Abstract
Background:
To assess the prevalence of myocardial microvascular dysfunction in rheumatoid arthritis (RA) patients without clinical cardiovascular disease (CVD), and its association with RA characteristics and measures of cardiac structure and function.
Methods:
Participants with RA underwent rest and vasodilator stress N-13 ammonia positron emission tomography and echocardiography. Global myocardial blood flow (MBF) was quantified at rest and during peak hyperemia. Myocardial flow reserve (MFR) was calculated as peak stress MBF/rest MBF. A small number of asymptomatic and symptomatic non-RA controls were also evaluated.
Results:
In RA patients, mean ± SD MFR was 2.9 ± 0.8, with 29% having reduced MFR (<2.5). Male sex and higher interleukin-6 were significantly associated with lower MFR, while use of tumor necrosis factor inhibitors was associated with higher MFR. Lower MFR was associated with higher LV mass index and higher LV volumes, but not with ejection fraction or diastolic dysfunction. RA and symptomatic controls had comparable MFR (mean ± SD: 2.9 ± 0.8 vs 2.55 ± 0.6, p=0.48). In contrast, MFR was higher in the asymptomatic controls (mean ± SD: 3.25 ± 0.7), although not statistically different.
Conclusion:
Reduced MFR was observed in a third of RA patients without clinical CVD and was associated with a measure of inflammation and with higher LV mass and volumes. MFR in RA patients was similar to controls referred for clinical scans (symptomatic controls). Whether reduced MFR contributes to the increased risk for heart failure in RA remains unknown.
Keywords: rheumatoid arthritis, biomarker, blood flow, perfusion, perfusion imaging
Journal Subject Terms: Computerized Tomography (CT), Echocardiography, Imaging, Nuclear Cardiology and PET
Rheumatoid arthritis (RA) is a chronic systemic inflammatory disease that is associated with higher mortality rates than in the general population (1). Cardiovascular disease (CVD), including heart failure (1, 2), is the leading cause of death in RA patients. RA patients have a two-fold higher risk of developing and dying from heart failure compared to individuals without RA , even after adjusting for coronary artery disease (CAD) (3). This observation suggests that factors other than flow limiting CAD alone may contribute to the pathogenesis of heart failure in RA(3) (4). However, the causal pathways associated with increased risk for heart failure in RA remain unclear.
In the general population, co-morbidities such as obesity (5) are associated with a state of low grade chronic systemic inflammation that is postulated to cause endothelial activation and damage resulting in dysfunction of the myocardial microvasculature (6). Microvascular dysfunction, in turn, is associated with LV remodeling with increase in LV mass, and fibrosis, features that predate and predict clinical heart failure (7). Insofar as RA is a disease of profound and chronic inflammation, we hypothesized that inflammatory pathways in RA may be associated with microvascular dysfunction and adverse changes in LV structure and function, independent of co-morbidities, yet there are few data to support this hypothesis to date (8, 9).
Microvascular dysfunction can be indirectly assessed by measuring myocardial blood flow (MBF) before and after vasodilation (i.e., ‘stress’). Cardiac positron emission tomography-computed tomography (PET-CT) is the gold standard non-invasive test for MBF (10, 11). The ratio of the MBF measured at maximal vasodilation over the MBF at rest is referred to as myocardial flow reserve (MFR) which is a measure of the vasodilatory reserve of the myocardium (12). Reduced MFR in the absence of flow limiting CAD is believed to reflect dysfunction in the myocardial microvasculature (10, 13).
In the current cross-sectional study, we assessed the prevalence of reduced MFR in a cohort of RA patients without clinical CVD and its association with RA characteristics and measures of LV structure and function. We hypothesized that myocardial microvascular dysfunction (reduced MFR) would be detectable in RA patients even in the absence of significant macrovascular disease (CAD), and would be associated with measures of inflammation, and with subclinical measures of LV remodeling such as higher LV mass, independent of conventional CV risk factors.
METHODS
The data, analytic methods and study materials will be made available to other researchers for purposes of reproducing the results or replicating the procedure. The material will be available directly from the author (JMB).
Study Design and Population
The study population consisted of participants in the RHeumatoid arthritis studY of THe Myocardium [RHYTHM], a cohort study to identify and evaluate factors associated with myocardial phenotypes in RA patients without clinical cardiovascular disease (CVD). The RHYTHM study has been previously described in detail (14). Participants enrolled between October 2011 (start of study) through September 2014 constituted the study population, Participants were recruited from the rheumatology clinics of Columbia University Medical Center and by referral from local rheumatologists. Inclusion criteria included age ≥ 18 years and fulfillment of the American College of Rheumatology 2010 classification criteria for RA (15). Exclusion criteria included: 1) any prior self-reported physician diagnosed CV event (myocardial infarction; angina; stroke or TIA; heart failure; prior CV procedure (e.g., coronary artery bypass graft, angioplasty, valve replacement, pacemaker); 2) contraindication to the vasodilators regadenoson and/or adenosine such as severe asthma; 3) active history of cancer.
Since MFR data in healthy controls are sparse and primarily limited to young men (16), we enrolled a small group of non-RA controls using the same inclusion and exclusion criteria as the RA participants other than the RA diagnosis (‘asymptomatic controls’). Given the difficulties in recruiting healthy individuals to a study involving radiation and vasodilation, we also retrospectively queried the Columbia University Medical Center/ New York Presbyterian Hospital (CUMC/NYP) nuclear medicine database to identify patients who were referred for a NH3-PET-CT study during the period 9/2016 – 4/2017 (‘symptomatic controls’); patients with a prior history of CAD, myocardial infarction or heart failure were excluded.
The study was approved by the CUMC/NYP Institutional Review Board. All subjects provided written informed consent prior to enrollment. All data acquired and analyzed in this report are from the baseline visit.
Imaging:
Myocardial Perfusion Imaging by 13N-Ammonia Positron Emission Tomography (PET) /Computed Tomography (CT) PET/CT
Patients refrained from caffeine and methylxanthine containing substances and drugs for 24 hours. The patients were studied on an MCT 64 PET/CT scanner (Siemens, Knoxville, TN). Myocardial blood flow (MBF) was measured during rest and maximal vasodilation with N-13 ammonia, as a perfusion tracer, as described previously (17). After transmission images, 10 mCi of intravenous N-13 ammonia was injected, list mode images were acquired for 10 minutes. Approximately 40 minutes later standard intravenous regadenoson or adenosine infusion was given. A second dose of N-13 ammonia was given at maximal vasodilation, and images were acquired in the same manner.
Absolute MBF (ml/g/min) was calculated from the dynamic rest and stress images with commercially available software (INVIA Corridor4DM; Ann Arbor, MI). Automated factor analysis was used to generate blood pool and tissue time activity curves. Regional and global rest and stress MBFs were computed by fitting the N-13 ammonia time activity curves to a 2-compartment tracer kinetic model. MFR was calculated as the ratio of absolute MBF measured at peak stress (i.e., during vasodilator-induced hyperemia) over that at rest (corrected for rate-pressure product as an index of baseline cardiac work). Per previous work, reduced MFR was defined as a ratio of less than 2.5, moderate reduction of less than 2.0, and severe reduction of MFR was defined as a ratio of less than 1.5 (18).
Coronary Artery Calcium (CAC)
CAC was assessed as a measure of macrovascular (coronary artery) atherosclerosis and quantified using the Agatston method (19). The presence of CAC was defined as an Agatston score of greater than zero. Given that a CAC score of ≥100 units is a strong predictor of future cardiovascular events (20), we further dichotomized the patients with a positive CAC score using this cutoff value.
