Abstract
Follicular atresia is a phenomenon that leads to evacuation of the ovary from the oocytes and the occurrence of menopause. The contribution of various types of cell death in atresia at different follicular developmental stages requires extensive investigation. In this study, we evaluated 3 types of programmed cell death (PCD), apoptosis, autophagy, and necrosis, in juvenile mouse ovary when we can observe all follicular stages as well as atresia. Ovaries from juvenile mice on the 21st post-natal (PN) day were prepared histologically for terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) to evaluate apoptosis and immunohistochemistry for beclin-1 to evaluate the autophagy marker. Necrotic cell death was also assessed by penetration of propidium iodide (PI). The count and percentage of the labeled follicles at different stages in the ovaries were evaluated and compared using the Kruskal-Wallis and Mann-Whitney tests. We detected TUNEL-positive granulosa cells in pre-antral and antral follicles but not in the primordial and primary follicles. Somatic cells and oocytes of primordial, primary, pre-antral and antral follicles reacted to beclin-1. The percentage of the PI-labeled primordial and primary follicles were significantly higher than the beclin-1 positive (P=0.01 and P=0.01). In conclusion, we showed that apoptosis, autophagy, and necrosis play a role in follicular atresia and the contributions of each one depends on the follicular stages. It was also demonstrated that necrosis happens particularly in the small follicles while in the large one, all three cell death types occurred with an equal ratio.
Key Words: Apoptosis, Autophagy, Mouse, Necrosis, Ovary
Introduction
Programmed cell death (PCD) plays an important role in the development of the fetal ovary and ovarian cycles. Nowadays, the cell death and its regulators in the ovary have been considered as a critical event (Sun et al., 2017). Three conventional types of PCD include apoptosis, autophagy and necrosis (Kutscher et al., 2017). The first type of PCD is apoptosis, with outstanding features including chromatin condensation and nuclear fragmentation without cell membrane and organelles disintegration (Saraste et al., 2000). The second type of cell death is autophagy, which is characterized by the formation of the double membrane structures called autophagosomes in the cytosol (Levine et al., 2005). Autophagy is generally regarded as a pro-survival process in the cells exposed to environmental stress. However, the long-term activation of this process may eventually lead to cell death (Gawriluk et al., 2011). The third type of cell death is necrosis. Although necrosis was the first term used to describe cell death, its role in the physiologic cell death is underestimated (Moreno-Gonzalez et al., 2016). The most distinctive feature of necrosis includes loss of the plasma membrane integrity. Compared to apoptosis as a clean and noiseless death without inflammatory responses, necrosis is a dirty and noisy cell death (Simon and Ramos, 2006). In recent years, molecular control of necrotic cell death has been studied and this type of PCD may occur in different organisms along with other types of PCD. Necrosis is often considered as cell death, which shows neither apoptotic nor autophagy characteristics (Ouyang et al., 2012).
Apoptosis plays several roles in ovary development including the germ cell death in the fetal period, follicular atresia after birth, death of the germinal epithelium during ovulation and luteolysis (Pru and Tilly, 2001). Although the dominant hypothesis suggests that apoptosis is the main type of cell death in the ovary, it may not be the only mechanism of follicular depletion. Evidence shows that apoptosis is not the only PCD that leads to follicular evacuation and the autophagy and necrosis may also have roles in such an event (Kutscher and Shaham, 2017).
Although a high incidence of follicular atresia occurs during neonatal to juvenile periods, it is mostly studied in the adult ovary (Tingen et al., 2009). Therefore, further studies are needed to find the types of cell death in the ovary during pre-puberty. Follicular atresia during pre-puberty is more complicated than the germ cells death in the fetal period because the follicle contains not only the germ cells, but also the somatic cells.
The ovary is not an inactive member in the juvenile mice. It is characterized by growth and development, along with the simultaneous destruction of the oocytes and follicles. During the third week post-natal (PN), mouse follicular development is in progress; therefore, primordial, primary, pre-antral and antral follicles can be found simultaneously. However, follicles degenerate upon reaching a certain size since the hormones allow the follicular development, but they are still unable to support the follicles to ovulate. Therefore, a large number of degenerated follicles can be found in the juvenile mouse ovary (Liew et al., 2017).
