Abstract
PIZZ alpha-1 antitrypsin deficiency (AATD) is an autosomal recessive disease affecting approximately 100,000 individuals in the United States and one of the most common hereditary causes of liver disease.1 The most common form of the disease results from a single base pair mutation (Glu342Lys), known as the “Z” mutation, that encodes a mutant protein (Z alpha-1 antritypsin [AAT]) that is prone to misfolding and is retained in the endoplasmic reticulum (ER) rather than appropriately secreted. Some of the retained mutant protein attains an unusual aggregated or polymerized conformation. Retained polymeric ZAAT aggregates are hepatotoxic and lead to downstream liver disease in a subset of PiZZ neonates and adults through a gain-of-function mechanism. PiZZ individuals are likewise highly predisposed to developing chronic obstructive pulmonary disease (COPD)/emphysema as a result of low circulating levels of AAT protein and associated protease-antiprotease imbalance. Much of our understanding of the molecular pathogenesis of AATD is based on studies employing either transgenic mice that express the mutant human Z allele or immortalized cell lines transduced to overexpress ZAAT. While they have been quite informative, these models fail to capture the patient-to-patient variability in disease phenotype that clinicians observe in their AATD patients, raising the question of whether alternative models might provide new insight. Induced pluripotent stem cells (iPSCs), first described in 2006, have the capacity to differentiate into a broad array of cell types from all 3 germ layers, including hepatocytes. Disease-specific iPSCs have been derived from patients with a variety of monogenic disorders and have been found to faithfully recapitulate features of such diseases as spinal muscular atrophy, familial dysautonomia, Rett syndrome, polycythemia vera, type 1A glycogen storage disease, familial hypercholesterolemia, long QT syndrome, and others. This discussion reviews the potential applications of iPSCs for understanding AATD-associated liver disease as well as for development of potential therapeutic strategies.
Keywords: alpha-1 antitrypsin deficiency, induced pluripotent stem cells, protein misfolding
Directed Differentiation of Induced Pluripotent Stem Cells to the Hepatic Lineage
The ability to isolate and culture human pluripotent stem cells, first with embryonic stem cells in the late 1990s and subsequently with induced pluripotent stem cells, has provided researchers with a tool to derive human hepatic cells in vitro via directed differentiation, an approach in which cells are sequentially exposed to developmental stage-appropriate signals to guide differentiation. The efficiency of directed differentiation protocols has substantially improved over time through the application of knowledge derived from developmental biology.1 For example, culture of undifferentiated pluripotent stem cells in the presence of TGF beta superfamily member and nodal analogue, Activin A, exposes cells to signals they would encounter in the anterior primitive streak of a gastrulating embryo thus inducing their differentiation to definitive endoderm, the germ layer from which hepatocytes as well as lung, thyroid, intestine, and pancreas are derived.2 The hepatic lineage is then specified via exposure of definitive endodermal cells to high levels of fibroblast growth factor and bone morphogenetic protein , resulting in the emergence of hepatoblasts that co-express alfa-fetoprotein (AFP) and albumin and serve as precursors for hepatocytes and biliary epithelial cells.3,4 Further exposure in culture to hepatocyte growth factor, dexamethasone, and oncostatin M then promotes hepatocyte maturation.5,6 As has been the case with other directed differentiation protocols, recent evidence has suggested that culture in 3-dimensional conditions likewise promotes the maturation of hepatocytes, resulting in increased expression of genes associated with liver function and a subset of cytochrome p450 genes together with increased expression of the asialo-glycoprotein protein receptor 1, a cell surface marker expressed by mature hepatocytes.6
Despite these advances, hepatic differentiation protocols have not been successful in reproducibly achieving levels of maturation comparable to adult primary hepatocytes, a challenge that has been broadly observed in pluripotent stem cell (PSC)- directed differentiation protocols.7 Limitations in hepatic differentiation protocols are illustrated both by the continued expression of immature markers, such as AFP, together with limited induction of Phase 1 and Phase 2 enzymes vital for drug metabolism. In addition, differences in the primary cells chosen for comparison has made it difficult to compare published protocols from different labs, resulting in uncertainty in their relative efficacy. The significance of these shortcomings with respect to the application of patient iPSC-hepatic cells to disease modeling, drug screens, or cell-based therapies is unclear but may vary based on the developmental stage at which hepatocytes acquire specific biological functions.
