Abstract
Detection of rare tumor cells circulating in the blood (CTCs) presents technical challenges. CellSearch, the only approved system for clinical use, fails to capture epithelial cell adhesion molecule‐negative CTCs such as malignant pleural mesothelioma (MPM). We have developed a novel microfluidic device (CTC‐chip) in which any Ab to capture CTCs is conjugated. The CTC‐chip was coated with an Ab against podoplanin that is abundantly expressed on MPM. Circulating tumor cell‐detection performance was evaluated in experimental models in which MPM cells were spiked in blood sampled from a healthy volunteer and in clinical samples drawn from MPM patients. The CTC‐chip showed superior CTC‐detection performance over CellSearch in experimental models (sensitivity, 63.3%‐64.5% vs 0%‐1.1%; P < .001) and in clinical samples (CTC‐positivity, 68.8% vs 6.3%; P < .001). A receiver operating characteristic (ROC) analysis showed that the CTC test provided a significant diagnostic performance in discrimination of unresectable disease from resectable disease (area under the ROC curve, 0.851; P = .003). The higher CTC count (≥2 cells/mL) was significantly associated with a poor prognosis (P = .030). The novel CTC‐chip enabled sensitive detection of CTCs, which provided significant diagnostic and prognostic information in MPM.
Keywords: circulating tumor cell, CTC‐chip, mesothelioma, podoplanin, prognosis
Abbreviations
- CFSE
carboxyfluorescein succinimidyl ester
- CK
cytokeratin
- CTC
circulating tumor cell
- EpCAM
epithelial cell adhesion molecule
- MPM
malignant pleural mesothelioma
- PD‐L1
programmed death ligand 1
- ROC
receiver operating characteristic
1. INTRODUCTION
Circulating tumor cells are tumor cells that are shed from tumor tissues and circulate in the blood. The can be found in the peripheral blood of cancer patients even when metastatic diseases are not clinically detectable with routine imaging techniques such as computed tomography, MRI, and PET scan. Accordingly, detection of CTCs could contribute to improvement in diagnosis and treatment decision‐making of cancer patients.1, 2, 3 In addition to direct quantitative analyses of tumor cells circulating in the blood, CTCs can be subject to further molecular and biological analyses. Despite these potential advantages, detection of rare CTCs contaminated in a large number of normal hematological cells can present technical challenges.2, 3, 4, 5 Among a variety of systems that have been developed for detection of CTCs, CellSearch (Merarini Silicon Biosystems, Huntington Valley, PA, USA) is the only system approved for clinical use.6 CellSearch is an automated system to isolate CTCs using an Ab against EpCAM that is abundantly expressed on epithelial cells.6, 7 CellSearch has provided highly reproducible results in detection of CTCs,6 and has been approved to monitor peripheral blood of patients with metastatic breast, prostate, and colorectal cancer in the USA.
Malignant pleural mesothelioma is a highly aggressive malignant tumor originating from the mesothelium.8, 9, 10 To evaluate the clinical utility of CTCs detected with CellSearch in MPM, we undertook a prospective study.11 The CTC test provided significant diagnostic performance in discrimination between MPM and nonmalignant pleural diseases such as asbestos pleurisy (P = .036). However, CTCs were detected only in 32.7% (34/104) of MPM patients, and the low sensitivity could mainly be due to EpCAM‐dependent capture of CTCs in CellSearch. Malignant pleural mesothelioma cells of mesothelial origin, not of epithelial origin, showed negative or low EpCAM expression, which might not be effectively captured with CellSearch.11 These results indicate a need for a more sensitive system to detect CTCs that can capture EpCAM‐negative tumor cells. Accordingly, we have developed a novel microfluidic device system in which CTCs are captured to numerous microposts coated with an Ab against an antigen expressed on target tumor cells (CTC‐chip).12, 13 The most important advantage of the novel CTC‐chip system is its capability of conjugating any Ab to capture CTCs (“universal” CTC‐chip), which could enable capture of a wide variety of CTCs by conjugating appropriate Abs. In fact, our previous experimental studies showed that MPM tumor cells were captured with the CTC‐chip coated with an Ab against podoplanin that was abundantly expressed on mesothelial cells.13, 14 However, the capture efficacy of tumor cells spiked in the blood remains less than 40%,13 which might not be enough for clinical application.
In the present study, we improved the capture efficiency up to 80% by optimizing conditions in preparing the CTC‐chip for capturing MPM cells, which provided an overwhelmingly higher performance than CellSearch. In addition, the CTC test showed a significant diagnostic performance in discrimination between early disease and advanced disease, and also provided a significant prognostic value in MPM. The novel CTC‐chip is the first promising system to detect CTCs that can be applied for clinical use in the diagnosis and treatment of MPM.
