Abstract
Calprotectin (CP, S100A8/S100A9 heterooligomer) is an abundant metal-sequestering host-defense protein expressed by neutrophils, other white blood cells, and epithelial cells. The apo protein is a S100A8/S100A9 heterodimer that contains two sites for transition metal binding at the S100A8/S100A9 interface: a His3Asp motif (site 1) and a His6 motif (site 2). In this chapter, we provide a step-by-step protocol for the overexpression and purification of the human and murine orthologues of CP that affords each apo heterodimer in high yield and purity. In these procedures, the S100A8 and S100A9 subunits are overexpressed in Escherichia. coli BL21(DE3), and each apo heterodimer is obtained following cell lysis, folding, column chromatography, and dialysis against Chelex resin to reduce metal contamination. Recent studies demonstrated that human CP coordinates Fe(II) and that the protein affects the redox speciation of Fe in solution. An Fe redox speciation assay employing ferrozine is described that demonstrates the ability of both the human and murine ortholuges of CP to shift the redox speciation of Fe from the ferric to the ferrous oxidation state over time.
Keywords: S100 protein, EF-hand, bacterial expression, Fe(II)-binding protein, iron redox speciation
1. Introduction
Calprotectin (CP, S100A8/S100A9 heterooligomer) is a member of the S100 family of Ca(II)-binding proteins and a topic of interest in the metal homeostasis community because of its contributions to the metal-withholding innate immune response, which is often termed nutritional immunity(1, 2). CP is a cytoplasmic protein produced by neutrophils, macrophages, monocytes, and epithelial cells. In response to infection, CP is released into the extracellular space where it sequesters essential nutrient metal ions to limit microbial growth. Our current understanding of its metal-sequestering function largely comes from mouse models of infectious disease(3–8), including studies that involve a S100A9−/− or CP knockout mouse(9), and molecular characterization of the recombinant human protein(10–20). A recombinant expression and purification of murine CP was recently reported, which provides a foundation for future evaluation of this orthologue(21).
Biochemical, biophysical and structural studies of human CP (hCP) demonstrated that the protein is a heterooligomer of S100A8 and S100A9 (10, 11, 22, 23). Each subunit possesses two EF-hand Ca(II)-binding sites: a canonical C-terminal EF-hand and a non-canonical N-terminal EF-hand(24). In the absence of Ca(II) and transition metal ions, hCP is a heterodimer(10). Two transition-metal-binding sites form at the dimer interface(11, 12, 25). Site 1 is a His3Asp motif composed of (A8)His83, (A8)His87, (A9)His20 and (A9)Asp30(11). Site 2 is a His6 motif composed of (A8)His17, (A8)His27, (A9)His91, (A9)His95, (A9)His103 and (A9)His105(13–15). The His6 site of hCP has gained significant attention in recent years because it can sequester a range of divalent first-row transition metal ions including Mn(II), Fe(II), Zn(II) and Ni(II)(1, 14–17, 19, 20). Like the human protein, murine CP (mCP) is also a heterooligomer of S100A8 and S100A9, and each subunit contains a canonical C-terminal EF-hand and a non-canonical N-terminal EF-hand domain. Amino acid sequence alignment of the human and murine S100A8 and S100A9 polypeptides indicates that the His3Asp and His6 sites are conserved(21).
Early studies of hCP revealed that Ca(II) binding causes two heterodimers to self-associate and form a heterotetramer (22, 23). In addition to this change in quaternary structure, Ca(II) binding enhances the transition metal affinities, antimicrobial activity, and proteolytic stability of hCP(1, 12, 26). Initial biochemical evaluation of mCP revealed that Ca(II) binding also causes this orthologue to form heterotetramers (21), and further investigations are required to decipher whether Ca(II) ions modulate other structural and functional properties of the protein. The studies of hCP provide the basis for a working model where CP responds to the high extracellular Ca(II) concentration (≈2 mM) at an infection site and becomes a tetramer with high transition metal affinities (1, 12).
In this chapter, we first present the overexpression and purification protocols for mCP and hCP-Ser, a cysteine-null variant [(S100A8(C42S)/S100A9(C3S)], which has been used extensively in biochemical, biophysical and structural studies of the human orthologue. These protocols are based on published work (12, 21), provide each protein as the apo heterodimer, and can be used for preparing native hCP as well as other mCP and hCP-Ser variants with single or multiple point mutations. Because the His6 site of hCP was recently shown to sequester Fe(II) and the protein was found to affect the redox speciation of Fe in aerobic solution (18, 19), we also provide a protocol for an Fe speciation assay that we first designed and utilized to study hCP-Ser(19). Herein, we extend this assay to mCP and report that this protein also shifts the redox speciation of Fe from Fe(III) to Fe(II) under aerobic conditions.
2. Materials
All chemicals are purchased from commercial suppliers and used as received.
All solutions are prepared using Milli-Q water (18.2 MΩ•cm, 22-μm filter).
Protein concentrations are determined using the calculated extinction coefficients of the S100A8/S100A9 (calprotectin) homodimer (Protparam: ε280 = 5960 M−1 cm−1 for mCP (mS100A8/mS100A9), 18,450 M−1cm−1 for human CP (hS100A8/hS100A9) and human CP-Ser (hS100A8(C42S)/hS100A9(C3S)). All concentrations reported are for the homodimer.
2.1. Preparation of expression plasmids
The synthetic genes for each protein subunit (mS100A8, mS100A9, hS100A8, hS100A9) are optimized for Escherichia coli codon usage and obtained from ATUM (formerly DNA2.0). These genes, as well as procedures for site-directed mutagenesis to obtain genes encoding hS100A8(C42S) and hS100A9(C3S), are described in the literature (12, 21). Protein nomenclature and expression plasmids are listed in Table 1.
The pET41a expression vector is obtained from Invitrogen.
The genes are inserted into the NdeI and XhoI restriction sites of pET41a, which affords the untagged, full-length proteins with no additional amino acids after IPTG-induced overexpression (12, 21).
The pET41a plasmids containing mS100A8, mS100A9, hS100A8(C42S), and hS100A9(C3S) are transformed into chemically-competent E. coli BL21(DE3) cells for overexpression.
