Abstract
Wild boars are a reservoir for many zoonotic pathogens and a good sentinel for surveillance of zoonotic viral infections, but collection of serum samples from wild boars in the field is sometimes difficult and requires special equipment and techniques. In this study, ELISA using meat juices extracted from the heart and diaphragm of wild boars, instead of serum samples, was performed to detect antibodies against zoonotic pathogens, Japanese encephalitis virus and hepatitis E virus. The results of ELISA using meat juice samples were significantly correlated with those using serum samples and meat juice contained one-fifth the antibodies of serum samples. As meat juice is easily collected from wild animals in the field without special equipment and techniques, ELISA using meat juice is a simple and superior method for serological survey of zoonosis among wild animals.
Keywords: ELISA, hepatitis E virus, Japanese encephalitis virus, meat juice, wild boar
Wild animals play an important role as reservoirs for many zoonoses [9]. In particular, wild boars are major reservoirs for zoonotic pathogens, such as Japanese encephalitis virus (JEV) and hepatitis E virus (HEV), in Japan [5, 19, 29].
JEV belongs to the family Flaviviridae, genus Flavivirus [14], and is a causative agent of Japanese encephalitis (JE) in humans. JEV is transmitted by Culex species mosquitoes and a leading cause of viral encephalitis in South, Southeast, and East Asia [27]. Pigs, wild boars and wild birds are reservoirs of this virus [15]. In Japan, only several cases of human infection have been recently reported, but many domestic pigs, dogs, monkeys, wild boars and wild raccoons are infected with JEV every year [19, 22, 23]. These results indicate that JEV is spreading among livestock, companion, and wild animals, and humans are protected from JE by a vaccination program under recommendation of the Japanese government.
HEV belongs to the family Hepeviridae, genus Orthohepevirus [24] and is a causative agent of hepatitis E in humans [7]. Basically, hepatitis E is an acute self-limiting disease, but rarely develops fulminant liver failure [7]. There are four genotypes known to infect humans; genotypes 1 and 2 circulate in developing countries and are transmitted via fecal-oral routes, and genotypes 3 and 4 are distributed in both developed and developing countries and are transmitted via meat consumption [25]. In developed countries, HEV, mainly genotypes 3 and 4, is recognized as a zoonosis and the main reservoirs are pigs, wild boars, and deer [3]. Recently, a number of HEV and HEV-related viruses have been reported from animals, such as wild boars, camels, rats, bats, moose, kestrels, ferrets and red foxes [1, 4, 6, 10,11,12,13, 20, 21, 28]. In Japan, the number of hepatitis E patients has been increasing, and the main infection sources are pigs and wild boars [5, 8].
Surveillance of zoonotic pathogens among reservoirs is one of the best methods to analyze the risk to humans. In Japan, national epidemiological surveillance of JEV infection has been performed using pig sera before and during the active mosquito season in order to alert the nation to the risk of JEV infection. Although domestic pigs are suitable as sentinels of JEV infection, pig farms are now largely separated from urban areas, and pigs are bred in enclosed spaces where mosquitoes cannot invade. Therefore, surveillance of pigs might not reflect the true risk of JEV infection in urban areas. On the other hand, wild boars have invaded human habitats and are not protected from mosquitoes, suggesting that wild boars are superior sentinels for surveillance of JEV.
Although serum samples are the best specimens for sero-surveillance, isolation of sera in the field is difficult because special equipment and techniques are required for serum collection. Meat juice could be collected by freezing and thawing of heart and diaphragm and contains antibodies against pathogens [2, 16, 18, 26]. As meat juice contains many other contaminants, virus-neutralization (VN) and hemagglutination-inhibition tests are difficult to perform. On the other hand, enzyme-linked immunosorbent assay (ELISA) is useful for detection of antibodies against specific pathogens in meat juice.
In this study, our established ELISA for JEV and HEV was evaluated using meat juice samples obtained from the hearts and diaphragms of wild boars, and the results were compared with those using serum samples.
MATERIALS AND METHODS
Collection of samples
Blood, heart, and diaphragm samples were collected from 46 wild boars from November 2016 to May 2017 in Yamaguchi Prefecture, Japan. These wild boars were hunted by hunters under permission of the local government. Blood was centrifuged at 2,000 × g for 10 min at 4°C, and supernatants were collected and stored as serum samples at −20°C until use.
