Abstract
The idea of tumor dormancy originated from clinical findings that recurrence of cancer occurs several years or even several decades after surgical resection of the primary tumor. Tumor mass dormancy was proposed as a model, where there is equal balance between increases in the number of cancer cells by proliferation and decreases as a result of cell death. Tumor mass dormancy includes angiogenic dormancy and immune‐mediated dormancy. Another emerging type of tumor dormancy is cellular dormancy in which cancer cells are in a quiescent state. Cellular dormancy is induced by cues such as the extracellular matrix environment, metastatic niches, a hypoxic microenvironment, and endoplasmic reticulum stress. Even the oncogenic pathways, on which active cancer cells depend for survival and growth, are suppressed in the dormant state. As tumor dormancy is one of the mechanisms of resistance against various cancer therapies, targeting dormant cancer cells should be considered for future treatment strategies.
1. INTRODUCTION
Tumor dormancy is classically defined as the arrest of tumor growth in the primary site or in metastatic dissemination. The concept of dormancy is derived from clinical findings that cancer recurs several years or even decades after surgical resection of the primary tumor, especially in breast and prostate cancers.1, 2 In tumor mass dormancy, there is a balance between the increase in cancer cells by proliferation and the decrease by cell death; whereas, in cellular dormancy, cancer cells are in a quiescent state (Figure 1). Here, we introduce the clinical relevancy and the mechanisms underlying tumor dormancy.
Figure 1.
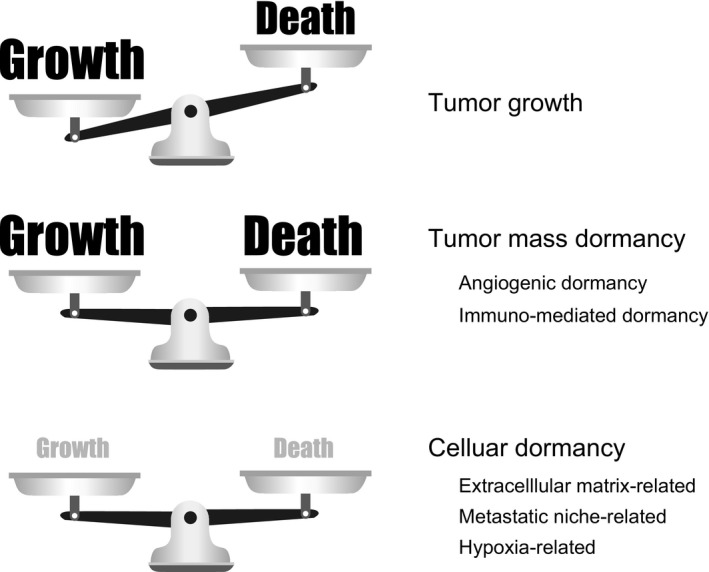
Tumor mass dormancy and cellular dormancy. The balance between the growth and death of cancer cells is illustrated. When the balance between growth and death is equal, the tumor is in tumor mass dormancy. In cellular dormancy, both growth and death are minimal. The underlying mechanisms are indicated
2. CLINICAL INSIGHTS
Breast cancers are divided into several subgroups based on receptor expression and proliferation activity. Relapse‐free survival times differ among the subtypes. In the human epidermal growth factor receptor 2 (HER2)‐positive or triple‐negative subtypes, which have high proliferation activity, the latent periods are shorter than for other subtypes. In these aggressive tumors, the frequency of late recurrence after 5 years is rather reduced3, 4 compared with other subtypes that may recur even after 20 years.5, 6 Estrogen receptor (ER)‐positive breast cancer is a clinical model of late recurrence. A clinical study was carried out evaluating adjuvant therapy with tamoxifen against ER‐positive breast cancer.7 Continuing tamoxifen for 10 years rather than stopping at 5 years further reduced recurrence and mortality, particularly after 10 years. These results indicate that ER‐positive cancer cells could be dormant and survive for more than 5 years; thus, prolonged treatment is required. Recurrences after long‐term latency are also reported in other cancers, such as melanoma and renal cell carcinoma.8, 9 In prostate cancer, there are some cases in which serum levels of prostate specific antigen (PSA) increase without apparent recurrence of the tumor after primary therapy; this is called “PSA recurrence”. The actual recurrences occur an average of 8 years after PSA recurrence.10 Thus, in cases of such late recurrence, cancer cells could have already disseminated at the time of or before surgical removal of the original tumors, and clinically recurred after being dormant for years.11
3. TUMOR MASS DORMANCY
To explain late recurrence, a model of “tumor mass dormancy” was proposed that assumed the balance between the increase in cancer cells by proliferation and the decrease by cell death was at equilibrium. Tumor mass dormancy can be achieved by two different mechanisms; angiogenic dormancy and immunomediated dormancy.
