Abstract
Objective:
To assess the impact of nurse-led Asha (Accredited Social Health Activist)-support behavioral and nutritional intervention among women living with HIV/AIDS (WLH/A) in rural India.
Design:
Cluster randomized-controlled trial.
Methods:
Sixteen Primary Health Centers serving WLH/A in Andhra Pradesh were grouped into four regional clusters that were randomly allocated into one of four arms. All four groups included Asha-support and consisted of: Asha-support only (control group); nutrition education; nutrition supplementation; and the combination of supplementation and education. Differences between baseline and 6-month follow-up for key physiological outcomes (body mass index [BMI], CD4 count) were analyzed using factorial mixed-models that accounted for geographic clustering.
Results:
At 6 months, all groups improved CD4 count: Asha only (mean difference score [D]=343.97, standard deviation [SD]=106.94), nutrition education (D=356.15, SD=0.69), nutrition supplement (D = 469.66, SD=116.0), and nutrition supplement and education (D=530.82, SD=128.56). In multivariable models, Asha-support plus nutrition and Asha-support support plus supplement interventions demonstrated independent significant improvements in CD4 count; the interaction term was significant (β=529.9; 95% confidence internal [CI]=512.0, 547.8; p=.006). BMI also increased for all groups: Asha only (D=0.95, SD=0.82), Asha plus nutrition education (D=1.28, SD=0.53), Asha plus nutrition supplement (D=2.38, SD=0.60), nutrition supplement and nutrition supplement and education (D=2.72=0.84). Nutrition supplementation and nutrition education demonstrated independent effects on BMI; the interaction term was not significant (β =.27, 95% CI=2.5, 2.7, p = 0.80).
Conclusion:
Interventions run by community workers were efficacious at improving physiological outcomes and may be beneficial at meeting critical healthcare needs of vulnerable WLH/A in India.
Keywords: Food insecurity, Social support, BMI, Body weight, Cluster RCT, Community interventions
INTRODUCTION
India has the third largest HIV-positive population in the world and 2.1 million Indians are infected [1, 2]. Rural areas carry a significantly larger burden compared to urban areas; in India and many other developing nations (DN), married women are particularly at risk for disease contraction [2, 3]. Moreover, women in India are at increased risk for malnutrition and food insecurity [4], which can negatively impact HIV treatment and worsen disease progression.
Nutrition is a critical component of immune functioning; nutrient, calorie, and protein deficits directly impair immune responses. In individuals with HIV/AIDS, this impairment is linked with reduced ability to absorb nutrition concurrent with increased metabolic need to fight the disease [5, 6]. This hastens CD4 decline, shortening survival time [7]. Inadequate protein intake causes catabolism of muscle tissue into amino acids to compensate for disease-related energy demands and structural repair of the body; in turn, muscle mass loss hastens disease advancement. Indeed, malnutrition and HIV have both compound and synergistic effects on the immune system [8], HIV disease progression, and vulnerability to AIDS-related illnesses [9, 10]. Prior research suggests nutrition training and/or food supplements can improve health outcomes for HIV-positive individuals over time [11]. Thus addressing deficits in nutrition is a critical component of facilitating optimal outcomes for women living with HIV/AIDS (WLH/A) in India and globally.
Addressing undernourishment in developing nations (DN) is a global challenge, and targeting nutrition deficits for WLH/A in rural India presents logistical, financial, and cultural challenges. Yet utilizing extant community resources can help with feasibility and dissemination of effective interventions. Prior work by our team in India has demonstrated that utilizing Asha (Accredited Social Health Activist)-based models, particularly those that integrate a nutritional component, may improve health-related outcomes in WLH/A [12, 13]. Asha are lay community healthcare workers trained to accomplish tasks typically placed in the hands of medical professionals, broadening the scope and feasibility of health-interventions in vulnerable populations.
We conducted a nurse-led, Asha-supported randomized control trial (RCT) to examine the effect of a behavioral intervention that included nutrition components (training and supplements) on physical health outcomes in HIV-positive women in rural India. We hypothesized that nutrition training and nutrition supplementation would have independent as well as interactive effects on key physical indicators of HIV progression (CD4 count, BMI, and lean weight gain) at 6-month follow up, compared to Asha-support alone.
METHODS
Study design, sample, and setting
We assessed the impact of a community-engaged, Asha-supported behavioral and nutritional intervention on key disease-related outcomes in WLH/A in Andhra Pradesh (AP), India (N = 600) using a prospective, cluster RCT. We implemented a 2×2 factorial design: 1) Asha-support (Asha+; control); 2) Asha support + nutrition training (Asha+ NEd+); 3) Asha support + nutrition supplements (Asha+ NSupl+); and 4) Asha support + nutrition training + nutrition supplements (Asha+NEd+NSupl+). The data presented herein are part of a larger parent study that includes mothers, their children, and additional metrics beyond the scope of the present analyses. We conducted research in the same region and were familiar with the geography, villages, location of Primary Care Centers (PHCs), and local population groups [12]. The four regional clusters were chosen to contain villages that were close to each other and yet distant from villages contained in other clusters.
WLH/A between ages of 18–50 with a verified HIV-positive diagnosis, antiretroviral therapy (ART)-prescription for the last three months, and who received care in one of the sixteen PHCs located in Nellore and Prakasam districts of AP were recruited for participation. Additional inclusion criteria included living with a child aged 3–8 (not relevant to the present analysis). Exclusionary factors included CD4 T-cell levels < 100 cells/mm3 and participation in previous Asha-interventions by our team. Baseline data collection began April 30, 2014, and enrollment was staggered every six months over a two-year period. The final cohort was enrolled on November 20, 2016. Follow-up assessment for each cohort occurred six months after baseline enrollment.
