Abstract
l‐Type amino acid transporter 1 (LAT1) disulfide linked to CD98 heavy chain (hc) is highly expressed in most cancer cells, but weakly expressed in normal cells. In the present study, we developed novel anti‐LAT1 mAbs and showed internalization activity, inhibitory effects of amino acid uptake and cell growth and antibody‐dependent cellular cytotoxicity, as well as in vivo antitumor effects in athymic mice. Furthermore, we examined the reactivity of mAbs with LAT1 of Macaca fascicularis to evaluate possible side‐effects of antihuman LAT1 mAbs in clinical trials. Antihuman LAT1 mAbs reacted with ACHN human and MK.P3 macaca kidney‐derived cells, and this reactivity was significantly decreased by siRNAs against LAT1. Macaca LAT1 cDNA was cloned from MK.P3, and only two amino acid differences between human and macaca LAT1 were seen. RH7777 rat hepatoma and HEK293 human embryonic kidney cells expressing macaca LAT1 were established as stable transfectants, and antihuman LAT1 mAbs were equivalently reactive against transfectants expressing human or macaca LAT1. Dual (high and low) avidity modes were detected in transfectants expressing macaca LAT1, MK.P3, ACHN and HCT116 human colon cancer cells, and KA values were increased by anti‐CD98hc mAb, suggesting anti‐LAT1 mAbs detect an epitope on LAT1‐CD98hc complexes on the cell surface. Based on these results, LAT1 may be a promising anticancer target and Macaca fascicularis can be used in preclinical studies with antihuman LAT1 mAbs.
Keywords: antitumor effect, avidity, LAT1, monoclonal antibody, non‐human primate
1. INTRODUCTION
CD98 was originally reported as a cell surface marker associated with lymphocyte activation, as defined by a 4F2 mAb,1 and subsequently identified as a unique molecule expressed by numerous cancer cells.2, 3, 4 CD98 with a molecular weight (MW) of 120~140 kDa (gp1252, 3) is composed of a glycosylated heavy chain (hc) with a MW of 80~100 kDa and non‐glycosylated light chains (lc) with a MW of 35~55 kDa.1, 2, 3, 4 CD98hc, also referred to solute carrier (SLC) 3A2, binds to six CD98lc of the SLC7A amino acid transporter family (SLC7A5; LAT1, 7A8; LAT2, 7A7; y+LAT1, 7A6; y+LAT2, 7A10; asc1 and 7A11; xCT).5, 6, 7, 8, 9, 10, 11 SLC7A11 (xCT) was identified as a molecule required for the maintenance of cancer stem cells (CSC).12 In particular, a variant form of CD44 (CD44v)12, 13, 14, 15, 16, 17 or human epidermal growth factor receptor 1 (HER1)18 associates with xCT in epithelial cancers or gliomas, respectively, and stabilizes xCT, resulting in the survival of CSC in unfavorable conditions for cancers such as oxidative stress due to anticancer drugs. In this context, we demonstrated the antitumor effects of a fully human anti‐CD44 mAb19 recognizing CD44v bound to xCT expressed by CSC.
l‐Type amino acid transporter 1 (LAT1)5, 6 is the first identified CD98lc, and cancers from several tissues express both CD98hc2, 3, 20, 21, 22, 23, 24, 25 and LAT125, 26, 27, 28, 29 proteins. The oncogenicity of CD98 was confirmed with wild‐type CD98hc30, 31, 32, 33 bound to CD98lc, but not with mutated CD98hc32 lacking the cysteine residue needed for the association with CD98lc.
These results suggest that molecular complexes between CD98hc and some CD98lc are oncogenic, and we recently identified CD98hc‐LAT1 as an oncogenic complex by targeted disruption of the chicken LAT1 gene.34 CD98‐LAT1 is therefore considered to be a promising target for cancer therapy. Although the major targets of existing anticancer therapeutic antibodies are receptor‐type tyrosine kinases, overexpression of anti‐HER1 and anti‐HER2 is limited to cancers from squamous35, 36 or glandular37, 38 epithelium, respectively. As CD98/LAT1 is expressed by almost all cancers irrespective of tissue origin,2, 3, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29 anti‐CD98/LAT1 therapeutic antibodies may be ideal against numerous human malignancies.
Although clinical trials with anti‐CD98hc therapeutic antibodies (KHK2898 and IGN523) were recently started,39 the cancer specificity of LAT1 is superior to that of CD98hc.40, 41 However, LAT1 is a 12‐pass membrane protein and has scarce extracellular domains, thus it was difficult to produce anti‐LAT1 mAb reactive to living cancer cells. We previously reported the production of the first (1st) anti‐LAT1 mAbs40, 41 and antitumor effects of the anti‐LAT1 mAb using a hybridoma therapy model system.34
We have recently prepared second (2nd)‐generation mAbs and reported anti‐LAT1‐based ImmunoPET detection of xenografted human cancers.42 In this study, we compared the reactivity between 1st‐ and 2nd‐generation anti‐LAT1 mAbs against cancer cells and analyzed the internalization activity and inhibitory effects of amino acid uptake and cell growth by 2nd‐generation mAbs. We then investigated the in vivo antitumor effects and antibody‐dependent cellular cytotoxicity (ADCC) of rat mAbs with splenocytes from athymic mice and of rat‐human chimeric anti‐LAT1 mAbs with human mononuclear cells from peripheral blood (hMNC‐PB) as effector cells. Furthermore, we examined the reactivity and avidity of antihuman LAT1 mAbs with LAT1 of Macaca fascicularis (crab‐eating monkey) cells and transfectants expressing macaca LAT1 to evaluate possible side effects of antihuman LAT1.
2. MATERIALS AND METHODS
2.1. Cell culture
Human colon (LS‐174T, HCT116), stomach (KATOIII), kidney (ACHN), lung (NCI‐H292, NCI‐H1944, A549), and uterine (HeLa) cancers, P3X63Ag8.653 mouse myeloma (ATCC, Manassas, VA, USA), OVTOKO human ovarian cancer (JCRB Cell Bank, Osaka, Japan), HEK293F (Invitrogen, Carlsbad, CA, USA), hMNC‐PB (PromoCell, Heidelberg, Germany), RH7777 rat hepatoma (donated by Dr K Chiba, Mitsubishi Tanabe Pharma, Yokohama, Japan), and MK.P3 macaca kidney (JCRB) cells were cultured in RD medium, which is a 1:1 mixture of DMEM medium and RPMI‐1640 medium (Nissui Pharmaceutical Co., Ltd, Tokyo, Japan) with 7% heat‐inactivated FBS (Nichirei Biosciences, Tokyo, Japan) in a humidified CO2 incubator (5% CO2) at 37°C.
2.2. Molecular cloning of macaca LAT1 cDNA
Macaca LAT1 cDNA was reverse‐transcribed with First Strand cDNA Synthesis kit (GE Healthcare, Uppsala, Sweden) from total RNA of MK.P3 cells prepared by Isogen II (Nippon Gene, Toyama, Japan), and cDNA was amplified by Q5 DNA polymerase (New England BioLabs, Tokyo, Japan) using a primer set for the amplification of full‐length macaca LAT1 mAbs.
2.3. Establishment of transfectant cells expressing macaca LAT1‐GFP
GFP was fused to full‐length macaca LAT1 in a pAcGFP vector (BD Biosciences, Mountain View, CA, USA). Transfection of macaca LAT1‐GFP vector into RH7777 or HEK293 cells was carried out using Lipofectamine 3000 (Invitrogen). Cells were selected with 400 μg/mL G418 (Nacalai Tesque, Kyoto, Japan), and clone‐sorted for cellular green fluorescence using a JSAN cell sorter (Bay Bioscience, Kobe, Japan).