Echocardiography
Transthoracic two-dimensional and real-time 3D-echocardiography (RT3DE) was performed using a commercially available system (iE 33; Philips, Andover, MA) by a registered cardiac sonographer according to a standardized protocol. LV end-diastolic (EDVI) and end-systolic (ESVI) volume indices, stroke volume (SV), and ejection fraction (LVEF) were measured by RT3DE using a commercially available software (Philips QLAB Advanced Quantification Software, version 8.1) as previously described (21). LV mass was also assessed by RT3DE by tracings of endocardial and epicardial borders. Left atrial volume and LV diastolic function were measured by 2-dimensional echocardiography as previously described (22). Briefly, in apical 4-chamber view, peak early (E) and late velocity (A) of mitral inflow were measured by pulsed-wave Doppler. Peak early diastolic velocity (E′) of the lateral and septal mitral annulus were evaluated by pulsed-wave Tissue-Doppler and averaged. The E/E’ ratio was calculated as an index of LV filling pressure. LV volumes, stroke volume, cardiac output, and LA volume were indexed by body surface area. LV mass was indexed by height2.7.
Clinical characteristics
Demographic and Cardiovascular characteristics:
Demographic, lifestyle characteristics and non-RA medications were assessed with a structured interview by trained evaluators. Resting blood pressure (BP) was measured 3 times in the seated position, and the average of the last 2 measurements was used. Hypertension was defined as a systolic BP of ≥140 mm Hg, a diastolic BP of ≥90 mm Hg, or use of antihypertensive medications. Diabetes was defined as a fasting serum glucose level of ≥126 mg/dl or use of antidiabetic medications. Body mass index was calculated by dividing the patient’s weight (in kilograms) by height (in square meters). RA disease duration was assessed by patient self-report of the date of diagnosis. Forty-four joints were examined for swelling and tenderness by the same trained assessor. RA disease activity was calculated with the Disease Activity Score for 28 joints (DAS28) using C-reactive protein (CRP) level(23). The Health Assessment Questionnaire (HAQ) was employed as a measure of self-reported disability(24). Current and past use of steroids and biologic and non-biologic disease-modifying anti-rheumatic drugs (DMARDs) were queried by detailed, examiner-administered questionnaires.
Laboratory Measurements:
Phlebotomy was performed on the morning of the PET/CT scan after overnight fast. Sera and plasma were separated by centrifugation and frozen at −80°C. Rheumatoid factor (RF) was measured by enzyme-linked immunosorbent assay (ELISA), with seropositivity defined at a level of ≥40 units (IBL America, Minneapolis, MN). Anti–cyclic citrullinated peptide antibody (anti-CCP) was measured by ELISA (using the CCP3 kit), with seropositivity defined at a level of ≥60 units (Inova Diagnostics, Woburn, MA). The levels of CRP and interleukin-6 (IL-6), and lipid panel were measured in the Biomarkers Core Laboratory of the Columbia University Medical Center Clinical and Translational Research Center (details available at http://irvinginstitute.columbia.edu/resources/biomarkers_core.html).
Statistical analysis:
In the RA patients only, the first set of analyses were performed to identify patient characteristics independently correlated with MFR; thus, MFR served as the primary outcome. In the second set of analyses in RA patients only, the goal was to investigate the association of MFR with myocardial parameters of structure and function; LVMI served as the primary outcome, and other parameters as secondary outcomes. Means and standard deviations for normally distributed, and medians and interquartile ranges for non-normally distributed variables, were calculated. For categorical variables, counts and percentages were calculated. Differences in continuous variables between RA groups were compared using student’s t-tests for normally distributed variables, or the Mann-Whitney-U test for continuous non-normally distributed variables. Categorical variables were compared using the Chi-square goodness of fit test or Fisher’s exact test, as appropriate.
Linear regression was used to model the associations of patient characteristics with MFR. Multivariable models were constructed by including any variable associated with MFR at p<0.2 in univariate models. Parsimonious models were constructed including only covariates that retained p< 0.2 in the prior more comprehensive model. Similar methods were used to model the associations of MFR with LVMI. We calculated variance inflation factors to ensure that collinear variables were not co-modelled and excluded non-contributory covariates using the Akaike’s Information Criterion for nested models. A p-value<0.05 was considered significant for final models. All analyses were performed using Stata version 12 (StataCorp, College Station, TX).
Results:
Participant Characteristics
RA Patients.
A total of 103 RA patients completed both the baseline PET/CT scan and echocardiogram. Twenty-seven patients (26%) were excluded from the analyses as a result of technical difficulties in measuring MBFs due to motion artifact or partial volume effects. Thus, the RA study population consisted of 76 subjects with complete data for MFR and echocardiographic measures. The demographic, RA and CV risk factor characteristics of excluded patients were not significantly different from the final participant group, except that they were more likely to be Hispanic and had a higher mean BMI (supplementary table S1). The baseline characteristics of the participants are summarized in Table 1. The majority of participants were middle aged (mean age 55± 13 years) women (79%). The mean BMI was 27± 5.3 kg/m2. The prevalences of conventional cardiovascular risk factors were: 42%, hypertension; 43%, hyperlipidemia; 11%, diabetes mellitus, and 8%, current smokers. Thirty-eight percent had a CAC score > 0, and 21% had a score > 100 units.
Table 1:
Baseline Characteristics of Participants
| Characteristics | Characteristics of all RA patients (n=76) |
RA patients 1st Tertile of IL-6* (n=26) |
RA patients 2nd Tertile of IL-6 (n=26) |
RA patients 3rd Tertile of IL-6 (n=24) |
p † value |
Non-RA Asymptomatic Controls (n=14) |
Non-RA Symptomatic Controls (n=34) |
|
|---|---|---|---|---|---|---|---|---|
| Demographic | ||||||||
| Mean Age in years (SD) | 55 (13) | 49 (15) | 59 (11) | 57 (11) | 0.029 | 51 (13) | 65 (10) | |
| Female, n (%) | 60 (79) | 22 (85) | 16 (62) | 22 (92) | 0.44 | 9 (69) | 15 (44) | |
| Race/Ethnicity | ||||||||
