Abstract
Subjective Visual Vertical (SVV) deviations have been correlated to abnormal cerebellar function in individuals diagnosed with Multiple Sclerosis (MS). It has been shown that individuals with MS have increased incidence of SVV abnormalities, yet this is not routinely tested in this population during physical therapy evaluation.
Objective:
To determine if there is a relationship between SVV and balance performance in people with MS who have cerebellar involvement. We hypothesize that individuals with greater SVV deviations will have worse balance performance.
Methods:
Fifteen females and 5 males [mean age 54.5 years old (± 7.03 SD)] with the diagnosis of MS and cerebellar involvement participated. Computerized SVV testing included rod and rod-and-frame conditions. None of the balance outcomes were correlated with the rod only condition. Because there was a difference in magnitude of results within the rod-and-frame condition, based on whether the frame was rotated clockwise (CW) or counter clockwise (CCW), they were analyzed independently.
Results:
For all 6 of the balance outcomes there was a statistically significant moderate correlation with SVV deviations when the frame was tilted CCW: Barthel Index (r=−.47, p=.018); Berg Balance Score (r=−.59, p=.003); gait velocity (r=−.52, p=.010); International Cooperative Ataxia Rating Scale (r=.56, p=.006); Scale for the Assessment and Rating of Ataxia (r=.62, p=.002); and Timed Up and Go (r=.58, p=.003). Interestingly, the Barthel Index was the only outcome that had statistical significance with a moderate correlation (r=−.66, p=.001) when the frame was rotated CW. In this cohort greater deviations during the rod-and-frame condition of SVV testing correlated with worse functional outcomes, especially when the frame was tilted CCW.
Conclusion:
Individuals with MS who demonstrate decreased balance performance may rely more heavily on visual backgrounds. Implementation of SVV assessment for individuals with MS may provide clinicians with valuable information to identify clinical interventions.
Keywords: Balance, multiple sclerosis, subjective visual vertical, vestibular rehabilitation
Introduction
In 1,065 middle-aged and older adults living with MS, 88% experienced impaired balance (M. Finlayson, Plow, & Cho, 2010). It has been reported that 52% of people with MS sustain at least one fall to the ground within a 6 month time frame (Finlayson, Peterson, & Cho, 2006). In addition, the sequelae of impaired balance and mobility negatively impacts quality of life for individuals with MS (Zwibel, 2009). The exact pathophysiology of the postural instability related to the diagnosis of MS is not clear but has been attributed to the location of the lesion. Additionally, the common disease manifestations of impaired cognition, vision, vestibular, motor, sensation, and coordination may contribute to balance impairments (Cameron, Horak, Herndon, & Bourdette, 2008).
The ability to perceive upright, or subjective visual vertical (SVV), is a function of the utricle and the superior vestibular nerve in the vestibular system and is a component of the ocular tilt reaction (Curthoys, Dai, & Halmagyi, 1991; MacNeilage, Banks, Berger, & Bulthoff, 2007; Tarnutzer, Bockisch, & Straumann, 2010; Vingerhoets, De Vrijer, Van Gisbergen, & Medendorp, 2009). When the otoliths are functioning normally, an illuminated rod can be adjusted within 0 ± 2 degrees from vertical during SVV assessment (Bohmer & Mast, 1999; Friedmann, 1970). In addition to perceiving upright, the otolith also functions during linear acceleration, eye movement control, and postural responses (Gresty & Bronstein, 1992). While other assessments of the perception of vertical have been related to balance control such as subjective postural vertical (SPV) and subjective haptic vertical (SHV), SVV is most likely the cause of postural instability in vestibular hypofunction (Bisdorff, Wolsley, Anastasopoulos, Bronstein, & Gresty, 1996).
Deviations of SVV have been noted in persons with MS (Versino et al., 2002) and it has been reported that 36% (n=50) of individuals with MS had an abnormal SVV (Crevits, Venhovens, Vanoutrive, & Debruyne, 2007; Serra, Derwenskus, Downey, & Leigh, 2003). There was a correlation between the SVV abnormality and disability (Crevits et al., 2007). Serra et al reported that people with MS who had the largest SVV deviations had more impaired cerebellar function which was speculated to be a result of underlying damage to the central otolithic pathways (Serra et al., 2003). However, the association of SVV related to balance performance in individuals with MS has not been documented. Additionally, there does not appear to be any prior work investigating the assessment of SVV in the presence of a misleading static visual reference (rod & frame illusion condition) in persons with MS who have well documented visual (internuclear opthalmoplegia, saccadic dysmetria, nystagmus, skew deviation, and abnormal smooth pursuit)(Graves & Balcer, 2010), SVV, and balance deficits.
The purpose of this study was to determine if there was a relationship between SVV (with and without static visual reference) and balance performance in people with MS who have cerebellar involvement. We hypothesized that individuals with greater SVV deviations would have worse balance performance.
Methods
Study Design.
