Abstract
Background:
Studies have indicated that the association of urbanicity at birth and during upbringing with schizophrenia may be driven by familial factors such as genetic liability. We used a population-based nested case-control study to assess whether polygenic risk score (PRS) for schizophrenia was associated with urbanicity at birth and at age 15, and to assess whether PRS and parental history of mental disorder together explained the association between urbanicity and schizophrenia.
Methods:
Data were drawn from Danish population registries. Cases born since 1981 and diagnosed with schizophrenia between 1994 and 2009 were matched to controls with the same sex and birthdate (1,549 pairs). Genome-wide data were obtained from the Danish Neonatal Screening Biobank and PRSs were calculated based on results of a separate, large meta-analysis.
Results:
Those with higher PRS were more likely reside in the capital compared to rural areas at age 15 (OR=1.19, 95%CI=1.01–1.40), but not at birth (OR=1.09, 95%CI=0.95–1.26). Adjustment for PRS produced almost no change in relative risks of schizophrenia associated with urbanicity at birth, but slightly attenuated those for urban residence at age 15. Additional adjustment for parental history led to slight attenuation of relative risks for urbanicity at birth (IRR for birth in capital=1.54, 95%CI=1.18–2.02; overall p=.016) and further attenuation of relative risks for urbanicity at age 15 (IRR for residence in capital=1.32, 95%CI=0.97–1.78; overall p=.148).
Conclusions:
While results regarding urbanicity during upbringing were somewhat equivocal, genetic liability as measured here does not appear to explain the association between urbanicity at birth and schizophrenia.
Introduction
The association between urbanicity and schizophrenia is long-established (March et al., 2008). Initial findings of greater first admission rates for schizophrenia in urban centers (Faris and Dunham, 1939) were subject to debate as to whether they reflected causal effects of urban residence on mental health or resulted from selection of ill or prodromal persons into more urban areas (Freeman, 1994, Lapouse et al., 1956, Pedersen, 2015, Eaton, 1974). Subsequently, a number of studies found that risk for schizophrenia is greater among those born or raised in more urban areas (Lewis et al., 1992, Marcelis et al., 1998, Mortensen et al., 1999, Pedersen and Mortensen, 2001a). Some reported that risk increased with the degree of urbanization of the place of birth or upbringing (Lewis et al., 1992, Pedersen and Mortensen, 2001b), and Pedersen and Mortensen (2001) reported a dose-response relationship between the duration of urban residence during upbringing and schizophrenia risk in Denmark (Pedersen and Mortensen, 2001a). These associations cannot be readily explained by selection of individuals into urban areas because individuals do not generally choose where they are born or raised. In addition, previous studies have shown that the finding persists after adjustment for family history of schizophrenia and other mental disorders, suggesting that they are not simply artefacts of selection of ill parents into more urban areas (Pedersen and Mortensen, 2001b). Although a few studies have reported discrepant findings (Suvisaari et al., 2000), evidence for this risk factor is generally regarded as strong, and is often invoked to illustrate the importance of the social environment in the etiology of schizophrenia (van Os et al., 2010, van Os et al., 2005).
A number of potential explanations for the urbanicity association have been investigated, including exposure to infections, environmental toxins, obstetric complications, vitamin D deficiency, cannabis, social processes, and stress (Kelly et al., 2010, Padhy et al., 2014). Unfortunately, explanations have not been clearly identified, as results for many candidate mechanisms are mixed (McGrath and Scott, 2006). One alternative explanation is that the association is induced by familial factors that increase both schizophrenia risk and the probability that a person will be born or raised in an urban area (Pedersen and Mortensen, 2006b). This possibility is supported by two findings. First, using information from Danish registries, Pedersen and Mortensen (2006) found that the place of birth of an individual’s nearest oldest sibling was associated with schizophrenia above and beyond the individual’s own place of birth or upbringing (Pedersen and Mortensen, 2006a). For example, among individuals brought up in rural areas, those whose older sibling was born in the capital had about 1.6 times the risk of schizophrenia as those whose older sibling was born in a rural area (Pedersen and Mortensen, 2006a). Second, a recent Swedish register-based study found that the association between population density during upbringing and schizophrenia was attenuated after accounting for unobserved familial factors within extended and nuclear families (Sariaslan et al., 2015). These findings are not completely incompatible with a causal effect of urbanicity; for example, they could be explained by exposures that accumulate in the family prior to an individual’s birth (Pedersen and Mortensen, 2006b). However, they are also compatible with a scenario in which the association is induced by another familial factor, such as genetic susceptibility. There is evidence for genetic effects on features of residential location during adulthood, such as urbanicity and deprivation (Whitfield et al., 2005, Sariaslan et al., 2016). If some genes that increase schizophrenia risk also predispose towards living (and raising children) in more urban locations, for example by influencing personality characteristics (Jokela et al., 2008), or if the chances of reproduction among those with genetic risk are greater in more urban areas, a spurious association between urbanicity and schizophrenia could result (Jablensky and Kalaydjieva, 2003). Controlling for family history would not entirely remove this influence because many people with schizophrenia do not have an affected first degree relative and family history captures only the end of a continuum of genetic risk (Yang et al., 2010). To our knowledge, no studies have assessed whether differences in genetic risk might explain the associations of urbanicity at birth and during upbringing with schizophrenia using measures of genetic liability other than family history. Here we used polygenic risk scores for schizophrenia, which provide individual-level continuous indices of genetic risk, to capture additional information about genetic liability in a population-based nested case-control study in Denmark.
Our aims were to (1) estimate the association between polygenic risk score and urbanicity to assess whether those born and raised in more urban areas have greater genetic susceptibility to schizophrenia; and (2) assess whether the associations of urban birth and upbringing with schizophrenia are explained by differences in genetic risk.