2. MATERIALS AND METHODS
2.1. Cell lines
Human mesothelioma cell line ACC‐MESO‐4 was purchased from the Riken BioResource Center (Tsukuba, Japan), and H226, H28, and MSTO‐211H were from ATCC (Manassas, VA, USA). These cells were cultured in RPMI‐1640 medium (Wako Pure Chemical Industries, Osaka, Japan) supplemented with 10% FBS (Invitrogen, San Diego, CA, USA) at 37°C and 5% CO2.
2.2. Flow cytometry
Cells were incubated with an antipodoplanin Ab (clone E1; Santa Cruz Biotechnology, Dallas, TX, USA). Then the cells were incubated with a goat anti‐mouse IgG Ab conjugated with FITC (BD Biosciences, San Jose, CA, USA). Flow cytometry analysis was carried out using an EC800 Cell Analyser (Sony, Tokyo, Japan) and FlowJo software (Tree Star, Ashland, OR, USA). The percentage of positive cells and the mean fluorescence intensity was determined by comparison with negative control.
2.3. Preparation of CTC‐chip
The CTC‐chip system was used after 2‐step coating with an Ab to capture CTCs as described previously (Figure S1).13 In brief, the chip was first incubated with a goat anti‐mouse IgG Ab (SouthernBiotech, Birmingham, AL, USA) overnight at 4°C. Thereafter, the chip was incubated with an antipodoplanin Ab (clone E1) to capture tumor cells for 1 hour at 4°C (podoplanin‐chip). After washing with PBS, the chip surface was kept wet.
In previous studies, both the concentration of the “base antibody” (anti‐mouse IgG Ab) coated over the chip surface and that of the “capture antibody” (antipodoplanin Ab) conjugated to the base Ab to capture tumor cells were 20 μg/mL.13 In the present study, higher concentrations of these Abs were tested to improve the cell‐capture efficacy (Figure S1).
2.4. Cell‐capture efficacy of CTC‐chip
Sample preparation and flow test to evaluate cell‐capture efficiency were carried out as described previously.13 In brief, tumor cells were labeled with CellTrace CFSE Cell Proliferation Kit (Life Technologies, Carlsbad, CA, USA), and were suspended either in PBS containing 5% BSA or in the blood sampled from a healthy volunteer at the concentration of 100 and 500 cells/mL. A cell suspension sample of 1 mL, which contains 100 or 500 tumor cells, was applied to the CTC‐chip.
Images and movies of cells in the CTC‐chip were monitored and recorded with a CKX41 fluorescence microscope (Olympus, Tokyo, Japan) and a digital video camera (Sony). The actual number of cells that were sent into the chip (N‐total) was determined by counting the number of cells that passed through the inlet of the CTC‐chip, and the number of captured cells (N‐captured) was also determined by counting CFSE‐labelled cells remained on the CTC‐chip. The cell capture efficiency was represented as N‐captured/N‐total.12, 13, 14 Experiments were carried out in triplicate.
2.5. Sensitivity to detect tumor cells: CTC‐chip vs CellSearch
ACC‐MESO‐4 cells, labeled with CFSE, were suspended in the blood sampled from a healthy volunteer at the concentration of 10 and 50 cells/mL. The peripheral blood was drawn in a sample tube (BD Vacutainer EDTA‐2K; BD, Franklin Lakes, NJ, USA) for the CTC‐chip, and in a designated sample tube (CellSave Preservative Tube; Merarini Silicon Biosystems, Huntington Valley, PA) for CellSearch. The cell suspension was applied to the CTC‐chip or to CellSearch (Figure S2). The sensitivity in detection of CTCs was defined as the percentage of the number of captured CFSE‐positive cells among the total number of tumor cells spiked in the suspension.
2.6. Clinical evaluation of CTCs in MPM patients
Peripheral blood was sampled from patients with a pathological diagnosis of MPM and served for detection of CTCs. For detection of CTCs with the CTC‐chip, peripheral blood was drawn to the sample tube (BD Vacutainer EDTA‐2K), and 1 mL blood was applied to the podoplanin‐chip. Cells captured on the CTC‐chip were stained with an antiwide spectrum CK Ab (rabbit polyclonal; Abcam, Cambridge, MA, USA) followed by incubation with anti‐rabbit IgG Ab‐conjugated Alexa Fluor 594 (Thermo Fisher Scientific, Waltham, MA, USA), an anti‐CD45 Ab (rat monoclonal, clone YTH24.5; Abcam) followed by incubation with anti‐rat IgG Ab‐conjugated Alexa Fluor 488 (Thermo Fisher Scientific), and with Hoechst33342 (Cell Signaling Technology, Danvers, MA, USA). Each cell with round to oval morphology, a Hoechst33342‐positive nucleus, positive staining for CK in the cytoplasm, and negative staining for CD45 was judged as a CTC. For detection of CTCs with CellSearch, 7.5 mL blood was drawn to the designated sample tube (CellSave Preservative Tube). Circulating tumor cells were quantitatively evaluated as described previously.6, 7 The number of CTCs in 1 mL blood was indicated as the CTC count for the CTC‐chip, and that in 7.5 mL blood was indicated as the CTC count for CellSearch.
