Abstract
Background
Anxious youth are at risk for negative peer interactions including peer victimization, and for suicidal ideation. However, data about the pattern of association between these two factors are scarce. In this study we examined the association between negative peer interactions and suicidal ideation in a sample of children and adolescents with anxiety disorders, and whether oxytocin, which has been shown to enhance the impact of social events, moderates the impact of negative peer interactions on suicidal ideation.
Method
Participants were 168 youths with primary anxiety disorders. All participants were assessed with semi-structured diagnostic interviews, and with self-report measures of suicidal ideation, negative peer interactions, anxiety, and depression. The anxious youths’ salivary oxytocin levels were measured with immunoassay.
Results
Thirty percent of the anxious youths reported suicidal ideation, with suicidal ideation severity associated with negative peer social interactions and depressive symptoms. Consistent with past data indicating that oxytocin enhances the impact of social events, the association between peer negative social interactions and suicidal ideation was stronger in youths with high oxytocin levels than in youths with low levels (i.e., moderation).
Limitations
Assessment focused on suicidal ideation and data on suicidal behavior were not available. Limitations inherent to immunoassay measurement of peripheral oxytocin levels are noted.
Conclusion
Negative peer interactions are associated with suicidal ideation in youth with anxiety disorders, and the association is stronger in youth with high oxytocin levels.
Keywords: Anxiety, Suicidal Ideation, Oxytocin, Child, Adolescent
INTRODUCTION
Suicide is among the top three causes of death for youth in the United States and has been increasing in incidence for the past several years (Centers for Disease Control, 2015). The latest Youth Risk Behavior Survey found 17.7% of youth reported suicidal ideation in the preceding twelve months, a rate that has been growing steadily since 2009 (Centers for Disease Control, 2017).
Data have accumulated from community and clinical samples that overall show an association between youth anxiety and suicidal ideation in studies that use carefully characterized samples and/or measures with well-established psychometrics, and even when controlling for depression (Boden, Fergusson, & Horwood, 2007; Carter, Silverman, Allen, & Ham, 2008; Cummings, Caporino, & Kendall, 2014; Esposito & Clum, 2002; Ghaziuddin, King, Naylor, & Ghaziuddin, 2000; Ruchkin, Schwab-Stone, Koposov, Vermeiren, & King, 2003; Woods, Silverman, Gentilini, Cunningham, & Grieger, 1991). In contrast, scant data exist that offer insights about factors that may contribute to, or help to explain, the association between youth anxiety and suicidal ideation (See, Hill, Castellanos, & Pettit, 2011 for a comprehensive review).
The present study addresses this gap in knowledge by examining two factors that have never been examined together in a study of youth anxiety and suicidal ideation, though research suggests intriguing associations may exist. Specifically, we examined negative peer social interactions, assessed from the perspective of the youth, and the role of the nonapeptide oxytocin in moderating the association of negative peer social interactions with suicidal ideation in youth with anxiety disorders.
Our examination of negative peer social interactions is guided by research findings showing, as meta-analyses have reported, that negative peer social interactions, including peer victimization, pose a significant risk factor for suicidal ideation in youth (van Geel, Vedder, & Tanilon, 2014), and that the peer interactions of anxious youth are characterized as more negative compared with their non-anxious counterparts (Cohen & Kendall, 2015; Hawker & Boulton, 2000; Scharfstein, Alfano, Beidel, & Wong, 2011). Also of note, longitudinal data indicate that negative peer social interactions mediate the emergence of depressive symptoms in anxious youth (Biggs, Nelson, & Sampilo, 2010). Depression is also linked to negative peer interactions, in a bidirectional manner. Specifically, negative peer interactions predict depression in youth (Hodges & Perry, 1999; MacPhee & Andrews, 2006; Seals & Young, 2003), and depressed youth report more negative peer interactions than non-depressed youth (Borelli & Prinstein, 2006; Sweeting, Young, West, & Der, 2006).
The nonapeptide oxytocin is implicated in social behavior and has been found to enhance the salience of both positive and negative social events (Averbeck, 2010; Bartz, Zaki, Bolger, & Ochsner, 2011; Gordon & Berson, 2018; McQuaid, McInnis, Matheson, & Anisman, 2016; Shamay-Tsoory, 2010) with genetic differences in oxytocin-related genes moderating the link between negative events and suicidal ideation (McQuaid et al., 2016). Although more research is needed to understand the mechanisms by which oxytocin enhances the salience of social events, research indicates that both social events and stressful events trigger oxytocin release, influencing oxytocin-receptor rich areas of the brain, and the connectivity between them (Crockford, Deschner, Ziegler, & Wittig, 2014). Negative peer social interactions combine both social and stressful elements, and as such may be particularly susceptible to oxytocin-enhancing effects.
In the current study we therefore investigated whether negative peer social interactions are associated with suicidal ideation in a sample of clinically anxious youth and whether youth oxytocin levels moderate the association between negative peer social interactions and suicidal ideation. We hypothesized that suicidal ideation would be associated with negative peer social interactions. We further hypothesized that peripheral oxytocin levels would moderate the association between negative peer social interactions and suicidal ideation, with stronger association in youth with high oxytocin levels, and weaker association in youth with low oxytocin levels.