Collection of meat juice
In order to obtain meat juice from heart and diaphragm, approximately 100 grams of meat samples were placed in a plastic bag (Ziploc; Asahi-kasei, Tokyo, Japan) and then frozen at −20°C. Frozen meat samples were thawed at room temperature for 5 hr and then meat juice were transferred to clean tubes and stored at −20°C until use [16].
Detection of anti-JEV antibody
Indirect ELISA was developed to detect anti-JEV antibodies from wild animals. JEV/sw/Chiba/88/2002 was isolated from a pig in Chiba Prefecture in 2002 and kindly provided by Dr. Takasaki at the National Institute of Infectious Diseases [17]. JEV was inoculated onto Vero cells and infected cells were lysed in RIPA buffer (1% sodium deoxycholate, 1% Triton X-100, 10 mM Tris-HCl pH 7.4, 150 mM sodium chloride, 0.5 mM ethylenediaminetetraacetic acid) after cytopathic effects were observed. Extract from mock-infected Vero cells was used as a control for the antigen. Protein concentrations were measured by Bradford assay (Bio-Rad, Hercules, CA, U.S.A.). Extracts were diluted with adsorption buffer (0.05 M carbonate-bicarbonate buffer, pH 9.6) to 5 µg per ml and then 100 µl of the diluent was added to a 96-well ELISA plate (Maxisorp; Thermo Fischer Scientific, Rockford, IL, U.S.A.). Plates were incubated for 2 hr at 37°C and then blocked with 1% Blockace (DS Pharma Biomedical, Osaka, Japan) in PBS for 30 min at 37°C. Serum samples were diluted to 1:100 with 0.4% Blockace in PBS containing 0.05% of Tween 20 (PBS-T). Meat juice samples were diluted to 1:10, 1:20 or 1:40 with 0.4% Blockace in PBS-T. After five washes with PBS-T, 100 µl of diluted sera or meat juice was added to each well in duplicate, followed by incubation for 30 min at 37°C. After five washes with PBS-T, 100 µl of diluted Peroxidase Conjugated Purified Recomb® Protein A/G (Thermo Fischer Scientific) was added, followed by incubation for 30 min at 37°C. After five washes with PBS-T, 100 µl of substrate reagent (HRP substrate kit; Bio-Rad) was added, followed by incubation with gentle shaking at room temperature for 30 min. Enzymatic reaction was stopped by adding 100 µl of 2% oxalic acid. Absorbance was measured at a wavelength of 415 nm. Absorbance of wells coated with extract from mock-infected cells was subtracted from that of JEV-infected cells. Absorbance greater than 0.623 was considered to be positive by comparison between this ELISA and VN test using wild boar sera.
Detection of anti-HEV antibody
Anti-HEV antibodies in serum and meat juice of heart and diaphragm were detected using our established ELISA [29]. Serum samples were used at dilutions of 1:100, and meat juice samples were used at dilutions of 1:10, 1:20 or 1:40. Absorbance greater than 0.437 was considered to be positive according to our previous report [29].
RESULTS
Collection of meat juice
From 46 wild boars, sera, heart, and diaphragm were collected. Meat juice was collected from all heart samples and from 36 of 46 diaphragm samples. About 10% the volume of meat was collected as meat juice, but only a small amount of meat juice was obtained from some meat samples.