The revised TNM criteria were used for evaluation of tumor staging.15 Podoplanin expression on tumor cells in pathological sections was evaluated with immunohistochemical staining using an antipodoplanin Ab (D2‐40; Nichirei Biosciences, Tokyo, Japan). This study was approved by the Ethics Committee of Medical Research, University of Occupational and Environmental Health (Kitakyushu, Japan).
2.7. Statistical analyses
The proportions of categorical data were compared by the χ2 test. Continuous data were compared using a nonparametric test (Mann‐Whitney U test). The Kaplan‐Meier method was used to estimate the probability of survival, with survival differences being analyzed by the log‐rank test. Differences were considered to be statistically significant for P‐value <.05. All statistical analyses were carried out with SPSS version 21 software (IBM, Armonk, NY, USA).
3. RESULTS
3.1. Improvement in cell‐capture efficiency at higher Ab concentrations
When ACC‐MESO‐4 cells were spiked in PBS, tumor cells were effectively captured by the podoplanin‐chip (average capture efficiency, 78.3%) prepared in the previous condition (base‐Ab concentration, 20 μg/mL; capture‐Ab concentration, 20 μg/mL) as shown in the previous study.13 When higher concentration of the base‐Ab and/or the capture‐Ab were used, the cell‐capture efficiency slightly improved and reached almost 100% (Figure 1).
Figure 1.
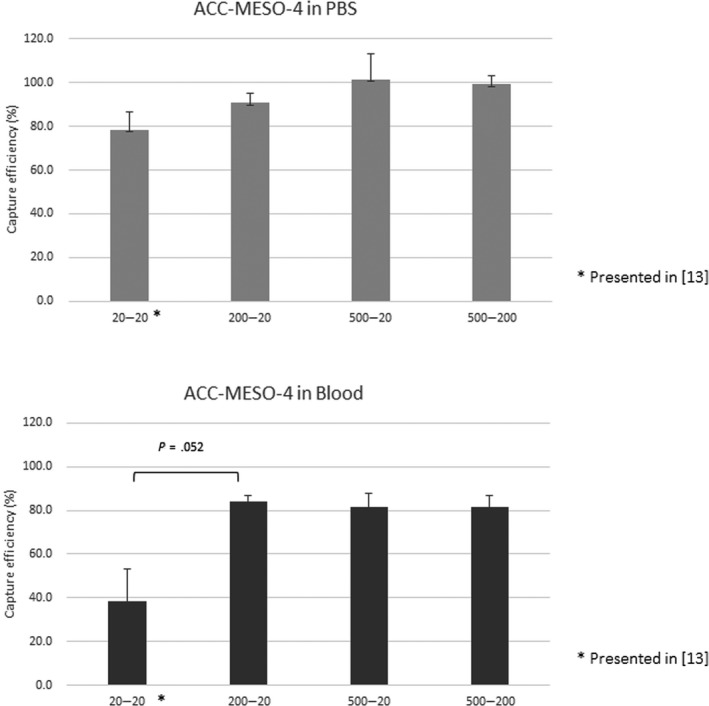
Cell‐capture efficacy for a mesothelioma cell line (ACC‐MESO‐4) using a novel microfluidic device to capture rare tumor cells circulating in the blood, the CTC‐chip, at several Ab concentrations. ACC‐MESO‐4 tumor cells were spiked in PBS (upper panel) or blood sampled from a healthy volunteer (lower panel). The cell suspension (500 cells/mL) served for evaluation of the cell‐capture efficacy at several concentrations of the base‐Ab and the capture‐Ab: 20 μg/mL and 20 μg/mL, respectively (20‐20); 200 μg/mL and 20 μg/mL, respectively (200‐20); 500 μg/mL and 20 μg/mL, respectively (500‐20); and 500 μg/mL and 200 μg/mL, respectively (500‐200). Experiments were carried out in triplicate. Error bar shows SD
When ACC‐MESO‐4 cells were spiked in the blood, tumor cells were not effectively captured by the podoplanin‐chip (average capture efficiency, 38.5%) prepared in the previous condition (base‐Ab concentration, 20 μg/mL; capture‐Ab concentration, 20 μg/mL) as shown in the previous study.13 When the base‐Ab concentration was increased to 200 μg/mL, the cell‐capture efficiency improved (average capture efficiency, 84.1%). However, even when a higher concentration (500 μg/mL) of base‐Ab was used in combination with a higher concentration of capture‐Ab (500 μg/mL), no higher cell‐capture efficiency was achieved (Figure 1). Based on these results, we determined the optimal concentrations of base‐Ab concentration (200 μg/mL) and capture‐Ab (20 μg/mL) in preparation of the podoplanin‐chip to capture MPM cells.
