Abstract
Background
During antiretroviral therapy, HIV RNA can be detected in Cerebrospinal Fluid (CSF) when it is undetectable in plasma, a condition termed “CSF viral escape”. The aim of the current study was to determine the prevalence and risk factors for CSF viral escape in two large cohorts in the USA.
Methods
A total of 1,264 HIV-infected volunteers enrolled in two US cohorts at their most recent visit between 2003 and 2011 were included in this cross-sectional analysis if their HIV RNA level in plasma was < 50 copies/mL (copies/mL) while receiving stable ART (> 6 months) and if they had HIV RNA measured in CSF at their most recent visit between 2003 and 2011. Potential risk factors were identified using univariable and multivariable regression.
Results
CSF viral escape was detected in 55 adults (4.4%; 95% CI: 3.4–5.6), who had a median CSF HIV RNA of 155 copies/mL (IQR: 80–283). Patients with or without CSF viral escape had similar rates of neurocognitive impairment (38.2 vs. 37.7%; p = 0.91). CSF viral escape was independently associated with the use of ritonavir-boosted protease inhibitors (OR: 2.0; 95% CI: 1.1–3.8) or unboosted atazanavir (OR: 5.1; 95% CI: 1.3–16.1), CSF pleocytosis (OR: 7.6; 95% CI: 4.2–13.7) and abnormal CSF total protein (OR: 2.1; 95% CI: 1.1–3.7).
Conclusions
In this large study of aviremic patients receiving ART, CSF viral escape was uncommon and was linked to evidence of CNS inflammation and the use of protease inhibitors, but not with worse neurocognitive performance.
Keywords: HIV-1, CSF viral escape, Neurocognitive impairment, HAND, ART
Summary:
Large study showing the prevalence of CSF viral escape in aviremic patients, and its association with both protease inhibitor (PI) use and CNS inflammation, but not with neurocognitive performance. Results have implications for our understanding of the clinical implications of CSF viral escape.
INTRODUCTION
The human immunodeficiency virus type-1 (HIV) is a neurotropic virus that can infect cells in the central nervous system (CNS)1. In some patients, HIV can infect glial cells and this may lead to severe CNS complications like HIV encephalitis (HIVE) or HIV-associated dementia (HAD)2.
Antiretroviral therapy (ART) can effectively suppress HIV replication, resulting in immune recovery, and protection against HIVE3 and severe HIV neurocognitive disorder (HAND)4. However, effective ART does not seem to consistently protect against mild HAND5. The explanation for this apparent discordance is debated. One explanation is that, during suppressive ART, mild HAND may result from persistent CNS inflammation6 associated with low-level HIV replication following suboptimal distribution of ART drugs into the CNS7.
If low-level HIV replication does occur in the CNS in the absence of systemic HIV replication, HIV RNA might be detected in cerebrospinal fluid (CSF) but not in blood. This discordance between the CSF and the blood, termed “CSF viral escape”, has been reported in several studies8–14. Still unknown are the prevalence of this condition in aviremic patients in a large cohort, the risk factors for CSF viral escape in such a cohort, and the relationship between CSF viral escape and HAND. To address these aims, we analysed CSF viral escape by combining data from two large, neuroAIDS-focused, US-based cohorts.
METHODS
Study design and settings
We designed a cross-sectional study to evaluate the prevalence of and the factors associated with CSF viral escape in HIV-infected participants with plasma viral suppression receiving ART who were enrolled in two large prospective US academic cohorts funded by the US National Institute of Mental Health (NIMH): the HIV Neurobehavioral Research Center (HNRC) cohort and the CNS HIV AntiRetroviral Therapy Effects Research (CHARTER) cohort.
Selection criteria
The study included all HIV-positive adults who were enrolled in the CHARTER or HNRP cohorts between 2003 and 2011, were taking at least three ART drugs for at least six months, had HIV RNA in plasma < 50 copies/mL and HIV RNA measured in CSF at the same assessment. We classified these patients into two groups according to the presence or absence of CSF viral escape, which was defined as HIV RNA in CSF > 50 copies/mL. For patients who had CSF viral escape at more than one visit, we selected data from the most recent visit at which CSF viral escape was present. For patients without detection of CSF viral escape, we selected data from the most recent visit.