Known Features of Alpha-1 Antitrypsin Deficiency-Associated Liver Disease
Careful work over a period of decades has resulted in a body of work describing the cellular and systemic consequences of protein misfolding arising from the pathogenic Z mutation. Our understanding of these consequences is outlined in the paragraphs that follow.
Polymerization and Accumulation of Misfolded Protein
Work performed in the early 1990s demonstrated that misfolded ZAAT molecules can polymerize within the ER via a mechanism referred to as loop-sheet polymerization7 wherein the reactive center loop of one ZAAT monomer can insert into the A-sheet of an adjacent monomer resulting in non-covalent binding. The misfolding of proteins and their predisposition to polymerization result in a variety of downstream consequences that together form the basis for the clinical disease.8 Polymerized ZAAT accumulates within hepatocytes of PiZZ patients and is poorly secreted. Retained ZAAT polymers form large inclusions, referred to as globules that occur within the ER of subsets of hepatocytes in affected individuals.7 These globules, likewise found in the PiZ transgenic mouse model of ZAAT-liver disease, stain positive with periodic acid Schiff (PAS) and are resistant to digestion with diastase, a pattern that differentiates them from other protein aggregates. ZAAT inclusions perturb the ER architecture and are physically segregated into compartments separate from the rest of the ER network that can nonetheless communicate with other inclusions and with the ER network itself.9 For reasons that are poorly understood, globules are not homogenously distributed and are found in only a subset of PiZZ patient hepatocytes.10 Studies in PiZ mice have demonstrated that hepatocytes lacking globules exhibit a proliferative advantage that varies with the relative number of globule-containing cells, leading to speculation that signaling between globule-containing and globule-devoid cells contributes to liver disease pathogenesis.10,11
Aberrant Protein Glycosylation and Secretion
Newly synthesized AAT protein is secreted into the rough ER where it is post-translationally modified prior to secretion. Steps in this process include cleavage of its signal peptide together with glycosylation at 3 distinct asparagine residues (numbers 46, 83, and 247) as the protein takes on a 3-dimensional conformation.12 Following additional trimming of glucose units to produce a monoglycosylated oligosaccharide, 13-16 mature AAT glycoproteins are released from the protein folding machinery and transported to the Golgi where terminal modification of the carbohydrate side chains occurs prior to secretion.12 While post-translational modifications and secretion of normally folded M protein occur very rapidly, glycosylation is altered and the overall kinetics of both glycosylation and secretion is significantly slowed for ZAAT protein.17-20 These processes have biological consequences as aberrant glycosylation has been associated with the proinflammatory properties of Z protein17 while delayed secretion is associated with the injurious intracellular accumulation of ZAAT polymers.
Autophagic Flux
Misfolded ZAAT not secreted or removed by ER-associated degradation (ERAD) accumulates in the ER and forms insoluble polymers that cannot be degraded proteasomally. ZAAT polymers, like other aggregated proteins, are degraded via an alternative degradation mechanism known as autophagy. Autophagy, commonly used to refer to the more specific term macroautophagy, is a homeostatic process of intracellular degradation in which subcellular structures are enveloped and degrade in double-membraned vacuoles known as autophagosomes.21 Autophagosomes fuse with lysosomes resulting in the degradation of their contents that can then be repurposed by the cell. Autophagy is best quantified through measurement of pathway activity in the cell that can be estimated via the relationship between autophagosome production and clearance, together known as autophagic flux.22 While flux is challenging to measure in human liver specimens, the formation of autophagic vacuoles is significantly increased in liver samples of PiZZ individuals, consistent with increased pathway activity. Autophagic activity quantified by flux has been documented in multiple cell-based models, including patient iPSC-hepatic cells, as well as in the PiZ mouse model of AATD liver disease.5,23-27
Endoplasmic Reticulum Stress Response
Expression of ZAAT protein in cell models is associated with activation of signaling pathways believed to be related to liver disease pathogenesis. One such pathway is the ER overload response (EOR) that been described in multiple ZAAT expression systems and that is characterized by induction of the transcription factor NF-κB.18,28 However, NF-κB activation is non-specific and can also indicate activation of a distinct signaling pathway known as the unfolded protein response (UPR). The UPR, activated in cells in response to cellular stresses such as accumulated misfolded proteins, involves global down-regulation of translation with associated up-regulation of genes that increase the ER protein folding capacity or degradative capacity via the ERAD pathway.29 Discordant findings have characterized the literature regarding the presence or absence of UPR activation in the setting of AATD, with activation not observed in primary mouse liver cells or immortalized human or mouse cells18 but found to occur in primary human peripheral blood monocytes.28 The reason for this discordance is uncertain but could be consistent with context specificity that varies with species, cell type, or developmental stage.
