, Craig CL, et al. Sitting time and mortality from all causes, cardiovascular disease, and cancer. Med Sci Sports Exerc. 2009;41(5):998–1005. 10.1249/MSS.0b013e3181930355. Medline:19346988 [DOI] [PubMed] [Google Scholar]
- 41. Colley RC, Garriguet D, Janssen I, et al. Physical activity of Canadian adults: accelerometer results from the 2007 to 2009 Canadian Health Measures Survey. Ottawa: Statistics Canada; 2011. [PubMed] [Google Scholar]
- 42. Tudor-Locke C, Bassett DR Jr.. How many steps/day are enough? Preliminary pedometer indices for public health. Sports Med. 2004;34(1):1–8. 10.2165/00007256-200434010-00001. Medline:14715035 [DOI] [PubMed] [Google Scholar]
- 43. Neven A, Vanderstraeten A, Janssens D, et al. Understanding walking activity in multiple sclerosis: step count, walking intensity and uninterrupted walking activity duration related to degree of disability. Neurol Sci. 2016;37(9):1483–90. 10.1007/s10072-016-2609-7. Medline:27207680 [DOI] [PubMed] [Google Scholar]
- 44. Mekary RA, Willett WC, Hu FB, et al. Isotemporal substitution paradigm for physical activity epidemiology and weight change. Am J Epidemiol. 2009;170(4):519–27. 10.1093/aje/kwp163. Medline:19584129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Buman MP, Winkler EA, Kurka JM, et al. Reallocating time to sleep, sedentary behaviors, or active behaviors: associations with cardiovascular disease risk biomarkers, NHANES 2005-2006. Am J Epidemiol. 2014;179(3):323–34. 10.1093/aje/kwt292. Medline:24318278 [DOI] [PubMed] [Google Scholar]
- 46. Hamer M, Stamatakis E, Steptoe A.. Effects of substituting sedentary time with physical activity on metabolic risk. Med Sci Sports Exerc. 2014;46(10):1946–50. 10.1249/MSS.0000000000000317. Medline:24674977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ekblom-Bak E, Ekblom Ö, Bergström G, et al. Isotemporal substitution of sedentary time by physical activity of different intensities and bout lengths, and its associations with metabolic risk. Eur J Prev Cardiol. 2016;23(9):967–74. 10.1177/2047487315619734. Medline:26635358 [DOI] [PubMed] [Google Scholar]
- 48. Buman MP, Hekler EB, Haskell WL, et al. Objective light-intensity physical activity associations with rated health in older adults. Am J Epidemiol. 2010;172(10):1155–65. 10.1093/aje/kwq249. Medline:20843864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. van Roekel EH, Bours MJ, Breedveld-Peters JJ, et al. Modeling how substitution of sedentary behavior with standing or physical activity is associated with health-related quality of life in colorectal cancer survivors. Cancer Causes Control. 2016;27(4):513–25. 10.1007/s10552-016-0725-6. Medline:26892604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Balboa-Castillo T, León-Muñoz LM, Graciani A, et al. Longitudinal association of physical activity and sedentary behavior during leisure time with health-related quality of life in community-dwelling older adults. Health Qual Life Outcomes. 2011;9(1):47–57. 10.1186/1477-7525-9-47. Medline:21708011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sandroff BM, Motl RW, Suh Y.. Accelerometer output and its association with energy expenditure in persons with multiple sclerosis. J Rehabil Res Dev. 2012;49(3):467–75. 10.1682/JRRD.2011.03.0063. Medline:22773205 [DOI] [PubMed] [Google Scholar]