Method
Study population
Danish population registry data were used to create a population-based nested case-control study. Three population registries were linked using a unique personal identifier assigned to all Danish residents: the Danish Civil Registration System (Pedersen et al., 2006), the Danish Neonatal Screening Biobank (Norgaard-Pedersen and Hougaard, 2007), and the Danish Psychiatric Central Research Register (Mors et al., 2011). The Civil Registration System was established in 1968 and contains dates of birth and death, place of birth and residence, and links to parents and siblings Danish residents are required to notify the government of address changes within five days; in Denmark it is unlikely that this mandatory information is not reported (Pedersen et al., 2006). The Neonatal Screening Biobank contains dried blood spot samples collected at birth from nearly all newborns born in Denmark since 1981. The Psychiatric Central Register contains all inpatient psychiatric diagnoses since 1969 and outpatient and emergency room visits since 1994. Diagnoses are those made by the treating clinician and are recorded as ICD-8 and ICD-10 codes. Denmark has free, universal healthcare coverage and no private psychiatric hospitals, making it extremely likely that severe mental disorder is captured in the registry. The study was approved by the Danish Data Protection Agency and the Danish Scientific Ethics Committee.
The study population included all singleton births from 1981 through 2000. This population was linked to the Psychiatric Central Register to identify incident cases of ICD-10 F20 schizophrenia first assigned from 1994 through September 2009. Cases were each matched to one randomly selected control with the same sex and birthdate who had not yet been diagnosed with schizophrenia at the time of the case’s diagnosis. DNA was extracted from the bloodspots, whole-genome amplified (in triplicate using the QiagenREPLI-g mini kit and the 3 separate reactions were pooled), and genotyped with Illumina Human 610-Quad BeadChip array or Illumina Infinium CoreExome beadchip (Agerbo et al., 2012, Meier et al., 2015). Details of genotyping and quality control have been published (Agerbo et al., 2012, Agerbo et al., 2015, Borglum et al., 2014, Meier et al., 2015). Principal Components Analysis was used to generate principal components to capture population ancestry and prevent confounding due to population stratification (Price et al., 2006). Related individuals, outliers (> +/− 4 sd) with respect to the first 10 principal components, and those whose sex in the Civil Registration System was inconsistent with genotype were removed, leaving 1,692 cases and 1,724 controls forming 1,549 complete matched pairs.
Polygenic Risk Score estimation
A meta-analysis was conducted of all Psychiatric Genetics Consortium samples (Schizophrenia Working Group of the Psychiatric Genomics Consortium, 2014), excluding the Danish sample, yielding a training sample of 34,600 cases and 45,968 controls. Single Nucleotide Polymorphisms (SNPs) were retained if their minor allele frequency was >= 10% and imputation information score >= 0.9 in both the training and target samples. Indels and SNPs in the extended MHC region, except for rs7746199, were excluded. Missing SNPs were imputed using the 1000 Genomes Project reference panel (Schizophrenia Working Group of the Psychiatric Genomics Consortium, 2014). Approximately 100,000 SNPs were selected from among those not in linkage disequilibrium (R2 values <= .1 in 500kb windows), preferentially retaining those that were most associated in any region. PRSs were calculated in the Danish sample using a p-value cutoff of <.05 following previous work (Agerbo et al., 2015, Wray et al., 2014, Schizophrenia Working Group of the Psychiatric Genomics Consortium, 2014). A PRS is a weighted sum of schizophrenia risk alleles, where the weights are the log odd ratios of disorder from the training sample. Higher PRS is associated with an increased risk of schizophrenia (Agerbo et al., 2015).
Urbanicity
We considered urbanicity at two time points: birth and age 15. Urbanicity at birth was measured using the mother’s address on each individual’s birthdate. Urbanicity at age 15 was used as a proxy for urbanicity during upbringing and was measured according to the individual’s address on his/her 15th birthday. Urbanicity was categorized by municipality into five levels, in keeping with prior studies: capital (Copenhagen), capital suburb, provincial city (city with >100,000 residents), provincial town (city with >10,000 residents), and rural areas. Those born or living in rural areas were used as reference groups. For analyses of urbanicity at age 15 we omitted those with a matching (onset) date before age 15, leaving 1,620 controls, 1,595 cases, and 1,456 complete pairs. These analyses also include an extra urbanicity level to accommodate those who lived in Greenland or abroad at age 15.
Parental characteristics
History of mental disorder in one or both parents before the matching date was gathered from the psychiatric registry and diagnoses were categorized hierarchically as follows: broad schizophrenia or bipolar disorder (ICD-10 F20-F29, F30-F31, or corresponding ICD-8 diagnoses (Pedersen et al., 2014)), another mental disorder (another ICD-10 F diagnosis or corresponding ICD-8 diagnosis), and none. Paternal age at the time of each individual’s birth was gathered from the CRS and categorized as follows: <=20, 21–25, 26–30, 31–35, 36–40, >40, and missing (due to a missing paternal link in the register, n=42). Parental place of birth was obtained from the civil registry and categorized as: both parents born in Denmark, only one parent born in Denmark, and both parents born abroad, in Greenland, or unknown.
Analysis
PRSs were standardized and adjusted for ancestry using the first 10 principal components in all analyses. Conditional logistic regression among the matched pairs was used to estimate incidence rate ratios (IRRs) of schizophrenia and 95% confidence intervals (CIs). Matched analyses adjust for age, sex, and date of birth by design. The mean PRS for each level of urban birth and residence was estimated in the entire sample, adjusted for the first 10 principal components as well as age, sex, and year of birth. Associations between PRS and being born or residing in an urban vs. a rural area were estimated using Generalized Estimating Equations among cases and controls residing in the capital or rural areas (the highest and lowest levels of urbanicity) at birth or age 15, and adjusted for the first 10 principal components and age, sex, and year of birth (Model 1) as well as other covariates (Models 2 and 3). Analyses were conducted using SAS version 9.4 (SAS Institute, Carey, NC).
Results
The sample consisted of 1,692 schizophrenia cases who were born in Denmark between 1981 and 2000, and 1,724 controls matched on age, sex, and date of birth. Characteristics of the 1,549 complete matched pairs are displayed in Table 1. PRS, urbanicity at birth, residence in the capital at age 15, parental psychiatric history, parental place of birth, and paternal age were associated with incidence of schizophrenia, similar to prior studies of the entire Danish population (Cantor-Graae and Pedersen, 2007, Mortensen et al., 1999, Pedersen and Mortensen, 2001a, Petersen et al., 2011). Details of the association between the PRS and schizophrenia in a subset of this cohort have been published previously (Agerbo et al., 2015).
