Abstract
Cardiac ischemia/reperfusion, loss of blood flow and its subsequent restoration, causes damage to the heart. Oxidative stress from ischemia/reperfusion leads to dysfunction and death of cardiomyocytes, increasing the risk of progression to heart failure. Alterations in mitochondrial dynamics, in particular mitochondrial fission, have been suggested to play a role in cardioprotection from oxidative stress. We tested the hypothesis that activation of RhoA regulates mitochondrial fission in cardiomyocytes. Our studies show that expression of constitutively active RhoA in cardiomyocytes increases phosphorylation of Dynamin-related protein 1 (Drp1) at serine-616, and leads to localization of Drp1 at mitochondria. Both responses are blocked by inhibition of Rho-associated Protein Kinase (ROCK). Endogenous RhoA activation by the GPCR agonist sphingosine-1-phosphate (S1P) also increases Drp1 phosphorylation and its mitochondrial translocation in a RhoA and ROCK dependent manner. Consistent with the role of mitochondrial Drp1 in fission, RhoA activation in cardiomyocytes leads to formation of smaller mitochondria and this is attenuated by inhibition of ROCK, by siRNA knockdown of Drp1 or by expression of a phosphorylation-deficient Drp1 S616A mutant. In addition, activation of RhoA prevents cell death in cardiomyocytes challenged by oxidative stress and this protection is blocked by siRNA knockdown of Drp1 or by Drp1 S616A expression. Taken together our findings demonstrate that RhoA activation can regulate Drp1 to induce mitochondrial fission and subsequent cellular protection, implicating regulation of fission as a novel mechanism contributing to RhoA-mediated cardioprotection.
Keywords: RhoA, Sphingosine-1-phosphate, Drp1, Mitochondria, Fission, Cardioprotection
1. Introduction
Cardiomyocyte death following cardiac ischemia/reperfusion (I/R) contributes to progression to heart failure [1], thus there is considerable interest in elucidating signaling pathways that sustain myocyte survival in the face of injury. We and others have previously demonstrated that signaling by activation of the small GTPase RhoA provides cardioprotection from ischemic injury [2–5]. RhoA regulates downstream phosphorylation cascades involved in protection from ischemic or oxidative stress through kinases such as Rho-associated Protein Kinase (ROCK), Protein Kinase B (Akt) and Protein Kinase D (PKD) [2, 3, 6]. Sphingosine-1-phosphate (S1P), a ligand released in response to injury, also elicits cardioprotection [7, 8] which we have shown to be associated with activation of RhoA [9, 10].
Mitochondria are dynamic organelles that undergo fusion and fission in response to changes in cellular environment [11, 12]. Mitochondrial fission precedes apoptosis [13, 14], but also occurs prior to more physiological responses such as mitosis [15], and has been suggested to provide protection from apoptosis [16]. A role for mitochondrial fission in cell survival is supported by evidence that inhibiting fission leads to cell death [17, 18]. Dynamin-related protein 1 (Drp1) is a GTPase that can translocate to the mitochondria and generate the force necessary for mitochondrial fission [19, 20]. Mitochondrial translocation of Drp1 can be increased or decreased through its post-translational modification, most clearly phosphorylation but also ubiquitination, SUMOylation, and S-nitrosylation [21]. Cardiac specific knockout of Drp1 results in mitochondrial dysfunction, cardiomyocyte death, and progression to lethal cardiomyopathy [18]. Similarly, a mutation in a Drp1 oligomerization domain induces cardiomyopathy in mice [22]. Heterozygous cardiomyocyte specific Drp1 knockouts are minimally affected under baseline conditions, but show enhanced dysfunction after fasting and increased infarct from ischemia/reperfusion (I/R), further indicating that Drp1 serves an adaptive role in response to stress [23].
Previous studies suggest the involvement of ROCK in regulation of Drp1 and mitochondrial fission [24–27]. It has not been determined, however, whether Drp1 serves as a downstream mediator of RhoA signaling and is a mechanism by which receptor activation can regulate mitochondrial dynamics. Here we use pharmacological and genetic approaches to provide evidence that activation of RhoA, through its heterologous expression or via GPCR mediated activation of endogenous RhoA, increases Drp1 serine-616 phosphorylation, Drp1 translocation to mitochondria, and mitochondrial fission. We further show that Drp1 participates in RhoA mediated cardioprotection from oxidative stress, implicating mitochondrial fission as a novel mechanism by which RhoA signaling can promote cardiomyocyte survival.
2. Materials and methods
2.1. Animals
All animal procedures were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee at the University of California San Diego. All experiments were performed on age-matched littermates.
2.2. Reagents
Predesigned rat siRNA and scrambled control siRNA were purchased from Qiagen and used at 3 μg per 1 × 106 cells. Cardiomyocytes were transfected with siRNA using DharmaFECT-1 transfection reagent (1:2.5 ratio) from Thermo Fisher Scientific based on the manufacturer’s instruction. The RhoA inhibitor C3 exoenzyme was obtained from Cytoskeleton (CT04). The ROCK inhibitor Y-27632 was purchased from Calbiochem. Mitochondrial fission inhibitor mdivi-1 was purchased from Sigma-Aldrich. S1P was obtained from Avanti Polar. CYM-51736, an allosteric agonist of S1PR3, was provided by Dr. Hugh Rosen (Scripps Research Institute, La Jolla, CA).
2.3. NRVM cell culture
Neonatal rat ventricular myocytes (NRVMs) were isolated from cardiac ventricles of 1- to 2-day-old Sprague-Dawley rat pups as described previously [28]. NRVMs were plated at a density of 3.0 × 104/cm2 on gelatin-coated dishes and maintained in Dulbecco-modified Eagle’s medium (DMEM) containing 15% fetal bovine serum overnight. Beginning the following day, cells were starved with serum-free DMEM for 24 h and, if applicable, transfected with siRNA for 48 h for further analysis.
For adenoviral expression in NRVMs, cells cultured in serum-free medium were subsequently infected with adenovirus for 16 h, unless otherwise stated. Adenovirus for the constitutively active (L63) RhoA, dominant-negative ROCK, Drp1 wildtype, and phosphorylation defective mutant Drp1 S616A constructs were generated as described previously [29]. Pharmacological inhibitors were added at the time of adenoviral infection.
2.4. Adeno-associated virus serotype 9 cardiac-targeted expression
AAV9 plasmids containing the gene for either a GFP control or the gene for (L63) RhoA, driven by a cardiomyocyte specific CMVMLC2v0.8 promoter, were prepared as previously described [30]. Mice were anesthetized with 2% isoflurane and then injected via lateral tail vein with 100 μl of AAV9 in lactated Ringer’s solution containing 1 × 1011 viral particles. Hearts were isolated at 2 weeks following AAV9 injection for analysis via mitochondrial isolation and Western blot.
2.5. Mitochondrial isolation
Isolation of mitochondrial fractions from NRVMs was performed as previously described [28]. Myocytes were washed with ice-cold PBS, resuspended in mitochondrial isolation buffer containing 420 mM mannitol, 140 mM sucrose, 2 mM EDTA, 20 mM HEPES (pH 7.4), 0.025% digitonin, 1 μM Na3VO4, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 0.5 mM PNPP (para-nitrophenyl phosphate), and 0.5 mM PMSF (phenylmethylsulfonyl fluoride), cells were broken up with a syringe and 25-gauge needle, and incubated on ice for 10 min. Samples were centrifuged twice at 700g for 10 min to spin down nuclei and cell debris. Clarified supernatants were spun at 12,000 g for 15 min to pellet mitochondria. The pellet was washed and resuspended in RIPA buffer as the mitochondrial fraction. The final supernatant was used as the cytosolic fraction.
For isolation of mitochondria from adult mouse hearts, the left ventricle was homogenized in mitochondrial isolation buffer described above, and incubated on ice for 10 min. Samples were then centrifuged twice at 700g for 10 min to spin down nuclei and cell debris. Clarified supernatants were spun at 10,000 g for 15 min to pellet mitochondria, which were resuspended in RIPA buffer.