Detection of anti-JEV antibodies in meat juice
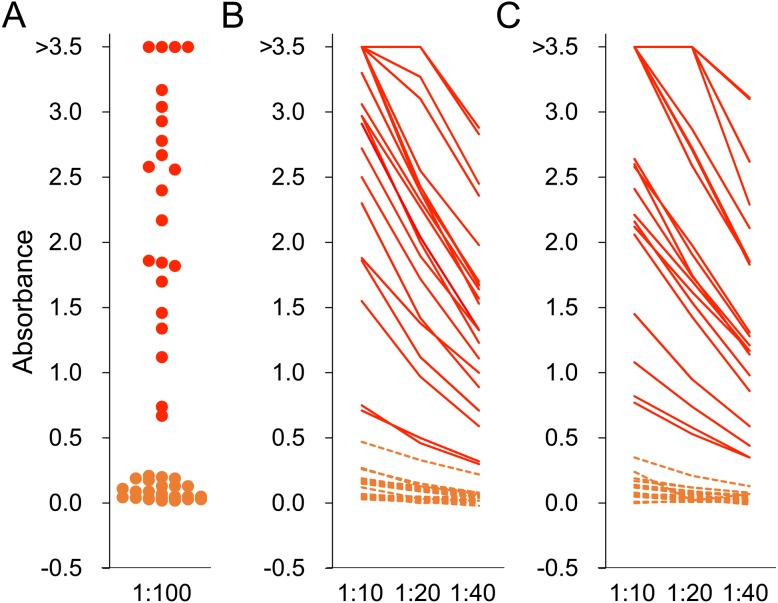
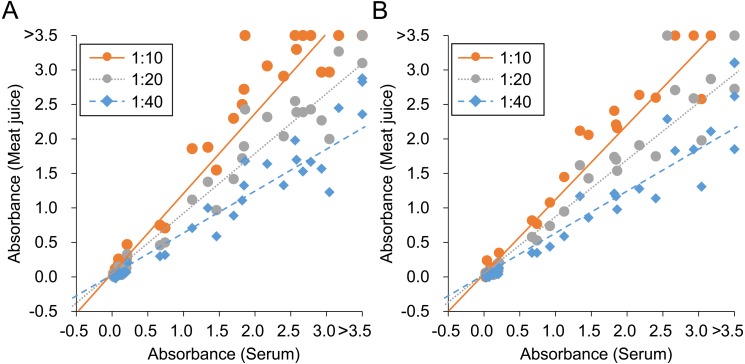
Twenty-two of 46 wild boars (48%) were seropositive using 100-fold diluted serum samples (Fig. 1). From meat juice of heart, 22 (48%), 20 (43%) and 19 (41%) of 46 wild boars were positive at 1:10, 1:20 and 1:40 dilution, respectively (Fig. 1). From meat juice of diaphragm, 19 (53%), 17 (47%) and 16 (44%) of 36 wild boars were positive at 1:10, 1:20, and 1:40 dilution, respectively (Fig. 1). In comparison with the results using serum samples, the sensitivity and specificity of meat juice samples were calculated. The sensitivity of 10-fold, 20-fold and 40-fold diluted heart samples was 100, 91 and 86%, respectively, and that for the diaphragm samples was 100, 89 and 84%, respectively. The specificity of all diluted meat juice was 100%. O.D. values of serum and each meat juice sample were plotted and the slopes of the line of best fit were calculated, giving 1.21, 0.91, and 0.63 at 1:10, 1:20, and 1:40 dilution for meat juice of heart, respectively (Fig. 3). Slopes of the line of best fit using meat juice of diaphragm samples were 1.13, 0.86, and 0.62 at 1:10, 1:20, and 1:40 dilution, respectively (Fig. 3). The correlation coefficient was 0.972, 0.983 and 0.968 at 1:10, 1:20, and 1:40 dilution of meat juice of heart, respectively. The correlation for diaphragm samples was 0.980, 0.973 and 0.956 at 1:10, 1:20, and 1:40 dilution, respectively.
Fig. 1.
ELISA for Japanese encephalitis virus. Serum (A) and meat juice of heart (B) and diaphragm (C) were used as first antibodies for ELISA. Serum was diluted to 1:100 and meat juice samples were diluted to 1:10, 1:20 and 1:40. Absorbance greater than 0.623 was judged to be positive. Red and orange colors indicate samples collected from wild boars positive and negative for anti-JEV antibody, respectively.
Fig. 3.
Correlation of absorbance values for Japanese encephalitis virus between serum and meat juice of heart (A) and diaphragm (B). Lines of best fit for each dilution of meat juice are also shown.
