Abstract
The purpose of the present study was to evaluate effects of aflatoxin B1 (AFB1) on the cell cycle and proliferation of splenic cells in chickens. A total of 144 one-day-old Cobb male chickens were randomly divided into 2 equal groups of 72 each and were fed on diets as follows: a control diet and a 0.6 mg/kg AFB1 diet for 21 days. The AFB1 diet reduced body weight, absolute weight and relative weight of the spleen in broilers. Histopathological lesions in AFB1 groups were characterized as slight congestion in red pulp and lymphocytic depletion in white pulp. Compared with the control group, the expression levels of ataxia–telangiectasia mutated (ATM), cyclin E1, cyclin-dependent kinases 6 (CDK6), CDK2, p53, p21 and cyclin B3 mRNA were significantly increased, while the mRNA expression levels of cyclin D1, cdc2 (CDK1), p16, p15 were significantly decreased in the AFB1 groups. Significantly decreased proliferating cell nuclear antigen (PCNA) expression and arrested G0G1 phases of the cell cycle were also seen in the AFB1 groups. In conclusion, dietary AFB1 could induce cell cycle blockage at G0G1 phase and impair the immune function of the spleen. Cyclin D1/CDK6 complex, which inhibits the activin/nodal signaling pathway, might play a significant role in the cell cycle arrest induced by AFB1.
Keywords: AFB1, G0G1phase, cell cycle arrest, mechanism, spleen, chicken
Introduction
Aflatoxin B1 (AFB1) is a type of mycotoxin that is classified as a Group I carcinogen to humans by the International Agency for Research on Cancer and is a mycotoxins produced typically during storage of crops1.It is commonly encountered and has potent carcinogenic, genotoxic, immunotoxic and other adverse effects in many animal species including poultry2, 3. An active intermediate products of AFB1, AFB1-exo-8, 9-epoxide, can bind with DNA to form the predominant trans-8, 9-dihydro-8-(N7-guanyl)-9-hydroxy-AFB1 (AFB1-N7-Gua) adduct, which causes DNA lesions4. Aflatoxin exposure is one of the multple of human hepatocellular carcinoma (HCC) development5.
Many researchers have reported that aflatoxin could affect many biological characteristics of intestine6-8, cecal tonsil9, thymus10, 11, spleen12, 13, bursa of Fabricius11, 14and kidney15 morphology in poultry, such as by causing histological lesions, oxidative stress, apoptosis, changes in T-cell subsets, and changes in cellular and humoral immune function16, 17.
AFB1-8, 9-exo-epoxide can affect every period of the cell cycle with its potent biological activity18, 19. The researches has demonstrated that AFB1 caused chick jejunum cells to be arrested at G2/M phase8, 20, renal cells to be arrested at G0/G1 phase21, increased percentages of chick thymocytes in the G2/M3 phase, and dose dependent accumulation in S phase in vitro in human cell lines22. AFB1 can impact a variety of cell cycle regulation gene, protein and related enzyme activities including p53 gene mutations and p16 gene methylation in tissue23, affecting activities of CDK4, CDK6 and Rb19, 24, 25, and reducing the amount of PCNA-positive cells8, 26. Bressac et al.27 and Hollestein et al.28 have demonstrated that dietary intake of aflatoxins was closely related to p53 gene mutations.
The spleen is the most important organ for antibacterial and antifungal immune reactivity29. AFB1 could decrease the number of CD4+ and CD8+ T cells in the spleens of mice30 and induce biomolecular oxidative damage and decrease cell proliferation of spleen mononuclear cells in rats31, 32, 33. Cell cycle arrest of splenocytes in chickens fed aflatoxin-contaminated corn has been reported34, but the exact molecular mechanism of cell cycle arrest in the spleen of chickens induced by AFB1 diet has not been elucidated. The results could help us to understand the molecule mechanism for the spleen immunosuppression attributable to AFB1.
Materials and Methods
Animals and diets
The experiment was performed with male Cobb chickens weighing 45 ± 5 g that were purchased from a commercial rearing farm (Wenjiang poultry farm, Chengdu, Sichuan Province, China).
A total of 144 one-day-old chickens were randomly divided into 2 groups, namely control group (0 mg/kg AFB1) and AFB1 group (0.6 mg/kg AFB1). All of the chickens were put into cages with three replicates per group and 24 birds per replicate. AFB1 was purchased from A6636, Sigma-Aldrich, St.Louis, MO, USA. Nutritional requirements were adequate according to National Research Council (National Research Council, 1994)35. The AFB1-contamin diet was produced by method similar to described by Kaoud36. In short, 27 mg AFB1 farinose solid was dissolved into 30 mL methanol completely, and then the 30 mL mixture was mixed into 45 kg corn-soybean basal diet to formulate AFB1 diet containing 0.6 mg/kg AFB1. Equivalent methanol was mixed into corn-soybean basal diet to produce a control diet. Then, methanol in diets was evaporated at 98°F (37°C). The concentrations of AFB1 were analyzed by HPLC (Waters, Milford, MA, USA) with fluorescence detection 2475 Fluorescence Detector (Waters, Milford, MA, USA) and were determined to be <0.001 mg/kg in the control diet and 0.601 mg/kg in the AFB1 diet. Chickens were housed in cages with electrically heated units and provided with water as well as the diets ad libitum for 21 days.
