Approximately 14% of the U.S. adult population has impaired kidney function1, 2. End-stage renal disease (ESRD) is characterized by extremely high mortality (20% per year), and a rate of cardiovascular (CV) death that is up to 100 times higher than that in the general population3, 4. The differences in longevity between the ESRD patients and the general population are striking5. Dialysis patients younger than 80 years old are expected to live less than one-third as long as their counterparts without ESRD. The United States Renal Data System (USRDS) report of 2017 noted that CV death was observed in 48% of all deaths in ESRD. The most common cause of death in dialysis patients is sudden cardiac death (SCD), 6–8 responsible for 37% of all deaths in this population9. There is a need to develop a strategy for the prevention of SCD in the ESRD population.
Adjudication of the mode of sudden death in ESRD is notoriously difficult, as ESRD patients are chronically ill, and are at increased risk of sudden non-arrhythmic death, such as pulmonary or air embolism, aortic dissection, or cerebral hemorrhage.10 On the other hand, bradyarrhythmic cardiac arrest due to withdrawal from dialysis (or after missed dialysis treatment) occurs due to arrhythmic death, which is expected and non-sudden. While there is still a controversy regarding a specific type of causative cardiac arrhythmia behind SCD in dialysis, without a doubt, clinically important arrhythmias are common in hemodialysis patients.11 Recent studies observed bradyarrhythmias more frequently than ventricular tachyarrhythmias.11 For additional details, we refer readers to several excellent reviews of SCD in dialysis.10, 12
The highest mortality in dialysis patients is observed during the first year of hemodialysis
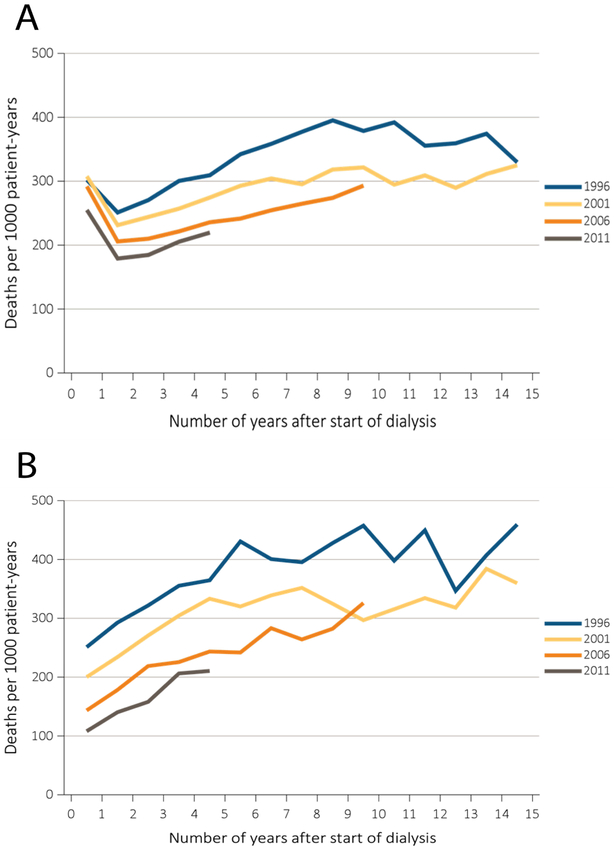
The highest mortality in dialysis patients is observed during the first year of hemodialysis13 (Figures 1 and 2). Dialysis vintage independently predicts mortality in patients on renal replacement therapy14-16. While in the past 15 years crude mortality rate decreased by 26% for dialysis patients, the pattern of mortality remains unchanged (Figure 1): there is a consistent increase in mortality in the first year after the onset of hemodialysis treatment. Moreover, the increase in mortality in incident hemodialysis patients is greater if patients are older (Figure 2). The reason for this increase in mortality within the first months after initiating hemodialysis is unknown. There are attempts to explain it by incomplete data reporting by the Center for Medicare & Medicaid Services.5 However, the fact that the sharp increase in mortality within the first 3 months after the beginning of hemodialysis represents a consistent pattern, observed over the course of 20 years (Figure 1), and is larger in older (vs. younger) patients (Figure 2) suggests that hemodialysis-related factors can be partially responsible for increased mortality after hemodialysis initiation. In this manuscript we discuss possible mechanisms behind increased mortality in incident hemodialysis, and put forward our hypothesis that should be tested in future prospective studies.
Figure 1.
Adjusted all-cause mortality by treatment modality, cohort (year of ESRD onset), and number of years after start of dialysis among incident (A) hemodialysis patients and (B) peritoneal dialysis patients, 1996, 2001, 2006, and 2011. From USRDS 2017 Annual Data Report. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the U.S. government.
Figure 2.
Adjusted mortality by treatment modality and number of months after treatment initiation among ESRD patients (A) under age 65 and (B) aged 65 and over, 2014. From USRDS 2017 Annual Data Report. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the U.S. government.