Detection of anti-HEV antibodies in meat juice
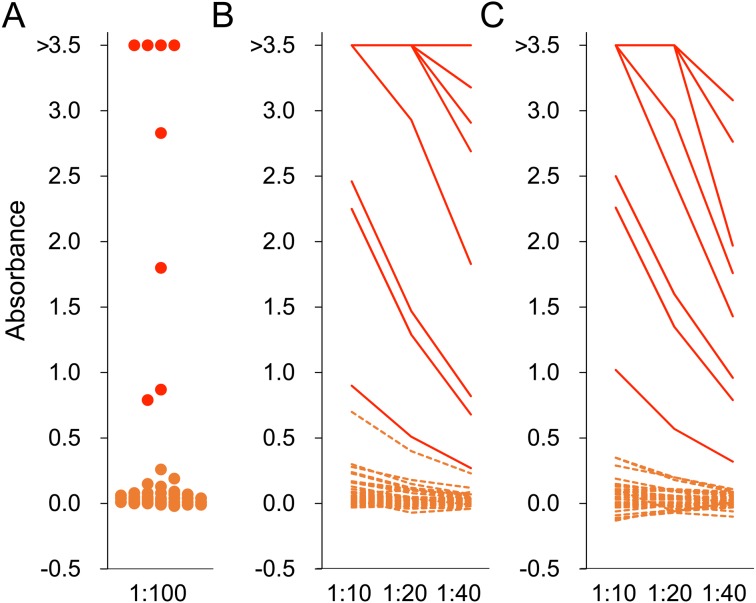
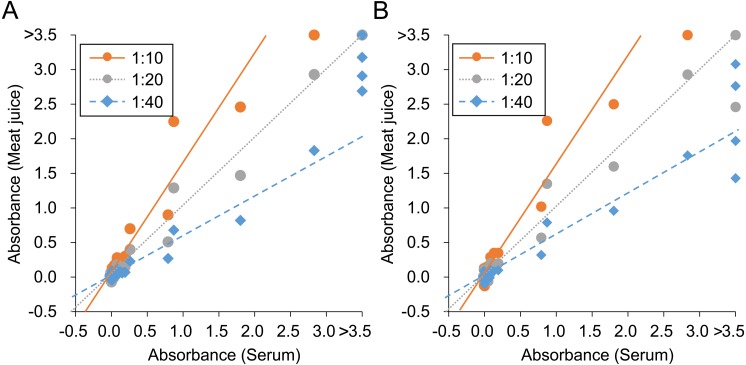
Eight of 46 wild boars (17%) were seropositive using 100-fold diluted serum samples (Fig. 2). From meat juice of heart, 9 (20%), 8 (17%) and 7 (15%) of 46 wild boars were positive at 1:10, 1:20 and 1:40 dilution, respectively (Fig. 2). From meat juice of diaphragm, 8 (22%), 8 (22%) and 7 (19%) of 36 wild boars were positive at 1:10, 1:20 and 1:40 dilution, respectively (Fig. 2). The sensitivity and specificity of meat juice samples were calculated for each dilution of heart and diaphragm meat juice samples. The specificity of 10-fold diluted heart samples was 97%, the sensitivity of 40-fold diluted heart samples was 88%, the sensitivity of 40-fold diluted diaphragm samples was 88%, and the other sensitivities and specificities were 100%. Slopes of the line of best fit were 1.58, 0.99 and 0.59 at 1:10, 1:20, and 1:40 dilution of meat juice of heart, respectively, and 1.58, 1.01 and 0.61 at 1:10, 1:20, and 1:40 dilution of meat juice of diaphragm, respectively (Fig. 4). The correlation coefficient was 0.978, 0.996 and 0.981 at 1:10, 1:20, and 1:40 dilution of meat juice of heart, respectively. The correlation for diaphragm samples was 0.976, 0.985 and 0.958 at 1:10, 1:20, and 1:40 dilution, respectively.
Fig. 2.
ELISA for hepatitis E virus. Serum (A) and meat juice of heart (B) and diaphragm (C) were used as first antibodies for ELISA. Serum was diluted to 1:100 and meat juice samples were diluted to 1:10, 1:20 and 1:40. Absorbance greater than 0.437 was judged to be positive. Red and orange colors indicate samples collected from wild boars positive and negative for anti-HEV antibody, respectively.
Fig. 4.
Correlation of absorbance values for hepatitis E virus between serum and meat juice of heart (A) and diaphragm (B). Lines of best fit for each dilution of meat juice are also shown.