2.4. Primary mAbs and polyclonal antibodies
First‐generation (SOL22 and SOL69),34, 40, 41 2nd‐generation (Ab1, Ab2,42 Ab3 and Ab4) antihuman LAT1 rat mAbs, antihuman LAT1 rat‐human chimeric mAbs (ChAb1 and ChAb3) reshaped from Ab1 and Ab3, respectively, anti‐HER1 chimeric mAb (Cetuximab; MerckSerono, Tokyo, Japan), antihuman CD98 rat mAb (HR3540, 41), antihuman xCT rat mAb (Ab3118), antihuman CD98 mouse mAb (HBJ1273, 43, 44, 45), antirat CD98 mouse mAb (B32, 43), antimouse CD98 rat mAb (MB87232), antimouse CD44v rat mAb (RM112, 13, 14), anti‐HER2 mouse mAb (SER446, 47), and anti‐GFP rabbit polyclonal antibodies (pAb) (produced in our laboratory) were used.
2.5. Animals
F344/N rats and KSN athymic (nude) mice were obtained from the Shimizu Animal Farm (Kyoto, Japan) and were maintained in the animal facility at Kindai University. All animals were maintained in specific pathogen‐free conditions. They were housed individually in plastic cages under a standard light/dark cycle (12‐hour light cycle starting at 7:00) at a constant temperature of 23 ± 1°C and had ad libitum access to food and water. All experiments were approved by the Committee for the Care and Use of Laboratory Animals at Kindai University (KAPS‐23‐004 and KAPS‐26‐019).
2.6. Flow cytometry (FCM)
Cells (1~5 × 105 cells) were incubated with the primary mAbs (10 μg/mL) for 1 hour on ice. Following two washes of cells with PBS containing 0.2% BSA, cells were incubated with phycoerythrin (PE)‐conjugated donkey antirat IgG (H+L) secondary pAb (Jackson ImmunoResearch, West Grove, PA, USA) for 45 minutes on ice. Following three washes with 0.2% BSA‐PBS, fluorescence intensity of individual cells was analyzed using an Accuri C6 or LSR‐Fortessa flow cytometer (Becton‐Dickinson, Franklin Lakes, NJ, USA). From the values of mean fluorescence intensity (MFI) with or without the primary mAbs, the subtracted (Δ) MFI or the ratio (+ mAb/− mAb) of MFI (rMFI) was calculated.
2.7. Production of novel antihuman LAT1 rat mAbs and chimeric rat‐human mAbs
Rats were s.c., i.p. or i.v. injected with RH7777 (3 × 107 cells) expressing human LAT1‐GFP six times at 2‐week intervals. Three days after the final immunization, the spleen of immunized rats was removed, and splenocytes (1 × 108 cells) were fused with P3X63Ag8.653 mouse myelomas (2 × 107 cells) using 50% polyethylene glycol 1540 (Roche, Penzberg, Germany). Hybridoma cells in ten 96‐well plates (BD Biosciences) were selected in RD medium containing hypoxanthine, aminopterin, thymidine (HAT; Thermo Fisher Scientific Inc., Waltham, MA, USA) with 7% FBS. Hybridoma antibodies were analyzed for reactivity against HEK293 cells expressing human LAT1‐GFP with FCM. Selected hybridoma cells were cloned by the limiting‐dilution method, and hybridoma clones (5 × 106 cells) in PBS were i.p. injected into KSN mice pretreated with 2,6,10,14‐tetramethylpentadecane (Pristane; Wako Pure Chemical Industries, Osaka, Japan). Approximately 10‐14 days after dosage, ascites fluid was collected, and mAbs were purified using Protein G Sepharose (GE Healthcare).
For the preparation of rat‐human chimeric anti‐LAT1 mAbs, reverse‐transcribed cDNA from variable regions of heavy and light chains of Ab1 or Ab3, which were cloned from RNA of hybridoma cells secreting rat IgG, were genetically reshaped to human IgG1, and subcloned into a pBud4.1 vector (Invitrogen) that had two separate cloning sites with independent promoters. This vector was transfected into HEK293 cells using 293fectin reagent (Invitrogen). Culture medium (2 L) containing chimeric rat‐human IgG was concentrated to 20 mL using a concentration apparatus (Millipore Corporation, Billerica, MA, USA) and precipitated by 50%‐saturated ammonium sulfate. Further purification was carried out by affinity chromatography with protein G Sepharose.
2.8. Internalization activity of anti‐LAT1 mAbs
Subconfluent HEK293 cells expressing human LAT1‐GFP in each well of six‐well plates (Corning, Corning, NY, USA) were cultured in the presence or absence of anti‐LAT1 mAbs at 37°C for 24 hours, fixed with 4% paraformaldehyde (Wako)‐PBS for 15 minutes, washed three times with PBS, and observed using a Biozero digital fluorescence microscope (Keyence, Osaka Japan) for cellular fluorescence.
HCT116 or NCI‐H1944 cells (1 × 105 cells in each sample) were suspended in 100 μL RD medium containing 7% FBS with or without anti‐LAT1 mAbs (10 μg/mL) and incubated for the indicated times at 37°C. After being washed with PBS, cells were mixed with 1:300 diluted PE‐conjugated antirat IgG (Jackson) in 1% BSA‐PBS for 30 minutes on ice. After being washed with PBS, fluorescence intensity of individual cells was analyzed by FCM.
2.9. In vivo antitumor effects of mAbs in athymic mice
KSN mice at 6 weeks of age were randomly separated into two groups (RM1 isotype‐control rat IgG2a or Ab1 anti‐LAT1 rat IgG2a). LS‐174T human colon cancer cells (5 × 106 in 200 μL PBS) were s.c. inoculated to mice, and visible tumors were confirmed in all mice. At this point (day 0), anti‐LAT1 mAb (Ab1) or isotype‐control mAb (100 μg in 500 μL PBS) was i.p. injected, followed by two additional injections of the same amount of mAb on day 8 and day 15. Size of each tumor was periodically measured and tumor volume (mm3) was calculated by the formula 0.4 × (length) × (width)2.
2.10. Effects of the anti‐LAT1 mAb on the uptake of branched chain amino acids
Subconfluent HeLa and HCT116 cells cultured for 12 hours in serum‐free RD medium with or without anti‐LAT1 mAb (Ab1) were recovered, and the cellular branched chain amino acids (BCAA) content was measured using a BCAA (Leu, Ile, Val) Colorimetric Assay Kit (BioVision, Milpitas, CA, USA) according to the manufacturer('s instructions. Briefly, cells (2 × 106) were homogenized in 100 μL of assay buffer and subjected to an enzyme reaction in which BCAA were oxidatively deaminated, producing NADH which reduces the probe, generating a color product (λmax = 450 nm). Absorbance at 450 nm was measured using a Model 550 microplate reader (Bio‐Rad, Hercules, CA, USA), and concentrations of cellular BCAA were calculated from the standard curve for leucine.
2.11. In vitro inhibitory effects on cell growth of cancer cells by anti‐LAT1 mAbs
HeLa, KATOIII or NCI‐H1944 cells (1 × 103 cells in each well of a 96‐well plate) were suspended in 100 μL serum‐free RD medium without the mAb or with isotype‐control rat IgG2a (RM1), antihuman CD98hc rat mAb (HR35), antihuman xCT rat IgG2a (Ab31), or anti‐LAT1 mAb (SOL69, Ab1 or Ab3) (0.02~20 μg/mL), and incubated for 4‐7 days at 37°C. WST‐8‐based CCK‐8 (Dojin Chemicals, Kumamoto, Japan) was added (5 μL/well), and absorbance at 450 nm was measured using a Model 550 microplate reader (Bio‐Rad).
2.12. Antibody‐dependent cellular cytotoxicity
Antibody‐dependent cellular cytotoxicity was measured by the lactate dehydrogenase (LDH)‐releasing assay, using a CytoTox 96 non‐Radioactive Cytotoxicity Assay kit (Promega Corporation, Madison, WI, USA) with phenol red‐free RD medium containing 7% heat‐inactivated FBS. Target cells (1 × 104 cells in each sample) were incubated with rat or rat‐human chimeric mAbs (10 μg/mL) for 15 minutes at 4°C. After washing, the target cells were cultured with splenocytes from nude mice (5 × 104 cells) or hMNC‐PB (5 × 105 cells) as effector cells in an incubator for 4~12 hours at 37°C. Cell lysis was confirmed by measuring the amount of LDH in culture supernatants and the optical density of the solution at 490 nm measured with a Model 550 Microplate Reader (Bio‐Rad). Maximum LDH release was determined by cell lysis in 0.2% Triton‐X100. Cytotoxicity was calculated as follows: % cytotoxicity = [(experimental release) − (effector spontaneous release) − (target spontaneous release)]/[(target maximum release) − (target spontaneous release)] × 100.