Ethics Statement
This study and its procedures were conducted according to the principles expressed in the Declaration of Helsinki. The Institutional Review Board of each participating academic medical centre approved the protocol and all procedures. All participants provided written informed consent.
Procedures
We collected and evaluated data for each participant from a single assessment. Each assessment included neuro-medical evaluation, venipuncture, lumbar puncture (within one hour of venipuncture) and comprehensive neurocognitive testing performed closing in time to day that CSF and plasma HIV RNA levels were determined.
From the neuro-medical evaluation we collected the following information: demographics, years of education, duration of HIV disease, AIDS diagnosis, CD4+ T-cell nadir, HCV serostatus, self-reported four-day ART adherence, duration of HIV suppression, duration of ART (for all regimens and for the current regimen), the CNS Penetration Effectiveness score (CPE, 2010 version19) at the time of the lumbar puncture, and the type of ART regimen: Any combination of nucleos(t)ide reverse transcriptase inhibitors (NRTI) plus a boosted protease inhibitor (PI)-based; unboosted atazanavir (ATV)-based; a non-nucleoside reverse transcriptase inhibitor (NNRTI)-based or raltegravir-based ART or a non-conventional ART combination of two or more third agents.
From the venipuncture the levels of haemoglobin, platelets, total serum protein, creatinine, hepatic transaminases, the CD4+ T-cell count and the level of HIV RNA (quantified using the Roche Amplicor assay, version 1.5. Lower limit of quantitation 50 copies/mL). When determinations were available, we also collected a sparse panel of inflammatory biomarkers (CCL2, TNF alpha, CXCL10 and/or IL-6). Quantification of these biomarkers was performed in duplicate by bead suspension array (Millipore, Billerica, MA).
From the lumbar puncture the levels of leukocytes, erythrocytes, total protein, glucose, HIV RNA (quantified using the Roche Amplicor assay, version 1.5. Lower limit of quantitation 50 copies/mL). Again when determinations were available, we collected the same panel of inflammatory biomarkers performed in plasma (CCL2, TNF alpha, CXCL10 and/or IL-6) using the same methodology. Genotypic resistance HIV testing against was not performed in CSF samples in these cohorts.
Finally, from the neurocognitive testing assessed using a comprehensive test battery that evaluated seven putative cognitive domains sensitive to the detection of HIV disease15 (the best available normative standards were used, corrected for the effects of age, education, sex, ethnicity and, when appropriate, practice16) we collected neurocognitive impairment rates determined, globally and by cognitive domains (2007 consensus guidelines for the diagnosis of HAND17) and neurocognitive performance quantified using the Global Deficit Score (GDS)18.
Statistical analysis
The main objective of our study was to estimate the prevalence of CSF viral escape. Our secondary objectives were: to identify which factors were independently associated with the presence of CSF viral escape and to determine if there was a relation between the presence of CSF viral escape and higher rates of neurocognitive impairment. An additional secondary objective was to determine if CSF viral escape was associated with higher levels of CCL2, TNF alpha, CXCL10 or IL-6 in blood or CSF. This objective was analysed in the subset of participants having at least one determination of CCL2, TNF alpha, CXCL10 or IL-6 in blood or CSF available.
The prevalence of CSF viral escape (and its 95% confidence interval [CI]) was calculated using the Wilson score method without continuity correction.
To determine which factors (including neurocognitive performance and inflammatory biomarkers) were associated with the presence of CSF viral escape, we used the Wilcoxon rank sum test or the t-test, depending on the characteristics of the distribution, to compare continuous variables and the Chi-square test to compare dichotomous variables between patients with and without CSF viral escape. Resulting comparison with a p value <0.10 were included in a multivariable regression logistic model to determine which of these factors were independently associated with the presence of CSF viral escape. We performed two models, one excluding and another including inflammatory biomarkers. The model excluding inflammatory biomarkers included all the participants of the study while the model including inflammatory biomarkers only included those participants with an available result for all biomarkers tested in blood and CSF. Factors from this models with a resulting p values < 0.05 were considered significant in all models.
Finally, the strength of the association between each independently associated factor and the presence of CSF viral escape was quantified using odds ratios (95% CI).