Flyers were posted in selected PHCs. Interested women met with a trained research staff who was blinded to the program hypotheses in a private location to receive study information, and complete an eligibility screener. Verified diagnosis of HIV was assessed by showing hospital-generated ART card. CD4+ T cell count was also collected during screening. As part of the larger 18-month study, WLH/A received cash compensation (USD $172-$182) to cover transportation costs, loss of pay for the time of enrollment, or childcare as needed.
All women who were interested in participating provided informed consent. A second informed consent was completed by all eligible participants based on the results of eligibility screening. The study was approved by University Human Subjects Protection Committees in the United States and the Ministry of Health in India.
Sample Size Calculation
Sample size was estimated according to estimated effect sizes for the parent study based on pilot study results [12–14]. Alpha was set to .01 to mitigate for multiple dependent variables. We estimated an intraclass correlation of .05 and a design effect of 2.45 (to account for potential PHC clustering effects) and a 90% attrition rate after 18 months (length of parent study). For each main effect of parent study, we estimated differences of about half a standard deviation (SD) in size (d=.48, medium effect size) between the outcome means of nutrition and supplement groups compared to Asha+. This suggested an overall sample size of 600 (150 per group).
Asha Selection and Training
Asha were selected from lay village women and eligibility included aged 20–50; 8th grade education or higher; interested in caring for women and children with HIV/AIDS; a history of community service; and residence in similar villages as the WLH/A.
Sixteen Asha were trained by the Primary Investigators (PI) and Project Director (PD). Asha training included education about study protocol, needs of WLH/A, basics of HIV/AIDS disease progression, importance of adherence to ART, strategies to assist coping with illness, and maintaining participant confidentiality. Four Asha were assigned to each program and trained per the program design. Quality assurance assessments were done quarterly. Nursing guidance and research staff were led by PI and nurses in the community. The Asha were employed full time and paid a monthly stipend of USD $54, consistent with typical salaries from the government for providing reproductive health-related services to women in these communities.
Overview of the Asha Nutrition Programs
Recruitment sties were grouped into four geographic locales. The four locals were then randomized to one of the four arms using computer-based sequencing by project statistician in the United States. Participants were assigned to groups by blinded research staff after initial assessment.
Program 1 (Asha+) served as the control group. Asha-support consisted of visiting 6–7 WLH/A weekly on a 1:1 basis, monitoring barriers to and recording of ART adherence, accompanying women to hospital and clinic visits, and providing assistance to accessing and understanding health care or prescribed treatments. Participants also engaged in group sessions delivered by healthcare providers on how to effectively seek support from Asha and physicians, nurses, social workers, etc, all about HIV/AIDS disease progression, treatment, maintaining healthy routines, and parenting. Additional modules included promotion of positive psychological states (e.g., dealing with family problems and promoting healthy relationships, taking active steps to improve life, creating a social network, finding joy in life, and seeking psychological assistance if necessary).
Program 2 (Asha+NEd+) sessions provided education about nutrient rich foods that were inexpensive, sharing recipes, and cooking classes to educate WLH/A on how to maintain nutritional value within cultural preferences. WLH/A were also taught where to purchase food and how to monitor food intake. Our nutrition experts, using the dietary guidelines for Indians, demonstrated healthy food selections, keeping in mind which foods could strengthen immune responses.
Program 3 (Asha+NSupl+) provided monthly food supplements that consisted of high protein dals (lentils). WLH/A received three packs of food monthly (i.e., 3kg of black gram and 3kg of pigeon peas). Food for the entire family was provided to ensure women themselves received adequate amounts. Per 100 grams, the black gram consisted of 347 calories, 24 grams of protein, 1.4 grams of fat, 59.6 grams of carbohydrate; and pigeon peas consisted of 335 calories, 22.3 grams of protein, 1.7 grams of fat and 57.6 grams of carbohydrate. The amount of food was calibrated to represent an additional 500kcal (bowl-sized serving) supplement to the daily diets of each woman. All nutritional intervention materials were purchased from a local provider. Each package of nutritional supplement was consistently mixed in prescribed amounts, weighed, and allotted to each participant by the provider and checked and divided into packs by the study manager.
Program 4 (Asha+NEd+NSupl+) combined all elements in Programs 1, 2 and 3.
Instruments
Many instruments in the structured questionnaire had been used with persons living with HIV (PLWH) in rural [13, 14] or urban [15–17] settings in southern India. Instrument translation was conducted in India by “Lingvopedia,” a professional language solutions company (http://lingvopedia.com).
Behavioral and psychosocial measures included: Quality of Life (QOL) assessed by the internationally validated Quality of Life Enjoyment and Satisfaction Questionnaire [4, 18]; Food insecurity by The Household Food Insecurity Access Scale [19]. This is widely used to measure food insecurity in India and elsewhere [20]; Depression by The Center for Epidemiological Studies Depression Scale (CES-D), short version [21], a 10-item scale with well-established reliability and validity [21] including in India [22]; Internalized stigma studied in Indian populations [15, 23] was represented by a 10-item scale that measured the extent to which respondents believed they deserved to be shunned (5 items, e.g., should avoid social interactions) or blamed/shamed because of HIV (5 items, e.g., shamed the family, feel guilty; [24] Social support derived from The MOS Social Support Survey [25]; Physical activity collected with the International Physical Activity Questionnaire was summarized into Metabolic Equivalents of Energy Expenditure (MET) minutes per week [26]; and Adherence to ART, was represented by a self-reported measure that assessed adherence to ART via a Visual Analogue Scale ranging from 0 to 100, with the number representing percent of pills taken in past month [17, 27].
