Abstract
Thyroid ultrasound screening of young residents in Fukushima Prefecture, Japan, showed a high detection rate of papillary thyroid carcinoma (PTC). Detailed morphological analysis of these tumors was not presented to date. This study sets out to evaluate changes in histopathological and invasive characteristics of Fukushima PTC with time after the nuclear accident of March 2011 in all available cases and in different age subgroups. Histological specimens of 115 PTCs from patients aged 18 years or younger at the time of the Fukushima Dai‐ichi Nuclear Power Plant accident, who underwent surgical resection at Fukushima Medical University during 2012‐2016, were reviewed. Patients were divided into those treated during the first 4 years after the accident (n = 78, shorter‐onset) or later (n = 37, longer‐onset). The whole group and 3 age subgroups: children (aged less than 15 years), adolescents (aged from 15 to less than 19 years), and young adults (aged from 19 years) at surgery were analyzed. No statistically significant time‐related changes in tumor structure or invasiveness were found in the whole group or in age‐matched subgroups. Statistically significant age‐related downtrend was observed for intrathyroid spread in the whole group of patients. The absence of temporal changes in tumor morphological characteristics and tumor invasiveness strongly suggests common etiology of the shorter‐ and longer‐onset Fukushima PTCs, which are unlikely related to the effect of exposure to very low doses of radiation.
Keywords: comparative study, Fukushima nuclear accident, mass screening, papillary thyroid carcinoma, surgical pathology
1. INTRODUCTION
The accident at Fukushima Dai‐ichi Nuclear Power Plant (NPP) occurred in March 2011 after the earthquake and tsunami in Japan, leading to a massive release of radionuclides into the environment. The amount of 131I from the Fukushima accident was about an order of magnitude lower than after the Chernobyl accident1, 2 and radiation doses to the thyroid in populations of affected regions, including children, were very low.3, 4 However, an uncertainty in potential exposure doses during the acute phase of the crisis in Fukushima and the experience of Chernobyl, after which a sharp increase in thyroid cancer incidence in children from the most affected regions was observed,5, 6, 7 led to the launch of the Fukushima Health Management Survey program.8 One of 4 detailed surveys, the Thyroid Ultrasound Examination (TUE) was started in October 2011.9 The aim of TUE was to undertake ultrasound screening of all children and adolescents of Fukushima Prefecture aged 18 years or younger as of April 1, 2011 (367 649 persons).
The first screening cycle, referred to as the “preliminary survey” was accomplished in March 2014, ie, 3 years after possible exposure to radiation, which would be insufficient for radiation effects on the thyroid (if any) to manifest, with the purpose to determine baseline thyroid disease incidence in the Prefecture. The second cycle, or the “first full‐scale survey,” was carried out from April 2014 to March 2016; the third cycle, “second full‐scale survey,” took place from May 2016 to March 2017, and currently the fourth cycle is in progress. The number of patients with malignant or suspected malignant nodules was 116 (among 300 473 examined), 71 (among 270 511), and 12 in the first, second, and third surveys, respectively.10, 11, 12 As of March 2016, of a total of 131 operated and pathologically confirmed thyroid cancer cases, 125 underwent surgery at Fukushima Medical University Hospital (Fukushima, Japan).13 Histopathologically, with a few exceptions, all cases of thyroid cancer in Fukushima were papillary thyroid carcinoma (PTC).
After Chernobyl, approximately 95% of thyroid malignancies were also PTC,14, 15, 16, 17 which displayed significant radiation dose‐dependent risk.18, 19 The increase in incidence of thyroid cancer in children was first registered in 1990, ie, 4 years after the accident,5, 6, 7 which is assumed to be the minimal period of latency after which radiation‐induced thyroid cancer can become detectable. Tumors diagnosed during the 1986‐1989 period are not considered to be related to radiation exposure.
From pathological studies, it is known that PTC detected 4‐5 years after the Chernobyl accident (tumors with the shortest latency) in Belarusian and Ukrainian children frequently had solid and solid‐follicular growth pattern, which is more characteristic for the youngest patients, whereas in the longer‐onset tumors the papillary structures were more common.20, 21, 22 The short‐latency PTCs were also morphologically more aggressive than tumors developing after a longer latency20, 21 or than sporadic PTC in age‐matched groups.15, 22, 23
In view of morphological differences between radiation‐related and sporadic PTC in Chernobyl countries, and its temporal morphological evolution, the objectives of this study were to undertake detailed histopathological analyses of structural components and invasive characteristics of Fukushima PTC to determine whether time‐dependent changes could be seen in the whole group or age subgroups of operated patients, and to evaluate age‐related trends.
2. MATERIALS AND METHODS
2.1. Patients
From 125 patients who underwent surgery at Fukushima Medical University Hospital, 119 cases of PTC were available for review by the authors TB, MI, and YH. Four cases (cribriform‐morular variant of PTC associated with familial adenomatous polyposis) were excluded from analysis, and the remaining 115 PTCs were included in the study. All paraffin sections of tumor and extratumoral tissues, as well as all paraffin sections of dissected lymph nodes stained with H&E for each case were reviewed.
The study was approved by the ethical committees of Fukushima Medical University nos. 1318, 1909, and 29195, which are guided by local policy, national law, and the World Medical Association Declaration of Helsinki. Written informed consent was obtained from all patients or their parents/guardians. Methods used were carried out in accordance with the approved guidelines.
2.2. Histopathology
Histological subtypes/variants of PTC were evaluated according to the 4th edition of the WHO histological classification of thyroid tumors.24 As this classifications indicates that classic (conventional) PTC also includes tumors with mixed structure, and the papillary architecture might not always overly present in those, PTCs under this study were additionally characterized according to the dominant growth pattern as papillary, follicular, and solid‐trabecular when the corresponding structure represented >50% of tumor section. In our opinion, classification by the dominant structural component rather than by histological variant would be more useful here once the particularities of tumor architecture are being addressed.16, 23, 25, 26 Note that the distribution of the dominant growth patterns correlates but does not coincide with that of histological PTC variants, which are important for tumor prognostication and long‐term management (see Table 1 for descriptive characteristics of cases in the study).