RESULTS
Patients’ characteristics
A total of 1,264 volunteers met the eligibility criteria for evaluation in this study, 675 (53.4%) from the CHARTER cohort and 589 (46.6%) from the HNRC cohort. Subjects were mostly middle-aged (mean age: 45.9±9.4 years) men (82%) of diverse ethnicities (European, non-Hispanic 49%, African 32%, European Hispanic 16%, other 3%). The mean duration of the current ART regimen was 1.06 years (SD ±0.6) with a mean total ART exposure of 6.9 years (SD ±5.1). Most (69.7%) had been diagnosed with AIDS and more than one in three (37.7%) had global neurocognitive impairment.
Prevalence of CSF viral escape
CSF viral escape was detected in 55 (4.4%) patients. Thirty-three were enrolled in the CHARTER cohort (4.9%) and 22 in the HNRC cohort (3.7%). The median CSF HIV RNA level was 155 copies/mL (IQR: 80–283 copies/mL). Nine patients had CSF HIV RNA > 500 copies/mL with four being > 1000 copies/mL. The prevalence estimate for the entire group was 4.4% (95% CI: 3.4–5.6).
Risk factors for CSF viral escape
Table 1 compares demographic, disease, laboratory, and treatment characteristics of subjects with or without CSF viral escape, excluding inflammatory biomarkers that were compared in table 2. CSF viral escape was associated with AIDS, lower nadir CD4+ T-cell count, longer duration of HIV disease, CSF pleocytosis (CSF leukocytes ≥ 5/μL), higher levels of platelets and serum total proteins, and abnormal CSF total protein level (CSF proteins > 45 mg/dL). CSF viral escape was also associated with use of either boosted PI or ATV (plus two NRTI) and, less frequently, NNRTI (plus two NRTI).
Table 1.
Characteristics of patients with and without CSF viral escape
| Presence of CSF viral escape n = 55 | Absence of CSF viral escape n = 1,209 | p value | |
|---|---|---|---|
| Demographics | |||
| Age (years), mean ± SD | 45.3 ± 7.5 | 46 ± 9.5 | 0.63 |
| Sex (male), n (%) | 43 (78.2) | 978 (82.0) | 0.49 |
| Ethnicity (European, non-Hispanic), n (%) | 25 (45.5) | 590 (49.5) | 0.69 |
| Years of education, mean ± SD | 13 ± 0.4 | 12.8 ± 0.1 | 0.66 |
| HIV-Related Medical History | |||
| Years of HIV infection, median (IQR) | 16.0 (11.6–19.9) | 12.7 (6.8–18.2) | 0.01 |
| HIV-undetectable (years), median (IQR) | 13.1 (6–37.7) | 18.6 (7.5–40.8) | 0.54 |
| Previous AIDS diagnosis (CDC), n (%) | 41 (83.7) | 775 (69.1) | 0.02 |
| Hepatitis C antibody positive, n (%) | 10 (22.2) | 219 (22.4) | 0.98 |
| CD4+ T-cells nadir (cells/mm3), median (IQR) | 71 (9–189) | 133 (28–240) | 0.03 |
| Current CD4/CD8 ratio, median (IQR) | 0.54 (0.28–0.86) | 0.62 (0.39–0.88) | 0.13 |
| ART-Related Medical History | |||
| Total duration of ART (years), median (IQR) | 6.5 (3.3–10.4) | 6.1 (2.9–9.8) | 0.42 |
| Duration of current ART (years), median (IQR) | 1.1 (0.5–2.5) | 1.5 (0.6–3.4) | 0.18 |
| CPE value (2010) < 6, n (%) | 5 (8.5) | 50 (4.16) | 0.16 |
| Adherence > 95% (last 4 days), n (%) | 44 (95.6) | 998 (91.2) | 0.29 |
| ART type | |||