METHODS
Study Sample
Participants were 168 youth (51% female) and their mothers, who presented consecutively to the Anxiety and Mood Disorders Program at the Yale Child Study Center and met DSM-5 criteria for a primary anxiety disorder. Youths were between the ages of 7–16 years (Mean=11 years, SD=2.8). This age range spans the modal period of onset for both suicidal ideation and anxiety disorders (Bolger et al., 1989; Costello, Egger, Copeland, Erkanli, & Angold, 2011; Costello, Mustillo, Erkanli, Keeler, & Angold, 2003; Kashani, Goddard, & Reid, 1989; Nock & Kazdin, 2002). The most common primary anxiety disorders in the sample were: generalized anxiety disorder (35%), social phobia (31%), separation anxiety disorder (19%), specific phobia (10%), panic disorder (3%), and agoraphobia (2%). Comorbidity among the anxiety disorders was high as is typical for clinically anxious youth (Chavira, Garland, Yeh, McCabe, & Hough, 2009; Costello et al., 2011; Costello et al., 2003; Franco, Saavedra, & Silverman, 2007): 29% met criteria for one anxiety disorder, 36% met criteria for two anxiety disorders, 27% met criteria for three anxiety disorders, and 8% met criteria for four or more anxiety disorders. Comorbid non-anxiety disorders were also common in the sample and included: attention deficit hyperactivity disorder (18%), oppositional defiant disorder (14%), obsessive-compulsive disorder (14%), depression (12%), and selective mutism (4%).
Measures and Procedures
The study was approved by the Yale Human Investigation Committee. Signed consent and assent were obtained from youths and their parents respectively, before all other study procedures.
Anxiety Disorder Interview Schedule, Child and Parent Versions
(ADIS-C/P) (Silverman & Albano, 1996). DSM-5 Diagnoses were determined using the ADIS-C/P, a semi-structured interview that was administered separately to children and parents. Final diagnoses were determined through the integration of information collected from both and agreed upon by expert consensus, including one of the authors of the ADIS C/P.
Suicidal Ideation Risk Scale (SIRS)
(Adrian, Miller, McCauley, & Vander Stoep, 2016). The SIRS sums youth responses to three items that assess thoughts about death and suicide, derived from the Mood and Feelings Questionnaire (Angold, Costello, Messer, & Pickles, 1995). The items are, ‘I thought life wasn’t worth living’; ‘I thought about death and dying’; and ‘I thought about killing myself’. Response options for each item are ‘Not True’; ‘Sometimes True’; and ‘True’, coded 0–2 respectively. The SIRS has been used in previous research to assess suicidal ideation in youth (e.g., Adrian et al., 2016). Internal consistency for the SIRS items was α=0.85. SIRS scores above zero indicate the presence of suicidal ideation, and moderate suicidal ideation has been associated with an average score of 0.73 in previous research (Adrian et al., 2016).
Friendship Questionnaire
(Bierman & McCauley, 1987). The Negative Interactions scale of the Friendship Questionnaire, completed by youth, queries a variety of negative social interactions and peer victimization. Items are rated on a 5-point scale from Never to Always and include for example ‘Is there someone who teases you and makes fun of you?’; ‘Is there someone who beats you up?’; ‘Is there someone who leaves you out and won’t include you in their fun?’; and ‘Is there someone who plays mean tricks on you?’. The scale has been used in previous research on the impact of social interactions on anxious youth and has good reliability and validity (e.g., Motoca, Williams, & Silverman, 2012). Internal consistency for the Friendship Questionnaire negative social interactions items was α=0.91.
Multidimensional Anxiety Scale for Children (MASC)
(March, Parker, Sullivan, Stallings, & Conners, 1997). Youth completed the 2nd edition of MASC, a 50-item rating scale that covers a broad range of anxiety symptoms. MASC has been shown to have good reliability and validity as a self-rated indicator of anxiety severity across the school age (March, Parker, Sullivan, Stallings, & Conners, 1997). Internal consistency for the MASC was α=0.88.
Child Depression Inventory (CDI)
(Kovacs, 1985). Youth completed the CDI, a 27-item rating scale that assesses the presence and severity of depressive symptoms in youth. CDI has been shown to have good reliability and validity in school-age youth (Kaslow, Rehm, & Siegel, 1984; Kovacs, 1985). Internal consistency for the CDI was 0.86
Oxytocin Levels.
Youth peripheral oxytocin levels were measured in saliva collected using salivettes (Sarstedt, Rommelsdorf, Germany), between 4PM and 5PM, after a 2-hour fast. Samples were stored at −20C until centrifuged twice, 2 days apart, at 40C at 1,500g for 20 min. Liquid samples were kept at −80C, lyophilized, and stored at −20C. On the assay day samples were reconstituted in water and concentrated × 4 before immunoassay with an ELISA kit from Enzo® (NY, USA). Measurements were performed in duplicate and concentrations were calculated using Matlab-7 according to relevant standard curves. The reliability of salivary oxytocin measurement is not well established, but studies have reported strong and significant associations between salivary oxytocin levels and plasma oxytocin levels for which reliability is better established (Feldman, Gordon, Schneiderman, Weisman, & Zagoory-Sharon, 2010; Grewen, Davenport, & Light, 2010; Weisman, Zagoory-Sharon, Schneiderman, Gordon, & Feldman, 2013). Two samples were collected from each youth, seven minutes apart. The two measurements were strongly correlated (r=0.8, p<.001) and the average of both measurements was used in analyses.