Table 1.
Descriptive clinicopathological characteristics of 115 cases of papillary thyroid carcinoma (PTC) detected after the nuclear accident of March 2011 in Fukushima, Japan
| Characteristics | Number or value | % or SD |
|---|---|---|
| Diagnosis time after accident | ||
| <4 y | 78 | 67.8 |
| ≥4 y | 37 | 32.2 |
| Age (range), y | 17.8 (9‐23) | 3.0a |
| <15 | 15 | 13.0 |
| ≥15‐<19 | 44 | 38.3 |
| ≥19 | 56 | 48.7 |
| Sex F/M (F:M ratio) | 73/42 (1.7:1) | 63.5 (F) |
| Tumor size (range), mm | 14.8 (6‐51) | 8.7a |
| ≤10 | 35 | 30.4 |
| >10‐≤20 | 61 | 53.0 |
| >20‐≤40 | 17 | 14.8 |
| >40 | 2 | 1.7 |
| pT (7th edition) | ||
| T1a | 20 | 17.4 |
| T1b | 41 | 35.7 |
| T2 | 5 | 4.3 |
| T3 | 49 | 42.6 |
| Histological variantb | ||
| Classic (conventional) papillary | 104 | 90.5 |
| Follicular | 5 | 4.3 |
| Solid | 4 | 3.5 |
| Diffuse sclerosing | 2 | 1.7 |
| Dominant growth pattern | ||
| Papillary | 81 | 70.5 |
| Follicular | 19 | 16.5 |
| Solid‐trabecular | 15 | 13.0 |
| Complete tumor capsule | 4c | 3.5 |
| Oxyphilic changes | 53 | 46.1 |
| Focal | 26 | 22.6 |
| Severe | 27 | 23.5 |
| Concomitant thyroid pathology | 47 | 39.2 |
| Nodular | 10 | 8.7 |
| Diffuse | 35 | 30.4 |
| Chronic thyroiditis | 33 | 28.7 |
| Intrathyroid spread | 56 | 48.7 |
| Extrathyroidal extension | 48 | 42.1 |
| Multifocality | 2 | 1.7 |
| Lymphatic/vascular invasion | 84 | 73.0 |
| Lymph node metastasis | 92 | 80.0 |
| N1a | 73 | 63.5 |
| N1b | 19 | 16.5 |
| Distant metastasis | 3 | 2.6 |
Standard deviation.
The numbers are specified according to the 4th edition of the WHO histological classification of thyroid tumors24 and may slightly differ from those reported previously according to the 7th edition of the Japanese general rules for the description of thyroid cancer.8, 13
All fully encapsulated PTCs were in adult patients aged ≥19 years at surgery
F, female; M, male.
Oxyphilic cell metaplasia was evaluated as focal or severe. Tumor stage was determined according to the 7th edition of TNM classification system, which assigns minimal extrathyroidal extension to the fat and connective tissue to the pT3 category.27 Only marked intrathyroid spread to the lobe(s) was considered positive, including diffuse sclerosing variant (DSV)‐like spread. For clearer identification of lymphatic/vascular invasion, sections were stained histochemically (Van Gieson staining) to visualize elastic lamina, or immunohistochemically for CD34 (blood vessel endothelium) and D2‐40 (lymphatic vessel endothelium). Tumor invasiveness was defined as morphological evidence of intrathyroid spread, extrathyroidal extension, tumor multifocality, lymphatic/vascular invasion, or metastatic process, either alone or in combination.
All cases were divided into 2 main groups to be compared: those operated during the period less than 4 years after the Fukushima NPP accident and those operated 4‐5 years after the accident (78 and 37 cases, respectively). We further considered subgroups of patients according to their age at surgery: children (patients aged less than 15 years, 9 and 6 cases, respectively); adolescents (patients aged from 15 to less than 19 years, 29 and 15 cases, respectively; pediatric cases (children and adolescents combined, 38 and 21 cases, respectively); and adults (patients aged 19 years or more at surgery (40 and 16 cases, respectively). Analyses were carried out for all cases in the subgroups, and for partially and nonencapsulated tumors combined, as fully encapsulated PTC could display less pronounced invasive features.14, 23 Encapsulated tumors in this series were observed only in adult patients.
2.3. Statistical analysis
Fisher's exact test for categorical data and Mann‐Whitney or Kruskal‐Wallis tests for quantitative measurements were used for univariate analysis. Multivariate logistic regression models were adjusted for age at surgery and sex. Analyses with very small numbers of outcomes (less than 5 per cell) or when quasi‐complete separation was observed were carried out using Firth's approach to bias‐reducing penalized maximum likelihood fit or exact logistic regression. Changes in characteristics across age groups were evaluated with the χ2 test (Cochran‐Armitage test) for trend for categorical and Jonckheere‐Terpstra test for quantitative data. Heterogeneity of age trends was evaluated using one degree of freedom likelihood ratio test for nested logistic regression models with and without an interaction term between onset indicator and age. Statistical assessments were undertaken using the 3.71 release of SAS Studio for the 9.4M5 version of SAS (SAS Institute, Cary, NC, USA) and/or IBM SPSS Statistics 24 software (IBM, Armonk, NY, USA). All tests were 2‐sided; P < .05 was considered statistically significant.
3. RESULTS
3.1. Clinicopathological characteristics of the whole PTC series
Mean age at surgery of patients diagnosed during the first 4 years after the Fukushima Dai‐ichi NPP accident (the earlier‐onset group) was 17.8 years, which was not different from the age of patients diagnosed later (the later‐onset group), 17.7 years old (Table 2 and Figure 1). Most patients were female in both groups (61.5% and 67.6%, respectively); sex ratio did not vary significantly between the groups. Most tumors were nonencapsulated or partially encapsulated (111/115, 96.5%). Four fully encapsulated PTCs (3.5%) occurred in adult patients only in the earlier‐onset group.