Table 1:
Sample characteristics for 1549 incident cases of schizophrenia and 1549 individually age- and sex-matched controls
| Characteristic | Cases N (%) |
Controls N (%) |
Incidence rate ratio1 IRR (95% CI) |
|||
|---|---|---|---|---|---|---|
| Sex | ||||||
| Male | 859 | (55.46) | 859 | (55.46) | - | |
| Female | 690 | (44.54) | 690 | (44.54) | - | |
| Age at matching, median (IQR) | 20 | (3.9) | 20 | (3.9) | - | |
| Polygenic risk score2, mean (SD) | 0.05 | (0.88) | ‒0.23 | (0.84) | 1.46 | (1.34,1.61) |
| Parental history of mental disorders | ||||||
| Schizophrenia or bipolar disorder | 107 | (6.91) | 39 | (2.52) | 3.23 | (2.24,4.77) |
| Other mental disorders | 355 | (22.92) | 167 | (10.78) | 2.60 | (2.12,3.21) |
| None | 1087 | (70.17) | 1343 | (86.70) | 1.00 | (ref) |
| Urbanicity of place of birth3 | ||||||
| Capital (Copenhagen) | 218 | (14.07) | 163 | (10.52) | 1.67 | (1.31,2.15) |
| Suburb of the capital | 249 | (16.07) | 221 | (14.27) | 1.38 | (1.10,1.73) |
| Provincial cities | 174 | (11.23) | 180 | (11.62) | 1.18 | (0.93,1.50) |
| Provincial towns | 436 | (28.15) | 414 | (26.73) | 1.28 | (1.06,1.54) |
| Rural areas | 472 | (30.47) | 571 | (36.86) | 1.00 | (ref) |
| Urbanicity of residence at age 154 | ||||||
| Capital (Copenhagen) | 148 | (10.16) | 103 | (7.07) | 1.58 | (1.20,2.09) |
| Suburb of the capital | 216 | (14.84) | 220 | (15.11) | 1.10 | (0.88,1.38) |
| Provincial cities | 143 | (9.82) | 147 | (10.10) | 1.08 | (0.84,1.40) |
| Provincial towns | 422 | (28.98) | 392 | (26.92) | 1.20 | (1.00,1.44) |
| Rural areas | 523 | (35.92) | 584 | (40.11) | 1.00 | (ref) |
| Greenland or other countries | 4 | (0.28) | 10 | (0.69) | 0.47 | (0.13,1.42) |
| Parental place of birth | ||||||
| Both parents born in Denmark | 1355 | (87.48) | 1431 | (92.38) | 1.00 | (ref) |
| One parent born in Denmark | 154 | (9.94) | 90 | (5.81) | 1.83 | (1.39,2.42) |
| Other5 | 40 | (2.58) | 28 | (1.81) | 1.47 | (0.91,2.43) |
| Paternal age at childbirth | ||||||
| 20 years or younger | 53 | (3.42) | 44 | (2.84) | 1.36 | (0.90,2.07) |
| 21–25 years | 305 | (19.69) | 260 | (16.79) | 1.32 | (1.07,1.62) |
| 26–30 years | 520 | (33.57) | 580 | (37.44) | 1.00 | (ref) |
| 31–35 years | 377 | (24.34) | 413 | (26.66) | 1.03 | (0.85,1.23) |
| 36–40 years | 186 | (12.01) | 168 | (10.85) | 1.23 | (0.97,1.56) |
| 41 years or older | 84 | (5.42) | 72 | (4.65) | 1.34 | (0.95,1.88) |
| Missing | 24 | (1.55) | 12 | (0.77) | 2.30 | (1.16,4.82) |
IRRs are adjusted for age, sex, and date of birth by design
Normalized to the sample
Provincial cities = municipalities having a town with more than 100,000 inhabitants; provincial towns= municipalities having a town with between 10,000 and 100,000 inhabitants; rural areas= other municipalities in Denmark (largest town has less than 10,000 inhabitants).
Only pairs whose date of matching (case onset) is after the 15th birthday (1 456 pairs).
Both parents born abroad, in Greenland or missing.
Figure 1 displays mean PRS, adjusted for ancestry, age, sex, and year of birth, according to urbanicity at birth and at age 15. PRS was highest among those born in the capital, decreased with decreasing urbanicity, and was lowest among those born in rural areas. The mean PRSs for each level of urbanicity at age 15 were similar to those at birth.
Figure 1:
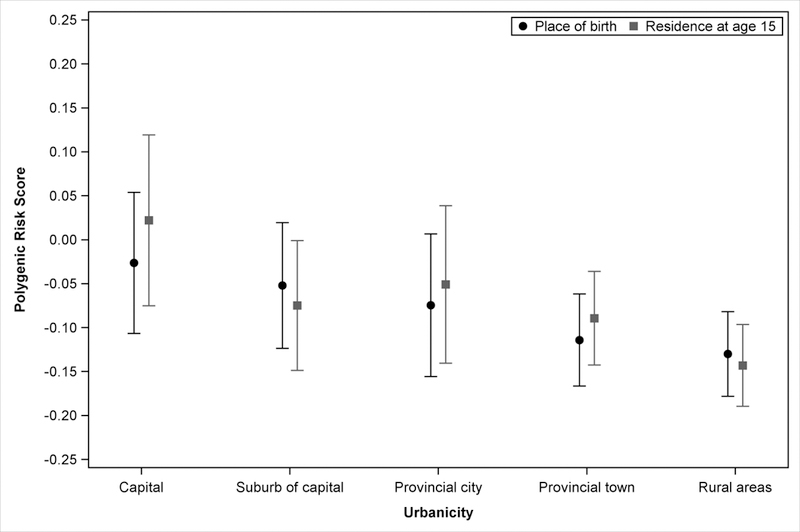
Mean polygenic risk score for schizophrenia, according to urbanicity at birth and at age 15, among 3,416 schizophrenia cases and controls. Polygenic risk score adjusted for the first 10 principal components as well as age, sex, and year of birth. Only those whose date of matching (case onset) is after the 15th birthday and who lived in Denmark at age 15 (n=3,214) are used when considering urbanicity at age 15.