Statistical Methods
Analyses were conducted in SPSS (version 24). Bivariate correlations and multiple regression were used to examine associations between the study variables. To test the hypothesis that oxytocin levels moderate the association between negative social interactions and suicidal ideation hierarchical multiple linear regression with suicidal ideation as the predicted variable was conducted. Youth age was included as covariate in the analysis, in light of two studies that have reported correlations between oxytocin levels and age in both anxious and typically-developing youth (Lebowitz et al., 2016; Modahl et al., 1998). In the first step, negative social interactions and oxytocin level were included as the predictor variables. In the second step, an interaction term was added, equal to the product of negative social interactions and oxytocin level. Significant change in explained variance in suicidal ideation when the interaction term is included in analysis provides evidence of moderation. The PROCESS macro for SPSS (Hayes, 2013) was then used to calculate simple slopes for the associations between negative social interactions and youth suicidal ideation at low (1 SD below the mean), moderate (mean), and high (1 SD above the mean) oxytocin level, and 95% confidence intervals for the effect sizes were calculated based on bootstrapping procedures with 5000 samples.
RESULTS
Table 1 presents clinical characteristics of the sample and associations between suicidal ideation and the other study variables. Overall, suicidal ideation was common among the anxious youth, with 30% endorsing at least one of the items on SIRS, and mean SIRS score was 0.8 (SD=1.5), comparable to the mean SIRS score associated with moderate risk of suicidal ideation in previous research (Adrian et al., 2016). Bivariate correlations revealed suicidal ideation was significantly correlated with negative social interactions, as well as with severity of anxiety and depression symptoms, and youth age, but not youth sex. Suicidal ideation was higher among youth with depression, generalized anxiety disorder, and panic disorder, compared to youth without (t=7.5, p<.001; t=2.4, p<.05; t=2.8, p<.01, respectively). No other diagnoses were associated with suicidal ideation.
Table 1.
Suicidal ideation in clinically anxious youth (N=168), associations with negative social interactions, clinical and demographic characteristics, and salivary oxytocin levels.
| Mean | (SD) | Correlation with Suicidal Ideation | |
|---|---|---|---|
| 0.8 | (1.5) | - | |
| Negative Social Interactions (FQ) | 1.7 | (0.67) | 0.32*** |
| Anxiety (MASC) | 70.9 | (25.3) | 0.28*** |
| Depression (CDI) | 16.8 | (8.7) | 0.55*** |
| Age | 11.1 | (2.8) | .19** |
| Sex (% female) | 51% | NS | |
| Salivary Oxytocin Level in pg/ml | 26.1 | 14.7 | 0.07 |
=p<001;
=p<.01;
SIRS=Suicide Ideation Risk Scale; FQ=Friendship Questionnaire (Negative Interactions); MASC=Multidimensional Anxiety Scale for Children; CDI=Child Depression Inventory.
Multiple linear regression, predicting suicidal ideation from age, negative social interactions, depression scores, and anxiety scores, resulted in a significant model. The overall model explained 34% of variance in suicidal ideation (adjusted R2=0.33, F=26.3, p<.001). Depression ratings and negative social interactions ratings contributed significantly to the model (Bstandardized=.52, p<.001, and Bstandardized=.18, p<.01, respectively). Youth age and anxiety scores did not significantly predict suicidal ideation in the multiple regression model, though both were significantly associated with suicidal ideation in bivariate correlations.
Salivary OT levels ranged from 4.5pg/ml to 75.8pg/ml (Mean=26.1pg/ml, SD=14.7), and were normally distributed with acceptable skewness (.69, SEM=.18) and kurtosis (.09, SEM=.37). Salivary oxytocin levels correlated positively with youth age (r=0.24, p<.01) and were not significantly correlated with suicidal ideation in bivariate correlation.
Hierarchical linear regression was conducted to test the hypothesis that oxytocin levels moderate the association between negative social interactions and suicidal ideation. Youth age was included as a covariate in the analysis as both oxytocin levels and suicidal ideation were significantly correlated with age. Results of the regression showed significant change in explained variance when the interaction term between negative social interactions and oxytocin level was included in the analysis, providing evidence of moderation. The first step, including negative social interactions and oxytocin level resulted in explained variance of R2=.19 for suicidal ideation and was significantly different from zero (F=9.34, p<.001). Both negative social interactions (Beta=.36, p<.001) and youth age (Beta=.23, p<.01) contributed significantly to the prediction of suicidal ideation, and oxytocin levels did not (Beta=.01, NS). The second step, with the addition of the interaction term equal to the product of negative social interaction and oxytocin level, increased the explained variance by 6.4%, which was significantly different from step 1 (Fchange=12.8, p<.001).
Examination of simple slopes for the associations between negative social interactions and suicidal ideation revealed that the effect size became larger with higher oxytocin levels. When oxytocin levels were low, the association between negative social interactions and suicidal ideation was not significant (β=.07, 95% CI: −.29 to .43, p=.68). For youth with moderate levels of oxytocin the association was significant (β=1.1, 95% CI: .55 to 1.6, p=<.001), and for youth with high oxytocin levels the association was between negative social interactions and suicidal ideation was strongest and most significant (β=.1.53, 95% CI: .81 to 2.25, p=<.001). Figure 1 plots the simple slopes for the interaction and shows that negative social interactions did not predict suicidal ideation in youth with low oxytocin levels, and did so at increasingly higher oxytocin levels.
Figure 1.