Table 2.
Clinicopathological characteristics of papillary thyroid carcinomas PTCs detected by ultrasound screening during the first 4 years or later after the Fukushima Nuclear Power Plant accident, all age groups
| Period after the accident | <4 y | ≥4 y | P value (uni)a | OR (95% CI) | P value (multi)b | ||
|---|---|---|---|---|---|---|---|
| Characteristics | n or value | % or SD | n or value | % or SD | |||
| Number of cases | 78 | 67.8 | 37 | 32.2 | |||
| Nonencapsulated | 74 | 64.3 | 37 | 32.2 | |||
| Tumor capsule | 4 | 5.1 | 0 | 0.0 | .304 | 0.21 (0.01‐5.71) | .356 |
| Sex (F:M ratio) | 48/30 (1.6:1) | 61.5 (F) | 25/12 (2.1:1) | 67.6 (F) | .679 | 1.28 (0.56‐2.92) | .551c |
| Nonencapsulated (F:M ratio) | 45/29 (1.6:1) | 60.8 (F) | 25/12 (2.1:1) | 67.6 (F) | .537 | 1.32 (0.58‐3.02) | .512 |
| Age, mean (range); years | 17.8 (9‐22) | 2.8 | 17.7 (10‐23) | 3.4 | .993 | 0.99 (0.87‐1.13) | .859d |
| Nonencapsulated | 17.7 (9‐22) | 2.8 | 17.7 (10‐23) | 3.4 | .828 | 1.00 (0.88‐1.14) | .982 |
| Tumor size, mean (range); mm | 15.1 (6‐41) | 8.2 | 14.2 (6‐51) | 9.6 | .188 | 0.99 (0.94‐1.04) | .678 |
| Nonencapsulated | 15.1 (6‐41) | 8.1 | 14.4 (6‐51) | 9.6 | .184 | 0.99 (0.94‐1.04) | .695 |
| Tumor size ≤10 mm | 22 | 28.2 | 13 | 35.1 | .517 | 1.41 (0.60‐3.28) | .432 |
| Nonencapsulated | 21 | 28.4 | 13 | 35.1 | .516 | 1.38 (0.59‐3.24) | .465 |
| Dominant growth pattern | |||||||
| Papillary | 52 | 66.7 | 29 | 78.4 | .274 | 1.82 (0.72‐4.56) | .204 |
| Nonencapsulated | 49 | 66.2 | 29 | 78.4 | .271 | 1.85 (0.73‐4.67) | .194 |
| Follicular | 13 | 16.7 | 6 | 16.2 | 1.000 | 0.99 (0.34‐2.89) | .978 |
| Nonencapsulated | 13 | 17.6 | 6 | 16.2 | 1.000 | 0.95 (0.32‐2.77) | .917 |
| Solid‐trabecular | 13 | 16.7 | 2 | 5.4 | .138 | 0.33 (0.08‐1.42) | .137 |
| Nonencapsulated | 12 | 16.2 | 2 | 5.4 | .136 | 0.33 (0.08‐1.44) | .141 |
| Oxyphilic changes | |||||||
| Focal | 18 | 23.1 | 8 | 21.6 | 1.000 | 0.93 (0.36‐2.39) | .884 |
| Nonencapsulated | 18 | 24.3 | 8 | 21.6 | .816 | 0.88 (0.34‐2.26) | .787 |
| Severe | 20 | 25.6 | 7 | 18.9 | .488 | 0.73 (0.28‐1.93) | .526 |
| Nonencapsulated | 19 | 25.7 | 7 | 18.9 | .484 | 0.72 (0.27‐1.91) | .506 |
| Concomitant thyroid pathology | |||||||
| Nodular | 8 | 10.3 | 2 | 5.4 | .497 | 0.53 (0.11‐2.53) | .428 |
| Nonencapsulated | 8 | 10.8 | 2 | 5.4 | .491 | 0.49 (0.10‐2.32) | .367 |
| Diffuse | 22 | 28.2 | 13 | 35.1 | .517 | 1.31 (0.55‐3.13) | .542 |
| Nonencapsulated | 22 | 29.7 | 13 | 35.1 | .665 | 1.22 (0.51‐2.92) | .657 |
| Chronic thyroiditis | 20 | 25.6 | 13 | 35.1 | .378 | 1.50 (0.61‐3.66) | .378 |
| Nonencapsulated | 20 | 27.0 | 13 | 35.1 | .388 | 1.39 (0.57‐3.41) | .473 |
| Invasiveness | |||||||
| Intrathyroid spread | 36 | 46.2 | 20 | 54.1 | .549 | 1.39 (0.61‐3.17) | .427 |
| Nonencapsulated | 36 | 48.6 | 20 | 54.1 | .688 | 1.28 (0.56‐2.91) | .556 |
| Extrathyroidal extension | 34 | 43.6 | 14 | 37.8 | .687 | 0.81 (0.36‐1.83) | .616 |
| Nonencapsulated | 34 | 45.9 | 14 | 37.8 | .542 | 0.75 (0.33‐1.69) | .484 |
| Multifocality (Tm) | 0 | 0.0 | 2 | 5.4 | .102 | 11.35 (0.27‐479.24) | .116 |
| Nonencapsulated | 0 | 0.0 | 2 | 5.4 | .109 | 10.86 (0.26‐459.06) | .122 |
| Lymphatic/vascular invasion | 56 | 71.8 | 28 | 75.7 | .823 | 1.7 | .754 |
| Nonencapsulated | 55 | 74.3 | 28 | 75.7 | 1.000 | 1.02 (0.41‐2.58) | .959 |
| Lymph node metastases (N1) | 61 | 78.2 | 31 | 83.8 | .620 | 1.53 (0.54‐4.34) | .427 |
| Nonencapsulated | 60 | 81.1 | 31 | 83.8 | .799 | 1.30 (0.44‐3.82) | .636 |
| Distant metastases (M1) | 3 | 3.8 | 0 | 0.0 | .550 | 0.30 (0.01‐9.34) | .493 |
| Nonencapsulated | 3 | 4.1 | 0 | 0.0 | .549 | 0.29 (0.01‐9.06) | .482 |
Univariate statistical tests.
