Abstract
Eukaryotic elongation factor 2 (eEF2) kinase (eEF2K) inhibits protein translation through the phosphorylation of its specific substrate, eEF2. We previously demonstrated that eEF2K expression increases in superior mesenteric artery from spontaneously hypertensive rats (SHR) and that eEF2K mediates development of hypertension in SHR. In addition, we recently revealed that A484954, a selective eEF2K inhibitor induced relaxation via opening smooth muscle inward rectifier K+ (Kir) channel in rat isolated superior mesenteric artery. Here, we further examined the effects of A484954 on contractility and blood pressure (BP) in rats. Isometric contraction of rat isolated superior mesenteric artery was measured. BP was measured by a carotid cannulation method. A484954 (10 µM) inhibited noradrenaline (NA)-induced contraction in a biphasic manner (magnitude of inhibition higher at high dose NA). A484954 also inhibited an α1-receptor agonist, phenylephrine-induced contraction, while it was not biphasic. Specifically, a β-receptor antagonist, propranolol (1 µM) prevented the A484954-mediated inhibition of NA (high-dose)-induced contraction. A484954 (10 µM) potentiated a β-receptor agonist, isoproterenol-induced relaxation, which was completely prevented by BaCl2 (1 mM), a Kir channel blocker. In vivo, A484954 (122 µg/kg) inhibited NA-induced increase of BP in rats. Another eEF2K inhibitor, NH125 (22 µg/kg) also inhibited the NA-induced BP increase in rats. In summary, it was concluded that A484954 lowers NA-induced BP rise perhaps through activation of β2-receptor-Kir channel and subsequent vasorelaxation via inhibiting eEF2K activity.
Keywords: blood pressure, β2-receptor, Kir channel, vasorelaxation
Calmodulin (CaM) regulates diverse cellular functions including muscle contraction, immune response, metabolism and nervous growth through the regulation of CaM-dependent proteins [8, 16]. Eukaryotic elongation factor 2 (eEF2) kinase (eEF2K) is one of the CaM-dependent protein kinases (CaMK) and is also known as CaMKIII. eEF2K inhibits protein translation via phosphorylating its specific substrate, eEF2 [1, 3, 9, 12, 13]. We previously demonstrated that expression of eEF2K increases in superior mesenteric artery from spontaneously hypertensive rats (SHR) and that eEF2K mediates development of hypertension in SHR in part through promoting reactive oxygen species-dependent inflammation, proliferation and migration of vascular smooth muscle cells [14, 15]. In addition, we showed that eEF2K mediates development of pulmonary arterial hypertension in part through promoting structural remodeling of pulmonary arterial wall [4]. 7-amino-1-cyclopropyl-3-ethyl-2,4-dioxo-1,2,3,4-tetrahydropyrido[2,3-d]pyrimidine-6-carboxamide (A484954), a cell-permeable small molecule, is a novel selective inhibitor of eEF2K identified from a chemical library using high-throughput screening [2]. We have recently revealed that A484954 induces relaxation in part through opening smooth muscle inward rectifier K+ (Kir) channel and also via endothelium-derived nitric oxide in rat isolated superior mesenteric artery [6]. Here we tested the hypothesis that A484954 causes vasorelaxation through the previously unrevealed mechanisms. Systemic blood pressure (BP) is mainly controlled by the peripheral artery resistance and cardiac output. Thus, in the present study, we also tested the hypothesis that A484954 affects systemic BP in rats.
MATERIALS AND METHODS
Materials
A484954 (Merck, Darmstadt, Germany), 1-benzyl-3-cetyl-2-methylmidazolium iodide (NH125) (Cayman, Ann Arbor, MI, U.S.A.), noradrenaline (NA), (±)-propranolol hydrochloride, L-phenylephrine hydrochloride, isoproterenol hydrochloride, BaCl2 (Sigma-Aldrich, St. Louis, MO, U.S.A.).
Tissue preparation
Male Wistar rats (5–9-week-old) were anesthetized with urethane (1.5 g/kg, i.p.) and euthanized by exsanguination. The superior mesenteric artery was isolated. After removal of fat and adventitia, the artery was cut into rings (1–1.5 mm in diameter) for the measurement of isometric contraction as described previously [6]. The endothelium was removed by rubbing an intimal surface with a flat face of a pair of forceps. Removal of the endothelium was confirmed by a lack of relaxation induced by acetylcholine (10 µM). Animal care and treatment were conducted in conformity with the institutional guidelines of The Kitasato University. Animal experiments were approved by the Institutional Animal Care and Use Committee of Kitasato University.