Next, other MPM cell lines were spiked in the blood, and cell‐capture efficiencies were examined. The H226 cells with positive podoplanin expression, as well as ACC‐MESO‐4 cells, were effectively captured with the optimized podoplanin‐chip (average capture efficiency, 76.3%). In contrast, H28 cells and MSTO‐211H cells showed low podoplanin expression and were not effectively captured with the podoplanin‐chip (average capture efficiency, 4.4% and 9.0%, respectively; Figure 2).
Figure 2.
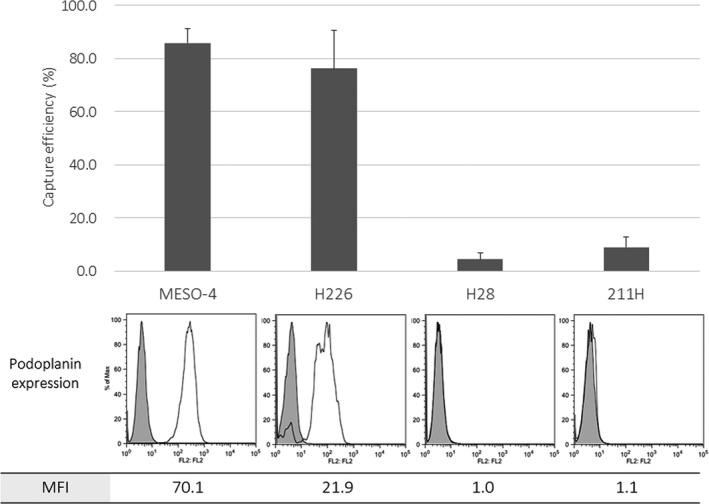
Cell‐capture efficacy for several mesothelioma cell lines using a novel microfluidic device to capture rare tumor cells circulating in the blood, the CTC‐chip. Several mesothelioma cells (ACC‐MESO‐4, H226, H28, and MSTO‐211H) were spiked in the blood sampled from a healthy volunteer. The cell suspension (100 cells/mL) was applied to the optimized podoplanin‐chip (base‐Ab concentration, 200 μg/mL; capture‐Ab concentration, 20 μg/mL). Podoplanin expression on each cell line was examined with flow cytometry, and the percentage of positive cells and the mean fluorescence intensity (MFI) are indicated. Experiments were carried out in triplicate. Error bar shows SD
3.1.1. Superior cell‐detection efficiency of the CTC‐chip over CellSearch
ACC‐MESO‐4 cells were successfully isolated from blood with the optimized podoplanin‐chip (Figure 3). When 10 cells and 50 cells were spiked in 1 mL blood, the average sensitivities to isolate and detect tumor cells with the podoplanin‐chip were 64.5% and 63.3%, respectively. In contrast, almost no tumor cells were detected with CellSearch (average sensitivity, 0% and 1.1%, respectively).
Figure 3.
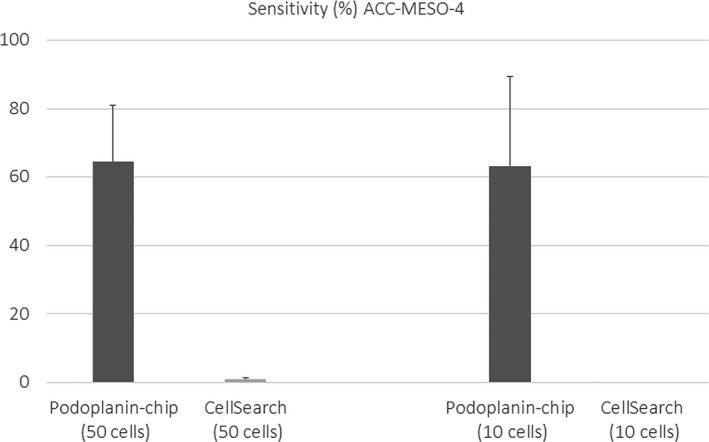
Comparison of sensitivity to detect mesothelioma cells spiked in the blood between 2 devices, CTC‐chip and CellSearch. The sensitivity is represented as the percentage of detected tumor cells among all spiked cells. Only a few tumor cells were detected with CellSearch. In contrast, a higher sensitivity was achieved with the CTC‐chip when either 10 cells or 50 cells were spiked in 1 mL blood. Experiments were carried out in duplicate. Error bar shows SD
A total of 16 peripheral blood samples drawn from 15 patients with MPM (11 samples from 11 patients with epithelioid type, 4 samples from 3 patients with sarcomatoid type, and 1 sample from 1 patient with sarcomatoid type) were subjected to quantitative analyses for CTCs by the podoplanin‐chip or by CellSearch. Only one CTC was detected in the peripheral blood (7.5 mL) sampled from an epithelioid‐MPM patient with CellSearch, and 16 CTCs were detected in the same blood sample (1 mL) with the podoplanin‐chip. No CTC was detected in the other 15 samples with CellSearch. In contrast, 11 samples were positive for CTCs with the podoplanin‐chip, which detected a significantly higher number of CTCs (Figure 4). Overall, the CTC‐positivity was significantly higher with the CTC‐chip than with CellSearch (68.5% vs 6.3%, P < .001).