The age of menopause depends on the rate of follicular atresia which leads to ovarian depletion from the oocytes. Changing lifestyle and marriage at old ages lead to the necessity to prolong the fertility period and delay the onset of menopause (Hart, 2016; de Kat and Broekmans, 2018). Involvement of different cell deaths in follicular atresia can help us to create new ways in the control of ovarian cell death and delay menopause. Due to limited information on the contribution of different death types in the atresia of different follicular stages, we intended to evaluate three main types of cell death in the mouse ovary, as an important animal model, not only when all types of follicles are present in the ovary but also when cell death is profuse.
Materials and Methods
Animals
In this project, we used the right ovaries of 20 mice. All animals were kept and handled according to the guidelines approved by the Ethics Committee of Shiraz University of Medical Sciences) ethical code: IR.SUMS.REC.1394.S592(. Animals were kept in the standard conditions (22 ± 1°C and a 12:12 light/dark cycle) with free access to food and water.
Tissue collection
The mice were euthanized by cervical dislocation. Ovaries from 21 days PN Balb/c mice were fixed in 10% buffered formalin. Five ovaries from 5 different animals were dehydrated in gradually increasing ethanol, cleared in xylene, and embedded in paraffin wax. The samples were sectioned at 5 µm thickness. The deparaffinized sections were stained with hematoxylin and eosin.
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) procedure
Apoptosis in ovaries was identified by TACS TdT DAB Kit (R&D, USA, and Catalog #4810-30-K). Paraffin-embedded sections form 5 ovaries were rehydrated and exposed to proteinase K for 30 min at 37°C; then, the endogenous peroxidase was blocked by incubation with quenching solution for 5 min at room temperature. The paraffin sections were incubated with labeling reaction mixture (Tdt dNTP mix and Tdt Enzyme) for 60 min at 37°C. Then, the samples were immersed in streptavidin-HRP solution for 10 min at 37°C. Thereafter, the sections were incubated in Diaminobenzidine for 7 min at room temperature. The samples were counterstained with methyl green.
Propidium iodide staining
Propidium iodide (PI) is a routine red fluorescent molecule that is used to evaluate the integrity of the cell membrane. Thus, this molecule can only penetrate to the cells with damaged cell membranes including necrotic cells. To keep the ovaries alive at the time of staining, the samples (n=5) were kept in Dulbecco Minimum Essential Medium (DMEM, Gibco, 11995) containing 1.5 µM/ml PI (Sigma, USA, P4170) and incubated at 37°C and 5% CO2 for 30 min in darkness. After the PI penetration, the culture medium was removed and the samples were washed in DMEM three times. The specimens were placed on a glass slide, mounted with mineral oil, and examined by fluorescent microscope. Due to the thickness of the specimens, immediately after taking photography, ovaries were embedded in OCT, sectioned at 5 µm thickness with cryomicrotome (Leica, USA, CM 1950) and stained with Hoechst (Sigma Aldrich, Germany, 33342) as a counterstain, the sections were again examined by fluorescent microscope.
Beclin -1 immunolocalization
Beclin-1 is a multifunctional protein that plays a key role in the autophagy. Five fresh ovaries from juvenile mice were embedded in OCT, sectioned at 5 µm thickness using cryomicrotome (Leica, USA, CM1950) and stored at -20°C until used for immunohistochemistry evaluation. The tissue sections were thawed for 1 h at room temperature; then, they were fixed with acetone for 15 min. Non-specific binding sites were blocked by phosphate buffer saline that contained 1% bovine serum albumin and 1% goat serum for 1h at room temperature. Sections were incubated overnight at 4°C with anti beclin-1 primary antibody (Abcam, USA, ab62557) at a dilution 1:50. Negative control sections were prepared in parallel by replacing the primary antibody with dilution medium. After washing, the sections were incubated with an anti-donkey antibody coupled to Alexa fluor 488 (Abcam, USA, ab150105) as the secondary antibody at the dilution 1:500 for 1 h at room temperature and darkness. Then, the samples were washed and counterstained with Hoechst (Sigma Aldrich, Germany, 33342).
Morphometric evaluation
Five ovaries from 5 different mice were evaluated for each test. The percentages of the follicles labeled with TUNEL, beclin-1, and PI were estimated by 3 randomly selected sections from the mouse ovaries and the labeled follicles were counted in 10 fields of each section and the percentage of the labeled follicles was estimated in different follicular stages.