3.1. Angiogenic dormancy
To support active proliferation, cancer cells consume large amounts of energy resources. To meet this necessity, tumor growth must be accompanied by the generation of a new network of blood vessels or angiogenesis.12 Angiogenesis is finely regulated by pro‐angiogenic factors and anti‐angiogenic factors.13 After the angiogenic switch, when the balance tilts to the pro‐angiogenic side, a tumor enters into the progression phase.14 Before the angiogenic switch, increased numbers of cancer cells cause cell death in a region distant from pre‐existing blood vessels because of a lack of oxygen and nutrients. The equilibrium between proliferation and death of cancer cells is called angiogenic dormancy. Involvement of angiogenic dormancy in the regulation of tumor growth was demonstrated in a large number of studies using mouse models.15, 16, 17
3.2. Immunomediated dormancy
Immune‐surveillance has long been known as a critical factor for suppression of tumor development and growth, and tumor cells in established tumors acquire the ability of immune‐evasion.18, 19 The immune system plays critical roles not only in avoiding the early establishment of tumors,20, 21, 22 but also in tumor mass dormancy. Using a spontaneous melanoma model in metallothionein (MT)‐ret/AAD mice, Eyles et al23, 24 showed that CD8+ T cells are involved in the maintenance of tumor dormancy at the metastatic site. In this model, suppression of the immune system by CD8+ antibody significantly promoted metastatic outgrowth. Genome‐wide single‐nucleotide polymorphism profiling showed that the patterns of genetic alterations are highly shared among primary and metastatic sites, indicating that metastases could be derived from tumor cells that disseminated from the primary tumor at an early time point. These results suggested that disseminated tumor cells would be dormant and avoid immune‐surveillance, and escape from the immune system resulted in metastatic outgrowth. Liang et al25 reported that an essential role for immune cells and their cytokines in suppressing tumor cell regrowth after irradiation and the disturbance of immune cells reversed the post‐radiation equilibrium.
4. CELLULAR DORMANCY
Cellular dormancy is another model of tumor dormancy.26, 27 Cellular dormancy is characterized by three features: (i) minimum proliferation; (ii) minimum death; and (iii) reversibility (Figure 1). Dormancy is ubiquitously found in living organisms, including bacteria, yeast, insects, and mammals as a survival strategy against a deteriorated environment. At the cellular level, the dormant status is observed in tissue stem cells28 and cancer. Cellular dormancy of cancer has mainly been studied in the context of late recurrence, especially metastasis, in which the microenvironment of the metastasis site plays a critical role. However, cellular dormancy can also exist in tumors growing at the primary site; for example, in the hypoxic regions (Figure 2). Given that dormant cells exist in tumors, they are likely resistant to conventional therapies such as anticancer drugs and radiation, which target actively proliferating cells. Various mechanisms of cellular dormancy have been reported (Figure 3).
Figure 2.
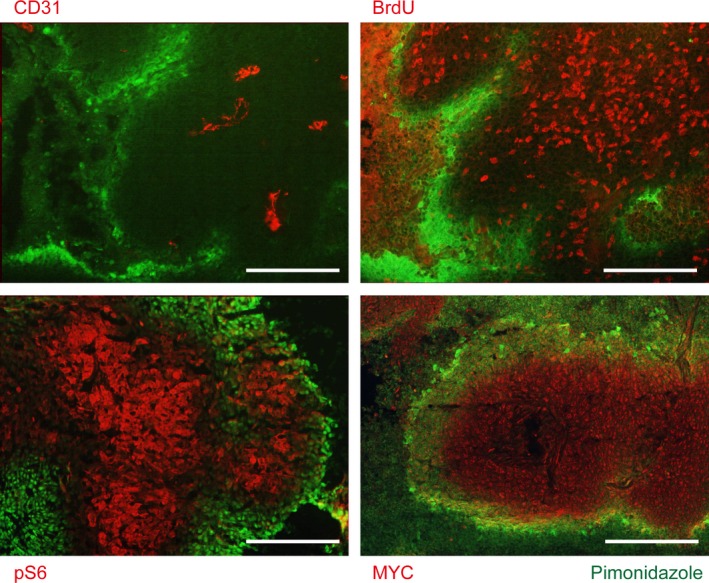
Cancer cells in hypoxic regions are inactive. Immunohistochemistry of a mouse xenograft tumor, generated by s.c. injection of HCT116, a colon cancer cell line (reprinted from Okuyama et al54). Primary antibodies are indicated for each panel. Pimonidazole staining (green) represents hypoxic regions; CD31, blood vessels; BrdU, proliferation; pS6, mTORC1 activity. Areas distant from the blood vessels, including the pimonidazole‐positive regions, are negative for BrdU, pS6, and MYC. Scale bar, 100 μm
Figure 3.