Figure 4.
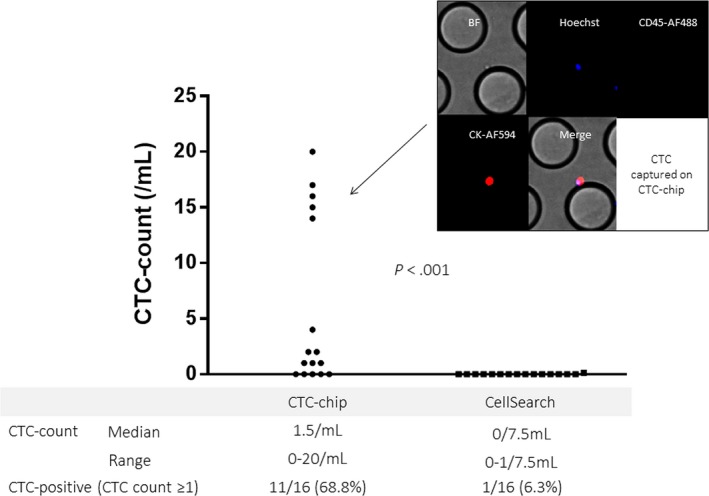
Circulating tumor cell (CTC) count in patients with malignant pleural mesothelioma (MPM) with the CTC‐chip and CellSearch. Among cells captured on the CTC‐chip, only cells with positive nuclear staining by Hoechst33342 (blue), positive cytoplasmic staining with cytokeratin (CK) by Alexa594 (red), and negative CD45 staining by Alexa488 (green) were judged as tumor cells. Blood samples (1 mL and 7.5 mL) from each patient with MPM were applied to the podoplanin‐chip and CellSearch, respectively. The CTC count is represented as the number of CTCs in 1 mL blood for the podoplanin‐chip and that in 7.5 mL blood for CellSearch. BF, bright field
3.2. Clinical implications of CTCs detected with the CTC‐chip
To evaluate the clinical implications of CTCs detected with the podoplanin‐chip, we further analyzed additional blood samples drawn from a total of 25 patients with MPM (Table 1). Overall, CTCs were detected in 16 patients (sensitivity, 64.0%), and there was no significant difference in the sensitivity to detect CTCs according to pathological subtypes of MPM (CTC‐positivity, 58.8% [10/17] for epithelioid type, 75.0% [3/4] for biphasic type, and 75.0% [3/4] for sarcomatoid type; P = .734).
Table 1.
Characteristics of patients with malignant pleural mesothelioma
| Characteristic | No. of patients | Percentage |
|---|---|---|
| Age, years | ||
| Median (range) | 72.0 (54‐83) | |
| Mean ± SD | 70.6 ± 8.4 | |
| Sex | ||
| Female | 4 | 16 |
| Male | 21 | 84 |
| Histology | ||
| Epithelioid | 17 | 68 |
| Biphasic | 4 | 16 |
| Sarcomatoid | 4 | 16 |
| Podoplanin expression in tumor tissue | ||
| Positive | 18 | 72 |
| Negative | 0 | 0.0 |
| Unknown | 7 | 28 |
| Stage | ||
| IA | 4 | 16 |
| IB | 7 | 28 |
| II, IIIA | 0 | 0.0 |
| IIIB | 10 | 40 |
| IV | 4 | 16 |
| Curative surgery | ||
| Performed (P/D in all patients) | 10 | 40 |
| CTC‐count with the CTC‐chip (per 1 mL) | ||
| 0 | 9 | 36 |
| 1 | 5 | 20 |
| 2 | 2 | 8 |
| 3 | 1 | 4 |
| 4 | 2 | 8 |
| 15, 16, 17, 20, 50, 96 | 1 each | 4 each |
CTC, circulating tumor cell; P/D, pleurectomy/decortication.
As expected, the sensitivity was significantly higher in advanced stages of diseases (CTC‐positivity, 0.0% [0/4] in stage IA, 42.9% [3/7] in stage IB, 56.3% [9/10] in stage IIIB, and 100.0% [4/4] in stage IV disease; P = .003) (Figure 5). As stage IIIB and IV diseases are generally recognized as “unresectable” disease, a ROC curve analysis was carried out to determine the optimal cut‐off value of CTC count in discrimination between “unresectable” disease (stage IIIB or IV disease) and “resectable” disease (stage IIIA or earlier). The area under ROC curve was 0.851 (95% confidence interval, 0.667‐1.000), indicating a significant diagnostic performance of the CTC‐test to predict “unresectable” disease in MPM (P = .003; Figure 5). The optimal cut‐off value of the CTC‐count was estimated as “2” which provided a very high specificity (90.9%) with a moderate sensitivity (64.3%). When the cut‐off value of “1” was used, the sensitivity was higher (92.3%) but the specificity was modest (72.7%). When patients were classified into high‐CTC and low‐CTC patients according to the cut‐off value of 2, high‐CTC (CTC‐count ≥2) was significantly associated with a poor prognosis (Figure 5).