Participants completed the Barthel Index self-report questionnaire (Mahoney & Barthel, 1965) followed by five balance and gait assessments including the Berg Balance Scale (BBS) (Berg, Wood-Dauphinee, Williams, & Maki, 1992; K. Berg, Wood-Dauphine, Williams, & Gayton, 1989), gait speed (Studenski et al., 2011), International Co-operative Ataxia Rating Scale (ICARS) (Schmitz-Hubsch, Tezenas du Montcel, et al., 2006), Scale for the Assessment and Rating of Ataxia (SARA) (Schmitz-Hubsch, du Montcel, et al., 2006), and Timed Up and Go (TUG) (Podsiadlo & Richardson, 1991). The use of a personal assistive device was permitted during gait assessment if the participant reported using a device during typical community ambulation. Rest breaks were allotted between tests and on an individual basis as needed. Following balance and gait testing, each participant completed computerized SVV testing. The total duration of the study visit was approximately 90 minutes.
Some of the balance measures included in this study were chosen based on prior work that aimed to standardize outcomes based on high clinical utility specifically for people with cerebellar ataxia. The ICARS was identified from a systematic review (Winser, Smith, Hale, Claydon, Whitney, et al., 2015) and the BBS, SARA, and TUG were identified from a Delphi survey study (Winser, Smith, Hale, Claydon, & Whitney, 2015).
Subjects.
Males and females between the ages of 18 to 65 years of age (mean age = 54.5 ± 7.03) with the diagnosis of MS and cerebellar involvement were eligible for participation. The diagnosis of multiple sclerosis was determined by a board-certified neurologist and cerebellar involvement was identified by: gait ataxia, limb ataxia, dysarthria, and/or nystagmus during screening procedures. Twenty individuals were recruited from the Multiple Sclerosis registry and the University of Pittsburgh Medical Center Neurology Department. Inclusion criteria were the ability to ambulate 10 meters without physical assistance of another person, but the use of an assistive device was permitted. The study sample was comprised of 15 females and 5 males (n=20), mean age 54.5 years old (± 7.03 SD). The Institutional Review Board at the University of Pittsburgh approved the study protocol (PRO13080051) and all participants provided informed consent.
Materials.
Each participant completed the Barthel Index questionnaire to obtain a self-report of performance of activities of daily living (ADL) (Collin, Wade, Davies, & Horne, 1988; Mahoney & Barthel, 1965). This instrument includes 10 ADL activities that are rated based on independence levels at which the individual can complete the mobility tasks. Higher scores are indicative of increased independence. Scores of 91–99 indicate slight dependence, 61–90 moderate dependence, 21–60 severe dependence, and 0–20 total dependence (Collin et al., 1988).
The Berg Balance Scale (BBS) is a 14-item outcome tool used to assess static and dynamic functional activities (Berg et al., 1992). The items are scored from 0 to 4 (worst to best) and when items are summed there is a maximum score of 56. The use of the BBS for individuals diagnosed with multiple sclerosis across the spectrum of clinical environments (acute care, inpatient rehabilitation, skilled nursing, outpatient, and home health) has been reported. A cut-off score of less than 45/56 identifies individuals at increased fall risk (Berg et al., 1992). The BBS has also been shown to discriminate fallers (mean score of 45.8) from non-fallers (mean score of 50.8) in individuals with MS (Dibble, Lopez-Lennon, Lake, Hoffmeister, & Gappmaier, 2013).
The 10-meter walk test was used to measure gait speed. Study participants were instructed to walk at their preferred speed for 10 meters. Time was recorded using a manual stopwatch for the middle 6 meters to account for acceleration and deceleration. The same assessor collected gait speed measurements for all of the study participants. The use of an assistive device was permitted and the assessor ambulated with the participant for safety. In older adults, gait speed has been associated with fall risk and overall mortality (Fritz & Lusardi, 2009; Studenski et al., 2011).
The ICARS is a 19 item outcome measure that quantifies impairment due to cerebellar ataxia (Trouillas et al., 1997). Four subscales including postural and gait disturbances, limb ataxia, dysarthria, and oculomotor disorders were assessed. Higher scores are indicative of worse impairment with a maximum score of 100. The ICARS has been validated for individuals with focal cerebellar lesions (Schoch et al., 2007).
The SARA outcome tool has been validated for individuals with ataxia (Weyer et al., 2007) and consists of eight items that attempt to quantify gait and ADL performance using a 40 point scale, where zero equates to no ataxia and 40 is equal to the most severe ataxia. In this assessment the participants were scored on walking, standing, sitting, speaking, and four coordination tasks.
For the TUG, participants were instructed to stand from a seated position in a chair with arm rests and walk at their preferred speed in a straight path for three meters, turn at the marked endpoint, walk back to the chair in a straight path, and sit on the chair (Podsiadlo & Richardson, 1991). Time was recorded in seconds. The use of an assistive device was permitted and the assessor walked with the participant for safety. The Berg Balance Scale, the use of walking aids, and the Timed Up and Go has been shown to predict falls in individuals with MS (Nilsagard, Lundholm, Denison, & Gunnarsson, 2009).