2.13. Knockdown of LAT1
Subconfluent ACHN and MK.P3 cells were seeded in each well of six‐well plates (Corning) at a density 3 × 105 cells/well and transfected with LAT1 siRNAs (30 pmol) in 2 mL medium, using Lipofectamine RNAiMAX (Invitrogen). After incubation for 72 hours, the effects of RNA interference on the expression of human LAT1 and CD98hc proteins in human and macaca cells were examined. Cells treated with mock (scramble) or LAT1 siRNAs (#1, #2, or #3) were stained with rat mAbs against human LAT1 (Ab1) or against CD98hc (HR35) followed by PE‐conjugated antirat IgG and analyzed for protein expression by FCM.
2.14. Immunoprecipitation analysis
Subconfluent cells in 10‐cm dishes (Corning) were washed with PBS and lysed on ice for 30 minutes in 3 mL lysis buffer containing 1% Nonidet P‐40 and protease inhibitor cocktail (Nacalai Tesque). Resulting extracts were centrifuged at 19 000 x g for 10 minutes at 4°C, and the supernatants were precleared with protein G Sepharose for 1 hour. Lysates were subjected to immunoprecipitation with antirat CD98hc (B3) or antihuman CD98hc (HBJ127) mouse mAb for 6 hours at room temperature, followed by the addition of protein G Sepharose and further incubation for 2 hours. Bead‐bound proteins were washed three times with 1% (v/v) Nonidet P‐40 in PBS, suspended in 1× SDS‐PAGE loading buffer, and boiled for 5 minutes, and the beads were then isolated by centrifugation. For immunoblotting analysis, proteins were solubilized in loading buffer, subjected to SDS‐PAGE (8% gels) and transferred to Immobilon‐P (Millipore), and exposed to rabbit anti‐GFP pAb. Immune complexes were detected by HRP‐conjugated VeriBlot (ab131366; Abcam, Cambridge, UK) and Chemi‐Lumi One Super (Nacalai Tesque).
2.15. Scatchard plot analysis
Avidity of antihuman LAT1 mAb in different cells was evaluated by Scatchard plot analysis. Cells were incubated with varying concentrations (3 ng~100 μg/mL) of mAbs with or without test mAbs (100 μg/mL) for 1 hour on ice. Following the removal of unbound mAbs by washing with 0.2% BSA‐PBS, cells were incubated with PE‐conjugated antirat IgG for 45 minutes on ice, and analyzed by FCM. From the values of MFI with or without the primary mAbs, the subtracted (Δ) MFI was calculated. ΔMFI/mAb concentrations were plotted against the ΔMFI, and dissociation constant: KD (nmol/L) and avidity constant: KA (M−1) were determined from the inclination of linear regression.
2.16. Statistical analysis
Experimental data were analyzed with Prism 7 for Windows (GraphPad Software, San Diego, CA, USA). Criteria for significance were P < .05 (*), .01 (**) and .001 (***).
3. RESULTS
3.1. Novel (2nd‐generation) antihuman LAT1 mAb
We previously reported the production of anti‐LAT1 mAbs,40, 41 designated as SOL22 and SOL69 (1st‐generation mAbs). To advance LAT1‐targeted cancer therapy, novel mAbs (Ab1~4; 2nd‐generation mAbs) were developed against transfectants expressing human LAT1‐GFP, and basic analyses on anti‐LAT1 (Ab2)‐based ImmunoPET40 were reported. All six anti‐LAT1 mAbs are of the rat IgG2a heavy chain subclass with κ‐type light chains. Details of mAb production were described in our previous reports.40, 41 Although the amino acid sequence in the complementarity‐determining region (CDR) between 1st‐ and 2nd‐generation mAbs differs, amino acid sequences within each generation were almost identical with very few amino acid differences in the CDR. Details for the novel mAbs were described in the patent (no. 9,725,519, USA and no. 6,421,371, Japan).
3.2. Comparison of 1st‐ and 2nd‐generation anti‐LAT1 mAbs
Anti‐LAT1 mAbs (SOL22, SOL69, Ab1 and Ab2) bound to HEK293 and RH7777 cells expressing human LAT1‐GFP in a GFP expression level‐dependent way, indicating that four anti‐LAT1 mAbs are specific for human LAT1 (Figure 1). Ab1 and Ab2 showed stronger reactions than SOL22 and SOL69 against HCT116 and LS‐174T human colon cancers (Figure 1), indicating that the 2nd‐generation mAbs have superior anticancer reactivity compared with the 1st‐generation mAbs.
Figure 1.
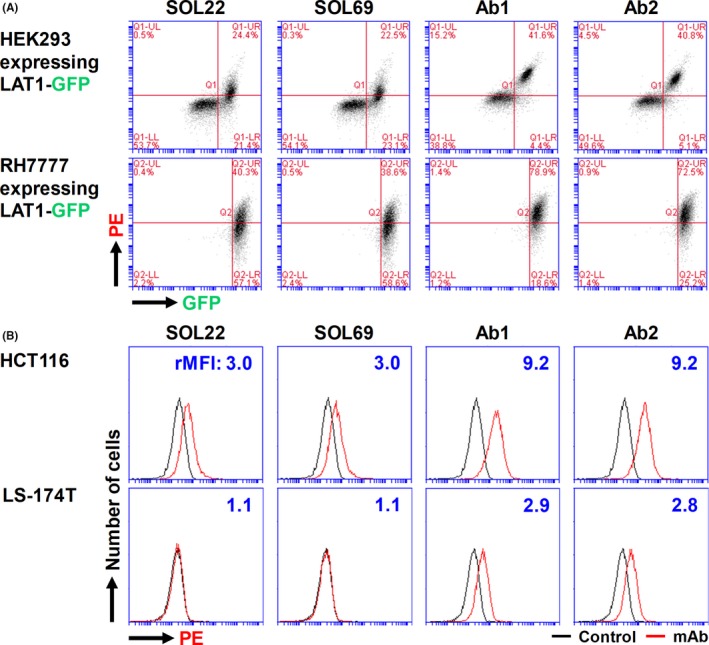
Specificity and reactivity of anti‐l‐type amino acid transporter 1 (anti‐LAT1) mAbs. Anti‐LAT1 rat mAb (SOL22, SOL69, Ab1 and Ab2) were compared for their binding activity against HEK293 and RH7777 expressing human LAT1‐GFP (A), and against human HCT116 and LS‐174T human colon cancer cells (B). Cells were stained with primary anti‐LAT1 rat mAbs, followed by secondary phycoerythrin‐conjugated antirat IgG, and analyzed by flow cytometry (FCM). Values in (B) indicate the ratio (+ mAb/− mAb) of mean fluorescence intensity (rMFI)
3.3. Internalization activity of mAbs
HEK293 expressing LAT1‐GFP were cultured with mAbs, and the internalization of LAT1‐GFP was analyzed (Figure 2A). Cell surface LAT1 proteins were reduced by all four anti‐LAT1 mAbs, and intracellular LAT1‐GFP proteins were observed (white arrows).
Figure 2.