Body weight and absolute and relative weight of spleen
At 7, 14, and 21 days of age, six chickens in each group were weighed and euthanized. The spleen was dissected from each chick immediately and weighed with electronic balance after removing the surrounding fat and connective tissue. The following formula was used to calculate the relative weight:
Relative weight (g/kg) = organ weight (g) / fasting weight of chick (kg)
Pathological observation
After weighing, spleens were fixed in 4% paraformaldehyde for 24 h and routinely processed in dehydration, transparent disposal, paraffin embedded and sectioned at 5 μm. Slides were stained with hematoxylin and eosin Y (H.E). Paraffin sections were also collected to perform immunohistochemistry. The histological structures of the tissues were observed and photographed with a digital camera (DS-Ri1, Nikon Instech Co., Ltd., Tokyo, Japan).
Cell cycle of the spleen by flow cytometry method
At 7, 14, and 21 days of age, six chickens in each group were euthanized, and the spleen was dissected from each animal an immediately minced with surgical scissors. The cell suspension was filtered through a 300-mesh nylon mesh. Then, the cells were washed and suspended in phosphate buffer at a concentration of 1 × 106 cells/mL (PBS, pH 7.2–7.4). 1 mL suspension was transferred to a 500 μL culture tube and centrifuged at 200× g for 5 min at 4°C. The supernatant was separated and discarded. Then, propidium iodide (5 μL, 51-66211E, BD Pharmingen, San Diego, CA, USA) was added into 100 μL cell suspension and incubated for 30 min at 4°C in a dark room. Finally, 500 μL PBS was added to each tube, and cells were analyzed by flow cytometry (FACSCalibur, BD, Franklin Lake, NJ, USA). The results were analyzed using the ModFit LT for Mac V3.0 computer program.
proliferating index (PI) = (S + G2M) / (G0G1 + S + G2M)
PCNA detection by the immunohistochemical method
The method of immunohistochemistry was applied according to the report by Fang et al.37 Spleen paraffin sections were dewaxed in xylene, rehydrated through a graded series of ethanol solutions and washed three times in distilled water and PBS (0.1 M, PH 7.2-7.4). Endogenous peroxidase activity was blocked by incubation with 3% H2O2 in methanol for 15 min. Following a wash with PBS, the sections were exposed to normal 10% goat sera for 30 min at 37°C to block nonspecific antibody binding. In a humidified chamber, 10 μg/mL primary antibodies rabbit anti-PCNA (bs-0754R, Wuhan Boster Bio-Engineering Limited Company, Wuhan, China) was applied sections for 20 h at 4°C. After three washings in PBS, the slices were exposed to 1% biotinylated goat anti-rabbit/mouse IgG secondary antibody (SA1030, Wuhan Boster Bio-engineering Limited Company, Wuhan, China) for 1 h at 37°C, and then incubated with streptavidin-biotin complex (SABC; Wuhan Boster Bio-engineering Limited Company, Wuhan, China) for 30 min at 37°C. Slides were visualized with 3,3’-diaminobenzidin. The slices were monitored microscopically and stopped by immersion in distilled water, as soon as brown staining was visible. Slices were lightly counterstained with hematoxylin, dehydrated in ethanol, cleared in xylene, and cover slipped. For negative control purposes, representative sections were processed in the same way by replacing primary antibodies in PBS. The stained sections were photographed with a digital camera (DS-Ri1, Nikon Instech Co., Ltd., Tokyo, Japan). Within a range of 1,000 times lens, 6 fields of view were randomly selected for each slice, and photos were taken for determination of integral optical density (IOD) value. The results were analyzed using Image-Pro Plus 6.0 software.
Quantitative real-time PCR
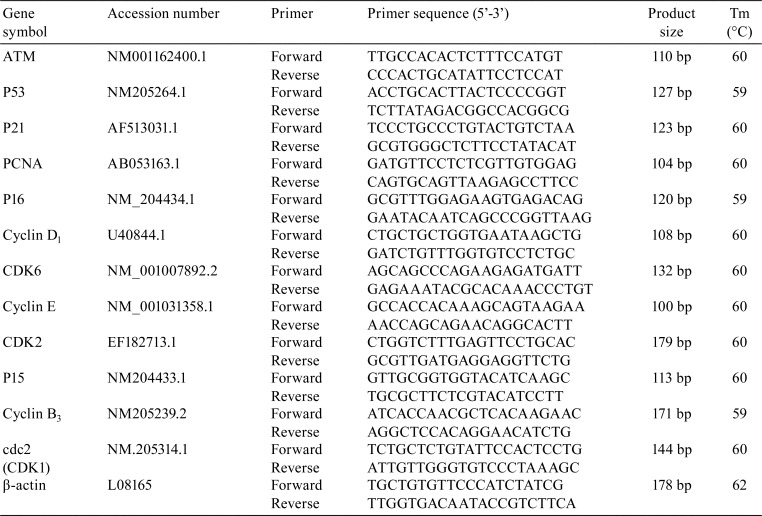
In order to comprehend the molecular mechanism of cell cycle arrest induced by AFB1, we continued our research to detect the mRNA expression of cell cyclins by quantitative real-time PCR (qRT-PCR). The spleens from six chickens in each group were remove at 7, 14, and 21 days of age and stored in liquid nitrogen. The spleens were crushed with pestle to homogenize them until they powdery, respectively. As previously described38, total RNA was extracted from the powder of the spleens using RNAiso Plus (9108/9109, Takara, Kusatsu, Japan). RNA concentrations and purity were checked by NanoDropTM One (Thermo Fisher Scientific, Waltham, MA, USA). Next, cDNA was synthesized using a PrimScript RT reagent Kit (RR047A, Takara, Kusatsu, Japan) according to the manufacturer’s protocol. The cDNA product was used as a template for qRT-PCR analysis. Sequences for target genes were obtained from the NCBI database. Oligonucleotide primers were designed using Primer 5 software and synthesized at Takara (Dalian, China; Table 1). All qRT-PCR reactions were performed using the SYBR® Premix Ex TaqT II system (DRR820A, Takara, Kusatsu, Japan) and a C1000 Touch (Bio-Rad Laboratories, Hercules, CA, USA). Chicken β-actin was used as an internal reference housekeeping gene. All data output from the qRT-PCR experiments were analyzed using the 2-ΔΔCT method39.