During computerized SVV testing, the participant’s ability to orient a fluorescent rod to a vertical position was assessed. Our protocol reflected other studies in the vestibular literature that investigated SVV rod and SVV rod-and-frame conditions (Guerraz et al., 2001; Pavlou, Davies, & Bronstein, 2006). Participants were seated at a distance of 80 cm away from the apparatus (as described by Guerraz et al., 2001) with chin support to maintain head stability in a neutral position. The center of the rod (40 × 0.5 cm) was positioned at eye level. For each of the trials the participant had 30 seconds to position the rod from an initial offset position of ± 40 deg into the perceived vertical position using a joystick control. The output generated from the device was assured by comparing verticality measurement from an electronic level inclinometer and calibration was completed prior to each testing session. A positive number indicated a deviation in the CW direction, while a negative number was indicative of a CCW deviation. During the rod and frame trials, the frame was rotated 28 degrees clockwise (CW) or counterclockwise (CCW). Aside from the illuminated rod or rod and frame, the testing room was completely dark. The end position of the rod was computed and recorded via a customized software program. Symptoms of dizziness, anxiety, and/or discomfort were monitored using the Subjective Units of Distress Scale which is a verbal analog scale that ranges from 0 (no symptoms) to 10 (worst imaginable symptoms). In between trials the participants view was occluded using darkened goggles. Each participant completed four trials of the rod condition and a total of 8 trials of rod and frame conditions (Figure 1).
Figure 1:
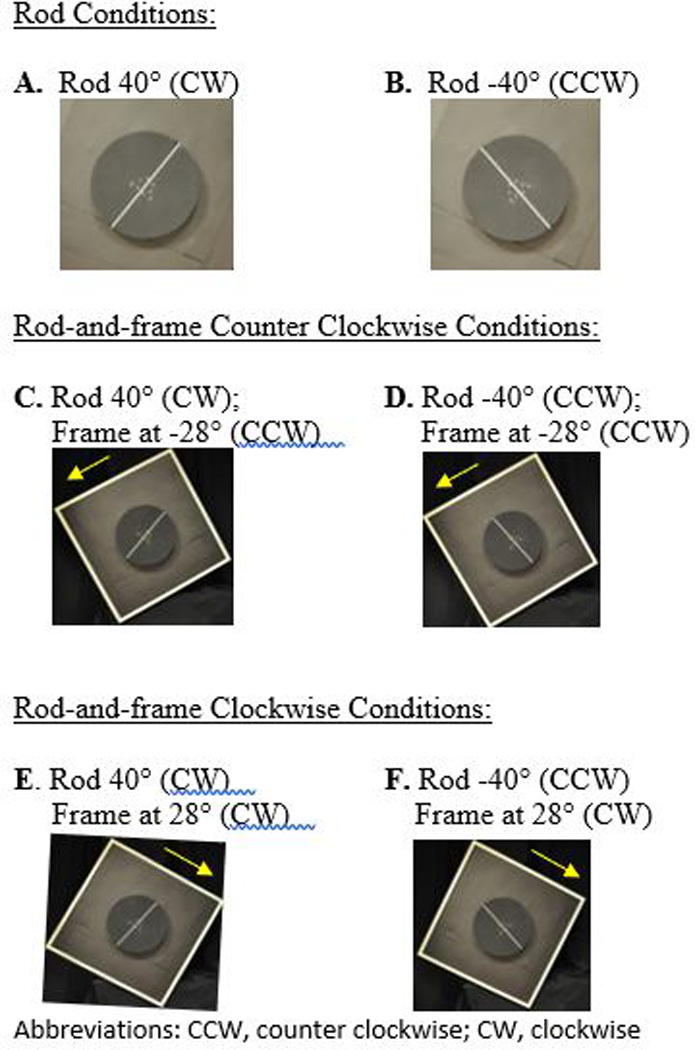
Subjective Visual Vertical Starting Position for A-F Conditions.
Procedure.
Statistical analysis was completed using IBM SPSS Statistics 22 (Armonk, NY: IBM Corp). Descriptive statistics were examined and the association of the performance variables were investigated using Pearson correlation. Spearman’s rho correlation was used to investigate the relationship between the SVV deviations and the performance measures. Analysis of variance was conducted to compare the magnitude of the deviations for the different SVV testing conditions. The significance level was set at p<0.05 and Sidak adjustment was used to correct for multiple comparisons. Paired t-test analysis was used to analyze differences in SVV deviations for CW and CCW trials for both rod and rod-and-frame conditions.
Results
Tables 1 and 2 present the mean values, standard deviations, and ranges for the outcome measure scores and SVV deviations respectively. As depicted in Table 1, the sample included individuals with significant balance impairments with a wide range of scores. The mean values for the rod condition yielded SVV deviations that were within normal limits, but the rod and frame condition SVV deviations exceeded normal limits (Table 2). None of the six functional outcome measures correlated with the rod only condition of the SVV testing (Table 3). All of the performance variables collected in this study were highly correlated to each other except for the Barthel Index (Table 4).