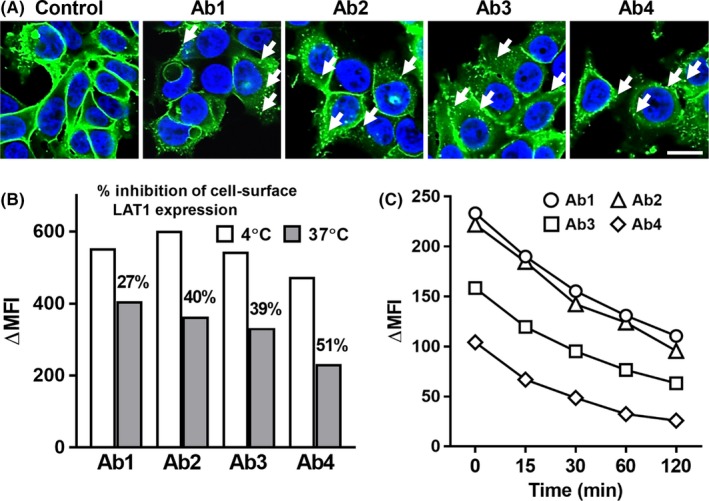
Internalization of l‐type amino acid transporter 1 (LAT1) proteins by anti‐LAT1 mAbs. A, HEK293 cells expressing human LAT1‐GFP were treated with anti‐LAT1 mAbs at 37°C for 24 h and analyzed by fluorescence microscopy. Scale bar, 20 μm. B, HCT116 cells were treated with anti‐LAT1 rat mAbs at 4°C or 37°C for 1 h, followed by incubation with phycoerythrin (PE)‐conjugated antirat IgG on ice and analyzed by FCM. C, NCI‐H1944 cells were treated with anti‐LAT1 rat mAbs at 37°C for the indicated times, followed by incubation with PE‐conjugated antirat IgG on ice, and analyzed by flow cytometry (FCM). MFI, mean fluorescence intensity
Internalization of LAT1 proteins was also seen in HCT116 colon (Figure 2B) and NCI‐H1944 lung (Figure 2C) cancers with antihuman LAT1 mAbs. Internalization of LAT1 proteins was observed at 15 minutes, and cell surface LAT1 proteins decreased time‐dependently until 120 minutes after the addition of mAbs (Figure 2C).
3.4. Antitumor effects of mAb on the growth of xenografted human cancer cells
Ab1 was evaluated for its antitumor effects against LS‐174T human colon cancer cells in an established tumor model.19, 48 In this systemic administration of the mAb to tumor‐bearing mice, we deliberately adopted i.p. injection to give an exact amount of mAb to each mouse. Tumor growth in Ab1‐treated mice was significantly inhibited (Figure 3).
Figure 3.
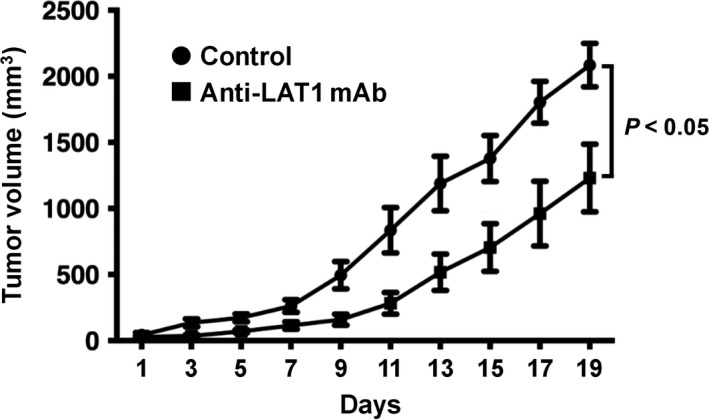
Antitumor effects of anti‐l‐type amino acid transporter 1 (anti‐LAT1) mAb in the established tumor model. Six days after LS‐174T human colon cancer cells (5 × 106) were s.c. inoculated into athymic mice, visible tumors were confirmed in all mice. At this point (day 0), anti‐LAT1 mAb (Ab1) or isotype control IgG mAb (100 μg) was i.p. injected, followed by two additional injections of the same amount of mAb on day 8 and day 15. Data were expressed as mean ± SEM, and statistical analysis was done using two‐way analysis of variance
3.5. In vitro effects of anti‐LAT1 mAb on BCAA uptake and growth of human cancer cells
To analyze the mechanisms of the in vivo antitumor effects by anti‐LAT1 mAb, mAb‐mediated in vitro inhibition of BCAA uptake and growth of human cancer cells was evaluated (Figure 4). Intracellular BCAA content significantly decreased in HeLa cells following anti‐LAT1 mAb treatment (Figure 4A). Anti‐CD98hc (SLC3A2) mAb and Ab1 (2nd‐generation mAb) showed superior antiproliferative effects compared with SOL69 (1st‐generation mAb) (Figure 4B). Against HeLa (B), KATOIII (C) and NCI‐H1944 (D) human cancer cells, anti‐LAT1 (SLC7A5) mAbs (Ab1 and Ab3) showed antiproliferative effects, although anti‐xCT (SLC7A11) mAb did not have any growth inhibitory effects against HeLa cells (Figure 4B).
Figure 4.
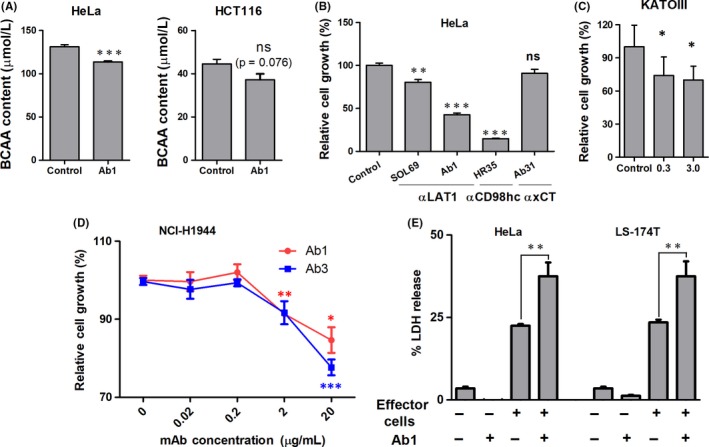
Effects of anti‐l‐type amino acid transporter 1 (anti‐LAT1) on branched chain amino acid (BCAA) uptake, cellular growth and mAb‐dependent cytotoxicity in the presence of mouse effector cells. A, BCAA concentrations of HeLa and HCT116 cancer cells cultured (12 h) with control (RM1) or anti‐LAT1 (Ab1) mAb (20 μg/mL) were evaluated. Growth inhibition of HeLa (B), KATOIII (C) and (D) NCI‐H1944 cells by anti‐LAT1 mAbs was evaluated by the WST‐8 assay. In (B), growth‐inhibitory effects of anti‐LAT1 (SOL69 and Ab1), anti‐CD98hc (HR35) or anti‐xCT (Ab31) mAb (20 μg/mL) were also evaluated with isotype control rat mAb (RM1). E, Antibody‐dependent cellular cytotoxicity activity of anti‐LAT1 rat mAb (Ab1) with splenocytes from athymic mice was evaluated in LS‐174T and HeLa cells. Effector to target (E/T) ratio was 5:1. Vertical bars show standard errors, and data were analyzed statistically by two‐sided Student's t tests. LDH, lactate dehydrogenase; ns, not significant. *P < .05, **P < .01 and ***P < .001.
3.6. Antibody‐dependent cellular cytotoxicity by rat mAbs and human‐rat chimeric mAbs
Anti‐LAT1 rat mAb‐mediated ADCC activity was examined as part of the analysis of the in vivo antitumor effects (Figure 4E). In LS174T and HeLa human cancer cells, Ab1 showed significant ADCC activity with splenocytes from athymic mice. Next, Ab1 and Ab3 rat mAbs were reshaped to ChAb1 and ChAb3 rat‐human chimeric mAbs and evaluated for ADCC activity against hMNC‐PB (human lymphocytes). Chimeric anti‐LAT1 mAbs showed strong ADCC activity against many human cancer cells from the colon, uterus, lung, and ovary (Figure 5A). Furthermore, the ADCC activity of anti‐LAT1 chimeric mAbs was markedly superior to chimeric anti‐HER1 (Cetuximab) against HCT116 (Figure 5B).
Figure 5.