| Non-Hispanic White, n (%) | 32 (42) | 16 (62) | 11 (42) | 5 (21) | 0.006 | 7 (50) | 29 (85) | |
| Non-Hispanic Black, n (%) | 13 (17) | 1 (4) | 4 (15) | 8 (33) | 2 (14) | 3 (9) | ||
| Hispanic, n (%) | 29 (38) | 8 (31) | 10 (39) | 11 (46) | 3 (21) | 2 (6) | ||
| Other, n (%) | 2 (3) | 2 (4) | 2 (4) | 0 (0) | 0 | 0 | ||
| Cardio-vascular risk factors | ||||||||
| Ever smoker, n (%) | 30 (39) | 10 (38) | 11 (42) | 9 (38) | 0.94 | 6 (43) | 2 (6) | |
| Current smoker, n (%) | 6 (8) | 1 (4) | 3 (12) | 2 (8) | 0.50 | 0 | 1 (3) | |
| Hypertension, n (%) | 32 (42) | 6 (23) | 12 (48) | 13 (54) | 0.024 | 1 (8) | 27 (79) | |
| Mean BMI (SD) in kg/m2 | 27.4 (5) | 25 (4) | 28.76 (5) | 28.71 (6) | 0.012 | 33.8 (5) | --- | |
| Diabetes, n (%) | 8 (11) | 0 (0.0) | 4 (16) | 4 (17) | 0.033 | 0 | 18 (53) | |
| Hyperlipidemia, n (%) | 33 (43) | 12 (46) | 14 (54) | 7 (29) | 0.22 | 7 (50) | 23 (68) | |
| RA characteristics | ||||||||
| Median RA Duration in years (IQR) | 7 (1.5-14) | 9 (3.8-15.) | 7.9 (0.6-11.2) | 13.5 (3.7-16.3) | 0.56 | |||
| RF or anti-CCP, n (%) | 54 (73) | 16 (62) | 18 (69.23) | 22 (91.67) | 0.013 | |||
| Median swollen joints (0-44) (IQR) | 10 (5-15) | 10 (5-15) | 9 (5-14) | 13 (8.5-17) | 0.11 | |||
| Median tender joints (0-42) (IQR) | 11.5 (4-19) | 11 (4-18) | 13 (4-21) | 14 (4-19.5) | 0.33 | |||
| Mean DAS28-CRP in units (SD) | 3.59 (1.23) | 3 (1.03) | 3.5 (1.37) | 4.1 (1.09) | <0.001 | |||
| Mean HAQ in units (IQR) | 1.06 (0.50-1.75) | .8 (0.80) | 1.18 (0.78) | 1.41 (0.72) | 0.0076 | |||
| Median AM stiffness in min (IQR) | 20 (3-60) | 29 (0-45) | 27 (2-30) | 59 (25-90) | 0.023 | |||
| Median CRP (mg/L) (IQR) | 1.83 (0.77-4.45) | 1.91 (0.44-1.76) | 4.31 (1.09-4.7) | 9.37 (2.06-9.68) | <0.001 | |||
| Median IL-6 (pg/mL) (IQR) | 2.24 (1.17-7.02) | 1.04 (0.85-1.27) | 2.26 (2.046-2.81) | 8.75 (7.03-17.1) | <0.001 | |||
| Current Treatment‡ (yes vs no) | ||||||||
| No DMARD or prednisone, n (%) | 5 (7) | 1 (3.85) | 2 (8.00) | 2 (8.33) | 0.50 | |||
| Non-biologic DMARD, n (%) | 58 (76) | 21 (81) | 22 (88) | 15 (62) | 0.15 | |||
| Methotrexate, n (%) | 47 (62) | 16 (62) | 20 (80) | 10 (42) | 0.16 | |||
| Biologic DMARDs, n (%) | 29 (38) | 10 (39) | 11 (44) | 8 (33) | 0.71 | |||
| TNF inhibitors, n (%) | 22 (29) | 8 (31) | 8 (31) | 6 (25) | 0.90 | |||
| Prednisone, n (%) | 27 (36) | 4 (15) | 8 (32) | 15 (63) | 0.001 | |||
| CAC score | ||||||||
| CAC score positive, n (%) | 29 (38) | 6 (23) | 13 (50) | 10 (42) | 0.16 | |||
| CAC score≥100, n (%) | 16 (25) | 2 (8) | 8 (31) | 6 (25) | 0.095 | |||
| Myocardial Blood Flow | ||||||||
| Flow at rest (SD), in mL/g/min | 1.04 (0.2) | 0.99 (0.21) | 1.02 (0.18) | 1.11 (0.19) | 0.044 | 0.94 (0.2) | 0.85 (0.2) | - |
| Flow hyperemic (SD), in mL/g/min | 2.92 (0.7) | 2.89 (0.70) | 3.00 (0.82) | 2.85 (0.67) | 0.83 | 2.98 (0.7) | 2.1 (0.4) | - |
| Mean MFR (SD) | 2.87 (0.7) | 3.00 (0.82) | 2.96 (0.71) | 2.62 (0.67) | 0.071 | 3.2 (0.7) | 2.5 (0.5) | - |
| Myocardial Structure | ||||||||
| LVMI (SD), per grams/ht2.7 | 29.5 (6) | 27.8 (4.8) | 29.3 (4.8) | 31.6(6.4) | 0.024 | - | - | - |
| End-Diastolic Volume Index (SD), in mL/m2 | 55.2 (11) | 57.9 (12.3) | 50.3 (8.8) | 57.6 (9.8) | 0.93 | - | - | - |
| End-Systolic Volume Index (SD), mL/m2 | 20.7 (5.7) | 21.8 (6.7) | 18.8(4.1) | 21.7 (5.9) | 0.94 | - | - | - |
| Myocardial Function | - | - | ||||||
| Ejection Fraction (%) | 63 (5) | 63.2 (4.04) | 62.6(5.1) | 62.8 (5.0) | 0.80 | - | - | - |
| Stroke Volume Index (SD), in mL/m2 | 34.7 (6.4) | 36.8(6.32) | 31.5 (6.09) | 36.0(5.35) | 0.66 | - | - | - |
| Cardiac Index (SD) in L/min/m2 | 2.31 (0.4) | 2.31 (0.33) | 2.11 (0.32) | 2.57 (0.47) | 0.051 | - | - | - |
| E/E’ (ratio) | 8.58 (2.3) | 7.73 (1.87) | 9.15 (2.57) | 8.86 (2.38) | 0.078 | |||
| Diastolic Dysfunction, yes v. no , n (%) | 36/74 (49) | 10/25 (40) | 14/25 (56) | 12/24 (50) | 0.48 |
Legend:
Lowest IL-6 tertile, 0.51-1.53 mg/mL; middle IL-6 tertile, 1.54-3.97 mg/mL; highest IL-6 tertile, 3.98-35.88 mg/mL).
The p values were obtained by Student’s t-test for normally distributed variables, and the Mann-Whitney-U test for continuous non-normally distributed variables, comparing the lowest and highest tertiles.
RA patients may be on more than one non-biologic disease-modifying anti-rheumatic drug (DMARD). SD: Standard Deviation, IQR: inter quantile range, RF: rheumatoid factor, CCP: cyclic citrullinated protein antibody, DAS: disease activity score, HAQ: health assessment questionnaire, AM: morning, CRP: C-reactive protein, IL-6: interleukin 6, CAC: coronary artery calcium, LVMI: left ventricle mass indexed to height2.73.
The median duration of RA was 7.1 years, and 73% of patients were seropositive for either RF or anti-CCP. The mean DAS28-CRP was in the moderate range (3.59 units) while the median HAQ score was 1.1, indicating mild-to-moderate disability for most patients. The majority of patients (88%) were on one or more DMARDs. Methotrexate was the most commonly used (61%) non biologic DMARD while tumor necrosis factor (TNF) inhibitors accounted for the majority of biologic use (76%) in the 38% who were on biologic DMARDS. Among the 32% of patients who were taking glucocorticoids (prednisone) at the time of data collection, the median daily dose was 5mg.
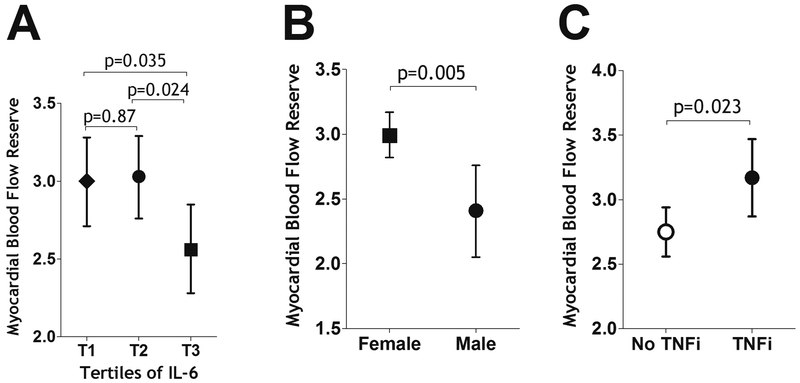
Characteristics of the RA patients are also shown as a function of level of interleukin-6 (IL-6), a key inflammatory cytokine associated with RA pathogenesis and disease activity (Table 1). Patients were compared in univariate analyses according to the highest tertile of IL-6 levels vs the lowest tertile. Compared with those with the lowest IL-6 levels, the group with the highest IL-6 levels had higher CRP levels (p < 0.001) , higher levels of clinical disease activity (measured by DAS28-CRP, p < 0.001), higher levels of disability (measured by the HAQ, p 0.007), were more likely to be on prednisone (p < 0.001), had a higher mean LVMI (p 0.024), and a non-significant trend towards a lower MFR (p 0.07) .
Non-RA Controls.
Fourteen asymptomatic controls were recruited using the same inclusion and exclusion criteria as the RA participants other than the RA diagnosis. From the nuclear medicine database, we identified 34 symptomatic controls. Not surprisingly, the symptomatic controls were more likely to be men and to have CV risk factors than the asymptomatic controls or the RA patients (Table 1).
MBF and MRF in RA Patients
The mean MBF in the RA group was 1.04 ± 0.20 mL/g/min at rest and 2.92 ± 0.73 mL/g/min during peak stress, yielding a mean MFR of 2.87 ± 0.75. Twenty-nine percent of RA patients had reduced MFR using a cut-off of < 2.5, 12% using a cutoff of < 2.0, and 4% had severely reduced MFR (cutoff of <1.5).