Statistical analysis
The mean values of the percentages of TUNEL, Beclin-1 and PI-positive follicles were analyzed by Kruskal-Wallis test and Mann-Whitney, with a significance level of P<0.05. SPSS version 16 was used for statistical analyses.
Results
Morphological features
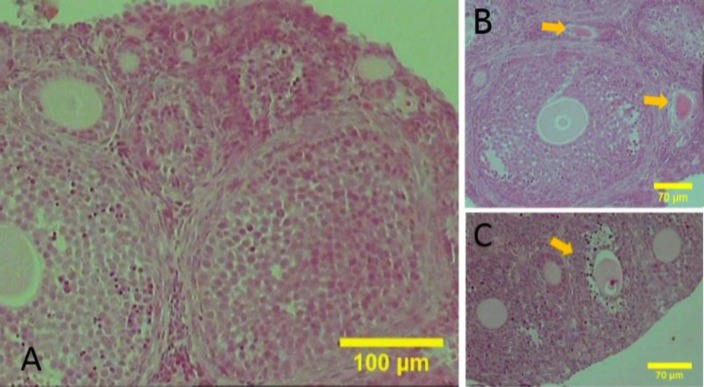
The morphology of the mouse ovary at 21 days after birth is shown in Fig. 1; the small, medium and large follicles were simultaneously observed. Small follicles, such as primordial and primary follicles, appear below the surface epithelium. The primordial follicles were characterized by an oocyte surrounded by squamous pre-granulosa cells; primary follicles were identified with a layer of cuboidal granulosa cells and pre-antral follicles characterized with two (as medium follicle) and more layers of granulosa cells (as large follicle). Antral follicles are characterized by the presence of the antral cavity between the granulosa cells (as large follicle). In medium and large follicles, the granulosa and theca layers were completely recognizable (Fig. 1).
Fig. 1.
The sections of the juvenile mice ovaries stained with haematoxylin and eosin. (A) The follicles are observed in different developmental stages, (B) Follicles in late stage of atresia are located at the adjacent antral follicle (yellow arrows), and (C) Antral follicle in early stage of atresia (yellow arrows) positive, but with less intensity (Fig. 5B).
Apoptosis detection
The results of the TUNEL procedure in juvenile mice ovary are shown in Fig. 2. TUNEL-positive granulosa cells were detected in the pre-antral and antral follicles. In the antral follicles, the apoptotic cells were present near the antral cavity. Theca cells were TUNEL-negative (Fig. 2). Apoptosis was not detected in the primordial and primary follicles (Fig. 3A). Some TUNEL-positive cells were also present in the surface epithelium (Fig. 3B).
Fig. 2.
Apoptosis is detected in the ovaries. TUNNEL-positive (brown) granulosa cells (arrows) were detected in the antral and pre-antral follicles
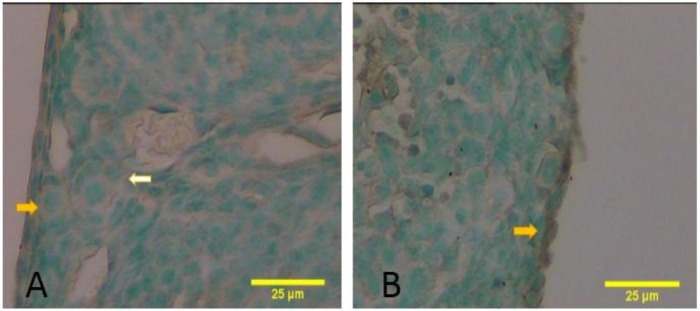
Fig. 3.