Measurement of isometric contraction
The contractility of arterial tissue was measured in normal physiological salt solution, which contained the following compositions (mM): 136.9 NaCl, 5.4 KCl, 1.5 CaCl2, 1.0 MgCl2, 23.8 NaHCO3, 5.6 glucose and 0.01 ethylene diamine tetraacetic acid. The high-K+ (72.7 mM) solution was prepared by replacing NaCl with equimolar KCl. These solutions were saturated with a 95% O2-5% CO2 mixture at 37°C and pH 7.4. Smooth muscle contraction was isometrically measured and digitally recorded using a force-displacement transducer (Nihon Kohden, Tokyo, Japan) and a PowerLab system (ADInstruments, Dunedin, New Zealand) as described previously [6]. Each preparation was mounted in a 3-ml organ bath under a resting tension of 0.5 g. After 30 min equilibration, each preparation was repeatedly exposed to high-K+ solution until the responses became stable (45–60 min). Concentration-response (contraction) curves were obtained by a cumulative addition of NA (1 nM-30 µM) or phenylephrine (1 nM-30 µM) (Fig. 1). A484954 (10 µM) or dimethyl sulfoxide (DMSO: vehicle control) was pretreated for 10 min before the addition of NA or phenylephrine. To examine the contribution of β2-receptor, a β-blocker, propranolol (1 µM) was pretreated for 5 min before the addition of A484954 (10 µM). Concentration-response (relaxation) curves were obtained by a cumulative addition of isoproterenol (1 nM-10 µM) to the arteries precontracted sub-maximally with NA (3 µM) (Fig. 2). To examine the contribution of Kir channel-mediated relaxation, a Kir channel blocker, BaCl2 (1 mM) was pretreated for 5 min before the addition of A484954 (10 µM).
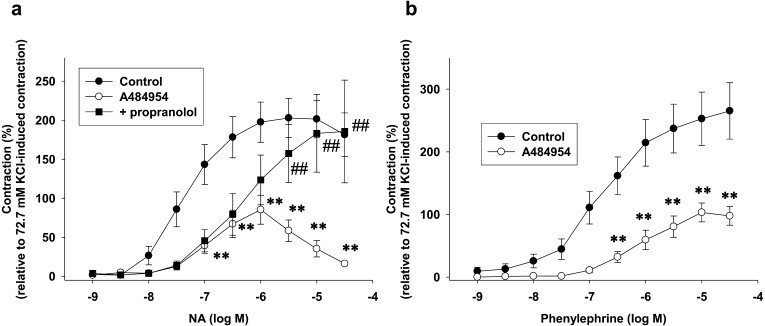
Fig. 1.
Effects of pretreatment with A484954 on adrenergic agonist [(a) noradrenaline (NA), (b) phenylephrine]-induced contraction in endothelium-denuded rat isolated superior mesenteric arteries. A484954 (10 µM) or dimethyl sulfoxide (DMSO: control) was pretreated for 10 min before the cumulative addition of (a) NA (1 nM–30 µM) or (b) phenylephrine (1 nM–30 µM). In (a), propranolol (1 µM) was pretreated for 5 min before the addition of A484954 (10 µM). Contraction was normalized to the high-K+ (72.7 mM)-induced contraction. The results were shown as mean ± SEM (a: n=5, b: n=7). **P<0.01 vs. control, ##P<0.01 vs. A484954.
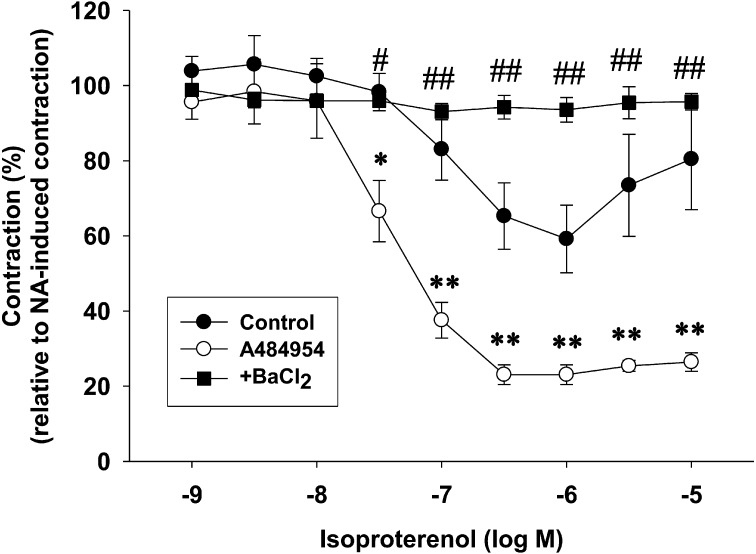
Fig. 2.