Biological measures included: Months living with HIV/AIDS, derived from asking subjects about month and year of HIV diagnosis; CD4+ T cell count from blood samples collected at screening with absolute numbers of CD4+ T cells obtained by multiplying percent CD4+ T cell count by total white blood cell count (determined at the Nellore District Hospital diagnostic laboratory using the Act Diff Coulter Analyzer); Height and weight was measured at baseline, and then monthly for six months by trained research staff who used a calibrated scale to weigh WLH/A while barefoot, and took heights using a stadiometer with body mass index (BMI) calculated by dividing weight in kilograms by height in meters squared; and Opportunistic infections (OI) were assessed by using a list of 8 common OI endorsed by our collaborating physicians that included tuberculosis, pneumocystic carini, Herpes Simplex Virus/Varicella Zoster, candida, fungal dermatoses, diarrhea, febrile illness, or other. Participants indicated whether they had experienced any of these OI in the previous 6 months, with a summation of endorsed OI into one index measure.
Statistical Methods
Interview data collected by interviewers on Android® tablets were uploaded at our study sites in AP and transferred to a Google cloud server hosted at UCLA campus. All data analyses were conducted using SAS 9.4® (Statistical Analysis System, Cary, NC). One-way ANOVA (for continuous variables) and Chi-Square tests (for categorical variables) assessed baseline differences according to intervention group. Variables that differed by treatment group (p < .10) were adjusted for in subsequent models.
For primary (CD4 count) and secondary outcomes (BMI and body weight), we computed outcome changes from baseline to 6 month. Within the mixed model specifications, we built contrasts that reflected the factorial design with outcome means for each factorial condition compared to control.
Crude mixed models assessed impact of factorial conditions on change since baseline observed on outcome variables. Final models were adjusted for all statistically significant covariates. Given the importance of 1) adherence to ART and 2) food insecurity as key factors for HIV outcomes and nutritional status [28], we present additional analyses, where results are stratified according to high/low adherence and food insecurity, both dichotomized at the median value for each variable.
RESULTS
Description of Overall Sample
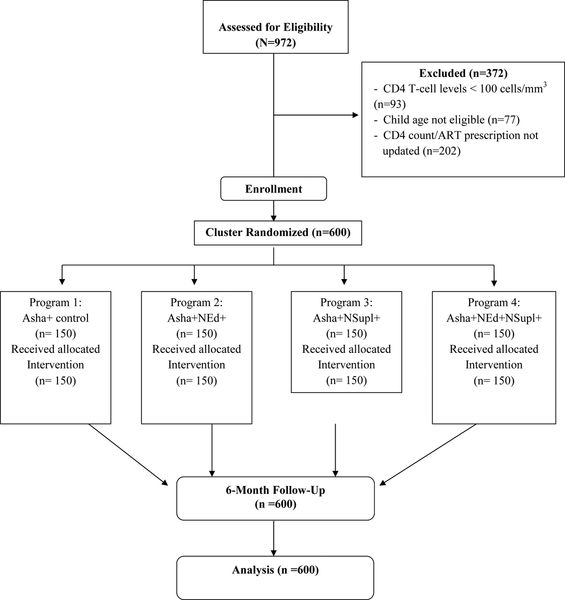
In total, 974 WLH/A were screened, with 600 recruited, 372 WLH/A were ineligible based on CD4 count/ART prescription not updated in the past 3 months, CD4 T-cell levels < 100 cells/mm3, and child age (Figure 1). Frequency distributions were described for the study population by group (Table 1). Participants were 34 years old on average; almost half of the population (49%) had no education. Participants experienced an average of 4.6 OI over the past 6 months. Average baseline CD4 count was 447. All women in the study had an ART prescription; at baseline, mean adherence for all groups was 30% in the past month (SD=13.23). At 6-month follow-up, 99.50% of the entire sample reported adherence > 95% in the past month (between group differences were not possible to analyze given ceiling effects).
Figure 1.
CONSORT Flow Diagram
Table 1.