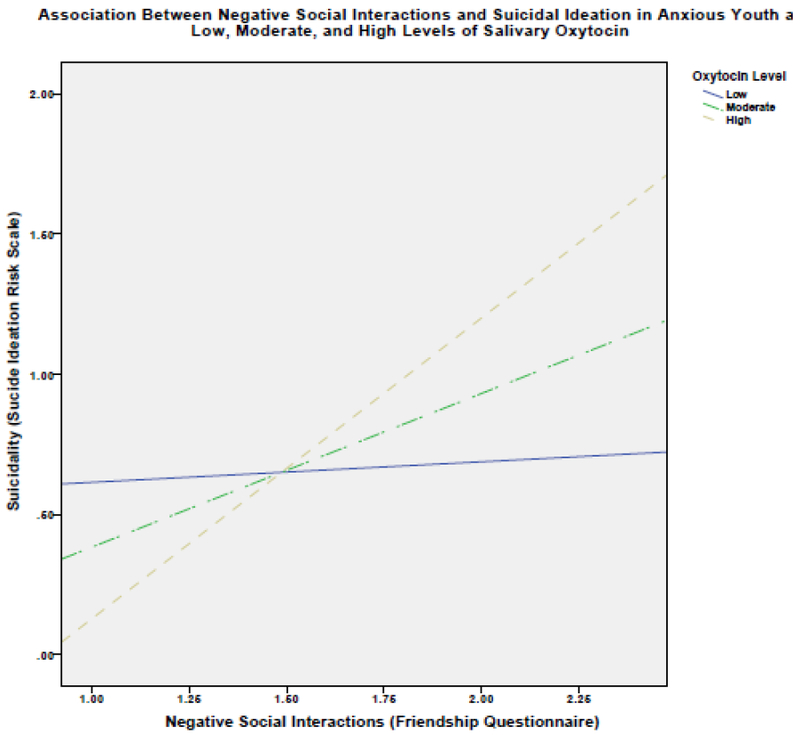
Association Between Negative Social Interactions and Suicidal Ideation in Anxious Youth at Low, Moderate, and High Levels of Salivary Oxytocin
DISCUSSION
Negative social interactions were associated with increased risk of suicidal ideation in anxious youth and were moderated by oxytocin levels. Suicidal ideation was also significantly associated with severity of anxiety and depression symptoms and with youth age. Examining the predictors of suicidal ideation together in multiple linear regression, depression and negative peer social interactions significantly predicted suicidal ideation, while age and anxiety scores no longer contributed significantly to the model.
Overall, these findings are in line with previous data linking anxiety to suicidal ideation in both community and clinical samples and have important clinical and research implications (Boden, Fergusson, & Horwood, 2007; Carter, Silverman, Allen, & Ham, 2008; Cummings, Caporino, & Kendall, 2014; Esposito & Clum, 2002; Ghaziuddin, King, Naylor, & Ghaziuddin, 2000; Ruchkin, Schwab-Stone, Koposov, Vermeiren, & King, 2003; Woods, Silverman, Gentilini, Cunningham, & Grieger, 1991). Negative social interactions and peer victimization are key aspects of youth experiences and are important to consider in evaluating risk factors for suicidal ideation or behavior. The importance of considering these aspects is compounded by the heightened prevalence of newer forms of peer victimization such as cyberbullying, which can be perpetrated anonymously at any time and place, and which a recent review found to be reported by as many as 42% of youth (Hamm et al., 2015).
This is the first study to show that oxytocin levels significantly moderate the association between negative peer social interactions and suicidal ideation in clinically anxious youth. As we predicted, higher levels of oxytocin were associated with a stronger link between negative social interactions and suicidal ideation. This is in line with research indicating that oxytocinergic functioning increases the salience and impact of social interactions and can amplify the impact of negative experiences (Averbeck, 2010; Chatzittofis, Nordstrom, Uvnas-Moberg, Asberg, & Jokinen, 2014; McQuaid et al., 2016). Animal studies and oxytocin administration studies in humans have begun to identify the mechanisms through which oxytocin impacts social perception, focusing on oxytocin-receptor rich areas of the brain implicated in social behavior, and the connectivity between them (e.g., Hurlemann & Scheele, 2016; Young, 2015). The results are also in line with recent findings from genetic research. The CD38 gene is required for oxytocin secretion and has been linked to differences in social processing (Jin et al., 2007; Kiss, Levy-Gigi, & Keri, 2011; Lerer et al., 2010; Munesue et al., 2010). A single nucleotide polymorphism on CD38 moderated the association between traumatic experiences and suicidal ideation in a recent study of college age students (McQuaid et al., 2016). The association was stronger among students with the genotype associated with higher oxytocin levels (AA). Additional research on the role of the oxytocinergic system for suicidal ideation is needed and may be best understood within the context of a diathesis-stress model, as has been proposed to integrate other findings on environmental and biological risk factors for suicidal behavior (Brodsky, 2016).
The study has several limitations that warrant consideration; two relate to our measurement approach. Specifically, our assessment was limited to suicidal ideation and did not incorporate broader aspects such as suicidal attempts (Silverman, Berman, Sanddal, O’Carroll, & Joiner, 2007). However, as the first study in this area, it was reasonable to begin with suicidal ideation given its high prevalence and relative ease in assessment. In the future it will be important to broaden the assessment to include other aspects of suicidal behavior. A potential limitation of establishing the presence of suicidal ideation using the SIRS subscale of MFQ is the possibility for youth to endorse the item ‘I thought about death and dying’ without suicidal intent (e.g., if a death in the family caused the child to think about death). The SIRS approach has been used in past research and our findings are similar to those previously reported. Nevertheless, additional research with more thorough assessment of suicidal ideation would be desirable.