2.6. Western blotting
Western blot analysis was performed according to protocols as previously described [28]. The primary antibodies used were Drp1 from BD Transduction Laboratories; phospho-Drp1 (Ser616), phospho-Drp1 (Ser637), RhoGDI, COX-IV, GAPDH, phospho-Akt (Ser473), and Akt from Cell Signaling Technology; RhoA from Santa Cruz; cofilin2, and phospho-cofilin2 (Ser3) from Millipore. Peroxidase-conjugated secondary antibodies (Sigma) were used at a dilution of 1:2000, and enhanced chemiluminescent substrate was from Thermo Scientific.
2.7. Isolated perfused heart (Langendorff)
Hearts from age-matched 2- to 4-month-old WT or cardiomyocyte-specific RhoA KO mice were removed quickly and perfused with modified Krebs-Henseleit buffer (118 mM NaCl, 4.7 mM KCl, 1.2 mM KH2PO4, 25 mM NaHCO3, 0.5 mM EDTA, 1.2 mM MgSO4, 11 mM glucose, 1.5 mM sodium pyruvate, and 2 mM CaCl2) in a Langendorff apparatus (Radnoti) at a constant pressure of 80 mmHg, as described previously [2]. Hearts were perfused for 15 min with and without addition of S1P to the Krebs-Henseleit buffer. Following perfusion, hearts were snap-frozen in liquid nitrogen prior to mitochondrial fractionation and subsequent analysis.
2.8. Fluorescence confocal microscopy
Cells plated on laminin-coated coverslips were incubated for 15 min with MitoTracker DeepRed (30 nM) and maintained in DMEM without Phenol Red. Fluorescent images were captured with a Leica SP5 confocal microscope, with a 40× oil immersion objective. Images were convolved with subsequent particle analysis of mitochondrial objects in ImageJ software (NIH) to quantify mitochondrial size by Form Factor and Aspect Ratio [31, 32]. Mitochondrial Form Factors and Aspect Ratios were averaged for each cell. Each experiment was performed 3–4 times, with 10 cells per condition quantified in each experiment.
2.9. Cell death enzyme-linked immunosorbent assay (ELISA)
DNA fragmentation indicative of apoptosis was assayed using the Cell Death Detection ELISAPLUS kit (Roche) according to the manufacturer’s instructions, as performed previously [2]. Cardiomyocytes were washed in ice-cold PBS and harvested in cytosolic extraction buffer containing 20 mM Tris pH 7.6, 3 mM EDTA, 3 mM EGTA, 125 mM NaCl, 20 mM β-glycerophosphate and 0.4% NP-40 plus protease and phosphatase inhibitors. Samples were then spun down and the supernatant was incubated with anti-histone-biotin and anti-DNA-peroxidase in a streptavidin-coated microplate for 2 h, washed 3 times, colorimetric substrate was added, and absorbance was measured at 405 nm.
2.10. Statistical analysis
All results are reported as means +/− standard error. Comparison of two groups with one variable was accomplished using an unpaired Student’s t-test, or if the control group was normalized to 1 with no variance, a paired t-test. Data from experiments with more than two groups with one variable were compared by one-way ANOVA followed by Tukey’s multiple comparison test, or repeated measures ANOVA followed by Tukey’s multiple comparison test if the control group was normalized to 1 with no variance. Probabilities ≤0.05 were considered significant.
3. Results
3.1. RhoA and S1P stimulate phosphorylation of Drp1 at serine-616
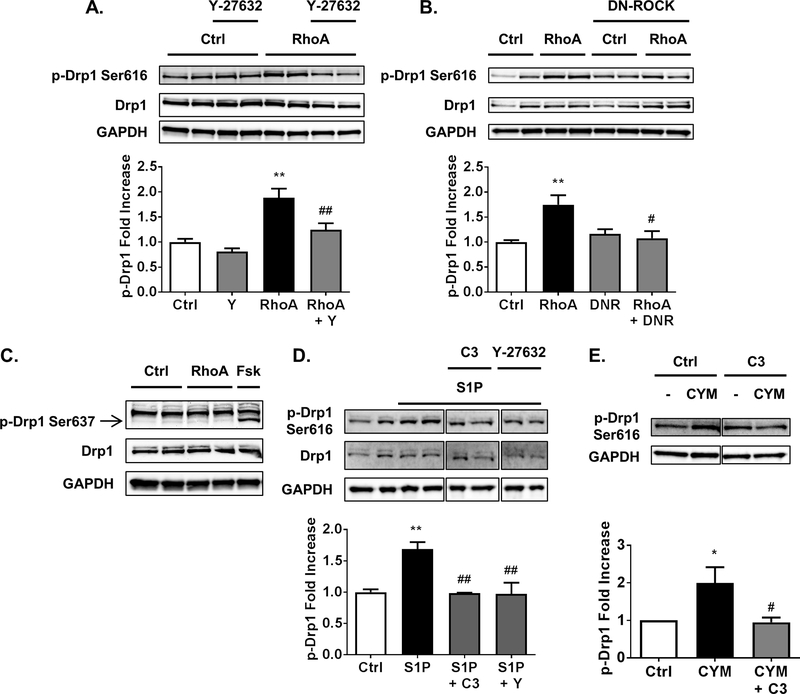
To establish whether activation of RhoA can regulate Drp1 we expressed constitutively active L63 RhoA adenovirus in cardiomyocytes. Expression of active RhoA increased phosphorylation of Drp1 at serine-616 (Fig. 1A-B), a site implicated in Drp1-dependent mitochondrial fission. To determine if ROCK is required for Drp1 phosphorylation we used the small molecule ROCK inhibitor Y-27632 (Fig. 1A), or co-expression of a dominant-negative ROCK adenovirus (Fig. 1B). Both means of inhibiting ROCK signaling prevented RhoA induced increases in Drp1 serine-616 phosphorylation (Fig. 1A-B). In contrast, phosphorylation of Drp1 at serine-637, a residue implicated in decreased mitochondrial fission, was not affected by expression of active RhoA (Fig. 1C), although increasing cAMP signaling with forskolin increased phosphorylation at this site, as previously reported [32].
Fig. 1.
RhoA activation through ROCK stimulates phosphorylation of Drp1 at serine-616. A, NRVMs adenovirally expressing activated RhoA or GFP control, in the absence or presence of 10 μM of ROCK inhibitor Y-27632. Whole cell lysates were analyzed by SDS-PAGE and Western blot for p-Drp1 at serine-616 and total Drp1 levels. **p < .01 vs. Ctrl, ##p < .01 vs. RhoA (n=5–6). B, NRVMs adenovirally expressing activated RhoA or GFP control, with and without co-expression of dominant-negative ROCK (DNR). Whole cell lysates were analyzed by SDS-PAGE and Western blot for p-Drp1 at serine-616 and total Drp1 levels. **p < .01 vs. Ctrl, #p < .05 vs. RhoA (n=4–5). C, A representative Western blot for p-Drp1 at serine-637 and total Drp1 in whole cell lysates from NRVMs adenovirally expressing activated RhoA or GFP control, or stimulated for 30 min with 10 μM forskolin (Fsk) as a positive control for PKA-dependent phosphorylation of Drp1 at serine-637. D, NRVMs were pretreated with C3 Rho inhibitor (1.5 μg/ml for 6 h pretreatment) or ROCK inhibitor Y-27632, then stimulated with 300 nM S1P for 30 min. Whole cell lysates were analyzed by SDS-PAGE and Western blot for p-Drp1 at serine-616 and total Drp1 levels. **p < .01 vs. Ctrl, ##p < .01 vs. S1P (n=4–6). E, NRVMs with or without pretreatment of C3 Rho inhibitor were stimulated by 10 μM of S1PR3 selective agonist CYM-51736 (CYM) for 30 min and cell lysates were subjected to Western blot for p-Drp1 at serine-616. *p < .05 vs. Ctrl, #p < .05 vs. CYM (n= 4).