Description of the Study Sample at Baseline by Intervention Group (N=600)
| Asha+ (Control) | Asha+ Nutrition Education | Asha+ Nutrition Supplements | Asha+ Nutrition Education and Supplements | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | M | SD | M | SD | M | SD | M | SD | p-value† |
| Age | 36.05 | 6.98 | 34.66 | 6.91 | 33.6 | 6.42 | 32.91 | 7.18 | .0006* |
| Number of Children | 1.83 | 0.73 | 1.78 | 0.78 | 1.91 | 0.93 | 1.92 | 0.76 | .37 |
| Monthly Income (INR) | 2110.00 | 758.9 | 2158.67 | 611.98 | 2137.33 | 626.97 | 2048.00 | 707.93 | .52 |
| Quality of Life | 0.26 | 0.31 | 0.34 | 0.25 | 0.27 | 0.33 | 0.33 | 0.24 | .03* |
| Percent Adherence | 30.37 | 12.75 | 32.00 | 14.35 | 28.60 | 12.99 | 30.50 | 12.69 | .18 |
| Number of OI‡ past 6 months | 4.61 | 1.35 | 4.52 | 1.35 | 4.58 | 0.96 | 4.62 | 1.13 | .88 |
| Time since HIV diagnosis (Yr) | 4.34 | 3.25 | 3.83 | 2.44 | 4.10 | 2.82 | 3.95 | 2.96 | .46 |
| Internalized Stigma | 2.24 | 0.41 | 2.30 | 0.16 | 2.33 | 0.19 | 2.32 | 0.15 | .02* |
| Summary CESD§ Depression | 9.12 | 3.11 | 9.09 | 3.06 | 9.16 | 3.13 | 9.35 | 3.04 | .88 |
| Summary Social Support | 1.13 | 0.41 | 1.06 | 0.12 | 1.06 | 0.08 | 1.06 | 0.08 | .004* |
| Summary Food Insecurity | 20.27 | 4.29 | 21.63 | 2.58 | 20.76 | 3.45 | 21.94 | 2.98 | .0001* |
| Total MET¶ min/wk | 4957.09 | 1873.23 | 4569.73 | 1416.82 | 4691.85 | 1718.60 | 4215.60 | 1539.27 | .001* |
| Body Mass Index | 19.81 | 3.78 | 20.20 | 3.97 | 20.55 | 4.67 | 19.86 | 4.16 | .38 |
| Weight (kg) | 45.30 | 9.66 | 46.42 | 9.65 | 47.43 | 11.22 | 45.84 | 10.86 | .33 |
| Height (cm) | 151.06 | 5.81 | 151.57 | 6.06 | 151.85 | 5.80 | 151.62 | 6.27 | .44 |
| Mom CD4 count | 442.57 | 272.66 | 459.61 | 284.48 | 426.19 | 275.34 | 461.29 | 262.75 | .65 |
| n | % | n | % | n | % | n | % | ||
| Marital status | |||||||||
| Married | 51 | 34.00 | 67 | 44.67 | 53 | 35.33 | 67 | 44.67 | |
| Divorced | 12 | 8.00 | 15 | 10.00 | 12 | 8.00 | 15 | 10.00 | |
| Widowed | 87 | 58.00 | 68 | 45.33 | 85 | 56.67 | 68 | 45.33 | .19 |
| Education | |||||||||
| None | 85 | 56.67 | 74 | 49.33 | 71 | 47.33 | 62 | 41.33 | |
| < 5 yrs | 25 | 16.67 | 29 | 19.33 | 25 | 16.67 | 19 | 12.67 | |
| 5 – 9 yrs | 24 | 16.00 | 31 | 20.67 | 33 | 22.00 | 35 | 23.33 | |
| ≥ 10 yrs | 16 | 10.67 | 16 | 10.67 | 21 | 14.00 | 34 | 22.67 | .04* |
| Religion | |||||||||
| Hindu | 110 | 73.33 | 116 | 77.33 | 104 | 69.33 | 109 | 72.67 | |
| Muslim | 17 | 11.33 | 5 | 3.33 | 12 | 8.00 | 10 | 6.67 | |
| Christian | 23 | 15.33 | 29 | 19.33 | 34 | 22.67 | 31 | 20.67 | .14 |
Evaluation of between group differences using one-way ANOVA for continuous variables and Chi-square test for categorical variables.
OI=Opportunistic Infections
CESD=Center for Epidemiological Studies of Depression Scale
MET=Metabolic Equivalent of Energy Expenditure
p-value <0.05
Table 1 shows results of continuous covariate means evaluated by ANOVA according to intervention group. Since randomization was not performed at individual level, some baseline group differences were expected. Age, QOL, summary social support, MET, and education were significantly different at baseline, and included as covariates in subsequent models. Adherence to ART was not significant (p=.18) at baseline, but was retained as a covariate given its clinical importance in relationship to the outcomes [28].
Two-by-two cross-tabulations evaluated categorical variable distributions at baseline according to intervention (Table 1). Deviations between observed and expected frequencies were assessed using Chi-Square test. Years of education was significantly different at baseline (p=.04), and included as a covariate.
Anthropometric and Immune Variable Outcomes
Crude mixed models were constructed with change in CD4 count from baseline as the primary outcome, and body weight and BMI changes as secondary outcome variables (Table 2 and 3). CD4 significantly improved compared to baseline due to nutrition education (β=443.5; 95% confidence interval [CI]= 431.1, 455.9; p = .0001), and nutrition supplementation (β= 500.2; 95% CI=487.8, 512.7; p = .0001), with a significant interaction (β=530.8; 95% CI=513.2, 548.4; p = .006) for combined nutrition supplementation and education (Table 3). These findings remained robust in fully adjusted models.
Table 2.
Mean of Immune and Anthropometric Outcomes by Intervention Group.
| Asha+ (Control) | Asha+ Nutrition Education | Asha+ Nutrition Supplements | Asha+ Nutrition Education and Nutrition Supplement | |||||
|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | M | SD | |
| Primary Outcome | ||||||||
| Baseline CD4 count | 442.57 | 272.66 | 459.61 | 284.48 | 426.19 | 275.34 | 461.29 | 262.75 |
| 6 Month CD4 count | 786.55 | 278.87 | 815.76 | 288.61 | 895.85 | 299.05 | 992.11 | 283.65 |
| Difference | 343.97 | 106.94 | 356.15 | 80.69 | 469.66 | 116.00 | 530.82 | 128.56 |
| Secondary Outcomes | ||||||||
| Baseline Weight (kg) | 45.30 | 9.66 | 46.42 | 9.65 | 47.43 | 11.22 | 45.84 | 10.86 |
| 6 Month Weight | 47.47 | 9.56 | 49.33 | 9.63 | 52.89 | 11.02 | 52.08 | 10.74 |
| Difference | 2.17 | 1.80 | 2.91 | 1.19 | 5.46 | 1.33 | 6.24 | 1.84 |
| Baseline BMI (kg/m2) | 19.81 | 3.78 | 20.20 | 3.97 | 20.55 | 4.67 | 19.86 | 4.16 |
| 6 Month BMI | 20.76 | 3.72 | 21.47 | 3.98 | 22.93 | 4.58 | 22.58 | 4.07 |
| Difference | 0.95 | 0.82 | 1.28 | 0.53 | 2.38 | 0.60 | 2.72 | 0.84 |
Table 3.