Also relating to assessment, salivary oxytocin has been increasingly used in research on the behavioral effects of oxytocin due to ease with which it can be obtained. Questions have arisen though about the validity of immunoassay oxytocin measurement and of peripheral markers as indicators of central oxytocinergic functioning. For example, there is debate about the optimal measurement methods and processes (e.g., use of extracted vs. unextracted samples; use of enzyme assays vs. radio-immunoassay), as well the fluid of choice in which to measure oxytocin (i.e., saliva vs. blood, urine or CSF) (Carter et al., 2007; Kagerbauer et al., 2013; Lebowitz, Gee, Pine, & Silverman, 2018; McCullough, Churchland, & Mendez, 2013; Szeto et al., 2011; Young & Anderson, 2010). These important questions notwithstanding, a wealth of data from numerous studies support the usefulness of measuring peripheral oxytocin levels, reporting highly stable oxytocin levels over periods of several months, and associations between peripheral oxytocin levels and behavioral indicators in line with what theory would predict, and pointing to coordination between peripheral and central oxytocinergic functioning (Crockford et al., 2014; Weisman et al., 2013). For example, a recent study compared central (cerebrospinal fluid) and peripheral oxytocin using four separate measurement methods and found positive correlation between the central and peripheral levels on three out of the four methods, including commercial enzyme immunoassay as was used in the current study (Lefevre et al., 2017).
Another limitation relates to our sampling frame. We focused, for this preliminary investigation, on clinic referred youth who presented with anxiety disorders, and we did not include other clinic samples or healthy’ controls. Future work is required using a broader sampling frame, to examine the patterns of associations in additional populations.
These limitations notwithstanding, the current paper provides important and preliminary data on the risk of negative peer social interactions for suicidal ideation in youth. The study also supports a role for the oxytocinergic system in modulating the effects negative social interactions, a novel neurobiological finding that underscores the need for more research on this and other neurobiological systems in youth suicidal ideation.
Table 2.
Results of hierarchical regression predicting suicidal ideation from negative social interactions, salivary oxytocin levels, youth age (Step 1) and including the interaction term equal to the product of negative social interactions and salivary oxytocin levels (Step 2).
| Step 1 | Beta | t | p |
|---|---|---|---|
| (Constant) | 3.59 | <.001 | |
| Negative Social Interactions | .362 | 5.03 | <.001 |
| Salivary Oxytocin Level | .01 | .143 | NS |
| Youth Age | .233 | 3.2 | <.01 |
| Step 2 | |||
| (Constant) | −2.18 | <.05 | |
| Negative Social Interactions | .18 | 1.68 | NS |
| Salivary Oxytocin Level | −.41 | −2.07 | <.05 |
| Youth Age | .239 | 3.32 | <01 |
| Interaction Term | .47 | 2.29 | <.01 |
Highlights.
We studied suicidal ideation, peer interactions, and oxytocin, in anxious youth.
Suicidal ideation was common in the anxious youth.
Negative peer interactions were associated with suicidal ideation.
Oxytocin moderated the link between negative interactions and suicidal ideation.
ACKNOWLEDGEMENTS
The authors are grateful to Ms. Alyssa Martino for her assistance in collecting these data. The authors are grateful to Mr. Steven Koproski for his assistance in this work.
FUNDING SOURCES
This work was supported by the National Institutes of Health (K23MH103555; ERL, R21MH113946; ERL/WKS) and the National Center for Advancing Translational Sciences (KL2TR000140; ERL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest The authors have no conflicts of interest to report.
REFERENCES
- Adrian M, Miller AB, McCauley E, & Vander Stoep A (2016). Suicidal ideation in early to middle adolescence: Sex-specific trajectories and predictors. J. Child Psychol. Psychiatry,.57(5), pp. doi: 10.1111/jcpp.1248426610726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angold A, Costello EJ, Messer SC, & Pickles A (1995). Development of a short questionnaire for use in epidemiological studies of depression in children and adolescents. Int. J. Methods Psychiatr. Res,.5(4), pp. [Google Scholar]
- Averbeck BB (2010). Oxytocin and the salience of social cues. Proc. Natl. Acad. Sci. U. S. A, 107(20), 9033–9034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartz JA, Zaki J, Bolger N, & Ochsner KN (2011). Social effects of oxytocin in humans: context and person matter. Trends Cogn Sci, 15(7), 301–309. doi: 10.1016/j.tics.2011.05.002 [DOI] [PubMed] [Google Scholar]
- Bierman KL, & McCauley E (1987). Children’s descriptions of their peer interactions: Useful information for clinical child assessment. J. Clin. Child Psychol,.16(1), pp. doi: 10.1207/s15374424jccp1601_2 [DOI] [Google Scholar]
