Abstract
The placenta plays a pivotal role in fetal growth, and placental dysfunction and injury are associated with embryo/fetal toxicity. Histological examination of the rat placenta for safety evaluation provides valuable clues to the mechanisms of this toxicity. However, the placenta has specific and complex biological features unlike those of other organs, and placental structure dramatically changes depending on the time during the gestation period. Thus, time-dependent histopathological examination of the rat placenta should be performed based on the understanding of normal developmental changes in morphology and function. The placentas of rats and humans are both anatomically classified as discoid and hemochorial types. However, there are differences between rats and humans in terms of placental histological structure, the fetal-maternal interface, and the function of the yolk sac. Therefore, extrapolation of placental toxicity from rats to humans should be done cautiously in the evaluation of risk factors. This review describes the development, morphology, physiology, and toxicological features of the rat placenta and the differences between the rat and human placenta to enable accurate evaluation of reproductive and developmental toxicity in studies.
Keywords: histopathology, human, placenta, rat, reproduction
Introduction
The placenta plays a pivotal role in fetal growth even though it is a temporary organ during pregnancy. It is the interface between the dam and developing embryo/fetus and is a multifunctional organ that serves as the liver, lung, gut, kidney, and endocrine/exocrine glands. Its functions include anchoring the developing fetus to the uterine wall, mediating maternal immune tolerance, hormone production, nutrient uptake, waste elimination, and gas exchange via the maternal blood supply during embryonic/fetal development1. Furthermore, the placenta serves as a protective barrier that protects the embryo/fetus against chemical injury. Placental dysfunction and injury have adverse effects on the maintenance of pregnancy and fetal development2. Detection of chemically induced placental damage in rats provides a valuable clue to the mechanisms of embryo/fetal toxicity in safety evaluation. Therefore, the placenta is an important organ for evaluating reproductive and developmental toxicity3.
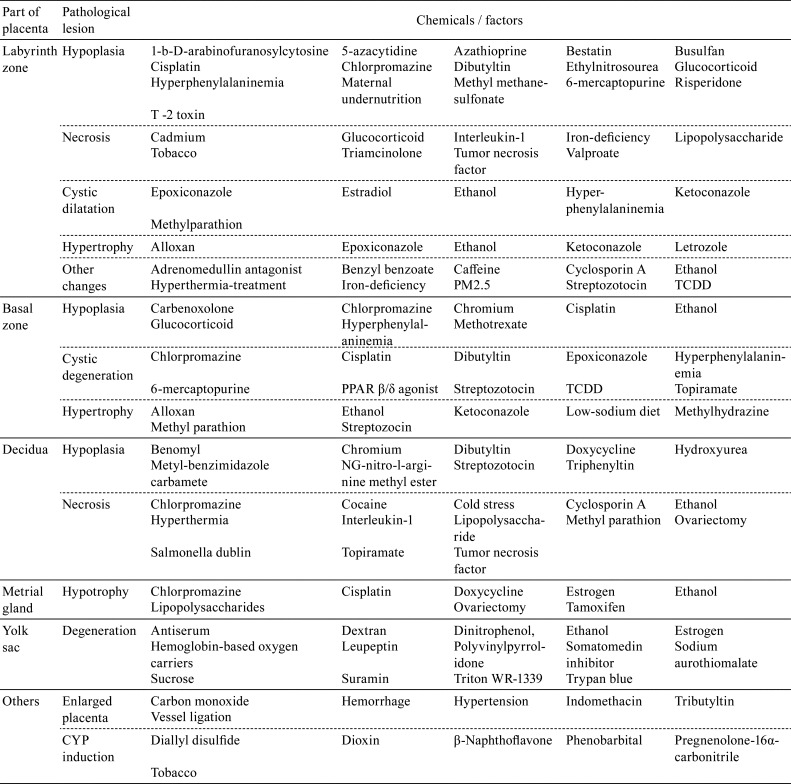
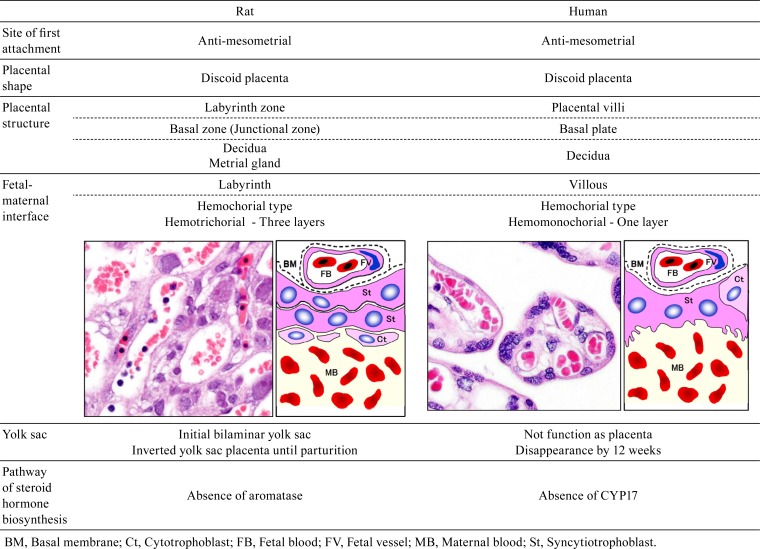
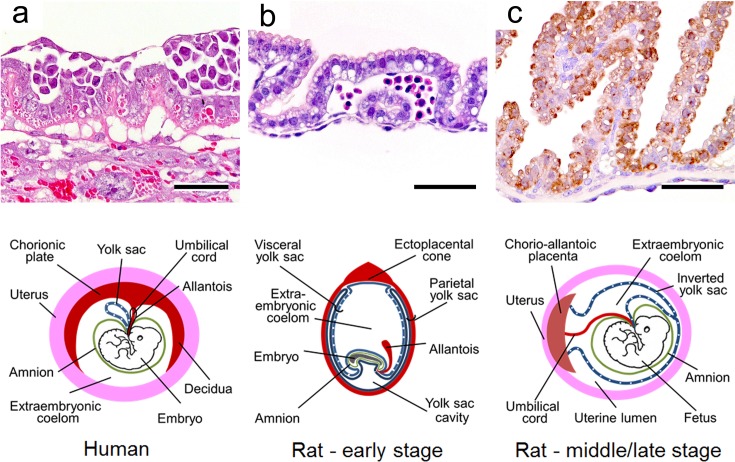
The large placental surface area comes in contact with a relatively large volume of maternal blood4. The placenta, which is rich in protein, may bioconcentrate chemical residues by means of protein binding and release these residues into the placental circulation. Due to these biological features, the placenta is vulnerable to toxicants, and placental toxicity has been reported for many chemicals and other factors (Table 1). However, the placenta has not received proper consideration as a target organ in safety evaluation of risks for dams and embryos/fetuses, because the placenta has the following complex biological features: a) a complicated structure composed of multiple tissues5, b) drastic changes in placental structure and function over time due to rapid development, and c) wide variations of placental structure among different animal species6. Additionally, the placentas of both rats and humans are anatomically classified as discoid and hemochorial types. However, there are differences between rats and humans in terms of the placental histological structure, the fetal-maternal interface, and the function of the yolk sac (Table 2). Therefore, extrapolation of placental toxicity from rats to humans should be done cautiously in the evaluation of risk factors. This review describes the development, morphology, physiology, and toxicological features of the rat placenta and the species-based differences between the rat and human placenta to enable accurate evaluation of the effect of reproductive and developmental toxicity in studies.
Table 1. Chemicals Toxic to the Placenta.
Table 2. Morphological Differences Between the Rat and Human Placenta.
Normal Development of the Rat Placenta
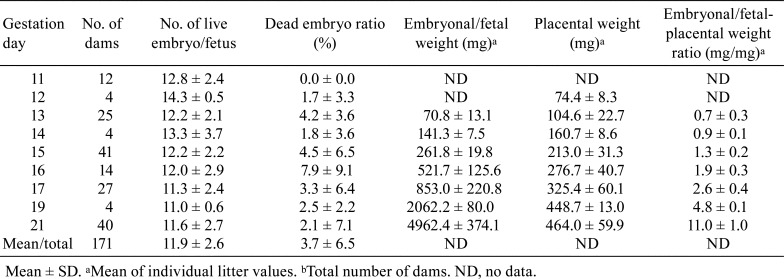
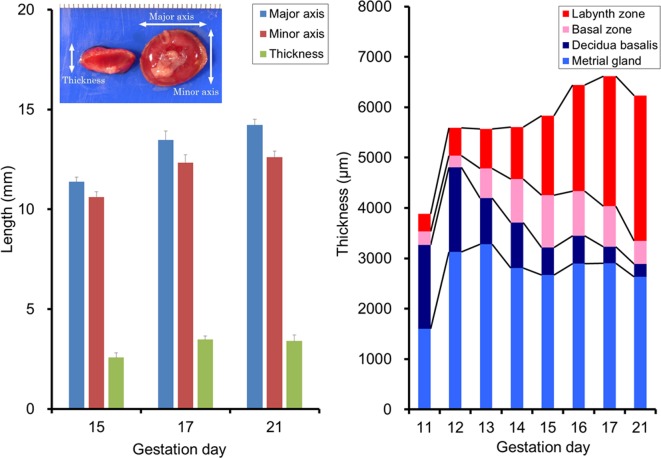
Table 3 shows the reproductive data (embryonal/fetal weight, placental weight, embryonal/fetal placental weight ratio, etc.) of 171 dams from gestation day (GD) 11 (GD 0 is designated as the day when the presence of a vaginal plug is identified) to GD 21 in control groups from our previous studies using Wistar Hannover rats. The placental weights gradually increase with pregnancy progression and reach a plateau on GD 19, whereas the fetal weights rapidly increase from GD 17 to GD 217, 8. The placental weight is approximately equal to the fetal weight on GD 15 and declines to one-fourth on GD 17 and one-tenth on GD 21. Figure 1 shows the time-dependent macroscopic changes in placental diameter in Crl:CD (SD) rats. The minor axis and thickness reach a plateau on GD 17, and the major axis gradually increases until GD 21.
Table 3. Reproductive Data of Wistar Han Rats During Pregnancy.
Fig. 1.
Time-dependent changes in diameter and thickness of each part of the placenta in rats. Left, placental diameter on GD 15, 17, and 21 in Crl:CD(SD) rats. Error bar represents SD. Right, thickness of each part of the placenta from GD 11 to GD 21 in Wistar Han rats.