In addition to examining responses to expression of active RhoA, we stimulated cardiomyocytes with the GPCR agonist sphingosine-1-phosphate (S1P) to activate endogenous RhoA [9]. S1P treatment also increased phosphorylation of Drp1 at serine-616 (Fig. 1D). Phosphorylation of Drp1 in response to S1P was dependent on both RhoA and ROCK, as indicated by attenuation of this response by functional RhoA inhibition with C3 exoenzyme and ROCK inhibition with Y-27632 respectively (Fig. 1D). We previously demonstrated that the S1PR3 is the S1P receptor subtype that selectively couples to RhoA activation in cardiomyocytes [10], and show here that the S1PR3 specific agonist CYM-51736 also increases phosphorylation of Drp1 at serine-616 (Fig. 1E), a response abolished by functional inhibition of RhoA signaling with C3.
3.2. RhoA and S1P increase Drp1 levels at the mitochondria
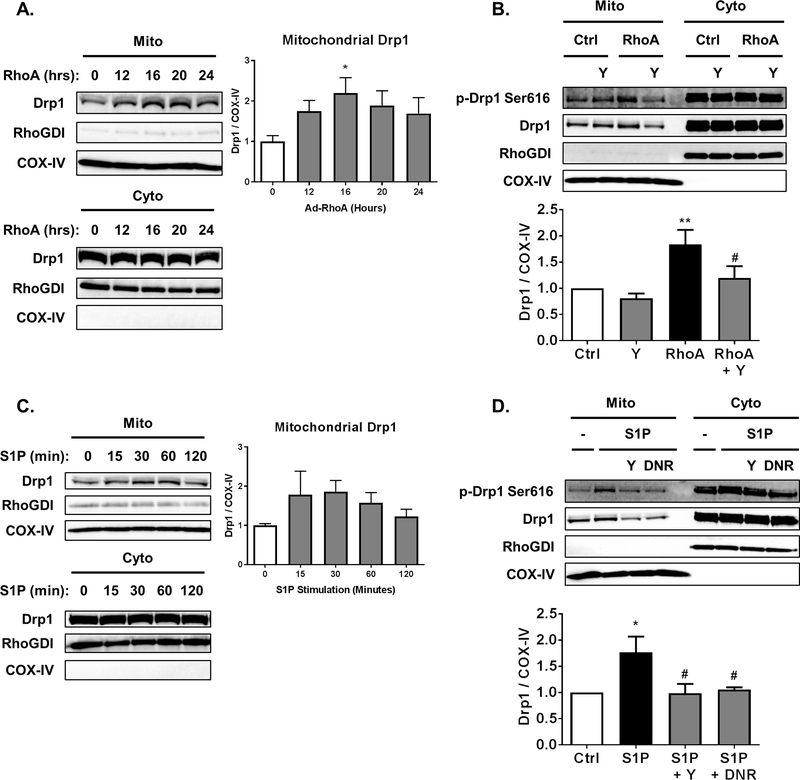
Phosphorylation of Drp1 at serine-616 has been associated with Drp1 translocation to the mitochondria. To determine if activation of RhoA increases mitochondrial levels of Drp1 we infected cardiomyocytes with adenovirus encoding active RhoA and harvested cells at various times. Mitochondrial fractions were prepared and RhoA activation was found to increase Drp1 in mitochondrial fractions 2-fold by 16 h following infection (Fig. 2A). This time point was used in all experiments, including demonstrating that ROCK inhibition with Y-27632 prevents RhoA-induced increases in mitochondrial Drp1 (Fig. 2B).
Fig. 2.
RhoA activation through ROCK increases mitochondrial levels of Drp1 in cardiomyocytes. A, NRVMs adenovirally expressing activated RhoA for the amount of time indicated following infection. Mitochondrial and cytosolic fractions analyzed by SDS-PAGE and Western blot for Drp1 in respective fractions. COX-IV and RhoGDI were used as controls for loading of mitochondrial and cytosolic fractions respectively. *p < .05 vs. 0 h (n=5) B, NRVMs adenovirally expressing activated RhoA or GFP control, in the absence or presence of 10 μM of ROCK inhibitor Y-27632. Mitochondrial and cytosolic fractions analyzed by SDS-PAGE and Western blot for Drp1 in respective fractions. **p < .01 vs. Ctrl, #p < .05 vs. RhoA (n=5). C, NRVMs with stimulation by 300nM S1P for the amount of time indicated. Mitochondrial and cytosolic fractions analyzed by SDS-PAGE and Western blot for Drp1 in respective fractions. D, NRVMs in the absence or presence of 10 μM of ROCK inhibitor Y-27632 (Y) or adenovirally expressing dominant-negative ROCK (DNR), were stimulated by 300 nM S1P for 30 min. Mitochondrial and cytosolic fractions analyzed by SDS-PAGE and Western blot for Drp1 in respective fractions. **p < .05 vs. Ctrl, #p < .05 vs. S1P (n=4).
To determine whether activation of endogenous RhoA is capable of increasing mitochondrial Drp1 cardiomyocytes were stimulated with S1P. Treatment with S1P for 30 min increased mitochondrial Drp1 approximately 1.5-fold (Fig. 2C). The response to S1P also required downstream ROCK signaling, as the ROCK inhibitor Y-27632 or adenoviral expression of dominant-negative ROCK prevented the increase of mitochondrial Drp1 (Fig. 2D).
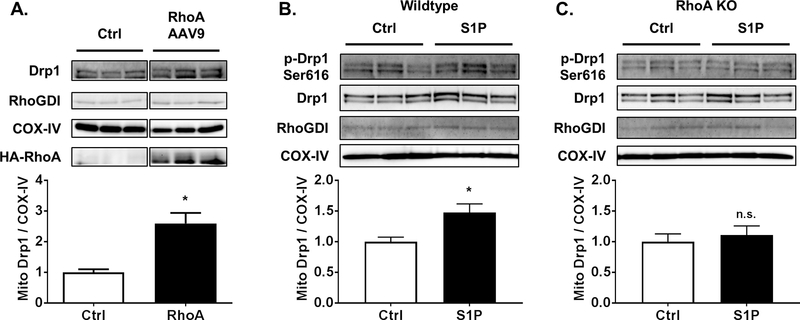
To determine if RhoA activation increases mitochondrial Drp1 in vivo, an AAV9 construct engineered to express constitutively activated RhoA was injected into the tail vein of mice. Hearts were harvested 2 weeks after injection to allow time for RhoA expression and shown to have increased levels of RhoA and of mitochondrial Drp1 (Fig. 3A). Isolated mouse hearts perfused with S1P for 15 min also had increased mitochondrial Drp1 (Fig. 3B). We then used hearts from cardiac specific RhoA KO mice [3] to establish the requirement for RhoA in S1P regulated Drp1 translocation in the perfused heart. When S1P was perfused into hearts isolated from RhoA KO mice no increase in mitochondrial Drp1 levels was observed, indicating that mitochondrial translocation of Drp1 due to ex vivo stimulation with S1P is a RhoA dependent event (Fig. 3C).
Fig. 3.
RhoA activation increases mitochondrial Drp1 levels in the adult mouse heart. A, Mice were injected with RhoA AAV9 or corresponding control for 2 weeks. Mitochondrial fractions of mouse hearts analyzed by SDS-PAGE and Western blot for Drp1. COX-IV and RhoGDI were used as mitochondrial and cytosolic markers respectively. *p < .05 vs. Ctrl (n=3). B, C, Mouse hearts from wildtype (B) or cardiac-specific RhoA knockouts (C) were subjected to ex vivo Langendorff perfusion with or without 300 nM S1P for 15 min. Mitochondrial fractions analyzed by SDS-PAGE and Western blot for Drp1. *p < .05 vs. Ctrl (n=7).