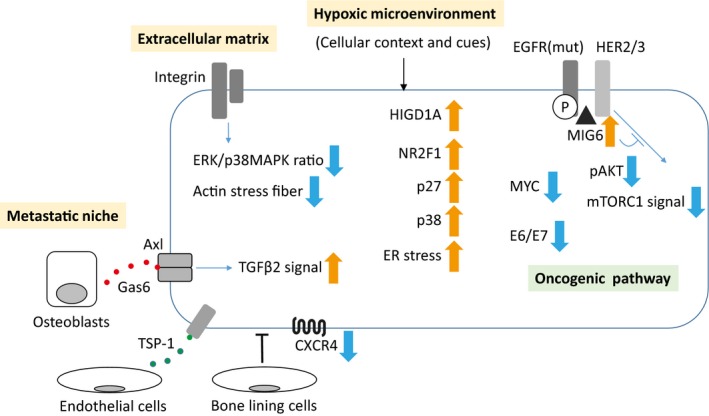
Mechanisms of cellular dormancy. There are various mechanisms underlying cellular dormancy. Black thin arrows, stimulation; blue thin arrows, intracellular signal; thick orange and blue arrows, up and down regulation, respectively, in terms of protein levels or activation of the molecule or the signal. EGFR, epidermal growth factor receptor; ER, endoplasmic reticulum; HER, human epidermal growth factor receptor; HIGD1A, hypoxia‐inducible gene domain family member 1A; mut, active mutant; P, phosphorylation; TGFβ2, transforming growth factor beta‐2
4.1. Extracellular matrix and dormancy
The microenvironment of the metastatic site in a distant organ should not be the same as that of the original tumor, and in such an aberrant milieu, cancer cells die or become dormant. The extracellular matrix is an important microenvironmental factor. Aguirre‐Ghiso et al29 reported that the ratio of active ERK and p38MAPK by attachment to the extracellular matrix is the molecular switch for cellular dormancy. Proliferation of human epidermoid carcinoma HEp3 cells is regulated by two pathways: (i) activation of ERK by urokinase‐plasminogen activator receptor (uPAR) and α5β1 integrin and; (ii) suppression of p38MAPK by fibronectin.30 When one of the pathways is impaired, the balance between ERK and p38MAPK is tilted to the growth suppression side and, consequently, HEp3 cells become dormant. By blocking p38MAPK, cancer cells are extricated from dormancy and resume proliferation.31 In breast cancer, inhibition of β1 integrin induced dormancy in vitro and in vivo.32, 33 The mouse breast cancer cell lines, D2.0R and D2A1, both grow exponentially in 2‐D culture conditions, but D2.0R fails to grow in 3‐D cultures and become dormant; this is compatible with the in vivo results that D2A1 forms lung metastasis within short periods, whereas D2.0R has a long latency.34 D2A1 cells proliferate in 3‐D cultures by producing fibronectin and forming actin stress fibers through activation of β1 integrin. Thus, the interaction between the extracellular matrix and cancer cells plays an important role in cellular dormancy.
4.2. Metastatic niche and dormancy
Prostate cancer cell lines can be dormant when cocultured with the pre‐osteoblastic cell line, MC3T3‐E1. Activated transforming growth factor beta‐2 (TGFβ2) signaling through Axl and Gas6, a ligand of Axl secreted by osteoblasts, plays a critical role in the induction of dormancy.35 Bone‐lining cells can be a niche (endosteal niche) for myeloma cells. Using in vivo imaging, Lawson et al36 showed that myeloma cells become dormant after attachment to the bone‐lining cells. Remodeling of the bone microenvironment by osteoclasts forces the myeloma cells to exit from dormancy and resume proliferation. Similarly, tumor necrosis factor alpha (TNF‐α) and interleukin (IL)‐6 remodel the bone microenvironment to cue the dormant metastatic breast cancer cells in the bone marrow to resume proliferation.37 Using coculture of breast cancer cells and endothelial cells, Ghajar et al38 showed that cancer cells become dormant when thrombospondin‐1 is produced by the stable microvasculature. Downregulation of CXCR4 in lung metastatic sites was also associated with dormant phenotype of a breast cancer cell line.39
4.3. Hypoxic microenvironment and dormancy
Disordered proliferation of cancer cells and disorganized angiogenesis make the cancer microenvironment hypoxic. Consumption of oxygen by tumor cells plays a critical role in generating a hypoxic microenvironment.40 Hypoxia induces a malignant phenotype and therapy resistance in cancer.41 We reported that vascular endothelial growth factor (Vegf ) is necessary for angiogenesis and the formation of pancreatic islet cell tumors in transgenic RIP1‐Tag2 mice,42 whereas tumors still exist in the Vegf null RIP1‐Tag2 mice in an hypoxia‐inducible factor (HIF)1α‐dependent way.43 The residual tumors are highly hypoxic with decreased proliferation and cell death. Both genetic and pharmacological inhibition of angiogenesis result in the tumors having an invasive phenotype.44 Using cell tracking experiments, Harada et al45 showed that the hypoxic cells are the origin of recurrence after irradiation in vivo.