Effects of pretreatment with A484954 on isoproterenol-induced relaxation in endothelium-denuded rat isolated superior mesenteric arteries. A484954 (10 µM) or DMSO was pretreated for 10 min before the addition NA (3 µM). Isoproterenol (1 nM–10 µM) was cumulative added after the contraction induced by NA (3 µM) had reached a steady state. BaCl2 was pretreated for 5 min before the addition of A484954 (10 µM). Contraction was normalized to the NA (3 µM)-induced pre-contraction. The results were shown as mean ± SEM (n=3). *P<0.05, **P<0.01 vs. Control, #P<0.05, ## P<0.01 vs. A484954.
Measurement of BP
BP and heart rate (HR) of male Wistar rats (7–9-week-old) were measured under urethane (1.5 g/kg, i.p.) anesthesia as described previously [5]. The catheter filled with a heparin-saline solution was inserted into carotid artery with a small incision, which was connected to a BP transducer (ADInstruments). Systolic BP (SBP), mean BP (MBP) and diastolic BP (DBP) were measured and recorded using a BP Amp (ADInstruments) and PowerLab system (ADInstruments). HR was calculated by a cyclic measurement of BP recording as described previously [5]. After A484954 (1.7–122 µg/kg), NH125 (0.3–22 µg/kg) or the same amount of DMSO (1–5%: control) was intravenously injected for 5 min through the catheter inserted into femoral vein, NA (0.01–20 µg/kg) was injected. The difference of BP and HR before and after the addition of drugs was calculated.
Statistics
Data were shown as mean ± standard error of the mean (SEM). Statistical evaluations were done with 2-way ANOVA followed by Bonferroni’s post-hoc test. Results were considered significant when P was less than 0.05.
RESULTS
Effects of pretreatment with A484954 on adrenergic agonist (NA or phenylephrine)-induced contraction in endothelium-denuded rat isolated superior mesenteric arteries
We first examined whether A484954 (10 µM) affects adrenergic agonist (NA or phenylephrine)-induced contraction in endothelium-denuded rat isolated superior mesenteric arteries. As shown in Fig. 1a, A484954 (10 µM) inhibited NA (1 nM-30 µM)-induced contraction in a biphasic manner [magnitude of inhibition higher at high dose NA (3–30 µM)] (n=5, P<0.01 vs. Control). A β-blocker, propranolol (1 µM) prevented the A484954-mediated inhibition of NA (high dose: 3–30 µM)-induced contraction (n=5, P<0.01 vs. A484954). As shown in Fig. 1b, A484954 (10 µM) inhibited a selective α1-receptor agonist, phenylephrine (1 nM-30 µM)-induced contraction, however, the inhibitory effect was not biphasic (n=7, P<0.01).
Effects of pretreatment with A484954 on isoproterenol-induced relaxation in endothelium-denuded rat isolated superior mesenteric arteries
We next examined whether A484954 (10 µM) affects a β-agonist, isoproterenol-induced relaxation in NA (3 µM)-precontracted endothelium-denuded superior mesenteric arteries. A484954 (10 µM) potentiated isoproterenol (30 nM-10 µM)-induced relaxation (Fig. 2, n=3, P<0.05, P<0.01 vs. Control). The A484954-induced potentiation of isoproterenol (30 nM-10 µM)-induced relaxation was completely inhibited by a Kir channel blocker, BaCl2 (1 mM, Fig. 2, n=3, P<0.05, P<0.01 vs. A484954).