Figure 5.
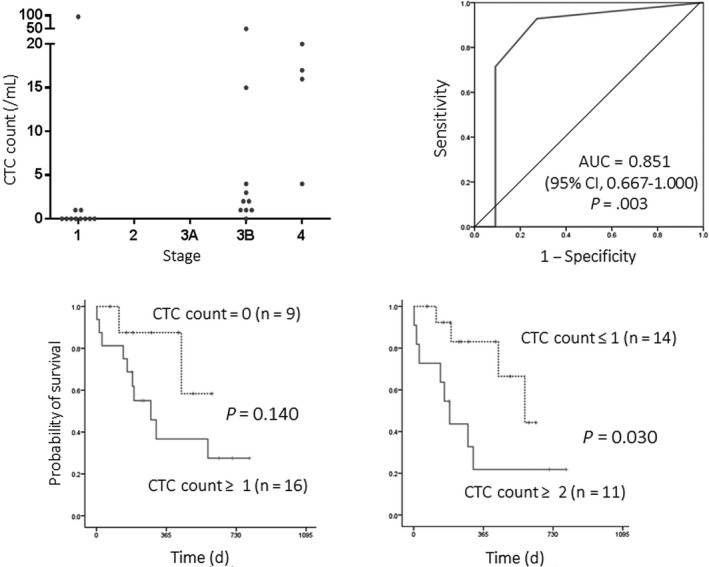
Clinical implications of circulating tumor cells (CTCs) detected with the CTC‐chip in malignant pleural mesothelioma (MPM). Top left panel, distribution of CTC count according to disease stage. CTCs were detected in peripheral blood sampled from 25 patients with MPM with the podoplanin‐chip. The number of CTCs (CTC count) was significantly higher in advanced stages of disease (stage IIIB and IV). Top right panel, receiver operating curve to predict “unresectable” disease (stage IIIB or IV). CTC count provided a significant diagnostic performance in discrimination of “unresectable” (stage IIIB or IV) disease from potentially “resectable” disease. Bottom panels, overall survival curves according to CTC count. Patients with high‐CTC tumor (CTC count ≥2) showed a significantly poorer prognosis than those with low‐CTC tumor (CTC count, 0 or 1)
The CTC‐count was longitudinally monitored during treatment in 2 patients. In both patients, the CTC‐count decreased after effective treatment (curative surgery followed by adjuvant chemotherapy in Case 1 or chemotherapy in Case 2; Figure S3, [Link]).
4. DISCUSSION
In the present study, we first optimized the novel CTC‐chip for effective capture of MPM cells by using higher concentrations of base‐Ab and capture‐Ab. Second, we examined the cell‐capture and cell‐detection performance of the optimized CTC‐chip in experimental models and showed the CTC‐chip had superior performance over CellSearch. Finally, the CTC‐chip enabled sensitive detection of CTCs in peripheral blood sampled from MPM patients, which provided a significant diagnostic performance in discrimination of “unresectable” disease from “resectable” disease and a significant prognostic value in MPM patients.
Effective capture of rare CTCs presents technical challenges. A common strategy is EpCAM‐dependent isolation, as is used in CellSearch, because tumor cells of epithelial origin express EpCAM but hematological cells do not. However, EpCAM‐dependent methods might not be applicable for isolation of EpCAM‐negative tumor cells, such as epithelial tumor cells undergoing epithelial‐mesenchymal transition or tumor cells of non‐epithelial origin. Among a variety of EpCAM‐independent methods including size‐based or density‐based separation,1, 2, 3 we adopted a microfluidic method (CTC‐chip). The original CTC‐chip, which was conjugated with an anti‐EpCAM Ab, could capture only EpCAM‐positive tumor cells.16 To overcome its EpCAM‐dependent limitations, we have developed a novel CTC‐chip to which any capture‐Ab can be easily conjugated.12, 13 In the present study, we optimized the CTC‐chip conjugated with the antipodoplanin Ab. When the capture‐Ab (20 μg/mL) was conjugated to the CTC‐chip after incubation with the base‐Ab at a higher concentration (200 μg/mL), tumor cells were effectively isolated from blood (cell‐capture efficiency, 84.1% for ACC‐MESO‐4 and 76.3% for H226; Figures 1 and 2). Moreover, in comparison with CellSearch, the optimized CTC‐chip achieved overwhelmingly superior performance to detect MPM cells not only in experimental models (Figure 3) but also in clinical samples drawn from MPM patients (Figure 4).