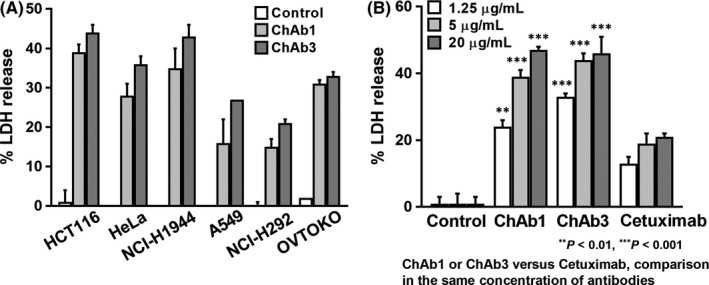
Antibody‐dependent cellular cytotoxicity (ADCC) of chimeric anti‐l‐type amino acid transporter 1 (anti‐LAT1) mAbs with human effector cells. A, ADCC activity of rat‐human chimeric anti‐LAT1 mAbs (ChAb1 and ChAb3) with human lymphocytes was evaluated against different human cancer cell lines. B, ADCC of anti‐LAT1 chimeric mAbs was compared with that of Cetuximab, an antihuman epidermal growth factor receptor 1 chimeric mAb. E/T ratio was 50:1. Vertical bars show standard errors, and data were analyzed statistically by two‐sided Student's t tests. LDH, lactate dehydrogenase
3.7. Cross‐reactivity of antihuman LAT1 mAbs with macaca LAT1 proteins
Evaluation of side‐effects of therapeutic mAbs is important for clinical use. However, mAbs against a given human protein (temporarily X) often do not react with X of other species. Indeed, antihuman LAT1 mAbs are specifically reactive with LAT1, but not with other human CD98lc,40, 41 or mouse LAT1.42 Therefore, we investigated the reactivity of Ab1 and Ab2 with LAT1 of M. fascicularis, which is generally used as non‐human primate for the preclinical evaluation of side‐effects. Ab1 and Ab2 were confirmed to be reactive against MK.P3 macaca kidney‐derived cells, and this reactivity was equivalent to that against ACHN human cells (Figure 6A).
Figure 6.
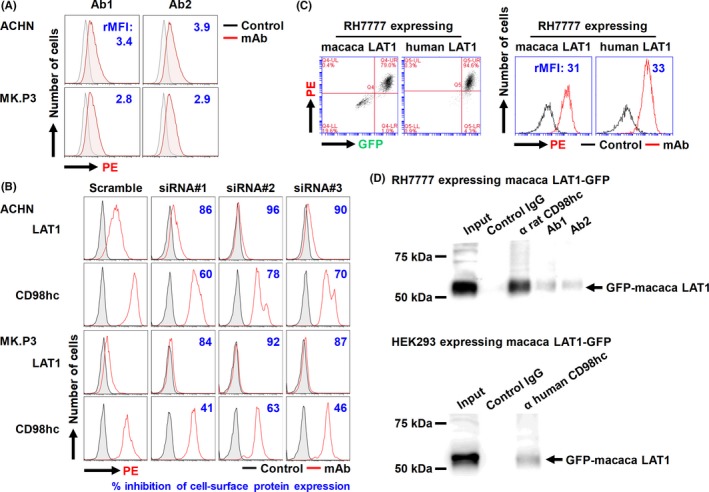
Specificity of antihuman l‐type amino acid transporter 1 (LAT1) mAbs in human and macaca cells. A, ACHN human and MK.P3 macaca cells were stained with antihuman LAT1 mAbs (Ab1 or Ab2), followed by phycoerythrin (PE)‐conjugated antirat IgG, and analyzed by flow cytometry (FCM). Values indicate the ratio (+ mAb/− mAb) of mean fluorescence intensity (rMFI). B, Effects of RNA interference on the expression of human LAT1 and CD98 heavy chain (CD98hc) proteins in human and macaca cells were analyzed. Cells were treated with mock (scramble) or LAT1 siRNA (#1, #2 or #3) for 72 h, stained with rat mAbs against human LAT1 (Ab1) or CD98hc (HR35), followed by PE‐conjugated antirat IgG, and analyzed for protein expression by FCM. C, Antihuman LAT1 mAb (Ab1) was evaluated for its reactivity against RH7777 rat cells expressing macaca (left) or human (right) LAT1‐GFP. D, Association of introduced macaca LAT1‐GFP with endogenous rat or human CD98hc was examined. Cell lysates from RH7777 (upper) or HEK293 (lower) expressing macaca LAT1 were subjected to immunoprecipitation with control IgG, anti‐LAT1, or anti‐CD98hc mAb, and the resulting precipitates, as well as the original cell lysates (input), were subjected to immunoblotting with anti‐GFP rabbit polyclonal antibodies
3.8. Novel mAbs specifically recognize human and macaca LAT1 proteins
To confirm that the reactivity of antihuman LAT1 mAbs with macaca cells was specific for macaca LAT1, we analyzed the effects of LAT1 siRNA on the binding of antihuman LAT1 mAb in ACHN and MK.P3 cells. Knockdown of LAT1 decreased the reactivity of antihuman LAT1 mAb with human and macaca cells. In both ACHN and MK.P3 cells, knockdown of LAT1 by the transfection of LAT1 siRNA reduced cell surface expression of CD98hc compared with mock‐transfection (Figure 6B). These results suggest that altered expression of LAT1 can change the expression of CD98hc.
3.9. Reactivity of mAbs with transfectants expressing macaca LAT1‐GFP
To substantiate the equivalent reactivity of antihuman LAT1 mAb against human and macaca LAT1, we established RH7777 cells expressing GFP‐fused human or macaca LAT1. Ab1, the antihuman LAT1 mAb, bound to both RH7777 transfectants in a GFP expression level‐dependent way, and the rMFI reflecting the relative amount of cell‐bound anti‐LAT1 mAb was almost equal in both RH7777 transfectants (Figure 6C).
3.10. Introduced macaca LAT1 associates with endogenous CD98hc
The possible association of macaca LAT1‐GFP proteins with endogenous CD98hc proteins was examined by immunoprecipitation with anti‐LAT1 or anti‐CD98hc mAb followed by western blotting with anti‐GFP pAb. Exogenously expressed macaca LAT1 bound to rat CD98hc in RH7777 rat cells and to human CD98hc in HEK293 human cells (Figure 6D).
3.11. Dual avidity modes of antihuman LAT1 mAbs
KD and KA of antihuman LAT1 mAbs against macaca and human LAT1 proteins were analyzed using MK.P3 macaca and ACHN human kidney cell lines to examine the detailed binding characteristics of antihuman LAT1 mAb. Antihuman LAT1 mAb (Ab1) showed dual (high: 3.1 × 109 M−1and low: 3.9 × 106 M−1) avidity modes for MK.P3 macaca cells (Figure 7A), similarly for ACHN human cells (Figure 7B).
Figure 7.
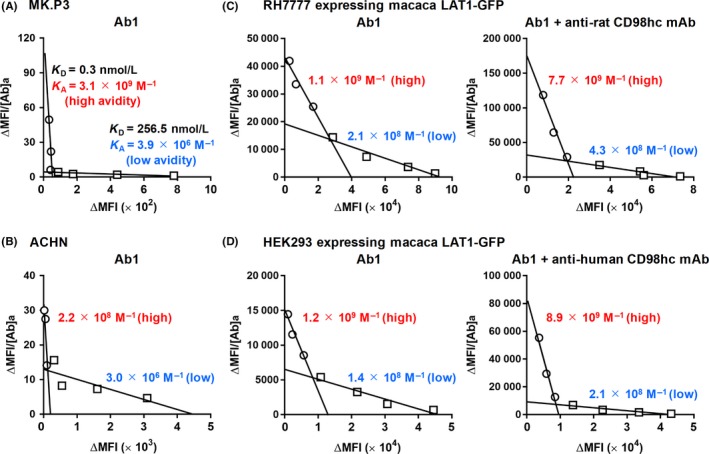
Scatchard plot analysis of antihuman l‐type amino acid transporter 1 (LAT1) mAbs with human and macaca cells. MK.P3 macaca (A) and ACHN human (B) kidney‐derived cells were reacted with varying amounts of antihuman LAT1 mAbs, followed by incubation with phycoerythrin (PE)‐conjugated antirat IgG, and analyzed by flow cytometry (FCM). From the values of mean fluorescence intensity (MFI) with or without primary mAbs, subtracted (Δ) MFI was calculated. ΔMFI/mAb concentrations were plotted against ΔMFI, and KD (nmol/L) and KA (M−1) were determined from the inclination of linear regression. To evaluate the effects of anti‐CD98 heavy chain (anti‐CD98hc) mAb on the avidity of anti‐LAT1 mAbs for macaca LAT1 proteins, KA of antihuman LAT1 mAbs against macaca LAT1 expressed in RH7777 (C) or HEK293 (D) cells were calculated with (right) or without (left) mAbs recognizing endogenous CD98hc. Values (red or blue) respectively, indicate KA (M−1) of high or low avidity of anti‐LAT1 mAbs
Next, to evaluate the effects of the anti‐CD98hc mAb on the avidity of the anti‐LAT1 mAb for macaca LAT1 proteins, KA of the antihuman LAT1 mAb against macaca LAT1 proteins expressed on RH7777 (C) or HEK293 (D) cells were determined with (right) or without (left) the mAb recognizing endogenous CD98hc proteins. As indicated by the KA values, both high and low avidity increased with the antirat CD98hc mAb in RH7777 rat cells, as was observed with antihuman CD98hc mAb in HEK293 human cells.