Embryology, Morphology, and Physiology of the Rat Placenta
The rat placenta is histologically divided into a fetal part and a maternal part5. The fetal part is composed of the labyrinth zone, basal zone (also referred to as the junctional zone), and yolk sac. The maternal part is composed of the decidua and metrial gland. Figure 1 and 2
Fig. 2.
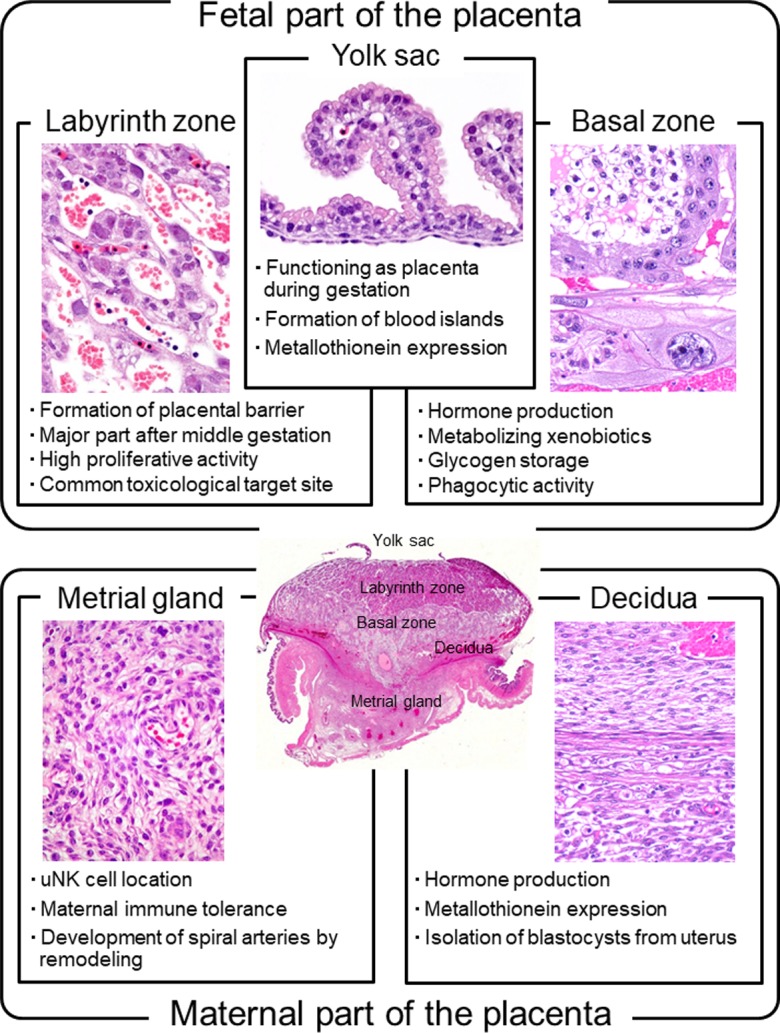
Biological features of each part of the placenta in rats.
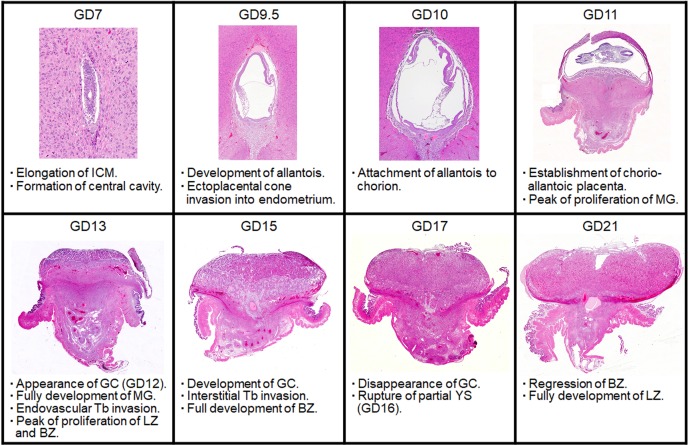
show the respective time-dependent thickness and biological features of each part of the placenta. Developmentally, the trophectoderm, which differentiates into the placenta, consists of the mural trophectoderm and polar trophectoderm. The mural trophectoderm surrounding the blastocyst cavity arrests cell division and differentiates into primary trophoblastic giant cells just after implantation. The polar trophectoderm forms the ectoplacental cone and invades the decidua. The edge and center of the ectoplacental cone differentiate into the basal zone (Fig. 3)9. The chorionic plate, which is the membrane of the ectoplacental cone on the embryonic side, differentiates into the labyrinth zone. In the endometrium, the decidua is derived from endometrial stromal cells by stimulation of decidualization. The decidua rapidly grows and fills the uterus. Primary decidualization takes place on the anti-mesometrial side in response to implantation of the blastocyst. This is followed by secondary decidualization on the mesometrial side. The metrial gland is composed of nodular aggregates of heterogeneous tissue that develop in the mesometrial triangle in the uterine wall9. Figure 4 shows the process of overall morphological development of the placenta with the events in each stage from GD 7 to GD 21.
Fig. 3.
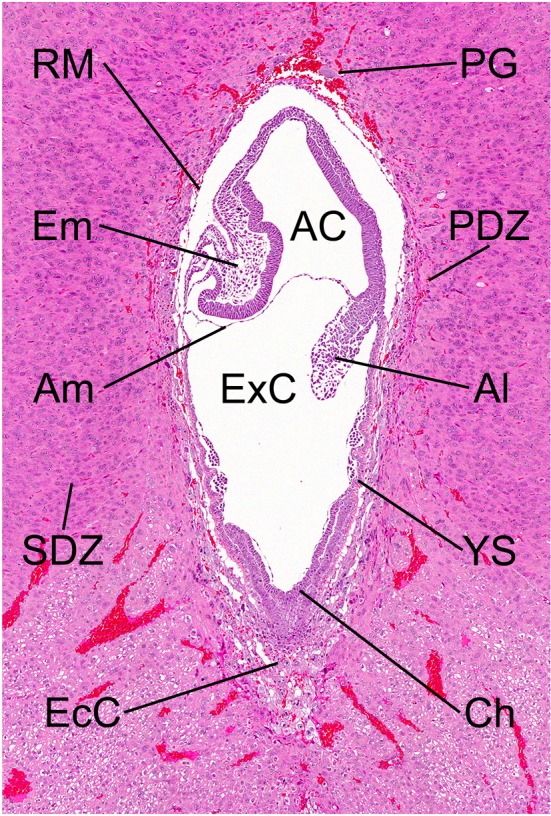
Morphology of the embryo on GD 9.5. HE stain. AC, amniotic cavity; Al, allantois; Am, amnion; Ch, chorionic plate; EcC, ectoplacental cone; Em, embryo; ExC, exocoelomic cavity; PDZ, primary decidual zone; PG, primary trophoblastic giant cell; RM, Reichert’s membrane; SDZ, secondary decidual zone; YS, yolk sac.
Fig. 4.
Morphological development of the rat placenta and histological events at each point. BZ, Basal zone; GC, Glycogen cell; ICM, Inner cell mass; LZ, Labyrinth zone; MG, Metrial gland; Tb, Trophoblast; YS, Yolk sac. HE stain.
1) Fetal part of the placenta
(1) Labyrinth zone
• Morphology
The labyrinth zone rests atop the flat, broad chorionic plate and is a thick vascular plate that serves as the terminus for the umbilical cord10. It is composed of the maternal sinusoids, trophoblastic septa, and fetal capillaries. In the trophoblastic septa, the outer trophectoderm, which comes into direct contact with the maternal blood, is referred to as the cytotrophoblast with a microvillous surface. Under this trophectoderm, there are two layers of syncytiotrophoblasts (syncytiotrophoblast I and syncytiotrophoblast II from the maternal sinusoid side) (Fig. 5 and 6). The continuity of the syncytiotrophoblast layer provides a placental barrier11. Connexin 26 is localized in the gap junctions connecting the two syncytiotrophoblast layers and allows these layers to act functionally as a single syncytial layer for the transfer of small molecules across the placental barrier12 (Fig. 5). The maternal sinusoids filled with blood cells are formed amid these cells. Developmentally, the trophectoderm layer appears on GD 10 and is occupied by syncytiotrophoblasts and cytotrophoblasts13. There is a decrease in the cellular density in the trophoblastic septa and an increase in the size of the cytotrophoblast with pregnancy progression (Fig. 6). The cellular proliferative activity of the trophoblasts is at its peak on GD 13 and then gradually decreases until GD 2114 (Fig. 7). In response to this, the labyrinth zone develops with advancing pregnancy and forms the majority of the fetal part of the placenta (Fig. 1).
Fig. 5.
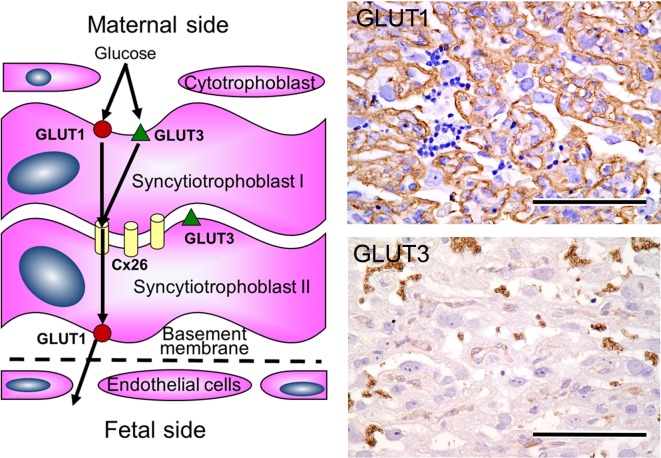
Expression of GLUT1 and GLUT3 in trophoblastic septa. Left, schema of trophoblastic septa. Pathway of glucose via GLUT1 and GLUT3. Upper right, immunohistochemical expression of GLUT1. Bar=100 µm. Lower right, immunohistochemical expression of GLUT3. Bar=100 µm.
Fig. 6.
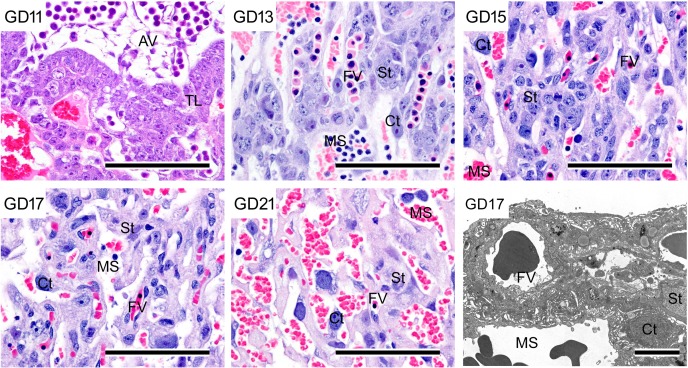
Morphological development and ultrastructure of trophoblastic septa in the labyrinth zone. Decrease in cellular density in trophoblastic septa and increase in size of cytotrophoblasts with pregnancy progression; HE stain. Bar=100 µm. Lower right, ultrastructure of trophoblastic septa. Bar=15 µm. AV, allantoic vessel; Ct, cytotrophoblast; FV, fetal vessel; MS, maternal sinusoid; St, syncytiotrophoblast; TL, trophectoderm layers.