Table 1. Sequence of Primers Used in qRT-PCR.
Statistical analysis
The significance of differences between two groups was analyzed by variance analysis, and results were expressed as mean ± standard deviation (X ± SD). The analysis was performed using the independent sample test in the IBM SPSS Statistics for Windows, Version 20.0 software (IBM Corp, Armonk, NY, USA). Difference were considered statistically significant at p<0.05 and markedly significant was considered at p<0.01.
Results
Body weight and absolute weight and relative weight of spleen
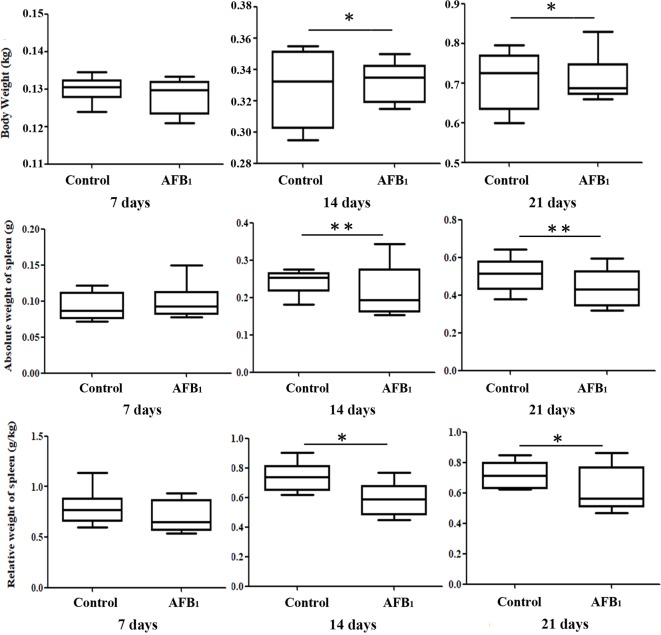
The effects of dietary AFB1 on body weight and absolute weight and relative weight of the spleen of chickens are shown in Fig. 1. No significant differences were observed in body weight or absolute weight and relative weight in the AFB1 group at 7 days of age. Compared with the control group, the AFB1 diet reduced body weight at 14 and 21 days of age (p<0.05). Meanwhile, the absolute and relative weight of the spleen in the AFB1 group were significantly lower than those in the control group at 14 and 21 days of age (p<0.05 or p<0.01).
Fig. 1.
The effects of dietary AFB1 on body weight and absolute weight and relative weight of the spleen. Notes: Data are presented with the means ± standard deviation (n = 6). *p<0.05 compared with control group, **p<0.01 compared with control group.
Pathological observation on spleen
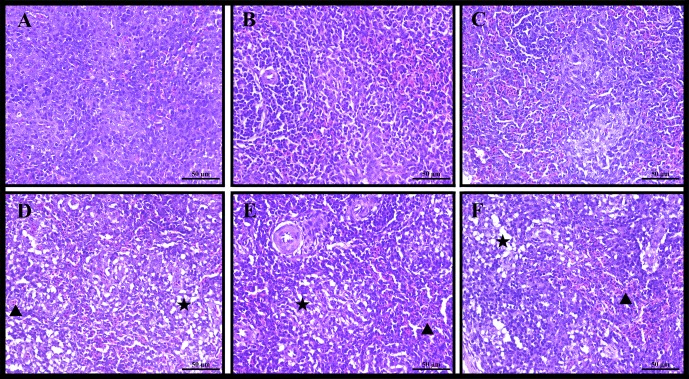
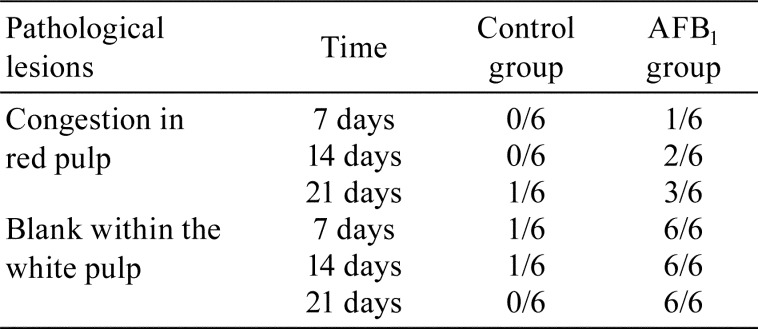
In the AFB1 group, lymphocyte density was mainly reduced in the white pulp, and loose arrangement of histocytes was observed when compared with those in the control group (Fig. 2). The main lesions of spleen were statistically analysed (Table 2). The results showed that AFB1 treatment led to spleen congestion in the red pulp in 1/6, 2/6 and 3/6 chickens at 7, 14, and 21 days of age, respectively, and loosely lined histocytes within the white pulp caused by a reduction in lymphocyte density in 6/6 chickens at 7–21 days of age.
Fig. 2.
Photomicrographs of hematoxylin and eosin stained chicken spleen. Notes: (A) the control group at 7 days of age; (B) the control group at 14 days of age; (C) the control group at 21 days of age; (D) the AFB1 group at 7 days of age; (E) the AFB1 group at 14 days of age; (F) the AFB1 group at 21 days of age; (Triangles) congestion in the red pulp; (Stars) lymphocytic depletion in white pulp. Bars = 50 μm.