The transcription factor HIF plays a central role in acute hypoxic response in cancer cells.46 Acute hypoxic responses have been intensively studied, using established cell lines fully supplied with nutrients except for oxygen for a short period, from several hours to 48 hours. Under such acute hypoxic conditions, glucose consumption drastically increases because of activated glycolysis (Figure 4). Under a microenvironment in which the glucose supply is limited, it is not likely that cancer cells will continue to consume large amounts of glucose. Thus, there should be other mechanisms different from the typical acute hypoxic response for the dormant cancer cells in hypoxia.
Figure 4.
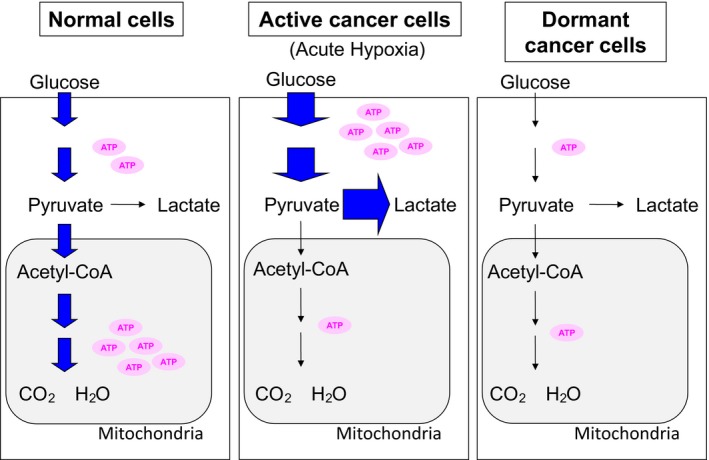
Glycolysis and oxidative phosphorylation in dormant cancer cells. Activation status of the metabolic pathways is expressed as thickening of arrows. Amount of ATP produced is illustrated as the number of ATP molecules
A pancreatic cancer cell line, AsPC‐1, was dormant in chronic hypoxia during in vitro experiments.40 The consumption of energy resources, such as oxygen and glucose, and ATP turnover are decreased in the dormant state. In contrast to the acute hypoxic response, the production of lactate, an end‐product of glycolysis, also decreased. Suppression of AKT activity is necessary for the dormancy of AsPC‐1 cells, as cells with forced expression of the active form of AKT could not be dormant, continued to consume energy resources, and died under chronic hypoxic conditions. Thus, suppressing cellular activity under a deteriorated microenvironment is beneficial for cancer cell survival.
Hypoxia‐inducible gene domain family member 1A (HIGD1A) is expressed near the necrotic region of solid tumors. As the HIGD1A promoter is methylated, the gene is not induced in hypoxia alone. Under glucose‐deprived conditions, DNA methyltransferase activity is suppressed, the HIGD1A promoter is demethylated, and HIGD1A is induced. HIGD1A suppresses oxidative phosphorylation, activates AMPK, and suppresses reactive oxygen species (ROS) production. Consequently, HIGD1A supports the survival of cancer cells under severely hypoxic conditions.47 The orphan nuclear receptor, NR2F1, plays a role in the maintenance of dormancy through SOX9, PARβ, and CDK inhibitors in HEp3 cells.48 Using patient‐derived xenograft models, Fluegen et al49 reported that an increase of p27 through NR2F1 and HIF1α is necessary for induction of dormancy in disseminated tumor cells using patient‐derived xenograft models.
4.4. Experimental platform for studying cellular dormancy of cancer in vitro
Establishing a model system has long been a critical concern for studying tumor dormancy in vitro. Recently, we developed a novel primary culture system of cancer cells, the cancer tissue‐originated spheroid (CTOS) method. The principle of the CTOS method is to maintain cell‐cell contact throughout the preparation and culture processes. CTOS retains the characteristics of the original patients’ tumors.50 In addition, CTOS can be reversibly dormant for at least 7 days in hypoxia without growth factor stimulation for colorectal cancer.40, 51 All of the examined CTOS derived from different patients can be dormant, suggesting that cellular dormancy is a common feature of cancer cells and is not restricted to particular cell lines.