Table 1:
Mean, standard deviation, and ranges of the self-report and functional performance scores.
| Variables | Mean ± SD | Range |
|---|---|---|
| Barthel Index | 89.25 ± 12.38 | 65 to 100 |
| Gait speed (m/s) | 0.84 ± 0.37 | 0.16 to 1.6 |
| BBS | 46 ± 8.7 | 27 to 56 |
| TUG (s) | 18.7 ± 14.4 | 6.8 to 63.2 |
| ICARS | 20.4 ± 14.1 | 4 to 44 |
| SARA | 7.8 ± 5.9 | 1 to 17 |
BBS: Berg Balance Scale; TUG: Timed Up and Go; ICARS: International Co-operative Ataxia Rating Scale; SARA: Scale for the Assessment and Rating of Ataxia.
Table 2:
Mean, standard deviation, and ranges of the subjective visual vertical conditions.
| Variables | Mean ± SD | Range |
|---|---|---|
| SVV: Rod CW | 1.35 ± 2.01 | −3.43 to 4.99 |
| SVV: Rod CCW | −0.29 ± 2.05 | −4.47 to 4.03 |
| SVV: Rod-and-frame (Frame CW, Rod CW) | 7.71 ± 10.72 | −13.64 to 27.64 |
| SVV: Rod-and-frame (Frame CW, Rod CCW) | 7.47 ± 10.26 | −8.60 to 25.87 |
| SVV: Rod-and-frame (Frame CCW, Rod CW) | −8.54 ± 10.32 | −32.32 to 6.89 |
| SVV: Rod-and-frame (Frame CCW, Rod CCW) | −10.28 ± 10.36 | −32.08 to 1.03 |
SVV: subjective visual vertical; CW: clockwise; CCW: counter clockwise
Table 3:
Correlation between performance variables.
| Gait Velocity |
BBS | TUG | ICARS | SARA | ||
|---|---|---|---|---|---|---|
| Barthel Index |
Pearson Correlation Sig. (2-tailed) |
0.30 p = 0.193 |
0.04 p = 0.856 |
− 0.11 p = 0.648 |
− 0.14 p = 0.544 |
− 0.11 p = 0.651 |
| Gait Velocity |
Pearson Correlation Sig. (2-tailed) |
0.82 p < 0.001 |
− 0.84 p < 0.001 |
− 0.83 p < 0.001 |
− 0.79 p < 0.001 |
|
| BBS | Pearson Correlation Sig. (2-tailed) |
− 0.80 p < 0.001 |
− 0.87 p < 0.001 |
− 0.89 p < 0.001 |
||
| TUG | Pearson Correlation Sig. (2-tailed) |
0.66 p = 0.002 |
0.66 p = 0.002 |
|||
| ICARS | Pearson Correlation Sig. (2-tailed) |
0.98 p < 0.001 |
BBS: Berg Balance Scale; TUG: Timed Up and Go; ICARS: International Co-operative Ataxia Rating Scale; SARA: Scale for the Assessment and Rating of Ataxia.
Table 4:
Spearman’s rho correlation of the averaged scores of the SVV Conditions and the Barthel Index, gait velocity, Berg Balance Scale, Timed Up and Go, ICARS, and SARA.
| Variables | SVV: Rod | SVV: Rod-and-frame (Frame CW) |
SVV: Rod-and-frame (Frame CCW) |
|---|---|---|---|
| Barthel Index |
r = −0.12 p = 0.31 |
r = −0.66* p ˂ .001 |
r = −0.47* p = 0.02 |
| Gait Velocity |
r = −0.04 p = 0.44 |
r = −0.37 p = 0.06 |
r = −0.52* p = 0.01 |
| Berg Balance Scale |
r = 0.09 p = 0.35 |
r = −0.40 p = 0.07 |
r = −0.59* p ˂ 0.001 |
| Timed Up and Go |
r = −0.08 p = 0.37 |
r = 0.28 p = 0.12 |
r = 0.58* p ˂ 0.001 |
| ICARS |
r = −0.09 p = 0.36 |
r = 0.20 p = 0.20 |
r = 0.56* p = 0.01 |
| SARA |
r = −0.05 p = 0.42 |
r = 0.30 p = 0.10 |
r = 0.62* p ˂ 0.001 |
Note:
Significant at P<0.05
Abbreviations: ICARS: International Co-operative Ataxia Rating Scale; SARA: Scale for the Assessment and Rating of Ataxia; SVV: subjective visual vertical; CW: clockwise; CCW: counter clockwise.
For all six of the outcomes there was a statistically significant moderate correlation with SVV deviations when the frame was tilted CCW: Barthel Index (r=−.473, p=.02); BBS (r=−.59, p=.003); gait velocity (r=−.52, p=.010); ICARS (r=.56, p=.006); SARA (r=.62, p=.002); TUG (r=.58, p=.003). Interestingly, the Barthel Index was the only outcome that demonstrated statistical significance with a moderate correlation when the frame was rotated CW (r=−.66, p=.001). Paired t-test analysis revealed that there was not a difference in SVV deviations when the rod was tilted CW or CCW during the rod condition, but there was a difference depending on whether the frame was tilted CW or CCW during rod-and-frame SVV testing. The magnitude of the rod-and-frame deviations were also notably greater than the deviations during the rod conditions.