Human-Induced Pluripotent Stem Cells Faithfully Model the Known Features of Alpha-1 Antitrypsin Deficiency-Associated Liver Disease
Polymerization and Accumulation of Misfolded Protein
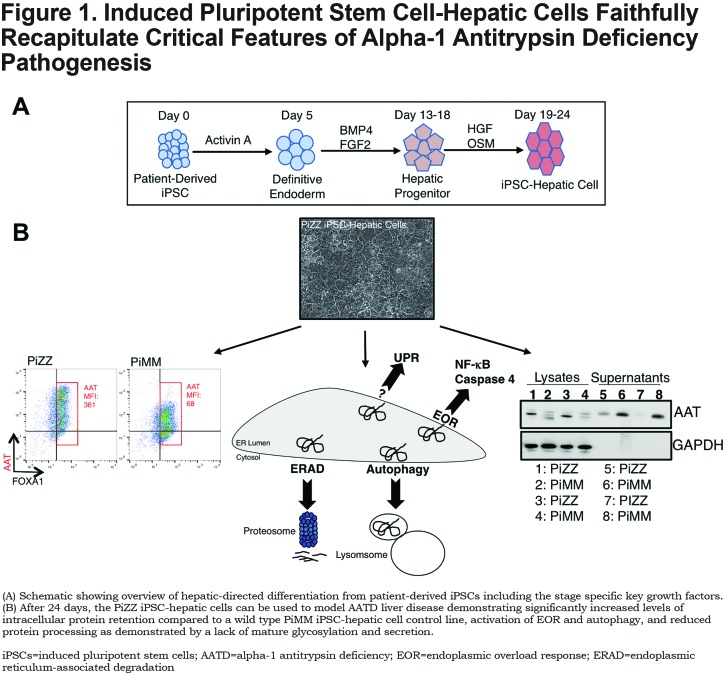
Work by our lab and others has demonstrated that AATD patient-derived iPSC-hepatic cells faithfully recapitulate features of a variety of metabolic liver diseases, including most of the known features of ZAAT-associated hepatocellular toxicity, as outlined in Figure 1. SERPINA1 is expressed in differentiating iPSCs upon reaching the hepatic progenitor stage with levels of expression increasing as cells mature further.5 In differentiating iPSCs derived from PiZZ patients homozygous for the Z allele, ZAAT protein accumulates intracellularly and forms polymers consistent with observations in the PiZZ human and PiZ mouse livers.5,30 Aggregated ZAAT likewise accumulates to form globular inclusions in PiZZ patient iPSC-hepatic cells that are localized predominantly within the rough endoplasmic reticulum.31 PiZZ patient iPSC-hepatic cell globules stain positive with PAS and are resistant to digestion with diastase as is the case in other model systems.32 In a limited number of iPSC lines derived from patients with severe liver disease, globular inclusions were identified in approximately 25% of iPSC-hepatic cells, a percentage greater than commonly observed in patient biopsy samples but consistent with their emergence in only a minority of hepatic cells even within diseased individuals.31
Aberrant Protein Glycosylation and Secretion
The kinetics of post-translational modifications of AAT protein can be quantified via the classical pulse-chase radiolabeling assay, in which nascent peptides are labeled with radioactive amino acids prior to immunoprecipitation and separation based on size.33 Glycosylated AAT protein is approximately 3kDa larger than the newly synthesized peptide, allowing for discrimination between glycosylated and non-glycosylated species. The delay in this process known to occur in ZAAT-expressing hepatocytes and the associated delay in ZAAT secretion has likewise been observed in PiZZ patient iPSC-hepatic cells by 2 groups of investigators.5,31 Evidence from these groups likewise demonstrates that the observed alterations in AAT processing and secretion are resolved with correction of the single base pair Z mutation in patient cells and that patient-to-patient differences in disease phenotype appear to be recapitulated in this model. Together, these findings underscore the potential utility of patient iPSCs as predictive and therapeutic tools for the AATD patient population. Further studies are needed to determine whether iPSC-derived cells recapitulate differences in fucosylation described in ZAAT relative to MAAT protein in patient blood samples.17
Autophagic Flux