Association of RA Patient Characteristics with MFR
Linear regression was used to model the associations of RA patient characteristics with MFR (Table 2). Characteristics with p values < 0.2 were carried into multivariable models shown in Table 3. As shown in Model 2 (Table 3) and Figures 1a and 1b, male sex and the highest tertile of IL-6 levels were significantly associated with lower (less favorable) MFR, while TNF inhibitor (but not other biologic) use was associated with higher (more favorable) MFR (Table 3, model 2, Figure 1c). Conventional CV risk factors were not independently associated with MFR in adjusted models, nor were CV pharmacotherapies including statins, beta blockers and angiotensin converting enzyme (ACE) inhibitors (all p values > 0.2, Table 3). CAC was not associated with MFR in univariate analyses (Table 2). In a sensitivity analysis in which CAC was added to multivariable model 2 in Table 3, it did not add to the prediction of MFR (unadjusted R2s, 0.192 and 0.196, without and with CAC, respectively). An additional sensitivity analysis using forward selection of the variables significant at the 0.20 level confirmed the above statistically significant associations with only nominal differences in p values (data not shown).
Table 2:
Univariate Associations of RA Participant Characteristics with Myocardial Flow Reserve (MFR) and Left Ventricular Mass Index (LVMI)
| Characteristics | MFR | LVMI | ||
|---|---|---|---|---|
| β | p | β | p | |
| Myocardial Blood Flow Reserve | n/a | n/a | −2.0586 | 0.014 |
| Age per year | −0.0090 | 0.16 | 0.1090 | 0.020 |
| Male vs. Female | −0.3970 | 0.06 | −0.0370 | 0.98 |
| Race/ethnicity | ||||
| Non-Hispanic white | ref | ref | ||
| Non-Hispanic black | 0.1470 | 0.55 | 4.0930 | 0.017 |
| Hispanic | −0.1540 | 0.42 | 4.9770 | <0.001 |
| Other | −0.9520 | 0.083 | 4.9650 | 0.18 |
| Ever smoking vs. never | 0.0400 | 0.82 | 0.9430 | 0.47 |
| Hypertension, yes v. no | −0.2630 | 0.13 | 3.2790 | 0.009 |
| Systolic Blood Pressure (mmHg) | −0.0043 | 0.38 | 0.1336 | <0.001 |
| Diastolic Blood Pressure (mmHg) | −0.0045 | 0.60 | 0.0455 | 0.47 |
| BMI, per kg/m2 | 0.0080 | 0.64 | 0.4050 | 0.001 |
| Diabetes, yes v. no | 0.1270 | 0.65 | 3.3960 | 0.10 |
| Total cholesterol, per mg/dL | 0.0020 | 0.32 | 0.0020 | 0.89 |
| LDL, per mg/dL | 0.0030 | 0.30 | 0.0160 | 0.43 |
| HDL, per mg/dL | −0.0200 | 0.50 | −0.1210 | 0.10 |
| log Triglycerides, per mg/dL | 0.3040 | 0.14 | 2.1600 | 0.17 |
| RA duration (square root), per year | 0.0130 | 0.81 | 0.4350 | 0.27 |
| RF or anti-CCP, yes v. no | −0.1780 | 0.36 | 3.2300 | 0.025 |
| Swollen Joint Count (0-42), per joint | −0.0020 | 0.86 | 0.2110 | 0.024 |
| Tender Joint Count (0-44), per joint | 0.0100 | 0.21 | 0.0910 | 0.13 |
| DAS28-CRP, per unit | 0.0770 | 0.28 | 1.0830 | 0.036 |
| log CRP, per mg/L | −0.0670 | 0.33 | 0.9270 | 0.06 |
| Median IL-6, per unit | −0.2710 | 0.12 | 2.33 | 0.072 |
| AM stiffness (square root), per min | −0.0240 | 0.30 | 0.2090 | 0.29 |
| HAQ, per unit | −0.0560 | 0.61 | 1.0300 | 0.20 |
| Biologic, yes v. no | ||||
| No biologic | ref | ref | ||
| TNF inhibitors | 0.3130 | 0.110 | −0.3060 | 0.83 |
| Non-TNF biologics | 0.2400 | 0.43 | 2.2300 | 0.32 |
| Methotrexate, yes v. no | −0.1721 | 0.34 | 0.5939 | 0.65 |
| Current prednisone, yes v. no | −0.2700 | 0.15 | 1.3090 | 0.34 |
| Current statin, yes v.no | 0.2344 | 0.29 | 1.6282 | 0.38 |
| Current ACE-inhibitor, yes v. no | 0.0054 | 0.98 | 4.3343 | 0.010 |
| Current beta-blocker, yes v. no | −0.2133 | 0.93 | 2.7045 | 0.18 |
| CAC score | ||||
| CAC score zero | ref | ref | ||
| CAC score 0-99 | −0.2590 | 0.28 | 2.0808 | 0.042 |
| CAC score ≥100 | −0.1010 | 0.64 | 3.8480 | |
Legend: Beta coefficients represent the average change in the MFR or LVMI per 1-unit higher value of the independent continuous variable of interest or for those with versus those without the independent dichotomous variable of interest., MFR: myocardial Blood Flow Reserve, LVMI: Left Ventricule Mass indexed for height2.73, SD: Standard Deviation, IQR: inter quantile range, RA: rheumatoid arthritis, RF: rheumatoid factor, CCP: cyclic citrullinated protein antibody, DAS: disease activity score, HAQ: health assessment questionnaire, AM: morning, CRP: C-reactive protein, IL-6: interleukin 6, CAC: coronary artery calcium.
Table 3:
Multivariable Associations of RA Participant Characteristics with MFR
| Myocardial blood flow reserve | Model 1 (n=76) |
Model 2 (n=76) |
||
|---|---|---|---|---|
| β | P | β | p | |
| Age, per year | −0.0003 | 0.96 | ||
| Male v. Female | −0.5147 | 0.018 | −0.5410 | 0.008 |
| Hypertension, yes v. no | −0.2858 | 0.12 | ||
| log triglycerides, per unit | 0.5483 | 0.017 | 0.3771 | 0.064 |
| Median IL-6, per unit | −0.2087 | 0.24 | −0.3325 | 0.043 |
| TNF inhibitor, yes v. no | 0.3916 | 0.032 | 0.4291 | 0.019 |
| Current prednisone use v. no use | −0.2745 | 0.14 | ||
| Prob>F | 0.0051 | 0.0037 | ||
| Unadjusted R2 | 0.2289 | 0.1921 | ||
Model 1 includes covariates from table 1 with p values < 0.2. Model 2 is a reduced model that includes all significant variables from Model 1 in addition to IL-6 given its biologic plausibility IL-6: interleukin 6. Prob>F is the overall probability for the model evaluated. Unadjusted R2 is the proportion of variance explained by each model.
Figure 1:
Adjusted Associations of MFR with IL-6 level, gender and use of TNF inhibitors in RA participants. Means and 95% confidence intervals are depicted. Adjusted analyses account for gender, log triglycerides, Il-6 and TNF inhibitor use, which were the only covariates independently associated with MFR in the multivariable models. IL-6: interleukin 6, TNF-i: TNF inhibitors.
Association of MFR with LV Structure and Function
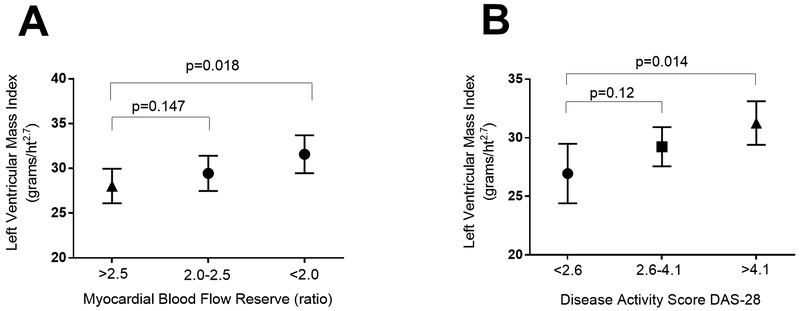
We next evaluated the association of MFR with LVMI. The mean LVMI was 29.5 ± 5.6 g/m2.7. None of the participants met criteria for elevated LVMI according to the definition of the American Society of Echocardiography (25). However, in univariate analyses, there was a strong inverse association of MFR with LVMI, with each unit lower MFR on average associated with 2.1 unit higher LVMI (beta: −2.0586, p=0.014; Table 2). In addition, several RA characteristics were significantly associated with higher LVMI, including seropositivity for RF or CCP, and DAS28 (Table 2). Non-RA characteristics significantly associated with higher LVMI were higher age, race, higher BMI, hypertension, and current use of an ACE-inhibitor (Table 2).