4.5. Suppression of the oncogenic pathway
The oncogene MYC is one of the most frequently dysregulated genes in many cancers.52 In mice with liver tissue‐specific tet‐off MYC gene expression, MYC is overexpressed in the absence of doxorubicin (Dox) treatment, and tumors are generated in the liver.53 After tumor formation, when MYC is inactivated by Dox treatment, the tumors stop growing and the cells revert to a normal liver cell appearance. After the withdrawal of Dox, tumor cells showed massive regrowth with reactivation of MYC. Interestingly, array comparative genomic hybridization analysis showed that the pattern of chromosomal gain and loss is almost the same between primary and recurrent tumors, indicating that the regrown tumor after MYC‐reactivation is not a de novo tumor but derived from the same clone as the tumors before MYC inactivation. These results suggest that the tumor cells are in a dormant state during MYC inactivation. Thus, inhibition of the oncogenic pathway, MYC in this case, induces dormancy.
MYC activation stimulates both oxygen and glucose consumption.54 We showed that MYC protein levels drastically decreased under glucose‐ and oxygen‐deprived conditions through enhancement of protein degradation. Cell death of colorectal cancer HCT116 cells is alleviated by suppression of MYC under prolonged glucose‐ and oxygen‐deprived conditions. MYC levels are frequently increased in colorectal cancer55 and decreased in hypoxia in CTOS (M. Inoue, unpubl. data). Regulation of MYC levels might also be involved in the induction of dormancy.
A driver mutation is a mutation within a gene that plays a critical role in cancer development and progression. Blocking the pathway of the driver mutation is the basis of molecular‐targeting therapy. We reported that CTOS from non‐small cell lung cancer patients with epidermal growth factor receptor (EGFR) mutations can be dormant in hypoxic conditions.51 Intracellular signaling of the EGFR pathway is suppressed in dormancy, whereas constitutive phosphorylation of EGFR is sustained. These dormant CTOS were resistant to an EGFR tyrosine kinase inhibitor. Unlike EGFR, phosphorylation of the ERBB family members HER2 and HER3 were remarkably suppressed. The intrinsic inhibitor of EGFR, MIG6 (ERRFI1/RALT/Gene33), blocks dimerization of ERBB family members by binding to the intracellular domain of EGFR. MIG6 is induced by hypoxia, and is necessary for dormancy in lung cancer cells harboring EGFR active mutations. In the Wnt1‐inducible fibroblast growth factor receptor (FGFR)‐driven breast cancer model, tumors become dormant when treated with FGFR inhibitors.56 Eventually, the tumors become resistant to the FGFR inhibitors by activation of EGFR. In a Dox‐inducible MMTV Neu mouse model, a decrease in HER2 signaling by withdrawal of Dox led to the tumor cells being dormant. Some of the cancer cells activated Notch signaling and resumed proliferation.57
Human papillomavirus is an oncogenic virus, and the E6/E7 product is important for oncogenesis. Cellular senescence is induced by suppression of E6/E7 under normoxic conditions, whereas cellular dormancy is induced under hypoxic conditions.58 Thus, suppression of the driver pathway leads to cancer cell death or senescence under the active state but not under the dormant state. Cellular dormancy of cancer cells is not a passive response; rather, it is regulated by active mechanisms.
4.6. Endoplasmic reticulum stress and dormancy
As mentioned above, some cells can be dormant only under some conditions. To be dormant, there should be a particular cellular context or cue, such as a hypoxic response. Another signal may be endoplasmic reticulum (ER) stress. In actively dividing cancer cells, protein synthesis that requires large amounts of energy resources is promoted. Protein synthesis and the key molecule, mTORC1, are downregulated in cancer cells cultured in hypoxia.59 When a colorectal cancer cell line, COLO320, was stimulated by insulin‐like growth factor (IGF) under hypoxic conditions, robust apoptosis was induced through an augmented ER stress response.60 Impaired protein folding under hypoxic conditions is responsible for the ER stress, and IGF might induce ER stress by stimulating aberrant protein synthesis and consequent cell death. Thus, too much ER stress must be avoided. Ranganathan et al61 reported that activation of p38 MAPK through ER stress is critical for induction of dormancy.