The the preparation of cell stocks of E. coli BL21(DE3), the cells are grown in LB to saturation, diluted1:1 into a 50 % glycerol solution, frozen in liquid nitrogen, and stored the cells at −80 °C.
Table 1:
Protein nomenclature and expression plasmids
| Protein | Abbreviation | Subunits | Expression plasmid | Ref* |
|---|---|---|---|---|
| Human | hCP | hS100A8 | pET41a-hS100A8 | (12) |
| calprotectin | hS100A9 | pET41a-hS100A9 | (12) | |
| Human | hCP-Ser | hS100A8(C42S) | pET41a-hS100A8(C42S) | (12) |
| calprotectin Cys⟶Ser variant |
hS100A9(C3S) | pET41a-hS100A9(C3S) | (12) | |
| Murine | mCP | mS100A8 | pET41a-mS100A8 | (21) |
| calprotectin | mS100A9 | pET41a-mS100A9 | (21) |
The original names for the plasmids containing the human S100A8 and S100A9 genes did not include “h” to designate “human.”
2.2. Commercial materials and preparation of reagents
To reduce metal ion contamination, plastic spatulas are used to transfer reagents.1
Stock solutions of metal ions and sodium citrate (100 mM) are prepared in nitric acidwashed volumetric glassware and transferred to polypropylene tubes for storage. 1
Stock solutions of Fe(III) (100 mM) are prepared from >99.99% trace metals basis anhydrous FeCl3, Milli-Q water, and trace metals basis 37% HCl.2
Stock solutions of Ca(II) (1 M) are prepared from 99.999% CaCl2 and Milli-Q water.
Other reagents utilized in this protocol are listed in Table 2.
Table 2:
Reagents and supplier information
| Purpose | Reagent | Supplier |
|---|---|---|
| Protein overexpression and purification |
Luria-Bertani (LB) | Beston Dickinson |
| Kanamycin sulfate | VWR | |
| Isopropyl β-d-1-thiogalactopyranoside (IPTG) | BACHEM | |
| 99.5 % 4-(2-hydroxyethyl)-1- piperazineethanesulfonic acid (HEPES) |
MilliporeSigma | |
| Sodium chloride | MilliporeSigma | |
| Sodium hydroxide | Macron | |
| Triton X-100 | EMD | |
| Ethylenediaminetetraacetic acid disodium salt dihydrate (EDTA) |
VWR | |
| Phenylmethylsulfonyl fluoride (PMSF) | VWR | |
| Dithiothreitol (DTT) | VWR | |
| Guanidinium hydrochloride (GuHCl) | MilliporeSigma | |
| Ammonium sulfate | MilliporeSigma | |
| Chelex resin | Bio-Rad | |
| Fe speciation assay |
Ultrol grade HEPES (free acid) | Calbiochem |
| TraceSELECT sodium chloride | MilliporeSigma | |
| 37% Hydrochloric acid (HCl), trace metals basis | MilliporeSigma | |
| >99.5% sodium citrate tribasic dihydrate | MilliporeSigma | |
| 99.99% anhydrous ferric chloride | MilliporeSigma | |
| >99.0% sodium ascorbate | MilliporeSigma | |
| >99.5% trichloroacetic acid | MilliporeSigma | |
| >99.99% Ammonium acetate | MilliporeSigma | |
| 97% 3-(2-Pyridyl)-5,6-diphenyl-1,2,4-triazine- p,p’-disulfonic acid monosodium salt hydrate (ferrozine) |
MilliporeSigma |
2.3. Ferrozine preparation.
For the Fe speciation assay, ferrozine is used to determine the Fe(II) content of solutions via detection of the [Fe(ferrozine)3]4- complex by optical absorption spectroscopy (19, 27, 28).
Stock solutions (≈100 mM, 20 mL) of ferrozine are prepared in Milli-Q water, aliquoted into 800-μL portions, and stored at −80 °C.
Each aliquot is thawed only once and subsequently diluted to the working concentration of 6.17 mM (10 mL) in 0.1 M HCl immediately before use.
2.4. Buffers for protein purification and the iron speciation assay.
Buffers employed for the purification of mCP and CP-Ser are prepared as indicated below with Milli-Q water and are subsequently filtered (0.2 μm) and stored at 4 °C. Buffer compositions are given in Table 3. For buffers used in the preparation of mCP or hCP, DTT (5 mM final concentration) is added to all buffers immediately before use because these proteins contain cysteine residues.3 The Fe speciation assay buffer is prepared from high purity reagents (see Section 2.2) in acid-washed volumetric glassware and subsequently transferred to a polypropylene container.1
Table 3:
Buffer composition
| Buffer (1 L) | Composition |
|---|---|
| lysis buffer A: | 50 mM Tris, 100 mM NaCl, 1 mM EDTA, 0.5 % Triton X-100, 1 mM PMSF*, pH 8.0 |
| lysis buffer B: | 50 mM Tris, 100 mM NaCl, 4 M GuHCl, pH 8.0 |
| dialysis buffer: | 20 mM HEPES, pH 8.0 |
| MonoQ buffer A: | 20 mM HEPES, pH 8.0 |
| MonoQ buffer B: | 20 mM HEPES, 1 M NaCl, pH 8.0 |
| S75 buffer: | 20 mM Tris, 100 mM NaCl, pH 8.0 |
| Fe speciation assay buffer: | 75 mM HEPES, 100 mM NaCl, 2 mM Ca(II)**, pH 7.0 |
PMSF is added immediately before use. It is dissolved in anhydrous ethanol before use or can be stored at −20 °C.
Ca(II) (80 μL of a 1 M stock solution) is added to 40 mL of the buffer before use.
2.5. Equipment for protein purification.
An ÄKTA purifier (GE Life Sciences) housed in a 4 °C room and equipped with a 150mL Superloop (GE Life Sciences) is employed for chromatographic purification.
Both human and murine CP orthologues are purified in two chromatographic steps: anion exchange chromatography using a MonoQ 10/100 GL column (GE Life Sciences) followed by size exclusion chromatography using a Superdex 75 10/300 GL column (GE Life Sciences).