Fig. 7.
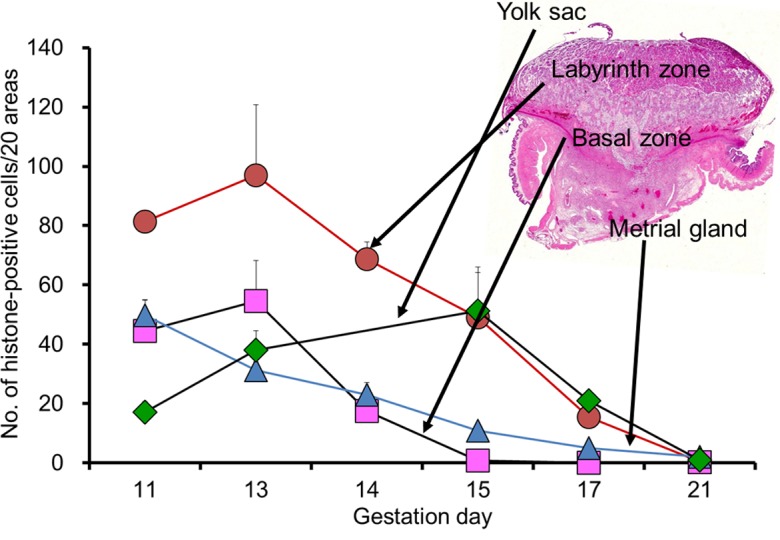
Cellular proliferative activity of each placental layer during pregnancy. Error bar represents SD.
• Physiological function and toxicological features
Syncytiotrophoblasts form a layer that separates the fetal circulation from the maternal circulation. These cells express several efflux and influx transporters that serve to control transfer of chemicals between the fetus and dam. In order to regulate the transfer process of chemicals, the plasma membranes are equipped with various transporters between the maternal and fetal side: multidrug resistance protein (Mdr)1a, Mdr1b, multidrug resistance-associated protein (Mrp)1, Mrp5, organic cation transporter (Oct)3, organic cation transporter novel (Octn)1, organic anion-transporting polypeptide (Oatp)3, Oatp12, four metal transporters (ZnT1, divalent metal transporter 1, Menkes, and Wilson), a prostaglandin, ATP-binding cassette sub-family G member (AbcG)8, equilibrative nucleoside transporter (ENT)1, and ENT212. In addition, glucose is the main fuel for growth and energy metabolism of fetoplacental tissues, and the transplacental movement of glucose is a stereospecific saturable and carrier-mediated process of facilitated diffusion15. The expression of two maternal-to-fetal glucose transporter isoforms has been demonstrated in rats, namely GLUT1 and GLUT3 on the trophoblastic septa16, 17. GLUT 1 is localized in the membrane of syncytiotrophoblast layer I facing the maternal blood side and is present in the membrane of syncytiotrophoblast II facing the fetal capillaries (Fig. 5). In contrast, GLUT3 is localized in the membrane of syncytiotrophoblast layer I facing the maternal blood side and is present in between syncytiotrophoblast layers I and II. The asymmetric distribution of GLUT3 across the placental barrier may suggest asymmetric transfer of glucose, which would be beneficial to provision of a stable milieu for fetal development while also preventing the loss of glucose at times of maternal hypoglycemia. The glucose transport capacity develops primarily due to an increase in the total number of glucose transporters found on the membranes of the trophoblasts. In addition, the expression of glucose transporters has been found to be increased as an adaptive change in small placentas18. Cytotrophoblasts and fetal endocapillary cells are nonfunctional for a placental barrier, since they are fenestrated. The cytotrophoblasts function by slowing the maternal blood flow and forming local regions of blood stasis behind the fenestrations that facilitate fetomaternal transport19.
The labyrinth zone plays a role in O2/CO2 exchange, providing nutrients for the fetus and removing waste products. Labyrinth zone damage has a high correlation with intrauterine growth retardation (IUGR)14. The labyrinth zone is vulnerable to the target site in placental toxicity because of high blood flow, high cellular proliferative activity, and long proliferation period as compared with other parts of the placenta. It has been reported that antiproliferative or apoptotic agents result in a small placenta and IUGR due to labyrinth zone hypoplasia20, 21, 22 (Table 1). Conversely, estrogen is an inhibitor of placental growth, and estrogen deficiency induces placental hypertrophy23, 24. The labyrinth zone is a hormone-dependent tissue, and aromatase inhibitors induce labyrinth zone hypertrophy, which is associated with an increase in mitosis of trophoblasts and the cystic dilatation of maternal sinuses due to the antiestrogenic effect25, 26.
(2) Basal zone
• Morphology
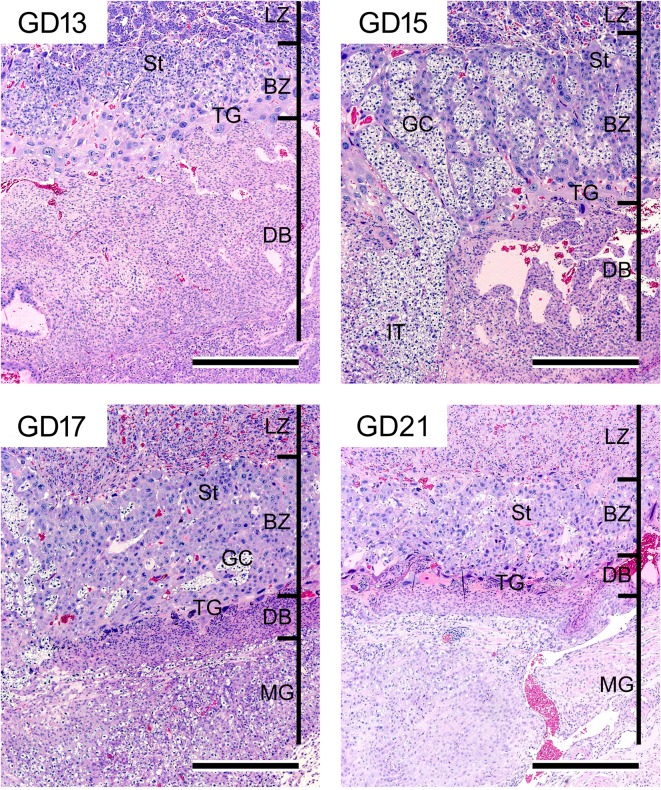
The basal zone forms just below the labyrinth zone and is composed of spongiotrophoblasts, glycogen cells, and (secondary) trophoblastic giant cells27. Developmentally, there are two types of trophoblastic giant cells. The primary trophoblastic giant cells derived from the mural trophectoderm are present outside Reichert’s membrane28 (Fig. 3). These cells are of paramount importance for successful implantation but are not components of the basal zone. The secondary trophoblastic giant cells (hereinafter referred to as trophoblastic giant cells) are formed from the edge of the trophoblastic ectoplacental cone29. These cells are large polyploid cells with a characteristically enormous, deeply divided nucleus. They provide the first surface layer for the ectoplacental cone on GD 10 and are located between the spongiotrophoblast layer and decidua basalis. The spongiotrophoblasts are derived from the outer layers of the ectoplacental cone on GD 10 and are located immediately above the trophoblastic giant cell layer. They are a main structural component of the basal zone. The glycogen cells, which accumulate glycogen-rich granules, transiently appear on GD 12 and form small clusters on GD 14. They develop into glycogen cell islands and comprise a large part of the basal zone on GD 15 and 16. They exhibit pyknosis after GD 17 with basal zone regression, and then almost all glycogen cells disappear on GD 21 (Fig. 8). The glycogen cells are reportedly derived from the spongiotrophoblasts. However, the same evidence suggests that glycogen cells are distinct from spongiotrophoblasts30. The cellular proliferative activity in the basal zone is at its peak on GD 13 and then nearly disappears until GD 15 (Fig. 7). In response to this, the basal zone fully develops on GD 15, and this gradually leads to regression before parturition (Fig. 8).
Fig. 8.
Morphology of the basal zone and decidua basalis from GD 13 to GD 21. Regression of the decidua basalis from GD 13. Full development of the basal zone with glycogen islet formation on GD 15 and then gradual regression. Penetration of interstitial trophoblasts through the decidua basalis into the metrial gland from GD 15. HE stain. Bar=500 µm. BZ, basal zone; DB, decidua basalis; GC, glycogen cell; IT, interstitial trophoblast; LZ, labyrinth zone; MG, metrial gland; St, spongiotrophoblast; TG, trophoblastic giant cell.
• Physiological function and toxicological features
The basal zone is a site of production of steroids and peptide hormones, which play an important role in the maintenance of pregnancy31. The peak placental progesterone production is on GD 11, and testosterone production reaches its maximum level on GD 1732. Synthesis of progesterone and testosterone falls precipitously from GD 17 onward, and this change reflects the reduced functional importance of the basal zone in association with the higher level of apoptosis in late pregnancy33. In ovariectomized rats treated with estrogen and progesterone, overgrowth of the basal zone is induced by a response to the hormonal imbalance34. The trophoblastic giant cells, spongiotrophoblasts, and invasive trophoblasts are capable of expressing components of the prolactin family35, 36. The spongiotrophoblasts, in particular, are a major source of them.
The basal zone plays an important functional role in metabolism of chemicals37. CYP3A1, a major component of the CYP system during pregnancy, is mainly located in trophoblastic giant cells. The expression of CYP3A1 is induced by diallyl disulfide38, pregnenolone 16-alpha-carbonitrile39, and phenobarbital40. In addition, CYP17 (17-alpha-hydroxylase/C17,20-lyase) is detected during the second half of pregnancy41, and is located in the spongiotrophoblasts and trophoblastic giant cells in the basal zone and in trophoblasts in the labyrinth zone.
The glycogen cells are the storage site for glycogen that is produced from maternal glucose42. This glycogen is suggested to be an important nutrient for the fetus. It is known that glycogen cells are increased in the basal zone with the expression of hypoxia in rats given a low-sodium diet43. The disappearance of glycogen cell islands is thought to meet the increased demand for glycogen as an energy substrate for the final period of fetal growth44, 45. Cystic degeneration describes abnormal retention of extensive cytoplasmic vacuolation within glycogen cells. It is induced by remaining glycogen cell islands that should regress and disappear at the end of pregnancy, as a result of placental developmental delay.
