Abstract
Lymph node (LN) dissection is a crucial procedure for cancer staging, diagnosis and treatment, and for predicting patient survival. Activation of lung metastatic lesions after LN dissection has been described for head and neck cancer and breast cancer. Preclinical studies have reported that dissection of a tumor‐bearing LN is involved in the activation and rapid growth of latent tumor metastases in distant organs, but it is also important to understand how normal (non‐tumor‐bearing) LN resection influences secondary cancer formation. Here, we describe how the resection of tumor‐bearing and non‐tumor‐bearing LN affects distant metastases in MXH10/Mo‐lpr/lpr mice. Tumor cells were administered intravenously and/or intranodally into the right subiliac lymph node (SiLN) to create a mouse model of lung metastasis. Luciferase imaging revealed that tumor cells in the lung were activated after resection of the SiLN, irrespective of whether it contained tumor cells. No luciferase activity was detected in the lungs of mice that did not undergo LN resection (excluding the intravenous inoculation group). Our results indicate that resection of an LN can activate distant metastases regardless of whether the LN contains tumor cells. Hence, lung metastatic lesions are suppressed while metastatic LN are present but activated after LN resection. If this phenomenon occurs in patients with cancer, it is likely that lung metastatic lesions may be activated by elective LN dissection in clinical N0 cases. The development of minimally invasive cancer therapy without surgery would help to minimize the risk of activation of distant metastatic lesions by LN resection.
Keywords: activation, lung metastasis, lymph node resection, mouse model, tumor cells
1. INTRODUCTION
Lymph node metastasis is a critical factor affecting the prognosis of patients with head and neck cancer and breast cancer. The classical theory proposes that lymph node metastasis occurs progressively from the upstream section to the downstream section of the lymphatic network, so that tumor cells eventually enter the blood circulation and produce distant metastases such as pulmonary metastasis.1 However, because only 2‐3 lymph nodes contain metastases in many cases of distant metastasis, the classical lymph node metastasis theory cannot easily explain the mechanism by which distant metastases are formed.2, 3, 4 Indeed, only a few cases of metastatic lesions in lymph nodes have been reported, but it has been assumed that lymph node metastasis plays an important role in the formation of distant metastasis.5, 6, 7 To elucidate the processes leading from lymph node metastasis to the formation of distant metastasis, our previous study examined the communication between the lymphatic network and blood circulation in a mouse model of lymph node metastasis in which the lymph nodes are of equivalent size to those of humans.8 We found numerous blood vessel branches that penetrated the lymph node capsule and communicated with blood vessels on the lymph node surface.8, 9
Because lymph node metastasis results from the proliferation of tumor cells that have invaded into the marginal sinus from the afferent lymphatic vessels, it is possible that these tumor cells invade the blood vessels penetrating the lymph node capsule and enter the blood circulation to reach distant organs, even during the early stages of lymph node metastasis. Our previous studies revealed that tumor cells migrated to the lungs in a relatively short period of time after their inoculation into a mouse lymph node.10, 11, 12 Therefore, we proposed a new concept of lymph node‐mediated hematogenous metastasis and have been working to clarify the mechanisms driving the onset of distant metastasis.9, 10 Papers published recently have supported the theory that lymph node vessels provide exit routes for metastatic tumor cells to the systemic circulation.13, 14 From another point of view, it has been shown in clinical studies that tumor cells attached to lung blood vessels are not activated for a considerable period of time, but these dormant tumor cells can be activated by surgical intervention, such as lymph node dissection.15, 16 In preliminary studies, we have created a mouse model in which dormant tumor cells in lung metastatic lesions are activated after lymph node dissection.10, 11, 12 In this model, the lung metastases were activated after excision of the lymph node into which the tumor cells had been inoculated. Because lymph node dissection is still considered to be the most effective treatment for lymph node metastasis, it is important to elucidate the mechanisms by which lymph node dissection activates distant metastatic lesions. In fact, if lymph node resection is confirmed to activate distant metastases in patients, it may be necessary to change the standard treatment regime for lymph node metastasis. In the present study, we have used a mouse model developed by us to further explore how lymph node resection might activate lung metastases.
2. MATERIALS AND METHODS
2.1. Ethics
The Institutional Animal Care and Use Committee of Tohoku University approved all the in vivo studies.
2.2. Mice
MXH10/Mo‐lpr/lpr (MXH10/Mo/lpr) mice17 were bred at the Institute for Animal Experimentation, Graduate School of Medicine, Tohoku University, Japan. Sixty‐six female and male mice (aged 12‐16 weeks) were used in this study, with no more than 4 animals housed per cage.
2.3. Cancer cells
Malignant fibrous histiocytoma‐like KM‐Luc/GFP cells,18 stably expressing a fusion of the luciferase (Luc) and enhanced green fluorescent protein (EGFP) genes, were used. On the inoculation day, cells were shown to test negative for Mycoplasma using a commercial kit (Lonza Rockland, Rockland, ME, USA).