2.6. Equipment for optical absorption spectroscopy.
A Beckman Coulter DU 800 spectrophotometer thermostatted at 25 °C with a Peltier temperature controller is used for optical absorption spectroscopy.
Plastic cuvettes (1-cm pathlength, polystyrene, VWR) are employed for optical absorption measurements during the Fe speciation assay. New cuvettes are used for each measurement. Acid-washed quartz cuvettes can also be used for these measurements.
Plastic cuvettes (1-cm pathlength, polystyrene, VWR) are used for monitoring the bacterial culture optical density at 600 nm (OD600, absorption at 600 nm).
Observed OD600 values of bacterial cultures are given throughout this protocol as benchmarks; however, these values will vary from instrument to instrument.
3. Methods
The methods for protein overexpression and purification described in this section are based on published procedures (12, 21) and can be employed to produce human and murine CP variants obtained by site-directed mutagenesis.
3.2. Protein preparation.
The protein purification procedures are described for overexpression carried out using a 1 L culture for each subunit. Typical yields are ≈60 mg / 2 L culture for CP-Ser and ≈45 mg / 2 L culture for mCP.
Day 1: Preparation for protein overexpression
Prepare and autoclave Luria-Bertani (LB) medium for protein overexpression according to the manufacturer’s instructions. Prepare 1 L LB medium in a 2-L baffled flask for each S100 subunit to be overexpressed. Cool the autoclaved medium and place the flasks in a 37 °C incubater-shaker or warm room overnight.
Prepare 50 mg/mL kanamycin by dissolving kanamycin sulfate in Milli-Q water. Filter the solution (0.2-μm syringe filter), collecting the filtrate in a polypropylene centrifuge tube, and store the solution at −20 °C. The pET41a vector contains a kanamycin resistance cassette.
Prepare 0.5 M IPTG by dissolving IPTG in Milli-Q water. Filter the solution (0.2-μm syringe filter), collect the filtrate in a polypropylene centrifuge tube, and store at −20 °C. Protein expression will be induced by IPTG addition on Day 2.
Prepare starter cultures of E. coli BL21(DE3) containing the desired pET41a expression plasmids. To a sterile 250-mL baffled flask, add 30 mL of LB and 50 μg/mL kanamycin. Inoculate the medium from a freezer stock (Section 2.1) of the E. coli overexpression strain or from a single colony that was grown on an agar plate. Incubate the cultures (37 °C, 150 rpm) for ≈16 h.
Day 2: Overexpression of SI00A8 and SI00A9 subunits
5. Measure the OD600 of the overnight culture, which should be >1.5.
6. Add 50 μg/mL kanamycin (final concentration, 1 mL of a 50 mg/mL stock solution in Milli-Q water) into the 2-L baffled flask containing 1 L of LB medium and dilute a 10-mL volume (1:100 dilution) of the overnight culture into the medium. Place the resulting culture in an incubator-shaker (37 °C, 150 rpm).
7. Monitor the OD600.
8. When OD600 ≈0.6–0.7, add 125 μΜ IPTG (250 μL of 0.5 M IPTG stock solution) to the culture.
9. Incubate the culture (37 °C, 150 rpm) for and additional 3.5–4 h after induction. At this time, measure the OD600 of the culture, which should be >1.5.
10. Harvest the cells by centrifugation (2246 g, 15 min, 4 °C), discard the supernatant, and transfer the cell pellet to a sterile 50-mL polypropylene centrifuge tube. Flash freeze the pellet in liquid nitrogen and store at −80 °C until use.
This procedure typically yields ≈2 g cells / 1 L culture (wet weight) for each subunit, which can be stored at −80 °C for over four months without an impact on protein yield. The overexpression can be evaluated by SDS-PAGE analysis (Coomassie stain) of the pre- and post-induction cell samples (whole cell lysate) using a 15% tricine gel (Figure 1). Successful overexpression of the S100A8 subunit should show an intense band at ≈11 kDa in the postinduction sample, corresponding to the S100A8 monomer. Successful overexpression of the S100A9 subunit should show an intense band at ≈13 kDa, corresponding to the S100A9 monomer. These overexpression conditions result in insoluble hS100A8, hS100A8(C42S), hS100A9, hS100A9(C3S), and mS100A8, and soluble mS100A9.
Figure 1.
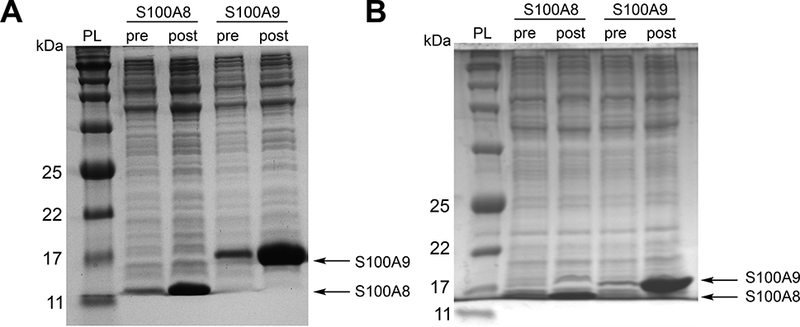
Representative 15% Tris-glycine SDS-PAGE gel of whole cell lysate of E. coli BL21(DE3) obtained from the overexpression of the S100A8 and S100A9 subunits of mCP (A)and hCP-Ser (B). Samples pre- and post-induction with IPTG are shown. The samples wereprepared using the B-PER reagent (Thermo Fisher Scientific, manufacturer protocol). The protein ladder (PL) is p7712S (New England Biolabs).
Day 3: Lysis and refolding of mCP
The following steps should be carried out at 4 °C and buffers should be equilibrated and stored at 4 °C (in a cold room) or on ice.
11. Thaw one cell pellet of mS100A8 and one mS100A9, each from a 1-L overexpression culture, on ice.
12. Add 116 mg DTT to 150 mL of lysis buffer A and stir to dissolve and afford a final concentration of 5 mM.4 Keep lysis buffer A on ice or in a 4 °C room.