As discussed above, ZAAT-expressing hepatocytes are unable to degrade polymeric ZAAT via the proteosomal degradation pathway but can do so at least in part via the autophagy pathway. The potential significance of this pathway for therapeutic manipulation was demonstrated in PiZ mice by Perlmutter and colleagues via the demonstration that the anti-seizure medication Carbamazepine, a member of a class of drugs that induce the autophagy pathway, reduced the number of hepatic PAS positive, diastase-resistant globules and likewise diminished hepatic fibrosis, a clinically significant downstream consequence of ZAAT proteotoxicity.34 Similar to observations made previously in mouse models and human cells, our group has found that autophagic flux is increased in PiZZ patient iPSC-hepatic cells relative to those differentiated from wild-type (WT) controls.5 Furthermore, this enhanced flux was further augmented in PiZZ but not WT iPSC-hepatic cells treated with carbamazepine and was associated with a reduction in intracellular AAT but not other hepatic proteins, such as AFP. These findings are in accord with other published data demonstrating reduced AAT immunofluorescent staining in iPSC-hepatic cells treated with carbamazepine.32
Endoplasmic Reticulum Stress Response
Accumulated ZAAT protein activates signaling pathways that have been overlapping but distinct in various models used to study AATD. To understand the downstream transcriptomic consequences of ZAAT production, we performed microarrays on PiZZ patient-derived iPSCs compared to WT iPSCs and embryonic stem cells during hepatic-directed differentiation.5 We found that there were no statistically significant differences in gene expression among the groups until reaching the hepatic stage of differentiation when AAT protein is expressed. To identify a transcriptomic signature of disease, we evaluated gene expression differences that emerged with differentiation between WT and PiZZ iPSCs and found a set of genes differentially expressed between these 2 groups upon reaching the hepatic stage. The second most differentially expressed gene on this list was caspase-4, the ER stress-specific caspase believed to be a homolog of caspase-12 in mice.35 We did not detect markers of UPR activation in our disease-specific signature, consistent with other models of hepatic ZAAT-associated signaling.18 Earlier stage liver progenitor cells evaluated by qPCR and western blot, however, did demonstrate up-regulation of ATF4 and sXBP-1 mRNA and increased sXBP-1 and Grp-78/BiP protein together with reduced IκBα protein, all consistent with UPR activation at this stage. Together, these results suggest that ZAAT expression activates the UPR in a stage/context-specific manner, consistent with the variability demonstrated in other models.
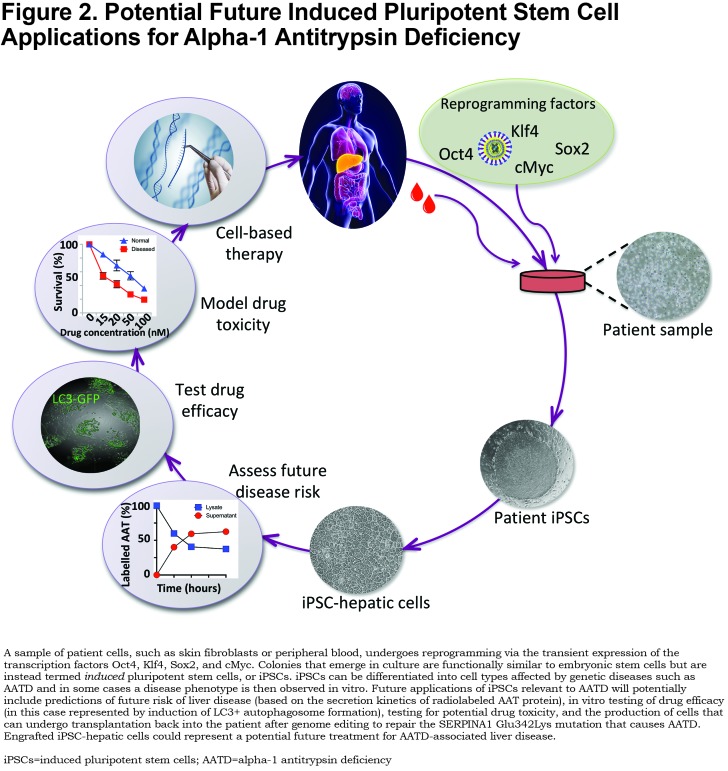
Future Directions for Induced Pluripotent Stem Cell-Based Studies of Alpha-1 Antitrypsin Deficiency