2.4. Induction of experimental tumor metastasis
The experimental procedures were performed under general anesthesia with 2.5% isoflurane in oxygen, and great efforts were made to minimize animal suffering, as previously reported.10, 11, 12 The time of inoculation was defined as 0 hour. A 60 μL volume of KM‐Luc/GFP cells suspended in PBS (1.0 × 106 cells/mL) was inoculated intravenously into the tail vein of the mouse. Intranodal inoculation of cells into the right subiliac lymph node (SiLN) was achieved by incision of the overlying skin and injection of 60 μL of KM‐Luc/GFP cells (vide supra) with a 27‐G injection needle. The needle was maintained in the same position for 1 min to prevent tumor cells leaking from the SiLN after its removal. The exposed SiLN was thoroughly irrigated with 20 mL of sterile normal saline, and excess fluid was aspirated (M‐20 aspirator; Tokyo M.I. Company, Tokyo, Japan). Wounds were closed with 5‐0 polyamide interrupted sutures (Neoblade N; Alfresa Pharma, Osaka, Japan). Accurate intranodal inoculation of KM‐Luc/GFP cells and the occurrence of metastasis were confirmed when luciferase activity exceeded 4 × 104 photons/s. The mice were divided into 7 groups (a‐g; vide infra) according to the inoculation and resection methods (Figure 1A), and the f and g groups were each further divided into 3 subgroups (Figure 1B). Thus, there were 11 groups in total with 6 mice per group (Figure 1B). No active randomization was applied to experimental groups before the tumor cell inoculation. The groups were as follows:
Figure 1.

Experimental interventions and groups. A, The experimental interventions (a) to (g) show the KM‐Luc/GFP cell inoculation methods and resection techniques used in each group. Syringe: tumor cell inoculation site; black‐filled scissors: resection site; white‐filled scissors: sham surgery site. B, Interventions used in each experimental group (a) RIN (intranodal inoculation into the right SiLN) control group (n = 6). Tumor cells would enter both the PALN via the afferent lymphatic vessel and the systemic circulation via the thoracoepigastric vein. b, IV (intravenous inoculation) group (n = 6), which provides a hematogenous route to the lung. This method has been used widely to induce metastasis in the lung. c, IV+RIN (intravenous inoculation and intranodal inoculation into the right SiLN) group. d, IV+RIN+RS (intravenous inoculation and intranodal inoculation into the right SiLN with sham surgery in the region of the right SiLN) group (n = 6), which was used to examine the effects of surgical invasion without lymph node resection. The sham surgery was conducted 72 hours after tumor cell inoculation. e, IV+RR (intravenous inoculation with right SiLN resection) group (n = 6), which was used to investigate the effect of lymph node resection during metastasis formation. The resection of the SiLN was conducted 72 hours after tumor cell inoculation. f, IV+RIN+LR (intravenous inoculation and intranodal inoculation into the right SiLN with left SiLN resection), which was used to observe the effects of normal (ie, non‐metastatic) lymph node resection. The group was divided into 3 subgroups (n = 6 per subgroup) according to the timing of the resection: f1 (6 hours after tumor cell inoculation), f2 (72 hours after tumor cell inoculation) and f3 (144 hours after tumor cell inoculation). g, IV+RIN+RR (intravenous inoculation and intranodal inoculation into the right SiLN with right SiLN resection) group, which was used to investigate the effects of metastatic lymph node resection. The group was divided into 3 subgroups (n = 6 per subgroup) according to the timing of the resection: g1 (6 hours after tumor cell inoculation), g2 (72 hours after tumor cell inoculation) and g3 (144 hours after tumor cell inoculation). PALN, proper axillary lymph node
RIN (intranodal inoculation into the right SiLN) group (n = 6), used as a control. Tumor cells would enter both the proper axillary lymph node (PALN) via the afferent lymphatic vessel and the systemic circulation via the thoracoepigastric vein.
IV (intravenous inoculation) group (n = 6), which provided the tumor cells with a hematogenous route to the lungs. In this group, tumor cells were administered intravenously but were not inoculated directly into a lymph node. This method has been widely used to induce metastasis in the lungs.
IV+RIN (intravenous inoculation and intranodal inoculation into the right SiLN) group (n = 6).
IV+RIN+RS (intravenous inoculation and intranodal inoculation into the right SiLN with sham surgery in the region of the right SiLN) group, which was used to examine the effect of surgical invasion without lymph node resection. The sham surgery was conducted 72 hours after tumor cell inoculation (n = 6).
IV+RR (intravenous inoculation with right SiLN resection) group, which was used to investigate the effect of lymph node resection during the formation of metastases. The resection of the SiLN was conducted 72 hours after tumor cell inoculation (n = 6).
IV+RIN+LR (intravenous inoculation and intranodal inoculation into the right SiLN with resection of the left SiLN), which was used to observe the effect of resection of a lymph node not containing tumor cells (ie, non‐metastatic lymph node). The group was divided into 3 subgroups (n = 6 per subgroup) according to the time at which resection was conducted: f1 (6 hours after tumor cell inoculation), f2 (72 hours after tumor cell inoculation) and f3 (144 hours after tumor cell inoculation).
IV+RIN+RR (intravenous inoculation and intranodal inoculation into the right SiLN with resection of the right SiLN) group, which was used to investigate the effect of resection of a metastatic lymph node. The group was divided into 3 subgroups (n = 6 per subgroup) according to the time at which resection was conducted: g1 (6 hours after tumor cell inoculation), g2 (72 hours after tumor cell inoculation) and g3 (144 hours after tumor cell inoculation).
2.5. Detection of metastasis in the proper axillary lymph node
Metastasis to the PALN was assessed using an in vivo bioluminescence imaging system (IVIS; Xenogen, Alameda, CA, USA)18, 19 at 6, 72, 144 and 216 hours post‐inoculation of tumor cells (n = 66). Each mouse was anesthetized with 2.5% isoflurane in oxygen, and 15 mg/kg luciferin (Promega, Madison, WI, USA) was injected intraperitoneally. At 10 minutes after the luciferin injection, bioluminescence images were captured for 30 seconds using the IVIS. Induction of metastasis was considered to have occurred if the luciferase intensity in the PALN was more than the background level (4 × 104 photons/s). In a minority of mice, metastasis to the PALN failed because of tumor cell blockage of the lymphatic vessels.