13. Suspend each cell pellet in 30 mL of lysis buffer A containing 5 mM DTT. Once the cell pellets are fully suspended, combine the suspensions to yield a 60-mL mixture containing both mS100A8 and mS100A9.
14. Transfer the combined suspension to a stainless steel beaker on ice. Lyse the cells by sonication on ice (40% amplitude, 2.5 min, 30 s on, 10 s off).
15. Centrifuge the mixture (22,000 g, 4 °C, 10 min). Transfer the supernatant, which contains soluble mS100A9, to a glass beaker on ice.
16. Repeat steps 13–15 for a total of two times. Keep the final pellets (containing insoluble mS100A8) on ice and combine the supernatant from each round (containing soluble mS100A9) in a glass beaker on ice.
17. To precipitate contaminating proteins from the combined supernatant containing soluble mS100A9, add ≈45 g of ammonium sulfate to the solution (≈120 mL) in one portion and stir rapidly for ≈1 h at 4 °C. This quantity of ammonium sulfate affords a final concentration of 60% ammonium sulfate.
18. Centrifuge the mixture obtained from step 17 (22,000 g, 4 °C, 20 min). Vacuum filter (Büchner funnel) and collect the filtrate on ice. The filtrate contains soluble mS100A9.
19. Add ≈38 g of ammonium sulfate to the combined filtrate in one portion to increase the ammonium sulfate concentration to 100%. Stir the mixture at 4 °C for ≈1 h to precipitate mS100A9.
20. Centrifuge the mixture obtained in step 19 (22,000 g, 4 °C, 20 min). Vacuum filter (Büchner funnel) and collect the precipitate, which contains mS100A9.
21. Add 77 mg DTT to 100 mL lysis buffer B,4 stir to dissolve, and keep the buffer on ice. Resuspend the mS100A8 pellets (step 16) and the mS100A9 precipitate (step 20) together in this solution by using a tissue homogenizer or by gently stirring the mixture with a stir bar at 4 °C.
22. Transfer the suspension to a stainless steel beaker and sonicate for 5 min (40% amplitude, 30 s on, 10 s off) on ice.
23. Centrifuge the mixture (22,000 g, 4 °C, 10 min). Transfer the supernatant to a dialysis bag (3500 MWCO) and dialyze against 3 × 4L of dialysis buffer containing 5 mM DTT at 4 °C for at least 12 h each.4
Day 3: Lysis and refolding of hCP-Ser
The following steps should be carried out at 4 °C and buffers should be equilibrated and stored at 4 °C (in a cold room) or on ice.
24. Thaw one cell pellet of hS100A8(C42S) and one hS100A9(C3S), each from a 1-L overexpression culture, on ice.
25. Keep 200 mL of lysis buffer A on ice or in a 4 °C room. Suspend each cell pellet in 30 mL (60 mL total) lysis buffer A. Once the cell pellets are fully suspended, combine the suspensions to yield a 60-mL mixture containing both hS100A8(C42S) and hS100A9(C3S).
26. Transfer the mixture to a stainless steel beaker on ice. Lyse the cells by sonication (40% amplitude, 2.5 min, 30 s on, 10 s off).
27. Centrifuge the crude lysate (22,000 g, 4 °C, 10 min). Discard the supernatant. The pellet contains the hS100A8(C42S) and hS100A9(C3S) subunits.
28. Repeat steps 25–27 for a total of three times.
29. Suspend the resulting cell pellets together in 100 mL lysis buffer B using a tissue homogenizer or by gently stirring the mixture with a stir bar at 4 °C.
30. Transfer the solution to a steel beaker on ice and sonicate for 5 min (40% amplitude, 30 s on, 10 s off).
31. Centrifuge the mixture (22,000 g, 4 °C, 10 min). Transfer the supernatant to a dialysis bag (3500 MWCO) and dialyze against 3 × 4 L of dialysis buffer at 4 °C for at least 12 h each.
Days 4 and 5: Change dialysis buffer
32. Change the dialysis buffer. If it is an mCP dialysis, add 5 mM DTT to the new dialysis buffer (3.1 g to 1 L dialysis buffer).
After dialysis, the refolded protein is purified by AEC and SEC – see Day 6.
33. The day before the chromatography steps, pre-equilibrate the S75 column with 1 column volume (CV) of S75 buffer.
Day 6: Protein purification of mCP and hCP-Ser
Over the course of the refolding process described above, some insoluble aggregates form and accumulate at the bottom of the dialysis bag as a white precipitate.
34. Centrifuge the dialysate to pellet the precipitate (22,000 g, 4°C, 10 min). Decant and vacuum filter the supernatant using a 0.2-μm bottle-top filter and collect the filtrate in a polypropylene bottle stored on ice.
35. Load the filtrate (≈110 mL; dialysis can cause some increase in the volume) solution onto the Superloop.
36. Equilibrate the MonoQ column with 2 CV Milli-Q water, 2 CV MonoQ buffer A, 2 CV MonoQ buffer B, 2 CV MonoQ buffer A. Use a flow rate of 2 mL/min. Include 5 mM DTT in the MonoQ A and B buffers when purifying a cysteine-containing protein.4
- 37. Load the column with the protein solution and elute at 2 mL/min using MonoQ buffer A and a gradient of:
-
0–15 % MonoQ buffer B over 15 CV for mCP (Figure 2A,C)OR
-
0–30% MonoQ buffer B over 30 CV for hCP-Ser (Figure 2B,D)Monitor protein elution at 280 nm and collect 5-mL fractions. SDS-PAGE analysis of the fractions is used to determine which fractions contain the CP heterodimer, which is evidenced by the presence of both the S100A8 and S100A9 subunits in about equal abundance on the gel (Figure 2). We typically perform two or three monoQ runs for a protein purification on this scale. The column is equilibrated (step 36) between runs.
-
38. Combine fractions containing both S100A8 and S100A9 (Figure 2) and concentrate the protein by centrifugation to ≈10 mL using an Amicon spin filter (10 kDa MWCO) and centrifuging (3210 g, ≈25 min, 4 °C).