Discussion
Larger deviations from vertical during the rod-and-frame condition of SVV testing correlated with worse balance scores when the frame was tilted CCW for our sample of persons living with MS. Even in healthy control groups a slight deviation in the direction of the frame tilt is expected based on previous findings (Beh, Beh, & Wenderoth, 1972; Guerraz et al., 2001). The tilted frame creates a visual context or reference that mismatches the gravitational vector and the perception of vertical is therefore challenged (Funk, Finke, Muller, Utz, & Kerkhoff, 2011). Guerraz et al. found that the average deviation in SVV rod-and-frame condition was 3.5 degrees, with a standard deviation of 3.8 degrees, for control subjects when the frame was tilted 28 degrees CW or CCW. Another study which tilted the frame 15 degrees yielded an average of 2.7 degrees for healthy controls (Funk et al., 2011). Within this study, 65% of the sample had SVV rod-and-frame deviations greater than 5 degrees when the frame was tilted both CW and CCW. The mean values and range of SVV deviations for the rod and frame conditions for this cohort further illustrate the abnormalities present.
Prior to conducting the study, we did not expect to observe a difference between the CW and CCW frame of reference deviations, but based on other findings in the literature we wanted to examine the effects of direction in which the frame was tilted. While the mean difference and ranges of SVV deviations are similar between the CW and CCW frame tilts, only the CCW tilt demonstrated statistical significance for association between the functional outcomes. One possible explanation for this finding could be that there may have been individual differences in lesion locations that lend towards specific roll tilt deviations. Recent investigation of SVV abnormalities in patients post ischemic brainstem stroke has speculated that crossed vestibulo-perceptive pathways carry the information that determines the roll of the SVV tilt (Yang et al., 2014). In the study by Yang et al. (2014), fifty percent of patients (41/82) with acute unilateral brainstem infarcts had abnormal SVV tilt, of which 76% (31/41) had ipsiversive tilt and 24% (10/41) had contraversive tilt. Their findings also showed that the ipsiversive tilts had a greater mean deviation of 7.04 degrees compared to mean tilt of 4.23 degrees for the contraversive deviations but the impact of static visual disturbance (rod and frame condition) was not assessed (Yang et al., 2014). Magnetic resonance imaging was used in the study by Yang et al. (2014) to understand the association between the specific lesion location and the roll tilt of the SVV deviation and the understanding of lesion location might be help interpret differences between frame tilted CW and CCW in future static visual disturbance SVV testing. Another study showed that 74% of people with cerebellar strokes (n = 31) had contraversive SVV deviations and within this sample, the dentate nucleus of the cerebellum was commonly damaged indicating that this structure has a role in perceiving vertical (Baier, Bense, & Dieterich, 2008). A limitation of our study was that we did not use medical imaging for lesion localization. We also did not have information to characterize the specific type of MS disease course for each participant.
In a study that examined perceptual asymmetry in people with Parkinson’s Disease (PD), the participants were instructed to point to the straight ahead position while their lower trunk was rotated 15 degrees clockwise and 15 degrees counterclockwise with the shoulders and head stabilized (Wright, Gurfinkel, King, & Horak, 2007). Wright et al. (2007) found that the participants with PD had great deviations from the straight ahead target when the lower trunk was rotated counterclockwise compared to clockwise. This led to the authors’ interpretation that perceptuomotor asymmetry in PD typically affects the left hemisphere (Wright et al., 2007). Similar asymmetric findings were observed in our study of people with MS, who showed a preponderance of CCW deviations that correlated to worse balance performance during the perceptual task of SVV with frame referencing.
Our results indicate that individuals with MS may have an increased visual dependence and this is supported by a recent report that found that participants with MS had an association between increased visual dependence for balance control and sensory loss as well as an association between increased cerebellar dysfunction and postural sway (McLoughlin, Barr, Crotty, Lord, & Sturnieks, 2015). Vision has a powerful influence on postural control typically in combination with proprioceptive and vestibular inputs. After damage to the vestibular apparatus, vision becomes more dominant as a sensory influence on postural control with more sway noted with conflicting visual inputs (Redfern, Yardley, & Bronstein, 2001). The presence of visual dependence is associated with worse clinical outcomes for people with balance impairments (Bronstein, 2013).
Symptoms of dizziness and imbalance experienced by individuals with MS are typically managed with medications and specialized balance and vestibular physical therapy (Hebert, Corboy, Manago, & Schenkman, 2011). Despite the relationship of SVV to balance, SVV is not typically tested, or currently recommended for this population during physical therapy care (Potter et al., 2014). Implementation of SVV assessment for individuals with MS may provide clinicians with additional insights related to balance function in persons living with MS. In persons with vestibular disorders, exposure to optokinetic stimulation has been shown to be an effective intervention for reductions in visual dependency (Pavlou et al., 2011) and could be considered for future studies for individuals with MS.