2.6. Induction of metastasis in the lungs after resection of the right subiliac lymph node
The experimental procedures were conducted as previously reported.10, 11, 12 Briefly, on the indicated days after inoculation, mice were placed in a supine position on a heated stage and anesthetized by inhalation of 2.5% isoflurane in oxygen. After depilation and skin disinfection, a minimally invasive approach was used for skin incision and exposure and extirpation of the tumor‐bearing or non‐tumor‐bearing SiLN. The tumor‐bearing or non‐tumor‐bearing SiLN was carefully removed with minimal disruption to the surrounding area, and a bipolar coagulator (Erbotom VI050C; ERBE Elektromedizin GmbH, Tübingen, Germany) and surgical microscope (Leica M80; Leica Microsystems GmbH, Wetzlar, Germany) were used to facilitate the resection process. The surgical area was thoroughly washed with 20 mL of sterile saline (37°C) to remove any tumor cells that had leaked from the SiLN. The skin wound was closed with 5‐0 polyamide interrupted sutures. The entire resection procedure was accomplished within 10 minutes. Survival of the mice was monitored, and the experiment was continued according to the protocol. In mice inoculated with tumor cells into the SiLN, the IVIS was used after surgery to determine whether the tumor‐bearing SiLN had been completely resected and whether metastasis had occurred in the lungs.
2.7. Detection of metastasis in the lung
Metastasis to the lungs was assessed using the IVIS18, 19 at 6, 72, 144 and 216 hours post‐inoculation, in the presence and absence of the SiLN (n = 66). Luciferin (15 mg/kg; Promega) was injected intraperitoneally into each mouse. Bioluminescence imaging was performed for 30 seconds, 10 minutes after the luciferin injection. Induction of metastasis was considered to have occurred if the luciferase intensity was more than the background level (4 × 104 photons/s). Although metastasis in the lung was not detected by in vivo bioluminescence imaging in some mice, it was subsequently confirmed by ex vivo bioluminescence imaging in these mice.
2.8. Ex vivo analysis
Mice from all 11 experimental groups were humanely euthanized under the inhalation of 2.5% isoflurane in oxygen at 216 hours post‐inoculation, and the lungs, PALN, spleen and liver were excised. Ex vivo bioluminescence imaging of the excised organs was performed immediately after the in vivo analyses for each mouse.
2.9. Adverse effects
Daily observations were made of food and water consumption, effects of anesthesia, postoperative complications and stool tests. Significant weight loss was considered to be >10% of body weight.
2.10. Histological analysis
The excised organs were fixed in 10% formaldehyde for 4 days at 4°C, dehydrated and embedded in paraffin. Specimens were serially sectioned (2 μm) and stained with Elastica‐Masson (EM) or H&E or immunostained for CD31 or Ki67. Tumor cell proliferation was evaluated using staining for Ki67. The Ki67 index in the lung was quantified using Image J (Bethesda, Maryland, USA).20 The hotspot method was used to calculate the CD31‐positive area in the lung. Four hotspot fields containing the highest amount of CD31‐positive staining were selected under low magnification (×10 or ×20), and the positively staining area was calculated as a percentage of the total area. Total tumor area in the lung was analyzed using cellSens 1.8.1 software (Olympus, Tokyo, Japan) to obtain the average diameter of the metastatic foci.
2.11. Statistical analysis
Statistical significance was determined using one‐way analyses of variance (ANOVA) Kruskal‐Wallis test, unless otherwise indicated. A P‐value ≤.5 was considered to be statistically significant. All measurements are presented as the mean ± SEM. Statistical analyses were carried out using Excel 2016 (Microsoft, Redmond, WA, USA) and Prism 7 (GraphPad Software, La Jolla, CA, USA).
3. RESULTS
3.1. Detection of metastasis in the lung and proper axillary lymph node
Figure 2 shows representative in vivo and ex vivo bioluminescence images, and Figure S1A and B show ex vivo luciferase activity in the lung and PALN, respectively. After intranodal inoculation into the rightSiLN (ie, RIN group), tumor cells migrated to the PALN via the lymphatic vessels and to the lung through the systemic blood circulation via the thoracoepigastric vein (Figure 1Aa). The incidence of metastasis in the lung and PALN was 17% and 50%, respectively (Table 1, Figure S1A and B). In the IV group (Figure 1Ab), in which tumor cells were directly inoculated into the tail vein, the incidence of metastasis in the lung and PALN was 100% and 0%, respectively (Table 1, Figure S1A and B).
Figure 2.