| NNRTI* + N(t)RTIs**, n (%) | 8 (14.6) | 438 (36.2) | < 0.01 |
| PI/r*** + N(t)RTIs**, n (%) | 31 (56.4) | 501 (41.5) | 0.03 |
| Unboosted atazanavir + N(t)RTIs**, n (%) | 4 (7.3) | 27 (2.2) | 0.05 |
| Raltegravir + N(t)RTIs**, n (%) | 0 (0) | 26 (2.1) | 0.13 |
| Other regimens****, n (%) | 12 (21.8) | 217 (18.0) | 0.47 |
| Blood Levels | |||
| CD4+ T-cell count (cells/mm3), median (IQR) | 506 (268–711) | 508 (340–710) | 0.68 |
| Haemoglobin (mg/dL), median (IQR) | 14 (12.9–15.1) | 14.4 (13.4–15.3) | 0.10 |
| Platelets (x103/mm3), median (IQR) | 232 (202–283) | 230 (189–273) | 0.06 |
| Total protein (mg/dL), median (IQR) | 7.7 (6.9–8.3) | 7.4 (7–7.9) | 0.01 |
| CSF Levels | |||
| HIV RNA (cop/mL), median (IQR) | 155 (80–283) | --- | --- |
| White Blood Cells (cells/mL), median (IQR) | 16.5 (4 – 33.2) | 2 (1 – 3) | --- |
| White blood cell pleocytosis (≥ 5 cells/mL), n (%) | 26 (49.1) | 122 (10.3) | < 0.01 |
| Red blood cells (cells/mL), median (IQR) | 4 (2.5–16.5) | 2 (0–18) | 0.29 |
| Glucose (mg/dL), median (IQR) | 62 (57.5–68.5) | 63 (58–68) | 0.74 |
| Total Protein, median (IQR) | 47 (31.5–55.5) | 38 (30–48) | --- |
| Elevated total protein (> 45 mg/dL), n (%) | 47 (31.5–55.5) | 38 (30–48) | < 0.01 |
| Neurocognitive Assessment | |||
| Neurocognitive impairment, n (%) | 21 (38.2) | 447 (37.7) | 0.91 |
| Global Deficit Score, median (IQR) | 0.4 (0.1–0.9) | 0.3 (0.1–0.7) | 0.94 |
NNRTI;
N(t)RTI;
PI/r;
Other regimens including the combination of two or more third agents.
Abbreviations: ART: antiretroviral therapy; CPE: CNS Penetration Effectiveness score; CSF: cerebrospinal fluid; IQR: interquartile range; NNRTI: non-nucleoside reverse transcriptase inhibitors; N(t)RTI: nucleos(t)ide reverse transcriptase inhibitor; PI/r: ritonavir-boosted protease inhibitors; SD: standard deviation.
Table 2.
Levels of CSF and blood inflammatory biomarkers in patients with and without CSF viral escape
| Presence of CSF viral escape | Absence of CSF viral escape | p value* | |||
|---|---|---|---|---|---|
| n | Median (IQR) | n | Median (IQR) | ||
| CSF Biomarkers | |||||
| Total protein, median (IQR) | 8 | 38.5 (33.0–54.5) | 284 | 42 (33–52.8) | 0.88 |
| CCL2, median (IQR) | 5 | 1,330 (719–2,153) | 233 | 1,151 (878–1,747) | 0.99 |
| TNF-α, median (IQR) | 7 | 7.1 (2.6–10.9) | 166 | 4.1 (2.7–5.5) | 0.19 |
| CXCL10, median (IQR) | 8 | 6,417 (4,468–14,551) | 288 | 2,771 (1,516–4585) | 0.01 |
| IL-6, median (IQR) | 7 | 13.7 (0.9–16.3) | 177 | 15.1 (9.8–23.8) | 0.22 |
| Blood Biomarkers | |||||
| Total protein, median (IQR) | 8 | 6.8 (6.7–8.2) | 284 | 7.5 (6.9–8.0) | 0.61 |
| CCL2, median (IQR) | 6 | 374 (310–398) | 163 | 382 (287–504) | 0.67 |
| TNF-α, median (IQR) | 6 | 17.1 (10.6–23.9) | 160 | 16.4 (10.3–24.1) | 0.98 |
| CXCL10, median (IQR) | 6 | 1,118 (728–2,099) | 161 | 1,040 (693–1,731) | 0.71 |
| IL-6, median (IQR) | 6 | 1.6 (0.3–2.0) | 160 | 3 (1.1–6.2) | 0.05 |
Abbreviation: CSF: cerebrospinal fluid. IQR: Interquartile range.