- Biggs BK, Nelson JM, & Sampilo ML (2010). Peer relations in the anxiety-depression link: test of a mediation model. Anxiety Stress Coping, 23(4), 431–447. [DOI] [PubMed] [Google Scholar]
- Boden JM, Fergusson DM, & Horwood LJ (2007). Anxiety disorders and suicidal behaviours in adolescence and young adulthood: findings from a longitudinal study. Psychol. Med, 37(3), 431–440. doi: 10.1017/S0033291706009147 [DOI] [PubMed] [Google Scholar]
- Bolger N, Downey G, Walker E, Steininger P, Id, & Bolger, N. O. h. o. o. (1989). The onset of suicidal ideation in childhood and adolescence. J. Youth Adol,.18(2), pp. doi: 10.1007/BF0213879924271685 [DOI] [PubMed] [Google Scholar]
- Borelli JL, & Prinstein MJ (2006). Reciprocal, longitudinal associations among adolescents’ negative feedback-seeking, depressive symptoms, and peer relations. J. Abnorm. Child Psychol, 34(2), 159–169. [DOI] [PubMed] [Google Scholar]
- Brodsky BS (2016). Early Childhood Environment and Genetic Interactions: the Diathesis for Suicidal Behavior. Curr Psychiatry Rep, 18(9), 86. doi: 10.1007/s11920-016-0716-z [DOI] [PubMed] [Google Scholar]
- Carter CS, Pournajafi-Nazarloo H, Kramer KM, Ziegler TE, White-Traut R, Bello D, & Schwertz D (2007). Oxytocin - Behavioral associations and potential as a salivary biomarker. Oral-Based Diagnostics, 1098, 312–322. doi: 10.1196/annals.1384.006DOI [DOI] [PubMed] [Google Scholar]
- Carter R, Silverman WK, Allen A, & Ham L (2008). Measures matter: the relative contribution of anxiety and depression to suicidal ideation in clinically referred anxious youth using brief versus full length questionnaires. Depress. Anxiety, 25(8), E27–35. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control. (2015). Injury Prevention & Control: Data & Statistics (WISQARS). Retrieved 03/14, 2018 https://www.cdc.gov/injury/wisqars/pdf/leading_causes_of_death_by_age_group_2015-a.pdf
- Centers for Disease Control. (2017). Youth Risk Behavior Surveillance - United States, 2015 Surveillance Summaries. Retrieved 03/14, 2018 https://www.cdc.gov/healthyyouth/data/yrbs/pdf/trends/2015_us_suicide_trend_yrbs.pdf
- Chatzittofis A, Nordstrom P, Uvnas-Moberg K, Asberg M, & Jokinen J (2014). CSF and plasma oxytocin levels in suicide attempters, the role of childhood trauma and revictimization. Neuro Endocrinol. Lett, 35(3), 213–217. [PubMed] [Google Scholar]
- Chavira DA, Garland A, Yeh M, McCabe K, & Hough RL (2009). Child anxiety disorders in public systems of care: comorbidity and service utilization. J. Behav. Health Serv. Res, 36(4), 492–504. doi: 10.1007/s11414-008-9139-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JS, & Kendall PC (2015). Peer victimization among children and adolescents with anxiety disorders. Child Psychiatry Hum. Dev,.46(3), pp. doi: 10.1007/s10578-014-0479-x25008188 [DOI] [PubMed] [Google Scholar]
- Costello EJ, Egger HL, Copeland W, Erkanli A, & Angold A (2011). The developmental epidemiology of anxiety disorders: Phenomenology, prevalence, and comorbidity. Anxiety disorders in children and adolescents: Research, assessment and intervention, 56–75. [Google Scholar]
- Costello EJ, Mustillo S, Erkanli A, Keeler G, & Angold A (2003). Prevalence and development of psychiatric disorders in childhood and adolescence. Arch. Gen. Psychiatry, 60(8), 837–844. doi: 10.1001/archpsyc.60.8.837 [DOI] [PubMed] [Google Scholar]
- Crockford C, Deschner T, Ziegler TE, & Wittig RM (2014). Endogenous peripheral oxytocin measures can give insight into the dynamics of social relationships: a review. Front. Behav. Neurosci, 8(68), 14. doi: 10.3389/fnbeh.2014.0006824672442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings CM, Caporino NE, & Kendall PC (2014). Comorbidity of anxiety and depression in children and adolescents: 20 years after. Psychol. Bull, 140(3), 816–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito CL, & Clum GA (2002). Psychiatric symptoms and their relationship to suicidal ideation in a high-risk adolescent community sample. J. Am. Acad. Child Adolesc. Psychiatry,.41(1), pp. doi: 10.1097/00004583-200201000-0001011800204 [DOI] [PubMed] [Google Scholar]
- Feldman R, Gordon I, Schneiderman I, Weisman O, & Zagoory-Sharon O (2010). Natural variations in maternal and paternal care are associated with systematic changes in oxytocin following parent-infant contact. Psychoneuroendocrinology, 35(8), 1133–1141. doi: 10.1016/j.psyneuen.2010.01.013 [DOI] [PubMed] [Google Scholar]
- Franco X, Saavedra LM, & Silverman WK (2007). External validation of comorbid patterns of anxiety disorders in children and adolescents. J. Anxiety Disord,.21(5), pp. doi: 10.1016/j.janxdis.2006.10.00217095184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaziuddin N, King CA, Naylor MW, & Ghaziuddin M (2000). Anxiety contributes to suicidality in depressed adolescents. Depress. Anxiety, 11(3), 134–138. [DOI] [PubMed] [Google Scholar]