We also indicated significant clinical relevance of CTCs detected with the CTC‐chip. The CTC count was significantly higher in advanced and “unresectable” stages (IIIB or IV) of disease, which provided a significant diagnostic performance to discriminate patients with unresectable disease from those with resectable disease (Figure 5). In addition, high‐CTC count (≥2) was significantly associated with poor prognosis (Figure 5), and the CTC‐count was decreased along with tumor resection and/or shrinkage (Figure S3, [Link]). These results could indicate that the CTC‐count evaluated with the novel CTC‐chip system is potentially useful as a biomarker in the diagnosis and treatment of MPM patients, which should be validated in future prospective studies.
In the present study, CTCs appear to be obtained more frequently in patients with sarcomatoid (75%, 3/4) and biphasic (75%, 3/4) subtypes, although the case numbers were small, which might suggest that sarcomatoid and biphasic subtypes are associated with aggressive tumor behavior representing frequent micrometastases. In our previous study using CellSearch,11 CTCs were detected in similar proportions of patients according to histologic subtypes (30.8% [24/78] in epithelial patients, 40.0% [4/10] in sarcomatoid patients, and 28.6% [2/7] in biphasic patients). These results might also indicate more aggressive behavior of sarcomatoid tumors, as only EpCAM‐positive CTCs, comprising a small subset of sarcomatoid tumor cells, can be captured with the CellSearch.
Despite these promising results, this study has several limitations. First, MPM cells with no or low podoplanin expression, such as H28 and MSTO‐211H, were not captured with the CTC‐chip conjugated with the antipodoplanin Ab, as expected (Figure 2). Podoplanin is a transmembrane receptor glycoprotein that is highly expressed on cells of mesothelial origin.17 The majority (90%‐100%) of mesotheliomas show positive staining of podoplanin and it is a most useful marker for the pathological diagnosis.18 However, Ordóñez reported that podoplanin expression was negative in all sarcomatoid mesothelioma cases (n = 4) whereas podoplanin expression was positive in the majority (86%, 25/29) of epithelioid mesothelioma cases. In addition, all biphasic mesothelioma cases (n = 4) showed positive podoplanin expression, but podoplanin expression was observed only in the epithelioid component.19 These results could indicate that not all tumor cells circulating in patients with MPM, especially in those with nonepithelioid subtypes, may express podoplanin. The CTC‐chip conjugated with an antipodoplanin Ab principally fails to capture podoplanin‐negative MPM cells. In fact, only a small number of CTCs were detected in some patients with advanced stages (stage IIIB/IV) of disease (Figure 5). In the present study, most tumor cells in tissue sections showed positive podoplanin expression, and a correlation between the number of CTCs detected with the CTC‐chip conjugated with the antipodoplanin Ab and the proportion of podoplanin‐positive tumor cells in tissue sections might not be actually analyzed. To capture such MPM cells, the use of CTC‐chip conjugated with multiple Abs such as an Ab against epidermal growth factor receptor in combination with the antipodoplanin Ab might be promising. Second, the peripheral blood sampled from each patient was immediately applied to the CTC‐chip. However, for use in daily clinical practice, blood samples could be stored in each clinic and be shipped to a laboratory for the CTC‐test. For the CTC‐test with CellSearch, each blood sample can be stabilized and stored in a specialized collection tube (CellSave Preservative Tube) for up to 96 hours at room temperature.6, 7, 11 For the CTC test with the novel CTC‐chip, the optimal pre‐analytical conditions for long‐term storage and shipment shall be explored in future studies. Finally, molecular profiling of CTCs is essential for further clinical applications. For example, expression of PD‐L1 on tumor cells is an important clinical marker to predict the efficacy of antitumor agents inhibiting programmed death 1 and PD‐L1.20 Expression of PD‐L1 on tumor cells can be drastically changed during the clinical course even in the same patient, which could be monitored real‐time as PD‐L1 expression on CTCs. Molecular analyses, such as genotyping, as well as protein expression profiling including PD‐L1 status are now tested for CTCs captured to the CTC‐chip.
In conclusion, the novel CTC‐chip enabled sensitive EpCAM‐independent detection of CTCs in MPM patients, which provided not only a significant diagnostic performance in discrimination of “unresectable” patients but also a significant prognostic value. Future prospective studies are warranted to validate the results.
CONFLICT OF INTEREST
The authors have no conflict of interest.
Supporting information
ACKNOWLEDGMENTS
We thank Eri Kawashima for her valuable technical assistance. This study was supported by Grants‐in‐Aid for Scientific Research (16K10697 and 16H01747) from the Japan Society for the Promotion of Science, by the Kaibara Morikazu Medical Science Promotion Foundation, and by the UOEH Research Grant for Promotion of Occupational Health.