39. Load all of the concentrated protein onto the Superloop.
40. Elute the heterodimer over 1 CV on the pre-equilibrated S75 column at a flow rate of 1 mL/min using S75 buffer (Figure 3). Include 5 mM DTT in the S75 buffer when purifying a cysteine-containing protein.4
41. Collect the fractions containing CP and dialyze for at least 12 h in 1 L S75 buffer containing ≈10 g Chelex at 4 °C. Include 5 mM DTT in this buffer when purifying a cysteine-containing protein.4
Figure 2.
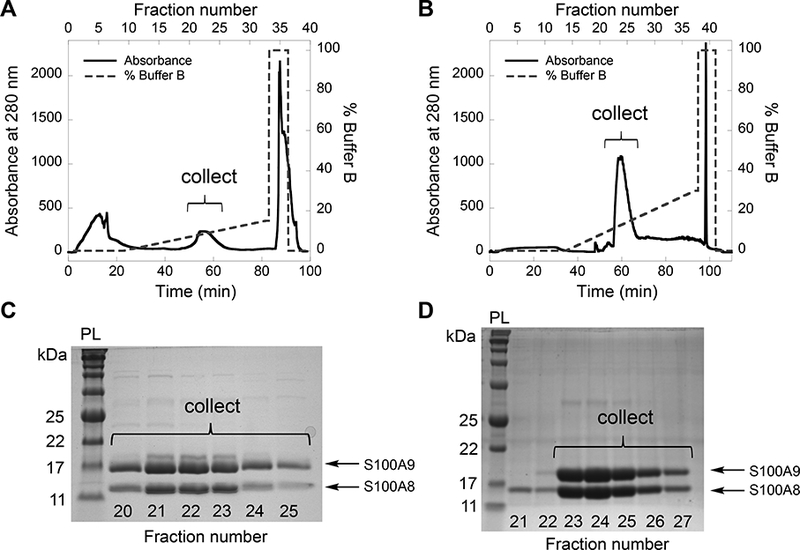
Purification of mCP (A, C) and CP-Ser (B, D) by anion exchange chromatography using a MonoQ column. Elution profiles (A, B) and corresponding 15% Tris-glycine SDS-PAGE gels of the fractions (C, D), indicating the fractions collected for subsequent purification. The protein ladder (PL) is p7712S (New England Biolabs).
Figure 3.
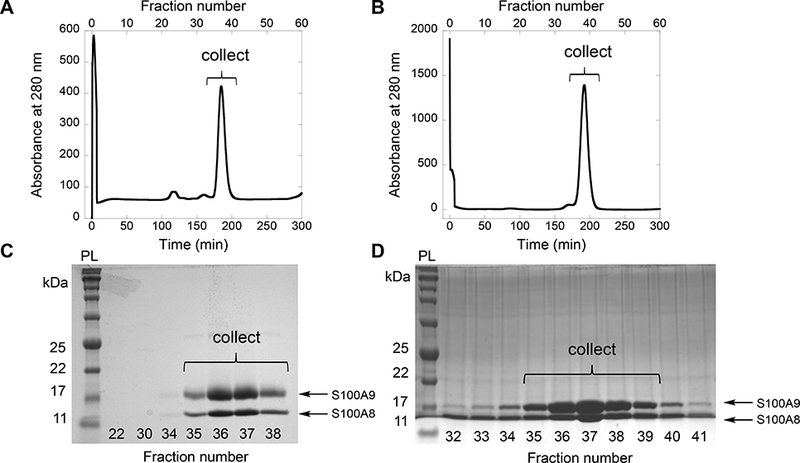
Purification of mCP (A, C) and CP-Ser (B, D) by size exclusion chromatography using a S75 column. Elution profiles (A, B) and corresponding 15% Tris-glycine SDS-PAGE gels of the fractions (C, D), indicating fractions collected for subsequent dialysis. The protein ladder (PL) is p7712s (New England Biolabs).
Day 7: Protein Storage
42. Concentrate the protein solution to >500 μM (≈3 mL). Aliquot the protein into microcentrifuge tubes, flash freeze the protein aliquots in liquid nitrogen, and store at −80 °C.
3.4. Biochemical characterization of calprotectin.
Following the purification of human or murine CP, we recommend employing standard biochemical techniques to evaluate the integrity of the purified protein (12). These methods include SDS-PAGE (Figure 4) to ascertain purity, analytical size exclusion chromatography to monitor oligomeric state, and metal analysis by inductively-coupled plasma mass spectrometry (ICP-MS). The experimental conditions for these procedures, as well as circular dichroism spectroscopy, are found in references 12 and 21.
Figure 4.
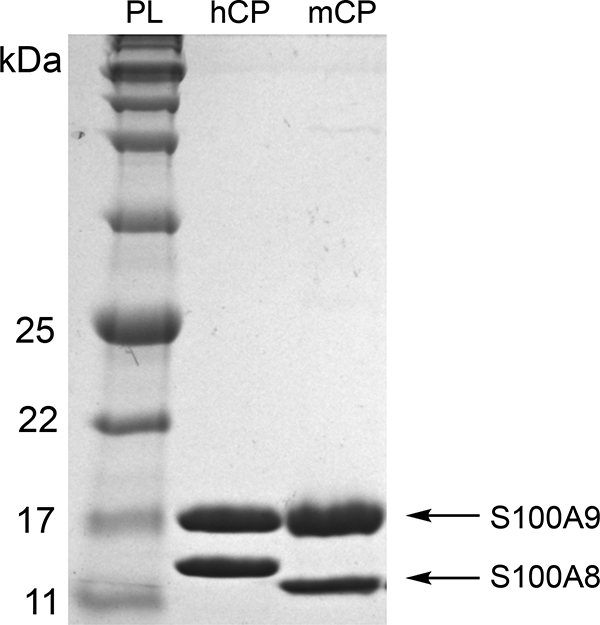
Representative 15% Tris-glycine SDS-PAGE gel of purified hCP-Ser (hCP) and mCP. The protein ladder (PL) is p7712S (New England Biolabs).
3.5. Fe speciation assay using ferrozine.
The Fe speciation assay allows quantification of Fe(II) as well as total Fe content in solutions containing this metal ion. The protocol described below is modified from prior reports(19, 27, 28).