3.3. RhoA induces Drp1 dependent mitochondrial fission
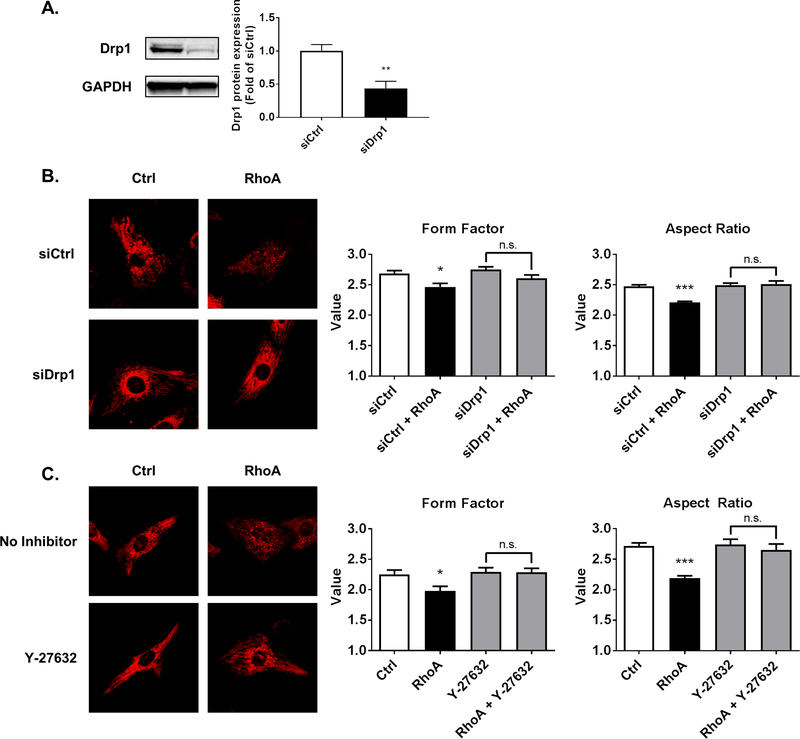
The ability of RhoA to regulate both phosphorylation and mitochondrial levels of Drp1 suggested that RhoA activation would lead to mitochondrial fission in cardiomyocytes. To examine this, mitochondria were visualized by confocal microscopy using MitoTracker DeepRed. RhoA expression was found to reduce mitochondrial size, assessed by calculation of form factor and aspect ratios, indicators of circularity used to determine mitochondrial size [31]. Knockdown of Drp1 with siRNA for 48 h, which caused ~60% reduction in Drp1 when assessed in whole cell lysates by Western blot (Fig. 4A), blocked the RhoA mediated decrease in mitochondrial size (Fig. 4B), indicating that Drp1 is required for RhoA induced mitochondrial fission in cardiomyocytes. Consistent with our finding of ROCK dependent Drp1 serine-616 phosphorylation and mitochondrial association, Y-27632 prevented RhoA dependent decreases in mitochondrial size (Fig. 4C).
Fig. 4.
RhoA activation induces mitochondrial fission in cardiomyocytes through ROCK and Drp1. A, NRVMs were transfected with either control siRNA (siCtrl) or siRNA for Drp1 (siDrp1) for 48 h. **p < .01 vs. siCtrl (n=6). B, Confocal microscopy of NRVMs treated with either control or Drp1 siRNA for 48 h, and subsequent adenoviral expression of either activated RhoA or control. MitoTracker DeepRed (30 nM) was loaded into cardiomyocytes to visualize mitochondria. Particle analysis was carried out to calculate form factor and aspect ratio as indicators of circularity to assess mitochondrial size. *p < .05 vs. siCtrl, ***p < .001 vs. siCtrl (n=40). C, Confocal microscopy images of NRVMs with adenoviral expression of either activated RhoA or control, and absence or presence of ROCK inhibitor Y-27632 (10 μM). MitoTracker DeepRed was used to visualize mitochondria. Particle analysis was carried out to calculate form factor and aspect ratio as indicators of circularity to assess mitochondrial size. *p < .05 vs. Ctrl, ***p < .001 vs. Ctrl (n=30).
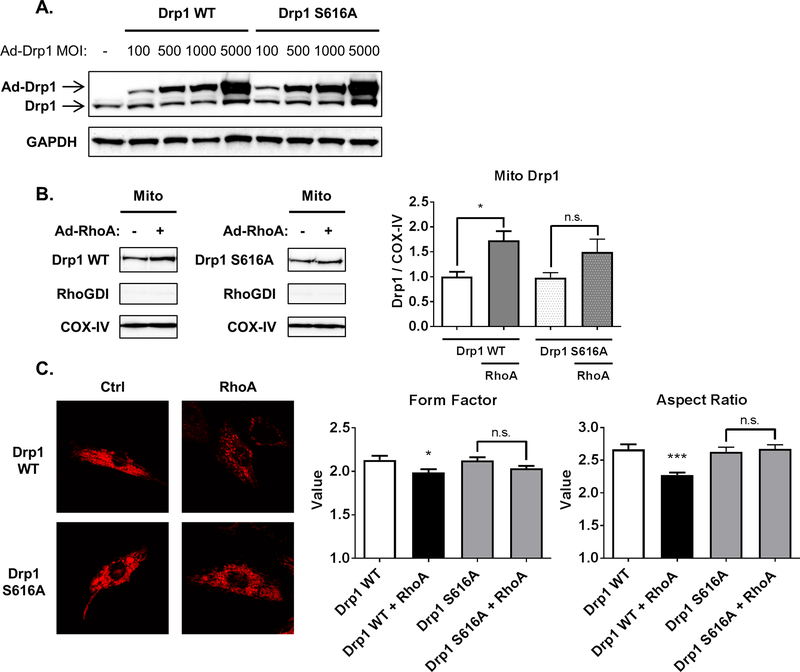
To further demonstrate a role for Drp1 phosphorylation at serine-616 in RhoA induced mitochondrial fission we constructed adenovirus expressing either Drp1 wildtype (Drp1 WT) or a non-phosphorylatable mutant, Drp1 S616A. Pilot studies were carried out using a range of MOIs to achieve similar expression levels for the two constructs (Fig. 5A). Expression of the Drp1 constructs at 500 MOI, which gives expression approximately 5 fold over endogenous levels, was used in further studies. As shown in Fig. 5B, Drp1 WT accumulated at mitochondria when co-expressed with active RhoA, consistent with the effects of RhoA expression on endogenous Drp1. In contrast there was not a significant increase in mitochondrial Drp1 S616A in response to RhoA (Fig. 5B). We further observed that RhoA increased mitochondrial fission, as indicated by decreased form factor and aspect ratios, in cells expressing Drp1 WT but not in cells expressing Drp1 S616A (Fig. 5C), consistent with the inability of RhoA activation to increase mitochondrial levels of Drp1 S616A.
Fig. 5.
Phosphorylation of Drp1 at serine-616 is required for RhoA-induced mitochondrial fission. A, NRVMs were infected with adenovirus expressing wildtype or S616A phosphorylation-defective mutant Drp1. Whole cell lysates were analyzed by Western blot for Drp1. B, NRVMs were infected with 500 MOI of adenovirus expressing Drp1 wildtype or S616A, and active RhoA or GFP control were adenovirally co-expressed. Mitochondrial fractions were analyzed by Western blot for Drp1, with COX-IV and RhoGDI as mitochondrial and cytosolic markers respectively. *p < .05 vs. Ctrl (n=6). C, Fluorescent confocal microscopy images of NRVMs with adenoviral expression of wildtype or S616A phosphorylation-defective mutant Drp1, and adenoviral co-expression of activated RhoA or control, using MitoTracker DeepRed to visualize mitochondria. Particle analysis was carried out to calculate form factor and aspect ratio as indicators of circularity to assess mitochondrial size. *p < .05 vs. Drp1 WT, ***p < .001 vs. Drp1 WT (n=30).