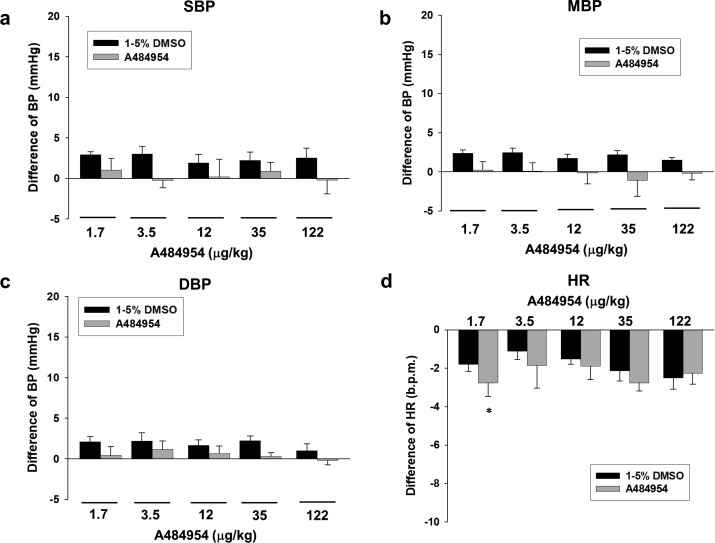
Effects of acute intravenous injection with A484954 on systemic BP and HR in rats
We further examined whether acute intravenous injection with A484954 alters BP and HR in rats. A484954 (1.7–122 µg/kg) alone injection had no influence on BP and HR in rats (Fig. 3, n=6).
Fig. 3.
Effects of A484954 alone injection on blood pressure (BP) and heart rate (HR) in rats. BP was measured by a carotid cannulation method. A484954 (1.7–122 µg/kg, 5 min-interval) or the same amount of DMSO (1–5%) was injected through femoral vein. Bar graph indicated the change of BP [(a) systolic BP (SBP), (b) mean BP (MBP) and (c) diastolic BP (DBP)] and (d) HR after injection with A484954 or DMSO. The results were shown as mean ± SEM (n=6). *P<0.05 vs. DMSO.
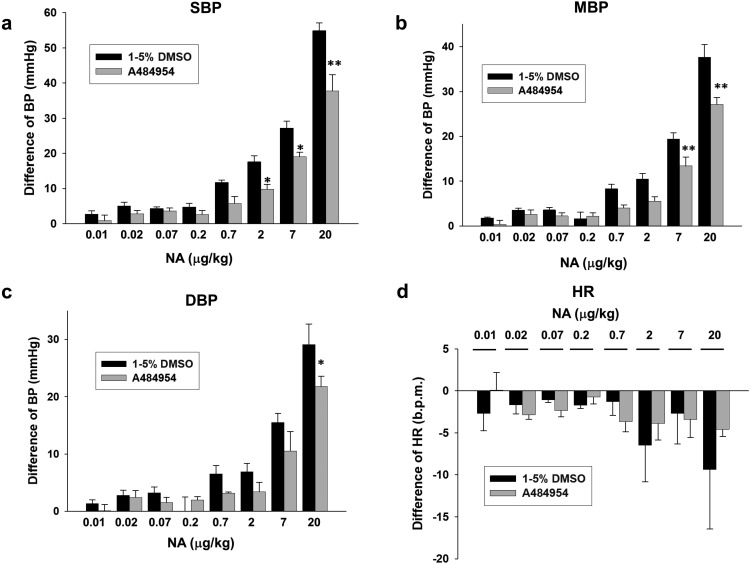
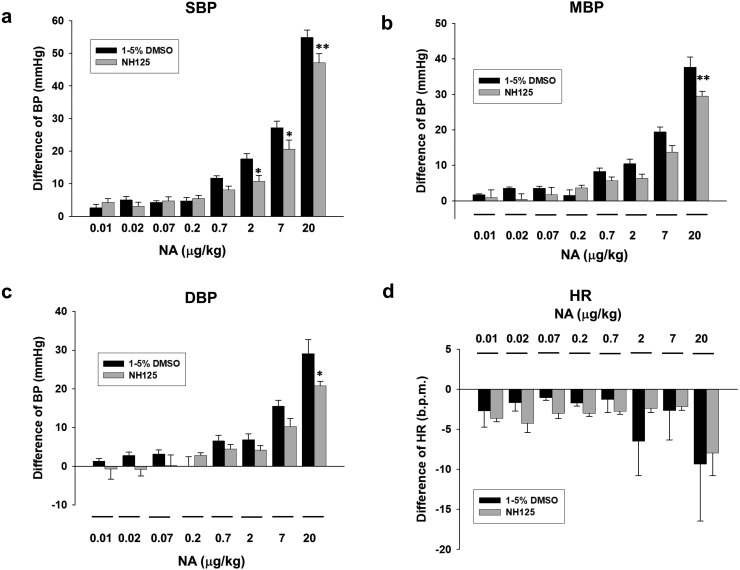
Effects of A484954 on NA-induced increase of BP in rats
We then examined effects of pretreatment with A484954 on NA-induced increase of BP in rats. A484954 (122 µg/kg) significantly inhibited NA (2–20 µg/kg)-induced increase of SBP (Fig. 4a, P<0.05, P<0.01), MBP (Fig. 4b, P<0.01) and DBP (Fig. 4c, P<0.05). NA slightly decreased HR in rats, which was not affected by A484954 (122 µg/kg) (Fig. 4d, n=6).