- Gordon I, & Berson Y (2018). Oxytocin modulates charismatic influence in groups. J. Exp. Psychol. Gen,.147(1), pp. doi: 10.1037/xge000037529072476 [DOI] [PubMed] [Google Scholar]
- Grewen KM, Davenport RE, & Light KC (2010). An investigation of plasma and salivary oxytocin responses in breast- and formula-feeding mothers of infants. Psychophysiology, 47(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm MP, Newton AS, Chisholm A, Shulhan J, Milne A, Sundar P, …Hartling L (2015). Prevalence and Effect of Cyberbullying on Children and Young People: A Scoping Review of Social Media Studies. JAMA pediatrics, 169(8), 770–777. [DOI] [PubMed] [Google Scholar]
- Hawker DSJ, & Boulton MJ (2000). Twenty years’ research on peer victimization and psychosocial maladjustment: A meta-analytic review of cross-sectional studies. Journal of Child Psychology and Psychiatry,.41(4), pp. doi: 10.1111/1469-7610.0062910836674 [DOI] [PubMed] [Google Scholar]
- Hayes AF (2013). Introduction to mediation, moderation, and conditional process analysis : a regression-based approach. New York: The Guilford Press. [Google Scholar]
- Hill RM, Castellanos D, & Pettit JW (2011). Suicide-related behaviors and anxiety in children and adolescents: a review. Clin. Psychol. Rev, 31(7), 1133–1144. doi: 10.1016/j.cpr.2011.07.008 [DOI] [PubMed] [Google Scholar]
- Hodges EV, & Perry DG (1999). Personal and interpersonal antecedents and consequences of victimization by peers. J. Pers. Soc. Psychol, 76(4), 677–685. [DOI] [PubMed] [Google Scholar]
- Hurlemann R, & Scheele D (2016). Dissecting the role of oxytocin in the formation and loss of social relationships. Biol. Psychiatry,.79(3), pp. doi: 10.1016/j.biopsych.2015.05.01326122876 [DOI] [PubMed] [Google Scholar]
- Jin D, Liu H-X, Hirai H, Torashima T, Nagai T, Lopatina O, …Higashida H (2007). CD38 is critical for social behaviour by regulating oxytocin secretion. Nature,.446(7131), pp. doi: 10.1038/nature0552617287729 [DOI] [PubMed] [Google Scholar]
- Kagerbauer SM, Martin J, Schuster T, Blobner M, Kochs EF, & Landgraf R (2013). Plasma oxytocin and vasopressin do not predict neuropeptide concentrations in human cerebrospinal fluid. J. Neuroendocrinol, 25(7). doi: 10.1111/jne.12038 [DOI] [PubMed] [Google Scholar]
- Kashani JH, Goddard P, & Reid JC (1989). Correlates of suicidal ideation in a community sample of children and adolescents. J. Am. Acad. Child Adolesc. Psychiatry,.28(6), pp. doi: 10.1097/00004583-198911000-000162808262 [DOI] [PubMed] [Google Scholar]
- Kaslow NJ, Rehm LP, & Siegel AW (1984). Social-cognitive and cognitive correlates of depression in children. J. Abnorm. Child Psychol,.12(4), pp. doi: 10.1007/BF009168536491065 [DOI] [PubMed] [Google Scholar]
- Kiss I, Levy-Gigi E, & Keri S (2011). CD 38 expression, attachment style and habituation of arousal in relation to trust-related oxytocin release. Biol. Psychol,.88(2–3), pp. doi: 10.1016/j.biopsycho.2011.08.00521893160 [DOI] [PubMed] [Google Scholar]
- Kovacs M (1985). The Children’s Depression, Inventory (CDI). Psychopharmacol. Bull, 21(4), 995–998. [PubMed] [Google Scholar]
- Lebowitz ER, Gee DG, Pine DS, & Silverman WK (2018). Implications of the research domain criteria project for childhood anxiety and its disorders. Clin. Psychol. Rev doi: 10.1016/j.cpr.2018.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebowitz ER, Leckman JF, Feldman R, Zagoory-Sharon O, McDonald N, & Silverman WK (2016). Salivary oxytocin in clinically anxious youth: Associations with separation anxiety and family accommodation. Psychoneuroendocrinology, 65, 35–43. doi: 10.1016/j.psyneuen.2015.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefevre A, Mottolese R, Dirheimer M, Mottolese C, Duhamel J-R, & Sirigu A (2017). A comparison of methods to measure central and peripheral oxytocin concentrations in human and non-human primates. Sci. Rep, 7(1), 17222. doi: 10.1038/s41598-017-17674-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerer E, Levi S, Israel S, Yaari M, Nemanov L, Mankuta D, …Ebstein RP O. h. o. o. X. (2010). Low CD38 expression in lymphoblastoid cells and haplotypes are both associated with autism in a family-based study. Autism Research,.3(6), pp. doi: 10.1002/aur.15621182206 [DOI] [PubMed] [Google Scholar]
- MacPhee AR, & Andrews JJW (2006). Risk factors for depression in early adolescence. Adolescence, 41(163), 435–466. [PubMed] [Google Scholar]
- March JS, Parker JD, Sullivan K, Stallings P, & Conners CK (1997). The Multidimensional Anxiety Scale for Children (MASC): factor structure, reliability, and validity. J. Am. Acad. Child Adolesc. Psychiatry, 36(4), 554–565. doi: 10.1097/00004583-199704000-00019 [DOI] [PubMed] [Google Scholar]