Covariate-adjusteda mixed model contrasting differences from baseline to 6-month on primary and secondary outcomes by Asha+ intervention groups (N=600)
| Primary Outcome | Secondary Outcomes | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD4 Count | Weight | BMI | ||||||||||
| Crude differenceb | Adjusted differenceb | Differenceb | Adjusted differenceb | Differenceb | Adjusted differenceb | |||||||
| β 95% CI | p- value | β 95% CI | p- value | β 95% CI | p- value | β 95% CI | p- value | β 95% CI | p- value | β 95% CI | p- value | |
| Asha+ Control | -- | -- | -- | -- | -- | -- | -- | |||||
| Asha + Nutrition Educationc | 443.5 (431.1, 455.9) | .0001 | 443.1 (394.6, 419.8) | .0001 | 4.6 (4.4, 4.8) | .0001 | 4.5 (4.3, 4.7) | .0001 | 2.0 (19, 2.1) | .0001 | 2.0 (1.9, 2.0) | .0001 |
| Asha+ Nutrition Supplementc | 500.2 (487.8, 512.7) | .0001 | 501.1 (448.6, 513.6) | .0001 | 5.9 (5.7, 6.0) | .0001 | 5.8 (5.6, 6.0) | .0001 | 2.6 (2.5, 2.6) | .0001 | 2.5 (2.4, 2.6) | .0001 |
| Asha+ Nutrition Supplement X Nutrition Educationd | 530.8 (513.2, 548.4) | .0063 | 529.9 (512.0, 547.8) | .0154 | 6.3 (6.0, 6.5) | .882 | 6.1 (5.9, 6.4) | .8576 | 2.7 (2.6, 2.8) | .8291 | 2.7 (2.5, 2.8) | .8022 |
Adjusted for all covariates significantly different at baseline (age, quality of life, total MET, internalized stigma, social support, food insecurity) and % ART adherence
Denotes difference from baseline to 6-month follow-up
Main effects are presented
Interaction term was calculated
The secondary outcomes were also evaluated using mixed models with the same factorial structure. Interaction terms for nutrition supplementation and nutrition education were not significantly associated with changes in body weight (β=6.1; 95% CI=5.9, 6.4; p = .883) nor change in body weight (β=2.7; 95% CI=2.6, 2.8; p=.829), which suggests that the combination of nutrition supplementation and education were not any more effective in promoting weight change than either alone. For both body weight and BMI, education (body weight: β=4.5; 95% CI=4.3, 4.7; p=.0001; BMI: β=2.0; 95% CI=1.9, 2.0; p=.0001) and supplementation (body weight: β=5.8; 95% CI=5.6, 6.0; p=.0001; BMI: β=2.6, 95% CI=2.5, 2.6; p=.0001) were statistically significant in an additive fashion.
A similar pattern of results was revealed for our secondary outcomes in the adjusted models. Education alone and supplementation alone were significantly associated with change in body weight and in BMI, which remained robust after adjusting for each covariate and all covariates together. Interaction terms between supplementation and education remained non-significant (Table 3).
We also assessed associations stratified by food insecurity and adherence (Tables 4 and supplement Table 4b). For CD4 count, among individuals with lower food insecurity (n = 311), the unadjusted supplementation and education interaction term was borderline significant (β=5.9.59; 95% CI=481.4, 537.7; p = .055), while adjusted term non-significant (β=509; 95% CI=480.1, 538.2; p = .16). In adjusted models, nutrition education alone (β=436.55; 95% CI=416.5, 456.6; p=.011) and nutrition supplementation alone (β=481.62; 95% CI=462.5, 500.8; p=.0001) were strongly associated with change in CD4 counts. Similar results were observed among individuals with above-median food insecurity, with the adjusted interaction for supplementation and education non-significant. Results for secondary outcome variables stratified by food insecurity showed results similar to what was observed with change in CD4 counts, although the unadjusted and adjusted interaction terms were non-significant.
Table 4.
Mixed model contrastinga differences from baseline to 6-month on primary and secondary outcomes by Asha+ intervention groups (N=600), stratified by food insecurityb
| Food Insecurity < 21% (n=311) | Food Insecurity ≥ 21% (n=289) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nutrition Education | Nutrition Supplement |
Interaction Nutrition Education x Nutrition Supplement |
Nutrition Education | Nutrition Supplement |
Interaction Nutrition Education x Nutrition Supplement |
|||||||
| β 95% CI | P- value | β 95% CI | P- value | β 95% CI | P- value | β 95% CI | P- value | β 95% CI | P- value | β 95% CI | P-value | |
| Primary Outcome | ||||||||||||
| CD4 Count | ||||||||||||
| Crude | 435.97 | 479.34 | 509.59 | 449.50 | 524.05 | 548.43 | ||||||
| Difference | 416., 455.7 | .01 06 | 460.7, 498.0 | .00 01 | 481.4, 537.7 | .0554 | 434.4, 464.6 | .00 38 | 507.9, 540.2 | .0001 | 527.3, 569.6 | .1 912 |
| Adjusted | 436.55 | 481.62 | 509.14 | 449.77 | 523.59 | 548.57 | ||||||
| Differencec | 416.5, 456.6 | .01 08 | 462.5, 500.8 | .00 01 | 480.1, 538.2 | .1615 | 434.2, 465.4 | .00 55 | 507.1, 540.1 | .0001 | 526.8, 570.3 | .1 849 |
| Secondary Outcomes | ||||||||||||
| Weight (kg) | ||||||||||||
| Crude | 4.20 | 5.34 | 5.53 | 4.89 | 6.36 | 6.82 | ||||||
| Difference | 3.9, 4.5 | .00 39 | 5.1, 5.6 | .00 01 | 5.1, 5.9 | .3383 | 4.7, 5.1 | .00 01 | 6.1, 6.6 | .0001 | 6.5, 7.1 | .5 637 |
| Adjusted | 4.16 | 5.31 | 5.44 | 4.83 | 6.35 | 6.73 | ||||||
| Differencec | 3.9, 4.4 | .01 56 | 5.0, 5.6 | .00 01 | 5.0, 5.9 | .3032 | 4.6, 5.0 | .00 01 | 6.1, 6.6 | .00 01 | 6.4, 7.0 | .6 499 |
| BMI (kg/m2) | ||||||||||||
| Crude | 1.84 | 2.34 | 2.43 | 2.14 | 2.76 | 3.00 | ||||||
| Difference | 1.7, 2.0 | .00 53 | 2.2, 2.5 | .00 01 | 2.3, 2.6 | .4645 | 2.0, 2.2 | .00 01 | 2.7, 2.9 | .0001 | 2.8, 3.1 | .6 120 |
| Adjusted | 1.82 | 2.33 | 2.40 | 2.11 | 2.75 | 2.92 | ||||||
| Differencec | 1.7, 1.9 | .01 81 | 2.2, 2.4 | .0001 | 2.2, 2.6 | .4187 | 2.0, 2.2 | .0001 | 2.6, 2.9 | .0001 | 2.8, 3.1 | .6748 |
Compared to Asha+;
Dichotomized at median.