The relationship between ataxia and SVV is logical as decreased control of the body’s movements, as occurs in ataxia, can lead to decreased balance. Given that balance is dependent upon the inputs received from the somatosensory, vestibular, and visual systems, it is not surprising that visual dependence (more or less) is related to postural control (Lacour et al., 1997). A relationship between postural deviations and distorted vestibular and visual spatial reference frames have been demonstrated in people with unilateral vestibular loss (Borel, Harlay, Magnan, & Lacour, 2001) and otolith dysfunction has been shown to underlie postural imbalance following minor head trauma (Basta, Todt, Scherer, Clarke, & Ernst, 2005).
Conclusion
Persons with MS with cerebellar findings had abnormal SVV results when tested with the rod and frame test. Worse SVV deviations appeared to be related to decrements in postural control.
Implications for Physiotherapy Practice
Using clinical tests of SVV may be warranted in order to identify vestibular deficits in persons with MS and cerebellar dysfunction.
Acknowledgements:
This research was supported by NIH Training in Auditory and Vestibular Neuroscience Grant #T32-DC011499. We acknowledge: Kaylee Klingensmith for her assistance in participant recruitment (University of Pittsburgh Multiple Sclerosis patient registry number: PRO12010609); Kathleen Brandfass, MS, PT and Margie O’Leary, RN and for their content expertise during the project planning; and Islam Zaydan, MD for recruitment assistance.
Footnotes
Conflict of Interest Statement:
The authors have no conflict of interest to declare.
References
- Baier B, Bense S, & Dieterich M (2008). Are signs of ocular tilt reaction in patients with cerebellar lesions mediated by the dentate nucleus? Brain, 131(6), 1445–1454. doi: 10.1093/brain/awn086 [DOI] [PubMed] [Google Scholar]
- Basta D, Todt I, Scherer H, Clarke A, & Ernst A (2005). Postural control in otolith disorders. Hum Mov Sci, 24(2), 268–279. doi: 10.1016/j.humov.2005.04.002 [DOI] [PubMed] [Google Scholar]
- Beh HC, Beh HC, & Wenderoth PM (1972). The effect of variation of frame shape on the angular function of the rod-and-frame illusion. Perception & psychophysics, 11(1), 35–37. doi: 10.3758/BF03212679 [DOI] [Google Scholar]
- Berg, Wood-Dauphinee, Williams, & Maki. (1992). Measuring balance in the elderly: validation of an instrument. Can J Public Health, 83 Suppl 2, S7–11. [PubMed] [Google Scholar]
- Berg K, Wood-Dauphine S, Williams JI, & Gayton D (1989). Measuring balance in the elderly: preliminary development of an instrument. Physiotherapy Canada, 41(6), 304–311. doi:doi: 10.3138/ptc.41.6.304 [DOI] [Google Scholar]
- Bisdorff AR, Wolsley CJ, Anastasopoulos D, Bronstein AM, & Gresty MA (1996). The perception of body verticality (subjective postural vertical) in peripheral and central vestibular disorders. Brain : a journal of neurology, 119 (Pt 5)(5), 1523–1534. doi: 10.1093/brain/119.5.1523 [DOI] [PubMed] [Google Scholar]
- Bohmer A, & Mast F (1999). Assessing otolith function by the subjective visual vertical. Ann N Y Acad Sci, 871, 221–231. [DOI] [PubMed] [Google Scholar]
- Borel L, Harlay F, Magnan J, & Lacour M (2001). How changes in vestibular and visual reference frames combine to modify body orientation in space. Neuroreport, 12(14), 3137–3141. [DOI] [PubMed] [Google Scholar]
- Bronstein AM (2013). Visual dependency and the visual control of balance in health and disease. Annals of Physical and Rehabilitation Medicine, 56, e104–e104. doi: 10.1016/j.rehab.2013.07.135 [DOI] [Google Scholar]
- Cameron MH, Horak FB, Herndon RR, & Bourdette D (2008). Imbalance in multiple sclerosis: a result of slowed spinal somatosensory conduction. Somatosens Mot Res, 25(2), 113–122. doi: 10.1080/08990220802131127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin C, Wade DT, Davies S, & Horne V (1988). The Barthel ADL Index: a reliability study. Int Disabil Stud, 10(2), 61–63. [DOI] [PubMed] [Google Scholar]
- Crevits L, Venhovens J, Vanoutrive J, & Debruyne J (2007). False perception of visual verticality in multiple sclerosis. Eur J Neurol, 14(2), 228–232. doi: 10.1111/j.1468-1331.2006.01636.x [DOI] [PubMed] [Google Scholar]