Wilcoxon Rank Sum Test was used in all comparisons
Multivariable modelling, excluding inflammatory biomarkers, was then performed to determine which of these factors were independently associated with CSF viral escape. We found that CSF pleocytosis (p < 0.01), an abnormal CSF total protein level (p = 0.02), and the use of PI-based ART (p = 0.02) or ATV-based ART (p = 0.02) were associated with CSF viral escape. The odds ratios (95% CI) for these associations were: CSF pleocytosis 7.6 (4.2–13.7), abnormal CSF total proteins 2.1 (1.1–3.7), PI-based ART 2.0 (1.1–3.8), and ATV-based ART 5.1 (1.3–16.1).
CSF biomarkers for CSF viral escape
Table 2 shows levels of CSF and blood inflammatory biomarkers in patients with and without CSF viral escape. From the 1264 participants in the study, 165 had levels of CCL2, TNF alpha, CXCL10 and IL-6 in blood and CSF available and 296 had at least one value of those biomarkers available.
Considering this subset of 296 participants with at least one determination of CCL2, TNF alpha, CXCL10 and IL-6 in blood or CSF, patients presenting CSF viral escape had higher median (IQR) levels of CSF CXCL10: 6417 (4468–14551) vs. 2771 (1516–4585) pg/mL, p=0.01; higher proportion of CSF pleocytosis: 50% vs. 12.3%, p<0.01; longer median (IQR) duration of HIV disease: 18.2 (13.3–23.8) vs. 12.9 (6.6–17.1) years, p=0.03; lower median (IQR) nadir CD4+ T-cell counts: 12 (6–107) vs. 115 (20.3–209) cells/mm3, p=0.03 and trend to have lower levels of IL-6 in blood: 3 (1.1–6.2) vs. 1.6 (0.3–2) pg/mL, p=0.05. Multivariable modelling found that only the levels of CSF CXCL10 were independently associated with CSF viral escape.
CSF viral escape and neurocognitive performance
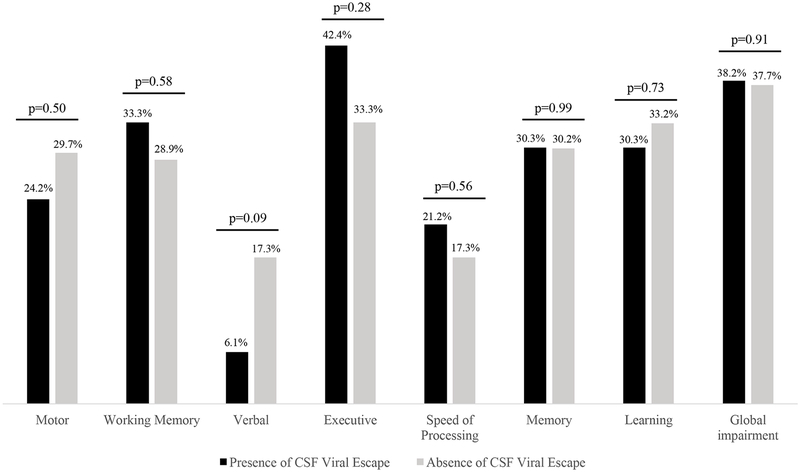
Neurocognitive performance was assessed in the 1,264 patients included in the study. Neurocognitive impairment was diagnosed in 21 patients (38.2%) with CSF viral escape and in 447 patients (37.7%) without (p = 0.91). The GDS values were also similar among patients with (0.4 [IQR: 0.1–0.9]) or without (0.3 [IQR: 0.1–0.7]) CSF viral escape (p = 0.94), as well as the results obtained in each neurocognitive domain tested (Figure). The proportion of patients with an abnormal Beck Depression Inventory was also similar using a cut-off for abnormality of 11 (p = 0.8) in patients with (47.6%) and without (45.6%) CSF viral escape.
Figure.
Rates of neurocognitive impairment in patients with and without CSF Viral Escape.
DISCUSSION
This large study with diverse population establishes and estimates the true prevalence and clinical implications of CSF viral escape in HIV-suppressed patients receiving ART based on a large cohort of well-characterized HIV-positive adults. In our study we found that the prevalence of CSF viral escape in aviremic patients on ART was 4.4%. CSF viral escape was more commonly seen in patients using PI-based ART or ATV-based ART than in those using other regimens such as NNRTI-based ART. CSF viral escape was not, however, associated with worse neurocognitive performance or impairment in this cross-sectional analysis.