Adjusted for all covariates significantly different at baseline (age, quality of life, total MET, internalized stigma, social support, food insecurity) and ART adherence. Difference=difference from baseline to 6 month follow-up.
Among individuals who were below the median of adherence (n=258), adjusted models of CD4 change from baseline to 6-months showed the unadjusted and adjusted interaction terms between supplementation and education was significant (adjusted: β=529.09; 95% CI=499.4, 558.8; p = .031), as was supplementation alone (adjusted: β=498.67; 95% CI=478.2, 519.1; p=.0001), with education alone marginally significant (adjusted: β=442.57, 95% CI= 420.7, 464.5; p=.077). Similar associations were observed for change in weight and BMI, although the interaction terms were non-significant (See supplement Table 4b).
DISCUSSION
We report results from a cluster-randomized-factorial-nutrition-controlled trial with a six-month intervention period. The effects of the nurse-led Asha-supported behavioral intervention that included nutrition education, nutritional supplementation, and both (nutrition and supplemented) were compared to a control group (i.e., Asha-support only) on key HIV-related outcomes (CD4 counts, weight and BMI). Overall, our findings demonstrate the importance of addressing the nutritional needs of individuals to improve HIV-related outcomes in addition to ART adherence. While all groups demonstrated improvements in ART adherence, those in the nutrition intervention groups experienced statistically significant improvements in outcomes compared to the control group (Asha-only).
Our results showed strong independent effects of nutrition education and nutrition supplementation, and moderate interactive education and supplementation effects on improved CD4 count six-months after baseline. Change in weight and BMI showed similar patterns, although the interaction between education and supplementation did not significantly exceed independent effects. These associations were further stratified according to food insecurity and adherence. The independent associations between change in CD4 counts and supplementation and CD4 and education were found to be stronger among individuals with greater food insecurity. Interactions between adherence and CD4 associations were not significantly different, and were marginally significantly different for weight and BMI.
Our findings suggest that the behavioral and nutrition-based interventions provided along with Asha-support may have important influences on improving immune-related outcomes in WLH/A in India. The combination of nutrition supplementation and education is particularly beneficial at improving immunity measured by increased CD4 count, especially for WLH/A who were below the median at baseline, although evaluation of interaction between adherence and intervention effects were non-significant. Our model could help address the often poor prognosis for HIV-positive individuals in DN where malnutrition is common [8]; adequate nutrition is a critical component of effective immune functioning and defense against OI [29]. Protein and nutrient deficiencies exacerbate immune impairments in HIV-positive individuals in a reciprocal relationship [8, 30]. Specifically, protein-calorie deficits are linked with emergence of viral infections and T-cell impairment, reduced activity of natural killer cells, and lymph tissue decay [30]. Our interventions, which targeted increasing protein intake, resulted in increased CD4 counts, one of the primary metrics of effective HIV treatment and immune functioning, over the 6-month follow-up.
Asha-supported nutrition supplementation and education both independently helped improve BMI and lean body mass over the 6-month follow-up. Body weight indices have been implicated in increasing relative-risk for death independent of CD4 counts [31]. This is likely due to increased resting metabolic rate in HIV-positive individuals, which necessitates increased caloric intake for weight maintenance to prevent wasting and untimely death [30]. Although BMI is not necessarily the ideal marker for malnutrition [30], in HIV-positive samples, low-BMI is a strong predictor of both morbidity and mortality [31, 32].
Our findings suggest that both Asha-supported nutrition supplements and education might help address the link between malnutrition and HIV progression and allow for antiviral therapy to more effectively address HIV symptoms. Perhaps somewhat surprisingly, both supplement alone and education alone improved CD4 count, BMI, and lean body mass, suggesting that education and supplements may each improve outcomes. This finding may help address the limitations noted in prior work examining nutrition supplementation as a component of HIV-treatment in DN [33]. Nutrition education, administered by lay community members, could serve as an alternative to nutrition supplementation in areas with limited capabilities to provide supplementation and serve as a more sustainable long-term solution.