Representative in vivo bioluminescence images of mice and ex vivo bioluminescence images of the lungs and proper axillary lymph node (PALN). The lungs and PALN were harvested at 216 hours post‐inoculation in all groups (n = 6 per group). a, RIN group: intranodal inoculation of the right SiLN without resection. b, IV group: intravenous inoculation through the tail vein. c, IV+RIN group: intravenous inoculation through the tail vein and intranodal inoculation of the right SiLN without resection. d, IV+RIN+RS‐72 hours group: intravenous inoculation through the tail vein and intranodal inoculation into the right SiLN with sham surgery performed in the region of the (tumor‐bearing) right SiLN at 72 hours post‐inoculation. e, IV+RR‐72 hours group: intravenous inoculation through the tail vein with resection of the normal (ie, non‐metastatic) right SiLN at 72 hours post‐inoculation. f1, IV+RIN+LR‐6 hours group: intravenous inoculation through the tail vein and intranodal inoculation into the right SiLN with resection of the non‐tumor‐bearing left SiLN at 6 hours post‐inoculation. f2, IV+RIN+LR‐72 hours group: intravenous inoculation through the tail vein and intranodal inoculation into the right SiLN with resection of the non‐tumor‐bearing left SiLN at 72 hours post‐inoculation. f3, IV+RIN+LR‐144 hours group: intravenous inoculation through the tail vein and intranodal inoculation into the right SiLN with resection of the non‐tumor‐bearing left SiLN at 144 hours post‐inoculation. g1, IV+RIN+RR‐6 hours group: intravenous inoculation through the tail vein and intranodal inoculation into the right SiLN with resection of the tumor‐bearing right SiLN at 6 hours post‐inoculation. g2, IV+RIN+RR‐72 hours group: intravenous inoculation through the tail vein and intranodal inoculation into the right SiLN with resection of the tumor‐bearing right SiLN at 72 hours post‐inoculation. g3, IV+RIN+RR‐144 hours group: intravenous inoculation through the tail vein and intranodal inoculation into the right SiLN with resection of the tumor‐bearing right SiLN at 144 hours post‐inoculation. No ex vivo luciferase activity was detected in the lung in the non‐resection groups, with the exception of the IV group. Tumor cells in the lung were activated after resection of the SiLN in the resection groups, excluding the IV+RR group. Tumor cells metastasized by the hematogenous route after inoculation into the tail vein in the IV and IV+RR groups; therefore, induction of lung metastasis was independent of lymph node removal. Ex vivo luciferase activity in the lung was highest in the IV+RR‐72 hours and the IV+RIN+RR6 hours groups. In the IV+RIN+LR groups, luciferase activity in the lung was highest when the resection was performed early (6 hours) and lowest when it was performed late (144 hours). Metastasis in the PALN was detected in the RIN and IV+RIN+RR groups. Metastasis in the PALN was not detected in the IV, IV+RR, IV+RIN or IV+RIN+RS groups
Table 1.
Conditions and subsequent results
| Group | n | Incidence of metastasisa | Average diameter of metastatic foci in the lung (μm) | ||||
|---|---|---|---|---|---|---|---|
| Lung | PALN | ||||||
| Metastasis (+)a | Percentage (%) | Metastasis (+) | Percentage (%) | ||||
| a | RIN | 6 | 1 | 17 | 3 | 50 | 19.3 ± 3.7 |
| b | IV | 6 | 6 | 100 | 0 | 0 | 64.0 ± 13.6 |
| c | IV+RIN | 6 | 1 | 17 | 1 | 17 | 5.5 ± 2.4 |
| d | IV+RIN+RS‐72 h | 6 | 2 | 33 | 0 | 0 | 14.0 ± 3.3 |
| e | IV+RR‐72 h | 6 | 6 | 100 | 0 | 0 | 137.5 ± 21.7 |
| f | |||||||
| 1 | IV+RIN+LR‐6 h | 6 | 5 | 83 | 0 | 0 | 71.4 ± 18.8 |
| 2 | IV+RIN+LR‐72 h | 6 | 6 | 100 | 1 | 17 | 65.9 ± 17.5 |
| 3 | IV+RIN+LR‐144 h | 6 | 5 | 83 | 0 | 0 | 103.0 ± 24.3 |
| g | |||||||
| 1 | IV+RIN+RR‐6 h | 6 | 5 | 83 | 3 | 50 | 96.9 ± 19.0 |
| 2 | IV+RIN+RR‐72 h | 6 | 6 | 100 | 3 | 50 | 90.8 ± 13.8 |
| 3 | IV+RIN+RR‐144 h | 6 | 6 | 100 | 2 | 33 | 121.5 ± 19.2 |
IV, intravenous inoculation into the tail vein; IVIS, in vivo bioluminescence imaging system; PALN, proper axillary lymph node; RIN, intranodal inoculation into the right SiLN.
The incidence of metastasis was determined by ex vivo bioluminescence in lung and PALN.
(+) means that metastasis was positive.
In the IV+RIN group (Figure 1Ac), the incidence of metastasis in the lung (17%) was lower than that in the IV group, and the incidence of metastasis in the PALN (17%) was lower than that in the RIN group (Table 1, Figure S1A and B). The incidence of metastasis in the lung in the IV+RIN+RS‐72 hours group (Figure 1Ad) was also lower than that in the IV group (33% vs 100%).
In the groups in which normal (non‐metastatic) lymph nodes were resected, ie, the IV+RR‐72 hours, IV+RIN+LR‐6 hours, IV+RIN+LR‐72 hours and IV+RIN+LR‐144 hours groups (Figure 1Ae,f), the incidence of metastasis in the lung was increased to 100%, 83%, 100% and 83%, respectively, and the incidence of metastasis in the PALN was suppressed to 0%, 0%, 17% and 0%, respectively (Table 1, Figure S1A and B). The incidence of lung metastasis in the above groups was dependent on the timing of the lymph node resection. In the groups in which tumor‐bearing lymph nodes were resected, ie, the IV+RIN+RR‐6 hours, IV+RIN+RR‐72 hours and IV+RIN+RR‐144 hours groups (Figure 1Ag), the incidence of metastasis in the lung increased to 83%, 100% and 100%, respectively, and the incidence of metastasis in the PALN decreased to 50%, 50% and 33%, respectively (Table 1, Figure S1A and B). The incidence of metastasis in the lung and PALN in the IV+RIN+RR groups was dependent on the timing of the resection of the tumor‐bearing lymph node. No statistically significant differences were observed in the activation of the lung metastasis between the groups. No statistically significant differences were found for metastasis in the PALN between the groups. These results suggest that resection of a lymph node increased the incidence of metastasis in the lung, irrespective of whether the lymph node contained tumor cells. In addition, resection of a tumor‐bearing lymph node (ie, a SiLN inoculated with tumor cells) induced metastasis to the downstream lymph node (PALN). The incidence of metastasis in the lung was dependent on the timing of lymph node resection regardless of whether tumor cells were absent or present. The incidence of metastasis in the PALN was independent of normal lymph node resection but dependent on the time at which a tumor‐bearing lymph node was resected. It should be noted that significant weight loss was not observed during these experiments (Figure S1C).