Multivariable modelling identified that CSF viral escape was associated with CSF pleocytosis and abnormal total protein levels in CSF. While regimens that contained a boosted PI or ATV had lower CPE values than other regimens (7.0 vs. 7.8; p < 0.0001), CPE values themselves were not associated with CSF viral escape.
Our results support smaller, previously published, cross-sectional studies showing low rates of CSF viral escape in treated, aviremic patients. In patients without neurocognitive symptoms, Eden et al.10 showed CSF viral escape in seven of 69 subjects (10%) who underwent lumbar puncture for research purposes. Rawson et al.11 identified CSF viral escape in nine of 69 patients (13%) who underwent lumbar puncture for clinical purposes, including investigation of HAND. Peluso et al.8 reported four cases, two of them showing levels of CSF HIV RNA between 40–50 copies/mL in 60 patients (6.7%). In addition to these reports, our study included a much larger number of patients from six US cities who underwent lumbar punctures for research purposes.
Few other reports of CSF viral escape in HIV-suppressed patients on ART have been published. In clinical patients with new-onset neurological symptoms, Canestri et al.9 reported eight cases of CSF viral escape from two Paris hospitals during five years, and Peluso et al.8 reported another five cases from four US and European hospitals. In patients treated with PI monotherapy in clinical trials, CSF viral escape was reported in another seven patients20–22.
Antiretroviral therapies with poor distribution into the CNS have previously been associated with CSF viral escape. Cusini et al.13 and Rawson et al.11, for instance, found that low CPE values were associated with CSF viral escape. Our study did not confirm this association, although we did find evidence of a drug effect: PI, a drug class with relatively worse distribution in the CNS compared with NNRTI, was associated with CSF viral escape. Prior reports that did not find an association with CPE did identify associations with specific antiretroviral drugs or classes of drug10,12,14, supporting the conclusion that CSF viral escape may occur in part due to suboptimal ART effectiveness in the CNS12–14. Another potential explanation for the association observed between PI and CSF viral escape could be related with the selection of PI-based ART in patients more difficult to treat (bad adherence, history of previous treatment failures…). Therefore, we cannot rule out that patients on a PI-based ART may have more non-controlled risk factors for developing CSF viral than patients on a NNRTI-based ART.
While CSF viral escape was not associated with worse neurocognitive performance in this cross-sectional analysis, it was associated with evidence of CNS inflammation, which has been linked with low-level HIV replication in CSF and worse CNS outcomes23,24. Our study supports previous reports indicating that CSF viral escape is associated with indicators of immune activation, such as high total protein levels and CSF pleocytosis. In addition to these findings, we found in the subgroup of participants with available CSF biomarkers that CSF viral escape was associated with higher levels of CXCL10.
CXCL10 is an interferon-induced protein that contributes to mediation of brain mononuclear inflammation in HIV25. Levels of CXCL10 in CSF correlate with leukocyte number and HIV RNA concentrations in CSF26,27, suggesting that this chemokine may indirectly increases production of HIV RNA in CSF via recruitment of activated target cells. Perpetuating this cycle, HIV RNA may then induce CXCL10 synthesis in response to the antiviral innate immune response28. While our findings support the role of CSF CXCL10 in CSF viral escape, our study cannot establish if CXCL10 elevations resulted from CSF viral escape or were a consequence of it (or both).
Despite this evidence of inflammation, CSF viral escape was not associated with worse neurocognitive performance in this cross-sectional analysis. There are two potential explanation for this lack of association. First, recent communications seem to support the transience of CSF viral escape in many cases24. Transient CSF viral escape cases is unlikely to impact on neurocognitive performance and only persistent cases associated persistent CSF inflammation are likely to produce neurocognitive impairment. Second, if any CSF viral escape case is, in fact, a risk factor for (or a consequence of) neurocognitive impairment, then our failure to identify an association may be due to the relatively small number of cases of CSF viral escape compared to the total sample size (i.e., type II error due to insufficient power), the cross-sectional design of our analyses, or the failure of these analyses to ensure that neurocognitively impaired and unimpaired subjects were balanced for conditions that might influence neurocognitive performance (e.g., severe substance use disorders). While these conditions were systematically reviewed and categorized in the CHARTER cohort, they were not in the HNRC cohort so were not included in this analysis.