Multivariable models showing crude and adjusted associations between patient characteristics and LVMI are summarized in Table 4. In the most parsimonious model (Model 2), MFR remained independently inversely associated with LVMI. Each unit lower MFR was associated, on average, with a 2.3 unit higher LVMI (Fig 2a). In addition, RA disease activity (DAS28-CRP) remained independently and positively associated with LVMI, as did race and current use of an ACE inhibitor (Fig 2b).
Table 4:
Multivariable Associations of RA Participant Characteristics with LVMI
| LV mass index | Model 1 n=72 |
Model 2 n=72 |
||
|---|---|---|---|---|
| β | p | β | P | |
| MFR global | −1.8403 | 0.007 | −2.0874 | 0.008 |
| Age, per year | 0.0894 | 0.49 | ||
| Race/ethnicity | ||||
| Non-Hispanic white | ref | Ref | ref | Ref |
| Non-Hispanic black | 1.5479 | 0.49 | 1.7415 | 0.343 |
| Hispanic | 3.6751 | 0.024 | 3.6076 | 0.013 |
| Hypertension, yes v no | −0.4099 | 0.81 | ||
| Diabetes, yes v. no | 1.076 | 065 | ||
| HDL, per mg/dL | −0.0129 | 0.73 | ||
| log triglycerides, per unit | −0.6970 | 0.72 | ||
| RF or anti-CCP, yes v. no | 2.5564 | 0.082 | 2.4703 | 0.063 |
| DAS28-CRP | ||||
| DAS28-CRP<2.6 | Ref | Ref | ref | Ref |
| DAS28-CRP 2.6-4.1 | 2.6664 | 0.12 | 2.3467 | 0.13 |
| DAS28-CRP>4.1 | 4.3491 | 0.032 | 4.1895 | 0.022 |
| HAQ, per unit | −1.9814 | 0.056 | −1.5833 | 0.062 |
| Median IL-6, per unit | 0.4385 | 0.75 | ||
| CAC score | ||||
| CAC score zero | Ref | ref | ||
| CAC score 1-99 | −1.2262 | 0.54 | ||
| CAC score ≥100 | −0.3529 | 0.87 | ||
| ACE-inhibitor use | 3.6773 | 0.075 | 4.6417 | 0.001 |
| Statin use | 0.7441 | 0.71 | ||
| Prob>F | 0.005 | <0.00001 | ||
| Unadjusted R2 | 0.4437 | 0.4094 | ||
Model 1 covariates are derived from table 2 where any variable with a p<0.2 was included. BMI was not included due to collinearity with LVMI which is indexed by height. Model 2 is a reduced model that includes all of the covariates from Model 1 with p values < 0.2. RF: rheumatoid factor, CCP: cyclic citrullinated protein antibody, DAS: disease activity score, HDL: high density lipoprotein, CRP: C-reactive protein, IL-6: interleukin 6, MFR: myocardial blood flow reserve, CAC: coronary artery calcium.
Figure 2:
Adjusted Associations of MFR and DAS-28 with LVMI in RA Participants. Means and 95% confidence intervals are depicted. Adjusted analyses account for race, seropositivity for either anti-CCP antibody or Rheumatoid factor, Health Assessment Questionnaire score, Disease Activity Score 28 (for Panel A), with the addition of Myocardial Blood Flow Reserve and ACE-inhibitor use (for Panel B) which were the only covariates independently associated with LVMI in the multivariable models
We also evaluated the independent association of MFR with other cardiac structure and function parameters, all of which were in the normal ranges except for diastolic dysfunction (present in 49% of the RA patients) (Table 1). Similar independent associations of reduced MFR with several of these measures of cardiac structure – specifically, higher left atrial volume index and higher end diastolic and systolic volume indexes- were observed (p < 0.05 for all; supplementary Tables S2 to S4). Regarding functional measures, LV ejection fraction (LVEF) was in the normal range for all patients, and there was no association of MFR with LVEF, nor with stroke volume (SV) or a measure of diastolic dysfunction (E/E’), in univariate analyses (supplementary Table S2). However, there was a small inverse association of MFR with cardiac index in univariate analyses (Table S2) which remained significant in multivariable analyses (Table S6). Sensitivity analyses for the multivariable models in Tables 3, 4, and S2, S3, S4, S5 and S6, using a forward selection procedure for variables significant at the 0.20 level, were performed and confirmed the above statistically significant associations with only nominal differences in p values (data not shown).
Comparison with controls
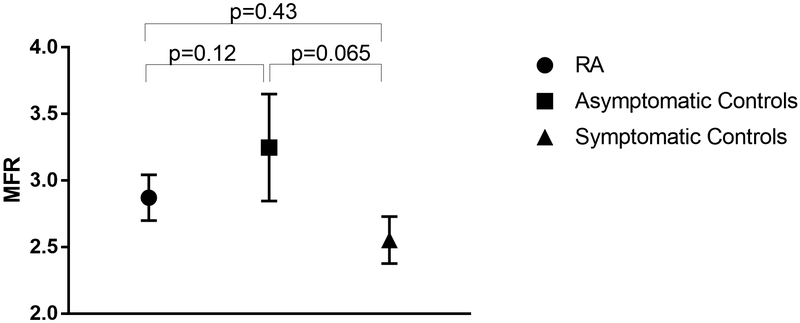
In asymptomatic controls, the mean MBF was 0.94± 0.21 mL/g/min at rest and 2.98 ± 0.66 mL/g/min during peak stress, yielding a mean MFR of 3.25 ± 0.7. In the symptomatic controls, the mean MBF was 0.85± 0.16 mL/g/min at rest and 2.12 ± 0.39 mL/g/min during peak stress, yielding a mean MFR of 2.55 ± 0.6 for symptomatic controls. While the mean MFR of the RA patients was numerically closer to that of the symptomatic controls, and less than that of asymptomatic controls, there were no statistically significant differences between the various groups after adjustment for hypertension and gender, the only variables significantly associated with MFR in the multivariable model (Figure 3).
Figure 3:
Comparison of MFR between RA patients and asymptomatic and symptomatic non-RA controls after adjustment for gender and hypertension. Means and 95% confidence intervals are depicted. Adjusted analyses account for hypertension and gender which were the only covariates independently associated with MFR in the multivariable models
Discussion:
Coronary arterial microcirculation to the myocardium cannot be assessed on routine coronary angiography. However, its functional (vasodilatory) capacity can be inferred indirectly from measurement of MBF and MFR, which, in the absence of significant macrovascular (coronary artery) disease, is assumed to represent microvascular perfusion. The current study is the largest to date of MBF and MFR in RA, which allowed a detailed assessment of associations of RA characteristics with MFR. It is also the first study in RA to evaluate the association of MFR with subclinical measures of LV structure and function. The major findings in this study are that reduced MFR was prevalent in approximately 30% of RA patients, that lower MFR was associated with measures of inflammation independent of conventional CV risk factors and CAC, and that lower MFR was independently associated with higher LVMI and higher volumes. We also found that RA patients had a mean MFR that was comparable to non-RA symptomatic controls referred for clinical NH3 PET-CT studies.