3.2. Histological analysis
Figure 3 shows representative lung sections stained with H&E or EM or immunostained for Ki67. Tumor cells were found in the pulmonary arteriol of the IV, IV+RR‐72 hours, IV+RIN+LR and IV+RIN+RR groups (Figure 3b1,e1‐k1). The boxes in the H&E‐stained sections highlight the regions shown for Ki67 immunostaining (Figure 3a2‐k2). No tumor foci in the lung were found in the RIN, IV+RIN or IV+RIN+RS‐72 hours groups. Several Ki67 positive cells were found in the tumor mass of dissection groups. Figure 4 shows the average diameter of the metastatic foci, total tumor area, Ki67 index and CD31‐positive area in the lung. The maximal average diameter of the metastatic foci (137.5 ± 21.7 μm) was detected in the lungs of the IV+RR‐72 hours group, and the smallest average diameter of the metastatic foci (5.5 ± 2.4 μm) was detected in the lungs of the IV+RIN group (Table 1 and Figure 4A). The average diameter of the metastatic foci in the lungs of the IV+RR‐72 hours group was higher than that of the IV group. Statistically significant differences were found for RIN vs IV, IV+RIN vs IV+RR‐72 hours, IV+RIN vs IV+RIN+LR‐6 hours, and IV+RIN+RS‐72 hours vs IV+RR‐72 hours. The formation of metastatic foci in the lung was not obviously dependent on whether a normal lymph node or tumor‐bearing lymph node was resected (Figure 4A). However, we found that later resection of the lymph node in the IV+RIN+LR and IV+RIN+RR groups appeared to be associated with the formation of larger metastatic foci.
Figure 3.
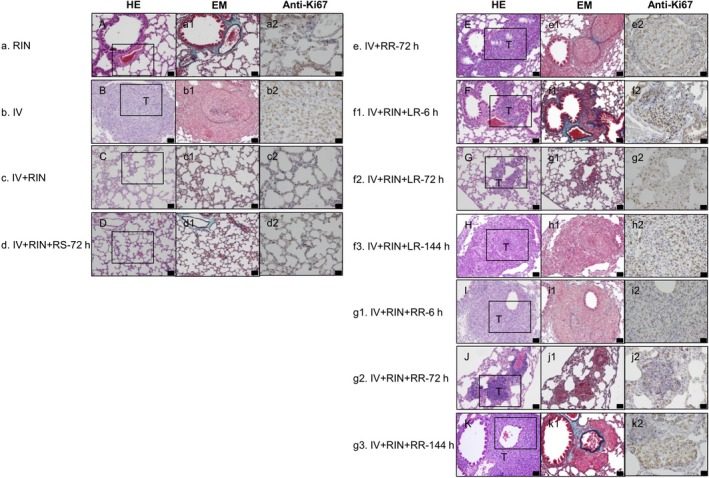
Histological analysis of the lung. a, RIN, b, IV, c, IV+RIN, d, IV+RIN+RS‐72 hours, e, IV+RR‐72 hours, f1, IV+RIN+LR‐6 hours, f2, IV+RIN+LR‐72 hours, f3, IV+RIN+LR‐144 hours, g1, IV+RIN+RR‐6 hours, g2, IV+RIN+RR‐72 hours, g3, IV+RIN+RR‐144 hours. A, C, D, The lung structure was normal in the RIN, IV+RIN and IV+RIN+RS groups. B, E‐K, Metastatic foci in the lung were observed in the IV, IV+RR, IV+RIN+LR and IV+RIN+RR groups. HE staining: A‐K, EM staining: a1‐k1, and immunostaining of Ki67: a2‐k2. The lungs were harvested at 216 hours post‐inoculation. Scale bar: 50 μm. Boxes in A‐K outline the region shown in a2‐k2. In the IV and IV+RR‐72 hours groups, proliferating tumor cells were found in the blood vessels after intravenous inoculation of the tumor cells. Tumor cells in the lung were activated after resection of the SiLN in the IV+RIN+LR and IV+RIN+RR groups. Tumor cell proliferation in the metastatic foci was higher in the groups showing notable lung metastasis than in the RIN, IV+RIN and IV+RIN+RS groups. EM, Elastica‐Masson
Figure 4.