Adjusted for sex and age unless otherwise specified.
Adjusted for age.
Adjusted for sex.
CI, confidence interval; F, female; M, male; OR, odds ratio.
Figure 1.
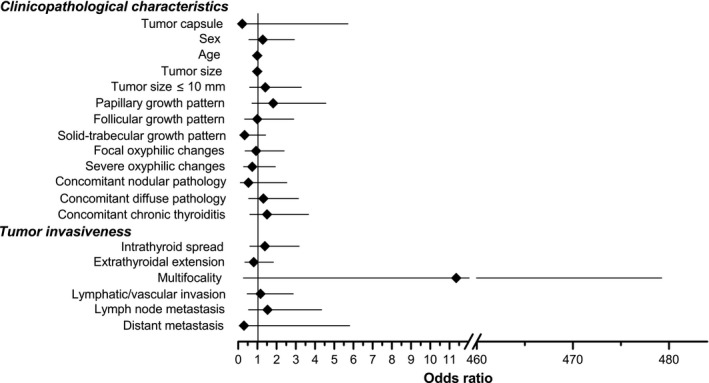
Statistical comparison of the earlier‐ and later‐onset papillary thyroid carcinomas detected during thyroid ultrasound examinations of young individuals in Fukushima Prefecture following the nuclear accident of March 2011. All cases (ie, nonencapsulated, partially encapsulated, and fully encapsulated) are shown. Characteristics of the earlier‐onset tumors were used as a reference. Plotted are odds ratios and their 95% confidence intervals obtained in multivariate logistic regression analyses
Mean tumor size did not change significantly with time after the accident in the whole group (15.1 vs 14.2 mm, P = .678). The proportion of tumors sized 10.0 mm or more increased with time from 28.2% to 35.1% but statistical significance was not reached (P = .432). Of importance, Fukushima micro‐PTCs were not indolent microcarcinomas as both in the earlier‐ and later‐onset groups these tumors displayed extrathyroidal extension in 40.9% and 46.2% (P = 1.000) and nodal disease in 77.3% and 92.3%, respectively (P = .377).
The distribution of dominant histological growth patterns did not show statistically significant changes over time, although a relative increase in the frequency of the papillary (from 66.7% to 78.4%, P = .204) and a decrease in the solid‐trabecular patterns (from 16.7% to 5.4%, P = .137) were seen. Among histological variants, the classic (conventional) PTC was the most prevalent (90.5% overall, Table 1) without significant differences between the earlier‐ and later‐onset groups (92.3% and 86.5%, respectively, P = .328). Histological variants such as follicular, solid, and DSV occurred in a minority cases.
Oxyphilic cell metaplasia was observed in 46.1% of cases cumulatively; no significant difference over time was detected (P = .884 and P = .526 for focal and severe changes, respectively, Table 2). Also, no differences between the 2 groups were observed for the presence of concomitant benign nodular thyroid pathology (10.3% vs 5.4%, P = .428) or chronic thyroiditis (25.6% vs 35.1%, P = .378).
Of importance, no differences in the frequencies of tumor invasive features such as intraglandular spread (P = .427), extrathyroidal extension (P = .616), lymphatic/vascular invasion (P = .754), and the presence of regional lymph node (P = .427) or distant metastases to the lung (P = .493) were found between the earlier‐ and later‐onset group (Figure 1 and Table 2).
The results of analyses of partially/nonencapsulated tumors were similar to those obtained for the whole group. As for fully encapsulated tumors, 3 out of 4 PTCs showed dominant papillary growth pattern and tumor capsule invasion. Only 1 fully encapsulated PTC was noninvasive. This tumor, however, had dominant solid growth pattern with severe oxyphilic cell metaplasia, not meeting the criteria for a noninvasive follicular thyroid neoplasm with papillary‐like nuclear features.24, 28 The size of fully encapsulated PTCs ranged from 6.7 to 32.6 mm, mean 15.8 mm, which was not significantly different from the nonencapsulated tumors for the whole series (14.8 mm, P = .945) or for young adult patients (13.6 mm, P = .874). Only 1 encapsulated PTC with dominant papillary growth pattern, capsular and vascular invasion, and lymph node metastases was sized less than 10 mm.
3.2. Analysis in age subgroups
Nearly identical results were obtained in separate analyses of age subgroups of children (Table S1, Figure S1), adolescents (Table S2, Figure S1), a combined children and adolescent group (the pediatric group, Table S3, Figure S1), and young adults (Table S4, Figure S1).
In the childhood subgroup (Table S1), mean tumor size appeared to be greater in the later‐onset PTCs, although not statistically significantly (14.8 vs 22.0 mm, P = .430). A possible explanation is that 1 tumor removed 4 years after the accident was a DSV characterized by numerous small tumor foci scattered throughout the thyroid gland. Tumor size in this case is the size of the greatest thyroid lobe, which was 35 mm, contributing to the mean size increase.
In adolescents (Table S2), a significant decrease in mean tumor size was observed over time on univariate (17.4 vs 11.2 mm, P = .007), but not on multivariate analysis (P = .061). This was paralleled by the greater frequency of small tumors sized 10 mm or less in the later‐onset PTCs (24.1% vs 60.0%, P = .026 on univariate and P = .059 on multivariate analysis, Table S2), which remained marginally significant in the combined pediatric subgroup on multivariate analysis (P = .056, Table S3).
In the adult subgroup, no significant changes in tumor size or frequency of small tumors were detected. The distribution of dominant growth patterns in adult patients displayed a tendency toward the increase in the frequency of tumors with dominant papillary structure and the decrease in the frequency of tumors with dominant solid‐trabecular patterns with time, which was seen on univariate statistical analysis but not in multivariate models (Table S4).