As shown in Figure 8, KA of the antihuman LAT1 mAb against HCT116 human colon cancer cells was measured in the absence (A) or presence of the test mAb recognizing HER2 (B), rat CD98hc (C), or human CD98 (D). Two avidity modes with high (1.5 × 1010 M−1) and low (6.7 × 107 M−1) KA values for anti‐LAT1 mAb (Ab1) were observed (A), demonstrating that anti‐LAT1 mAb could recognized the epitope on LAT1 proteins in multimeric LAT1‐related complexes. These dual avidities increased with antihuman CD98hc mAb to high (3.3 × 1010 M−1) and low (1.5 × 108 M−1) (D), but were not affected by anti‐HER2 (B) or antirat CD98hc (C) mAb, suggesting that the emergence of high and low avidity modes was caused, at least in part, by the association of LAT1 and CD98hc.
Figure 8.
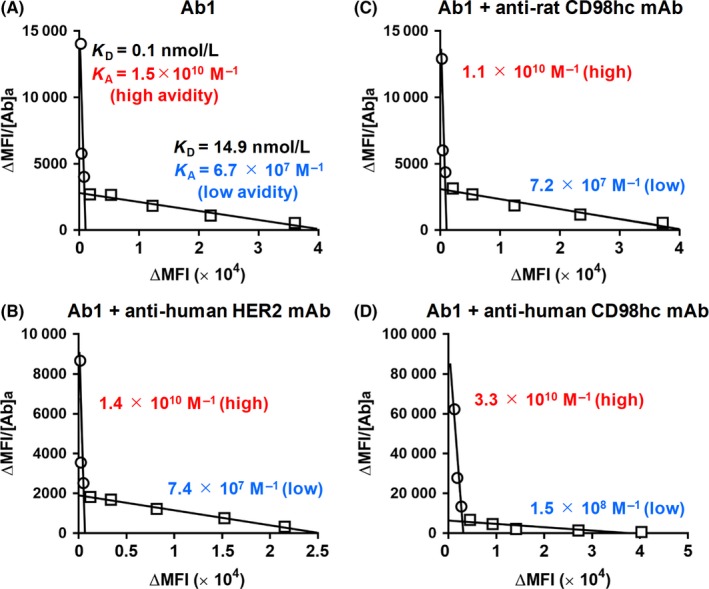
Scatchard plot analysis of anti‐l‐type amino acid transporter 1 (anti‐LAT1) mAbs with HCT116 cells. KA of anti‐LAT1 mAbs against HCT116 cells was determined by Scatchard plot analysis in the absence (A) or presence of mAbs recognizing human epidermal growth factor receptor 2 (HER2) (B), rat CD98 heavy chain (CD98hc) (C), or human CD98hc (D). HCT116 cells were reacted with varying amounts of anti‐LAT1 mAbs, followed by incubation with phycoerythrin‐conjugated antirat IgG, and were then analyzed by flow cytometry (FCM). MFI, mean fluorescence intensity
4. DISCUSSION
The CD98hc‐LAT1 complex is regarded as a promising molecular target for cancer therapy, and antibody therapy against CD98hc is now in progress.39 We previously showed that the cancer specificity of LAT1 is superior to that of CD98hc40, 41 by analyses with 1st‐generation anti‐LAT1 mAbs, and we recently developed 2nd‐generation anti‐LAT1 mAbs.42
The 2nd‐generation anti‐LAT1 mAbs had markedly stronger reactivity against HEK293 and RH7777 cells expressing human LAT1‐GFP, HCT116 and LS‐174T human colon cancer cells than the 1st‐generation mAbs (Figure 1).
In the present study, we examined in vitro mAb‐dependent internalization of LAT1 proteins (Figure 2) and in vivo growth inhibition of LS174T cancer cells in athymic mice by systemic Ab1 treatment (Figure 3). LAT1/CD98hc has been implicated in cancer growth through mammalian target of rapamycin (mTOR) signaling;49 therefore, we speculate that inhibition of mTOR signaling or the starving effects of amino acids were due to induced internalization of LAT1 by Ab1. In this context, we examined the effects of anti‐LAT1 mAb on the intracellular concentrations of BCAA because LAT1 is a transporter of large neutral amino acids including BCAA.5 In vitro inhibitory effects on BCAA uptake into cancer cells in the presence of anti‐LAT1 mAb were observed in this study (Figure 4A). Although we previously reported growth inhibition of cancer cells by anti‐CD98hc mouse mAb,43, 50 in vitro growth inhibition of human cancer cells by anti‐LAT1 rat mAb (Figure 4B‐D) in addition to anti‐CD98hc rat mAb, was observed in this study (Figure 4B). Moreover, ADCC may be an additional antitumor mechanism in this xenograft mouse model because Ab1 has shown ADCC activity against LS‐174T and HeLa cells in the presence of splenocytes from athymic mice (Figure 4E). We also expect our anti‐LAT1 mAbs to exert ADCC‐mediated therapeutic effects on human malignancies because rat‐human chimeric ChAb1 and ChAb3 had stronger ADCC activity than Cetuximab, the anti‐HER1 mAb, using human lymphocytes as effector cells (Figure 5).
As antihuman LAT1 mAbs are not reactive against mouse LAT1,42 possible side‐effects of anti‐LAT1 mAbs therapy were unable to be evaluated in the mouse system. Therefore, we planned the analysis of cross‐species reactions of antihuman LAT1 mAbs against LAT1 proteins in human and non‐human primates. Macaca fascicularis is an invaluable animal in biomedical research because the macaque species is phylogenetically close to the human species. Antihuman LAT1 mAbs reacted to MK.P3 macaca‐derived cells (Figure 6A), and this reactivity was specifically knocked down by LAT1 siRNA (Figure 6B), showing that antihuman LAT1 mAb specifically react to macaca LAT1.
To substantiate the equivalent reactivity of macaca and human LAT1 proteins against the antihuman LAT1 mAb, macaca LAT1 cDNA was cloned from MK.P3 cells to establish transfectants expressing macaca LAT1 proteins. Nucleotide and amino acid sequence identity between human and macaca LAT1 was 96.6% (1473/1524) and 99.6% (506/508), respectively, as compared with human LAT1. The nucleotide and amino acid sequence identity between human and mouse LAT1 was 86% and 89%, respectively, as compared with human LAT1. Antihuman LAT1 mAb showed almost equal reactivity against both RH7777 transfectants expressing human or macaca LAT1 proteins (Figure 6C); therefore, M. fascicularis was confirmed to be applicable for the evaluation of side‐effects in preclinical tests before clinical trials of anti‐LAT1 mAbs.