In the general population, it is hypothesized that a pro-inflammatory state associated with aging and co-morbidities such as diabetes and obesity is the first in a sequence of events associated with endothelial and microvascular dysfunction which in turns promotes LV hypertrophy, remodeling, and stiffness, leading to heart failure (6, 26-29). There is growing evidence that RA patients without clinical CVD have a higher prevalence of endothelial dysfunction than matched non-RA controls (30), even at the early stages of disease (31). However, studies assessing microvascular dysfunction at the myocardial level in RA are rare and limited to small number of patients (8, 9, 31, 32).
The recognized gold standard methodology for noninvasive assessment of microvascular dysfunction is cardiac PET-CT which provides accurate and reproducible quantification of MBF (11). This modality has been applied to RA patients in only one prior study (9) wherein a combined group of 13 SLE and 12 RA patients with ≤ 1 CV risk factor and minimal to no CAD had a 34% lower mean MFR than a healthy control group (9). This study was too small to investigate the association of MFR with LV structure and function, or with patient characteristics other than disease duration. MBF can also be quantified by MRI with first pass gadolinium but these authors are not aware of any published data using this quantitative technique in RA. There is one small study in which 2 of 18 RA patients (8) had qualitatively assessed first pass defects in gadolinium perfusion that did not map to the territory of a coronary artery, suggesting microvascular disease.
In our RA patients without clinical CVD nearly 30% of patients had reduced MFR (<2.5). MFR measurements in healthy controls are sparse but a recent study in 77 healthy men (mean age 36 ± 4) reported a mean MFR of 4.1 ±1.3 and less than 1% had MFR <2.5 (16). Historical data in patients suspected of coronary artery disease indicate that a MFR<2.5 may reflect two possibilities: 1) coronary artery stenosis > 50%, or 2) microvascular dysfunction in the absence of significant stenosis on coronary artery angiography (18). Although we did not perform coronary angiography to rule out coronary artery stenosis, the vast majority of our RA cohort had no, or mild, coronary atherosclerosis as measured by CACs of zero or less than 100, respectively; moreover, adjustment for CAC score did not alter the relationships of MFR to IL-6 or TNF inhibitor use. Thus, we speculate that the low MFRs in our RA patients more likely reflect microvascular disease. Interestingly, the mean MFR in our RA cohort was similar to non-RA controls referred for clinical PET-CT scans (symptomatic controls) despite the non-RA controls having higher levels of conventional CV risk factors, older age and greater proportion of men. The highest mean MFR was in the non-RA controls who were recruited using the same criteria as the RA group (the asymptomatic controls). Although their mean MFR was arithmetically higher than that of the RA group, it did not reach statistical significance.
In the general population, CV risk factors are associated with lower MFRs (33). In our RA patient population, we confirmed the association of lower MFR with male gender, as previously reported in the general population (34). Interestingly, there was no association of MFR, in either univariate or multivariable analyses, with other conventional CV risk factors such as tobacco use, hyperlipidemia or diabetes mellitus, which are known to be associated with microvascular dysfunction in the general population (12). These findings suggest the possibility that microvascular dysfunction in the RA population may be driven, at least in part, by determinants other than conventional cardiovascular risk factors.
In the general population, elevated levels of circulating pro-inflammatory cytokines, particularly IL-6 and TNF, are independent predictors of the incidence and severity of heart failure (35, 36) and have been implicated in the pathogenesis of endothelial and microvascular dysfunction (37-39). Our data in this RA cohort showed lower MFRs in patients with the highest IL-6 levels, and higher MFRs in those receiving inhibitors of TNF. These observations are consistent with inflammation being a determinant of microvascular disease in RA, and are consistent with several small interventional studies reporting improvement of endothelial dysfunction with anti-TNF therapy (40-43). However, because our study was cross-sectional, causality cannot be inferred. Prednisone, which is known to be atherogenic, was not associated with lower MFR in this cohort but residual confounding cannot be ruled out. Likewise, an association of each DMARD class with MFR could not be evaluated due to small numbers.
We performed echocardiography in our RA participants to assess whether lower MFR was associated with measures of LV remodeling. In the general population, increased LVMI in particular has been identified as a subclinical phenotype of LV remodeling in the pathway to the development of heart failure(44). The association of myocardial microvascular dysfunction with LV remodeling is hypothesized to be triggered by inflammation-induced endothelial injury(45, 46) which results in cardiomyocyte hypertrophy (26), concentric LV remodeling and LV wall stiffness (26). These same echocardiographic findings are also reported in a significant proportion of RA patients without clinical CVD (26-28). Progressive increase in LVMI precedes heart failure in the general population, and is a potent predictor of sudden cardiac death, even in subsets with normal ejection fraction(47, 48). We observed a statistically significant association of lower MFR with higher LVMI in our RA cohort, although none of the LVMIs met cut-off levels for high LVMI (25). That we observed similar independent associations of reduced MFR with other structural LV parameters, namely with higher LV end diastolic and systolic volumes, further supports an association of microvascular dysfunction with LV remodeling in RA.
Our results did not show a significant association of lower MFR with most measures of systolic and diastolic function, except for a modest relationship with cardiac index. This may reflect a true lack of association or that structural changes precede functional changes; indeed, we excluded patients with clinical heart failure.
The strengths of our study are the relatively large sample size, use of the gold standard for quantifying MBF, concomitant assessment of sensitive measures of subclinical cardiac structure and function, and concomitant assessment of a measure of severity of coronary artery disease. Another strength is the inclusion of patients with and without CV risk factors as well as patients with early and late RA, thus accurately reflecting the population of RA patients managed in clinical practice.
There are several limitations to our study. Because of the cross sectional nature of our study design, we could not assess a causative link between microvascular dysfunction and LV mass. Our patients did not undergo coronary angiography to rule out flow limiting coronary artery disease, however we measured CAC which is a validated surrogate measure of atherosclerosis. Despite statistical adjustments, there may be residual confounding by medications and unmeasured variables.
Conclusion
Reduced MFR was prevalent in approximately one third of RA patients and was associated with a higher LVMI, as well as measures of inflammation independent of conventional CV risk factors. Whether these factors, in turn, contribute to the increased risk for heart failure in patients with RA compared to controls will require large longitudinal studies.
Supplementary Material
Acknowledgments:
The authors would like to thank the RHYTHM study staff (Afshin Zartoshti, Janine Rose, Rachel Broderick, Rachelle Morgenstern, Thania Perez) for their hard work and dedication, and the participants in the RHYTHM study who graciously agreed to participate in this research. We also thank Drs. Alice Chu, Anna Broder, Teja Kapoor, Edward Dwyer, Elizabeth Mayer, Katherine Nickerson, Jane Kang, Laura Geraldino, and others for generously recommending their patients for this study.
Sources of Funding: Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases under Award Number AR-050026 (JMB), the Rheumatology Research Foundation under Award Number CU15-0082 (JMB), and by the National Institutes of Health National Center for Translational Science Clinical and Translational Science Award (CTSA) grant UL1 TR000040, of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosures: Dr. Russo is currently a full-time employee of Novartis Institutes, Basel, Switzerland. Dr Tugcu is currently a full time employee of Bristol Myers Squibb, Lawrenceville, NJ. Their contributions to this work were performed while both were full-time faculty members at Columbia University.