There are a variety of key, unanswered questions that iPSC-based studies may be well-suited to address, partially summarized in Figure 2. Our understanding of AATD disease pathogenesis remains incomplete. In addition to the established features of AATD-associated liver disease enumerated above, recent literature has suggested additional mechanisms through which ZAAT proteotoxicity may perturb hepatocellular function and thereby lead to clinical disease. For instance, regional heterogeneity of hepatocellular phenotype, referred to as zonation,36,37 has been found to be perturbed in liver tissue of PiZ mice and PiZZ patients and is associated with a defect in ureagenesis.38 The significance of these findings for patients has yet to be elucidated and represents an area where iPSCs could provide validation and potentially further our understanding of a potentially significant disease mechanism.
Ira Fox and colleagues demonstrated differences in the secretion kinetics of ZAAT that varied between PiZZ individuals with and without known liver disease.31 This work was in accord with studies from this group 2 decades earlier featuring primary skin fibroblasts that together suggest the presence of genetic cofactors that influence the kinetics of ZAAT secretion and may thereby confer risk of developing liver disease.20 If confirmed, these findings suggest the possibility that patient iPSCs could be used to predict an individual’s future risk of developing liver disease, an exciting proposition since there is currently no way to know which PiZZ patients are likely to develop this complication. The prospect that patient-derived iPSCs serve as genetic surrogates for patients themselves likewise supports potential cell-based in vitro clinical trials that could serve as an adjunct to traditional clinical studies. To be successful, “clinical trials in a dish” would require curated pools of iPSCs harboring genetic diversity representative of the applicable patient population.39 Whether differences in the drug responsiveness of iPSC-derived cell types such as hepatocytes predict the response of an individual patient’s hepatocytes in vivo is an important question that will require head-to-head testing of cell types, likely in the context of a clinical trial.
Similarly, published work has suggested that PiZZ iPSC-hepatic cells exhibit broad sensitivity to toxicity from commonly-used medications.5 This finding is in keeping with work demonstrating that ZAAT polymer expression renders cells more sensitive to UPR activation in response to a “second hit”, such as treatment with tunicamycin or in response to low glucose.40 While the mechanistic underpinnings of this drug-induced toxicity in cells that did not express adult levels of CYP450 enzymes were not defined, the findings suggest the possibility that iPSCs could represent a platform to predict individualized risk of drug toxicity, when combined with protocols capable of achieving this level of maturation.
Along these same lines, recent literature has demonstrated the applicability of patient iPSCs as a platform for drug screening.41 While other, immortalized, cell lines could similarly serve this purpose, the genetic and phenotypic diversity that patient iPSCs potentially represent could make them well suited as a primary or confirmatory tool for this application.