The sections of the juvenile mice ovaries stained by TUNEL reaction and counterstained methyl green. (A) TUNNEL-negative primordial (yellow arrow) and primary follicles (white arrow) were detected, and (B) TUNNEL-positive cells (arrows) can be also found in the ovarian surface epithelium
Beclin-1 expression
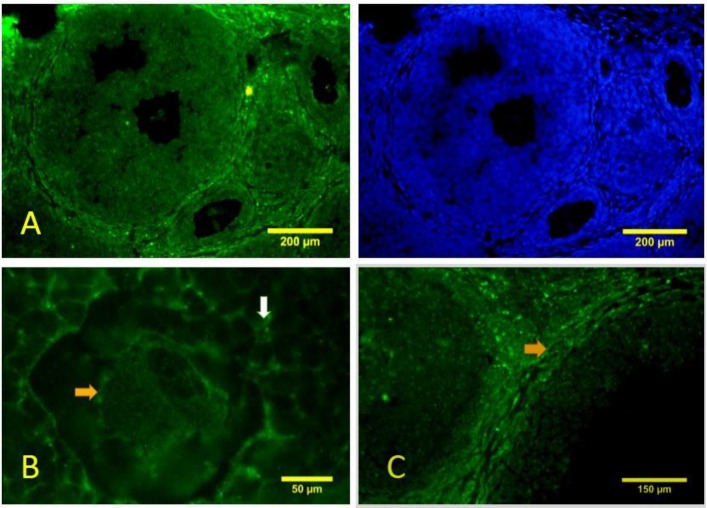
In the immature mice ovary, beclin-1 immunostaining was shown in both oocyte and somatic cells of the primordial and primary follicles (Figs. 4A-B). Autophagic vacuoles were observed as green spots throughout the cytoplasm. Although beciln 1 is mainly localized in the cytoplasm, some cells including the oocytes and somatic cells exhibited nuclear expression. This finding is probably due to the diffusion of antigen during fixation or the presence of nuclear proteins. Theca cells in pre-antral and antral follicles showed recognizable amounts of beciln 1 (Figs. 5A, C). The granulosa and oocytes of such follicles were also
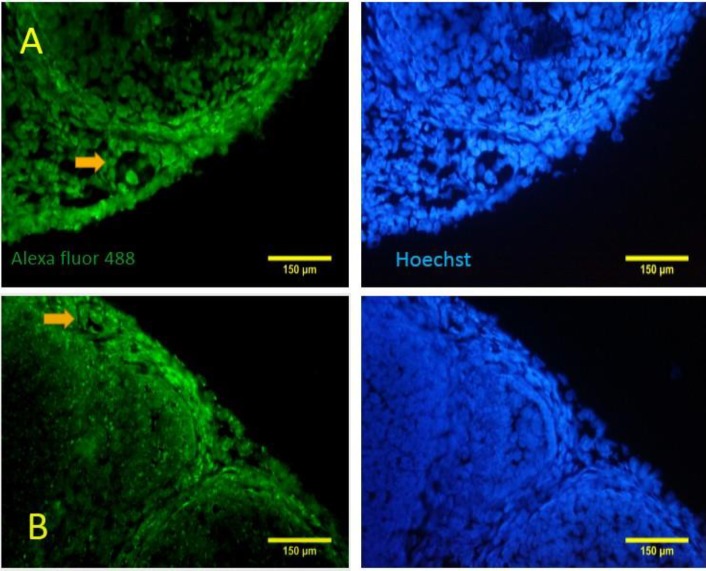
Fig. 4.
Immunohistochemistry for beclin-1 shows that autophagy has happened in the primordial and primary follicles. The nuclei are stained with Hoechst. (A) A primary follicle (arrow) with beclin-1 expression in the oocyte and somatic cells, (B) A primordial follicle (arrow) is reacted to beclin-1 for both oocyte and squamous somatic cells
Fig. 5.