Quantification of the histological analyses. A, Average diameter of the metastatic foci in the lung was quantified from HE‐stained sections. A statistically significant difference was found between the RIN and IV groups (Kruskal‐Wallis test: *P < .05, RIN vs IV), IV+RIN and IV+RR‐72 hours groups (Kruskal‐Wallis test: **P < .01, IV+RIN vs IV+RR‐72 hours), IV+RIN and IV+RIN+LR‐6 hours groups (Kruskal‐Wallis test: *P < .05, IV+RIN vs IV+RIN+LR‐6 hours), and IV+RIN+RS‐72 hours and IV+RR‐72 hours groups (Kruskal‐Wallis test: *P < .05, IV+RIN+RS‐72 hours vs IV+RR‐72 hours). Data are presented as the mean ± SEM. B, The total tumor area in the lung was quantified from HE‐stained sections. A statistically significant difference was found between the RIN and IV+RIN groups (Kruskal‐Wallis test: ***P < .001, RIN vs IV+RIN), RIN and IV+RIN+RS‐72 hours groups (Kruskal‐Wallis test: **P < .01, RIN vs IV+RIN+RS‐72 hours), IV+RIN and IV+RR‐72 hours groups (Kruskal‐Wallis test: *P < .05, IV+RIN vs IV+RR‐72 hours) and IV+RIN+RS‐72 hours and IV+RR‐72 hours groups (Kruskal‐Wallis test: **P < .01, IV+RIN+RS‐72 hours vs IV+RR‐72 hours). Data are presented as the mean ± SEM. C, Ki67 index in the lung was quantified from sections immunostained for Ki67. There were statistically significant differences between the RIN and IV+RR‐72 hours groups (Kruskal‐Wallis test: ***P < .001, RIN vs IV+RR‐72 hours), RIN and IV+RIN+RR‐6 hours (Kruskal‐Wallis test: *P < .05, RIN vs IV+RIN+RR‐6 hours) and RIN and IV+RIN+RR‐144 hours groups (Kruskal‐Wallis test: **P < .01, RIN vs IV+RIN+RR‐144 hours). Data are presented as the mean ± SEM. D, CD31‐positive area quantified from sections immunostained for CD31. No statistically significant differences were detected. Data are presented as the mean ± SEM
As shown in Figure 4B, the smallest tumor area was found in the lungs of the RIN, IV+RIN and IV+RIN+RS groups, and the tumor area was increased in all the resection groups. The maximal tumor area was found in the IV+RR and IV+RIN+RR‐6 hours groups (Figure 4B). In the IV+RIN+LR group, the total tumor area appeared to increase as the timing of the resection of the normal SiLN was delayed (Figure 4B). A statistically significant difference was found for RIN vs IV+RIN, RIN vs IV+RIN+RS‐72 hours, IV+RIN vs IV+RR‐72 hours, and IV+RIN+RS‐72 hours vs IV+RR‐72 hours.
Figure 4C shows the Ki67 index in the lung. Tumor cell proliferation was numerically higher in the IV+RR group than in the other experimental groups. A statistically significant difference was found for RIN vs IV+RR‐72 hours, RIN vs IV+RIN+RR‐6 hours, and RIN vs IV+RIN+RR‐144 hours.
Figure 4D shows the CD31‐positive area in the lung (CD31‐immunostained sections are shown in Figure S2a2‐k2). No significant differences were detected between groups. However, the minimal CD31‐positive area was observed in the IV+RIN+RS group, whereas the maximal CD31‐positive area was detected in the SiLN resection groups (IV+RIN+LR and IV+RIN+RR). Although the CD31‐positive area appeared to increase in the lymph node resection groups, this increase was independent of the resection time.
Sections of the PALN stained with H&E or immunostained for Ki67 are shown in Figure S2. Metastasis was detected in the PALN of the RIN, IV+RIN+RS‐72 hours, IV+RIN+RR and IV+RIN+LR groups. Histologically, no tumor cells were found in the liver and spleen in sections stained with H&E in all the groups (Figure S3).
4. DISCUSSION
Activation of lung metastatic lesions after lymph node resection has been reported in clinical studies of head and neck cancer and breast cancer.3, 21 We have proposed a new concept of lymph node‐mediated hematogenous metastasis in which tumor cells invade the blood vessels of the lymph node capsule and then induce metastasis in distant organs during the early stages of lymph node metastasis.10, 12 Our previous studies have revealed that tumor cells reach the lungs in a relatively short period of time after their inoculation into mouse lymph nodes.9, 10, 12 Although it has been confirmed that lung metastatic lesions are activated by resection of lymph nodes inoculated with tumor cells, the underlying mechanisms remain unknown.10, 12 In the present study, we used a mouse model to study lung metastasis activation by lymph node resection and explore which conditions promoted the activation of lung metastases (Figure 2).
In the RIN group, in which tumor cells were inoculated into the SiLN, lung metastasis occurred infrequently with only small metastatic foci observed (Figure 2a and Table 1). In the IV group, which received an intravenous inoculation of tumor cells, lung metastasis was confirmed in all cases with clear evidence of metastatic foci (Figure 2 and Table 1). However, in the IV+RIN group, which was inoculated with tumor cells both intravenously and into the SiLN, lung metastasis was less extensive than that in the IV group (Figure 2 and Table 1). Although this finding suggests that the presence of tumor cells in a lymph node (ie, lymph node metastasis) may suppress the development and/or progression of lung metastasis, the underlying mechanism is unknown. Engel et al22 report that randomized clinical trials (ie, the highest level of clinical evidence) did not reveal a survival benefit of lymph node dissection. Engel's team have evaluated the prognosis associated with a positive lymph node and the growth rate of a positive lymph node, estimating the time of first and subsequent lymph node infiltrations,23, 24 and their data support the basic hypothesis that a positive lymph node does not metastasize.22, 25 In contrast, in our study, activation of lung metastasis was not induced by sham surgery but was induced by excision of lymph nodes irrespective of whether they had been inoculated with tumor cells (Figure 2 and Table 1). There was no evidence that the period of tumor cell proliferation or the size of the metastatic lesion in the lymph node defined the level of activation of lung metastasis. To summarize the above findings, lung metastasis (after intravenous inoculation of tumor cells) was suppressed to some extent during the period in which a metastatic lymph node was present, but tumor cells in the lung arteriols were activated by resection of a lymph node irrespective of whether the lymph node contained tumor cells (Figures 2 and 3 and Table 1).
If the same phenomenon were to occur in patients with cancer, it is possible that lung metastasis would be activated by elective resection of lymph nodes in clinical N0 cases. Therefore, if elective lymph node dissection activates potential microscopic distant metastases, which are not detectable using current diagnostic imaging systems, cancer radio‐chemotherapy without surgical treatment might be considered a more desirable option in patients with N0 disease. However, considering the adverse effects and the uncertainty of the response, it would not be realistic to recommend cancer radio‐chemotherapy without surgery to patients with N0 disease.