No significant changes in the frequencies of oxyphilic cell metaplasia, any type of concomitant thyroid pathology and, importantly, of invasive features were found in any age subgroup (Tables [Link], [Link], [Link], Figure S1).
3.3. Age‐related trends of histopathological characteristics of PTC
The availability of 3 age subgroups in the study enabled trend analysis. Several morphologic tumor characteristics were found to vary with age in the entire series, as well as in the earlier‐ and later‐onset groups (Table 3).
Table 3.
Age‐related trends for papillary thyroid carcinomas detected by ultrasound screening in Fukushima Prefecture, Japan, after the nuclear power plant accident
| Characteristics | All cases | <4 y | >4 y | P heterogeneity | |||
|---|---|---|---|---|---|---|---|
| P trend | Direction | P trend | Direction | P trend | Direction | ||
| Tumor capsule | .063 | ↑a | .073 | ↑ | ND | ND | |
| Sex (F:M ratio) | .778 | .979 | .717 | .796 | |||
| Nonencapsulated | .710 | .886 | .717 | .845 | |||
| Tumor size, mean | .024 | ↓b | .026 | ↓ | .502 | .751 | |
| Nonencapsulated | .021 | ↓ | .026 | ↓ | .502 | .766 | |
| Tumor size ≤10 mm | .060 | ↑ | .023 | ↑ | .819 | .083 | |
| Nonencapsulated | .052 | ↑ | .019 | ↑ | .819 | .084 | |
| Dominant growth pattern | |||||||
| Papillary | .364 | 0.562 | .005 | ↑ | .034 | ||
| Nonencapsulated | .481 | .506 | .005 | ↑ | .028 | ||
| Follicular | .088 | ↓ | .608 | .027 | ↓ | .291 | |
| Nonencapsulated | .217 | .744 | .027 | ↓ | .239 | ||
| Solid‐trabecular | .515 | .212 | .126 | .174 | |||
| Nonencapsulated | .671 | .233 | .126 | .176 | |||
| Oxyphilic changes | |||||||
| Focal | .176 | .106 | .930 | .432 | |||
| Nonencapsulated | .244 | .164 | .930 | .507 | |||
| Severe | .171 | .441 | .227 | .590 | |||
| Nonencapsulated | .168 | .428 | .227 | .597 | |||
| Concomitant thyroid pathology | |||||||
| Nodular | .222 | .127 | .591 | .201 | |||
| Nonencapsulated | .210 | .096 | ↑ | .591 | .188 | ||
| Diffuse | .062 | ↓ | .036 | ↓ | .809 | .313 | |
| Nonencapsulated | .101 | .065 | ↓ | .809 | .388 | ||
| Chronic thyroiditis | .047 | ↓ | .025 | ↓ | .809 | .268 | |
| Nonencapsulated | .077 | ↓ | .046 | ↓ | .809 | .333 | |
| Invasiveness | |||||||
| Intrathyroid spread | .008 | ↓ | .006 | ↓ | .527 | .332 | |
| Nonencapsulated | .020 | ↓ | .017 | ↓ | .527 | .421 | |
| Extrathyroidal extension | .065 | ↓ | .245 | .102 | .491 | ||
| Nonencapsulated | .120 | .419 | .102 | .386 | |||
| Multifocality (Tm) | .470 | NDc | .591 | ND | |||
| Nonencapsulated | .500 | ND | .591 | ND | |||
| Lymphatic/vascular invasion | .238 | .235 | .766 | .688 | |||
| Nonencapsulated | .408 | .428 | .766 | .838 | |||
| Lymph node metastases (N1) | .466 | .923 | .110 | .288 | |||
| Nonencapsulated | .199 | .636 | .110 | .415 | |||
| Distant metastases (M1) | .374 | .309 | ND | .871 | |||
| Nonencapsulated | .406 | .352 | ND | .901 | |||
An uptrend (frequency or value increases with age), indicated for P trend <.1.
A downtrend (frequency or value decreases with age), indicated for P trend <.1.
F, female; M, male; ND, not determined; values in bold indicate statistical significance.
In the whole group, a statistically significant trend towards the decrease in mean tumor size (both all cases and nonencapsulated PTC) was seen (P trend = .024 and P trend = .021, respectively). Age‐related downtrends were also found for concomitant chronic thyroiditis (P trend = .047) and intrathyroid spread, whose frequency declined in all cases (P trend = .008) and nonencapsulated PTCs (P trend = .020). No significant age‐related trends were detected for other histopathological characteristics.
Among PTCs removed less than 4 years after the accident (earlier‐onset group), similar significant downtrends were observed for the above parameters. In addition, there was an uptrend for PTC sized 10 mm or more (both all cases and nonencapsulated, P trend = .023 and P trend = .019, respectively).
In the later‐onset group, significant age trends were observed only for the dominant growth patterns: an uptrend for dominant papillary (P trend = .005 for both all and nonencapsulated PTCs) and downtrend for dominant follicular growth patterns (P trend = .027 for both all and nonencapsulated PTCs).
Age‐related trends for most histopathological characteristics did not show heterogeneity between earlier‐ and later‐onset groups (Table 3). The increase in frequency of the dominant papillary growth pattern for all and nonencapsulated PTCs (P het = .034 and P het = .028, respectively) were the only trends that were more pronounced in the later‐ than in earlier‐onset PTC.
4. DISCUSSION
In this work, we examined temporal patterns of histopathological changes of Fukushima PTC bearing in mind that radiation‐related short‐latency PTC detected 4‐5 years after the Chernobyl accident was characterized by the high frequency of solid and solid‐follicular growth patterns and morphological features of higher aggressiveness in comparison with sporadic PTC in age‐matched groups.15, 22, 23
Our analyses showed that most characteristics of PTC did not change significantly over time since the Fukushima NPP accident (see Figure 1). The only meaningful findings, complementing each other, were the decrease in tumor size and the increase in frequency of small PTCs measuring 10 mm or less in the later‐onset tumors in adolescents, seen on univariate statistical analysis. Similar changes, however, were not observed in childhood or in adult patients’ subgroups.