Table 2 shows the associations of PRS and parental history with being born or residing (at age 15) in the capital compared to a rural area, under different model adjustments. Regarding place of birth, each standard deviation increase in the PRS was associated with 1.14-fold greater odds (95%CI=1.00–1.31) of being born in the capital compared to a rural area, although estimates were not significantly different from unity after adjustment (Table 2, Models 2 and 3). Regarding place of upbringing, each standard deviation increase in PRS was associated with a 1.24-fold (95%CI=1.06–1.46) increase in the odds of residing in the capital compared to a rural area at age 15. This estimate changed only slightly after adjustment for parental history of mental disorder (Model 2 OR=1.20, 95%CI=1.02–1.41) and for paternal age and parental place of birth (Model 3 OR=1.19, 95%CI=1.01–1.40). Parental history of mental disorder was associated with greater odds of birth and residence in the capital (Table 2).
Table 2:
Associations of polygenic risk score for schizophrenia with being born in the capital and living in the capital at age 15, compared to being born or living in rural areas
| Place of birth |
||||||
|---|---|---|---|---|---|---|
| Capital N (%) |
Rural areas1 N (%) |
Model 12 OR (95% CI) |
Model 23 OR (95% CI) |
Model 34 OR (95% CI) |
||
| Polygenic risk score5, mean (SD) | 0.06 (0.89) | -0.18 (0.85) | 1.14 (1.00,1.31) | 1.10 (0.95,1.26) | 1.09 (0.95,1.26) | |
| Parental history of mental disorders | ||||||
| Schizophrenia or bipolar disorder | 32 (7.75) | 34 (2.99) | 2.81 (1.68,4.68) | 2.75 (1.57,4.81) | 2.78 (1.58,4.91) | |
| Other mental disorders | 83 (20.10) | 171 (15.01) | 1.58 (1.17,2.12) | 1.59 (1.17,2.16) | 1.55 (1.13,2.12) | |
| No diagnosis of mental disorders | 298 (72.15) | 934 (82.00) | 1.00 Ref | 1.00 Ref | 1.00 Ref | |
| Place of residence at age 156 | ||||||
|
Capital N (%) |
Rural areas1 N (%) |
Model 1 OR (95% CI) |
Model 2 OR (95% CI) |
Model 3 OR (95% CI) |
||
| Polygenic risk score5, mean (SD) | 0.13 (0.91) | -0.19 (0.84) | 1.24 (1.06,1.46) | 1.20 (1.02,1.41) | 1.19 (1.01,1.40) | |
| Parental history of mental disorders | ||||||
| Schizophrenia or bipolar disorder | 17 (6.14) | 45 (3.71) | 1.90 (1.05,3.42) | 1.86 (1.00,3.45) | 1.90 (1.02,3.53) | |
| Other mental disorders | 67 (24.19) | 189 (15.57) | 1.79 (1.30,2.47) | 1.73 (1.24,2.42) | 1.71 (1.21,2.41) | |
| No diagnosis of mental disorders | 193 (69.68) | 980 (80.72) | 1.00 Ref | 1.00 Ref | 1.00 Ref | |
Note: Analysis includes only those residing in the lowest and highest levels of urbanicity at either birth or age 15.
Municipalities in Denmark where largest town has less than 10,000 inhabitants.
Model 1: Adjusted for sex, age, year of birth, and the first 10 principal components.
Model 2: Model 1, plus polygenic risk score and parental history of mental disorder are adjusted for one another.
Model 3: Model 2 additionally adjusted for parental place of birth and paternal age at childbirth.
Normalized to the sample
Only those aged 15 or older at the time of matching are considered for the analysis with place of residence at age 15.
Versions of Figure 1 and Table 2 calculated among cases and controls separately are provided in the Supplement. However, these results may possibly be influenced by collider-stratification bias (Cole et al., 2010).
Associations between urbanicity and schizophrenia under different adjustments are displayed in Table 3. The first column (Model 1) displays associations adjusted for age, sex, and date of birth by the matched design, as in Table 1. Compared to birth in rural areas, birth at higher levels of urbanicity was associated with greater risk of schizophrenia, with the exception of birth in provincial cities (Table 3, top). The greatest risk was for birth in the capital (IRR=1.67, 95%CI=1.31–2.15). Adjustment for the PRS resulted in no change in the IRR for birth in the capital and minimal changes to the IRRs for other urbanicity levels (Table 3, top, Model 2). The overall association between urbanicity and schizophrenia remained in this model (p=.001). Subsequent adjustment for parental history slightly attenuated the IRR for birth in the capital (IRR=1.54, 95%CI=1.18–2.02) and capital suburb (IRR=1.25, 95%CI=0.99–1.59); other estimates were minimally affected (Table 3, top, Model 3). Final adjustment for paternal age and parental place of birth produced minimal change in estimates (Table 3, top, Model 4). The overall association between urbanicity and schizophrenia remained in Models 3 (p=.016) and 4 (p=.028).
Table 3:
Associations of urbanicity at birth and during upbringing with the risk of schizophrenia under different adjustments.
| Model 11 IRR (95% CI) |
Model 22 IRR (95% CI) |
Model 33 IRR (95% CI) |
Model 44 IRR (95% CI) |
|
|---|---|---|---|---|
| Urbanicity at birth5 | ||||
| Capital (Copenhagen) | 1.67 (1.31,2.15) | 1.67 (1.29,2.17) | 1.54 (1.18,2.02) | 1.51 (1.16,1.98) |
| Suburb of the capital | 1.38 (1.10,1.73) | 1.35 (1.08,1.71) | 1.25 (0.99,1.59) | 1.25 (0.98,1.59) |
| Provincial cities | 1.18 (0.93,1.50) | 1.16 (0.91,1.50) | 1.17 (0.91,1.52) | 1.17 (0.90,1.52) |
| Provincial towns | 1.28 (1.06,1.54) | 1.31 (1.08,1.60) | 1.28 (1.05,1.56) | 1.26 (1.03,1.54) |
| Rural areas | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Urbanicity at age 156 | ||||
| Capital (Copenhagen) | 1.58 (1.20,2.09) | 1.47 (1.10,1.97) | 1.32 (0.97,1.78) | 1.30 (0.96,1.77) |
| Suburb of the capital | 1.10 (0.88,1.38) | 1.07 (0.85,1.36) | 1.01 (0.79,1.28) | 0.99 (0.78,1.27) |
| Provincial cities | 1.08 (0.84,1.40) | 1.02 (0.79,1.33) | 0.96 (0.73,1.26) | 0.97 (0.73,1.27) |
| Provincial towns | 1.20 (1.00,1.44) | 1.19 (0.98,1.43) | 1.15 (0.94,1.39) | 1.13 (0.93,1.38) |
| Rural areas | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
Model 1: Adjusted for sex, age and date of birth by design.