The trophoblastic giant cells have phagocytic activity, which mediates the implantation and invasion of the conceptus into the endometrial stroma28. This expression tends to decrease with advancing pregnancy, and the phagocytosis in the final stages of pregnancy reportedly is quite reduced46. The trophoblastic giant cells also secrete several factors that control placental growth and maternal reactions to pregnancy. In addition, they are immunologically specialized, and express none of the major histocompatibility complex determinants on their plasma membranes. It is thought that the trophoblastic giant cells play the role of an immunologically neutral buffer zone at the interface between fetal and maternal tissues. As described above, the basal zone consists of 3 different kinds of tissues that develop different functions and regress at different times. Thus, chemically induced lesions in the basal zone largely tend to differ depending on the chemical administration period as compared with other parts of the placenta.
(3) Yolk sac
• Morphological structure
The yolk sac is an extraembryonic membrane surrounding the embryo and is composed of epithelial and mesodermal cells. Developmentally, the yolk sac is derived from embryonic endoderm and mesoderm. It becomes enlarged and surrounds the entire embryo. It is divided into the visceral yolk sac surrounding the embryo with the amnion and the parietal yolk sac attached to the rim of the chorionic plate (Fig. 3, 9). The parietal yolk sac is lined with Reichert’s membrane between the endoderm and trophoblasts47. Reichert’s membrane is a rodent-specific and acellular thin membrane.
In early development, the blood islands are formed on the surface of the visceral yolk sac and are the first site of embryonic hematopoiesis48. They subsequently develop into the yolk sac circulation on GD 10. The inside of the visceral yolk sac becomes exposed to the intrauterine cavity and becomes an inverted yolk sac placenta when the parietal yolk sac ruptures on GD 1649 (Fig. 9). The cellular proliferative activity of the yolk sac is at its peak on GD 15 and then gradually decreases until GD 21 (Fig. 7).
Fig. 9.
Morphology of the parietal and visceral yolk sac in rats and morphological differences in the yolk sac between rats and humans. a. Parietal yolk sac on GD 9.5 in rats. HE stain. Bar = 50 µm. b. Visceral yolk sac on GD 9.5 in rats. HE stain. Bar = 50 μm. c. Metallothionein expression in the visceral yolk sac on GD 17 in rats. Bar = 50 µm. Lower schema. Morphological differences of yolk sac between humans and rats (early stage and middle/late stage).
• Physiological function and toxicological features
During an early stage, the visceral and parietal yolk sacs play a role as a transient placenta that performs endocytosis of nutrient materials50. This yolk sac placenta is also involved with endocrine, metabolic, immunologic, secretory, excretory, and hematopoietic functions51. It is the only route for major transport between dam and embryo until establishment of the chorioallantoic placenta on GD 11.549. Furthermore, the visceral yolk sac even maintains placental functions, as the inverted yolk sac placenta, until just before parturition5. The inverted yolk sac placenta is a specific biological structure in rodents and rabbits. Thus, impaired structural and functional development of the yolk sac contributes to embryo/fetal toxicity and teratogenicity in rats52, 53. In addition, the presence of metallothionein in the yolk sac protects the fetus from heavy metals. Expression of metallothionein is detected in epithelial cytoplasm from GD 9. It gradually increases until GD 19 and then slightly decreases on GD 2154 (Fig. 9).
2) Maternal part of the placenta
(1) Decidua
• Morphology
The decidua is derived from the endometrial stroma and is divided into the decidua capsularis (anti-mesometrial side), decidua parietalis (lateral side), and decidua basalis (mesometrial side) (Fig. 10). Developmentally, the decidual cells surrounding the blastocyst initially form the primary decidual zone. Subsequently, the more loosely packed decidual cells around the primary decidual zone form the secondary decidual zone55 (Fig. 3). The primary decidual zone degenerates progressively, and placental and embryonic growth slowly replaces the secondary decidual zone, which develops into a thin layer comprised of the decidua capsularis and decidua parietalis. The decidua capsularis consistently regresses from GD 8 onward and completely ruptures by GD 17-18. Rupture of the decidua capsularis exposes the inverted yolk sac placenta to the reformed uterine epithelium, which is completely restored by GD 1556. The decidua basalis is located at the base of the placenta and is an important site for maternal angiogenesis. Most of the development of the decidua basalis occurs during early pregnancy, and the decidua basalis undergoes regression from GD 11 onward. Therefore, the sensitivity of the decidua to chemicals in the organogenesis period is generally lower than that of other parts of the placenta. It is known that the regression of the decidua is mediated by apoptosis57. As hemorrhage and edema are sometimes observed in the regression process of the decidua, it is necessary to distinguish them from toxicological lesions. Histologically, the decidual cells are of 2 types: large cells with a large round nucleus and smaller cells with a small nucleus and periodic acid-Schiff-positive vacuolated cytoplasm. The large cells disappear on GD 14, but the small cells remain until the end of pregnancy58.
Fig. 10.
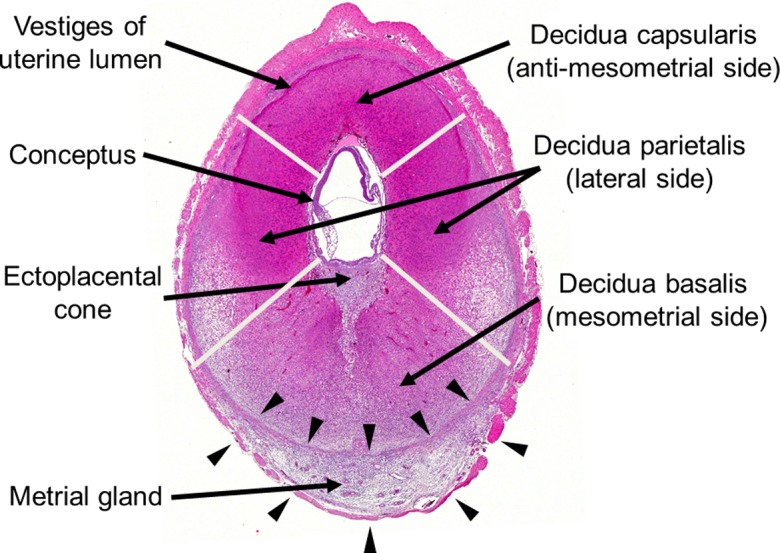
Transverse sections of the decidua and metrial gland on GD 9.5. Division of the decidua into the decidua capsularis, decidua parietalis, and decidua basalis. The metrial gland in the mesometrial triangle with borders on two smooth muscle layers (arrows). HE stain.
• Physiological function and toxicological features
The decidua has a variety of functions: it acts as a physical barrier to isolate the implanting blastocyst from maternal tissue, as a nutrient source as a result of accumulation of glycogen and/or lipid, and as a producer of hormones, such as prolactin, prostaglandin, steroid, relaxin, etc.47. The decidua is a hormone-dependent tissue. Estradiol plays an important role in the growth and differentiation of endometrial stromal cells into decidual cells. In addition, progesterone and prolactin are considered essential for decidual development. Excess estrogen administration in pseudopregnant rats has an inhibitory effect on decidual growth59. A reduction in serum progesterone levels also suppresses the decidual cell response in pseudopregnant rats60. Estrogen receptors (ER)α and ERβ are expressed in the decidua and are differentially regulated by prolactin and steroids61.
The decidua is an important tissue in protection against heavy metals. Metallothionein is present in the decidua from GD 9 onward, increases until GD 15 in tissue surrounding the embryo/fetus53, and then decreases with progression of placental development.
(2) Metrial gland
• Morphology
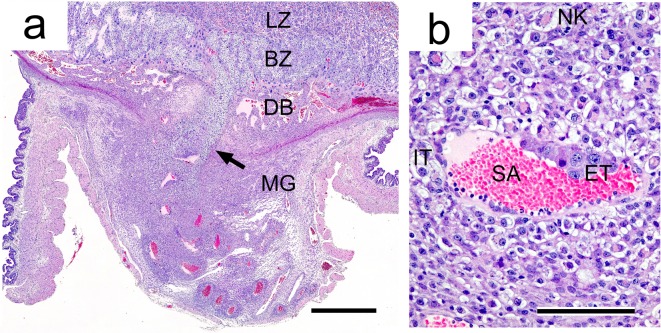
The metrial gland occupies a space in the mesometrial triangle and borders on two smooth muscle layers in the early gestation period. These muscle layers are disrupted as the metrial gland develops62 (Fig. 10, 11). The metrial gland is composed of uterine natural killer (uNK) cells, decidualized endometrial stromal cells, invasive trophoblasts, blood vessels (including spiral arteries), and fibroblasts63. Developmentally, the cellular proliferative activity in the metrial gland is at its peak on GD 11 or 12 and then gradually decreases toward late gestation (Fig. 7). In response, the metrial gland fully develops on GD 13 and comprises the majority of the maternal part of the placenta until parturition8, 64 (Fig. 1).
Two types of trophoblasts from the basal zone are present in the metrial gland65 (Fig. 11). Interstitial trophoblasts penetrate through the decidua basalis and myometrium on GD 15 and are often situated in perivascular locations of the metrial gland11, 44. They are one of the main cell types constituting the metrial gland after invasion. Endovascular trophoblasts invade into the spiral arteries, where they replace endothelial cells from GD 13 or 14 onward66.
Fig. 11.
Morphology of the metrial gland. a. Invasion of interstitial trophoblasts (arrow) into the metrial gland on GD 15. HE stain. Bar=900 µm. b. Invasion of endovascular trophoblasts into spiral arteries and localization of interstitial trophoblasts around spiral arteries in stroma. HE stain. Bar=100 µm. BZ, basal zone; DB, decidua basalis; ET, endovascular trophoblast; IT, interstitial trophoblasts; LZ, labyrinth zone; MG, metrial gland; SA, spiral artery; NK, uNK cell.
• Physiological function and toxicological features
The metrial gland is a unique decidual tissue characterized by the appearance of uNK cells and is rodent specific. Development of the metrial gland is inhibited in connection with the reduced proliferative activity of uNK cells caused by tamoxifen67, estrogen68, and ovariectomy69. In uNK gene knockout mice (TgE26 mice), the mesometrial triangle area does not develop into the metrial gland, and the absence of uNK cells results in a small placenta and poor reproductive performance70. The importance of progesterone in uNK cell differentiation and proliferation has been shown in studies on the effects of ovariectomy with replacement progesterone treatment62, 71. On the other hand, impaired basal zone development leads to metrial gland hypoplasia14, 22, 72, since invasion of interstitial trophoblasts derived from the basal zone is inhibited73. Thus, it is thought that the metrial gland is a hormone-dependent tissue74, and its development is also affected by uNK cell proliferation and basal zone development.