An increase in the frequency of micro‐PTCs after repeated screening examination has been reported in the Belarusian‐American and Ukrainian‐American Chernobyl cohorts,25, 29 ascribed to active health surveillance. In our opinion, a somewhat increased detection rate of small‐size tumors in Fukushima TUE in adolescents in the later‐onset group could also be explained, at least in part, by the repeated screening. Further screenings will probably shed light if similar changes would be seen in other age subgroups.
It is important to emphasize that micro‐PTCs in this study were not incidental “occult” tumors just because they were detected by ultrasound in asymptomatic patients. The high rates of extrathyroidal extension to the fat and connective tissue and nodal disease attest to the justified treatment tactics that included surgery in view of the patients’ young age and the risk of rapid progression of tumors with such invasive characteristics.30, 31, 32, 33
It should be noted that in 2017, the criteria for ascribing well‐differentiated thyroid carcinomas pT3 category were revised in the 8th edition of the TNM classification.34 According to this classification, tumors larger than 40 mm confined to the thyroid belong to the pT3a category. The new pT3b category includes tumors of any size, but only if there are signs of tumor extension to the muscle surrounding the thyroid. In the series under study, no tumors extending to the muscle (pT3b = 0) and only 2 PTCs larger than 40 mm (pT3a = 2, tumors sized 40.5 and 51 mm) were observed. Consequently, if the 8th edition of the TNM classification was applied, 47 out of 49 PTCs would migrate from the pT3 to pT1a, pT1b, and pT2 categories. However, in our opinion, even the minimal extrathyroidal extension deserved attention in the analysis of PTC invasiveness undertaken in this study.
We did not observe significant changes in mean tumor size in the whole series, which was 15.1 and 14.2 mm in the earlier‐ and later‐onset PTC. Noteworthy nevertheless, that it was substantially smaller than that of sporadic PTCs from young patients from Japan. In previous studies, mean tumor size was 25.0 mm in children younger than 15 years at operation, and 18.0 mm in adolescents aged 15 years or older at surgery.35 Another study reported sizes of 24.0 mm in children younger than 15 years at surgery and 21.9 mm in adolescents aged 15‐18 years at surgery.26 We explain these observations by the screening in Fukushima as all previous works involved tumors from nonscreened individuals. In addition, it appears that before the NPP accident, thyroid cancers in young patients were usually detected at a more advanced stage. In this regard, a comparative histopathological analysis of Fukushima PTC and age‐matched sporadic PTC from Japan would be useful.
Mean tumor size tended to decrease with age in patients from Fukushima (see Table 3). In our previous study of mutational profile of Fukushima PTC, the most prevalent BRAF V600E mutation (found in 63.2% of cases) was positively correlated with patients’ age, ie, displaying an increase in prevalence from children to adults.36, 37 Furthermore, BRAF V600E was significantly associated with a smaller tumor size and a higher chance of occurring in micro‐PTC. On the contrary, gene rearrangements (combined with unknown genetic alteration) were more prevalent in the tumors of younger patients and associated with larger tumor size. These observations underscore age‐related difference in the molecular pathogenesis of PTC and indicate that BRAF V600E‐positive tumors might grow more slowly than those harboring other types of activated oncogenes (or even stop growing). Future molecular analysis, currently underway, is expected to reveal whether there is a shift from point mutations to gene rearrangements in the tumors operated 4‐5 years and on after the accident if a scenario of radiation‐related carcinogenesis similar to that in Chernobyl would be taking place. According to our histopathological analysis, however, the frequency of PTCs with the dominant papillary growth pattern remained prevalent in all subgroups regardless of time since the accident. It was even increased in young adults who underwent surgery 4‐5 years after the accident, whereas the frequency of tumors with the solid‐trabecular structure decreased (Table S4) in contrast to Chernobyl PTCs. These observations suggest that the “radiation nature” of later‐onset tumors in Fukushima residents would be unlikely.
Morphological features of tumor aggressiveness, such as intrathyroid spread, extrathyroidal extension, lymphatic/vascular invasion, and frequency of regional lymph node metastases, did not change in the whole series or in age‐matched subgroups. This is also at variance with findings in Chernobyl, where a significant difference in tumor aggressiveness was seen between radiation‐related and sporadic PTC in age‐matched groups.15, 22 These facts again advocate a similar etiology of the earlier‐ and later‐onset PTC in Fukushima.
Our analysis of age trends detected a significant decrease in the frequency of intrathyroid spread (in all PTCs and earlier‐onset group). In general, tumor aggressiveness at presentation is known to decline with patients’ age, both in radiogenic and sporadic PTC.22, 23 This might point at the common mechanisms underlying lesser tumor aggressiveness in older patients regardless of etiology; these may involve, eg, difference from younger patients’ spectrum of somatic mutations driving carcinogenesis.
We acknowledge that our study has some limitations, primarily due to the inherent characteristics of the studied group of patients. In particular, the group of children included a small number of subjects. Furthermore, while the number of childhood cases in the later‐onset group could increase (3 additional patients aged less than 15 years were diagnosed in the second full‐scale survey),12 the number of earlier‐onset tumors will not. The same consideration applies to all other age subgroups described in the study. This circumstance, however, does not impede statistical analysis, although due to insufficient statistical power some subtle changes, perhaps, could not be revealed. However, our results show that no strong effects corresponding to morphological changes could be expected on comparison of the earlier‐ and later‐onset tumors either in individual age subgroups or in the combined pediatric or the whole group.