Table 2. Incidence of Major Lesions in Spleen (n=6).
Cell cycle phase of splenocytes
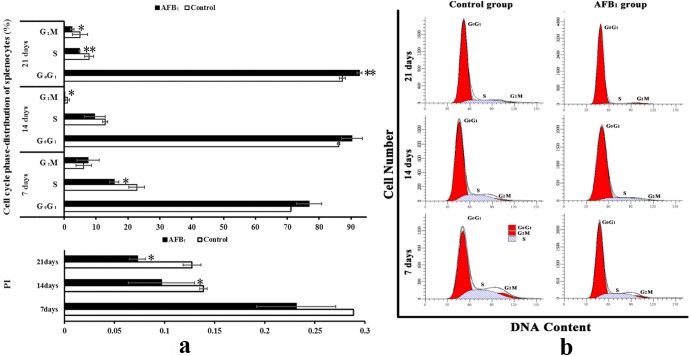
The percentages of cells in the G2M phase in the AFB1 group were significantly lower than those in the control group at 14 and 21 days of age (p<0.05). The percentages of cells in S phase in the AFB1 group were significantly lower than those in the control group at 7 and 21 days of age (p<0.05 or p<0.01). Furthermore, changes of the G0G1 phase were obvious. The percentage of cells in the G0G1 phase in the AFB1 group was markedly increased at 21 days of age, when compared with the control group (p<0.01). The proliferating index (PI) value was significantly decreased in the AFB1 group at 14 and 21 days of age (p<0.05) when compared with the control group (Fig. 3). Histograms obtained by cytometer analysis show that the cell peaks of S and G2M phases are obviously lower and that the cell peaks of G0G1 phase are higher in the AFB1 group than those in the control group, especially at 14 and 21 days of age.
Fig. 3.
Cell cycle phase distribution of splenic cell. a: Effect of AFB1 on cell cycle phase distribution of spleen in chicken (%). b: Histogram of splenocyte cell cycle by flow cytometry. Notes: Data are presented with the means ± standard deviation (n = 6). *p<0.05 compared with control group, **p<0.01 compared with control group.
PCNA expression by immunohistochemical method
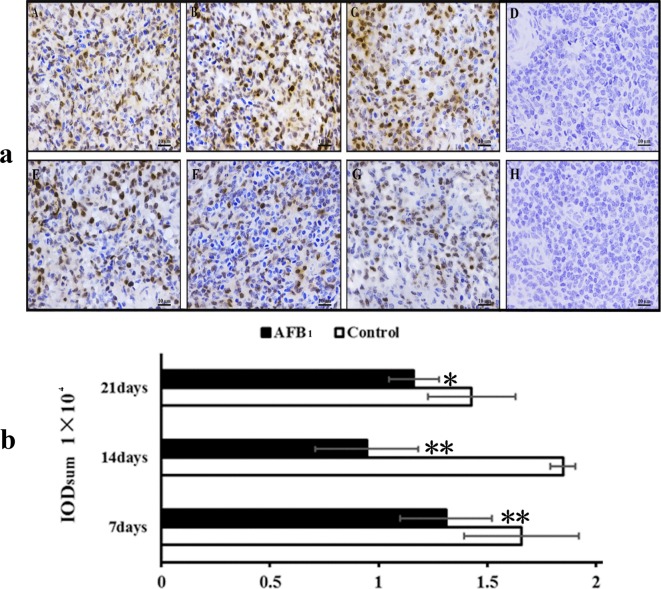
Brownish-yellow staining indicated the expression of PCNA expression. Control sections showed a negative reaction, as shown in Fig. 4. The PCNA protein expression in the spleen of the AFB1 group was obviously lower than that in the control group. PCNA expression was slightly increased at 21 days compared to 14 days treatment. When compared with the control group, the Integrated optical density (IOD sum) of PCNA protein expression in the AFB1 group was significantly decreased at 7, 14, and 21 days of age (p<0.05 or p<0.01).
Fig. 4.
Immunohistochemistry of the spleen. a: Expression of PCNA protein by immunohistochemistry in the spleen. b: Integrated optical density (IOD sum) of PCNA protein expression in the spleen. Notes: A, B, and C showing the control group at 7, 14, and 21 days respectively; E, F, and H showing the AFB1 group at 7, 14, and 21 days respectively; D and H showing negative control in the control group and the AFB1 group respectively. Bars = 10 μm. Data are presented with the mean ± standard deviation (n = 10). * means p<0.05 compared with the control group, ** means p<0.01 compared with the control group.