Finally, the only curative treatment for AATD has long been organ transplantation, a procedure with limited availability and considerable morbidity that carries with it the life-long burden of immunosuppression. Seminal work from Japan has demonstrated the transplantation of patient iPSC-derived differentiated cells to restore function to damaged tissue.42 Potential roadblocks to widespread use of this approach remain to be surmounted, including potential off-target effects of genome editing to correct disease-causing mutations and the potential risk associated with inadvertent transplantation of pluripotent cells.39 However, recent studies demonstrating a proliferative advantage for gene-corrected MAAT hepatocytes over diseased ZAAT hepatocytes suggest that transplantation of cells edited to correct the Z mutation may have utility for AATD and could represent a cure for the disease that to this point has remained elusive.43
Abbreviations
alpha-1 antitrypsin, AATD; alpha-1 antitrypsin, AAT; endoplasmic reticulum, ER; chronic obstructive pulmonary disease, COPD; induced pluripotent stem cells, iPSCs; alfa-fetoprotein, ATP; pluripotent stem cells, PSCs; periodic acid Schiff, PAS; ER-associated degradation, ERAD; ER overload response, EOR; unfolded protein response, UPR; wild-type, WT
References
- 1.Murry CE,Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell. 2008;132(4):661-680. doi: https://doi.org/10.1016/j.cell.2008.02.008 [DOI] [PubMed] [Google Scholar]
- 2.Gadue P,Huber T,Nostro M,Kattman S,Keller G. Germ layer induction from embryonic stem cells. Exp Hematol. 2005;33(9):955-964. doi: https://doi.org/10.1016/j.exphem.2005.06.009 [DOI] [PubMed] [Google Scholar]
- 3.Gouon-Evans V,Bousemant L,Gadue P,et al. BMP-4 is required for hepatic specification of mouse embryonic stem cell-derived definitive endoderm. Nat Biotechnol. 2006;24:402-1411. doi: https://doi.org/10.1038/nbt1258 [DOI] [PubMed] [Google Scholar]
- 4.Si-Tayeb K,Noto FK,Nagaoka M,et al. Highly efficient generation of human hepatocyte-like cells from induced pluripotent stem cells. Hepatology. 2010;51(1):297-305. doi: https://doi.org/10.1002/hep.23354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilson AA,Ying L,Leisa M,et al. Emergence of a stage-dependent human liver disease signature with directed differentiation of alpha-1 antitrypsin-deficient iPS cells. Stem Cell Reports. 2015;4(5):873-885. doi: https://doi.org/10.1016/j.stemcr.2015.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ogawa S,Surapisitchat J,Virtanen C,et al. Three-dimensional culture and cAMP signaling promote the maturation of human pluripotent stem cell-derived hepatocytes. Development. 2013;140:3285-3296. doi: https://doi.org/10.1242/dev.090266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lomas DA,Evans DL,Finch JT,Carrell RW. The mechanism of Z alpha 1-antitrypsin accumulation in the liver. Nature. 1992;357:605-607. doi: https://doi.org/10.1038/357605a0 [DOI] [PubMed] [Google Scholar]
- 8.Lomas DA,Hurst JR,Gooptu B. Update on alpha-1 antitrypsin deficiency: New therapies. J Hepatol. 2016;65(2):413-424. doi: https://doi.org/10.1016/j.jhep.2016.03.010 [DOI] [PubMed] [Google Scholar]
- 9.Dickens JA,Ordóñez A,Chambers JE,et al. The endoplasmic reticulum remains functionally connected by vesicular transport after its fragmentation in cells expressing Z-α1-antitrypsin. FASEB J. 2016;30(12):4083-4097. doi: https://doi.org/10.1096/fj.201600430R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perlmutter DH,Brodsky JL,Balistreri WF,Trapnell BC. Molecular pathogenesis of alpha-1-antitrypsin deficiency-associated liver disease: A meeting review. Hepatology. 2007;45(5):1313-1323. doi: https://doi.org/10.1002/hep.21628 [DOI] [PubMed] [Google Scholar]
- 11.Rudnick DA,Liao Y,Jae-Koo A,et al. Analyses of hepatocellular proliferation in a mouse model of alpha-1 antitrypsin deficiency. Hepatology. 2004;39(4):1045-1055. doi: https://doi.org/10.1002/hep.20118 [DOI] [PubMed] [Google Scholar]