Day 1: Preparation of samples
Combine 1 mL of the 100 mM FeCl3 stock solution (Section 2.2) with l mL of the 100 mM sodium citrate stock solution (Section 2.2) to afford a 50 mM Fe(III)-citrate solution (2 mL).
Dilute l mL of the 50 mM Fe(III)-citrate solution with 9 mL Milli-Q water to afford a 5 mM Fe(III)-citrate solution (10 mL).
Buffer-exchange mCP and hCP-Ser into the Fe speciation assay buffer (4x, 500 μL spin filter).5
Prepare 5-mL samples that contain both 30 μM Fe(III)-citrate and 20 μM protein in 15-mL polypropylene tubes by diluting the 5 mM Fe(III)-citrate solution (step 2) and the buffer-exchanged protein (step 3) into the Fe speciation assay buffer. Cap the tubes and incubate in an incubator shaker (30 °C, 150 rpm).
Prepare Fe standards for a standard curve. Dilute the 50 mM Fe(III)-citrate stock solution (step 1) with Fe speciation assay buffer to afford 10-mL aliquots of 32 μM and 25 μM Fe(III)-citrate. Serially dilute the 32 μΜ Fe(III)-citrate solution (1:1 dilution series) with Fe speciation assay buffer to prepare standards containing 16, 8, 4, and 2 μM Fe(III)-citrate. The standard curve is generated using standards containing 32, 25, 16, 8, 4, 2 and μΜ Fe.
Day 1: Fe speciation assay of calibration standards
6. Before initiating the assay, set a heating block to 95 °C and turn on the optical absorption spectrophotometer.
7. Prepare 0.4 M TCA and 1.3 M ammonium acetate solutions in Milli-Q water (or thaw a solution prepared the same day and stored at −20 °C).6
8. Thaw an aliquot of 100 mM ferrozine.
9. Prepare 0.2 M HCl (10 mL) in a 15-mL polypropylene tube by diluting 166.6 μL of 37% HCl into 10 mL of Milli-Q water.7
10. Prepare 40 mL 1.5 mM sodium ascorbate in 0.2 M HCl. Dilute 667 μL 37% HCl into 40 mL of Milli-Q water and add 12 mg sodium ascorbate. This solution must be prepared immediately before use.6,7
11. Prepare 6.17 mM ferrozine in 0.1 M HCl. Dilute 83.3 μL 37% HCl and 617 μL 100 mM ferrozine into 10 mL of Milli-Q water in a 15-mL polypropylene centrifuge tube.6,7
12. Transfer 200 μL of each standard solution to a separate 1.7-mL microcentrifuge tube. The total Fe content of these standards will be quantified. Ascorbate will be added to each sample to reduce all Fe(III) to Fe(II) before absorption of the Fe(II)-ferrozine complex is measured by optical absorption spectroscopy. Label each these tubes with an A.
13. Add 200 μL of 1.5 mM ascorbate solution to each tube labeled A.
14. Add 200 μL of 0.4 M TCA to each tube labeled A.
15. Vortex each tube labeled A for 10 s.
16. Heat all tubes labeled A for 5 min at 95 °C.
17. Centrifuge all tubes labeled A (15,000 g, 5 min, 4 °C).
18. Transfer 300 μL of each supernatant to a new microcentrifuge tube.
19. Add 400 μL of 1.3 M ammonium acetate to each tube.
20. Add 100 μL of 6.17 mM ferrozine in 0.1 M HCl to each tube. Vortex to mix.
21. Transfer 500 μL of each solution to disposable semi-micro polystyrene cuvettes.
22. Blank the optical absorption spectrometer using Milli-Q water and collect the optical absorption spectrum of each solution (Figure 5A).
23. Plot A562 vs [Fetotal] of the calibration standards and apply a linear regression to generate a calibration curve (Figure 5B).
Figure 5.
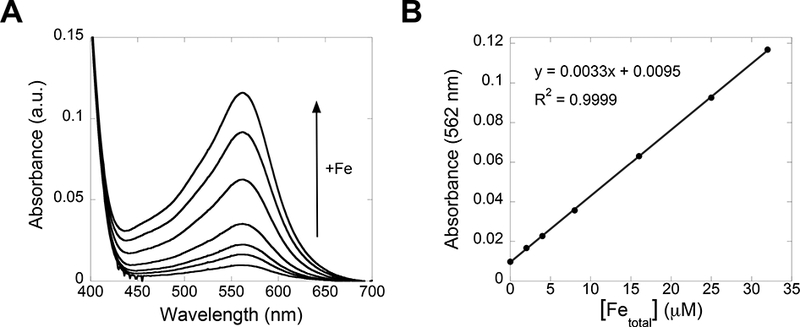
Absorbance profile (A) of Fe standards in Fe speciation assay buffer (0, 2, 4, 8, 16, 25, and 32 μM total Fe). A calibration curve is generated by plotting A562 vs. [Fetotal] and applying a linear regression fit (B).
Days 1–4: Fe speciation assay of protein samples
This assay is conducted at 0, 24, 48, and 72 h after the protein assay setup.
24. On each day, complete steps 6–11 from Fe speciation assay of calibration standards, above.
25. Immediately after preparing the samples that contain both Fe(III)-citrate and protein (Day 1: Preparation of samples, step 4), and on each day after, add 200 μL of each sample to each of two microcentrifuge tubes, labeled A and B, respectively. Samples labeled A will be treated with an ascorbate solution to reduce all Fe to Fe(II) and quantity total Fe. Samples labeled B will not be treated with ascorbate to quantify Fe(II).
26. Add 200 μL of 0.2 M HCl to all tubes labeled B.
27. Add 200 μL of 1.5 mM ascorbate solution to all tubes labeled A.
28. Add 200 μL of 0.4 M TCA to all tubes labeled A and B.
29. Vortex each tube for 10 s.
30. Heat all tubes for 5 min at 95 °C.
31. Centrifuge all tubes (15,000 g, 5 min, 4 °C). A small, white protein pellet is observed in the bottom of the tubes containing protein.