The mRNA expression of cell cyclins by qRT-PCR
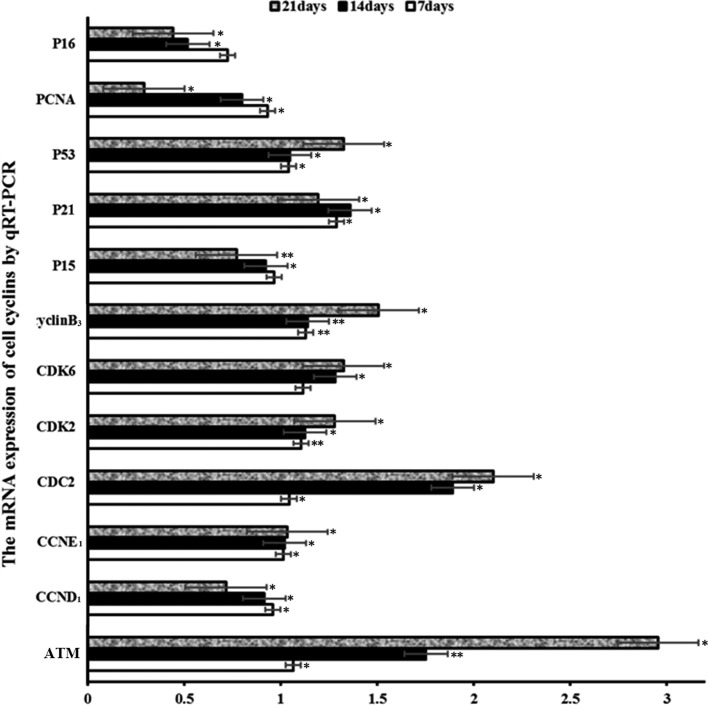
At 7 days of AFB1 treatment, the mRNA expression levels of ATM, CDK2, cdc2, p53, p21, cyclin E1, and cyclin B3 were obviously increased (p<0.05 or p<0.01) compared with the control group, and the mRNA expression levels of cdk6 were increased. Furthermore, the mRNA expression levels of cyclin D1 in the AFB1 group were obviously decreased (p<0.05), whereas the mRNA expression levels of P15, P16, and PCNA showed no obvious changes (P>0.05).
At 14 and 21 days of AFB1 treatment, the mRNA expression levels of ATM, CDK2, cdc2, CDK6, p53, p21, cyclin E1, and cyclin B3 were obviously increased (p<0.05 or p<0.01). Furthermore, the mRNA expression levels of cyclin D1, P15, P16, and PCNA in the AFB1 group were obviously decreased (p<0.05 or p<0.01; Fig. 5).
Fig. 5.
The levels of the ATM, cyclinB3, cyclinD1, CyclinE1, cdc2, CDK6, CDK2, p53, p21, p15, p16, and PCNA mRNA expression in the spleen. Notes: Data are presented with the means ± standard deviation (n = 6). *p<0.05 compared with control group, **p<0.01 compared with control group.
Discussion
Compared with the control group, the AFB1 diet reduced chicken weight at 14 and 21 days, indicating that AFB1 diet inhibited chicken growth performance. In the present study, the relative weight of the spleen was used to judge the development status and degree of pathologic change in spleen. At 14 and 21 days of age, the relative weight of the spleen in AFB1 group was significantly lower than that in the control group, which was consistent with the results of Chen et al.12 and Quist et al.40 However, it was not consistent the results of Peng et al.34 Who showed obvious congestion red pulp of the spleen in chicks exposed to corn with containing of AFB1 and AFB2. In this study, the results of histopathological observation suggested that the decreased weight and relative weight of the spleen might be due to lymphocytes depletion, as a decreased number of lymphocytes and loosely lined histocytes in the lymphatic nodules and periarterial lymphatic sheath were observed. The results of histopathological observation were also similar to the results of other researchers12, 34. Numerous studies and reports have documented that aflatoxin could inhibit the development of thymus10, 11, 14, 41. The loosely lined histocytes within the white pulp were suspected of resulting from the decreased number of lymphocytes.
In the present study the S phase and G2M phase were obviously decreased, and the G0G1 phase was markedly increased at 21 days of age. These results indicated that 0.6 mg/kg AFB1 could induce G0G1 phase arrest in chickens splenocytes, which was in line with previous research34. However, feeding with AFB-contaminated could lead to G2M and G0G1 phase blockage in the spleens of chickens14. Zhang et al.8 demonstrated that AFB1 caused jejunal cells to be arrested at G2/M phase. Scott et al.3 reported that AFB1 treatment increased the percentages of chick thymocytes in the G2/M. Ricordy et al.22 showed that human cell lines exposed AFB1 toxin for 24 h caused dose-dependent accumulation in S phase in vitro. Differences in animal species, viscera, cell type and mycotoxin type may cause different characteristics of cell cycle arrest.
Several reports have already suggested cellular and humoral immune function changes induced by AFB1 exposure 6, 7, 9, 10, 11, 12, 13, 14, 41 and AFB1-induced cell arrest, which can be impacted by a variety of cell cycle regulation genes. However, the exact molecular mechanism of AFB1-induced cell cycle arrest in the spleen of chickens has not been elucidated so far. The experimental results from this study could enrich the knowledge concerning the molecular mechanism of AFB1-induce immunosuppression. Further studies are needed on protein levels.