Uterine natural killer cells are the main component of the metrial gland and are also known as granulated metrial gland (GMG) cells in rodents. Decidualized endometrial stromal cells produce signals that trigger recruitment and guide uNK cell differentiation to express a different phenotype from that of circulating NK cells75. The uNK cells play critical roles in maternal immune tolerance at the maternal-fetal interface by facilitating the pairing of fetal leukocyte antigen and maternal killer inhibitory receptors76. In addition, they produce protease that destroys the spiral arterial basement membranes and produce vascular endothelial growth factor that stimulates endothelial cell proliferation in order to promote vascular remodeling and angiogenesis77, 78. Insufficient uNK cell activation contributes to poor spiral arterial remodeling79. Failure of spiral arterial remodeling increases maternal blood pressure80 and reduces subsequent placental perfusion. Therefore, alterations of uNK cell function and inadequate remodeling of spiral arteries in the metrial gland are linked to pregnancy-associated diseases such as preeclampsia, IUGR, and premature pregnancy termination81, 82, 83.
Comparison with Human Placenta
1) Fetal part of the placenta
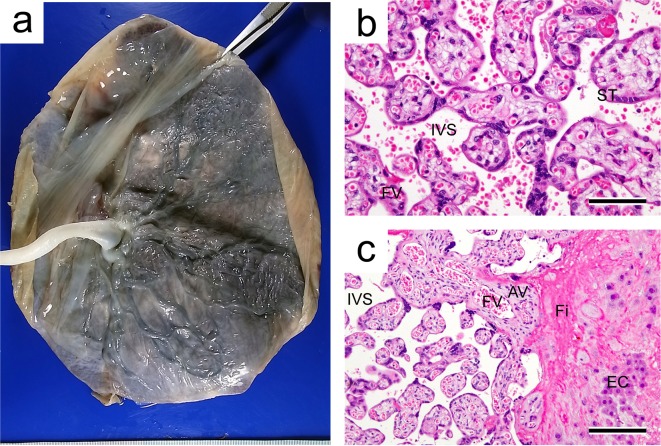
The placental villi that show many tree-like projections in humans are functionally analogous to the labyrinth zone in rats. The placentas of humans and rats are categorized as the hemochorial type, as the trophoblasts of both are directly bathed in maternal blood84 (Fig. 12). The fetal-maternal interface in humans is comprised of a single syncytiotrophoblast layer and single cytotrophoblast layer and is categorized as hemomonochorial. In contrast, the interface in rats is comprised of three layers and is therefore hemotrichorial (Table 2). This difference between rats and humans might affect fetal-maternal exchange processes in the placental barrier85.
Fig. 12.
Human placenta. a. Macrograph of a placenta at term pregnancy. b. Placental villi. HE stain. Bar=75 µm. c. Surface of the basal plate with extravillous cytotrophoblast invasion and fibrinoid deposition. HE stain. Bar=150 µm. AV, anchoring villus; EC, extravillous cytotrophoblast; Fi, fibrinoid; FV, fetal vessel; IVS, intervillous space; St, syncytiotrophoblast.
The basal plate is the bottom of the intervillous space and represents part of the maternal-fetal junction. It is composed of an admixture of extravillous trophoblasts, various endometrial stromal cells, decidual cells, blood vessels (including spiral arteries), and endometrial glands with fibrinoid overlying the myometrium86 (Fig. 12). Multinucleated trophoblastic giant cells are present in the deep basal plate at the myometrial border. The basal plate is roughly analogous to the basal zone in rats, based on to its anatomical location85, but the morphology is not necessarily similar. Therefore, caution is required in extrapolation of toxicological effects on the basal zone from the rat placenta to the human placenta.
The yolk sac in humans does not enlarge and remains as a stalk only. The yolk sac floats like a balloon within the exocoelomic cavity and never becomes directly apposed to the chorionic plate, maternal uterine wall, or uterine lumen87 (Fig. 9). It is thought that the human yolk sac does not function as a placenta in the sense of being able to transfer nutrients between maternal and embryonal circulations86, 88, 89. Therefore, embryo toxicity induced by yolk sac dysfunction during the period before chorioallantoic placenta formation in rats cannot necessarily be extrapolated to humans. Toxicologically, this is one of the important species differences between rat and human placentas90. Caution is therefore also required in extrapolation of embryo toxicity from rats to humans during the period before chorioallantoic placenta formation91. In addition, there is no inverted yolk sac placenta in humans. Thus, an animal model that does not rely upon an inverted yolk sac for embryonic nutrition should be used to elucidate any potential risk of developmental toxicity caused by chemicals in humans.89
2) Maternal part of the placenta
In humans, the decidua, which underlies the basal plate, is the site of implantation. The spiral arteries that connect the maternal uterine arteries to the intervillous space penetrate through the decidua. The decidua in humans is analogous to the decidua and metrial gland in rats. There are three types of decidual cells in humans: small predecidual swollen fibroblasts, large undifferentiated decidual cells, and large differentiated decidual cells. These decidual cells are isolated from each other by an investment of fibrinoid and are linked by fewer gap junctions than are found in rats47. The decidua contains many uNK cells, which are also known as large granular lymphocytes in humans. Many of these are aggregated around the spiral arteries92 and play a role in the control of trophoblast invasion and vascular remodeling93. Therefore, the uNK cells in humans have approximately the same function as those in rats82. In humans, preeclampsia is believed to result in part from inadequate maternal blood flow to the implantation site associated with angiogenesis inhibition and inadequate remodeling of spiral arteries94, 95. The histological feature of preeclampsia is restricted invasion of spiral arteries into the placental bed, with endovascular trophoblast invasion limited to the decidual vessels96. Tamoxifen induces the angiogenesis inhibition and inadequate remodeling of spiral arteries in the metrial glands in rats67.
3) Steroid hormone biosynthesis
There are species differences in the steroid hormone biosynthetic pathway in the placenta that result from differences in enzyme expression associated with this pathway (Fig. 13). In humans, the synthesis of pregnenolone and progesterone from cholesterol is carried out in the placenta. However, pregnenolone is converted to dehydroepiandrosterone in the adrenal gland of fetuses, as CYP17 is absent from the human placenta97. Dehydroepiandrosterone is transported into the placenta and is then converted to estrogen98. In contrast, pregnenolone synthesized from cholesterol is converted to androgen in the rat, as CYP17 is present in the rat placenta99. However, as aromatase is not present in the rat placenta, estrogen is not synthesized in the placentas but is instead synthesized in ovary. The androgen in the rat placentas might contribute to estrogen synthesis in the ovaries. Therefore, caution is also required in extrapolation of toxicological effects on the steroidogenic pathway from rat to human placentas.
Fig. 13.
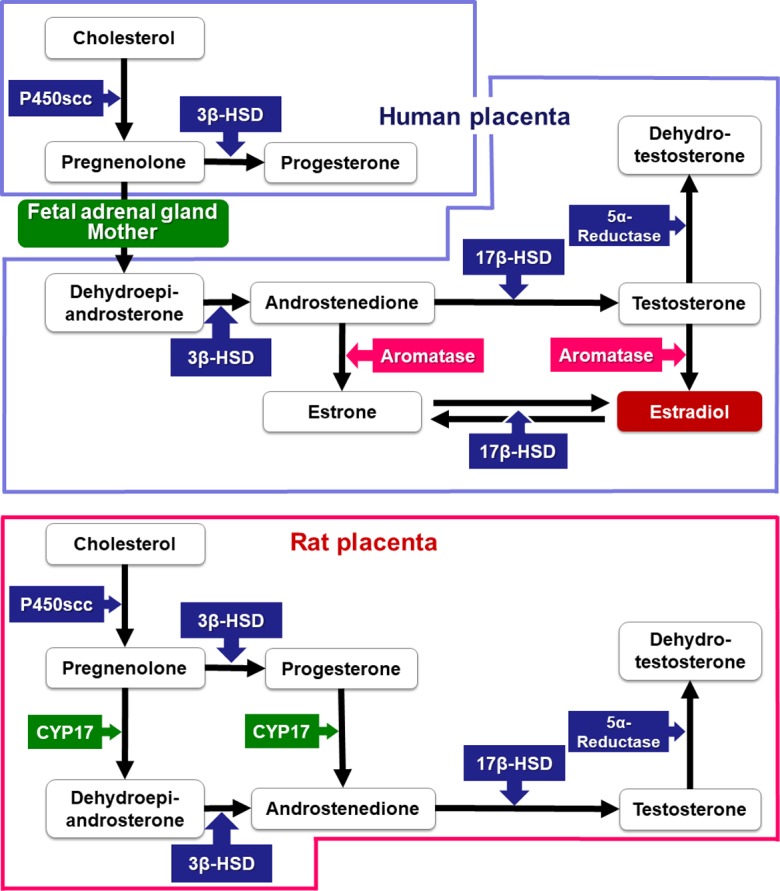
Pathway of steroid hormone biosynthesis in rats and humans.
Toxicological Significance of Placental Weight Change
Placental weight shows a high correlation with placental transport and metabolic mechanisms that qualitatively and quantitatively affect placental-fetal nutrient exchange100. In humans, a strong relationship has been reported between fetal and placental weight101. In rats, this relationship at GD 21 is the basis of our previous placental toxicity studies using Wistar Hannover rats, as shown in Fig. 14. The correlation is present except when there is a significant increase in placental weight. Conversely, placental weight is strongly affected by placental histopathological lesions (Fig. 15). In particular, placental weight reflects changes in the labyrinth zone in middle and late gestation, since the labyrinth zone becomes a major part of the placenta with advancing pregnancy. An increase in placental weight is induced by the adaptive response to a circulatory disturbance in the labyrinth zone102, hypertrophy of the labyrinth zone26 and basal zone24, or a reduction in the number of fetuses to less than 6103. A decrease in placental weight is mainly induced by hypoplasia of the labyrinth zone, resulting from apoptosis and/or necrosis of trophoblasts, and usually leads to IUGR. However, despite the fact that placental weight was reduced to about 70% of that of the control group in a previous study, it was found that a compensatory response by the placenta might prevent IUGR from being induced18. Moreover, it is difficult to detect hypoplasia of the metrial gland from placental weight changes despite induction of IUGR, because the placentas are separated at the basal zone and decidua basalis and are removed from the uterine wall for placental weight measurement. Therefore, although histopathological examination of the placenta is not generally performed in reproductive and developmental toxicity studies, not only placental weight but also placental histopathology should be considered in order to accurately evaluate the secondary effects of placental damage on embryo/fetal toxicity.