3.4. Drp1 is required for RhoA-mediated cardioprotection of cardiomyocytes from oxidative stress
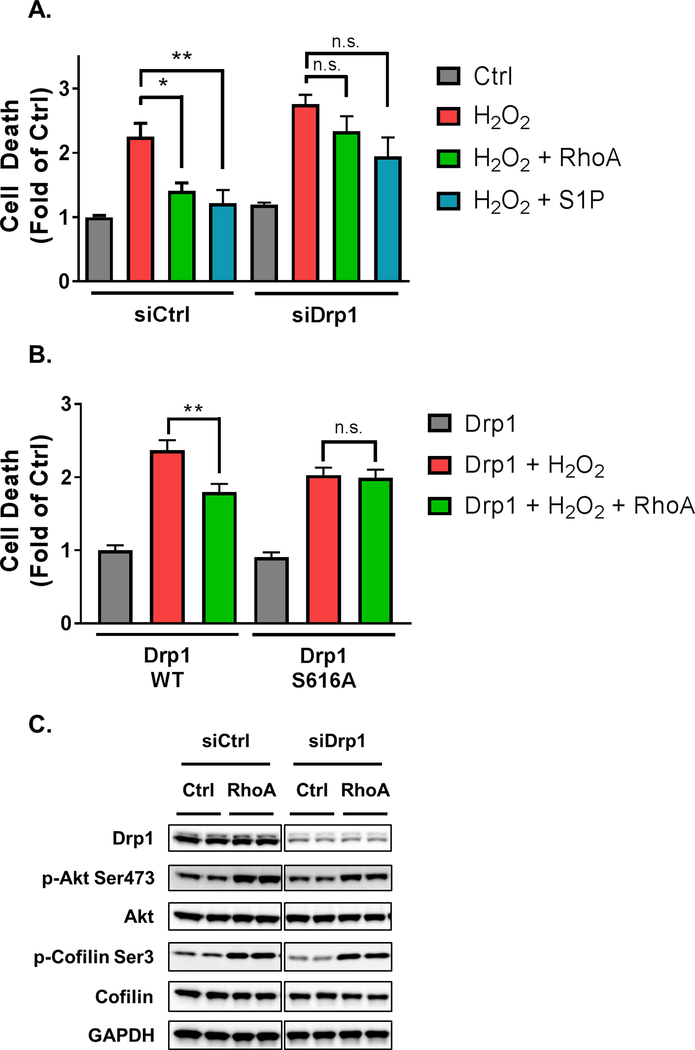
Sustained mitochondrial fission through Drp1 has been implicated in cardiac cell death [33], but studies on heterozygous Drp1 knockout mice suggest that Drp1 dependent fission is protective against ischemia/reperfusion injury [23]. Thus we considered whether the ability of RhoA signaling to protect cardiomyocytes against oxidative stress [9] could derive in part from its ability to regulate Drp1. We induced oxidative stress by hydrogen peroxide treatment since we have previously established that RhoA expression or S1P treatment protect against cell death in this model [2, 9]. The ability of either RhoA or S1P to protect cardiomyocytes against cell death was significantly diminished when cardiomyocyte Drp1 was knocked down with siRNA (Fig. 6A). Protection of cardiomyocytes by RhoA was also observed when Drp1 WT was expressed, but not with expression of Drp1 S616A (Fig. 6B). Expression of Drp1 S616A had a modestly protective effect, but this was independent of RhoA activation. To provide further evidence for a role of Drp1-mediated fission in the cardioprotective effect of RhoA we utilized mdivi-1, a pharmacological inhibitor of Drp1 dependent fission. Prolonged treatment with 50 μM mdivi-1 induces cardiomyocyte death [23]. However, when cells were treated with a lower dose of mdivi-1 (10 μM) to limit its pro-apoptotic effects, it was found to attenuate RhoA mediated protection (Supplemental Fig. 1). Thus Drp1 dependent mitochondrial fission is a critical mechanism underlying RhoA-mediated cardioprotection. Drp1 knockdown did not affect RhoA induced changes in phosphorylation of Akt or cofilin, markers of pathways we have previously implicated in cardioprotection (Fig. 6C). Thus the requirement for Drp1 in RhoA mediated mitochondrial fission defines a novel pathway independent of known RhoA dependent kinase cascades that protect against oxidative stress.
Fig. 6.
Drp1 contributes to RhoA-mediated cardioprotection from oxidative stress. A, Following 48 h of control or Drp1 siRNA treatment, NRVMs were infected with adenovirus expressing activated RhoA or GFP control for 12 h and treated with or without S1P for 30 min. NRVMs were then treated with 50 μM H2O2 for 16 h. Cell death was quantified by Cell Death Detection ELISAPLUS POD assay of nucleus-free lysates. *p < .05 vs. H2O2, **p < .01 vs. H2O2 in control (n=6). B, NRVMs were infected with adenovirus expressing wildtype or S616A phosphorylation-defective mutant Drp1, and adenoviral co-expression of activated RhoA or GFP control for 24 h. NRVMs were then treated with 50 μM H2O2 for 16 h. Cell death was quantified by Cell Death Detection ELISAPLUS POD assay of nucleus-free lysates. **p < .01 vs. Drp1 + H2O2 (n=6). C, NRVMs were treated with control or Drp1 siRNA knockdown for 48 h. Subsequently, NRVMs were infected with adenovirus expressing activated RhoA or GFP control, and whole cell lysates were analyzed by Western blot for Drp1 to confirm knockdown, and of p-Akt, total Akt, p-cofilin, and total cofilin to assess phosphorylation of these proteins with and without Drp1 siRNA.
4. Discussion
We show here, through studies using either adenoviral expression of constitutively active RhoA or stimulation of RhoA with the GPCR ligand S1P, that RhoA activation in cardiomyocytes regulates Drp1 phosphorylation at serine-616 through a ROCK dependent pathway. Using these same approaches, we demonstrate that RhoA activation increases mitochondrial levels of Drp1 in both cardiomyocytes and the whole heart. We further show that activation of RhoA induces mitochondrial fission in cardiomyocytes in a Drp1 dependent manner as indicated through studies using siRNA knockdown. Experiments using expression of phosphomutant Drp1 indicate that RhoA induced Drp1 translocation and fission both require the phosphorylation of Drp1 at serine-616. Lastly using a cellular model of cardiomyocyte death we demonstrate, via siRNA knockdown or expression of a nonphosphorylatable Drp1 mutant, that Drp1 dependent fission contributes to RhoA-mediated cardioprotection against oxidative stress. To our knowledge, this is the first demonstration that a RhoA signaling pathway can elicit Drp1 dependent mitochondrial fission to provide protection against oxidative stress in cardiomyocytes.
4.1. Drp1 is phosphorylated and localized to mitochondria in response to RhoA and ROCK
Our studies demonstrate that RhoA activation, achieved through adenoviral expression of active RhoA or S1P treatment, leads to Drp1 phosphorylation at serine-616. Phosphorylation at this site appears to be a point of convergence for regulation of Drp1 by multiple kinase cascades: Drp1 has been reported to be phosphorylated at serine-616 through Cdk1, PKCδ, and CaMKII [15, 34, 35]. These kinases were implicated in non-cardiomyocytes, except in the case of CaMKII, and none of these kinases are likely downstream targets of RhoA signaling. Our studies demonstrate that Drp1 phosphorylation by RhoA is blocked by pharmacological or genetic interventions that inhibit ROCK, consistent with the finding that pharmacological inhibition of ROCK decreases Drp1 phosphorylation at serine-616 in hearts of mice subjected to endotoxemia [36].
We did not observe effects of RhoA signaling on serine-637 phosphorylation (Fig. 1C). This contrasts with a study using podocytes subjected to hyperglycemia where ROCK was implicated in phosphorylation of Drp1 at serine-637 [37]. PKD, another downstream target of RhoA explored in our previous work [3, 9, 10], has also been suggested to phosphorylate Drp1 at serine-637 in cardiomyocytes [38]. However, serine-637 phosphorylation of Drp1 was not affected by activation of RhoA nor was the RhoA-induced increase in phosphorylation of Drp1 at serine-616 blocked by a PKD inhibitor (data not shown). Thus activation of RhoA in cardiomyocytes does not lead to phosphorylation of Drp1 at serine-637 or phosphorylation of Drp1 through PKD signaling.
Our previous work demonstrates that RhoA signaling in cardiomyocytes regulates the kinases ROCK, Akt, and PKD [2, 3, 6, 9]. We considered possible involvement of Akt downstream of ROCK [2, 6], in the regulation of Drp1; however, phosphorylation of Drp1 by S1P was not blocked by an Akt inhibitor or a PKD inhibitor (data not shown). In addition, direct signaling by ROCK was recently suggested by its immunoprecipitation with Drp1 [37]. In light of these findings we propose that ROCK is the kinase responsible for transducing RhoA activation to Drp1 phosphorylation.