References:
- 1.Gonzalez A, Maradit Kremers H, Crowson CS, Nicola PJ, Davis JM 3rd, Therneau TM, Roger VL, Gabriel SE. The widening mortality gap between rheumatoid arthritis patients and the general population. Arthritis Rheum. 2007;56(11):3583–7. doi: 10.1002/art.22979. PubMed PMID: 17968923. [DOI] [PubMed] [Google Scholar]
- 2.Nicola PJ, Crowson CS, Maradit-Kremers H, Ballman KV, Roger VL, Jacobsen SJ, Gabriel SE. Contribution of congestive heart failure and ischemic heart disease to excess mortality in rheumatoid arthritis. Arthritis Rheum. 2006;54:60–7. doi: 10.1002/art.21560. PubMed PMID: 16385496. [DOI] [PubMed] [Google Scholar]
- 3.Wright K, Crowson CS, Gabriel SE. Cardiovascular comorbidity in rheumatic diseases: a focus on heart failure. Heart failure clinics. 2014;10:339–52. doi: 10.1016/j.hfc.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis JM 3rd, Roger VL, Crowson CS, Kremers HM, Therneau TM, Gabriel SE. The presentation and outcome of heart failure in patients with rheumatoid arthritis differs from that in the general population. Arthritis Rheum. 2008;58:2603–11. doi: 10.1002/art.23798. PubMed PMID: 18759286; PMCID: 2631416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mentz RJ, Kelly JP, von Lueder TG, Voors AA, Lam CS, Cowie MR, Kjeldsen K, Jankowska EA, Atar D, Butler J, Fiuzat M, Zannad F, Pitt B, O’Connor CM. Noncardiac comorbidities in heart failure with reduced versus preserved ejection fraction. Journal of the American College of Cardiology. 2014;64:2281–93. doi: 10.1016/j.jacc.2014.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cahill PA, Redmond EM. Vascular endothelium - Gatekeeper of vessel health. Atherosclerosis. 2016;248:97–109. doi: 10.1016/j.atherosclerosis.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown RA, Shantsila E, Varma C, Lip GY. Current understanding of atherogenesis. The American journal of medicine. 2016. doi: 10.1016/j.amjmed.2016.10.022. [DOI] [PubMed] [Google Scholar]
- 8.Kobayashi Y, Giles JT, Hirano M, Yokoe I, Nakajima Y, Bathon JM, Lima JA, Kobayashi H. Assessment of myocardial abnormalities in rheumatoid arthritis using a comprehensive cardiac magnetic resonance approach: a pilot study. Arthritis Res Ther. 2010;12:R171. doi: 10.1186/ar3131. PubMed PMID: 20836862; PMCID: 2990998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Recio-Mayoral A, Mason JC, Kaski JC, Rubens MB, Harari OA, Camici PG. Chronic inflammation and coronary microvascular dysfunction in patients without risk factors for coronary artery disease. Eur Heart J. 2009;30:1837–43. doi: 10.1093/eurheartj/ehp205. PubMed PMID: 19502228. [DOI] [PubMed] [Google Scholar]
- 10.Waller AH, Blankstein R, Kwong RY, Di Carli MF. Myocardial blood flow quantification for evaluation of coronary artery disease by positron emission tomography, cardiac magnetic resonance imaging, and computed tomography. Curr Cardiol Rep 2014;16:483. doi: 10.1007/s11886-014-0483-6. PubMed PMID: 24718671; PMCID: 4053657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaufmann PA, Camici PG. Myocardial blood flow measurement by PET: technical aspects and clinical applications. J Nucl Med. 2005;46:75–88. PubMed PMID: 15632037. [PubMed] [Google Scholar]
- 12.Camici PG, Crea F. Coronary microvascular dysfunction. The New England journal of medicine. 2007;356:830–40. doi: 10.1056/NEJMra061889. [DOI] [PubMed] [Google Scholar]
- 13.Murthy VL, Naya M, Foster CR, Hainer J, Gaber M, Di Carli G, Blankstein R, Dorbala S, Sitek A, Pencina MJ, Di Carli MF. Improved cardiac risk assessment with noninvasive measures of coronary flow reserve. Circulation. 2011;124:2215–24. doi: 10.1161/CIRCULATIONAHA.111.050427. PubMed PMID: 22007073; PMCID: 3495106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Winchester R, Giles JT, Nativ S, Downer K, Zhang H- Z, Bag-Ozbek A, Zartoshti A, Bokhari S, Bathon JM. Association of Elevations of Specific T Cell and Monocyte Subpopulations in Rheumatoid Arthritis With Subclinical Coronary Artery Atherosclerosis. Arthritis & rheumatology (Hoboken, NJ). 2016;68:92–102. doi: 10.1002/art.39419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd, Birnbaum NS, Burmester GR, Bykerk VP, Cohen MD, Combe B, Costenbader KH, Dougados M, Emery P, Ferraccioli G, Hazes JM, Hobbs K, Huizinga TW, Kavanaugh A, Kay J, Kvien TK, Laing T, Mease P, Menard HA, Moreland LW, Naden RL, Pincus T, Smolen JS, Stanislawska-Biernat E, Symmons D, Tak PP, Upchurch KS, Vencovsky J, Wolfe F, Hawker G. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569–81. doi: 10.1002/art.27584. PubMed PMID: 20872595. [DOI] [PubMed] [Google Scholar]
- 16.Uusitalo V, Saraste A, Kajander S, Luotolahti M, Wendelin-Saarenhovi M, Sundell J, Raitakari O, Knuuti J. The association between coronary flow reserve and development of coronary calcifications: a follow-up study for 11 years in healthy young men. European heart journal cardiovascular Imaging. 2013;14:812–8. doi: 10.1093/ehjci/jes301. [DOI] [PubMed] [Google Scholar]
- 17.Rosas E, Slomka PJ, García-Rojas L, Calleja R, Jácome R, Jiménez-Santos M, Romero E, Meave A, Berman DS. Functional Impact of Coronary Stenosis Observed on Coronary Computed Tomography Angiography: Comparison with 13N-Ammonia PET. Archives of Medical Research. 2010;41:642–8. doi: 10.1016/j.arcmed.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 18.Schindler TH, Schelbert HR, Quercioli A, Dilsizian V. Cardiac PET imaging for the detection and monitoring of coronary artery disease and microvascular health. JACC Cardiovascular imaging. 2010;3:623–40. doi: 10.1016/j.jcmg.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 19.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. Journal of the American College of Cardiology. 1990;15:827–32. [DOI] [PubMed] [Google Scholar]
- 20.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, O’Leary DH, Tracy R, Watson K, Wong ND, Kronmal RA. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. The New England journal of medicine. 2008;358:1336–45. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 21.Russo C, Jin Z, Homma S, Rundek T, Elkind MSV, Sacco RL, Tullio MR. Relationship of Multidirectional Myocardial Strain with Radial Thickening and Ejection Fraction and Impact of Left Ventricular Hypertrophy: A Study in a Community Based Cohort. Echocardiography. 2013;30:794–802. doi: 10.1111/echo.12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Russo C, Jin Z, Homma S, Rundek T, Elkind MS, Sacco RL, Di Tullio MR. Effect of obesity and overweight on left ventricular diastolic function: a community-based study in an elderly cohort. Journal of the American College of Cardiology. 2011;57:1368–74. doi: 10.1016/j.jacc.2010.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wells G, Becker JC, Teng J, Dougados M, Schiff M, Smolen J, Aletaha D, van Riel P. Validation of the 28-joint Disease Activity Score (DAS28) and European League Against Rheumatism response criteria based on C-reactive protein against disease progression in patients with rheumatoid arthritis, and comparison with the DAS28 based on erythrocyte sedimentation rate. Annals of the rheumatic diseases. 2009;68:954–60. doi: 10.1136/ard.2007.084459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fries JF, Spitz P, Kraines RG, Holman HR. Measurement of patient outcome in arthritis. Arthritis and rheumatism. 1980;23:137–45. [DOI] [PubMed] [Google Scholar]