5. FINAL REMARKS
Although the phenomenon of dormancy is becoming increasingly recognized in the field of cancer research, the mechanisms for it are still largely unknown. One of the reasons is that “dormancy” includes many different states that depend on cell type; nutrient levels, oxygen, and growth factors; extracellular matrix; and so on. Even if the final status is the same “dormancy”, the mechanisms of induction are different. Creating a model system still remains a critical issue. Recent advances in cell culture and imaging technology will enable detailed studies, although late recurrence in patients after several years or decades would not be the same as in mouse models or culture models. Finding specific markers of dormancy will enable cell tagging and cell fate tracking, which is necessary for assessing the role of dormant cells in various therapies. Developing drugs that disturb the induction of dormancy, kill dormant cells, or block regrowth might be other options for cancer therapy. Combination with current drugs, including molecular‐targeting drugs, and radiation therapy might enhance the efficacy of the treatments. Recent advances in cancer immunology will also accelerate understanding of immunomediated dormancy.
CONFLICTS OF INTEREST
Masahiro Inoue belongs to the Department of Clinical Bio‐Resource Research and Development, which is sponsored by KBBM.
ACKNOWLEDGMENT
This work was partly supported by JSPS Grant‐in‐Aid for Scientific Research on Innovative Areas (26111005) to MI.
Endo H, Inoue M. Dormancy in cancer. Cancer Sci. 2019;110:474–480. 10.1111/cas.13917
REFERENCES
- 1. Retsky M, Demicheli R. Multimodal hazard rate for relapse in breast cancer: quality of data and calibration of computer simulation. Cancers (Basel). 2014;6:2343‐2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Weckermann D, Muller P, Wawroschek F, Harzmann R, Riethmuller G, Schlimok G. Disseminated cytokeratin positive tumor cells in the bone marrow of patients with prostate cancer: detection and prognostic value. J Urol. 2001;166:699‐703. [PubMed] [Google Scholar]
- 3. Dent R, Trudeau M, Pritchard KI, et al. Triple‐negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429‐4434. [DOI] [PubMed] [Google Scholar]
- 4. Romero A, Prat A, Garcia‐Saenz JA, et al. Assignment of tumor subtype by genomic testing and pathologic‐based approximations: implications on patient's management and therapy selection. Clin Transl Oncol. 2014;16:386‐394. [DOI] [PubMed] [Google Scholar]
- 5. Prat A, Pineda E, Adamo B, et al. Clinical implications of the intrinsic molecular subtypes of breast cancer. Breast. 2015;24(Suppl 2):S26‐S35. [DOI] [PubMed] [Google Scholar]
- 6. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15‐year survival: an overview of the randomised trials. Lancet. 2005;365:1687‐1717. [DOI] [PubMed] [Google Scholar]
- 7. Davies C, Pan H, Godwin J, et al. Long‐term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor‐positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381:805‐816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tsao H, Cosimi AB, Sober AJ. Ultra‐late recurrence (15 years or longer) of cutaneous melanoma. Cancer. 1997;79:2361‐2370. [PubMed] [Google Scholar]
- 9. McNichols DW, Segura JW, DeWeerd JH. Renal cell carcinoma: long‐term survival and late recurrence. J Urol. 1981;126:17‐23. [DOI] [PubMed] [Google Scholar]
- 10. Freedland SJ, Moul JW. Prostate specific antigen recurrence after definitive therapy. J Urol. 2007;177:1985‐1991. [DOI] [PubMed] [Google Scholar]
- 11. Sosa MS, Bragado P, Aguirre‐Ghiso JA. Mechanisms of disseminated cancer cell dormancy: an awakening field. Nat Rev Cancer. 2014;14:611‐622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182‐1186. [DOI] [PubMed] [Google Scholar]
- 13. Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353‐364. [DOI] [PubMed] [Google Scholar]
- 14. Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401‐410. [DOI] [PubMed] [Google Scholar]
- 15. Holmgren L, O'Reilly MS, Folkman J. Dormancy of micrometastases: balanced proliferation and apoptosis in the presence of angiogenesis suppression. Nat Med. 1995;1:149‐153. [DOI] [PubMed] [Google Scholar]
- 16. Giuriato S, Ryeom S, Fan AC, et al. Sustained regression of tumors upon MYC inactivation requires p53 or thrombospondin‐1 to reverse the angiogenic switch. Proc Natl Acad Sci USA. 2006;103:16266‐16271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Indraccolo S, Stievano L, Minuzzo S, et al. Interruption of tumor dormancy by a transient angiogenic burst within the tumor microenvironment. Proc Natl Acad Sci USA. 2006;103:4216‐4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Farrar JD, Katz KH, Windsor J, et al. Cancer dormancy. VII. A regulatory role for CD8+ T cells and IFN‐gamma in establishing and maintaining the tumor‐dormant state. J Immunol. 1999;162:2842‐2849. [PubMed] [Google Scholar]