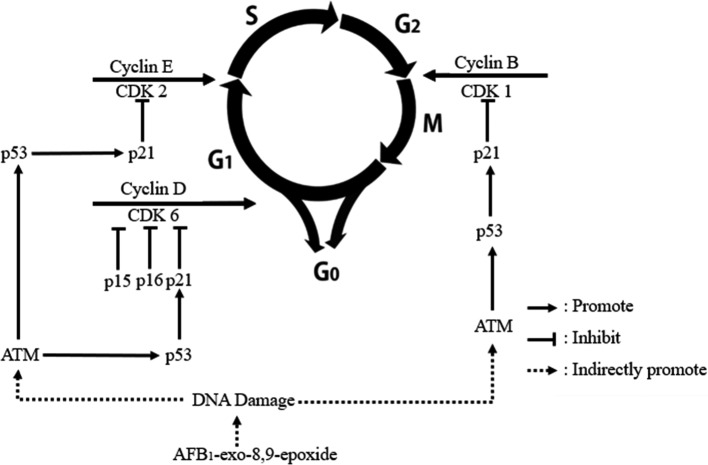
The active intermediate products of AFB1 causes DNA lesions and DNA damage (including double-strand breaks), which can activate ATM42. ATM initiates cell cycle arrest, DNA repair, or apoptosis by phosphorylating downstream targets. When ATM phosphorylates p5342, which is a tumo-suppressor gene integrating numerous signals to control cell life and death, the p53 protein can directly stimulate the expression of p21WAF1/CIP1, which is an inhibitor of cyclin-dependent kinases (CDKs) and p21WAF1/CIP1, and inhibit the G1 to S and G2 to M43. Besides, p53 can indirectly sequester cyclin B1/CDK1 complexes and help to maintain a G2 block44. The ATM(ATR)/CHK2(CHK1)–p53/MDM2-p21 pathway is the dominant checkpoint response to DNA damage in mammalian cells traversing through the G145. In this study, our results showed that AFB1 caused an increase in ATM, p53, and p21 mRNA expression. Therefore, we speculated that dietary AFB1 could induce G1 arrest by activating ATM(ATR)/CHK2(CHK1)–p53/MDM2-p21 pathway. Meanwhile, p21WAF1 complexed with CDKs can inhibit their kinase activity43. pl5 inhibits cyclin D/CDK6 without causing the dissociation of this complex46. The P16 family inhibits cell growth by inhibiting the activities of CDK6 and CDK4 kinases24. P21WAF1 boun to PCNA can directly inhibit DNA synthesis. Progression through the G1 is mediated by cyclin D/CDK646, and CDK2/cyclin E were thought to exclusively promote the G1S transition47, and the cyclin B/CDK1 kinase is a G2 checkpoint45. Our results showed that AFB1 caused an increase in CDK2, CDK6, cyclin E, and cyclin B3 mRNA expression and a decrease in cyclin D1, p15, p16, PCNA, and CDK1 mRNA expression as well as PCNA proteins, indicating that AFB1 induced G0G1 phase arrest via ATM-p53-p21-cyclin D/CDK6 route.
Conclusions
In summary, 0.6 mg/kg AFB1 in the diet inhibited development of the chicken’s spleen by causing G0G1 cell cycle arrest. The mRNA expression levels of ATM, p53, p21, and CDK6 were all increased, while the mRNA expression of cyclin D1 was decreased, suggesting that cyclin D1 mRNA expression may play an important role during G0G1 cell cycle arrest of splenocytes induced by AFB1. The results and above mention discussion suggest that G0G1 phase arrest of splenocytes could be induced via activation ATM-p53-p21-cyclin D/CDK6 route, and the proposed mechanisms are shown in Fig. 6.
Fig. 6.
Schematic diagram of the proposed the molecular mechanism of AFB1 arrest cell cycle in chicken spleen.
Disclosure of Potential Conflicts of Interest
The authors declare that they have no conflict of interest.
Acknowledgments
This work was supported by the program for Changjiang scholars, the University Innovative Research Team (IRT 0848), the Education Department of Sichuan Province (2012FZ0066 and 2013FZ0072).
References
- 1.Bryden WL. Mycotoxins in the food chain: human health implications. Asia Pac J Clin Nutr. 16(Suppl 1): 95–101. 2007. [PubMed] [Google Scholar]
- 2.Kalpana S, Aggarwal M, Srinivasa Rao G, and Malik JK. Effects of aflatoxin B1 on tissue residues of enrofloxacin and its metabolite ciprofloxacin in broiler chickens. Environ Toxicol Pharmacol. 33: 121–126. 2012. [DOI] [PubMed] [Google Scholar]
- 3.Scott TR, Rowland SM, Rodgers RS, and Bodine AB. Genetic selection for aflatoxin B1 resistance influences chicken T-cell and thymocyte proliferation. Dev Comp Immunol. 15: 383–391. 1991. [DOI] [PubMed] [Google Scholar]
- 4.Denissenko MF, Cahill J, Koudriakova TB, Gerber N, and Pfeifer GP. Quantitation and mapping of aflatoxin B1-induced DNA damage in genomic DNA using aflatoxin B1-8,9-epoxide and microsomal activation systems. Mutat Res. 425: 205–211. 1999. [DOI] [PubMed] [Google Scholar]
- 5.Aguilar F, Hussain SP, and Cerutti P. Aflatoxin B1 induces the transversion of G-->T in codon 249 of the p53 tumor suppressor gene in human hepatocytes. Proc Natl Acad Sci USA. 90: 8586–8590. 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang M, Peng X, Fang J, Cui H, Yu Z, and Chen Z. Effects of aflatoxin b1 on T-cell subsets and mRNA expression of cytokines in the intestine of broilers. Int J Mol Sci. 16: 6945–6959. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He Y, Fang J, Peng X, Cui H, Zuo Z, Deng J, Chen Z, Lai W, Shu G, and Tang L. Effects of sodium selenite on aflatoxin B1-induced decrease of ileac T cell and the mRNA contents of IL-2, IL-6, and TNF-α in broilers. Biol Trace Elem Res. 159: 167–173. 2014. [DOI] [PubMed] [Google Scholar]
- 8.Zhang S, Peng X, Fang J, Cui H, Zuo Z, and Chen Z. Effects of aflatoxin B1 exposure and sodium selenite supplementation on the histology, cell proliferation, and cell cycle of jejunum in broilers. Biol Trace Elem Res. 160: 32–40. 2014. [DOI] [PubMed] [Google Scholar]