In the present study, we found high and low avidity modes between antihuman LAT1 mAb and human or macaca cells (Figures 7 and 8). We purposely used the term “avidity” rather than “affinity” because the experiments were carried out with divalent mAbs bound to LAT1 on living cells, and we regarded the reciprocal of KD (nmol/L) = KA (M−1) as an index of avidity. Regarding the multiple affinity states, the interleukin (IL)‐2 receptor has three forms that show different affinities for IL‐2:51 the low‐affinity monomeric IL‐2Rα, the intermediate‐affinity dimeric IL‐2Rβγ, and the high‐affinity trimeric IL‐2Rαβγ. IL‐2β homodimers also show high affinity against IL‐2 compared with IL‐2β monomers.52 Anti‐LAT1 mAbs bind to LAT1 proteins in different modes in molecular complexes, and LAT1‐CD98hc complexes may be responsible for the low‐ or high‐avidity binding of anti‐LAT1 mAbs because the avidity of the anti‐LAT1 mAbs increased with the anti‐CD98hc mAb (Figures 7 and 8). In this context, we are planning antibody therapy using anti‐LAT1 and anti‐CD98hc mAbs.
The target molecules of existing anticancer therapeutic antibodies are divided into: (i) receptor‐type tyrosine kinases; (ii) differentiation antigens; (iii) angiogenesis‐related molecules; and (iv) immune checkpoint molecules. In this context, we have recently reported a novel therapy targeting lymphangiogenesis, but not angiogenesis, using an anti‐LYVE‐1 mAb.53 At present, many transporters are not considered to be target molecules for cancer therapy; however, this study strongly suggests that inhibition of cancer metabolism by mAbs against amino acid transporters will play a significant role in future cancer therapies.
CONFLICTS OF INTEREST
Masakazu Tamura, Yuki Abe and Toshinori Aagatsuma (Daiichi Sankyo Co., Ltd) received annual research funds and annual remuneration from Daiichi Sankyo Co., Ltd. The other authors declare that they have no conflicts of interest.
ACKNOWLEDGMENTS
This study was done in part by a research grant (J14072) sponsored by Daiichi Sankyo Co., Ltd. We acknowledge Akitaka Yamasaki, Natsumi Hayashi and Rikuto Miyake for their technical assistance in the experiments required for this revised version.
Ueda S, Hayashi H, Miyamoto T, et al. Anti‐tumor effects of mAb to l ‐type amino acid transporter 1 (LAT1) bound to human and monkey LAT1 with dual avidity modes. Cancer Sci. 2019;110:674–685. 10.1111/cas.13908
REFERENCES
- 1. Haynes BF, Hemler ME, Mann DL, et al. Characterization of a monoclonal antibody (4F2) that binds to human monocytes and to a subset of activated lymphocytes. J Immunol. 1981;126:1409‐1414. [PubMed] [Google Scholar]
- 2. Hashimoto Y, Masuko T, Yagita H, Endo N, Kanazawa J, Tazawa J. A proliferation‐associated rat cell surface antigen recognized by a murine monoclonal antibody. Gan. 1983;74:819‐821. [PubMed] [Google Scholar]
- 3. Yagita H, Masuko T, Takahashi N, Hashimoto Y. Monoclonal antibodies that inhibit activation and proliferation of lymphocytes. I. Expression of the antigen on monocytes and activated lymphocytes. J Immunol. 1986;136:2055‐2061. [PubMed] [Google Scholar]
- 4. Lüscher B, Rousseaux M, Lees R, MacDonald HR, Bron C. Cell surface glycoproteins involved in the stimulation of interleukin 1‐dependent interleukin 2 production by a subline of EL4 thymoma cells. II. Structure, biosynthesis, and maturation. J Immunol. 1985;135:3951‐3957. [PubMed] [Google Scholar]
- 5. Mastroberardino L, Spindler B, Pfeiffer R, et al. Amino‐acid transport by heterodimers of 4F2hc/CD98 and members of a permease family. Nature. 1998;395:288‐291. [DOI] [PubMed] [Google Scholar]
- 6. Kanai Y, Segawa H, Miyamoto K, Uchino H, Takeda E, Endou H. Expression cloning and characterization of a transporter for large neutral amino acids activated by the heavy chain of 4F2 antigen (CD98). J Biol Chem. 1998;273:23629‐23632. [DOI] [PubMed] [Google Scholar]
- 7. Segawa H, Fukasawa Y, Miyamoto K, Takeda E, Endou H, Kanai Y. Identification and functional characterization of a Na+‐independent neutral amino acid transporter with broad substrate selectivity. J Biol Chem. 1999;274:19745‐19751. [DOI] [PubMed] [Google Scholar]
- 8. Pineda M, Fernández E, Torrents D, et al. Identification of a membrane protein, LAT‐2, that co‐expresses with 4F2 heavy chain, an L‐type amino acid transport activity with broad specificity for small and large zwitterionic amino acids. J Biol Chem. 1999;274:19738‐19744. [DOI] [PubMed] [Google Scholar]
- 9. Rossier G, Meier C, Bauch C, et al. LAT2, a new basolateral 4F2hc/CD98‐associated amino acid transporter of kidney and intestine. J Biol Chem. 1999;274:34948‐34954. [DOI] [PubMed] [Google Scholar]
- 10. Torrents D, Estévez R, Pineda M, et al. Identification and characterization of a membrane protein (y+L amino acid transporter‐1) that associates with 4F2hc to encode the amino acid transport activity y+L. A candidate gene for lysinuric protein intolerance. J Biol Chem. 1998;273:32437‐32445. [DOI] [PubMed] [Google Scholar]
- 11. Pfeiffer R, Rossier G, Spindler B, Meier C, Kühn L, Verrey F. Amino acid transport of y+L‐type by heterodimers of 4F2hc/CD98 and members of the glycoprotein‐associated amino acid transporter family. EMBO J. 1999;18:49‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ishimoto T, Nagano O, Yae T, et al. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc− and thereby promotes tumor growth. Cancer Cell. 2011;19:387‐400. [DOI] [PubMed] [Google Scholar]
- 13. Ishimoto T, Oshima H, Oshima M, et al. CD44+ slow‐cycling tumor cell expansion is triggered by cooperative actions of Wnt and prostaglandin E2 in gastric tumorigenesis. Cancer Sci. 2010;101:673‐678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yae T, Tsuchihashi K, Ishimoto T, et al. Alternative splicing of CD44 mRNA by ESRP1 enhances lung colonization of metastatic cancer cell. Nat Commun. 2012;3:883. [DOI] [PubMed] [Google Scholar]
- 15. Yoshikawa M, Tsuchihashi K, Ishimoto T, et al. xCT inhibition depletes CD44v‐expressing tumor cells that are resistant to EGFR‐targeted therapy in head and neck squamous cell carcinoma. Cancer Res. 2013;73:1855‐1866. [DOI] [PubMed] [Google Scholar]
- 16. Wada T, Ishimoto T, Seishima R, et al. Functional role of CD44v‐xCT system in the development of spasmolytic polypeptide‐expressing metaplasia. Cancer Sci. 2013;104:1323‐1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Thanee M, Loilome W, Techasen A, et al. CD44 variant‐dependent redox status regulation in liver fluke‐associated cholangiocarcinoma: a target for cholangiocarcinoma treatment. Cancer Sci. 2016;107:991‐1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tsuchihashi K, Okazaki S, Ohmura M, et al. The EGF receptor promotes the malignant potential of glioma by regulating amino acid transport system xc(−). Cancer Res. 2016;76:2954‐2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Masuko K, Okazaki S, Satoh M, et al. Anti‐tumor effect against human cancer xenografts by a fully human monoclonal antibody to a variant 8‐epitope of CD44R1 expressed on cancer stem cells. PLoS ONE. 2012;7:e29728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. El Ansari R, Craze ML, Diez‐Rodriguez M, et al. The multifunctional solute carrier 3A2 (SLC3A2) confers a poor prognosis in the highly proliferative breast cancer subtypes. Br J Cancer. 2018;2:1115‐1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wu B, Wang Y, Yang XM, et al. Basigin‐mediated redistribution of CD98 promotes cell spreading and tumorigenicity in hepatocellular carcinoma. J Exp Clin Cancer Res. 2015;34:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. D')Agostino S, Lanzillotta D, Varano M, et al. The receptor protein tyrosine phosphatase PTPRJ negatively modulates the CD98hc oncoprotein in lung cancer cells. Oncotarget. 