- Curthoys IS, Dai MJ, & Halmagyi GM (1991). Human ocular torsional position before and after unilateral vestibular neurectomy. Experimental Brain Research, 85(1), 218–225. [DOI] [PubMed] [Google Scholar]
- Dibble LE, Lopez-Lennon C, Lake W, Hoffmeister C, & Gappmaier E (2013). Utility of Disease-Specific Measures and Clinical Balance Tests in Prediction of Falls in Persons With Multiple Sclerosis. Journal of Neurologic Physical Therapy, 37(3), 99–104. doi: 10.1097/NPT.0b013e3182a18460 [DOI] [PubMed] [Google Scholar]
- Finlayson Peterson, & Cho. (2006). Risk factors for falling among people aged 45 to 90 years with multiple sclerosis. Arch Phys Med Rehabil, 87(9), 1274–1279; quiz 1287. doi: 10.1016/j.apmr.2006.06.002 [DOI] [PubMed] [Google Scholar]
- Finlayson M, Plow M, & Cho C (2010). Use of physical therapy services among middle-aged and older adults with multiple sclerosis. Phys Ther, 90(11), 1607–1618. doi: 10.2522/ptj.20100072 [DOI] [PubMed] [Google Scholar]
- Friedmann G (1970). The judgement of the visual vertical and horizontal with peripheral and central vestibular lesions. Brain, 93(2), 313–328. [DOI] [PubMed] [Google Scholar]
- Fritz S, & Lusardi M (2009). White paper: “walking speed: the sixth vital sign”. J Geriatr Phys Ther, 32(2), 46–49. [PubMed] [Google Scholar]
- Funk J, Finke K, Muller HJ, Utz KS, & Kerkhoff G (2011). Visual context modulates the subjective vertical in neglect: evidence for an increased rod-and-frame-effect. Neuroscience, 173, 124–134. doi: 10.1016/j.neuroscience.2010.10.067 [DOI] [PubMed] [Google Scholar]
- Graves J, & Balcer LJ (2010). Eye disorders in patients with multiple sclerosis: natural history and management. Clin Ophthalmol, 4, 1409–1422. doi: 10.2147/opth.s6383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresty MA, & Bronstein AM (1992). Testing otolith function. Br J Audiol, 26(2), 125–136. [DOI] [PubMed] [Google Scholar]
- Guerraz M, Yardley L, Bertholon P, Pollak L, Rudge P, Gresty MA, & Bronstein AM (2001). Visual vertigo: symptom assessment, spatial orientation and postural control. Brain, 124(Pt 8), 1646–1656. [DOI] [PubMed] [Google Scholar]
- Hebert JR, Corboy JR, Manago MM, & Schenkman M (2011). Effects of vestibular rehabilitation on multiple sclerosis-related fatigue and upright postural control: a randomized controlled trial. Phys Ther, 91(8), 1166–1183. doi: 10.2522/ptj.20100399 [DOI] [PubMed] [Google Scholar]
- Lacour M, Barthelemy J, Borel L, Magnan J, Xerri C, Chays A, & Ouaknine M (1997). Sensory strategies in human postural control before and after unilateral vestibular neurotomy. Experimental Brain Research, 115(2), 300–310. [DOI] [PubMed] [Google Scholar]
- MacNeilage PR, Banks MS, Berger DR, & Bulthoff HH (2007). A Bayesian model of the disambiguation of gravitoinertial force by visual cues. Experimental Brain Research, 179(2), 263–290. doi: 10.1007/s00221-006-0792-0 [DOI] [PubMed] [Google Scholar]
- Mahoney FI, & Barthel DW (1965). FUNCTIONAL EVALUATION: THE BARTHEL INDEX. Md State Med J, 14, 61–65. [PubMed] [Google Scholar]
- McLoughlin J, Barr C, Crotty M, Lord SR, & Sturnieks DL (2015). Association of Postural Sway with Disability Status and Cerebellar Dysfunction in People with Multiple Sclerosis: A Preliminary Study. Int J MS Care, 17(3), 146–151. doi: 10.7224/1537-2073.2014-003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsagard Y, Lundholm C, Denison E, & Gunnarsson LG (2009). Predicting accidental falls in people with multiple sclerosis -- a longitudinal study. Clin Rehabil, 23(3), 259–269. doi: 10.1177/0269215508095087 [DOI] [PubMed] [Google Scholar]
- Pavlou M, Davies RA, & Bronstein AM (2006). The assessment of increased sensitivity to visual stimuli in patients with chronic dizziness. J Vestib Res, 16(4–5), 223–231. [PubMed] [Google Scholar]
- Pavlou M, Quinn C, Murray K, Spyridakou C, Faldon M, & Bronstein AM (2011). The effect of repeated visual motion stimuli on visual dependence and postural control in normal subjects. Gait Posture, 33(1), 113–118. doi: 10.1016/j.gaitpost.2010.10.085 [DOI] [PubMed] [Google Scholar]
- Podsiadlo D, & Richardson S (1991). The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc, 39(2), 142–148. [DOI] [PubMed] [Google Scholar]