Fig. 4.
Effects of A484954 on NA-induced increase of BP in rats. NA (0.01–20 µg/kg) was cumulatively added 10 min after injection with A484954 (122 µg/kg) or DMSO. Bar graph indicated the change of BP [(a) SBP, (b) MBP and (c) DBP] and (d) HR after injection with NA. The results were shown as mean ± SEM (n=6). *P<0.05, **P<0.01 vs. DMSO.
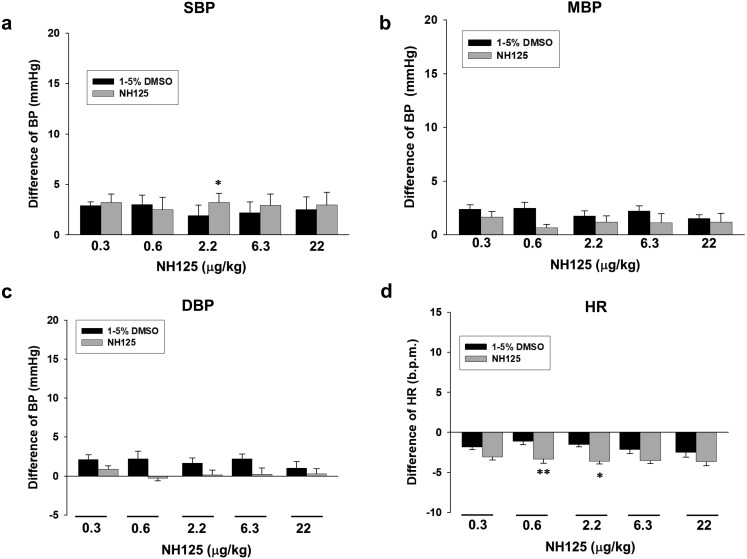
Effects of acute intravenous injection with NH125 on BP and HR in rats
We next examined effects of acute intravenous injection with another eEF2K inhibitor, NH125 on BP and HR in rats. NH125 (0.3–22 µg/kg) alone injection had no big influence on BP and HR in rats [Fig. 5, n=6 (DMSO), n=5 (NH125)].
Fig. 5.
Effects of NH125 alone injection on BP and HR in rats. NH125 (0.3–22 µg/kg, 5 min-interval) or the same amount of DMSO was injected through femoral vein. Bar graph indicated the change of BP [(a) SBP, (b) MBP and (c) DBP] and (d) HR after injection with NH125 or DMSO. The results were shown as mean ± SEM [n=6 (DMSO), n=5 (NH125)]. *P<0.05, **P<0.01 vs. DMSO.
Effects of NH125 on NA-induced increase of BP in rats
We finally examined effects of pretreatment with NH125 on NA-induced increase of BP in rats. NH125 (22 µg/kg) significantly inhibited NA (2–20 µg/kg)-induced increase of SBP (Fig. 6a, P<0.05, P<0.01), MBP (Fig. 6b, P<0.01) and DBP (Fig. 6c, P<0.05). NA slightly decreased HR in rats, which was not affected by NH125 (22 µg/kg) [Fig. 6d, n=6 (DMSO), n=5 (NH125)].
Fig. 6.
Effects of NH125 on NA-induced increase of BP in rats. NA (0.01−20 µg/kg) was cumulatively added 10 min after injection with NH125 (22 µg/kg) or DMSO (control). Bar graph indicated the change of BP [(a) SBP, (b) MBP and (c) DBP] and (d) HR after injection with NA. The results were shown as mean ± SEM [n=6 (DMSO), n=5 (NH125)]. *P<0.05, **P<0.01 vs. DMSO.