Model 2: Model 1 additionally adjusted for the polygenic risk score and the first 10 principal components
Model 3: Model 2 additionally adjusted for parental history of mental disorders.
Model 4: Model 3 additionally adjusted for parental place of birth and paternal age at childbirth.
Provincial cities = municipalities having a town with more than 100,000 inhabitants; provincial towns= municipalities having a town with between 10,000 and 100,000 inhabitants; rural areas= other municipalities in Denmark (largest town has less than 10,000 inhabitants).
Only includes pairs whose date of matching (case onset) is after the 15th birthday (1,456 pairs).
Residence in the capital at age 15 was also associated with greater risk of schizophrenia compared to residence in rural areas (IRR=1.58, 95%CI=1.20–2.09). Adjustment for the PRS produced a slight change in this estimates (IRR=1.47, 95%CI=1.10–1.97), but other estimates were minimally affected (Table 3, bottom). The overall association between urbanicity and schizophrenia was still present under this adjustment (p=.037). Further adjustment for parental history of mental disorders attenuated this estimate slightly more (IRR=1.32, 95%CI=0.97–1.78). Final adjustment for paternal age and parental place of birth (Model 4) resulted in little additional change. The overall association between urbanicity and schizophrenia was attenuated in Models 3 (p=.148) and 4 (p=.151). There were no statistical interactions between PRS and urbanicity at birth (p=.208) or age 15 (p=.140).
Discussion
In this study we included information on polygenic risk for schizophrenia, along with information on parental history of mental disorder, to assess whether the association between urbanicity and schizophrenia may be confounded by genetic liability. We found that genetic liability, as we measured it, does not appear to explain the association between urbanicity at birth and schizophrenia. There was no association in the full sample between PRS and birth in the capital compared to rural areas, the highest and lowest levels of urbanicity. Furthermore, adjusting the urbanicity-schizophrenia associations for the PRS had little effect on the magnitude of risk ratios. The changes in risk ratio estimates that resulted from adjusting for both measures of genetic liability simultaneously were slight, and urbanicity at birth was still associated with schizophrenia under full adjustment. These results using a direct index of genetic liability corroborate findings from prior studies that used family history alone (e.g., Pedersen and Mortensen, 2001b, Mortensen et al., 2010) in concluding that the association between urbanicity at birth and schizophrenia is not confounded by genetic liability.
Our results regarding urbanicity at age 15 were somewhat less straightforward than to those for urbanicity at birth. While we found some evidence in support of confounding due to genetic liability, risk ratio estimates were only partially attenuated when genetic liability was controlled for. Therefore, while a degree of confounding may be present, it may be inappropriate to conclude that the association between urbanicity during upbringing and schizophrenia is entirely explained by genetic risk. Additional studies using larger samples with urbanicity measured during upbringing may be necessary to confirm the magnitude of confounding present. Knowledge of which windows of exposure are more likely to reflect causal associations may help to narrow down the list of candidate mechanisms and constituent causes that explain the urbanicity associations.
“Passive” gene-environment correlation refers to scenarios in which a child “inherits” both gene and environment from his or her parents (Jaffee and Price, 2007). This is the type of correlation we assessed in our first aim, as both PRS and place of residence were “inherited” by the cases and controls. However, in this context passive correlation could be driven by a second mechanism, selective gene-environment correlation, operating among parents. Selective gene-environment correlation occurs when a person’s genetically influenced traits cause him/her to seek out certain environments (Jaffee and Price, 2007). For example, if parents with certain genetically-influenced personality traits or preferences are more likely to have or raise children in more urban areas, this could give rise to passive gene-environment correlation among offspring. This study was designed assuming that selective migration by parents could occur before a child’s birth or at any point during upbringing. However, we lacked genetic and behavioral information on parents with which we might have assessed this process directly. Other factors may also influence parents’ choice of residential location, including children’s behaviors or characteristics, which may themselves be genetically influenced. We are unable to distinguish between these (possibly co-occurring) influences in our data. Regardless of the mechanism(s) involved, the strength of PRS-urbanicity correlation in this study appeared to be modest. Interestingly, the association between PRS and urban birth appeared to be stronger in controls, who represent the underlying population, than in cases (see Supplement). Nevertheless, there was no evidence that the PRS confounded the association between urbanicity at birth and schizophrenia. Other explanations for the familial basis of the association, such as shared environmental factors or exposures that are transmitted between family members, have been discussed previously (Pedersen and Mortensen, 2006a). Continued research including direct measurement of hypothesized mechanisms that are consistent with the familial basis for the association may be warranted (Pedersen and Mortensen, 2006b).
A small number of prior studies have investigated PRS for schizophrenia in relation to other established risk factors. Sariaslan et al. found that PRS for schizophrenia, calculated among a population-based twin sample not selected for mental disorder, predicted living in deprived neighborhoods in adulthood and that the association between schizophrenia and neighborhood deprivation in adulthood was explained by shared genetic influences (Sariaslan et al., 2016). However, other interpretations of these results have been put forth (Gage et al., 2016). A prior Danish study of schizophrenia PRS in relation to socioeconomic status found no evidence that PRS explained the association between parental socioeconomic status at birth and schizophrenia (Agerbo et al., 2015). Power et al. reported that schizophrenia PRS was associated with ever vs. never cannabis use and quantity of use in adult twins, although French et al. did not find associations between PRS and cannabis use in 3 adolescent and youth samples (French et al., 2015, Power et al., 2014). Mehta et al. reported a U-shaped association between schizophrenia PRS and age at first childbirth among women, consistent with the U-shaped association between maternal age and risk of schizophrenia in offspring (Mehta et al., 2016). Finally, a prior study using the current dataset indicated no association between schizophrenia PRS and risk of infection among controls (Benros et al., 2016).