This study has several limitations. First, we included patients from two cohorts that aimed to evaluate neurocognition in HIV-positive adults. Such cohorts may be subject to referral bias, which could inflate our prevalence estimates. We believe this possibility is unlikely, considering that there is not a clear link between neurocognitive performance and CSF viral escape. However, the number of events was relatively small and type II error is possible. Second, the cross-sectional design prevented us from determining the persistence of CSF viral escape or the temporal relationship between intrathecal inflammation and CSF viral escape. Third, participants differed in the duration of follow-up, which could have influenced our results since longer duration of follow-up was associated with CSF viral escape. Adjusting our analyses for the duration of follow-up at least partially accounted for the influence of this factor on our prevalence estimate of CSF viral escape.
Fourth, the number of prior neurocognitive assessments performed was also not homogeneous in all patients. To reduce the potential bias of this, we applied practice effect correction to our neurocognitive assessments16. Fifth, our analyses did not account for other neuropsychiatric conditions that can affect neurocognitive performance since these were not similarly summarized in both cohorts. If subjects who did not have CSF viral escape were more likely to have one or more of these conditions than subjects who did have CSF viral escape, this could confound our neurocognitive analyses.
Our results are important because they indicate that CSF viral escape is associated with higher levels of CNS inflammation, although this does not appear to increase the risk of neurocognitive impairment in this study. Longitudinal studies are needed to evaluate the relation between CSF viral escape, CNS inflammation, and long-term CNS outcomes.
In conclusion, CSF viral escape appears to be an uncommon condition that occurs in approximately one in 20 patients taking suppressive ART and is linked with evidence of CNS inflammation and the use of PI, but not worse neurocognitive performance. Our findings do not support routine CSF examination to evaluate patients for CSF viral escape.
REFERENCES
- 1.Burdo TH, Lackner A, Williams KC. Monocyte/macrophages and their role in & HIV neuropathogenesis. Immunol Rev. 2013;254:102–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McArthur JC, Hoover DR, Bacellar H, et al. Dementia in AIDS patients: incidence and risk factors. Neurology. 1993;43:2245–52. [DOI] [PubMed] [Google Scholar]
- 3.Langford D, Marquie-Beck J, de Almeida S, et al. Relationship of antiretroviral treatment to post mortem brain tissue viral load in human immunodeficiency virus-infected patients. J Neurovirology. 2006;12:100–7. [DOI] [PubMed] [Google Scholar]
- 4.Dilley JW, Schwarcz S, Loeb L, Hsu L, Nelson K, Scheer S. The decline of incident cases of HIV-associated neurological disorders in San Francisco, 1991–2003. AIDS. 2005;19:634–5. [DOI] [PubMed] [Google Scholar]
- 5.Heaton RK, Clifford DB, Franklin BS. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy. CHARTER study. Neurology. 2010;75:2087–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anthony IC, Ranage SN, Carnie FW, et al. Influence of HAART on HIV-related CNS disease and neuroinflammation. J Neuropathol Exp Neurol. 2005;64:529–36. [DOI] [PubMed] [Google Scholar]
- 7.Letendre S, Marquie-Beck J, Capparelli E, et al. Validation of the CNS Penetration-Effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch Neurol. 2008;65:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peluso MJ, Ferretti F, Peterson J, et al. Cerebrospinal fluid HIV escape associated with progressive neurologic dysfunction in patients on antiretroviral therapy with well controlled plasma viral load. AIDS. 2012;26:1765–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Canestri A, Lescure FX, Jaureguiberry S. Discordance between cerebral spinal fluid and plasma HIV replication in patients with neurological symptoms who are receiving suppressive antiretroviral therapy. Clin Infect Dis. 2010;50:773–8. [DOI] [PubMed] [Google Scholar]