DISCUSSION
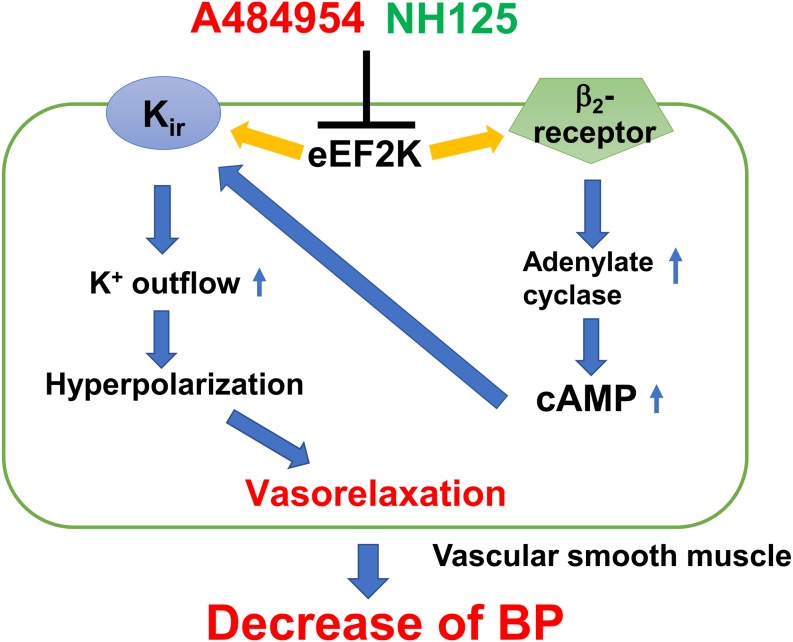
We examined effects of an eEF2K inhibitor on contractility of rat isolated superior mesenteric artery and blood pressure in rats, and the major findings are as follows; 1) A484954 (10 µM) inhibited NA (1 nM-30 µM)-induced contraction in a biphasic manner [magnitude of inhibition higher at high dose NA (3–30 µM)] in endothelium-denuded artery. The A484954-mediated inhibition of NA (high dose: 3–30 µM)-induced contraction was prevented by a β-blocker, propranolol (1 µM). In addition, A484954 (10 µM) inhibited a selective α1-receptor agonist, phenylephrine (1 nM-30 µM)-induced contraction, however, the inhibitory effect was not biphasic (Fig. 1). 2) A484954 (10 µM) potentiated a β-receptor agonist, isoproterenol (30 nM-10 µM)-induced relaxation. The A484954-induced potentiation of isoproterenol-induced relaxation was completely inhibited by a Kir channel blocker, BaCl2 (1 mM) (Fig. 2). 3) A484954 (1.7–122 µg/kg) alone injection had no influence on BP and HR (Fig. 3), while A484954 (122 µg/kg) significantly inhibited NA (2–20 µg/kg)-induced increase of BP (Fig. 4). 4) Another eEF2K inhibitor, NH125 (0.3–22 µg/kg) alone injection had no influence on BP and HR (Fig. 5), while NH125 (22 µg/kg) significantly inhibited NA (2–20 µg/kg)-induced increase of BP (Fig. 6). In summary, it was concluded that A484954 lowers NA-induced BP rise through activation of β2-receptor-Kir channel and subsequent vasorelaxation perhaps through inhibiting eEF2K activity (Fig. 7).
Fig. 7.
Summary of the present results. An eEF2K inhibitor (A484954 and NH125) lowers NA-induced BP rise perhaps through the activation of β2-receptor-Kir channel and subsequent vasorelaxation through inhibiting eEF2K activity.
The dose of A484954 (122 µg/kg, i.v.) and NH125 (22 µg/kg, i.v.) that we used in this study could be estimated 10 µM (A484954) and 1 µM (NH125), respectively, because it was reported that a total blood volume was 12 ml in 200 g body weight rat [7]. We previously reveled in vitro study that A484954 (10 µM) induced relaxation thorough opening smooth muscle Kir channel in rat isolated superior mesenteric artery [6]. The same concentration (10 µM) of A484954 also inhibited platelet-derived growth factor-BB-induced proliferation and migration of vascular smooth muscle cells [15]. Moreover, we previously showed in vitro study that NH125 (1 µM) inhibited tumor necrosis factor-α-induced inflammation in human umbilical vein endothelial cell [14]. In vivo, we previously reveled that long-term treatment with a higher dose of A484954 (2.5 mg/kg/day, i.p.) prevented development of pulmonary arterial hypertension partly through inhibiting structural remodeling of pulmonary arterial wall in monocrotaline-induced pulmonary hypertensive rats [4]. In addition, we previously showed that long-term treatment with a higher dose of NH125 (500 µg/kg/day, s.c.) prevented SBP rise partly through inhibiting vascular inflammation and structural remodeling of mesenteric arterial wall [14]. Considering the concentrations of A484954 and NH125 that we used in the previous experiments performed both in vitro and in vivo, the dose of A484954 and NH125 used in this study might be within the appropriate ranges.