Results also provide support for an Asha-based approach to HIV-intervention in DN. WLH/A in India face a number of challenges regarding receipt of adequate care [34]. These difficulties include lack of resources, restricted mobility to reach facilities, stigma, and apathetic or outright abusive behavior by healthcare workers [35]. In contrast, Asha are members of the community who provide emotional as well as logistical and medical support, which serve as an important bridge between WLH/A and the treatment community [36]. Thus, Asha-approach may improve intervention efficacy, bolstering trust and adherence. The improvement in primary and secondary outcomes for both intervention groups in combination with Asha-support suggests the value of Asha across treatment modalities. Future research should explore this approach in other population (e.g., Africa, other locations in India) where women face similar logistical and psychological barriers.
Although our study was able to demonstrate the effectiveness of lay-administered nutrition education and supplementation on HIV-related outcomes, some limitations exist. Randomization was not conducted on an individual level resulting in group differences according to geographic stratification. Contamination of intervention conditions across sites was possible, despite the geographic distance between sites and limited mobility of the population. Moreover, although interviewers and Asha were trained not to discuss other intervention conditions and all group training sessions were held on specific days devoted to one of the four conditions, intervention fidelity could not be completely verified. However, interviewers and Asha were repeatedly queried about their communication with study participants to insure minimal contamination. Finally, although our covariate-adjusted models sought to control for a number of potential confounders, it is possible that improvements in primary and secondary outcomes could result from other components of the Asha-intervention that were not specifically tested (e.g., social support from women in cooking classes, extra time spent with Asha).
Conclusions
Our findings demonstrate the importance of integrating improved nutrition into effective HIV care for WLH/A in India. Moreover, results suggest that targeting nutrition is beneficial for women who are especially food insecure in this setting. While the combination of nutrition education and supplementation were superior to either alone at increasing CD4 counts, both exhibited strong independent effects on this immune marker as well as secondary outcomes (i.e., weight, BMI). Importantly, this intervention was administered by trained lay members of the community, providing an approach that may be scalable, economically viable, and clinically efficacious to improve life for HIV-positive women in vulnerable communities.
Supplementary Material
Acknowledgement
This study was funded by the National Institute of Mental Health (NIMH) through grant R01MH098728.
A.N. designed the research study and contributed to methods and discussion. C.C. developed the nutrition education intervention, analyzed the data, and contributed to the statistical methods, results and discussion sections. K.Y. contributed to methods and measures. D.G. contributed to introduction and discussion sections. L.M. and M.K. contributed to review of literatures. M.E. and S.S. contributed to the editing and final review of the manuscript. All authors have read and approved the final manuscript.
Source of Funding: This study is funded by the National Institute of Mental Health (NIMH) through grant R01MH098728.
Clinical Trial Registration Number: This study is registered with ClinicalTrials.gov NCT02136082 (URL: https://clinicaltrials.gov/ct2/show/NCT02136082)
Footnotes
Conflict of Interest: The authors declare that they have no competing interests.
References
- 1.NACO. National AIDS Control Organisation (NACO) Annual Report 2016–17. New Dehli, India. Available at: http://naco.gov.in/documents/annual-reports [Google Scholar]
- 2.Paranjape RS, Challacombe SJ. HIV/AIDS in India: An overview of the Indian epidemic. Oral Dis 2016; 22:10–14. [DOI] [PubMed] [Google Scholar]
- 3.Mothi SN, Lala MM, Tappuni AR. HIV/AIDS in women and children in India. Oral Dis 2016; 22:19–24. [DOI] [PubMed] [Google Scholar]
- 4.Heylen E, Panicker ST, Chandy S, Steward WT, Ekstrand ML. Food Insecurity and Its Relation to Psychological Well-Being Among South Indian People Living with HIV. AIDS Behav 2015; 19:1548–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chandra RK, Kumari S. Nutrition and immunity: An overview. J Nutr 1993; 124:1436–1441. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. Consultation on Nutrition and HIV/AIDS in Africa: Evidence, lessons and recommendations for action, 2005. Geneva, Switzerland: Available at: http://www.who.int/nutrition/topics/consultation_nutrition_and_hivaids/en/ [Google Scholar]
- 7.Weiser SD, Young SL, Cohen CR, Kushel MB, Tsai AC, Tien PC, et al. Conceptual framework for understanding the bidirectional links between food insecurity and HIV/AIDS. Am J Clin Nutr 2011; 94:1729S–1739S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anabwani G, Navario P. Nutrition and HIV/AIDS in sub-Saharan Africa: An overview. Nutrition 2005; 21:96–99. [DOI] [PubMed] [Google Scholar]
- 9.Mahlungulu S, Grobler LA, Visser ME, Volmink J. Nutritional interventions for reducing morbidity and mortality in people with HIV. Cochrane Database Syst Rev 2007; 18:CD004536. [DOI] [PubMed] [Google Scholar]
- 10.Kotler DP. Wasting Syndrome: Nutritional Support in HIV Infection. AIDS Res Hum Retrovir 1994; 10:931–934. [DOI] [PubMed] [Google Scholar]