Yoneda K, Kuwata T, Chikaishi Y, et al. Detection of circulating tumor cells with a novel microfluidic system in malignant pleural mesothelioma. Cancer Sci. 2019;110:726–733. 10.1111/cas.13895
Funding information
Japan Society for the Promotion of Science, Grant/Award Number: 16K10697, 16H01747; Kaibara Morikazu Medical Science Promotion Foundation University of Occupational and Environmental Health
REFERENCES
- 1. Alix‐Panabières C, Riethdorf S, Pantel K. Circulating tumor cells and bone marrow micrometastasis. Clin Cancer Res. 2008;14:5013‐5021. [DOI] [PubMed] [Google Scholar]
- 2. Siravegna G, Marsoni S, Siena S, et al. Integrating liquid biopsies into the management of cancer. Nat Rev Clin Oncol. 2017;14:531‐548. [DOI] [PubMed] [Google Scholar]
- 3. Cabel L, Proudhon C, Gortais H, et al. Circulating tumor cells: clinical validity and utility. Int J Clin Oncol. 2017;22:421‐430. [DOI] [PubMed] [Google Scholar]
- 4. Tanaka F, Yoneda K. Adjuvant therapy following surgery in non‐small cell lung cancer (NSCLC). Surg Today. 2016;46:25‐37. [DOI] [PubMed] [Google Scholar]
- 5. Yoneda K, Imanishi N, Ichiki Y, et al. A liquid biopsy in primary lung cancer. Surg Today. 2019;49:1‐14. [DOI] [PubMed] [Google Scholar]
- 6. Allard WJ, Matera J, Miller MC, et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy or patients with nonmalignant diseases. Clin Cancer Res. 2004;10:6897‐6904. [DOI] [PubMed] [Google Scholar]
- 7. Tanaka F, Yoneda K, Kondo N, et al. Circulating tumor cell as a diagnostic marker in primary lung cancer. Clin Cancer Res. 2009;15:6980‐6986. [DOI] [PubMed] [Google Scholar]
- 8. Ismail‐Khan R, Robinson LA, Williams CC Jr, et al. Malignant pleural mesothelioma: a comprehensive review. Cancer Control. 2006;13:255‐263. [DOI] [PubMed] [Google Scholar]
- 9. Tsiouris A, Walesby RK. Malignant pleural mesothelioma: current concepts in treatment. Nat Clin Pract Oncol. 2007;4:344‐352. [DOI] [PubMed] [Google Scholar]
- 10. Lin RT, Takahashi K, Karjalainen A, et al. Ecological association between asbestos‐related diseases and historical asbestos consumption: an international analysis. Lancet. 2007;369:844‐849. [DOI] [PubMed] [Google Scholar]
- 11. Yoneda K, Tanaka F, Kondo N, et al. Circulating tumor cells (CTCs) in malignant pleural mesothelioma (MPM). Ann Surg Oncol. 2014;21(suppl 4):S472‐S480. [DOI] [PubMed] [Google Scholar]
- 12. Ohnaga T, Shimada Y, Moriyama M, et al. Polymeric microfluidic devices exhibiting sufficient capture of cancer cell line for isolation of circulating tumor cells. Biomed Microdevices. 2013;15:611‐616. [DOI] [PubMed] [Google Scholar]
- 13. Chikaishi Y, Yoneda K, Ohnaga T, et al. Capture of EpCAM‐negative mesothelioma cells with a “Universal CTC‐Chip”. Oncol Rep. 2017;37:77‐82. [DOI] [PubMed] [Google Scholar]
- 14. Yoneda K, Chikaishi Y, Kuwata T, et al. Capture of mesothelioma cells with ‘universal’ CTC‐chip. Oncol Lett. 2018;15:2635‐2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rusch VW, Chansky K, Kindler HL, et al. IASLC Staging and Prognostic Factors Committee, advisory boards, and participating institutions. The IASLC mesothelioma staging project: proposals for the M descriptors and for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for mesothelioma. J Thorac Oncol. 2016;11:2112‐2119. [DOI] [PubMed] [Google Scholar]
- 16. Nagrath S, Sequist LV, Maheswaran S, et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature. 2007;450:1235‐1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Krishnan H, Rayes J, Miyashita T, et al. Podoplanin – an emerging cancer biomarker and therapeutic target. Cancer Sci. 2018;109:1292‐1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Husain AN, Colby TV, Ordóñez NG, et al. Guidelines for pathologic diagnosis of malignant mesothelioma: 2017 update of the consensus statement from the International Mesothelioma Interest Group. Arch Pathol Lab Med. 2018;142:89‐108. [DOI] [PubMed] [Google Scholar]
- 19. Ordóñez NG. D2‐40 and podoplanin are highly specific and sensitive immunohistochemical markers of epithelioid malignant mesothelioma. Hum Pathol. 2005;36:372‐380. [DOI] [PubMed] [Google Scholar]
- 20. Yu H, Boyle TA, Zhou C, et al. PD‐L1 expression in lung cancer. J Thorac Oncol. 2016;11:964‐975. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