- 19. Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137‐148. [DOI] [PubMed] [Google Scholar]
- 20. Shankaran V, Ikeda H, Bruce AT, et al. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107‐1111. [DOI] [PubMed] [Google Scholar]
- 21. Girardi M, Oppenheim DE, Steele CR, et al. Regulation of cutaneous malignancy by gammadelta T cells. Science. 2001;294:605‐609. [DOI] [PubMed] [Google Scholar]
- 22. Hayakawa Y, Rovero S, Forni G, Smyth MJ. Alpha‐galactosylceramide (KRN7000) suppression of chemical‐ and oncogene‐dependent carcinogenesis. Proc Natl Acad Sci USA. 2003;100:9464‐9469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lengagne R, Graff‐Dubois S, Garcette M, et al. Distinct role for CD8 T cells toward cutaneous tumors and visceral metastases. J Immunol. 2007;180:130‐137. [DOI] [PubMed] [Google Scholar]
- 24. Eyles J, Puaux AL, Wang X, et al. Tumor cells disseminate early, but immunosurveillance limits metastatic outgrowth, in a mouse model of melanoma. J Clin Invest. 2010;120:2030‐2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liang H, Deng L, Chmura S, et al. Radiation‐induced equilibrium is a balance between tumor cell proliferation and T cell‐mediated killing. J Immunol. 2013;190:5874‐5881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yeh AC, Ramaswamy S. Mechanisms of cancer cell dormancy–another hallmark of cancer? Can Res. 2015;75:5014‐5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vera‐Ramirez L, Hunter KW. Tumor cell dormancy as an adaptive cell stress response mechanism. F1000Res. 2017;6:2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Takubo K, Goda N, Yamada W, et al. Regulation of the HIF‐1alpha level is essential for hematopoietic stem cells. Cell Stem Cell. 2010;7:391‐402. [DOI] [PubMed] [Google Scholar]
- 29. Aguirre‐Ghiso JA, Kovalski K, Ossowski L. Tumor dormancy induced by downregulation of urokinase receptor in human carcinoma involves integrin and MAPK signaling. J Cell Biol. 1999;147:89‐104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Aguirre‐Ghiso JA, Liu D, Mignatti A, Kovalski K, Ossowski L. Urokinase receptor and fibronectin regulate the ERK(MAPK) to p38(MAPK) activity ratios that determine carcinoma cell proliferation or dormancy in vivo. Mol Biol Cell. 2001;12:863‐879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Aguirre‐Ghiso JA, Estrada Y, Liu D, Ossowski L. ERK(MAPK) activity as a determinant of tumor growth and dormancy; regulation by p38(SAPK). Can Res. 2003;63:1684‐1695. [PubMed] [Google Scholar]
- 32. Weaver VM, Petersen OW, Wang F, et al. Reversion of the malignant phenotype of human breast cells in three‐dimensional culture and in vivo by integrin blocking antibodies. J Cell Biol. 1997;137:231‐245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. White DE, Kurpios NA, Zuo D, et al. Targeted disruption of beta1‐integrin in a transgenic mouse model of human breast cancer reveals an essential role in mammary tumor induction. Cancer Cell. 2004;6:159‐170. [DOI] [PubMed] [Google Scholar]
- 34. Barkan D, Kleinman H, Simmons JL, et al. Inhibition of metastatic outgrowth from single dormant tumor cells by targeting the cytoskeleton. Can Res. 2008;68:6241‐6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yumoto K, Eber MR, Wang J, et al. Axl is required for TGF‐beta2‐induced dormancy of prostate cancer cells in the bone marrow. Sci Rep. 2016;6:36520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lawson MA, McDonald MM, Kovacic N, et al. Osteoclasts control reactivation of dormant myeloma cells by remodelling the endosteal niche. Nat Commun. 2015;6:8983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sosnoski DM, Norgard RJ, Grove CD, Foster SJ, Mastro AM. Dormancy and growth of metastatic breast cancer cells in a bone‐like microenvironment. Clin Exp Metastasis. 2015;32:335‐344. [DOI] [PubMed] [Google Scholar]
- 38. Ghajar CM, Peinado H, Mori H, et al. The perivascular niche regulates breast tumour dormancy. Nat Cell Biol. 2013;15:807‐817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nobutani K, Shimono Y, Mizutani K, et al. Downregulation of CXCR4 in metastasized breast cancer cells and implication in their dormancy. PLoS ONE. 2015;10:e0130032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Endo H, Okuyama H, Ohue M, Inoue M. Dormancy of cancer cells with suppression of AKT activity contributes to survival in chronic hypoxia. PLoS ONE. 2014;9:e98858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pouyssegur J, Dayan F, Mazure NM. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature. 2006;441:437‐443. [DOI] [PubMed] [Google Scholar]