Fig. 14.
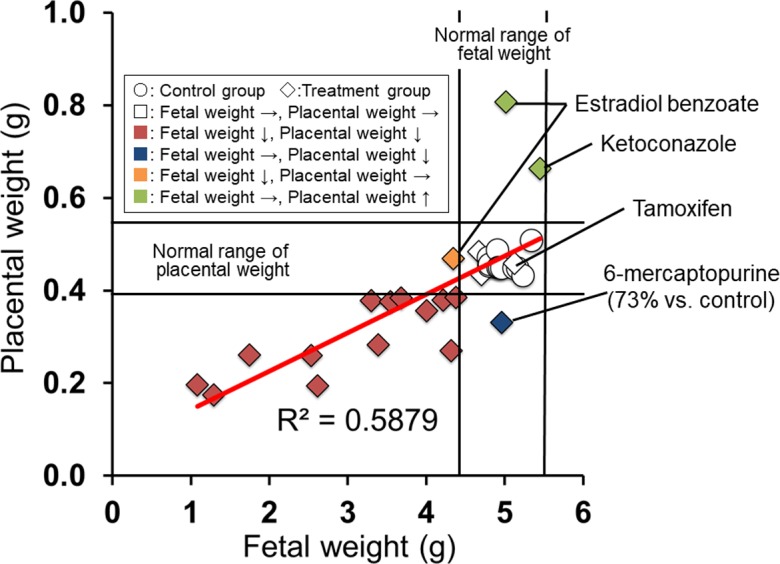
Relationship between fetal and placental weight. Normal range of weight, mean ± 3 SD.
Fig. 15.
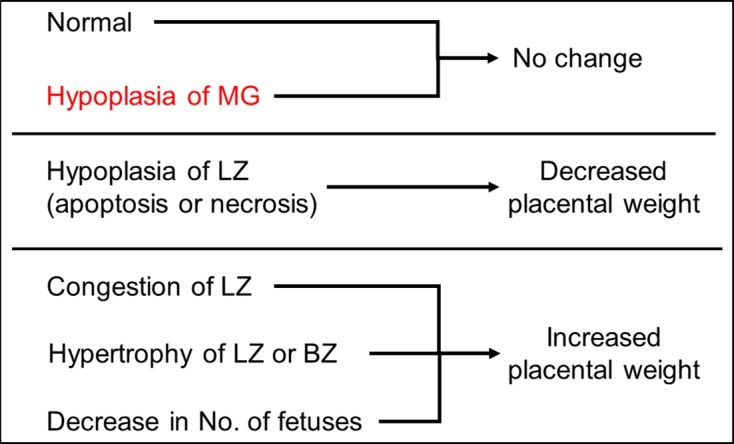
Pathogenesis of placental weight changes. BZ, basal zone; LZ, labyrinth zone; MG, metrial gland.
Conclusion
The placenta plays a pivotal role in fetal growth, and placental dysfunction and injury are associated with embryo/fetal toxicity. Although the placental weight change is one of the most important indexes of placental toxicity in reproductive and developmental toxicity studies, the placental weight assessment alone is not always enough to evaluate placental toxicity. In order to accurately evaluate reproductive and developmental toxicity studies, it is important to identify the histopathological changes in each part of the placenta and to elucidate the mechanism of placental toxicity through molecular investigation (Fig. 16). The placenta is toxicologically susceptible to various chemicals because of its high blood flow, rapid growth, and sex-hormone dependence. The placenta has these complex toxicological features, which are unlike those of other organs, and placental toxicity depends on the timing of pregnancy. Thus, the time-dependent histopathological examination of the rat placenta should be based on understanding of normal developmental changes in morphology and function. In addition, extrapolation of placental toxicity from rats to humans should be done cautiously in evaluation of risk factors because of species differences.
Fig. 16.
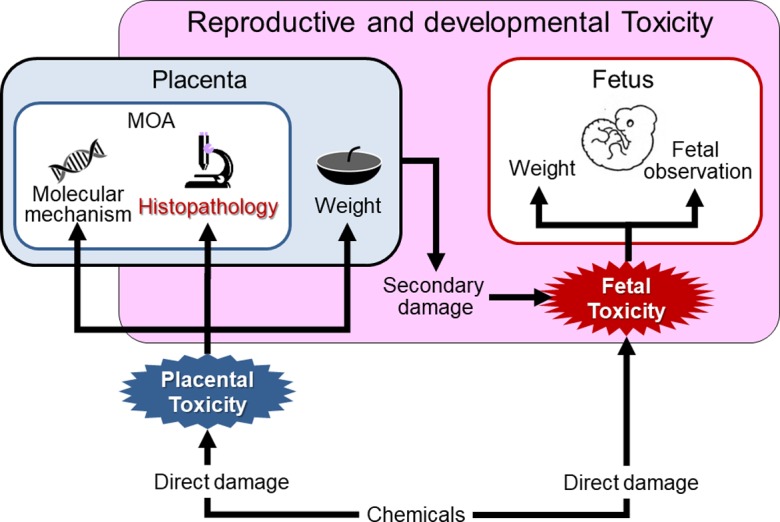
Significance of placental histopathology in reproductive and developmental toxicity studies.
Disclosure of Potential Conflicts of Interest
The authors declare that there is no conflict of interest.
Acknowledgments
The authors would like to thank Ms. Kaori Maejima, Ms. Hiromi Asako, Ms. Yukiko Sudo, Mr. Atsushi Funakoshi, Mr. Makoto Tsuchiya and Mr. Yoshinori Tanaka (Nissan Chemical Corporation.) for their excellent technical assistance.
Reference
- 1.Bauer MK, Harding JE, Bassett NS, Breier BH, Oliver MH, Gallaher BH, Evans PC, Woodall SM, and Gluckman PD. Fetal growth and placental function. Mol Cell Endocrinol. 140: 115–120. 1998. [DOI] [PubMed] [Google Scholar]
- 2.Sankaran S, and Kyle PM. Aetiology and pathogenesis of IUGR. Best Pract Res Clin Obstet Gynaecol. 23: 765–777. 2009. [DOI] [PubMed] [Google Scholar]
- 3.Goodman DR, James RC, and Harbison RD. Placental toxicology. Food Chem Toxicol. 20: 123–128. 1982. [DOI] [PubMed] [Google Scholar]
- 4.Gupta RK, and Gupta RC. Placental toxicity. In: Reproductive and Developmental Toxicology, 2nd ed. RC Gupta (ed). Elsevier, London. 1301–1325. 2017. [Google Scholar]
- 5.Furukawa S, Hayashi S, Usuda K, Abe M, Hagio S, and Ogawa I. Toxicological pathology in the rat placenta. J Toxicol Pathol. 24: 95–111. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Furukawa S, Kuroda Y, and Sugiyama A. A comparison of the histological structure of the placenta in experimental animals. J Toxicol Pathol. 27: 11–18. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cline JM, Dixon D, Ernerudh J, Faas MM, Göhner C, Häger JD, Markert UR, Pfarrer C, Svensson-Arvelund J, and Buse E. The placenta in toxicology. Part III: Pathologic assessment of the placenta. Toxicol Pathol. 42: 339–344. 2014. [DOI] [PubMed] [Google Scholar]
- 8.Furukawa S, Hayashi S, Abe M, Hagio S, Irie K, Kuroda Y, Ogawa I, and Sugiyama A. Background data on developmental parameters during the gestation period in rats. J Toxicol Pathol. 26: 83–88. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cross JC. How to make a placenta: mechanisms of trophoblast cell differentiation in mice--a review. Placenta. 26(Suppl A): S3–S9. 2005. [DOI] [PubMed] [Google Scholar]
- 10.Bolon B. Pathology analysis of the placenta. In: The Guide to Investigation of Mouse Pregnancy, 1st ed. BA Croy, AT Yamada, FJ DeMayo, SL Adamson (eds). Elsevier, Amsterdam. 175–188. 2014. [Google Scholar]
- 11.Georgiades P, Ferguson-Smith AC, and Burton GJ. Comparative developmental anatomy of the murine and human definitive placentae. Placenta. 23: 3–19. 2002. [DOI] [PubMed] [Google Scholar]
- 12.Leazer TM, and Klaassen CD. The presence of xenobiotic transporters in rat placenta. Drug Metab Dispos. 31: 153–167. 2003. [DOI] [PubMed] [Google Scholar]
- 13.Davies J, and Glasser SR. Histological and fine structural observations on the placenta of the rat. Acta Anat (Basel). 69: 542–608. 1968. [DOI] [PubMed] [Google Scholar]
- 14.Furukawa S, Tsuji N, Hayashi S, Abe M, Hagio S, Yamagishi Y, Kuroda Y, and Sugiyama A. Histomorphological comparison of rat placentas by different timing of chlorpromazine-administration. Exp Toxicol Pathol. 67: 443–452. 2015. [DOI] [PubMed] [Google Scholar]
- 15.Wheeler PD, and Yudilevich DL. Effect of insulin, prostaglandin E1 and uptake inhibitors on glucose transport in the perfused guinea-pig placenta. J Dev Physiol. 11: 159–169. 1989. [PubMed] [Google Scholar]
- 16.Shin BC, Fujikura K, Suzuki T, Tanaka S, and Takata K. Glucose transporter GLUT3 in the rat placental barrier: a possible machinery for the transplacental transfer of glucose. Endocrinology. 138: 3997–4004. 1997. [DOI] [PubMed] [Google Scholar]
- 17.Takata K, and Hirano H. Mechanism of glucose transport across the human and rat placental barrier: a review. Microsc Res Tech. 38: 145–152. 1997. [DOI] [PubMed] [Google Scholar]
- 18.Furukawa S, Hayashi S, Usuda K, Abe M, and Ogawa I. The relationship between fetal growth restriction and small placenta in 6-mercaptopurine exposed rat. Exp Toxicol Pathol. 63: 89–95. 2011. [DOI] [PubMed] [Google Scholar]
- 19.Enders AC. A comparative study of the fine structure of the trophoblast in hemochorial placentas. Am J Anat. 116: 29–67. 1965. [DOI] [PubMed] [Google Scholar]
- 20.Furukawa S, Usuda K, Abe M, Hayashi S, and Ogawa I. Busulfan-induced apoptosis in rat placenta. Exp Toxicol Pathol. 59: 97–103. 2007. [DOI] [PubMed] [Google Scholar]
- 21.Furukawa S, Usuda K, Abe M, Hayashi S, and Ogawa I. Effect of 6-mercaptopurine on rat placenta. J Vet Med Sci. 70: 551–556. 2008. [DOI] [PubMed] [Google Scholar]
- 22.Furukawa S, Hayashi S, Usuda K, Abe M, Hagio S, and Ogawa I. Effect of cisplatin on rat placenta development. Exp Toxicol Pathol. 65: 211–217. 2013. [DOI] [PubMed] [Google Scholar]
- 23.Bartholomeusz RK, Bruce NW, and Lynch AM. Embryo survival, and fetal and placental growth following elevation of maternal estradiol blood concentrations in the rat. Biol Reprod. 61: 46–50. 1999. [DOI] [PubMed] [Google Scholar]
- 24.Csapo A, Dray F, and Erdos T. Letter: Oestradiol 17beta: inhibitor of placental growth. Lancet. 2: 51–52. 1974. [DOI] [PubMed] [Google Scholar]
- 25.Rey Moreno MC, Fussell KC, Gröters S, Schneider S, Strauss V, Stinchcombe S, Fegert I, Veras M, and van Ravenzwaay B. Epoxiconazole-induced degeneration in rat placenta and the effects of estradiol supplementation. Birth Defects Res B Dev Reprod Toxicol. 98: 208–221. 2013. [DOI] [PubMed] [Google Scholar]
- 26.Furukawa S, Hayashi S, Usuda K, Abe M, and Ogawa I. Histopathological effect of ketoconazole on rat placenta. J Vet Med Sci. 70: 1179–1184. 2008. [DOI] [PubMed] [Google Scholar]
- 27.Jollie WP. Fine structural changes in the junctional zone of the rat placenta with increasing gestational age. J Ultrastruct Res. 12: 420–438. 1965. [DOI] [PubMed] [Google Scholar]
- 28.Cross J. Trophoblast cell fate specification. In: Biology and pathology of trophoblast, Functions and Evolution, 1st ed. A Moffett, C Loke, A McLaren (eds). Cambridge University Press, Cambridge. 3–14. 2006. [Google Scholar]
- 29.Kaufmann MH. Origin, properties and fate of trophoblast in the mouse. In: Biology of Trophoblast, 1st ed. C Loke, A Whyte (eds). Elsevier, Amsterdam. 23–70. 1983. [Google Scholar]