- 9.Liu C, Jiang M, Fang J, Peng X, and Cui H. Inhibitory effects of dietary aflatoxin B1 on cytokines expression and T-cell subsets in the cecal tonsil of broiler chickens. Span J Agric Res. 14: e05SC03 2016. [Google Scholar]
- 10.Chen K, Shu G, Peng X, Fang J, Cui H, Chen J, Wang F, Chen Z, Zuo Z, Deng J, Geng Y, and Lai W. Protective role of sodium selenite on histopathological lesions, decreased T-cell subsets and increased apoptosis of thymus in broilers intoxicated with aflatoxin B1. Food Chem Toxicol. 59: 446–454. 2013. [DOI] [PubMed] [Google Scholar]
- 11.Peng X, Bai S, Ding X, Zeng Q, Zhang K, and Fang J. Pathological changes in the immune organs of broiler chickens fed on corn naturally contaminated with aflatoxins B1 and B2. Avian Pathol. 44: 192–199. 2015. [DOI] [PubMed] [Google Scholar]
- 12.Chen K, Peng X, Fang J, Cui H, Zuo Z, Deng J, Chen Z, Geng Y, Lai W, Tang L, and Yang Q. Effects of dietary selenium on histopathological changes and T cells of spleen in broilers exposed to aflatoxin B1. Int J Environ Res Public Health. 11: 1904–1913. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang F, Shu G, Peng X, Fang J, Chen K, Cui H, Chen Z, Zuo Z, Deng J, Geng Y, and Lai W. Protective effects of sodium selenite against aflatoxin B1-induced oxidative stress and apoptosis in broiler spleen. Int J Environ Res Public Health. 10: 2834–2844. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peng X, Bai S, Ding X, and Zhang K. Pathological Impairment, cell cycle arrest and apoptosis of thymus and bursa of fabricius induced by aflatoxin-contaminated corn in broilers. Int J Environ Res Public Health. 14: 77–88. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liang N, Wang F, Peng X, Fang J, Cui H, Chen Z, Lai W, Zhou Y, and Geng Y. Effect of sodium selenite on pathological changes and renal functions in broilers fed a diet containing aflatoxin B1. Int J Environ Res Public Health. 12: 11196–11208. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campbell ML, Jr., May JD, Huff WE, and Doerr JA. Evaluation of immunity of young broiler chickens during simultaneous aflatoxicosis and ochratoxicosis. Poult Sci. 62: 2138–2144. 1983. [DOI] [PubMed] [Google Scholar]
- 17.Ghosh RC, Chauhan HVS, and Jha GJ. Suppression of cell-mediated immunity by purified aflatoxin B1 in broiler chicks. Vet Immunol Immunopathol. 28: 165–172. 1991. [DOI] [PubMed] [Google Scholar]
- 18.Bahari A, Mehrzad J, Mahmoudi M, Bassami MR, and Dehghani H. Cytochrome P450 isoforms are differently up-regulated in aflatoxin B1-exposed human lymphocytes and monocytes. Immunopharmacol Immunotoxicol. 36: 1–10. 2014. [DOI] [PubMed] [Google Scholar]
- 19.Bbosa GS, Kitya D, Lubega A, Ogwal-Okeng J, Anokbonggo WW, and Kyegombe DB. Review of the biological and health effects of aflatoxins on body organs and body systems. In: Aflatoxins-Recent Advances and Future Prospects. M Razzaghi-Abyaneh (ed). IntechOpen, London. 239–265, 2013. [Google Scholar]
- 20.Yin H, Jiang M, Peng X, Cui H, Zhou Y, He M, Zuo Z, Ouyang P, Fan J, and Fang J. The molecular mechanism of G2/M cell cycle arrest induced by AFB1 in the jejunum. Oncotarget. 7: 35592–35606. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu Z, Wang F, Liang N, Wang C, Peng X, Fang J, Cui H, Jameel Mughal M, and Lai W. Effect of selenium supplementation on apoptosis and cell cycle blockage of renal cells in broilers fed a diet containing aflatoxin B1. Biol Trace Elem Res. 168: 242–251. 2015. [DOI] [PubMed] [Google Scholar]
- 22.Ricordy R, Gensabella G, Cacci E, and Augusti-Tocco G. Impairment of cell cycle progression by aflatoxin B1 in human cell lines. Mutagenesis. 17: 241–249. 2002. [DOI] [PubMed] [Google Scholar]
- 23.Zhang YJ, Rossner P, Jr , Chen Y, Agrawal M, Wang Q, Wang L, Ahsan H, Yu MW, Lee PH, and Santella RM. Aflatoxin B1 and polycyclic aromatic hydrocarbon adducts, p53 mutations and p16 methylation in liver tissue and plasma of hepatocellular carcinoma patients. Int J Cancer. 119: 985–991. 2006. [DOI] [PubMed] [Google Scholar]
- 24.Guan KL, Jenkins CW, Li Y, O’Keefe CL, Noh S, Wu X, Zariwala M, Matera AG, and Xiong Y. Isolation and characterization of p19INK4d, a p16-related inhibitor specific to CDK6 and CDK4. Mol Biol Cell. 7: 57–70. 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xue KX. [Molecular genetic and epigenetic mechanisms of hepatocarcinogenesis]. Chin J Cancer. 24: 757–768. 2005. (in Chinese) [PubMed] [Google Scholar]