In earlier studies, we examined the communication between the lymphatic network and blood circulation in a mouse model of lymph node metastasis.8, 9 Interestingly, we observed several blood vessel branches that penetrated the lymph node capsule.8, 9 In addition, the findings indicated that tumor cells invaded from the afferent lymphatic vessels into the marginal sinuses of the lymph nodes and infiltrated the vessel branches penetrating the lymph node capsule at an early stage of tumor cell proliferation.8, 9 We have considered previously described phenomena that might account for the mechanisms of distant metastasis onset and proposed a theory of metastasis termed lymph node‐mediated hematogenous metastasis.8, 9 In the present study, tumor cells were inoculated into the lymph node and/or injected intravenously into the tail vein, and lung metastasis was confirmed after the removal of the lymph node, regardless of whether or not it contained tumor cells. Our current understanding is that intravenously and intranodally inoculated tumor cells reach lung blood vessels and remain there in an inactive mode. The activation of latent metastatic cells after lymph node resection indicates that an immunological response had initially prevented the progression of metastasis in the lung and that lymph node resection had disrupted this immunological response. Thus, latent metastasis in the lung implies that lymph node‐mediated hematogenous metastasis had occurred. Tumor latency is a stage of tumor development that is not associated with clinical symptoms. Two mechanisms have been implicated in tumor latency: one involves a single disseminated tumor cell undergoing growth arrest and is called tumor cell dormancy, and the other involves cell cycle arrest and is termed tumor mass dormancy.26, 27, 28 The observation of Ki67‐positive cells suggests that, in certain niches, dormant cells are activated to divide. Additional research is needed to investigate the immune responses and cell cycle changes that occur after tumor cell inoculation and lymph node resection. We do not know which molecules are involved in the activation of tumor cells in the lung after resection. Recently, we observed that lysine oxidase expression was significantly higher in lymph node resection groups than in the control group.12 These findings are consistent with the results of previous studies,29, 30 suggesting that high lysine oxidase expression in metastatic sites is closely associated with tumor cell colonization and metastasis.
One limitation of the present study was that only one cell line (KM‐Luc/GFP) and mouse strain (MXH10/Mo/lpr) were used. Further experiments utilizing different cell lines and mouse strains are needed to confirm the present results. Another potential limitation was that our experimental model of lung metastasis (generated using intravenous or intranodal injection of tumor cells) did not fully replicate the process of systemic metastasis that occurs in patients with cancer. However, the results obtained using this model may still be relevant to cancer metastasis in humans. In addition, it will be important to elucidate the molecular mechanisms underlying the activation of latent pulmonary metastases by the resection of tumor‐bearing and tumor‐free lymph nodes.
Development of a minimally invasive treatment to prevent lymph node‐mediated hematogenous metastasis is desirable, and we have previously reported (using the same animal model) that anticancer drugs can be injected into an upstream lymph node to treat micrometastasis in a downstream lymph node.31, 32, 33 A lymphatic anticancer drug delivery system can remove the origin of lymph node‐mediated hematogenous metastasis by targeting micrometastases in lymph nodes, avoiding surgical intervention and preventing the risk of activation of distant metastatic lesions by lymph node resection. Therefore, this novel cancer chemotherapy approach may be a promising technique that should be further investigated in future studies.
In conclusion, tumor‐bearing or non‐tumor‐bearing lymph node resection/biopsy could be responsible for an increased incidence of secondary cancer foci. The resection/biopsy of metastatic lymph nodes may increase metastasis via hematogenous and lymphogenous pathways if the lymph nodes contain tumor cells. Removal of healthy lymph nodes may induce metastases in distant organs if the organs contain latent tumor cells. It is important to develop an improved diagnostic method that can diagnose cancer at an early stage to help limit its progression. The development of minimally invasive cancer therapy without surgical intervention would potentially remove the risk of activation of distant metastatic lesions by lymph node resection. Thus, intranodal or intralymphatic inoculation of de‐activated tumor cells may potentially lead to lymphatic system vaccination.
CONFLICTS OF INTEREST
The authors have no conflicts of interest.
Supporting information
ACKNOWLEDGMENTS
The authors would like to thank T. Sato for technical assistance and the Biomedical Research Core of Tohoku University Graduate School of Medicine for technical support.