- 12.Brantly M,Nukiwa T,Crystal RG. Molecular basis of alpha-1-antitrypsin deficiency. Am J Med. 1988;84(6):13-31. doi: https://doi.org/10.1016/S0002-9343(88)80066-4 [DOI] [PubMed] [Google Scholar]
- 13.Sifers RN. Intracellular processing of α1-antitrypsin. Proc Am Thorac Soc. 2010;7(6):376-380. doi: https://doi.org/10.1513/pats.201001-011AW [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sifers RN,Hardick CP,Woo SL. Disruption of the 290-342 salt bridge is not responsible for the secretory defect of the PiZ alpha 1-antitrypsin variant. J Biol Chem. 1989;264:2997-3001. [PubMed] [Google Scholar]
- 15.Ellgaard L,Molinari M,Helenius A. Setting the standards: quality control in the secretory pathway. Science. 1999;286(5446):1882-1888. doi: https://doi.org/10.1126/science.286.5446.1882 [DOI] [PubMed] [Google Scholar]
- 16.Plemper RK,Wolf DH. Endoplasmic reticulum degradation. Reverse protein transport and its end in the proteasome. Mol Biol Rep. 1999;26(1-2):125-130. doi: https://doi.org/10.1023/A:1006913215484 [DOI] [PubMed] [Google Scholar]
- 17.McCarthy C,Saldova R,O'Brien ME,et al. Increased outer arm and core fucose residues on the N-glycans of mutated alpha-1 antitrypsin protein from alpha-1 antitrypsin deficient individuals. J Proteome Res. 2014;13(2):596-605. doi: https://doi.org/10.1021/pr400752t [DOI] [PubMed] [Google Scholar]
- 18.Hidvegi T,Schmidt BZ,Hale P,Perlmutter DH. Accumulation of mutant alpha-1 antitrypsin Z in the endoplasmic reticulum activates caspases-4 and -12, NFkB, and BAP31 but not the unfolded protein response. J Biol Chem. 2005;280:39002-39015. doi: https://doi.org/10.1074/jbc.M508652200 [DOI] [PubMed] [Google Scholar]
- 19.Graham KS,Le A,Sifers RN. Accumulation of the insoluble PiZ variant of human alpha-1 antitrypsin within the hepatic endoplasmic reticulum does not elevate the steady-state level of grp78/BiP. J Biol Chem. 1990;265:20463-20468. [PubMed] [Google Scholar]
- 20.Wu Y,Whitman J,Molmenti E,Moore K,Hippenmeyer P,Perlmutter DH. A lag in intracellular degradation of mutant alpha-1 antitrypsin correlates with the liver disease phenotype in homozygous PiZZ alpha-1 antitrypsin deficiency. PNAS. 1994;91(19):9014-9018. doi: https://doi.org/10.1073/pnas.91.19.9014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi AMK,Ryter SW,Levine B. Autophagy in human health and disease. New Eng J Med. 2013;368:651-662. doi: https://doi.org/10.1056/NEJMra1205406 [DOI] [PubMed] [Google Scholar]
- 22.Klionsky DJ,Abdalla FC,Abeliovich H,et al. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy. 2012;8(4):445-544. doi: https://doi.org/10.4161/auto.19496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teckman JH,Perlmutter DH. Retention of mutant alpha(1)-antitrypsin Z in endoplasmic reticulum is associated with an autophagic response. Am J Physiol Gastrointest Liver Physiol. 2000;279(5):G961-G974. doi: https://doi.org/10.1152/ajpgi.2000.279.5.G961 [DOI] [PubMed] [Google Scholar]
- 24.An J-K,Blomenkamp K,Lindblad D,Teckman JH. Quantitative isolation of α1AT mutant Z protein polymers from human and mouse livers and the effect of heat. Hepatology. 2005;41(1):160-167. doi: https://doi.org/10.1002/hep.20508 [DOI] [PubMed] [Google Scholar]
- 25.Teckman JH. Mitochondrial autophagy and injury in the liver in α1-antitrypsin deficiency. Am J Physiol Gastrointest Liver Physiol. 2004;286(5):G851-G862. doi: https://doi.org/10.1152/ajpgi.00175.2003 [DOI] [PubMed] [Google Scholar]
- 26.Granell S,Baldini G. Inclusion bodies and autophagosomes: are ER-derived protective organelles different than classical autophagosomes?. Autophagy. 2008;4(3):375-377. [DOI] [PubMed] [Google Scholar]
- 27.Teckman JH,An J-K,Loethen S,Perlmutter DH. Fasting in alpha-1 antitrypsin deficient liver: constitutive activation of autophagy. Am J Physiol Gastrointest Liver Physiol. 2002;283(5):G1156-G1165. doi: https://doi.org/10.1152/ajpgi.00041.2002 [DOI] [PubMed] [Google Scholar]