We also show that localization of Drp1 to mitochondria is mediated through ROCK in cardiomyocytes. Our studies using phosphomutant Drp1 support the hypothesis that phosphorylation at serine-616 is required for regulation of the mitochondrial translocation of Drp1 [34, 35]. Thus activation of RhoA and its downstream kinase, ROCK, defines a new pathway by which phosphorylation of Drp1 can be regulated in cardiomyocytes to transduce environmental signals to mitochondria.
4.2. Activation of RhoA by S1P mediates regulation of Drp1
Our findings with expression of active RhoA are recapitulated by GPCR stimulation with S1P or an S1PR3 agonist, both of which we have shown to lead to activation of RhoA [9, 10]. Both agonists increase phosphorylated Drp1 in a RhoA/ROCK dependent manner. Mitochondrial Drp1 was also increased in both cardiomyocytes and isolated hearts treated with S1P. GPCR pathways previously reported to stimulate Drp1 translocation to the mitochondria are the Gαs coupled β-adrenergic [35, 39] and the Gαq coupled α-adrenergic [40] receptors. Notably, studies showing responses to agonists for these other GPCRs in primary cardiomyocytes have examined changes in Drp1 phosphorylation, localization, or function after 6–48 h of stimulation, when additional signaling cascades or changes in gene expression could be activated [35, 40]. In contrast, S1PR3 couples through a different family of heterotrimeric G-proteins, Gα12/13, to activate RhoA signaling in both cardiomyocytes and other cardiac cells [10, 41], and the finding that S1P can stimulate RhoA-mediated Drp1 phosphorylation and translocation within 15 min of treatment provides important new insights into the role of acute activation of a GPCR signaling pathway in regulation of Drp1 in cardiomyocytes.
4.3. Mitochondrial fission induced by RhoA through regulation of Drp1 in cardiomyocytes
Expression of constitutively active RhoA results in changes in mitochondrial morphology consistent with increased fission. We demonstrated through pharmacological inhibition that ROCK is required for RhoA to elicit fission in cardiomyocytes, consistent with evidence for a role of ROCK in mitochondrial fission in neuronal and cancer cells [25, 26]. The siRNA knockdown of Drp1 prevented development of this phenotype, indicating that RhoA leads to mitochondrial fission through Drp1. Mitochondrial size was not altered in cardiomyocytes by Drp1 knockdown alone, in contrast to the mitochondrial enlargement observed in hearts of Drp1 knockouts [18]. This likely reflects the more limited extent and time of knockdown with siRNA versus that in genetic knockout models, as well as differences in the in vivo mouse versus isolated rat cardiomyocyte models. In a similar vein, expression of mutant Drp1 S616A, which appears to act as a dominant negative in blocking RhoA mediated Drp1 translocation and fission, did not alter mitochondrial size in the cardiomyocyte. This is consistent with an observation in HeLa cells where Drp1 serine-616 mutants had no basal effect on mitochondrial size [42]. The fact that there are no significant basal changes in mitochondrial dynamics in our model provided the opportunity to more specifically focus on the importance of activating RhoA signaling pathways on Drp1 mediated mitochondrial fission.
Previous studies have shown that Drp1 dependent mitochondrial fission occurs following I/R in the heart [33, 43], and we have demonstrated that RhoA activation is a signaling event that occurs in response to I/R [3, 9]. There is abundant evidence that S1P is generated in the heart in response to ischemic injury [7, 8]. These events have not been directly linked, but our evidence for regulation of Drp1 by RhoA activation in the heart suggests that the Drp1 dependent mitochondrial fission that occurs in response to I/R could be mediated through S1P generation and signaling through RhoA.
4.4. Mitochondrial mechanism of Drp1 dependent cardioprotection by RhoA
Extending our previous work delineating pathways by which S1P and RhoA protect cardiomyocytes [2, 9], we show here that siRNA knockdown of Drp1 significantly attenuates RhoA mediated cardioprotection. This is consistent with studies in which heterozygous cardiac Drp1 knockout mice were reported to have increased infarct from I/R [23] and increased apoptosis following pressure overload [44], suggesting that Drp1 serves a protective function in the response to these interventions. Inhibiting Drp1 dependent fission in cardiomyocytes during exercise was also recently shown to prevent β-adrenergic receptor mediated increases in mitochondrial respiration capacity [39]. Thus in the setting of exercise, Drp1 dependent fission serves as an adaptive physiological response to enhance mitochondrial function. Notably the fission observed with exercise is not associated with mitochondrial depolarization and concurrent cardiomyocyte cell death [39]. Likewise, activation of RhoA to levels used in the current study does not lead to mitochondrial membrane depolarization or increased opening of the mitochondrial permeability transition pore [45] and S1P stimulation preserves mitochondrial membrane potential in cardiomyocytes under oxidative stress [9]. Thus we propose that moderate levels of RhoA activation, as occur through S1P signaling, provide cardioprotection through mitochondrial fission without triggering mitochondrial dysfunction. This is in contrast to effects of chronic β-adrenergic receptor activation or inflammatory sepsis in which both increases in fission as well as mitochondrial depolarization and/or cell death are observed [33, 35, 46].
We previously reported that S1P signaling through RhoA in cardiomyocytes stimulates survival pathways, including FAK/Akt [2, 6] and PKD [9]. Drp1 dependent fission joins these as protective mechanisms regulated through RhoA signaling. All three signaling pathways eventually converge at the mitochondria. Akt signaling inhibits mitochondrial permeability transition pore opening to protect against dysfunction in cardiomyocytes [47]. PKD signals to mitochondria by inhibiting translocation of the pro-apoptotic protein Bax [9]. Drp1 dependent fission can segregate damaged from healthy mitochondria, preventing accumulation of oxidized mitochondrial proteins and dysfunction [48]. Thus rather than a “single” pro-survival pathway, a combination of anti-apoptotic signaling along with pathways that inhibit mitochondrial dysfunction may be optimal for sustaining cardiomyocytes under ischemic or oxidative stress. We suggest that these RhoA dependent pathways represent integrated mechanisms by which RhoA activation can protect against mitochondrial dysfunction in the immediate aftermath of cardiomyocyte injury.
Currently, potential roles played by RhoA mediated regulation of Drp1 beyond mitochondrial fission that may further explain the involvement of Drp1 in RhoA dependent mitochondrial protection have not been explored. There is interplay between mitochondrial fission and mitophagy signals in the heart [49], although there are conflicting observations as to whether knockout of Drp1 leads to increased [18, 50] or decreased mitophagy [23, 44]. Drp1 also regulates mitochondrial respiration in cardiomyocytes [51, 52]. Thus, it will be of interest in future studies to determine if activation of RhoA facilitates mitophagy and/or mitochondrial respiration as part of a broader mitochondrial quality control program in the cardiomyocyte.
5. Conclusion
In summary, we have shown that increases in RhoA activity, whether by expression of active RhoA or by activation of endogenous RhoA in response to the agonist S1P, regulate the mitochondrial fission protein Drp1. The consequent changes observed include Drp1 phosphorylation, mitochondrial translocation, and Drp1 dependent mitochondrial fission. Drp1 and mitochondrial fission also play a role in the ability of RhoA to protect cardiomyocytes from oxidative stress induced cell death.
Supplementary Material
Acknowledgements
We thank Jeffrey Smith, Melissa Barlow, and Hoyoung Moon for excellent technical assistance. This work was supported by National Institutes of Health Grants T32HL007444 and F32HL140851 (CSB), R37HL028143 (JHB), and R56HL097037 (SM).
Footnotes
Conflicts of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.clim.2018.03.017.