In conclusion, our study shows that PTCs detected in Fukushima Prefecture during mass ultrasound thyroid screening of young individuals do not display specific qualitative changes in tumor histopathology or morphological features of tumor aggressiveness over time. The similarity of pathological characteristics between tumors removed within 4 years after the Fukushima Dai‐ichi NPP accident and 4‐5 years after it strongly suggests their common etiology, which is unlikely related to radiation.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
ACKNOWLEDGMENTS
We acknowledge the commitment of the staff of Fukushima Medical University's Department of Diagnostic Pathology, who prepared all pathological material for the study, and of the Department of Thyroid Endocrinology, who operated on the patients. We also gratefully acknowledge the confirmation of diagnoses provided by the members of the Japanese Pathology Panel: A. Sakamoto (Chairman), M. Hirokawa, M. Ito, and H. Naganuma. This work was supported in part by KAKENHI Grant Number 16H02774 from the Japan Society for the Promotion of Science and H29‐Gantaisaku‐006 Grant from The Ministry of Health, Labour and Welfare of the Government of Japan.
Suzuki S, Bogdanova TI, Saenko VA, et al. Histopathological analysis of papillary thyroid carcinoma detected during ultrasound screening examinations in Fukushima. Cancer Sci. 2019;110:817–827. 10.1111/cas.13912
Suzuki, Bogdanova, and Saenko contributed equally to this work.
Contributor Information
Shinichi Suzuki, Email: shsuzuki@fmu.ac.jp.
Vladimir A. Saenko, Email: saenko@nagasaki-u.ac.jp.
REFERENCES
- 1. United Nations Scientific Committee on the Effects of Atomic Radiation . UNSCEAR 2000 Report to the General Assembly, with scientific annexes. Volume II, Annex J: Exposures and effects of the Chernobyl accident. 2000. http://www.unscear.org/docs/publications/2000/UNSCEAR_2000_Annex-J.pdf. Accessed July 26, 2018.
- 2. United Nations Scientific Committee on the Effects of Atomic Radiation: Sources and effects of ionizing radiation. UNSCEAR 2013 Report to the General Assembly with scientific annexes. Volume I, Annex A: Levels and effects of radiation exposure due to the nuclear accident after the 2011 Great east‐Japan earthquake and tsunami. 2014. http://www.unscear.org/docs/reports/2013/13-85418_Report_2013_Annex_A.pdf. Accessed July 26, 2018.
- 3. Nagataki S, Takamura N, Kamiya K, et al. Measurements of individual radiation doses in residents living around the Fukushima Nuclear Power Plant. Radiat Res. 2013;180:439‐447. [DOI] [PubMed] [Google Scholar]
- 4. Nagataki S, Takamura N. Radioactive doses – predicted and actual – and likely health effects. Clin Oncol. 2016;28:245‐254. [DOI] [PubMed] [Google Scholar]
- 5. Jacob P, Bogdanova TI, Buglova E, et al. Thyroid cancer among Ukrainians and Belarusians who were children or adolescents at the time of the Chernobyl accident. J Radiol Prot. 2006;26:51‐67. [DOI] [PubMed] [Google Scholar]
- 6. Kazakov VS, Demidchik EP, Astakhova LN. Thyroid cancer after Chernobyl. Nature. 1992;359:21. [DOI] [PubMed] [Google Scholar]
- 7. Likhtarev IA, Sobolev BG, Kairo IA, et al. Thyroid cancer in the Ukraine. Nature. 1995;375:365. [DOI] [PubMed] [Google Scholar]
- 8. Yamashita S, Suzuki S, Suzuki S, et al. Lessons from Fukushima: latest findings of thyroid cancer after the Fukushima nuclear power plant accident. Thyroid. 2018;28:11‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Suzuki S, Yamashita S, Fukushima T, et al. The protocol and preliminary baseline survey results of the thyroid ultrasound examination in Fukushima [Rapid Communication]. Endocr J. 2016;63:315‐321. [DOI] [PubMed] [Google Scholar]
- 10. Radiation Medical Science Center FMU . Thyroid Ultrasound Examination (Preliminary Baseline Screening). Supplemental Report of the FY 2016 Survey. 2017. http://fmu-global.jp/download/thyroid-ultrasound-examination-first-full-scale-thyroid-screening-program-5/?wpdmdl=2690. Accessed July 26, 2018.
- 11. Radiation Medical Science Center FMU . Report of Second‐Round Thyroid Ultrasound Examinations (First Full‐Scale Thyroid Screening Program). 2017. http://fmu-global.jp/download/thyroid-ultrasound-examination-first-full-scale-thyroid-screening-program-5/?wpdmdl=2692. Accessed July 26, 2018.
- 12. Radiation Medical Science Center FMU . Report of Third‐Round Thyroid Ultrasound Examinations (Second Full‐Scale Thyroid Screening Program). 2018. http://fmu-global.jp/download/31st_report-of-third-round-thyroid-ultrasound-examinations/?wpdmdl=4375. Accessed July 26, 2018.