- 26.Zychowski KE, Hoffmann AR, Ly HJ, Pohlenz C, Buentello A, Romoser A, Gatlin DM, and Phillips TD. The effect of aflatoxin-B1 on red drum (Sciaenops ocellatus) and assessment of dietary supplementation of NovaSil for the prevention of aflatoxicosis. Toxins (Basel). 5: 1555–1573. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bressac B, Puisieux A, Kew M, Volkmann M, Bozcall S, Mura JB, de la Monte S, Carlson R, Blum M, Wands J, Takahashi H, Weizsacker F, Galun E, Kar S, Carr B, Schroder C, Erken E, Varinli S, Rustgi V, Prat J, Toda G, Koch H, Liang X, Tang Z, Shouval D, Lee H, Vyas G, Sarosi I, and Ozturk D. p53 mutation in hepatocellular carcinoma after aflatoxin exposure. Lancet. 338: 1356–1359. 1991. [DOI] [PubMed] [Google Scholar]
- 28.Hollstein MC, Wild CP, Bleicher F, Chutimataewin S, Harris CC, Srivatanakul P, and Montesano R. p53 mutations and aflatoxin B1 exposure in hepatocellular carcinoma patients from Thailand. Int J Cancer. 53: 51–55. 1993. [DOI] [PubMed] [Google Scholar]
- 29.Mebius RE, and Kraal G. Structure and function of the spleen. Nat Rev Immunol. 5: 606–616. 2005. [DOI] [PubMed] [Google Scholar]
- 30.Sabourin PJ, Price JA, Casbohm SL, Perry MR, Tuttle RS, Rogers JV, Rowell KS, Estep JE, and Sabourin CL. Evaluation of acute immunotoxicity of aerosolized aflatoxin bin female C57BL/6N mice. J Immunotoxicol. 3: 11–20. 2006. [DOI] [PubMed] [Google Scholar]
- 31.Hinton DM, Myers MJ, Raybourne RA, Francke-Carroll S, Sotomayor RE, Shaddock J, Warbritton A, and Chou MW. Immunotoxicity of aflatoxin B1 in rats: effects on lymphocytes and the inflammatory response in a chronic intermittent dosing study. Toxicol Sci. 73: 362–377. 2003. [DOI] [PubMed] [Google Scholar]
- 32.Mary VS, Theumer MG, Arias SL, and Rubinstein HR. Reactive oxygen species sources and biomolecular oxidative damage induced by aflatoxin B1 and fumonisin B1 in rat spleen mononuclear cells. Toxicology. 302: 299–307. 2012. [DOI] [PubMed] [Google Scholar]
- 33.Theumer MG, López AG, Masih DT, Chulze SN, and Rubinstein HR. Immunobiological effects of AFB1 and AFB1-FB1 mixture in experimental subchronic mycotoxicoses in rats. Toxicology. 186: 159–170. 2003. [DOI] [PubMed] [Google Scholar]
- 34.Peng X, Zhang K, Bai S, Ding X, Zeng Q, Yang J, Fang J, and Chen K. Histological lesions, cell cycle arrest, apoptosis and T cell subsets changes of spleen in chicken fed aflatoxin-contaminated corn. Int J Environ Res Public Health. 11: 8567–8580. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dale N. National research council nutrient requirements of poultry - ninth revised edition. J Appl Poult Res. 3: 101 1994. [Google Scholar]
- 36.Kaoud AH. Innovative methods for the amelioration of aflatoxin (AFB1) effect in broiler chicks. Sci J Appl Res. 1: 16–21. 2012. [Google Scholar]
- 37.Fang J, Cui H, Peng X, Chen Z, He M, and Tang L. Developmental changes in cell proliferation and apoptosis in the normal duck thymus. Anat Histol Embryol. 40: 457–465. 2011. [DOI] [PubMed] [Google Scholar]
- 38.Wu B, Cui H, Peng X, Fang J, Zuo Z, Deng J, and Huang J. Dietary nickel chloride induces oxidative stress, apoptosis and alters Bax/Bcl-2 and caspase-3 mRNA expression in the cecal tonsil of broilers. Food Chem Toxicol. 63: 18–29. 2014. [DOI] [PubMed] [Google Scholar]
- 39.Livak KJ, and Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔC(T) Method. Methods. 25: 402–408. 2001. [DOI] [PubMed] [Google Scholar]
- 40.Quist CF, Bounous DI, Kilburn JV, Nettles VF, and Wyatt RD. The effect of dietary aflatoxin on wild turkey poults. J Wildl Dis. 36: 436–444. 2000. [DOI] [PubMed] [Google Scholar]
- 41.Guo SN, Liao SQ, Su RS, Lin RQ, Chen YZ, Tang ZX, Wu H, and Shi DY. Influence of longdan xiegan decoction on body weights and immune organ indexes in ducklings intoxicated with aflatoxin B1. J Anim Vet Adv. 11: 1162–1165. 2012. [Google Scholar]
- 42.Lee JH, and Paull TT. ATM activation by DNA double-strand breaks through the Mre11-Rad50-Nbs1 complex. Science. 308: 551–554. 2005. [DOI] [PubMed] [Google Scholar]
- 43.Harada K, and Ogden GR. An overview of the cell cycle arrest protein, p21WAF1. Oral Oncol. 36: 3–7. 2000. [DOI] [PubMed] [Google Scholar]
- 44.Vogelstein B, Lane D, and Levine AJ. Surfing the p53 network. Nature. 408: 307–310. 2000. [DOI] [PubMed] [Google Scholar]
- 45.Kastan MB, and Bartek J. Cell-cycle checkpoints and cancer. Nature. 432: 316–323. 2004. [DOI] [PubMed] [Google Scholar]
- 46.Reynisdóttir I, and Massagué J. The subcellular locations of p15(Ink4b) and p27(Kip1) coordinate their inhibitory interactions with cdk4 and cdk2. Genes Dev. 11: 492–503. 1997. [DOI] [PubMed] [Google Scholar]
- 47.Maddika S, Ande SR, Panigrahi S, Paranjothy T, Weglarczyk K, Zuse A, Eshraghi M, Manda KD, Wiechec E, and Los M. Cell survival, cell death and cell cycle pathways are interconnected: implications for cancer therapy. Drug Resist Updat. 10: 13–29. 2007. [DOI] [PubMed] [Google Scholar]