- 42. Inoue M, Hager JH, Ferrara N, Gerber HP, Hanahan D. VEGF‐A has a critical, nonredundant role in angiogenic switching and pancreatic beta cell carcinogenesis. Cancer Cell. 2002;1:193‐202. [DOI] [PubMed] [Google Scholar]
- 43. Takeda T, Okuyama H, Nishizawa Y, Tomita S, Inoue M. Hypoxia inducible factor‐1alpha is necessary for invasive phenotype in Vegf‐deleted islet cell tumors. Sci Rep. 2012;2:494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Paez‐Ribes M, Allen E, Hudock J, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15:220‐231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Harada H, Inoue M, Itasaka S, et al. Cancer cells that survive radiation therapy acquire HIF‐1 activity and translocate towards tumour blood vessels. Nat Commun. 2012;3:783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Semenza GL. Targeting HIF‐1 for cancer therapy. Nat Rev Cancer. 2003;3:721‐732. [DOI] [PubMed] [Google Scholar]
- 47. Ameri K, Jahangiri A, Rajah AM, et al. HIGD1A regulates oxygen consumption, ROS production, and AMPK activity during glucose deprivation to modulate cell survival and tumor growth. Cell Rep. 2015;10:891‐899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sosa MS, Parikh F, Maia AG, et al. NR2F1 controls tumour cell dormancy via SOX9‐ and RARbeta‐driven quiescence programmes. Nat Commun. 2015;6:6170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fluegen G, Avivar‐Valderas A, Wang Y, et al. Phenotypic heterogeneity of disseminated tumour cells is preset by primary tumour hypoxic microenvironments. Nat Cell Biol. 2017;19:120‐132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kondo J, Endo H, Okuyama H, et al. Retaining cell‐cell contact enables preparation and culture of spheroids composed of pure primary cancer cells from colorectal cancer. Proc Natl Acad Sci USA. 2011;108:6235‐6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Endo H, Okami J, Okuyama H, Nishizawa Y, Imamura F, Inoue M. The induction of MIG6 under hypoxic conditions is critical for dormancy in primary cultured lung cancer cells with activating EGFR mutations. Oncogene. 2017;36:2824‐2834. [DOI] [PubMed] [Google Scholar]
- 52. Dang CV. c‐Myc target genes involved in cell growth, apoptosis, and metabolism. Mol Cell Biol. 1999;19:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Shachaf CM, Kopelman AM, Arvanitis C, et al. MYC inactivation uncovers pluripotent differentiation and tumour dormancy in hepatocellular cancer. Nature. 2004;431:1112‐1117. [DOI] [PubMed] [Google Scholar]
- 54. Okuyama H, Endo H, Akashika T, Kato K, Inoue M. Downregulation of c‐MYC protein levels contributes to cancer cell survival under dual deficiency of oxygen and glucose. Cancer Res. 2010;70:10213‐10223. [DOI] [PubMed] [Google Scholar]
- 55. Sikora K, Chan S, Evan G, et al. c‐myc oncogene expression in colorectal cancer. Cancer. 1987;59:1289‐1295. [DOI] [PubMed] [Google Scholar]
- 56. Holdman XB, Welte T, Rajapakshe K, et al. Upregulation of EGFR signaling is correlated with tumor stroma remodeling and tumor recurrence in FGFR1‐driven breast cancer. Breast Cancer Res. 2015;17:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Abravanel DL, Belka GK, Pan TC, et al. Notch promotes recurrence of dormant tumor cells following HER2/neu‐targeted therapy. J Clin Invest. 2015;125:2484‐2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hoppe‐Seyler K, Bossler F, Lohrey C, et al. Induction of dormancy in hypoxic human papillomavirus‐positive cancer cells. Proc Natl Acad Sci USA. 2017;114:E990‐E998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Arsham AM, Howell JJ, Simon MC. A novel hypoxia‐inducible factor‐independent hypoxic response regulating mammalian target of rapamycin and its targets. J Biol Chem. 2003;278:29655‐29660. [DOI] [PubMed] [Google Scholar]
- 60. Endo H, Murata K, Mukai M, Ishikawa O, Inoue M. Activation of insulin‐like growth factor signaling induces apoptotic cell death under prolonged hypoxia by enhancing endoplasmic reticulum stress response. Cancer Res. 2007;67:8095‐8103. [DOI] [PubMed] [Google Scholar]
- 61. Ranganathan AC, Zhang L, Adam AP, Aguirre‐Ghiso JA. Functional coupling of p38‐induced up‐regulation of BiP and activation of RNA‐dependent protein kinase‐like endoplasmic reticulum kinase to drug resistance of dormant carcinoma cells. Can Res. 2006;66:1702‐1711. [DOI] [PMC free article] [PubMed] [Google Scholar]