It is known that the adrenergic actions in vascular smooth muscle are mediated through α1- and β2-adrenergic receptors. When an agonist binds to the α1-receptor, phospholipase C is activated and inositol trisphosphate (IP3) is produced. This IP3 through binding to the IP3 receptor can release Ca2+ from sarcoplasmic reticulum, leading to a rise in cytosolic Ca2+ concentration, which contracts blood vessel. On the other hand, cAMP concentration in the cell rises due to the activation of adenylate cyclase when an agonist binds to the β2-receptor. This rise in cAMP concentration activates protein kinase A (PKA), which relaxes blood vessel. Thus, in vascular smooth muscle, α1-adrenergic action induces vasoconstriction, while β2-adrenergic action induces vasorelaxation. In this study, we reveled that A484954 (10 µM) inhibited an α1- and β2-adrenergic receptor agonist, NA-induced contraction in a biphasic manner, while an inhibitory effect of A484954 on a selective α1-receptor agonist, phenylephrine-induced contraction was not biphasic. It was also shown that the A484954-mediated inhibition of NA (high dose: 3–30 µM)-induced contraction was specifically prevented by a β-blocker, propranolol (1 µM). In addition, we previously revealed that A484954 caused relaxation via opening smooth muscle Kir channel in rat isolated superior mesenteric artery [6]. Taken together, it is suggested that A484954 inhibited low dose NA (1 nM-3 µM)-induced contraction via directly opening Kir channel, while it inhibited high dose NA (3–30 µM)-induced contraction via activation of β2-adrenergic receptor in addition to Kir channel.
In the present study, we showed that A484954 (10 µM) potentiated a β-receptor agonist, isoproterenol (30 nM-10 µM)-induced relaxation, which was completely prevented by a Kir channel blocker, BaCl2 (1 mM). When an agonist binds to the β2-receptor, the cAMP concentration in the cell rises through adenylate cyclase activation. It was reported that adenosine, a vasodilator stimulates Kir2 channel through an increase in cAMP concentration [10]. The cAMP-PKA signal increases Kir current in rabbit coronary arterial smooth muscle cell [11]. These reports suggest that A484954-induced potentiation of β2-receptor agonist-induced relaxation was mediated through the activation of Kir channel via cAMP/PKA pathways.
We reveled that the pretreatment with A484954 inhibited NA-induced increase of systemic BP in rats. It is suggested that the inhibitory effect of A484954 on BP was mediated via vasorelaxation through the activation of β2-receptor and Kir channel. We also showed that another eEF2K inhibitor, NH125 inhibited NA-induced increase of BP in rats. eEF2K is one of the CaMKs. It is known that NH125 inhibits the activity of eEF2K by a CaM-competitive manner, while A484954 inhibits the activity of eEF2K by an ATP-competitive but CaM-independent manner [2]. Since two kinds of kinase inhibitors with different site of actions prevented the increase of BP, it is suggested that the effects were mediated perhaps through inhibiting eEF2K activity.
In conclusion, we for the first time revealed that A484954 lowers NA-induced BP rise perhaps through the activation of β2-receptor-Kir channel and subsequent vasorelaxation via inhibiting eEF2K activity. It was expected that A484954 contributes to progress drug discovery research for cardiovascular diseases including hypertension.
Acknowledgments
This study was supported by the grant from School of Veterinary Medicine, The Kitasato University.