2018;9:23334‐23348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Poettler M, Unseld M, Braemswig K, Haitel A, Zielinski CC, Prager GW. CD98hc (SLC3A2) drives integrin‐dependent renal cancer cell behavior. Mol Cancer. 2013;12:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bajaj J, Konuma T, Lytle NK, et al. CD98‐mediated adhesive signaling enables the establishment and propagation of acute myelogenous leukemia. Cancer Cell. 2016;30:792‐805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Haining Z, Kawai N, Miyake K, et al. Relation of LAT1/4F2hc expression with pathological grade, proliferation and angiogenesis in human gliomas. BMC Clin Pathol. 2012;12:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. El Ansari R, Craze ML, Miligy I, et al. The amino acid transporter SLC7A5 confers a poor prognosis in the highly proliferative breast cancer subtypes and is a key therapeutic target in luminal B tumours. Breast Cancer Res. 2018;20:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xu M, Sakamoto S, Matsushima J, et al. Up‐regulation of LAT1 during antiandrogen therapy contributes to progression in prostate cancer cells. J Urol. 2016;195:1588‐1597. [DOI] [PubMed] [Google Scholar]
- 28. Ohshima Y, Kaira K, Yamaguchi A, et al. Efficacy of system L amino acid transporter 1 inhibition as a therapeutic target in esophageal squamous cell carcinoma. Cancer Sci. 2016;107:1499‐1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hirano K, Uno K, Kuwabara H, et al. Expression of L‐type amino acid transporter 1 in various skin lesions. Pathol Res Pract. 2014;210:634‐639. [DOI] [PubMed] [Google Scholar]
- 30. Hara K, Kudoh H, Enomoto T, Hashimoto Y, Masuko T. Malignant transformation of NIH3T3 cells by overexpression of early lymphocyte activation antigen CD98. Biochem Biophys Res Commun. 1999;262:720‐725. [DOI] [PubMed] [Google Scholar]
- 31. Hara K, Kudoh H, Enomoto T, Hashimoto Y, Masuko T. Enhanced tumorigenicity caused by truncation of the extracellular domain of GP125/CD98 heavy chain. Oncogene. 2000;19:6209‐6215. [DOI] [PubMed] [Google Scholar]
- 32. Shishido T, Uno S, Kamohara M, et al. Transformation of BALB3T3 cells caused by over‐expression of rat CD98 heavy chain (HC) requires its association with light chain: mis‐sense mutation in a cysteine residue of CD98HC eliminates its transforming activity. Int J Cancer. 2000;87:311‐316. [DOI] [PubMed] [Google Scholar]
- 33. Hara K, Ueda S, Ohno Y, et al. NIH3T3 cells overexpressing CD98 heavy chain resist early G1 arrest and apoptosis induced by serum starvation. Cancer Sci. 2012;103:1460‐1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ohkawa M, Ohno Y, Masuko K, et al. Oncogenicity of L‐type amino‐acid transporter 1 (LAT1) revealed by targeted gene disruption in chicken DT40 cells: LAT1 is a promising molecular target for human cancer therapy. Biochem Biophys Res Commun. 2011;406:649‐655. [DOI] [PubMed] [Google Scholar]
- 35. Ullrich A, Coussens L, Hayflick JS, et al. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. Nature. 1984;309:418‐425. [DOI] [PubMed] [Google Scholar]
- 36. Merlino GT, Xu YH, Ishii S, et al. Amplification and enhanced expression of the epidermal growth factor receptor gene in A431 human carcinoma cells. Science. 1984;224:417‐419. [DOI] [PubMed] [Google Scholar]
- 37. Semba K, Kamata N, Toyoshima K, Yamamoto T. A v‐erbB‐related protooncogene, c‐erbB‐2, is distinct from the c‐erbB‐1/epidermal growth factor‐receptor gene and is amplified in a human salivary gland adenocarcinoma. Proc Natl Acad Sci USA. 1985;82:6497‐6501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yokota J, Yamamoto T, Toyoshima K, et al. Amplification of c‐erbB‐2 oncogene in human adenocarcinomas in vivo. Lancet. 1986;1:765‐767. [DOI] [PubMed] [Google Scholar]
- 39. Hayes GM, Chinn L, Cantor JM, et al. Antitumor activity of an anti‐CD98 antibody. Int J Cancer. 2015;137:710‐720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ohno Y, Suda K, Masuko K, Yagi H, Hashimoto Y, Masuko T. Production and characterization of highly tumor‐specific rat monoclonal antibodies recognizing the extracellular domain of human L‐type amino‐acid transporter 1. Cancer Sci. 2008;99:1000‐1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Masuko T, Ohno Y, Masuko K, et al. Towards therapeutic antibodies to membrane oncoproteins by a robust strategy using rats immunized with transfectants expressing target molecules fused to green fluorescent protein. Cancer Sci. 2011;102:25‐35. [DOI] [PubMed] [Google Scholar]
- 42. Ikotun OF, Marquez BV, Huang C, et al. Imaging the L‐type amino acid transporter‐1 (LAT1) with Zr‐89 immunoPET. PLoS ONE. 2013;8:e77476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yagita H, Masuko T, Hashimoto Y. Inhibition of tumor cell growth in vitro by murine monoclonal antibodies that recognize a proliferation‐associated cell surface antigen system in rats and humans. Cancer Res. 1986;46:1478‐1484. [PubMed] [Google Scholar]
- 44. Masuko T, Abe J, Yagita H, Hashimoto Y. Human bladder cancer cell‐surface antigens recognized by murine monoclonal antibodies raised against T24 bladder cancer cells. Jpn J Cancer Res. 1985;76:386‐394. [PubMed] [Google Scholar]
- 45. Tanaka T, Suzuki S, Masuko T, Hashimoto Y. In vitro targeting and cytotoxicity of adriamycin in liposomes bearing monoclonal antibody against rat or human gp125 cell proliferation‐associated antigen. Jpn J Cancer Res. 1989;80:380‐386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Masuko T, Sugahara K, Kozono M, et al. A murine monoclonal antibody that recognizes an extracellular domain of the human c‐erbB‐2 protooncogene product. Jpn J Cancer Res. 1989;80:10‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nishimura T, Nakamura Y, Tsukamoto H, et al. Human c‐erbB‐2 proto‐oncogene product as a target for bispecific‐antibody‐directed adoptive tumor immunotherapy. Int J Cancer. 1992;50:800‐804. [DOI] [PubMed] [Google Scholar]
- 48. Kasprzyk PG, Song SU, Di Fiore PP, King CR. Therapy of an animal model of human gastric cancer using a combination of anti‐erbB‐2 monoclonal antibodies. Cancer Res. 1992;52:2771‐2776. [PubMed] [Google Scholar]
- 49. Nicklin P, Bergman P, Zhang B, et al. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell. 2009;136:521‐534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Saito M, Kondo M, Ohshima M, et al. Identification of anti‐CD98 antibody mimotopes for inducing antibodies with antitumor activity by mimotope immunization. Cancer Sci. 2014;105:396‐401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Asao H, Takeshita T, Ishii N, Kumaki S, Nakamura M, Sugamura K. Reconstitution of functional interleukin 2 receptor complexes on fibroblastoid cells: involvement of the cytoplasmic domain of the gamma chain in two distinct signaling pathways. Proc Natl Acad Sci USA. 1993;90:4127‐4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pillet AH, Juffroy O, Mazard‐Pasquier V, et al. Human IL‐Rβ chains form IL‐2 binding homodimers. Eur Cytokine Netw. 2008;19:49‐59. [DOI] [PubMed] [Google Scholar]
- 53. Hara Y, Torii R, Ueda S, et al. Inhibition of tumor formation and metastasis by a monoclonal antibody against lymphatic vessel endothelial hyaluronan receptor 1. Cancer Sci. 2018;109:3171‐3182. [DOI] [PMC free article] [PubMed] [Google Scholar]