To our knowledge, this is the first study to use a polygenic measure to assess the role of genetic liability in explaining differences in schizophrenia risk according to urbanicity at birth and during upbringing. Strengths of this study include the nested case-control design, which estimates incidence in a nation-wide population-based cohort. Urbanicity was measured prospectively and independently of the outcome, without relying on self-reports or recall. The psychiatric registry provides national coverage of psychiatric diagnoses, enabling us to include information on parental history of psychiatric disorder in addition to PRS.
This study has a number of limitations. First, although the discovery sample used in this study was large (Schizophrenia Working Group of the Psychiatric Genomics Consortium, 2014), PRSs for schizophrenia tend to have low individual predictive ability and they capture only proportions of variance in a trait due to common SNPs (Purcell et al., 2009). We cannot rule out that use of a more optimal measure of genetic liability could have fully explained the urbanicity-schizophrenia associations. However, we combined the PRS with parental history information to capture information on genetic liability, rather than relying on the PRS alone.
Second, as mentioned above, the only information on parents available in this study was psychiatric history. Although this is helpful for quantifying genetic risk to offspring, it tells us little about parental characteristics that may influence choice of residence. Direct measures on parents, such as genetic information, behavioral data, or personality characteristics, would enable direct assessment of hypothesized mechanisms of gene-environment correlation. Such information was unfortunately not available in this study.
Third, because the Neonatal Screening Biobank started in 1981, the cases and controls were relatively young at the end of follow-up. This resulted in a relatively smaller sample that may have affected our ability to detect adjusted associations between urbanicity and Schizophrenia. The slightly smaller base associations we found between urbanicity and schizophrenia compared to older and larger Danish cohorts (Pedersen and Mortensen, 2001b) are not ideal for assessing confounding and may reduce the applicability of our findings to earlier cohorts. In addition, the cases and controls have not yet completely passed through the age period of heightened risk for schizophrenia, meaning that our findings may not apply to those with onset later in adulthood.
Other limitations include the use of a slightly smaller sample for investigation of urbanicity at age 15, which may preclude direct comparability with the investigation of urbanicity at birth. The psychiatric registry contains diagnoses given by treating clinicians, although studies have indicated that schizophrenia diagnoses are valid (Jakobsen et al., 2008, Uggerby et al., 2013). Finally, this study was conducted among a specific population and may not generalize to other settings.
We assessed the role of genetic liability to schizophrenia, indexed by both polygenic risk score and parental history of mental disorder, in the association of urbanicity at birth and during upbringing with schizophrenia. While results regarding urbanicity during upbringing may be suggestive of a degree of confounding, we found that genetic liability did not explain the association between urbanicity at birth and schizophrenia. Research is needed to identify the mechanisms or constituent causes that comprise this ubiquitous exposure.
Supplementary Material
Acknowledgements
We acknowledge the work of the Schizophrenia Working Group of the Psychiatric Genetics Consortium.
Financial support
This study was supported by the Intramural Research Program of the National Institute of Mental Health, the Danish Strategic Research Council, the Faculty of Health Sciences at Aarhus University, the Lundbeck Foundation Initiative for Integrative Psychiatric Research (iPSYCH), the Stanley Medical Research Institute, and a European Research Council advanced grant (Dr. Mortensen; GA294838). Genotyping was supported by funding from a philanthropic gift to the Stanley Center for Psychiatric Research at the Broad Institute. The funders of the study had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Results from this study were presented at the International Congress on Schizophrenia Research on March 25, 2017.
Disclaimer: The views and opinions expressed in this article are those of the authors and should not be construed to represent the views of any of the sponsoring organizations, agencies, or the US government.
Conflict of Interest: None.
Ethical Standards: The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
References
- AGERBO E, MORTENSEN PB, WIUF C, PEDERSEN MS, MCGRATH J, HOLLEGAARD MV, NORGAARD-PEDERSEN B, HOUGAARD DM, MORS O & PEDERSEN CB 2012. Modelling the contribution of family history and variation in single nucleotide polymorphisms to risk of schizophrenia: a Danish national birth cohort-based study. Schizophr Res, 134, 246–52. [DOI] [PubMed] [Google Scholar]
- AGERBO E, SULLIVAN PF, VILHJALMSSON BJ, PEDERSEN CB, MORS O, BORGLUM AD, HOUGAARD DM, HOLLEGAARD MV, MEIER S, MATTHEISEN M, RIPKE S, WRAY NR & MORTENSEN PB 2015. Polygenic Risk Score, Parental Socioeconomic Status, Family History of Psychiatric Disorders, and the Risk for Schizophrenia: A Danish Population-Based Study and Meta-analysis. JAMA Psychiatry, 72, 635–41. [DOI] [PubMed] [Google Scholar]