REFERENCES
- 1.Adaikkan C., Taha E., Barrera I., David O., Rosenblum K.2018. Calcium/calmodulin-dependent protein kinase II and eukaryotic elongation factor 2 kinase pathways mediate the antidepressant action of ketamine. Biol. Psychiatry 84: 65–75. doi: 10.1016/j.biopsych.2017.11.028 [DOI] [PubMed] [Google Scholar]
- 2.Chen Z., Gopalakrishnan S. M., Bui M. H., Soni N. B., Warrior U., Johnson E. F., Donnelly J. B., Glaser K. B.2011. 1-Benzyl-3-cetyl-2-methylimidazolium iodide (NH125) induces phosphorylation of eukaryotic elongation factor-2 (eEF2): a cautionary note on the anticancer mechanism of an eEF2 kinase inhibitor. J. Biol. Chem. 286: 43951–43958. doi: 10.1074/jbc.M111.301291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deng Z., Luo P., Lai W., Song T., Peng J., Wei H. K.2017. Myostatin inhibits eEF2K-eEF2 by regulating AMPK to suppress protein synthesis. Biochem. Biophys. Res. Commun. 494: 278–284. doi: 10.1016/j.bbrc.2017.10.040 [DOI] [PubMed] [Google Scholar]
- 4.Kameshima S., Kazama K., Okada M., Yamawaki H.2015. Eukaryotic elongation factor 2 kinase mediates monocrotaline-induced pulmonary arterial hypertension via reactive oxygen species-dependent vascular remodeling. Am. J. Physiol. Heart Circ. Physiol. 308: H1298–H1305. doi: 10.1152/ajpheart.00864.2014 [DOI] [PubMed] [Google Scholar]
- 5.Kazama K., Okada M., Hara Y., Yamawaki H.2013. A novel adipocytokine, omentin, inhibits agonists-induced increases of blood pressure in rats. J. Vet. Med. Sci. 75: 1029–1034. doi: 10.1292/jvms.12-0537 [DOI] [PubMed] [Google Scholar]
- 6.Kodama T., Okada M., Yamawaki H.2018. Mechanisms underlying the relaxation by A484954, a eukaryotic elongation factor 2 kinase inhibitor, in rat isolated mesenteric artery. J. Pharmacol. Sci. 137: 86–92. doi: 10.1016/j.jphs.2018.04.006 [DOI] [PubMed] [Google Scholar]
- 7.Lee H. B., Blaufox M. D.1985. Blood volume in the rat. J. Nucl. Med. 26: 72–76. [PubMed] [Google Scholar]
- 8.Lei M., Xu J., Gao Q., Minobe E., Kameyama M., Hao L.2018. PKA phosphorylation of Cav1.2 channel modulates the interaction of calmodulin with the C terminal tail of the channel. J. Pharmacol. Sci. 137: 187–194. doi: 10.1016/j.jphs.2018.05.010 [DOI] [PubMed] [Google Scholar]
- 9.Nairn A. C., Palfrey H. C.1987. Identification of the major Mr 100,000 substrate for calmodulin-dependent protein kinase III in mammalian cells as elongation factor-2. J. Biol. Chem. 262: 17299–17303. [PubMed] [Google Scholar]
- 10.Park W. S., Han J., Earm Y. E.2008. Physiological role of inward rectifier K(+) channels in vascular smooth muscle cells. Pflugers Arch. 457: 137–147. doi: 10.1007/s00424-008-0512-7 [DOI] [PubMed] [Google Scholar]
- 11.Park W. S., Han J., Kim N., Ko J. H., Kim S. J., Earm Y. E.2005. Activation of inward rectifier K+ channels by hypoxia in rabbit coronary arterial smooth muscle cells. Am. J. Physiol. Heart Circ. Physiol. 289: H2461–H2467. doi: 10.1152/ajpheart.00331.2005 [DOI] [PubMed] [Google Scholar]
- 12.Price N. T., Redpath N. T., Severinov K. V., Campbell D. G., Russell J. M., Proud C. G.1991. Identification of the phosphorylation sites in elongation factor-2 from rabbit reticulocytes. FEBS Lett. 282: 253–258. doi: 10.1016/0014-5793(91)80489-P [DOI] [PubMed] [Google Scholar]
- 13.Ryazanov A. G., Ward M. D., Mendola C. E., Pavur K. S., Dorovkov M. V., Wiedmann M., Erdjument-Bromage H., Tempst P., Parmer T. G., Prostko C. R., Germino F. J., Hait W. N.1997. Identification of a new class of protein kinases represented by eukaryotic elongation factor-2 kinase. Proc. Natl. Acad. Sci. U.S.A. 94: 4884–4889. doi: 10.1073/pnas.94.10.4884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Usui T., Okada M., Hara Y., Yamawaki H.2013. Eukaryotic elongation factor 2 kinase regulates the development of hypertension through oxidative stress-dependent vascular inflammation. Am. J. Physiol. Heart Circ. Physiol. 305: H756–H768. doi: 10.1152/ajpheart.00373.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Usui T., Nijima R., Sakatsume T., Otani K., Kameshima S., Okada M., Yamawaki H.2015. Eukaryotic elongation factor 2 kinase controls proliferation and migration of vascular smooth muscle cells. Acta Physiol. (Oxf.) 213: 472–480. doi: 10.1111/apha.12354 [DOI] [PubMed] [Google Scholar]
- 16.Xu J., Yabuki Y., Yu M., Fukunaga K.2018. T-type calcium channel enhancer SAK3 produces anti-depressant-like effects by promoting adult hippocampal neurogenesis in olfactory bulbectomized mice. J. Pharmacol. Sci. 137: 333–341. doi: 10.1016/j.jphs.2018.07.006 [DOI] [PubMed] [Google Scholar]