Immunohistochemistry for beclin-1. (A) Lower magnification of the mouse ovary shows that beclin-1-positive cells are prominent in the theca layer of the pre-antral and antral follicles, (B) Higher magnification of the oocyte of antral follicle shows that the oopelasm and granulosa cells labeled for beclin-1 (yellow and white arrows, respectively), and (C) The theca cells with high intensity for beclin-1 (arrow) can also be detected
Necrosis
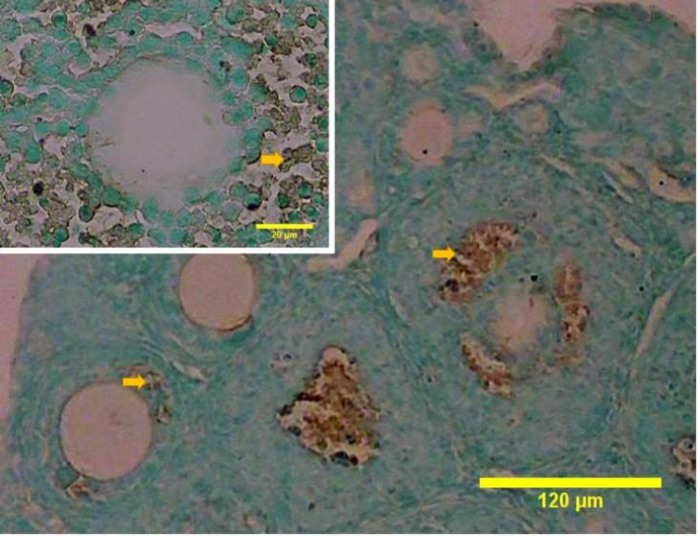
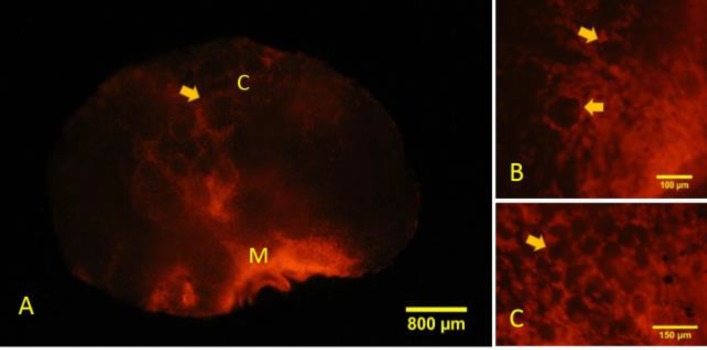
Necrotic cells with ruptured cell membrane were detected in the juvenile mouse ovary, as shown by PI absorption. Whole organ evaluation of the ovaries showed that necrotic cell death presented in both cortical and medullary regions with higher frequency in the medulla. In the primordial and primary follicles, necrotic cell death was seen in the follicular cells, while the oocytes of these follicles were negative (Fig. 6).
Fig. 6.
Whole-mount staining of the ovary reveals areas of propidium iodide (PI) absorption in the cortical and medullary regions. (A) Necrosis has happened mainly in the medullary region. M: Medulla, and C: Cortex. The arrow shows follicle in cortical region, (B) Somatic cells (arrow) of primary follicles are stained with PI, and (C) Somatic cells in the primordial follicles (arrow) also show necrosis
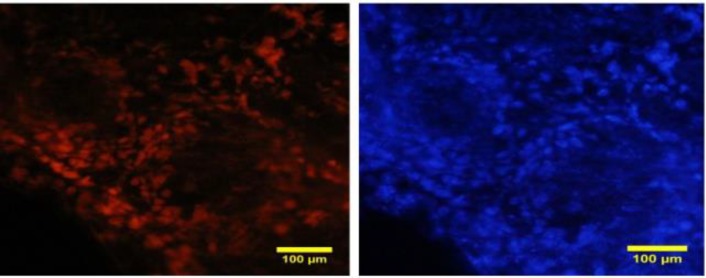
In ovarian sections, necrosis appeared only in the somatic cells in pre-antral and antral follicles. The oocyte did not absorb PI while both granulosa and theca cells absorbed the dye with higher intensity in theca cells (Fig. 7).
Fig. 7.
Sections of the ovary pre-exposed to propidium iodide (PI) are counterstained with Hoechst. Necrosis is shown in the theca and granulosa cells in large follicles
The contribution of cell death types in each follicle stage
The mean value of the percentage of primordial (6.36 ± 0.10%) and primary (3.10 ± 0.01%) follicles labeled with PI was significantly higher compared with those labeled with Beclin-1 in the matched stages (2.26 ± 0.10% and 2.12 ± 0.02%, respectively) (both P=0.01, Table 1). The percentages of PI, beclin-1 and TUNEL-labeled follicles in pre-antral and antral stages were the same (Table 1).
Table 1.