- BENROS ME, TRABJERG BB, MEIER S, MATTHEISEN M, MORTENSEN PB, MORS O, BORGLUM AD, HOUGAARD DM, NORGAARD-PEDERSEN B, NORDENTOFT M & AGERBO E 2016. Influence of Polygenic Risk Scores on the Association Between Infections and Schizophrenia. Biol Psychiatry. [DOI] [PubMed]
- BORGLUM AD, DEMONTIS D, GROVE J, PALLESEN J, HOLLEGAARD MV, PEDERSEN CB, HEDEMAND A, MATTHEISEN M, INVESTIGATORS G, UITTERLINDEN A, NYEGAARD M, ORNTOFT T, WIUF C, DIDRIKSEN M, NORDENTOFT M, NOTHEN MM, RIETSCHEL M, OPHOFF RA, CICHON S, YOLKEN RH, HOUGAARD DM, MORTENSEN PB & MORS O 2014. Genome-wide study of association and interaction with maternal cytomegalovirus infection suggests new schizophrenia loci. Mol Psychiatry, 19, 325–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CANTOR-GRAAE E & PEDERSEN CB 2007. Risk of schizophrenia in second-generation immigrants: a Danish population-based cohort study. Psychological Medicine, 37, 485–494. [DOI] [PubMed] [Google Scholar]
- COLE SR, PLATT RW, SCHISTERMAN EF, CHU HT, WESTREICH D, RICHARDSON D & POOLE C 2010. Illustrating bias due to conditioning on a collider. International Journal of Epidemiology, 39, 417–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EATON WW 1974. Residence, Social Class, and Schizophrenia. Journal of Health and Social Behavior, 15, 289–299. [PubMed] [Google Scholar]
- FARIS REL & DUNHAM HW 1939. Mental Disorders in Urban Areas; an Ecological Study of Schizophrenia and Other Psychoses, New York, Hafner. [Google Scholar]
- FREEMAN H 1994. Schizophrenia and City Residence. British Journal of Psychiatry, 164, 39–50. [PubMed] [Google Scholar]
- FRENCH L, GRAY C, LEONARD G, PERRON M, PIKE GB, RICHER L, SEGUIN JR, VEILLETTE S, EVANS CJ, ARTIGES E, BANASCHEWSKI T, BOKDE AL, BROMBERG U, BRUEHL R, BUCHEL C, CATTRELL A, CONROD PJ, FLOR H, FROUIN V, GALLINAT J, GARAVAN H, GOWLAND P, HEINZ A, LEMAITRE H, MARTINOT JL, NEES F, ORFANOS DP, PANGELINAN MM, POUSTKA L, RIETSCHEL M, SMOLKA MN, WALTER H, WHELAN R, TIMPSON NJ, SCHUMANN G, SMITH GD, PAUSOVA Z & PAUS T 2015. Early Cannabis Use, Polygenic Risk Score for Schizophrenia, and Brain Maturation in Adolescence. Jama Psychiatry, 72, 1002–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAGE S, SMITH GD & MUNAFÒ M 2016. Schizophrenia and neighbourhood deprivation. Translational Psychiatry, 6, e979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JABLENSKY AV & KALAYDJIEVA LV 2003. Genetic epidemiology of schizophrenia: Phenotypes, risk factors, and reproductive behavior. American Journal of Psychiatry, 160, 425–429. [DOI] [PubMed] [Google Scholar]
- JAFFEE SR & PRICE TS 2007. Gene-environment correlations: a review of the evidence and implications for prevention of mental illness. Molecular Psychiatry, 12, 432–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAKOBSEN KD, HANSEN T, DAM H, LARSEN EB, GETHER U & WERGE T 2008. Reliability of clinical ICD-10 diagnoses among electroconvulsive therapy patients with chronic affective disorders. European Journal of Psychiatry, 22, 161–172. [Google Scholar]
- JOKELA M, ELOVAINIO M, KIVIMAKI M & KELTIKANGAS-JARVINEN L 2008. Temperament and Migration Patterns in Finland. Psychological Science, 19, 831–837. [DOI] [PubMed] [Google Scholar]
- KELLY BD, O’CALLAGHAN E, WADDINGTON JL, FEENEY L, BROWNE S, SCULLY PJ, CLARKE M, QUINN JF, MCTIGUE O, MORGAN MG, KINSELLA A & LARKIN C 2010. Schizophrenia and the city: A review of literature and prospective study of psychosis and urbanicity in Ireland. Schizophrenia Research, 116, 75–89. [DOI] [PubMed] [Google Scholar]
- LAPOUSE R, MONK MA & TERRIS M 1956. The Drift Hypothesis and Socioeconomic Differentials in Schizophrenia. American Journal of Public Health and the Nations Health, 46, 978–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEWIS G, DAVID A, ANDREASSON S & ALLEBECK P 1992. Schizophrenia and City Life. Lancet, 340, 137–140. [DOI] [PubMed] [Google Scholar]
- MARCELIS M, NAVARRO-MATEU F, MURRAY R, SELTEN JP & VAN OS J 1998. Urbanization and psychosis: a study of 1942–1978 birth cohorts in The Netherlands. Psychological Medicine, 28, 871–879. [DOI] [PubMed] [Google Scholar]
- MARCH D, HATCH SL, MORGAN C, KIRKBRIDE JB, BRESNAHAN M, FEARON P & SUSSER E 2008. Psychosis and Place. Epidemiologic Reviews, 30, 84–100. [DOI] [PubMed] [Google Scholar]
- MCGRATH J & SCOTT J 2006. Urban birth and risk of schizophrenia: a worrying example of epidemiology where the data are stronger than the hypotheses. Epidemiologia E Psichiatria Sociale-an International Journal for Epidemiology and Psychiatric Sciences, 15, 243–246. [PubMed] [Google Scholar]
- MEHTA D, TROPF FC, GRATTEN J, BAKSHI A, ZHU ZH, BACANU SA, HEMANI G, MAGNUSSON PKE, BARBAN N, ESKO T, METSPALU A, SNIEDER H, MOWRY BJ, KENDLER KS, YANG J, VISSCHER PM, MCGRATH JJ, MILLS MC, WRAY NR, LEE SH, CONSORTIUM, P. G., STUDY, L. C. & TWINSUK 2016. Evidence for Genetic Overlap Between Schizophrenia and Age at First Birth in Women. Jama Psychiatry, 73, 497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEIER SM, AGERBO E, MAIER R, PEDERSEN CB, LANG M, GROVE J, HOLLEGAARD MV, DEMONTIS D, TRABJERG BB, HJORTHOJ C, RIPKE S, DEGENHARDT F, NOTHEN MM, RUJESCU D, MAIER W, MOO DSSCZC, WERGE T, MORS O, HOUGAARD DM, BORGLUM AD, WRAY NR, RIETSCHEL M, NORDENTOFT M, MORTENSEN PB & MATTHEISEN M 2015. High loading of polygenic risk in cases with chronic schizophrenia. Mol Psychiatry. [DOI] [PubMed]
- MORS O, PERTO GP & MORTENSEN PB 2011. The Danish Psychiatric Central Research Register. Scandinavian Journal of Public Health, 39, 54–57. [DOI] [PubMed] [Google Scholar]