References
- [1].Moe GW, Marin-Garcia J, Role of cell death in the progression of heart failure, Heart Fail. Rev 21 (2) (2016) 157–167. [DOI] [PubMed] [Google Scholar]
- [2].Del Re DP, Miyamoto S, Brown JH, Focal adhesion kinase as a RhoA-activable signaling scaffold mediating Akt activation and cardiomyocyte protection, J. Biol. Chem 283 (51) (2008) 35622–35629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Xiang SY, Vanhoutte D, Del Re DP, Purcell NH, Ling H, Banerjee I, Bossuyt J, Lang RA, Zheng Y, Matkovich SJ, Miyamoto S, Molkentin JD, Dorn GW 2nd, Brown JH, RhoA protects the mouse heart against ischemia/reperfusion injury, J. Clin. Invest 121 (8) (2011) 3269–3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Mozzicato S, Joshi BV, Jacobson KA, Liang BT, Role of direct RhoA-phospholipase D1 interaction in mediating adenosine-induced protection from cardiac ischemia, FASEB J. 18 (2) (2004) 406–408. [DOI] [PubMed] [Google Scholar]
- [5].Krijnen PA, Sipkens JA, Molling JW, Rauwerda JA, Stehouwer CD, Muller A, Paulus WJ, van Nieuw Amerongen GP, Hack CE, Verhoeven AJ, van Hinsbergh VW, Niessen HW, Inhibition of Rho-ROCK signaling induces apoptotic and non-apoptotic PS exposure in cardiomyocytes via inhibition of flippase, J. Mol. Cell. Cardiol 49 (5) (2010) 781–790. [DOI] [PubMed] [Google Scholar]
- [6].Zhao X, Ding EY, Yu OM, Xiang SY, Tan-Sah VP, Yung BS, Hedgpeth J, Neubig RR, Lau LF, Brown JH, Miyamoto S, Induction of the matricellular protein CCN1 through RhoA and MRTF-A contributes to ischemic cardioprotection, J. Mol. Cell. Cardiol 75 (2014) 152–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Theilmeier G, Schmidt C, Herrmann J, Keul P, Schafers M, Herrgott I, Mersmann J, Larmann J, Hermann S, Stypmann J, Schober O, Hildebrand R, Schulz R, Heusch G, Haude M, von Wnuck Lipinski K, Herzog C, Schmitz M, Erbel R, Chun J, Levkau B, High-density lipoproteins and their constituent, sphingosine-1-phosphate, directly protect the heart against ischemia/reperfusion injury in vivo via the S1P3 lysophospholipid receptor, Circulation 114 (13) (2006) 1403–1409. [DOI] [PubMed] [Google Scholar]
- [8].Vessey DA, Li L, Honbo N, Karliner JS, Sphingosine 1-phosphate is an important endogenous cardioprotectant released by ischemic pre- and postconditioning, Am. J. Physiol. Heart Circ. Physiol 297 (4) (2009) H1429–H1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Xiang SY, Ouyang K, Yung BS, Miyamoto S, Smrcka AV, Chen J, Brown J. Heller, PLCepsilon, PKD1, and SSH1L transduce RhoA signaling to protect mitochondria from oxidative stress in the heart, Sci. Signal 6 (306) (2013) ra108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Yung BS, Brand CS, Xiang SY, Gray CB, Means CK, Rosen H, Chun J, Purcell NH, Brown JH, Miyamoto S, Selective coupling of the S1P3 receptor subtype to S1P-mediated RhoA activation and cardioprotection, J. Mol. Cell. Cardiol 103 (2017) 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Youle RJ, van der Bliek AM, Mitochondrial fission, fusion, and stress, Science 337 (6098) (2012) 1062–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Dorn GW 2nd, Kitsis RN, The mitochondrial dynamism-mitophagy-cell death interactome: multiple roles performed by members of a mitochondrial molecular ensemble, Circ. Res 116 (1) (2015) 167–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Frank S, Gaume B, Bergmann-Leitner ES, Leitner WW, Robert EG, Catez F, Smith CL, Youle RJ, The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis, Dev. Cell 1 (4) (2001) 515–525. [DOI] [PubMed] [Google Scholar]
- [14].Xu W, Jing L, Wang Q, Lin CC, Chen X, Diao J, Liu Y, Sun X, Bax-PGAM5-LDrp1 complex is required for intrinsic apoptosis execution, Oncotarget 6 (30) (2015) 30017–30034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Taguchi N, Ishihara N, Jofuku A, Oka T, Mihara K, Mitotic phosphorylation of dynamin-related GTPase Drp1 participates in mitochondrial fission, J. Biol. Chem 282 (15) (2007) 11521–11529. [DOI] [PubMed] [Google Scholar]
- [16].Szabadkai G, Simoni AM, Chami M, Wieckowski MR, Youle RJ, Rizzuto R, Drp-1-dependent division of the mitochondrial network blocks intraorganellar Ca2+ waves and protects against Ca2+−mediated apoptosis, Mol. Cell 16 (1) (2004) 59–68. [DOI] [PubMed] [Google Scholar]
- [17].Parone PA, Da Cruz S, Tondera D, Mattenberger Y, James DI, Maechler P, Barja F, Martinou JC, Preventing mitochondrial fission impairs mitochondrial function and leads to loss of mitochondrial DNA, PLoS One 3 (9) (2008) e3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Song M, Mihara K, Chen Y, Scorrano L, Dorn GW 2nd, Mitochondrial fission and fusion factors reciprocally orchestrate mitophagic culling in mouse hearts and cultured fibroblasts, Cell Metab. 21 (2) (2015) 273–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ingerman E, Perkins EM, Marino M, Mears JA, Mccaffery JM, Hinshaw JE, Nunnari J, Dnm1 forms spirals that are structurally tailored to fit mitochondria, J. Cell Biol 170 (7) (2005) 1021–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Smirnova E, Griparic L, Shurland DL, van der Bliek AM, Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells, Mol. Biol. Cell 12 (8) (2001) 2245–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Chang CR, Blackstone C, Dynamic regulation of mitochondrial fission through modification of the dynamin-related protein Drp1, Ann. N. Y. Acad. Sci 1201 (2010) 34–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Cahill TJ, Leo V, Kelly M, Stockenhuber A, Kennedy NW, Bao L, Cereghetti GM, Harper AR, Czibik G, Liao C, Bellahcene M, Steeples V, Ghaffari S, Yavari A, Mayer A, Poulton J, Ferguson DJ, Scorrano L, Hettiarachchi NT, Peers C, Boyle J, Hill RB, Simmons A, Watkins H, Dear TN, Ashrafian H, Resistance of dynamin-related protein 1 oligomers to disassembly impairs mitophagy, resulting in myocardial inflammation and heart failure, J. Biol. Chem 291 (49) (2016) 25762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ikeda Y, Shirakabe A, Maejima Y, Zhai P, Sciarretta S, Toli J, Nomura M, Mihara K, Egashira K, Ohishi M, Abdellatif M, Sadoshima J, Endogenous Drp1 mediates mitochondrial autophagy and protects the heart against energy stress, Circ. Res 116 (2) (2015) 264–278. [DOI] [PubMed] [Google Scholar]
- [24].Liu MY, Jin J, Li SL, Yan J, Zhen CL, Gao JL, Zhang YH, Zhang YQ, Shen X, Zhang LS, Wei YY, Zhao Y, Wang CG, Bai YL, Dong DL, Mitochondrial fission of smooth muscle cells is involved in artery constriction, Hypertension 68 (5) (2016) 1245–1254. [DOI] [PubMed] [Google Scholar]