DISCUSSION
In this study, we successfully detected anti-JEV and anti-HEV antibodies in meat juice from wild boars.
Heart and diaphragm samples were collected from 46 wild boars captured in Japan. From all 46 heart samples, we obtained meat juice, but from diaphragm samples, only 36 of 46 yielded meat juice. Thus, heart is a more suitable sample for collection of meat juice than diaphragm.
Meat juice samples were examined by ELISA for detection of anti-JEV and anti-HEV antibodies. The results indicated that the sensitivities and specificities of ELISA using both sources of meat juice were almost 100%. Meat juice is therefore suitable for ELISA.
The slope of the line of best fit for each dilution of meat juice sample in comparison with 100-fold diluted serum samples was calculated. Slopes using 20-fold diluted meat juice samples were almost 1.0. This indicates that meat juice samples contain approximately one-fifth the anti-viral antibodies of serum. For ELISA using meat juice, a 1:20 dilution is recommended, as serum samples are generally diluted to 1:100 in our ELISA system.
In conclusion, meat juice is a good specimen for detection of anti-JEV and anti-HEV antibodies from wild boars. Furthermore, as our established ELISA used protein A/G for detection of immunoglobulins, this ELISA using meat juice must be applicable to detection of anti-viral antibodies from other mammalian species. Our ELISA system using meat juice and protein A/G is expected to be widely suitable for surveillance of infectious diseases among wild animals.
Acknowledgments
Sample collection from wild boras was performed by the Hohoku hunting club, Yamaguchi Prefecture. This study was funded by the Ministry of Health, Labour and Welfare (MHLW) (H30-shokuhin-ippan-004, H27-shokuhin-ippan-011) and The Morinaga Foundation for Health & Nutrition.
REFERENCES
- 1.Bodewes R., van der Giessen J., Haagmans B. L., Osterhaus A. D. M. E., Smits S. L.2013. Identification of multiple novel viruses, including a parvovirus and a hepevirus, in feces of red foxes. J. Virol. 87: 7758–7764. doi: 10.1128/JVI.00568-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coelho C., Lopes A. P., Mesquita J. R., Cardoso L., Vieira-Pinto M.2015. Toxoplasma gondii infection in hunted wild boars (sus scrofa): Heart meat juice as an alternative sample to serum for the detection of antibodies. EcoHealth 12: 685–688. doi: 10.1007/s10393-015-1073-9 [DOI] [PubMed] [Google Scholar]
- 3.Doceul V., Bagdassarian E., Demange A., Pavio N.2016. Zoonotic hepatitis E virus: Classification, animal reservoirs and transmission routes. Viruses 8: 270. doi: 10.3390/v8100270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drexler J. F., Seelen A., Corman V. M., Fumie Tateno A., Cottontail V., Melim Zerbinati R., Gloza-Rausch F., Klose S. M., Adu-Sarkodie Y., Oppong S. K., Kalko E. K., Osterman A., Rasche A., Adam A., Müller M. A., Ulrich R. G., Leroy E. M., Lukashev A. N., Drosten C.2012. Bats worldwide carry hepatitis E virus-related viruses that form a putative novel genus within the family Hepeviridae. J. Virol. 86: 9134–9147. doi: 10.1128/JVI.00800-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hara Y., Terada Y., Yonemitsu K., Shimoda H., Noguchi K., Suzuki K., Maeda K.2014. High prevalence of hepatitis E virus in wild boar (Sus scrofa) in Yamaguchi Prefecture, Japan. J. Wildl. Dis. 50: 378–383. doi: 10.7589/2013-06-144 [DOI] [PubMed] [Google Scholar]
- 6.Johne R., Plenge-Bönig A., Hess M., Ulrich R. G., Reetz J., Schielke A.2010. Detection of a novel hepatitis E-like virus in faeces of wild rats using a nested broad-spectrum RT-PCR. J. Gen. Virol. 91: 750–758. doi: 10.1099/vir.0.016584-0 [DOI] [PubMed] [Google Scholar]