- 30.Coan PM, Conroy N, Burton GJ, and Ferguson-Smith AC. Origin and characteristics of glycogen cells in the developing murine placenta. Dev Dyn. 235: 3280–3294. 2006. [DOI] [PubMed] [Google Scholar]
- 31.Shiota K, Hirosawa M, Hattori N, Itonor S, Miura R, Noda K, Takahashi M, and Ogawa T. Structural and functional aspects of placental lactogens (PLs) and ovarian 20α-hydroxysteroid dehydrogenase (20α-HSD) in the rat. Endocr J. 41: S43–S56. 1994. [Google Scholar]
- 32.Matt DW, and MacDonald GJ. In vitro progesterone and testosterone production by the rat placenta during pregnancy. Endocrinology. 115: 741–747. 1984. [DOI] [PubMed] [Google Scholar]
- 33.Matt DW, and Macdonald GJ. Placental steroid production by the basal and labyrinth zones during the latter third of gestation in the rat. Biol Reprod. 32: 969–977. 1985. [DOI] [PubMed] [Google Scholar]
- 34.Chan SW, and Leathem JH. Placental steriodogenesis in the rat: comparison of normal and giant placentae. Endocrinology. 100: 1418–1422. 1977. [DOI] [PubMed] [Google Scholar]
- 35.Ain R, Canham LN, and Soares MJ. Gestation stage-dependent intrauterine trophoblast cell invasion in the rat and mouse: novel endocrine phenotype and regulation. Dev Biol. 260: 176–190. 2003. [DOI] [PubMed] [Google Scholar]
- 36.Soares MJ. The prolactin and growth hormone families: pregnancy-specific hormones/cytokines at the maternal-fetal interface. Reprod Biol Endocrinol. 2: 51 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ejiri N, Katayama KI, Nakayama H, and Doi K. Expression of cytochrome P450 (CYP) isozymes in rat placenta through pregnancy. Exp Toxicol Pathol. 53: 387–391. 2001. [DOI] [PubMed] [Google Scholar]
- 38.Kim SH, Lee IC, Baek HS, Moon C, Kim SH, Yoo JC, Shin IS, and Kim JC. Induction of cytochrome P450 3A1 expression by diallyl disulfide: protective effects against cyclophosphamide-induced embryo-fetal developmental toxicity. Food Chem Toxicol. 69: 312–319. 2014. [DOI] [PubMed] [Google Scholar]
- 39.Ejiri N, Katayama K, and Doi K. Induction of CYP3A1 by dexamethasone and pregnenolone-16alpha-carbonitrile in pregnant rat and fetal livers and placenta. Exp Toxicol Pathol. 54: 273–279. 2003. [DOI] [PubMed] [Google Scholar]
- 40.Ejiri N, Katayama K, and Doi K. Induction of cytochrome P450 isozymes by phenobarbital in pregnant rat and fetal livers and placenta. Exp Mol Pathol. 78: 150–155. 2005. [DOI] [PubMed] [Google Scholar]
- 41.Yokoi H, Tsuruo Y, Nihira M, and Ishimura K. Light and electron microscopic immunocytochemistry on the localization of 17 alpha-hydroxylase/C17,20-lyase (P450c17) in the rat placenta. J Med Invest. 44: 155–162. 1998. [PubMed] [Google Scholar]
- 42.Nishimura T, Takanohashi T, Tomi M, Horikoshi M, Higuchi K, Sai Y, and Nakashima E. Evaluation of rat in vivo fetal-to-maternal transfer clearances of various xenobiotics by umbilical perfusion. J Pharm Sci. 102: 3356–3363. 2013. [DOI] [PubMed] [Google Scholar]
- 43.Bibeau K, Sicotte B, Béland M, Bhat M, Gaboury L, Couture R, St-Louis J, and Brochu M. Placental underperfusion in a rat model of intrauterine growth restriction induced by a reduced plasma volume expansion. PLoS One. 11: e0145982 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vercruysse L, Caluwaerts S, Luyten C, and Pijnenborg R. Interstitial trophoblast invasion in the decidua and mesometrial triangle during the last third of pregnancy in the rat. Placenta. 27: 22–33. 2006. [DOI] [PubMed] [Google Scholar]
- 45.Sferruzzi-Perri AN, Macpherson AM, Roberts CT, and Robertson SA. Csf2 null mutation alters placental gene expression and trophoblast glycogen cell and giant cell abundance in mice. Biol Reprod. 81: 207–221. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Levario-Carrillo M, Olave ME, Corral DC, Alderete JG, Gagioti SM, and Bevilacqua E. Placental morphology of rats prenatally exposed to methyl parathion. Exp Toxicol Pathol. 55: 489–496. 2004. [DOI] [PubMed] [Google Scholar]
- 47.Wooding P, and Burton G. Implantation, maternofetal exchange and vascular relationships. In: Comparative Placentation. Structures, Functions and Evolution, 1st ed. P Wooding and G Burton (eds). Springer-Verlag, Berlin. 1–81. 2008. [Google Scholar]
- 48.Palis J, McGrath KE, and Kingsley PD. Initiation of hematopoiesis and vasculogenesis in murine yolk sac explants. Blood. 86: 156–163. 1995. [PubMed] [Google Scholar]
- 49.Jollie WP. Development, morphology, and function of the yolk-sac placenta of laboratory rodents. Teratology. 41: 361–381. 1990. [DOI] [PubMed] [Google Scholar]
- 50.Zohn IE, and Sarkar AA. The visceral yolk sac endoderm provides for absorption of nutrients to the embryo during neurulation. Birth Defects Res A Clin Mol Teratol. 88: 593–600. 2010. [DOI] [PubMed] [Google Scholar]
- 51.Beckman DA, Koszalka TR, Jensen M, and Brent RL. Experimental manipulation of the rodent visceral yolk sac. Teratology. 41: 395–404. 1990. [DOI] [PubMed] [Google Scholar]
- 52.Rogers JM, Daston GP, Ebron MT, Carver B, Stefanadis JG, and Grabowski CT. Studies on the mechanism of trypan blue teratogenicity in the rat developing in vivo and in vitro. Teratology. 31: 389–399. 1985. [DOI] [PubMed] [Google Scholar]
- 53.Xu Y, Xiao R, and Li Y. Effect of ethanol on the development of visceral yolk sac. Hum Reprod. 20: 2509–2516. 2005. [DOI] [PubMed] [Google Scholar]
- 54.Furukawa S, Usuda K, Abe M, Hayashi S, and Ogawa I. Histological expression of metallothionein in the developing rat placenta. J Toxicol Pathol. 21: 223–227. 2008. [Google Scholar]
- 55.Dey SK, and Lim H. Implantation. In: Knobil and Neill’s Physiology of reproductive, 3rd ed. JD Neill (ed). Academic press, Amsterdam. 147–188. 2006. [Google Scholar]
- 56.Welsh AO, and Enders AC. Occlusion and reformation of the rat uterine lumen during pregnancy. Am J Anat. 167: 463–477. 1983. [DOI] [PubMed] [Google Scholar]
- 57.Dai D, Moulton BC, and Ogle TF. Regression of the decidualized mesometrium and decidual cell apoptosis are associated with a shift in expression of Bcl2 family members. Biol Reprod. 63: 188–195. 2000. [DOI] [PubMed] [Google Scholar]
- 58.de Rijk EP, van Esch E, and Flik G. Pregnancy dating in the rat: placental morphology and maternal blood parameters. Toxicol Pathol. 30: 271–282. 2002. [DOI] [PubMed] [Google Scholar]
- 59.Yochim JM, and De Feo VJ. Control of decidual growth in the rat by steroid hormones of the ovary. Endocrinology. 71: 134–142. 1962. [DOI] [PubMed] [Google Scholar]
- 60.Harazono A, and Ema M. Suppression of decidual cell response induced by dibutyltin dichloride in pseudopregnant rats: as a cause of early embryonic loss. Reprod Toxicol. 17: 393–399. 2003. [DOI] [PubMed] [Google Scholar]
- 61.Tessier C, Deb S, Prigent-Tessier A, Ferguson-Gottschall S, Gibori GB, Shiu RP, and Gibori G. Estrogen receptors alpha and beta in rat decidua cells: cell-specific expression and differential regulation by steroid hormones and prolactin. Endocrinology. 141: 3842–3851. 2000. [DOI] [PubMed] [Google Scholar]
- 62.Peel S. Granulated metrial gland cells. Advances in Anatomy Embryology and Cell Biology. Springer-Verlag, Berlin. 1989. [DOI] [PubMed] [Google Scholar]
- 63.Picut CA, Swanson CL, Parker RF, Scully KL, and Parker GA. The metrial gland in the rat and its similarities to granular cell tumors. Toxicol Pathol. 37: 474–480. 2009. [DOI] [PubMed] [Google Scholar]
- 64.Fukazawa Y, Yamamura Y, Sato T, Iguchi T, and Ohta Y. Mode of cell death in the rat metrial gland during peripartum regression. Anat Rec. 252: 369–377. 1998. [DOI] [PubMed] [Google Scholar]
- 65.Pijnenborg R, and Vercruysse L. Animal models of deep trophoblast invasion. In: Placental Bed Disorders 1st ed. R Pijnenborg, I Brosens and R Romero (eds). Cambridge University Press, Cambridge. 127–140. 2010. [Google Scholar]