- 11.Ahoua L, Umutoni C, Huerga H, Minetti A, Szumilin E, Balkan S, et al. Nutrition outcomes of HIV-infected malnourished adults treated with ready-to-use therapeutic food in sub-Saharan Africa: A longitudinal study. J Int AIDS Soc 2011; 14:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nyamathi A, Sinha S, Ganguly KK, Ramakrishna P, Suresh P, Carpenter CL. Impact of protein supplementation and care and support on body composition and CD4 count among HIV-infected women living in rural India: Results from a randomized pilot clinical trial. AIDS Behav 2013; 17:2011–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nyamathi A, Hanson AY, Salem BE, Sinha S, Ganguly KK, Leake B, et al. Impact of a rural village women (Asha) intervention on adherence to antiretroviral therapy in southern India. Nurs Res 2012; 61:353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nyamathi A, Salem B, Ernst EJ, Keenan C, Suresh P, Sinha S, et al. Correlates of Adherence Among Rural Indian Women Living With HIV/AIDS. J HIV AIDS Soc Serv 2012; 11:327–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steward WT, Chandy S, Singh G, Panicker ST, Osmand TA, Heylen E, et al. Depression is not an inevitable outcome of disclosure avoidance: HIV stigma and mental health in a cohort of HIV-infected individuals from Southern India. Psychol Health Med 2011; 16:74–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ekstrand ML, Shet A, Chandy S, Singh G, Shamsundar R, Madhavan V, et al. Suboptimal adherence associated with virological failure and resistance mutations to first-line highly active antiretroviral therapy (HAART) in Bangalore, India. Int Health 2011; 3:27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ekstrand ML, Chandy S, Heylen E, Steward W, Singh G. Developing useful highly active antiretroviral therapy adherence measures for India: the Prerana study. J Acquir Immune Defic Syndr 2010; 53:415–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Endicott J, Nee J, Harrison W, Blumenthal R. Quality of Life Enjoyment and Satisfaction Questionnaire: a new measure. Psychopharmacol Bull 1993; 29:321–326. [PubMed] [Google Scholar]
- 19.Coates J, Swindale A, Bilinsky P. Household Food Insecurity Access Scale (HFIAS) for Measurement of Household Food Access: Indicator Guide (v.3). Washington, D.C., United States, 2007. [Google Scholar]
- 20.Puello VV, Orellana SA, Samur AE. Food insecurity among elderly people in 15 districts of the Great Santiago area; an unresolved issue. Nutr Hosp 2013; 28:1430–1437. [DOI] [PubMed] [Google Scholar]
- 21.Radloff L The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas 1977; 1:385–401. [Google Scholar]
- 22.Nyamathi A, Heravian A, Zolt-Gilburne J, Sinha S, Ganguly K, Liu E, et al. Correlates of depression among rural women living with AIDS in Southern India. Issues Ment Health Nurs 2011; 32:385–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ekstrand ML, Bharat S, Ramakrishna J, Heylen E. Blame, symbolic stigma and HIV misconceptions are associated with support for coercive measures in urban India. AIDS Behav 2012; 16:700–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steward WT, Herek GM, Ramakrishna J, Bharat S, Chandy S, Wrubel J, et al. HIV-related stigma: adapting a theoretical framework for use in India. Soc Sci Med 2008; 67:1225–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med 1991; 32:705–714. [DOI] [PubMed] [Google Scholar]
- 26.Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 2003; 35:1381–1395. [DOI] [PubMed] [Google Scholar]
- 27.Giordano TP, Guzman D, Clark R, Charlebois ED, Bangsberg DR. Measuring adherence to antiretroviral therapy in a diverse population using a visual analogue scale. HIV Clin Trials 2004; 5:74–79. [DOI] [PubMed] [Google Scholar]
- 28.McDermott AY, Terrin N, Wanke C, Skinner S, Tchetgen E, Shevitz AH. CD4+ cell count, viral load, and highly active antiretroviral therapy use are independent predictors of body composition alterations in HIV-infected adults: a longitudinal study. Clin Infect Dis 2005; 41:1662–1670. [DOI] [PubMed] [Google Scholar]
- 29.Scrimshaw NS, Sangiovanni JP. Synergism of nutrition, infection, and immunity: an overview. Am J Clin Nutr 1997; 66:464S–477S. [DOI] [PubMed] [Google Scholar]
- 30.Koethe JR, Chi BH, Megazzini KM, Heimburger DC, Stringer JSA. Macronutrient Supplementation for Malnourished HIV‐Infected Adults: A Review of the Evidence in Resource‐Adequate and Resource‐Constrained Settings. Clin Infect Dis 2009; 49:787–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guenter P, Muurahainen N, Simons G, Kosok A, Cohan GR, Rudenstein R, et al. Relationships among nutritional status, disease progression, and survival in HIV infection. J Acquir Immune Defic Syndr 1993; 6:1130–1138. [PubMed] [Google Scholar]
- 32.Jones CY, Hogan JW, Snyder B, Klein RS, Rompalo A, Schuman P, et al. Overweight and human immunodeficiency virus (HIV) progression in women: associations HIV disease progression and changes in body mass index in women in the HIV epidemiology research study cohort. Clin Infect Dis 2003; 37(Suppl 2):S69–S80. [DOI] [PubMed] [Google Scholar]
- 33.Byron E, Gillespie S, Nangami M. Integrating nutrition security with treatment of people living with HIV: Lessons from Kenya. Food Nutr Bull 2008; 29:87–97. [DOI] [PubMed] [Google Scholar]
- 34.Srivastava N, Nyamathi AM, Sinha S, Carpenter C, Satyanarayana V, Ramakrishnan P, et al. Women living with AIDS in rural Southern India: Perspectives on mental health and lay health care worker support. J HIV AIDS Soc Serv 2017; 16:170–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nyamathi A, Ekstrand M, Heylen E, Ramakrishna P, Yadav K, Sinha S, et al. Relationships Among Adherence and Physical and Mental Health Among Women Living with HIV in Rural India. AIDS Behav 2018: 22:867–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nyamathi AM, Ekstrand M, Yadav K, Ramakrishna P, Heylen E, Carpenter CL, et al. Women living with AIDS in rural Southern India: Perspectives on mental health and lay health care worker support. J Assoc Nurses AIDS Care 2016; 37:412–425. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.