Sukhbaatar A, Mori S, Saiki Y, Takahashi T, Horii A, Kodama T. Lymph node resection induces the activation of tumor cells in the lungs. Cancer Sci. 2019;110:509–518. 10.1111/cas.13898
REFERENCES
- 1. Halsted WSI. The results of radical operations for the cure of carcinoma of the breast. Ann Surg. 1907;46:1‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cady B. Basic principles in surgical oncology. Arch Surg. 1997;132:338‐346. [DOI] [PubMed] [Google Scholar]
- 3. Cho JK, Hyun SH, Choi N, et al. Significance of lymph node metastasis in cancer dissemination of head and neck cancer. Transl Oncol. 2015;8:119‐125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kawada K, Taketo MM. Significance and mechanism of lymph node metastasis in cancer progression. Cancer Res. 2011;71:1214‐1218. [DOI] [PubMed] [Google Scholar]
- 5. Hellman S. Karnofsky Memorial Lecture. Natural history of small breast cancers. J Clin Oncol. 1994;12:2229‐2234. [DOI] [PubMed] [Google Scholar]
- 6. Stacker SA, Williams SP, Karnezis T, Shayan R, Fox SB, Achen MG. Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nat Rev Cancer. 2014;14:159‐172. [DOI] [PubMed] [Google Scholar]
- 7. Reintgen D, Cruse CW, Wells K, et al. The orderly progression of melanoma nodal metastases. Ann Surg. 1994;220:759‐767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shao L, Takeda K, Kato S, Mori S, Kodama T. Communication between lymphatic and venous systems in mice. J Immunol Methods. 2015;424:100‐105. [DOI] [PubMed] [Google Scholar]
- 9. Takeda K, Mori S, Kodama T. Study of fluid dynamics reveals direct communications between lymphatic vessels and venous blood vessels at lymph nodes of mice. J Immunol Methods. 2017;445:1‐9. [DOI] [PubMed] [Google Scholar]
- 10. Shao L, Ouchi T, Sakamoto M, Mori S, Kodama T. Activation of latent metastases in the lung after resection of a metastatic lymph node in a lymph node metastasis mouse model. Biochem Biophys Res Commun. 2015;460:543‐548. [DOI] [PubMed] [Google Scholar]
- 11. Ouchi T, Sukhbaatar A, Horie S, et al. Superselective drug delivery using doxorubicin‐encapsulated liposomes and ultrasound in a mouse model of lung metastasis activation. Ultrasound Med Biol. 2018;44:1818‐1827. [DOI] [PubMed] [Google Scholar]
- 12. Zheng J, Jia L, Mori S, Kodama T. Evaluation of metastatic niches in distant organs after surgical removal of tumor‐bearing lymph nodes. BMC Cancer. 2018;18:608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brown M, Assen FP, Leithner A, et al. Lymph node blood vessels provide exit routes for metastatic tumor cell dissemination in mice. Science. 2018;359:1408‐1411. [DOI] [PubMed] [Google Scholar]
- 14. Pereira ER, Kedrin D, Seano G, et al. Lymph node metastases can invade local blood vessels, exit the node, and colonize distant organs in mice. Science. 2018;359:1403‐1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Aguirre‐Ghiso JA, Sosa MS. Emerging topics on disseminated cancer cell dormancy and the paradigm of metastasis. Ann Rev Cancer Biol. 2018;2:377‐393. [Google Scholar]
- 16. Gao XL, Zhang M, Tang YL, Liang XH. Cancer cell dormancy: mechanisms and implications of cancer recurrence and metastasis. Onco Targets Ther. 2017;10:5219‐5228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shao L, Mori S, Yagishita Y, et al. Lymphatic mapping of mice with systemic lymphoproliferative disorder: usefulness as an inter‐lymph node metastasis model of cancer. J Immunol Methods. 2013;389:69‐78. [DOI] [PubMed] [Google Scholar]
- 18. Li L, Mori S, Sakamoto M, Takahashi S, Kodama T. Mouse model of lymph node metastasis via afferent lymphatic vessels for development of imaging modalities. PLoS ONE. 2013;8:e55797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li L, Mori S, Kodama M, Sakamoto M, Takahashi S, Kodama T. Enhanced sonographic imaging to diagnose lymph node metastasis: importance of blood vessel volume and density. Cancer Res. 2013;73:2082‐2092. [DOI] [PubMed] [Google Scholar]
- 20. Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671‐675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pavlidis N, Khaled H, Gaafar R. A mini review on cancer of unknown primary site: a clinical puzzle for the oncologists. J Adv Res. 2015;6:375‐382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Engel J, Emeny RT, Holzel D. Positive lymph nodes do not metastasize. Cancer Metastasis Rev. 2012;31:235‐246. [DOI] [PubMed] [Google Scholar]
- 23. Veronesi U, Marubini E, Mariani L, Valagussa P, Zucali R. The dissection of internal mammary nodes does not improve the survival of breast cancer patients. 30‐year results of a randomised trial. Eur J Cancer. 1999;35:1320‐1325. [DOI] [PubMed] [Google Scholar]
- 24. Hartgrink HH, van de Velde CJ, Putter H, et al. Extended lymph node dissection for gastric cancer: who may benefit? Final results of the randomized Dutch gastric cancer group trial. J Clin Oncol. 2004;22:2069‐2077. [DOI] [PubMed] [Google Scholar]
- 25. Morton DL, Thompson JF, Cochran AJ, et al. Sentinel‐node biopsy or nodal observation in melanoma. N Engl J Med. 2006;355:1307‐1317. [DOI] [PubMed] [Google Scholar]
- 26. Yeh AC, Ramaswamy S. Mechanisms of cancer cell dormancy – another hallmark of cancer? Cancer Res. 2015;75:5014‐5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gomis RR, Gawrzak S. Tumor cell dormancy. Mol Oncol. 2017;11:62‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Aguirre‐Ghiso JA. Models, mechanisms and clinical evidence for cancer dormancy. Nat Rev Cancer. 2007;7:834‐846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Erler JT, Bennewith KL, Cox TR, et al. Hypoxia‐induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell. 2009;15:35‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Perryman L, Erler JT. Lysyl oxidase in cancer research. Future Oncol. 2014;10:1709‐1717. [DOI] [PubMed] [Google Scholar]
- 31. Fujii H, Horie S, Takeda K, Mori S, Kodama T. Optimal range of injection rates for a lymphatic drug delivery system. J Biophotonics. 2018;11:e201700401. [DOI] [PubMed] [Google Scholar]
- 32. Kodama T, Matsuki D, Tada A, Takeda K, Mori S. New concept for the prevention and treatment of metastatic lymph nodes using chemotherapy administered via the lymphatic network. Sci Rep. 2016;6:32506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tada A, Horie S, Mori S, Kodama T. Therapeutic effect of cisplatin given with a lymphatic drug delivery system on false‐negative metastatic lymph nodes. Cancer Sci. 2017;108:2115‐2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