32. Transfer 300 μL of the supernatant to new microcentrifuge tubes.
33. Add 400 μL of 1.3 M ammonium acetate to each tube.
34. Add 100 μL of 6.17 mM ferrozine in 0.1 M HCl to each tube. Vortex to mix.
35. Transfer 500 μL of each solution to disposable semi-micro polystyrene cuvettes.
36. Blank the optical absorption spectrometer using Milli-Q water and collect the optical absorption spectrum of each solution 400–700 nm (Figure 6A,B). Record the absorbance of the Fe(II)-ferrozine complex in each solution at 562 nm.
37. Use the calibration curve to determine the [Fe(II)] and [Fetotal] in the mCP and CP-Ser samples. Subtract the measured [Fe(II)] from the [Fetotal] to determine the [Fe(III)]. Plot the concentrations of Fe(II), Fe(III), and total Fe for the samples over days 0–3 (Figure 7).8
Figure 6.
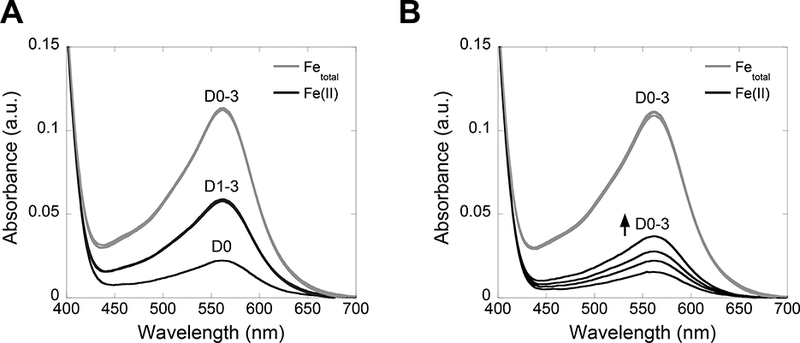
Absorbance profiles of mCP (A) and hCP-Ser (B) samples analyzed by the Fe speciation assay. Spectra obtained for the Fetotal quantification samples (tubes labeled A, gray traces) and the Fe(II) quantification samples (tubes labeled B, black traces) over days 0–3 (D0–3) are shown.
Figure 7.
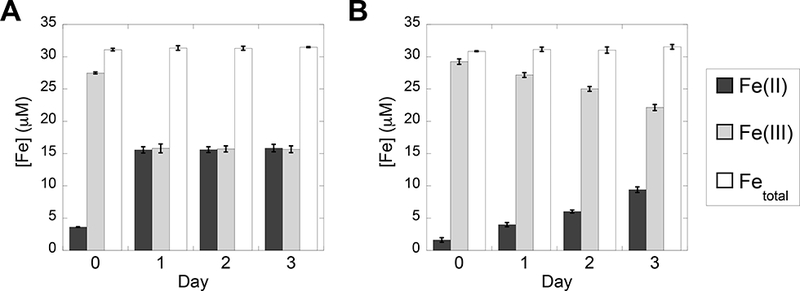
Bar plots showing the concentrations of Fe(II), Fe(III), and total Fe in solutions containing mCP (A) and hCP-Ser (B) quantified on days 0–3 using the Fe speciation assay (average ± SDM, n = 3).
4. Notes
Both human and murine CP coordinate transition metal ions with high affinity. Thus, precautions must be taken to reduce metal contamination when working with these proteins and to ensure the apo heterodimers are obtained from the purification. Because calcium ions affect the quaternary structure and transition metal affinities of CP, precautions must be taken to avoid calcium contaminations during the preparation of the apo heterodimer. After column purification, the protein is dialyzed in buffer containing Chelex resin to limit metal ion contamination. Additionally, for solution studies such as the Fe speciation assay, high purity reagents are employed and precautions are taken to avoid metal ion contamination. These precautions include the use of plastic spatulas to transfer reagents, the exclusive use of Milli-Q water, and the use of acid-washed glassware for the preparation of buffer and metal ion solutions. Because metal ions can leach from glassware, solutions are transferred to polypropylene containers for long-term storage.
Anhydrous FeCl3 is purchased in an ampule and is hygroscopic. Upon opening the ampule, the powder should be weighed and the stock solution prepared immediately.
If the protein contains one or more cysteine residues, 5 mM DTT is included in all buffers used for protein purification(12, 13, 21). This modification is employed for purifying hCP, which contains two cysteine residues, as well as variants of mCP, and should be applicable for variants that have cysteine residues incorporated at non-native positions by site-directed mutagenesis. It is important to determine whether DTT in the storage buffer must be removed prior to an experiment. Note that DTT can affect the redox speciation of metal ions like Fe and Cu.
DTT is not infinitely stable and thus buffer containing DTT should not be stored for extended periods of time because DTT is not stable. We recommend storing buffers without DTT and adding DTT powder to buffers immediately before use.
To set up the Fe speciation assay, proteins are buffer-exchanged into the Fe speciation assay buffer. Remember that mCP is stored in buffer that contains 5 mM DTT, and this reducing agent must be removed before setting up the Fe speciation assay.
We strongly recommend that the TCA, ammonium acetate, and sodium ascorbate reagents used in the ferrozine assay are prepared immediately before use. Ferrozine solutions can be stored at −80 °C and thawed daily for use in the assay.
We strongly recommend using high purity reagents for the iron speciation assay, including trace metals basis HCl solution, to avoid metal contamination. In particular, we have found that use of lower purity HCl compromises the Fe speciation assay.
We recommend using at least 30 μM total Fe concentration in the assay. This concentration will provide absorbance values > 0.1 a.u. when measuring the total iron content of the solution with a 1-cm pathlength cuvette.
Acknowledgements
Our current studies of calprotectin are supported by the National Science Foundation (CHE-1352132) and the National Institutes of Health (R01GM118695 and R01GM126376).