Mean value of the percentage of the follicles labeled with TUNNEL, beclin-1, and propidium iodide (PI) in the juvenile mouse ovaries
| Stages of follicles | TUNNEL-positive Follicles (mean%±SE) (total number: labeled follicles) |
Beclin-1-labeled Follicles (mean%±SE) (total number: labeled follicles) |
PI-labeled follicles (mean%±SE) (total number: labeled follicles) |
|---|---|---|---|
| Primordial | 0 (330:0) | 2.26 ± 0.10* (296: 7) | 6.36 ± 0.10 (304:19) |
| Primary | 0 (215:0) | 2.12 ± 0.02* (180:4) | 3.10 ± 0.01 (202:7) |
| Pre-antral | 1.50 ± 0.13 (180:3) | 1.84 ± 0.21 (168:3) | 1.76 ± 0.06 (131:2) |
| Antral | 3.13 ± 0.04 (150:5) | 3.81 ± 0.21 (157:6) | 3.53 ± 0.14 (169:6) |
Significant differences with the PI-labeled follicles (P<0.05)
Discussion
During development, the ovary undergoes a variety of permanent changes such as follicular growth and death. Follicular atresia is usually described as a reduction in the number of follicles regardless of the cell death mechanisms. The contribution of each type of cell death in depletion of follicular reserve is unclear. Evaluation of three conventional cell deaths that play roles in homeostasis can be useful in identifying the involved mechanisms in depletion of follicular reserve. The innovation of this work is evaluation of three types of cell death in all stages of fulliculogenesis. Especially in relation to necrosis, we suggested a new and simple method for evaluating the areas of necrosis in the immature mouse ovary. The role of necrosis in the mammalian ovary hemostasis requires extensive examination.
The data from the current study showed that apoptosis occurs predominantly in the granulosa cells of pre-antral and antral follicles, but not in the oocyte. This result confirmed the previous study that revealed the occurrence of apoptosis in the granulosa cells of the mouse ovary (Zucker and Jeffay, 2006). TUNEL-positive granulosa cells are localized in clusters near the cavity of the antrum; this is in agreement with the study done by Amsterdam et al. (2003). Probably similar to the thymocytes, the granulosa cells also showed the train for apoptosis undergoing physical and chemical signals from the environment (Zucker and Jeffay, 2006). We also observed TUNEL-positive cells in the surface epithelium, which is in agreement with Murdoch et al.’s results (2000). It is believed that follicular atresia is fundamentally mediated by apoptosis in the granulosa cells (YU et al., 2004). Apoptotic death has been detected in in-vitro oocyte maturation in rats and it was suggested that the different oocyte death types happen in vitro versus in vivo (Tiwari et al., 2015). In fact, apoptosis may not be the dominant type of cell death in vivo; therefore, the role of apoptosis in the follicular atresia should be reconsidered (Jenkins et al., 2013). We found necrosis and autophagy, but not apoptosis, in theca cell of the preantral and antral follicles. Along with our data, a report showed that apoptosis is not seen in the theca layer at the early and mid-stage of the porcine follicular atresia (Manabe et al., 2004). Evaluation of gene expression profile in the theca cells of bovine atretic follicles determined stopping the cell cycle marker expression; in contrast, in the granulosa cells, the apoptotic genes were up-regulated (Hatzirodos et al., 2014a).
Necrotic death is characterized by inflammatory response. Inflammation plays a role in the repair of the tissues and destruction of the pathogens. Investigation of gene expression profile in the granulosa cells of the bovine antral follicles during atresia showed that not only is cell death involved in atresia, but also an active cellular infiltration similar to the wound healing occurs (Hatzirodos et al., 2014b). These findings may support our observation that necrotic death occurs in both granulosa and theca cells of the atretic follicles.
Gaytan et al. (2008) reported that in adult human, beclin-1 expression is limited to theca cell, while the granulosa cells were negative. In contrast, we found that both granulosa and oocyte expressed beclin-1. Along with our result, the other studies reported other autophagic markers such as acid phosphatase or Lamp 1, as lysosomal membrane protein in the oocytes of immature rats (Escobar et al., 2008; Omari et al., 2015). This contradictory result is probably due to the differences in the species or the age as the follicular atresia is age dependent.
Our results indicated that apoptosis was not involved in the cell death of the primordial and primary follicles, while the autophagy happened in both granulosa and oocyte of these follicles. Necrosis was also observed in the somatic cells of these follicles. Thousands of primordial follicles disappeared in the pre-pubertal mouse ovary. In mice, the number of primordial follicles decreases from 10000 on day 6 to 3000 on day 45 PN (Tingen et al., 2009). During this period, the morphological and biochemical markers of apoptosis were not observed in the primordial follicles (Tingen et al., 2009). This may be due to the limitation of apoptotic evaluation techniques or the participation of other types of cell death (Tingen et al., 2009). The electron microscopic study revealed granulosa cells with necrosis and autophagy phenotypes in quail atretic antral follicles (D’Herde et al., 1996); our histochemical observations in mice were also in agreement with the previous study on quail.