- 25.Mizukoshi K, Takeuchi M, Nagata Y, Addetia K, Lang RM, Akashi YJ, Otsuji Y. Normal Values of Left Ventricular Mass Index Assessed by Transthoracic Three-Dimensional Echocardiography. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2016;29:51–61. doi: 10.1016/j.echo.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 26.Aslam F, Bandeali SJ, Khan NA, Alam M. Diastolic dysfunction in rheumatoid arthritis: a meta-analysis and systematic review. Arthritis care & research. 2013;65:534–43. Epub 2012/09/25. doi: 10.1002/acr.21861. PubMed PMID: 23002032. [DOI] [PubMed] [Google Scholar]
- 27.Neglia D, Michelassi C, Trivieri M, Sambuceti G, Giorgetti A, Pratali L, Gallopin M, Salvadori P, Sorace O, Carpeggiani C. Prognostic role of myocardial blood flow impairment in idiopathic left ventricular dysfunction. Circulation. 2002;105:186–93. doi: 10.1161/hc0202.102119. [DOI] [PubMed] [Google Scholar]
- 28.Majmudar MD, Murthy VL, Shah RV, Kolli S, Mousavi N, Foster CR, Hainer J, Blankstein R, Dorbala S, Sitek A, Stevenson LW, Mehra MR, Carli MF. Quantification of coronary flow reserve in patients with ischaemic and non-ischaemic cardiomyopathy and its association with clinical outcomes. European Heart Journal - Cardiovascular Imaging. 2015;16. doi: 10.1093/ehjci/jev012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giamouzis G, Schelbert EB, Butler J. Growing Evidence Linking Microvascular Dysfunction With Heart Failure With Preserved Ejection Fraction. Journal of the American Heart Association. 2016;5. doi: 10.1161/JAHA.116.003259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Minno M, Ambrosino P, Lupoli R, Minno A, Tasso M, Peluso R, Tremoli E. Clinical assessment of endothelial function in patients with rheumatoid arthritis: A meta-analysis of literature studies. European journal of internal medicine. 2015;26:835–42. doi: 10.1016/j.ejim.2015.10.016. [DOI] [PubMed] [Google Scholar]
- 31.Turiel M, Atzeni F, Tomasoni L, de Portu S. Non-invasive assessment of coronary flow reserve and ADMA levels: a case–control study of early rheumatoid arthritis patients. 2009. doi: 10.1093/rheumatology/kep082. [DOI] [PubMed] [Google Scholar]
- 32.Ciftci O, Yilmaz S, Topcu S, Caliskan M, Gullu H, Erdogan D, Pamuk BO, Yildirir A, Muderrisoglu H. Impaired coronary microvascular function and increased intima-media thickness in rheumatoid arthritis. Atherosclerosis. 2008;198:332–7. doi: 10.1016/j.atherosclerosis.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 33.Prior JO, Quiñones MJ, Hernandez-Pampaloni M, Facta AD, Schindler TH, Sayre JW, Hsueh WA, Schelbert HR. Coronary circulatory dysfunction in insulin resistance, impaired glucose tolerance, and type 2 diabetes mellitus. Circulation. 2005;111:2291–8. doi: 10.1161/01.CIR.0000164232.62768.51. [DOI] [PubMed] [Google Scholar]
- 34.Patel MB, Bui LP, Kirkeeide RL, Gould LK. Imaging Microvascular Dysfunction and Mechanisms for Female-Male Differences in CAD. JACC: Cardiovascular Imaging. 2016;9:465–82. doi: 10.1016/j.jcmg.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 35.Deswal A, Petersen NJ, Feldman AM, Young JB. Cytokines and cytokine receptors in advanced heart failure an analysis of the cytokine database from the Vesnarinone Trial (VEST). Circulation. 2001. doi: 10.1161/01.CIR.103.16.2055. [DOI] [PubMed] [Google Scholar]
- 36.Gonzalez-Juanatey C, Llorca J, Testa A, Revuelta J, Garcia-Porrua C, Gonzalez-Gay MA. Increased prevalence of severe subclinical atherosclerotic findings in long-term treated rheumatoid arthritis patients without clinically evident atherosclerotic disease. Medicine (Baltimore). 2003;82:407–13. doi: 10.1097/01.md.0000101572.76273.60. PubMed PMID: 14663290. [DOI] [PubMed] [Google Scholar]
- 37.van Heerebeek L, Hamdani N, Falcao-Pires I, Leite-Moreira AF, Begieneman MP, Bronzwaer JG, van der Velden J, Stienen GJ, Laarman GJ, Somsen A, Verheugt FW, Niessen HW, Paulus WJ. Low myocardial protein kinase G activity in heart failure with preserved ejection fraction. Circulation. 2012;126:830–9. Epub 2012/07/19. doi: 10.1161/circulationaha.111.076075. PubMed PMID: 22806632. [DOI] [PubMed] [Google Scholar]
- 38.van Heerebeek L, Franssen CP, Hamdani N, Verheugt FW, Somsen GA, Paulus WJ. Molecular and cellular basis for diastolic dysfunction. Current heart failure reports. 2012;9:293–302. Epub 2012/08/29. doi: 10.1007/s11897-012-0109-5. PubMed PMID: 22926993. [DOI] [PubMed] [Google Scholar]
- 39.Westermann D, Lindner D, Kasner M, Zietsch C, Savvatis K, Escher F, von Schlippenbach J, Skurk C, Steendijk P, Riad A, Poller W, Schultheiss HP, Tschope C. Cardiac inflammation contributes to changes in the extracellular matrix in patients with heart failure and normal ejection fraction. Circulation Heart failure. 2011;4:44–52. Epub 2010/11/16. doi: 10.1161/circheartfailure.109.931451. PubMed PMID: 21075869. [DOI] [PubMed] [Google Scholar]
- 40.Hürlimann D, Forster A, Noll G, Enseleit F, Chenevard R, Distler O, Béchir M, Spieker LE, Neidhart M, Michel BA, Gay RE, Lüscher TF, Gay S, Ruschitzka F. Anti-tumor necrosis factor-alpha treatment improves endothelial function in patients with rheumatoid arthritis. Circulation. 2002;106:2184–7. doi: 10.1161/01.CIR.0000037521.71373.44. [DOI] [PubMed] [Google Scholar]
- 41.Bilsborough W, Keen H, Taylor A, O’Driscoll GJ, Arnolda L, Green DJ. Anti-tumour necrosis factor-alpha therapy over conventional therapy improves endothelial function in adults with rheumatoid arthritis. Rheumatology International. 2006;26:1125–31. doi: 10.1007/s00296-006-0147-y. PubMed PMID: WOS:000240715400011. [DOI] [PubMed] [Google Scholar]
- 42.Datta D, Ferrell WR, Sturrock RD, Jadhav ST, Sattar N. Inflammatory suppression rapidly attenuates microvascular dysfunction in rheumatoid arthritis. Atherosclerosis. 2007;192:391–5. doi: 10.1016/j.atherosclerosis.2006.05.034. PubMed PMID: WOS:000247225900023. [DOI] [PubMed] [Google Scholar]
- 43.Sidiropoulos PI, Siakka P, Pagonidis K, Raptopoulou A, Kritikos H, Tsetis D, Boumpas DT. Sustained improvement of vascular endothelial function during anti-TNF treatment in rheumatoid arthritis patients. Scandinavian Journal of Rheumatology. 2009;38:6–10. doi: 10.1080/03009740802363768. PubMed PMID: WOS:000263093900002. [DOI] [PubMed] [Google Scholar]
- 44.Cheng S, Vasan RS. Advances in the epidemiology of heart failure and left ventricular remodeling. Circulation. 2011;124:9. doi: 10.1161/CIRCULATIONAHA.111.070235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brutsaert DL, Meulemans AL, Sipido KR, Sys SU. Effects of damaging the endocardial surface on the mechanical performance of isolated cardiac muscle. Circulation research. 1988;62:358–66. doi: 10.1161/01.RES.62.2.358. [DOI] [PubMed] [Google Scholar]
- 46.Paulus WJ, Vantrimpont PJ, Shah AM. Paracrine Coronary Endothelial Control of Left Ventricular Function in Humans. Circulation. 1995;92:2119–26. doi: 10.1161/01.cir.92.8.2119. [DOI] [PubMed] [Google Scholar]
- 47.Stevens SM, Reinier K, Chugh SS. Increased left ventricular mass as a predictor of sudden cardiac death: is it time to put it to the test? Circulation Arrhythmia and electrophysiology. 2013;6:212–7. doi: 10.1161/CIRCEP.112.974931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reinier K, Dervan C, Singh T, Uy-Evanado A, Lai S, Gunson K, Jui J, Chugh SS. Increased left ventricular mass and decreased left ventricular systolic function have independent pathways to ventricular arrhythmogenesis in coronary artery disease. Heart rhythm : the official journal of the Heart Rhythm Society. 2011;8:1177–82. doi: 10.1016/j.hrthm.2011.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.