References
- 1.Zygiel EM, Nolan EM (2018) Transition metal sequestration by host-defense protein calprotectin. Ann Rev Biochem 87: 621–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hood MI, Skaar EP (2012) Nutritional immunity: transition metals at the pathogen-host interface. Nat Rev Microbiol 10(8): 525–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corbin BD, Seeley EH, Raab A et al. (2008) Metal chelation and inhibition of bacterial growth in tissue abscesses. Science 319(5865): 962–965 [DOI] [PubMed] [Google Scholar]
- 4.Hood MI, Mortensen BL, Moore JL et al. (2012) Identification of an Acinetobacter baumannii zinc acquisition system that facilitates resistance to calprotectin-mediated zinc sequestration. PLoS Pathog. 8(12): e1003068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu JZ, Jellbauer S, Poe AJ et al. (2012) Zinc sequestration by the neutrophil protein calprotectin enhances Salmonella growth in the inflamed gut. Cell Host Microbe 11(3): 227–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Jong HK, Achouiti A, Koh GC et al. (2015) Expression and function of S100A8/A9 (calprotectin) in human typhoid fever and the murine Salmonella model. PLoS Negl. Trop. Dis 9(4): e0003663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wakeman CA, Moore JL, Noto MJ et al. (2016) The innate immune protein calprotectin promotes Pseudomonas aeruginosa and Staphylococcus aureus interaction. Nat. Commun 7: 11951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark HL, Jhingran A, Sun Y et al. (2016) Zinc and manganese chelation by neutrophil S100A8/A9 (calprotectin) limits extracellular Aspergillus fumigatus hyphal growth and corneal infection. J. Immunol 196(1): 336–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hobbs JA, May R, Tanousis K et al. (2003) Myeloid cell function in MRP-14 (S100A9) null mice. Mol. Cell. Biol 23(7): 2564–2576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hunter MJ, Chazin WJ (1998) High level expression and dimer characterization of the S100 EF-hand proteins, migration inhibitory factor-related proteins 8 and 14. J. Biol. Chem 273(20): 12427–12435 [DOI] [PubMed] [Google Scholar]
- 11.Korndörfer IP, Brueckner F, Skerra A (2007) The crystal structure of the human (S100A8/S100A9)2 heterotetramer, calprotectin, illustrates how conformational changes of interacting α-helices can determine specific association of two EF-hand proteins. J. Mol. Biol 370(5): 887–898 [DOI] [PubMed] [Google Scholar]
- 12.Brophy MB, Hayden JA, Nolan EM (2012) Calcium ion gradients modulate the zinc affinity and antibacterial activity of human calprotectin. J. Am. Chem. Soc 134(43): 18089–18100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brophy MB, Nakashige TG, Gaillard A et al. (2013) Contributions of the S100A9 C-terminal tail to high-affinity Mn(II) chelation by the host-defense protein human calprotectin. J. Am. Chem. Soc 135(47): 17804–17817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Damo SM, Kehl-Fie TE, Sugitani N et al. (2013) Molecular basis for manganese sequestration by calprotectin and roles in the innate immune response to invading bacterial pathogens. Proc. Natl. Acad. Sci 110(10): 3841–3846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gagnon DM, Brophy MB, Bowman SEJ et al. (2015) Manganese binding properties of human calprotectin under conditions of high and low calcium: X-ray crystallographic and advanced electron paramagnetic resonance spectroscopic analysis. J. Am. Chem. Soc 137(8): 3004–3016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayden JA, Brophy MB, Nolan EM (2013) High-affinity manganese coordination by human calprotectin is calcium-dependent and requires the histidine-rich site formed at the dimer interface. J. Am. Chem. Soc 135(2): 775–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakashige TG, Stephan JR, Cunden LS et al. (2016) The hexahistidine motif of host defense protein human calprotectin contributes to zinc withholding and its functional versatility. J. Am. Chem. Soc 138(37): 12243–12251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakashige TG, Zhang B, Krebs C et al. (2015) Human calprotectin is an iron-sequestering host-defense protein. Nat. Chem. Biol 11(10): 765–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakashige TG, Nolan EM (2017) Human calprotectin affects the redox speciation of iron. Metallomics 9: 1086–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakashige TG, Zygiel EM, Drennan CL et al. (2017) Nickel sequestration by the hostdefense protein human calprotectin. J. Am. Chem. Soc 139: 8828–8836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hadley RC, Gu Y, Nolan EM 2018) Initial biochemical and functional evaluation of murine calprotectin reveals Ca(II)-dependence and its ability to chelate multiple nutrient transition metal ions. Biochemistry 57(19): 2846–2856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vogl T, Roth J, Sorg C et al. (1999) Calcium-induced noncovalently linked tetramers of MRP8 and MRP14 detected by ultraviolet matrix-assisted laser desorption/ionization mass spectrometry. J. Am. Soc. Mass Spectrom 10(11): 1124–1130 [DOI] [PubMed] [Google Scholar]
- 23.Strupat K, Rogniaux H, Van Dorsselaer A et al. (2000) Calcium-induced noncovalently linked tetramers of MRP8 and MRP14 are confirmed by electrospray ionization-mass analysis. J. Am. Soc. Mass Spectrom 11(9): 780–788 [DOI] [PubMed] [Google Scholar]
- 24.Gifford JL, Walsh MP, Vogel HJ (2007) Structures and metal-ion-binding properties of the Ca2+-binding helix-loop-helix EF-hand motifs. Biochem. J 405(2): 199–221 [DOI] [PubMed] [Google Scholar]
- 25.Kehl-Fie TE, Chitayat S, Hood MI et al. (2011) Nutrient metal sequestration by calprotectin inhibits bacterial superoxide defense, enhancing neutrophil killing of Staphylococcus aureus. Cell Host Microbe. 10(2): 158–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stephan JR, Nolan EM (2016) Calcium-induced tetramerization and zinc chelation shield human calprotectin from degradation by host and bacterial extracellular proteases. Chem. Sci 7(3): 1962–1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stookey LL (1970) Ferrozine- a new spectrophotometric reagent for iron. Anal. Chem 42(7): 779–781 [Google Scholar]
- 28.Carter P (1971) Spectrophotometric Determination of serum iron at the submicrogram level with a new reagent (ferrozine). Anal. Biochem 40: 450–458 [DOI] [PubMed] [Google Scholar]