In conclusion, our results indicated that three types of PCD participated in follicular atresia in the juvenile mouse ovary. Although somatic cells showed all kinds of cell death, in the oocyte, only autophagic marker was expressed. In primordial and primary follicles, necrosis had a significantly greater contribution in atresia than autophagy, while apoptosis was not involved. Our study revealed that in the pre-antral and antral follicular atresia, all three types of cell death had an equal contribution. We recommend flow cytometry and electron microscopy method as a gold standard to confirm the types of cell death in the ovary.
Acknowledgements
The authors would like to thank the Vice Chancellery of Research, Shiraz University of Medical Sciences, for its financial support. The authors wish to thank the Research Consultation Center (RCC) at Shiraz University of Medical Sciences for their assistance in editing this manuscript.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Amsterdam, A , Sasson, R;Keren-Tal, I , Aharoni, D;Dantes, A;Rimon, E , Land, A , Cohen, T , Dor, Yand Hirsh, L Alternative pathways of ovarian apoptosis: death for life. Biochem. Pharmacol. 2003;66:1355–1362. doi: 10.1016/s0006-2952(03)00485-4. [DOI] [PubMed] [Google Scholar]
- 2.de Kat AC, Broekmans FJM. Female age and reproductive chances. In: Stoop Dominic., editor. Preventing age related fertility loss. 1st Edn. Switzerland: Springer International Publishing; 2018. pp. 1–10. [Google Scholar]
- 3.D’Herde, K , De Prest, Band Roels, F Subtypes of active cell death in the granulosa of ovarian atretic follicles in the quail (Coturnix coturnix japonica) Rep. Nut. Dev. 1996;36:175–189. doi: 10.1051/rnd:19960203. [DOI] [PubMed] [Google Scholar]
- 4.Escobar, ML , Echeverría, OM , Ortíz, R , Vázquez-Nin, GH Combined apoptosis and autophagy, the process that eliminates the oocytes of atretic follicles in immature rats. Apoptosis. 2008;13:1253–1266. doi: 10.1007/s10495-008-0248-z. [DOI] [PubMed] [Google Scholar]
- 5.Gawriluk, TR;Hale, AN , Flaws, JA;Dillon, CP;Green, DR , Rucker, EB Autophagy is a cell survival program for female germ cells in the murine ovary. Reproduction. 2011;141:759–765. doi: 10.1530/REP-10-0489. [DOI] [PubMed] [Google Scholar]
- 6.Gaytán, M , Morales, C , Sánchez-Criado, JE , Gaytán, F Immunolocalization of beclin 1, a bcl-2-binding, autophagy-related protein, in the human ovary: possible relation to life span of corpus luteum. Cell Tissue Res. 2008;331:509–517. doi: 10.1007/s00441-007-0531-2. [DOI] [PubMed] [Google Scholar]
- 7.Hart, RJ Physiological aspects of female fertility: role of the environment, modern lifestyle, and genetics. Physiol. Rev. 2016;96:873–909. doi: 10.1152/physrev.00023.2015. [DOI] [PubMed] [Google Scholar]
- 8.Hatzirodos, N , Hummitzsch, K;Irving-Rodgers, HF , Harland, ML , Morris, SE , Rodgers, RJ Transcriptome profiling of granulosa cells from bovine ovarian follicles during atresia. BMC Genomics. 2014a;15:40. doi: 10.1186/1471-2164-15-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hatzirodos, N , Irving-Rodgers, HF , Hummitzsch, K , Rodgers, RJ Transcriptome profiling of the theca interna from bovine ovarian follicles during atresia. PLoS One. 2014b;9:e99706. doi: 10.1371/journal.pone.0099706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jenkins, VK , Timmons, AK , McCall, K Diversity of cell death pathways: insight from the fly ovary. Trends Cell Biol. 2013;23:567–574. doi: 10.1016/j.tcb.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kutscher, LM , Shaham, S Non-apoptotic cell death in animal development. Cell Death Differ. 2017;24:1326–1336. doi: 10.1038/cdd.2017.