- [25].Martorell-Riera A, Segarra-Mondejar M, Reina M, Martinez-Estrada OM, Soriano FX, Mitochondrial fragmentation in excitotoxicity requires ROCK activation, Cell Cycle 14 (9) (2015) 1365–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Li G, Zhou J, Budhraja A, Hu X, Chen Y, Cheng Q, Liu L, Zhou T, Li P, Liu E, Gao N, Mitochondrial translocation and interaction of cofilin and Drp1 are required for erucin-induced mitochondrial fission and apoptosis, Oncotarget 6 (3) (2015) 1834–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kim JE, Ryu HJ, Kim MJ, Kang TC, LIM kinase-2 induces programmed necrotic neuronal death via dysfunction of DRP1-mediated mitochondrial fission, Cell Death Differ. 21 (7) (2014) 1036–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Miyamoto S, Purcell NH, Smith JM, Gao T, Whittaker R, Huang K, Castillo R, Glembotski CC, Sussman MA, Newton AC, Brown JH, PHLPP-1 negatively regulates Akt activity and survival in the heart, Circ. Res 107 (4) (2010) 476–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hoshijima M, Sah VP, Wang Y, Chien KR, Brown JH, The low molecular weight GTPase rho regulates myofibril formation and organization in neonatal rat ventricular myocytes Involvement of Rho kinase, J. Biol. Chem 273 (13) (1998) 7725–7730. [DOI] [PubMed] [Google Scholar]
- [30].Doroudgar S, Quijada P, Konstandin M, Ilves K, Broughton K, Khalafalla FG, Casillas A, Nguyen K, Gude N, Toko H, Ornelas L, Thuerauf DJ, Glembotski CC, Sussman MA, Volkers M, S100A4 protects the myocardium against ischemic stress, J. Mol. Cell. Cardiol 100 (2016) 54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Yu T, Sheu SS, Robotham JL, Yoon Y, Mitochondrial fission mediates high glucose-induced cell death through elevated production of reactive oxygen species, Cardiovasc. Res 79 (2) (2008) 341–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Merrill RA, Dagda RK, Dickey AS, Cribbs JT, Green SH, Usachev YM, Strack S, Mechanism of neuroprotective mitochondrial remodeling by PKA/AKAP1, PLoS Biol. 9 (4) (2011) e1000612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Zaja I, Bai X, Liu Y, Kikuchi C, Dosenovic S, Yan Y, Canfield SG, Bosnjak ZJ, Cdk1, PKCdelta and calcineurin-mediated Drp1 pathway contributes to mitochondrial fission-induced cardiomyocyte death, Biochem. Biophys. Res. Commun 453 (4) (2014) 710–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Qi X, Disatnik MH, Shen N, Sobel RA, Mochly-Rosen D, Aberrant mitochondrial fission in neurons induced by protein kinase C{delta} under oxidative stress conditions in vivo, Mol. Biol. Cell 22 (2) (2011) 256–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Xu S, Wang P, Zhang H, Gong G, Gutierrez Cortes N, Zhu W, Yoon Y, Tian R, Wang W, CaMKII induces permeability transition through Drp1 phosphorylation during chronic beta-AR stimulation, Nat. Commun 7 (2016) 13189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Preau S, Delguste F, Yu Y, Remy-Jouet I, Richard V, Saulnier F, Boulanger E, Neviere R, Endotoxemia engages the RhoA kinase pathway to impair cardiac function by altering cytoskeleton, Mitochondrial Fission, and Autophagy, Antioxid Redox Signal 24 (10) (2016) 529–542. [DOI] [PubMed] [Google Scholar]
- [37].Wang W, Wang Y, Long J, Wang J, Haudek SB, Overbeek P, Chang BH, Schumacker PT, Danesh FR, Mitochondrial fission triggered by hyperglycemia is mediated by ROCK1 activation in podocytes and endothelial cells, Cell Metab. 15 (2) (2012) 186–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Jhun BS, SM OUJ, Adaniya T.J. Mancini, Cao JL, King ME, Landi AK, Ma H, Shin M, Yang D, Xu X, Yoon Y, Choudhary G, Clements RT, Mende U, Sheu SS, Protein kinase D activation induces mitochondrial fragmentation and dysfunction in cardiomyocytes, J. Physiol 596 (5) (2018) 827–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Coronado M, Fajardo G, Nguyen K, Zhao M, Kooiker KB, Jung G, Hu DQ, Reddy S, Sandoval E, Stotland A, Gottlieb RA, Bernstein D, Physiological mitochondrial fragmentation is a normal cardiac adaptation to increased energy demand, Circ. Res 122 (2) (2018) 282–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Pennanen C, Parra V, Lopez-Crisosto C, Morales PE, Del Campo A, Gutierrez T, Rivera-Mejias P, Kuzmicic J, Chiong M, Zorzano A, Rothermel BA, Lavandero S, Mitochondrial fission is required for cardiomyocyte hypertrophy mediated by a Ca2+−calcineurin signaling pathway, J. Cell Sci 127 (Pt 12) (2014) 2659–2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Castaldi A, Chesini GP, Taylor AE, Sussman MA, Brown JH, Purcell NH, Sphingosine 1-phosphate elicits RhoA-dependent proliferation and MRTF-A mediated gene induction in CPCs, Cell. Signal 28 (8) (2016) 871–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Cereghetti GM, Stangherlin A, De Brito O. Martins, Chang CR, Blackstone C, Bernardi P, Scorrano L, Dephosphorylation by calcineurin regulates translocation of Drp1 to mitochondria, Proc. Natl. Acad. Sci. U. S. A 105 (41) (2008) 15803–15808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Sharp WW, Fang YH, Han M, Zhang HJ, Hong Z, Banathy A, Morrow E, Ryan JJ, Archer SL, Dynamin-related protein 1 (Drp1)-mediated diastolic dysfunction in myocardial ischemia-reperfusion injury: therapeutic benefits of Drp1 inhibition to reduce mitochondrial fission, FASEB J. 28 (1) (2014) 316–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Shirakabe A, Zhai P, Ikeda Y, Saito T, Maejima Y, Hsu CP, Nomura M, Egashira K, Levine B, Sadoshima J, Drp1-dependent mitochondrial autophagy plays a protective role against pressure overload-induced mitochondrial dysfunction and heart failure, Circulation 133 (13) (2016) 1249–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Del Re DP, Miyamoto S, Brown JH, RhoA/Rho kinase up-regulate Bax to activate a mitochondrial death pathway and induce cardiomyocyte apoptosis, J. Biol. Chem 282 (11) (2007) 8069–8078. [DOI] [PubMed] [Google Scholar]
- [46].Shen YL, Shi YZ, Chen GG, Wang LL, Zheng MZ, Jin HF, Chen YY, TNF-alpha induces Drp1-mediated mitochondrial fragmentation during inflammatory cardiomyocyte injury, Int. J. Mol. Med 41 (4) (2018) 2317–2327. [DOI] [PubMed] [Google Scholar]
- [47].Miyamoto S, Murphy AN, Brown JH, Akt mediates mitochondrial protection in cardiomyocytes through phosphorylation of mitochondrial hexokinase-II, Cell Death Differ. 15 (3) (2008) 521–529. [DOI] [PubMed] [Google Scholar]
- [48].Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, Stiles L, Haigh SE, Katz S, Las G, Alroy J, Wu M, Py BF, Yuan J, Deeney JT, Corkey BE, Shirihai OS, Fission and selective fusion govern mitochondrial segregation and elimination by autophagy, EMBO J. 27 (2) (2008) 433–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Lee Y, Lee HY, Hanna RA, Gustafsson AB, Mitochondrial autophagy by Bnip3 involves Drp1-mediated mitochondrial fission and recruitment of Parkin in cardiac myocytes, Am. J. Physiol. Heart Circ. Physiol 301 (5) (2011) H1924–H1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Song M, Gong G, Burelle Y, Gustafsson AB, Kitsis RN, Matkovich SJ, Dorn GW 2nd, Interdependence of Parkin-mediated Mitophagy and mitochondrial fission in adult mouse hearts, Circ. Res 117 (4) (2015) 346–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Kageyama Y, Hoshijima M, Seo K, Bedja D, Sysa-Shah P, Andrabi SA, Chen W, Hoke A, Dawson VL, Dawson TM, Gabrielson K, Kass DA, Iijima M, Sesaki H, Parkin-independent mitophagy requires Drp1 and maintains the integrity of mammalian heart and brain, EMBO J. 33 (23) (2014) 2798–2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Zhang H, Wang P, Bisetto S, Yoon Y, Chen Q, Sheu SS, Wang W, A novel fission-independent role of dynamin-related protein 1 in cardiac mitochondrial respiration, Cardiovasc. Res 113 (2) (2017) 160–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.