- 13. Suzuki S. The features of childhood and adolescent thyroid cancer after Fukushima nuclear power plant accident In: Yamashita S, Thomas G, eds. Thyroid Cancer and Nuclear Accidents Long‐term After Effects of Chernobyl and Fukushima. London, UK: Academic Press, Elsevier Inc.; 2017:155‐163. [Google Scholar]
- 14. Bogdanova T, Zurnadzhy L, LiVolsi VA, et al. Thyroid cancer pathology in Ukraine after Chernobyl In: Tronko M, Bogdanova T, Saenko V, et al., eds. Thyroid Cancer in Ukraine After Chernobyl Dosimetry, Epidemiology, Pathology, Molecular Biology. Nagasaki: IN‐TEX; 2014:65‐108. [Google Scholar]
- 15. Fridman M, Lam AK, Krasko O, et al. Morphological and clinical presentation of papillary thyroid carcinoma in children and adolescents of Belarus: the influence of radiation exposure and the source of irradiation. Exp Mol Pathol. 2015;98:527‐531. [DOI] [PubMed] [Google Scholar]
- 16. Bogdanova T, Saenko V, Shpak V, et al. Long‐term analysis of the incidence and histopathology of thyroid cancer in Ukraine in adult patients who were children and adolescents at the time of the Chernobyl accident In Yamashita S, Thomas G, eds. Thyroid Cancer and Nuclear Accidents Long term After Effects of Chernobyl and Fukushima. London, UK: Academic Press, Elsevier Inc.; 2017:67‐76. [Google Scholar]
- 17. Demidchik YE, Fridman MV, Mankovskaya S, et al. Post‐Chernobyl pediatric papillary thyroid carcinoma in Belarus: histopathological features, treatment strategy, and long‐term outcome In: Yamashita S, Thomas G, eds. Thyroid Cancer and Nuclear Accidents Long term After Effects of Chernobyl and Fukushima. London, UK: Academic Press, Elsevier Inc.; 2017:49‐58. [Google Scholar]
- 18. Brenner AV, Tronko MD, Hatch M, et al. I‐131 dose response for incident thyroid cancers in Ukraine related to the Chornobyl accident. Environ Health Perspect. 2011;119:933‐939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tronko M, Brenner AV, Bogdanova T, et al. Thyroid neoplasia risk is increased nearly 30 years after the Chernobyl accident. Int J Cancer. 2017;141:1585‐1588. [DOI] [PubMed] [Google Scholar]
- 20. Williams ED, Abrosimov A, Bogdanova T, et al. Thyroid carcinoma after Chernobyl latent period, morphology and aggressiveness. Br J Cancer. 2004;90:2219‐2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Williams D. Twenty years’ experience with post‐Chernobyl thyroid cancer. Best Pract Res Clin Endocrinol Metab. 2008;22:1061‐1073. [DOI] [PubMed] [Google Scholar]
- 22. Bogdanova T, Saenko V, Zurnadzhy L, et al. Comparative pathological analysis of papillary thyroid carcinoma in age‐matched groups of patients born before and after Chernobyl In: Tronko M, Bogdanova T, Saenko V, et al., eds. Thyroid Cancer in Ukraine After Chernobyl Dosimetry, Epidemiology, Pathology, Molecular Biology. Nagasaki, Japan: IN‐TEX; 2014:109‐135. [Google Scholar]
- 23. Bogdanova TI, Saenko VA, Brenner AV, et al. Comparative histopathologic analysis of “radiogenic” and “sporadic” papillary thyroid carcinoma: patients born before and after the Chernobyl accident. Thyroid. 2018;28:880‐890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lloyd RV, Osamura RY, Kloppel G, et al. WHO Classification of Tumours of Endocrine Organs, 4th edn Lyon, France: IARC Press; 2017. [Google Scholar]
- 25. Bogdanova TI, Zurnadzhy LY, Nikiforov YE, et al. Histopathological features of papillary thyroid carcinomas detected during four screening examinations of a Ukrainian‐American cohort. Br J Cancer. 2015;113:1556‐1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bogdanova TI, Saenko VA, Hirokawa M, et al. Comparative histopathological analysis of sporadic pediatric papillary thyroid carcinoma from Japan and Ukraine. Endocr J. 2017;64:977‐993. [DOI] [PubMed] [Google Scholar]
- 27. Sobin LH, Gospodarowicz MK, Wittekind C, et al. TNM Classification of Malignant Tumours, 7th edn Hoboken, NJ: Wiley‐Blackwell; 2010. [Google Scholar]
- 28. Nikiforov YE, Seethala RR, Tallini G, et al. Nomenclature revision for encapsulated follicular variant of papillary thyroid carcinoma: a paradigm shift to reduce overtreatment of indolent tumors. JAMA Oncol. 2016;2:1023‐1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zablotska LB, Nadyrov EA, Rozhko AV, et al. Analysis of thyroid malignant pathologic findings identified during 3 rounds of screening (1997‐2008) of a cohort of children and adolescents from belarus exposed to radioiodines after the Chernobyl accident. Cancer. 2015;121:457‐466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ito Y, Miyauchi A, Kihara M, et al. Patient age is significantly related to the progression of papillary microcarcinoma of the thyroid under observation. Thyroid. 2014;24:27‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ito Y, Miyauchi A, Kihara M, et al. Prognostic values of clinical lymph node metastasis and macroscopic extrathyroid extension in papillary thyroid carcinoma. Endocr J. 2014;61:745‐750. [DOI] [PubMed] [Google Scholar]
- 32. Hirokawa M, Kudo T, Ota H, et al. Pathological characteristics of low‐risk papillary thyroid microcarcinoma with progression during active surveillance. Endocr J. 2016;63:805‐810. [DOI] [PubMed] [Google Scholar]
- 33. Su HK, Wenig BM, Haser GC, et al. Inter‐observer variation in the pathologic identification of minimal extrathyroidal extension in papillary thyroid carcinoma. Thyroid. 2016;26:512‐517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Brierley JD, Gospodarowicz MK, Wittekind C, eds. TNM Classification of Malignant Tumours. 8th edn Hoboken, NJ: Wiley‐Blackwell; 2017. [Google Scholar]
- 35. Enomoto Y, Enomoto K, Uchino S, et al. Clinical features, treatment, and long‐term outcome of papillary thyroid cancer in children and adolescents without radiation exposure. World J Surg. 2012;36:1241‐1246. [DOI] [PubMed] [Google Scholar]
- 36. Mitsutake N, Fukushima T, Matsuse M, et al. BRAF(V600E) mutation is highly prevalent in thyroid carcinomas in the young population in Fukushima: a different oncogenic profile from Chernobyl. Sci Rep. 2015;5:16976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Iyama K, Matsuse M, Mitsutake N, et al. Identification of three novel fusion oncogenes, SQSTM1/NTRK3, AFAP1L2/RET, and PPFIBP2/RET, in thyroid cancers of young patients in Fukushima. Thyroid. 2017;27:811‐818. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