- 7.Kamar N., Dalton H. R., Abravanel F., Izopet J.2014. Hepatitis E virus infection. Clin. Microbiol. Rev. 27: 116–138. doi: 10.1128/CMR.00057-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanayama A., Arima Y., Yamagishi T., Kinoshita H., Sunagawa T., Yahata Y., Matsui T., Ishii K., Wakita T., Oishi K.2015. Epidemiology of domestically acquired hepatitis E virus infection in Japan: assessment of the nationally reported surveillance data, 2007−2013. J. Med. Microbiol. 64: 752–758. doi: 10.1099/jmm.0.000084 [DOI] [PubMed] [Google Scholar]
- 9.Kruse H., kirkemo A. M., Handeland K.2004. Wildlife as source of zoonotic infections. Emerg. Infect. Dis. 10: 2067–2072. doi: 10.3201/eid1012.040707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li T. C., Ami Y., Suzaki Y., Yasuda S. P., Yoshimatsu K., Arikawa J., Takeda N., Takaji W.2013. Characterization of full genome of rat hepatitis E virus strain from Vietnam. Emerg. Infect. Dis. 19: 115–118. doi: 10.3201/eid1901.121007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li T. C., Yang T., Ami Y., Suzaki Y., Shirakura M., Kishida N., Asanuma H., Takeda N., Takaji W.2014. Complete genome of hepatitis E virus from laboratory ferrets. Emerg. Infect. Dis. 20: 709–712. doi: 10.3201/eid2004.131815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li T. C., Zhou X., Yoshizaki S., Ami Y., Suzaki Y., Nakamura T., Takeda N., Wakita T.2016. Production of infectious dromedary camel hepatitis E virus by a reverse genetic system: Potential for zoonotic infection. J. Hepatol. 65: 1104–1111. doi: 10.1016/j.jhep.2016.07.013 [DOI] [PubMed] [Google Scholar]
- 13.Lin J., Karlsson M., Olofson A. S., Belák S., Malmsten J., Dalin A. M., Widén F., Norder H.2015. High prevalence of hepatitis E virus in Swedish moose −a phylogenetic characterization and comparison of the virus from different regions. PLoS One 10: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mackenzie J. S., Barrett A. D. T., Deubel V.2002. The Japanese encephalitis serological group of flaviviruses: a brief introduction to the group. Curr. Top. Microbiol. Immunol. 267: 1–10. [DOI] [PubMed] [Google Scholar]
- 15.Mansfield K. L., Hernández-Triana L. M., Banyard A. C., Fooks A. R., Johnson N.2017. Japanese encephalitis virus infection, diagnosis and control in domestic animals. Vet. Microbiol. 201: 85–92. doi: 10.1016/j.vetmic.2017.01.014 [DOI] [PubMed] [Google Scholar]
- 16.Meemken D., Tangemann A. H., Meermeier D., Gundlach S., Mischok D., Greiner M., Klein G., Blaha T.2014. Establishment of serological herd profiles for zoonoses and production diseases in pigs by “meat juice multi-serology”. Prev. Vet. Med. 113: 589–598. doi: 10.1016/j.prevetmed.2013.12.006 [DOI] [PubMed] [Google Scholar]
- 17.Nerome R., Tajima S., Takasaki T., Yoshida T., Kotaki A., Lim C. K., Ito M., Sugiyama A., Yamauchi A., Yano T., Kameyama T., Morishita I., Kuwayama M., Ogawa T., Sahara K., Ikegaya A., Kanda M., Hosoya Y., Itokazu K., Onishi H., Chiya S., Yoshida Y., Tabei Y., Katsuki K., Tabata K., Harada S., Kurane I.2007. Molecular epidemiological analyses of Japanese encephalitis virus isolates from swine in Japan from 2002 to 2004. J. Gen. Virol. 88: 2762–2768. doi: 10.1099/vir.0.82941-0 [DOI] [PubMed] [Google Scholar]
- 18.Nielsen B., Ekeroth L., Bager F., Lind P.1998. Use of muscle fluid as a source of antibodies for serologic detection of Salmonella infection in slaughter pig herds. J. Vet. Diagn. Invest. 10: 158–163. doi: 10.1177/104063879801000207 [DOI] [PubMed] [Google Scholar]