- March JS, Parker JDA, Sullivan K, Stallings P, & Conners CK (1997). The Multidimensional Anxiety Scale for Children (MASC): Factor structure, reliability, and validity. J. Am. Acad. Child Adolesc. Psychiatry, 36(4). doi: 10.1097/00004583-199704000-00019 [DOI] [PubMed] [Google Scholar]
- McCullough ME, Churchland PS, & Mendez AJ (2013). Problems with measuring peripheral oxytocin: Can the data on oxytocin and human behavior be trusted? Neurosci. Biobehav. Rev, 37(8). doi: 10.1016/j.neubiorev.2013.04.018 [DOI] [PubMed] [Google Scholar]
- McQuaid RJ, McInnis OA, Matheson K, & Anisman H (2016). Oxytocin and social sensitivity: Gene polymorphisms in relation to depressive symptoms and suicidal ideation. Front. Hum. Neurosci, 10(358), 9. doi: 10.3389/fnhum.2016.0035827486392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modahl C, Green L, Fein D, Morris M, Waterhouse L, Feinstein C, & Levin H (1998). Plasma oxytocin levels in autistic children. Biol. Psychiatry, 43(4), 270–277. [DOI] [PubMed] [Google Scholar]
- Motoca LM, Williams S, & Silverman WK (2012). Social skills as a mediator between anxiety symptoms and peer interactions among children and adolescents. J. Clin. Child Adolesc. Psychol, 41(3), 329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munesue T, Yokoyama S, Nakamura K, Anitha A, Yamada K, Hayashi K, …Higashida H O. h. o. o. (2010). Two genetic variants of CD38 in subjects with autism spectrum disorder and controls. Neurosci. Res,.67(2), pp. doi: 10.1016/j.neures.2010.03.00420435366 [DOI] [PubMed] [Google Scholar]
- Nock MK, & Kazdin AE (2002). Examination of affective, cognitive, and behavioral factors and suicide-related outcomes in children and young adolescents. J. Clin. Child Adolesc. Psychol,.31(1), pp. doi: 10.1207/15374420275344166611845650 [DOI] [PubMed] [Google Scholar]
- Ruchkin VV, Schwab-Stone M, Koposov RA, Vermeiren R, & King RA (2003). Suicidal ideations and attempts in juvenile delinquents. J. Child Psychol. Psychiatry,.44(7), pp. doi: 10.1111/1469-7610.0019014531588 [DOI] [PubMed] [Google Scholar]
- Scharfstein L, Alfano C, Beidel D, & Wong N (2011). Children with generalized anxiety disorder do not have peer problems, just fewer friends. Child Psychiatry Hum. Dev, 42(6), 712–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals D, & Young J (2003). Bullying and victimization: prevalence and relationship to gender, grade level, ethnicity, self-esteem, and depression. Adolescence, 38(152), 735–747. [PubMed] [Google Scholar]
- Shamay-Tsoory SG (2010). Oxytocin, social salience, and social approach. Biol. Psychiatry,.67(6), pp. doi: 10.1016/j.biopsych.2009.11.020 [DOI] [PubMed] [Google Scholar]
- Silverman MM, Berman AL, Sanddal ND, O’Carroll PW, & Joiner TE Jr. (2007). Rebuilding the Tower of Babel: A revised nomenclature for the study of suicide and suicidal behaviors: Part II: Suicide-related ideations, communications and behaviors. Suicide Life Threat. Behav, 37(3). doi: 10.1521/suli.2007.37.3.264 [DOI] [PubMed] [Google Scholar]
- Silverman WK, & Albano AM (1996). Anxiety Disorders Interview Schedule (ADIS-IV) Parent Interview Schedule. New York: Oxford University Press. [Google Scholar]
- Sweeting H, Young R, West P, & Der G (2006). Peer victimization and depression in early-mid adolescence: a longitudinal study. The British journal of educational psychology, 76(Pt 3), 577–594. [DOI] [PubMed] [Google Scholar]
- Szeto A, McCabe PM, Nation DA, Tabak BA, Rossetti MA, McCullough ME, …Mendez AJ (2011). Evaluation of enzyme immunoassay and radioimmunoassay methods for the measurement of plasma oxytocin. Psychosom. Med, 73(5). doi: 10.1097/PSY.0b013e31821df0c2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Geel M, Vedder P, & Tanilon J (2014). Relationship between peer victimization, cyberbullying, and suicide in children and adolescents: a meta-analysis. JAMA Pediatr, 168(5), 435–442. doi: 10.1001/jamapediatrics.2013.4143 [DOI] [PubMed] [Google Scholar]
- Weisman O, Zagoory-Sharon O, Schneiderman I, Gordon I, & Feldman R (2013). Plasma oxytocin distributions in a large cohort of women and men and their gender-specific associations with anxiety. Psychoneuroendocrinology, 38(5), 694–701. doi: 10.1016/j.psyneuen.2012.08.011 [DOI] [PubMed] [Google Scholar]
- Woods PJ, Silverman ES, Gentilini JM, Cunningham DK, & Grieger RM (1991). Cognitive variables related to suicidal contemplation in adolescents with implications for long-range prevention. J. Ration. Emot. Cogn. Behav. Ther,.9(4), pp. doi: 10.1007/BF01263157 [DOI] [Google Scholar]
- Young LJ (2015). Oxytocin, social cognition and psychiatry. Neuropsychopharmacology,.40(1), pp. doi: 10.1038/npp.2014.18625482173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SN, & Anderson GM (2010). Bioanalytical inaccuracy: A threat to the integrity and efficiency of research. J. Psychiatry Neurosci, 35(1). doi: 10.1503/jpn.090171 [DOI] [PMC free article] [PubMed] [Google Scholar]