- 10.Edén A, Fuchs D, Hagberg L, et al. HIV-1 viral escape in cerebrospinal fluid of subjects on suppressive antiretroviral treatment. J Infect Dis. 2010;202:1819–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rawson T, Muir D, Mackie NE, Garvey LJ, Everitt A, Winston A. Factors associated with cerebrospinal fluid HIV RNA in HIV infected subjects undergoing lumbar puncture examination in a clinical setting. J Infect. 2012;65:239–45. [DOI] [PubMed] [Google Scholar]
- 12.Pinnetti C, Lorenzini P, Forbici F, et al. CSF viral escape in patients without neurological disorders: prevalence and associated factors. 21st CROI; Boston, MA: March 3–6, 2014. [Abstract 443]. [Google Scholar]
- 13.Cusini A, Vernazza PL, Yerly S, et al. Higher CNS penetration-effectiveness of long-term combination antiretroviral therapy is associated with better HIV-1 viral suppression in cerebrospinal fluid. J Acquir Immune Defic Syndr. 2013;62:28–35. [DOI] [PubMed] [Google Scholar]
- 14.Nightingale S, Geretti AM, Fisher M, et al. HIV-1 RNA detection in cerebrospinal fluid in those on antiretroviral therapy experiencing incomplete viral suppression in plasma. 21st CROI; Boston, MA: March 3–6, 2014. [Abstract 442]. [Google Scholar]
- 15.Heaton RK, Clifford DB, Franklin DR Jr, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75:2087–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cysique LA, Franklin D Jr, Abramson I, et al. Normative data and validation of a regression based summary score for assessing meaningful neuropsychological change. J Clin Exp Neuropsychol. 2011;33:505–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carey CL, Woods SP, Gonzalez R, et al. Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. J Clin Exp Neuropsychol. 2004;26:307–19. [DOI] [PubMed] [Google Scholar]
- 19.Letendre SL, Ellis RJ, Ances BM, McCutchan JA. Neurologic complications of HIV disease and their treatment. Top HIV Med. 2010;18:45–55. [PMC free article] [PubMed] [Google Scholar]
- 20.Gutmann C, Cusini A, Günthard HF, et al. Randomized controlled study demonstrating failure of LPV/r monotherapy in HIV: the role of compartment and CD4-nadir. AIDS. 2010;24:2347–54. [DOI] [PubMed] [Google Scholar]
- 21.Katlama C, Valantin MA, Algarte-Genin M, et al. Efficacy of darunavir/ritonavir maintenance monotherapy in patients with HIV-1 viral suppression: a randomized open-label, noninferiority trial, MONOI-ANRS 136. AIDS. 2010;24:2365–74. [DOI] [PubMed] [Google Scholar]
- 22.Yeh RF, Letendre S, Novak IS et al. 14th CROI Los Angeles, California: February 25–28, 2007. [Abstract 381]. [Google Scholar]
- 23.Sattler FR, He J, Letendre S, et al. Abdominal obesity contributes to neurocognitive impairment in HIV-infected patients with increased inflammation and immune activation. J Acquir Immune Defic Syndr. 2015;68:281–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edén A, Nilsson S, Hagberg L, et al. Asymptomatic Cerebrospinal Fluid HIV-1 Viral Blips and Viral Escape During Antiretroviral Therapy: A Longitudinal Study. J Infect Dis. 2016;214:1822–5. [DOI] [PubMed] [Google Scholar]
- 25.Kolb SA, Sporer B, Lahrtz F, Koedel U, Pfister HW, Fontana A. Identification of a T cell chemotactic factor in the cerebrospinal fluid of HIV-1-infected individuals as interferon-gamma inducible protein 10. J Neuroimmunol. 1999;93:172–81. [DOI] [PubMed] [Google Scholar]
- 26.Cinque P, Bestetti A, Marenzi R, et al. Cerebrospinal fluid interferon-g-inducible protein 10 (IP-10, CXCL10) in HIV-1 infection. J Neuroimmunol. 2005;168:154–63. [DOI] [PubMed] [Google Scholar]
- 27.Schrier RD, Hong S, Crescini M, et al. Cerebrospinal Fluid (CSF) CD8+ T-Cells That Express Interferon-Gamma Contribute to HIV Associated Neurocognitive Disorders (HAND). PLoS One. 2015;10:e0116526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mailova E, Bertley FM, Huang Q, et al. HIV-1 Tat reprograms immature dendritic cells to express chemoattractants for activated T cells and macrophages. Nat Med. 2003:9;191–7. [DOI] [PubMed] [Google Scholar]