- Potter K, Cohen ET, Allen DD, Bennett SE, Brandfass KG, Widener GL, & Yorke AM (2014). Outcome measures for individuals with multiple sclerosis: recommendations from the American Physical Therapy Association Neurology Section task force. Phys Ther, 94(5), 593–608. doi: 10.2522/ptj.20130149 [DOI] [PubMed] [Google Scholar]
- Redfern MS, Yardley L, & Bronstein AM (2001). Visual influences on balance. J Anxiety Disord, 15(1–2), 81–94. [DOI] [PubMed] [Google Scholar]
- Schmitz-Hubsch T, du Montcel ST, Baliko L, Berciano J, Boesch S, Depondt C, … Fancellu R (2006). Scale for the assessment and rating of ataxia: development of a new clinical scale. Neurology, 66(11), 1717–1720. doi: 10.1212/01.wnl.0000219042.60538.92 [DOI] [PubMed] [Google Scholar]
- Schmitz-Hubsch T, Tezenas du Montcel S, Baliko L, Boesch S, Bonato S, Fancellu R, … Klockgether T (2006). Reliability and validity of the International Cooperative Ataxia Rating Scale: a study in 156 spinocerebellar ataxia patients. Mov Disord, 21(5), 699–704. doi: 10.1002/mds.20781 [DOI] [PubMed] [Google Scholar]
- Schoch B, Regel JP, Frings M, Gerwig M, Maschke M, Neuhäuser M, & Timmann D (2007). Reliability and validity of ICARS in focal cerebellar lesions. Movement Disorders, 22(15), 2162–2169. doi: 10.1002/mds.21543 [DOI] [PubMed] [Google Scholar]
- Serra A, Derwenskus J, Downey DL, & Leigh RJ (2003). Role of eye movement examination and subjective visual vertical in clinical evaluation of multiple sclerosis. J Neurol, 250(5), 569–575. doi: 10.1007/s00415-003-1038-8 [DOI] [PubMed] [Google Scholar]
- Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, … Guralnik J (2011). Gait speed and survival in older adults. Jama, 305(1), 50–58. doi: 10.1001/jama.2010.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarnutzer AA, Bockisch CJ, & Straumann D (2010). Roll-dependent modulation of the subjective visual vertical: contributions of head- and trunk-based signals. J Neurophysiol, 103(2), 934–941. doi: 10.1152/jn.00407.2009 [DOI] [PubMed] [Google Scholar]
- Trouillas P, Takayanagi T, Hallett M, Currier RD, Subramony SH, Wessel K, … Manyam B (1997). International Cooperative Ataxia Rating Scale for pharmacological assessment of the cerebellar syndrome. The Ataxia Neuropharmacology Committee of the World Federation of Neurology. J Neurol Sci, 145(2), 205–211. [DOI] [PubMed] [Google Scholar]
- Versino M, Colnaghi S, Callieco R, Bergamaschi R, Romani A, & Cosi V (2002). Vestibular evoked myogenic potentials in multiple sclerosis patients. Clinical Neurophysiology, 113(9), 1464–1469. doi: 10.1016/S1388-2457(02)00155-4 [DOI] [PubMed] [Google Scholar]
- Vingerhoets RAA, De Vrijer M, Van Gisbergen JAM, & Medendorp WP (2009). Fusion of Visual and Vestibular Tilt Cues in the Perception of Visual Vertical. Journal of Neurophysiology, 101(3), 1321–1333. doi: 10.1152/jn.90725.2008 [DOI] [PubMed] [Google Scholar]
- Weyer A, Abele M, Schmitz-Hübsch T, Schoch B, Frings M, Timmann D, & Klockgether T (2007). Reliability and validity of the scale for the assessment and rating of ataxia: A study in 64 ataxia patients. Movement Disorders, 22(11), 1633–1637. doi: 10.1002/mds.21544 [DOI] [PubMed] [Google Scholar]
- Winser SJ, Smith C, Hale LA, Claydon LS, & Whitney SL (2015). Balance outcome measures in cerebellar ataxia: a Delphi survey. Disabil Rehabil, 37(2), 165–170. doi: 10.3109/09638288.2014.913709 [DOI] [PubMed] [Google Scholar]
- Winser SJ, Smith CM, Hale LA, Claydon LS, Whitney SL, & Mehta P (2015). Systematic review of the psychometric properties of balance measures for cerebellar ataxia. Clin Rehabil, 29(1), 69–79. doi: 10.1177/0269215514536412 [DOI] [PubMed] [Google Scholar]
- Wright WG, Gurfinkel V, King L, & Horak F (2007). Parkinson’s disease shows perceptuomotor asymmetry unrelated to motor symptoms. Neurosci Lett, 417(1), 10–15. doi: 10.1016/j.neulet.2007.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T-H, Oh S-Y, Kwak K, Lee J-M, Shin B-S, & Jeong S-K (2014). Topology of brainstem lesions associated with subjective visual vertical tilt. Neurology, 82(22), 1968–1975. doi: 10.1212/WNL.0000000000000480 [DOI] [PubMed] [Google Scholar]
- Zwibel H (2009). Contribution of impaired mobility and general symptoms to the burden of multiple sclerosis. Advances in Therapy, 26(12), 1043–1057. doi: 10.1007/s12325-009-0082-x [DOI] [PubMed] [Google Scholar]