- 28.Lawless MW,Greene CM,Mulgrew A,Taggert CC,O’Neil SJ,McElvaney NG. Activation of endoplasmic reticulum-specific stress responses associated with the conformational disease Z α-1-antitrypsin deficiency. J Immunol. 2004;172(9):5722-5726. doi: https://doi.org/10.4049/jimmunol.172.9.5722 [DOI] [PubMed] [Google Scholar]
- 29.Xu C. Endoplasmic reticulum stress: cell life and death decisions. J Clin Invest. 2005;115:2656-2664. doi: https://doi.org/10.1172/JCI26373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rashid ST,Corbineau S,Hannan N,et al. Modeling inherited metabolic disorders of the liver using human induced pluripotent stem cells. J Clin Invest. 2010;120:3127-3136. doi: https://doi.org/10.1172/JCI43122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tafaleng EN,Chakraborty S,Han B,et al. Induced pluripotent stem cells model personalized variations in liver disease resulting from. Hepatology. 2015;62(1):147-157. doi: https://doi.org/10.1002/hep.27753/suppinfo [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi SM,Kim Y,Shim JS,et al. Efficient drug screening and gene correction for treating liver disease using patient-specific stem cells. Hepatology. 2013;57(6):2458-2468. doi: https://doi.org/10.1002/hep.26237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Caro LG,Palade GE. Protein synthesis, storage and discharge in the pancreatic exocrine cell. An autoradiographic study. J Cell Biol. 1964;20(3):473-495. doi: https://doi.org/10.1083/jcb.20.3.473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hidvegi T,Ewing M,Hale P,et al. An autophagy-enhancing drug promotes degradation of mutant alpha1 antitrypsin z and reduces hepatic fibrosis. Science. 2010;329(5988):229-232. doi:https://doi.org/10.1126/science.1190354 [DOI] [PubMed] [Google Scholar]
- 35.Hitomi J,Katayama T,Eguchi Y,et al. Involvement of caspase-4 in endoplasmic reticulum stress-induced apoptosis and Aβ-induced cell death. J Cell Biol. 2004;165(3):347-356. doi: https://doi.org/10.1083/jcb.200310015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gebhardt R,Matz-Soja M. Liver zonation: Novel aspects of its regulation and its impact on homeostasis. World J Gastroenteral. 2014;20(26):8491-8415. doi: https://doi.org/10.3748/wjg.v20.i26.8491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Halpern KB,Shenhav R,Matcovitch-Natan O,et al. Single-cell spatial reconstruction reveals global division of labour in the mammalian liver. Nature. 2017;542:352-356. doi: https://doi.org/10.1038/nature21065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Piccolo P,Amunziata P,Soria LR,et al. Down-regulation of hepatocyte nuclear factor-4α and defective zonation in livers expressing mutant Z α1-antitrypsin. Hepatology. 2017;66(1):124-135. doi: https://doi.org/10.1002/hep.29160 [DOI] [PubMed] [Google Scholar]
- 39.Neofytou E,O'Brien CG,Couture LA,Wu JC. Hurdles to clinical translation of human induced pluripotent stem cells. J Clin Invest. 2015;125:2551-2557. doi: https://doi.org/10.1172/JCI80575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ordó-ez A,Snapp EL,Tan L,Miranda E,Marciniak SJ,Lomas DA. Endoplasmic reticulum polymers impair luminal protein mobility and sensitize to cellular stress in alpha 1-antitrypsin deficiency. Hepatology. 2013;57(5):2049-2060. doi: https://doi.org/10.1002/hep.26173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cayo MA,Mallanna Sk,Di Furio F,et al. A drug screen using human iPSC-derived hepatocyte-like cells reveals cardiac glycosides as a potential treatment for hypercholesterolemia. Cell Stem Cell. 2017;20(4):478-489.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mandai M,Watanabe A,Kurimaoto Y,et al. Autologous induced stem-cell-derived retinal cells for macular degeneration. New Eng J Med. 2017;376:1038-1046. doi: https://doi.org/10.1056/NEJMoa1608368 [DOI] [PubMed] [Google Scholar]
- 43.Borel F,Tang Q,Gernoux G,et al. Survival advantage of both human hepatocyte xenografts and genome-edited hepatocytes for treatment of alpha-1 antitrypsin deficiency. Molecular Therapy. 2017;25(11):2477-2489. doi: https://doi.org/10.1016/j.ymthe.2017.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]