- 19.Ohno Y., Sato H., Suzuki K., Yokoyama M., Uni S., Shibasaki T., Sashika M., Inokuma H., Kai K., Maeda K.2009. Detection of antibodies against Japanese encephalitis virus in raccoons, raccoon dogs and wild boars in Japan. J. Vet. Med. Sci. 71: 1035–1039. doi: 10.1292/jvms.71.1035 [DOI] [PubMed] [Google Scholar]
- 20.Reuter G., Boros Á., Mátics R., Kapusinszky B., Delwart E., Pankovics P.2016. Divergent hepatitis E virus in birds of prey, common kestrel (Falco tinnunculus) and red-footed falcon (F. vespertinus), Hungary. Infect. Genet. Evol. 43: 343–346. doi: 10.1016/j.meegid.2016.06.013 [DOI] [PubMed] [Google Scholar]
- 21.Sato Y., Sato H., Naka K., Furuya S., Tsukiji H., Kitagawa K., Sonoda Y., Usui T., Sakamoto H., Yoshino S., Shimizu Y., Takahashi M., Nagashima S., Jirintai, Nishizawa T., Okamoto H.2011. A nationwide survey of hepatitis E virus (HEV) infection in wild boars in Japan: identification of boar HEV strains of genotypes 3 and 4 and unrecognized genotypes. Arch. Virol. 156: 1345–1358. doi: 10.1007/s00705-011-0988-x [DOI] [PubMed] [Google Scholar]
- 22.Shimoda H., Inthong N., Noguchi K., Terada Y., Nagao Y., Shimojima M., Takasaki T., Rerkamnuaychoke W., Maeda K.2013. Development and application of an indirect enzyme-linked immunosorbent assay for serological survey of Japanese encephalitis virus infection in dogs. J. Virol. Methods 187: 85–89. doi: 10.1016/j.jviromet.2012.09.022 [DOI] [PubMed] [Google Scholar]
- 23.Shimoda H., Saito A., Noguchi K., Terada Y., Kuwata R., Akari H., Takasaki T., Maeda K.2014. Seroprevalence of Japanese encephalitis virus infection in captive Japanese macaques (Macaca fuscata). Primates 55: 441–445. doi: 10.1007/s10329-014-0421-7 [DOI] [PubMed] [Google Scholar]
- 24.Smith D. B., Simmonds P., Jameel S., Emerson S. U., Harrison T. J., Meng X. J., Okamoto H., Van der Poel W. H. M., Purdy M. A. and International Committee on Taxonomy of Viruses Hepeviridae Study Group. 2014. Consensus proposals for classification of the family Hepeviridae. J. Gen. Virol. 95: 2223–2232. doi: 10.1099/vir.0.068429-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Teshale E. H., Hu D. J.2011. Hepatitis E: Epidemiology and prevention. World J. Hepatol. 3: 285–291. doi: 10.4254/wjh.v3.i12.285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wacheck S., Werres C., Mohn U., Dorn S., Soutschek E., Fredriksson-Ahomaa M., Märtlbauer E.2012. Detection of IgM and IgG against hepatitis E virus in serum and meat juice samples from pigs at slaughter in Bavaria, Germany. Foodborne Pathog. Dis. 9: 655–660. doi: 10.1089/fpd.2012.1141 [DOI] [PubMed] [Google Scholar]
- 27.Wang H., Liang G.2015. Epidemiology of Japanese encephalitis: past, present, and future prospects. Ther. Clin. Risk Manag. 11: 435–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woo P. C. Y., Lau S. K. P., Teng J. L. L., Tsang A. K. L., Joseph M., Wong E. Y. M., Tang Y., Sivakumar S., Xie J., Bai R., Wernery R., Wernery U., Yuen K. Y.2014. New hepatitis E virus genotype in camels, the Middle East. Emerg. Infect. Dis. 20: 1044–1048. doi: 10.3201/eid2006.140140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yonemitsu K., Terada Y., Kuwata R., Nguyen D., Shiranaga N., Tono S., Matsukane T., Yokoyama M., Suzuki K., Shimoda H., Takano A., Muto M., Maeda K.2016. Simple and specific method for detection of antibodies against hepatitis E virus in mammalian species. J. Virol. Methods 238: 56–61. doi: 10.1016/j.jviromet.2016.07.030 [DOI] [PubMed] [Google Scholar]