- MORTENSEN PB, PEDERSEN CB, WESTERGAARD T, WOHLFAHRT J, EWALD H, MORS O, ANDERSEN PK & MELBYE M 1999. Effects of family history and place and season of birth on the risk of schizophrenia. New England Journal of Medicine, 340, 603–608. [DOI] [PubMed] [Google Scholar]
- MORTENSEN PB, PEDERSEN MG & PEDERSEN CB 2010. Psychiatric family history and schizophrenia risk in Denmark: which mental disorders are relevant? Psychological Medicine, 40, 201–210. [DOI] [PubMed] [Google Scholar]
- NORGAARD-PEDERSEN B & HOUGAARD DM 2007. Storage policies and use of the Danish Newborn Screening Biobank. Journal of Inherited Metabolic Disease, 30, 530–536. [DOI] [PubMed] [Google Scholar]
- PADHY SK, SARKAR S, DAVULURI T & PATRA BN 2014. Urban living and psychosis - An overview. Asian Journal of Psychiatry, 12, 17–22. [DOI] [PubMed] [Google Scholar]
- PEDERSEN CB 2015. Persons with schizophrenia migrate towards urban areas due to the development of their disorder or its prodromata. Schizophrenia Research, 168, 204–208. [DOI] [PubMed] [Google Scholar]
- PEDERSEN CB, GOTZSCHE H, MOLLER JO & MORTENSEN PB 2006. The Danish Civil Registration System - A cohort of eight million persons. Danish Medical Bulletin, 53, 441–449. [PubMed] [Google Scholar]
- PEDERSEN CB, MORS O, BERTELSEN A, WALTOFT BL, AGERBO E, MCGRATH JJ, MORTENSEN PB & EATON WW 2014. A Comprehensive Nationwide Study of the Incidence Rate and Lifetime Risk for Treated Mental Disorders. JAMA Psychiatry, 71, 573–581. [DOI] [PubMed] [Google Scholar]
- PEDERSEN CB & MORTENSEN PB 2001a. Evidence of a dose-response relationship between urbanicity during upbringing and schizophrenia risk. Archives of General Psychiatry, 58, 1039–1046. [DOI] [PubMed] [Google Scholar]
- PEDERSEN CB & MORTENSEN PB 2001b. Family history, place and season of birth as risk factors for schizophrenia in Denmark: a replication and reanalysis. British Journal of Psychiatry, 179, 46–52. [DOI] [PubMed] [Google Scholar]
- PEDERSEN CB & MORTENSEN PB 2006a. Are the cause(s) responsible for urban-rural differences in schizophrenia risk rooted in families or in individuals? American Journal of Epidemiology, 163, 971–978. [DOI] [PubMed] [Google Scholar]
- PEDERSEN CB & MORTENSEN PB 2006b. Why factors rooted in the family may solely explain the urban-rural differences in schizophrenia risk estimates. Epidemiologia E Psichiatria Sociale-an International Journal for Epidemiology and Psychiatric Sciences, 15, 247–251. [PubMed] [Google Scholar]
- PETERSEN L, MORTENSEN PB & PEDERSEN CB 2011. Paternal Age at Birth of First Child and Risk of Schizophrenia. American Journal of Psychiatry, 168, 82–88. [DOI] [PubMed] [Google Scholar]
- POWER RA, VERWEIJ KJH, ZUHAIR M, MONTGOMERY GW, HENDERS AK, HEATH AC, MADDEN PAF, MEDLAND SE, WRAY NR & MARTIN NG 2014. Genetic predisposition to schizophrenia associated with increased use of cannabis. Molecular Psychiatry, 19, 1201–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRICE AL, PATTERSON NJ, PLENGE RM, WEINBLATT ME, SHADICK NA & REICH D 2006. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet, 38, 904–9. [DOI] [PubMed] [Google Scholar]
- PURCELL SM, WRAY NR, STONE JL, VISSCHER PM, O’DONOVAN MC, SULLIVAN PF, SKLAR P, RUDERFER DM, MCQUILLIN A, MORRIS DW & O’DUSHLAINE CT 2009. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature, 460, 748–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SARIASLAN A, FAZEL S, D’ONOFRIO BM, LANGSTROM N, LARSSON H, BERGEN SE, KUJA-HALKOLA R & LICHTENSTEIN P 2016. Schizophrenia and subsequent neighborhood deprivation: revisiting the social drift hypothesis using population, twin and molecular genetic data. Transl Psychiatry, 6, e796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SARIASLAN A, LARSSON H, D’ONOFRIO B, LANGSTROM N, FAZEL S & LICHTENSTEIN P 2015. Does Population Density and Neighborhood Deprivation Predict Schizophrenia? A Nationwide Swedish Family-Based Study of 2.4 Million Individuals. Schizophrenia Bulletin, 41, 494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHIZOPHRENIA WORKING GROUP OF THE PSYCHIATRIC GENOMICS CONSORTIUM 2014. Biological insights from 108 schizophrenia-associated genetic loci. Nature, 511, 421–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUVISAARI JM, HAUKKA JK, TANSKANEN AJ & LONNQVIST JK 2000. Decreasing seasonal variation of births in schizophrenia. Psychological Medicine, 30, 315–324. [DOI] [PubMed] [Google Scholar]
- UGGERBY P, OSTERGAARD SD, ROGE R, CORRELL CU & NIELSEN J 2013. The validity of the schizophrenia diagnosis in the Danish Psychiatric Central Research Register is good. Danish Medical Journal, 60. [PubMed] [Google Scholar]
- VAN OS J, KENIS G & RUTTEN BPF 2010. The environment and schizophrenia. Nature, 468, 203–212. [DOI] [PubMed] [Google Scholar]
- VAN OS J, KRABBENDAM L, MYIN-GERMEYS I & DELESPAUL P 2005. The schizophrenia envirome. Current Opinion in Psychiatry, 18, 141–145. [DOI] [PubMed] [Google Scholar]
- WHITFIELD JB, ZHU G, HEATH AC & MARTIN NG 2005. Choice of residential location: Chance, family influences, or genes? Twin Research and Human Genetics, 8, 22–26. [DOI] [PubMed] [Google Scholar]
- WRAY NR, LEE SH, MEHTA D, VINKHUYZEN AA, DUDBRIDGE F & MIDDELDORP CM 2014. Research review: Polygenic methods and their application to psychiatric traits. J Child Psychol Psychiatry, 55, 1068–87. [DOI] [PubMed] [Google Scholar]
- YANG JA, VISSCHER PM & WRAY NR 2010. Sporadic cases are the norm for complex disease. European Journal of Human Genetics, 18, 1039–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