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levine, B , Yuan, J Autophagy in cell death: an innocent convict? J. Clin. Invest. 2005;115:2679–2688. doi: 10.1172/JCI26390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liew, SH , Nguyen, QN , Strasser, A , Findlay, JK , Hutt, KJ The ovarian reserve is depleted during puberty in a hormonally driven process dependent on the pro-apoptotic protein BMF. Cell Death Dis. 2017;8:e2971. doi: 10.1038/cddis.2017.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manabe, N , Goto, Y , Matsuda-Minehata, F , Inoue, N , Maeda, A , Sakamaki, K , Miyano, T Regulation mechanism of selective atresia in porcine follicles: regulation of granulosa cell apoptosis during atresia. J. Repro. Dev. 2004;50:493–514. doi: 10.1262/jrd.50.493. [DOI] [PubMed] [Google Scholar]
- 15.Moreno-Gonzalez, G , Vandenabeele, P , Krysko, DV Necroptosis: a novel cell death modality and its potential relevance for critical care medicine. Am. J. Respir. Crit. Care Med. 2016;194:415–428. doi: 10.1164/rccm.201510-2106CI. [DOI] [PubMed] [Google Scholar]
- 16.Murdoch, WJ Proteolytic and cellular death mechanisms in ovulatory ovarian rupture. Biol. Signals Recept. 2000;9:102–114. doi: 10.1159/000014629. [DOI] [PubMed] [Google Scholar]
- 17.Omari, S , Waters, M , Naranian, T , Kim, K , Perumalsamy, AL , Chi, M , Greenblatt, E , Moley, KH , Opferman, JT , Jurisicova, A Mcl-1 is a key regulator of the ovarian reserve. Cell Death Dis. 2015;6 doi: 10.1038/cddis.2015.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ouyang, L , Shi, Z , Zhao, S , Wang, FT , Zhou, TT , Liu, B andBao, JK Programmed cell death pathways in cancer: a review of apoptosis, autophagy and programmed necrosis. Cell Proliferation. 2012;45:487–498. doi: 10.1111/j.1365-2184.2012.00845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pru, JKand , Tilly, JL Programmed cell death in the ovary: insights and future prospects using genetic technologies. Mol. Endocrinol. 2001;15:845–853. doi: 10.1210/mend.15.6.0646. [DOI] [PubMed] [Google Scholar]
- 20.Saraste, A , Pulkki, K Morphologic and biochemical hallmarks of apoptosis. Cardiovasc. Res. 2000;45:528–537. doi: 10.1016/s0008-6363(99)00384-3. [DOI] [PubMed] [Google Scholar]
- 21.Simon, CR , Ramos, RGP Death by fine tunning. Braz. J. Morphol. Sci. 2006;23:3–13. [Google Scholar]
- 22.Sun, YC , Sun, XF , Dyce, PW , Shen, W andChen, H The role of germ cell loss during primordial follicle assembly: a review of current advances. Int. J. Biol. Sci. 2017;13:449–457. doi: 10.7150/ijbs.18836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tingen, CM , Bristol-Gould, SK , Kiesewetter, SE , Wellington, JT , Shea, L andWoodruff, TK Prepubertal primordial follicle loss in mice is not due to classical apoptotic pathways. Biol. Rep. 2009;81:16–25. doi: 10.1095/biolreprod.108.074898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tiwari, M , Prasad, S , Tripathi, A , Pandey, AN , Ali, I , Singh, AK , Shrivastav, TG andChaube, SK Apoptosis in mammalian oocytes: a review. Apoptosis. 2015;20:1019–1025. doi: 10.1007/s10495-015-1136-y. [DOI] [PubMed] [Google Scholar]
- 25.Yu, YS , Sui, HS , Han, ZB , Li, W , Luo, MJ , Tan, JH Apoptosis in granulosa cells during follicular atresia: relationship with steroids and insulin-like growth factors. Cell Res. 2004;14:341–346. doi: 10.1038/sj.cr.7290234. [DOI] [PubMed] [Google Scholar]
- 26.Zucker, RM , Jeffay, SC Confocal laser scanning microscopy of whole mouse ovaries: excellent morphology, apoptosis detection, and spectroscopy. Cytometry. 2006;69:930–939. doi: 10.1002/cyto.a.20315. [DOI] [PubMed] [Google Scholar]