- 66.Caluwaerts S, Vercruysse L, Luyten C, and Pijnenborg R. Endovascular trophoblast invasion and associated structural changes in uterine spiral arteries of the pregnant rat. Placenta. 26: 574–584. 2005. [DOI] [PubMed] [Google Scholar]
- 67.Furukawa S, Hayashi S, Usuda K, Abe M, and Ogawa I. The impairment of metrial gland development in tamoxifen exposed rats. Exp Toxicol Pathol. 64: 121–126. 2012. [DOI] [PubMed] [Google Scholar]
- 68.Furukawa S, Hayashi S, Usuda K, Abe M, Hagio S, Kuroda Y, and Ogawa I. Effect of estrogen on rat placental development depending on gestation stage. Exp Toxicol Pathol. 65: 695–702. 2013. [DOI] [PubMed] [Google Scholar]
- 69.Peel S, and Bulmer D. The effects of late ovariectomy on the proliferation and differentiation of the uterus of the pregnant rat. J Anat. 119: 569–578. 1975. [PMC free article] [PubMed] [Google Scholar]
- 70.Guimond MJ, Luross JA, Wang B, Terhorst C, Danial S, and Croy BA. Absence of natural killer cells during murine pregnancy is associated with reproductive compromise in TgE26 mice. Biol Reprod. 56: 169–179. 1997. [DOI] [PubMed] [Google Scholar]
- 71.Martel D, Monier MN, Roche D, De Feo VJ, and Psychoyos A. Hormonal dependence of the metrial gland: further studies on oestradiol and progesterone receptor levels in the rat. J Endocrinol. 120: 465–472. 1989. [DOI] [PubMed] [Google Scholar]
- 72.Furukawa S, Hayashi S, Abe M, Hagio S, Irie K, Kuroda Y, Ogawa I, and Sugiyama A. Effect of chlorpromazine on rat placenta development. Exp Toxicol Pathol. 66: 41–47. 2014. [DOI] [PubMed] [Google Scholar]
- 73.Sibai B, Dekker G, and Kupferminc M. Pre-eclampsia. Lancet. 365: 785–799. 2005. [DOI] [PubMed] [Google Scholar]
- 74.Selye H, Borduas A, and Masson G. Studies concerning the hormonal control of deciduomata and metrial glands. Anat Rec. 82: 199–209. 1942. [Google Scholar]
- 75.Croy BA, Chantakru S, Esadeg S, Ashkar AA, and Wei Q. Decidual natural killer cells: key regulators of placental development (a review). J Reprod Immunol. 57: 151–168. 2002. [DOI] [PubMed] [Google Scholar]
- 76.Riley JK, and Yokoyama WM. NK cell tolerance and the maternal-fetal interface. Am J Reprod Immunol. 59: 371–387. 2008. [DOI] [PubMed] [Google Scholar]
- 77.Bilinski MJ, Thorne JG, Oh MJ, Leonard S, Murrant C, Tayade C, and Croy BA. Uterine NK cells in murine pregnancy. Reprod Biomed Online. 16: 218–226. 2008. [DOI] [PubMed] [Google Scholar]
- 78.Kaloglu C, and Bulut HE. Vascular endothelial growth factor production by rat granulated metrial gland cells and their morphological features in normal and pathological conditions. Reprod Fertil Dev. 19: 341–350. 2007. [DOI] [PubMed] [Google Scholar]
- 79.Zhang J, Chen Z, Smith GN, and Croy BA. Natural killer cell-triggered vascular transformation: maternal care before birth? Cell Mol Immunol. 8: 1–11. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Carlström M, Wentzel P, Skøtt O, Persson AE, and Eriksson UJ. Angiogenesis inhibition causes hypertension and placental dysfunction in a rat model of preeclampsia. J Hypertens. 27: 829–837. 2009. [DOI] [PubMed] [Google Scholar]
- 81.Cross JC, Hemberger M, Lu Y, Nozaki T, Whiteley K, Masutani M, and Adamson SL. Trophoblast functions, angiogenesis and remodeling of the maternal vasculature in the placenta. Mol Cell Endocrinol. 187: 207–212. 2002. [DOI] [PubMed] [Google Scholar]
- 82.Eriksson M, Basu S, and Sentman CL. NK cells and pregnancy. In: Immunology of Pregnancy, 1st ed. G Mor (ed). Springer-Verlag, New York. 84–95. 2006. [Google Scholar]
- 83.Soares MJ, Chakraborty D, Karim Rumi MA, Konno T, and Renaud SJ. Rat placentation: an experimental model for investigating the hemochorial maternal-fetal interface. Placenta. 33: 233–243. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Knipp GT, Audus KL, and Soares MJ. Nutrient transport across the placenta. Adv Drug Deliv Rev. 38: 41–58. 1999. [DOI] [PubMed] [Google Scholar]
- 85.Pence L, and Telugu BP. In Animal Models and Human Reproduction, 1st ed. S Heide (ed). John Wiley & Sons, Inc, Hoboken. 65–90. 2017. [Google Scholar]
- 86.Boyd KL, Muehlenbachs A, Rendi MH, Garcia RL, and Gibson-KN. Female Reproductive System. In: Comparative Anatomy and Histology; A Mouse Rat, and Human Atlas. 2nd ed. PM Treuting, SM Dintzis, and KS Montine (eds). Academic Pres, Amsterdam. 324–333. 2017. [Google Scholar]
- 87.Carney EW, Scialli AR, Watson RE, and DeSesso JM. Mechanisms regulating toxicant disposition to the embryo during early pregnancy: an interspecies comparison. Birth Defects Res C Embryo Today. 72: 345–360. 2004. [DOI] [PubMed] [Google Scholar]
- 88.DeSesso JM, Williams AL, Ahuja A, Bowman CJ, and Hurtt ME. The placenta, transfer of immunoglobulins, and safety assessment of biopharmaceuticals in pregnancy. Crit Rev Toxicol. 42: 185–210. 2012. [DOI] [PubMed] [Google Scholar]
- 89.Stump DG, Holson JF, Harris C, Pearce LB, Watson RE, and DeSesso JM. Developmental toxicity in rats of a hemoglobin-based oxygen carrier results from impeded function of the inverted visceral yolk sac. Reprod Toxicol. 52: 108–117. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Maranghi F, Macrì C, Ricciardi C, Stazi AV, and Mantovani A. Evaluation of the placenta: suggestions for a greater role in developmental toxicology. Adv Exp Med Biol. 444: 129–136. 1998. [DOI] [PubMed] [Google Scholar]
- 91.Furukawa S, Tsuji N, Kobayashi Y, Yamagishi Y, Hayashi S, Abe M, Kuroda Y, Kimura M, Hayakawa C, and Sugiyama A. Effect of dibutyltin on placental and fetal toxicity in rat. J Toxicol Sci. 42: 741–753. 2017. [DOI] [PubMed] [Google Scholar]
- 92.Bulmer JN, Williams PJ, and Lash GE. Immune cells in the placental bed. Int J Dev Biol. 54: 281–294. 2010. [DOI] [PubMed] [Google Scholar]
- 93.Bulmer JN, and Lash GE. Human uterine natural killer cells: a reappraisal. Mol Immunol. 42: 511–521. 2005. [DOI] [PubMed] [Google Scholar]
- 94.Naeye RL. Pregnancy hypertension, placental evidences of low uteroplacental blood flow, and spontaneous premature delivery. Hum Pathol. 20: 441–444. 1989. [DOI] [PubMed] [Google Scholar]
- 95.Plaisier M. Decidualisation and angiogenesis. Best Pract Res Clin Obstet Gynaecol. 25: 259–271. 2011. [DOI] [PubMed] [Google Scholar]
- 96.Lyall F. The placenta in preeclampsia. In: The Placenta: From Development to Disease, 1st ed. HH Kay, DM Nelson, Y Wang. (eds). Wiley-Blackwell, Oxford. 246–252. 2011. [Google Scholar]
- 97.Payne AH, and Hales DB. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr Rev. 25: 947–970. 2004. [DOI] [PubMed] [Google Scholar]
- 98.Miller WL. Steroid hormone biosynthesis and actions in the materno-feto-placental unit. Clin Perinatol. 25: 799–817. 1998. [PubMed] [Google Scholar]
- 99.Durkee TJ, McLean MP, Hales DB, Payne AH, Waterman MR, Khan I, and Gibori G. P450(17 alpha) and P450SCC gene expression and regulation in the rat placenta. Endocrinology. 130: 1309–1317. 1992. [DOI] [PubMed] [Google Scholar]
- 100.Hay WW., Jr. The placenta. Not just a conduit for maternal fuels. Diabetes. 40(Suppl 2): 44–50. 1991. [DOI] [PubMed] [Google Scholar]
- 101.Cetin I, and Taricco E. Clinical causes and aspects of placental insufficiency. In: The Placenta and Human Developmental Programming, 1st ed. GJ Burton, DJP Barker, A Moffett and K Thornburg (eds). University Press, Cambridge. 114–125. 2011. [Google Scholar]
- 102.Ichikawa A, and Tamada H. Ketoconazole-induced estrogen deficiency causes transient decrease in placental blood flow associated with hypoxia and later placental weight gain in rats. Reprod Toxicol. 63: 62–69. 2016. [DOI] [PubMed] [Google Scholar]
- 103.Csapo AI, and Wiest WG. Plasma steroid levels and ovariectomy-induced placental hypertrophy in rats. Endocrinology. 93: 1173–1177. 1973. [DOI] [PubMed] [Google Scholar]