Hemodialysis treatment is associated with plastic chemical exposure
Dialysis tubing and catheters are commonly manufactured using polyvinyl chloride (PVC) polymers. Made of vinyl chloride monomers, PVC is naturally a hard plastic that can be softened with plasticizer chemicals, most notably those from the phthalate class. Phthalate plasticizers soften PVC by embedding between polymer segments, effectively disrupting complete polymerization. Since phthalate plasticizers do not covalently bond to the PVC matrix, they are highly susceptible to leaching when in contact with lipophilic substances, including blood.17 Indeed, phthalate chemical concentrations have been shown to increase in patient blood samples during a 4-hour dialysis session.18 Di(2-ethylhexyl) phthalate (DEHP) remains the most widely used plasticizer in the manufacturing of dialysis tubing circuits, since mass production of DEHP is economical and its chemical properties are ideal for the manufacturing of flexible plastic tubing.19
In addition to PVC plastics, track-etched polycarbonate plastic is often used in the manufacturing of dialysis membranes.20 Bisphenol-A (BPA) is the most commonly used monomer in polycarbonate plastics, and as such, BPA is present in dialysis circuit coatings and membranes.20 BPA is also used in epoxy resins (coatings and adhesives), which can result in a chemical residue21 within PVC medical devices.
Dialysis patients are often exposed to exceedingly high concentrations18, 22 of DEHP and BPA, due to extended periods of contact between these plastic medical devices and the patient’s blood. Moreover, previously reported serum concentrations of DEHP may in fact be underestimated, since DEHP has been shown to incorporate into red blood cell plasma membranes.23 Phthalates can also deposit into adipose tissue,24 which could result in extended chemical exposures – even after a dialysis session is completed. Incidental exposure to plastic additives may be a modifiable factor, since studies have shown that the degree of chemical leaching is influenced by environmental conditions, including temperature, contact time, circuit coatings, and flow rate.25
Plastic chemical exposure alters cardiac electrophysiology and autonomic regulation
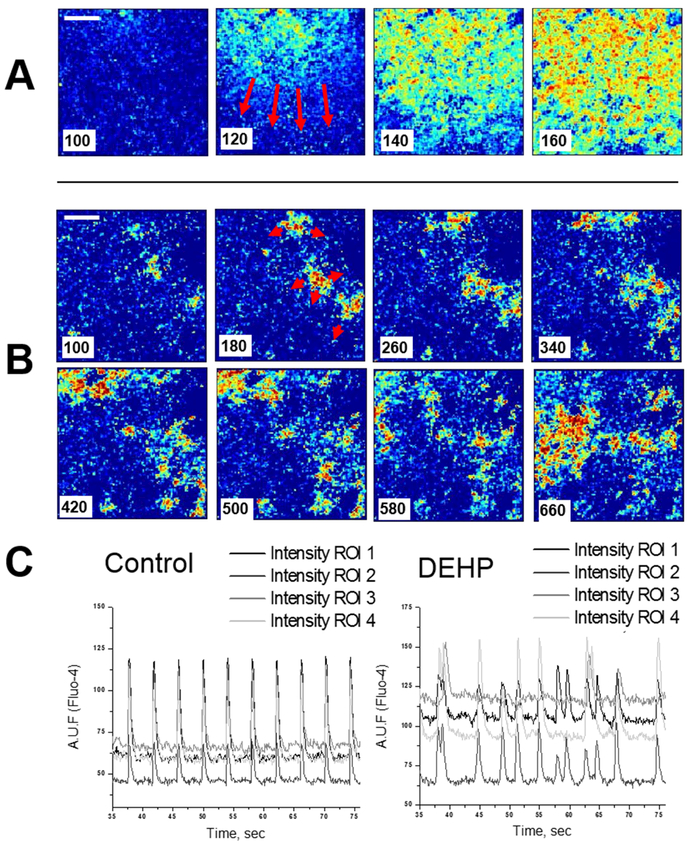
Studies have shown that plastic chemicals are not universally biocompatible. A causal relationship has been identified between exposure to phthalate and bisphenol chemicals and altered cardiovascular and autonomic function.17 Clinically relevant DEHP concentrations have been shown to impair electrical conduction in cardiomyocytes, resulting in an arrhythomogenic phenotype (Figure 3). Specifically, DEHP-treatment caused asynchronous cell beating and decreased conduction velocity across confluent cardiomyocyte layers. These effects were attributed to a loss of gap junctional connexin-43,26 a transmembrane protein necessary for coordinated depolarization. DEHP exposure modifies cardiac electrical conduction in isolated perfused rat hearts, decreases heart rate and causes prolongation of PR and QT intervals.27 DEHP exposure can also cause negative inotropic and chronotropic effects 27-29, hypotension, and cardiac arrest (in animal studies).30 To the best of our knowledge, causal effect of DEHP/BPA exposure on SCD has not been previously studied in clinical studies. Recent studies have shown that in vivo phthalate exposure also alters autonomic regulation (Figure 4), resulting in decreased heart rate variability, increased sympathetic tone, and exaggerated cardiovascular reactivity and recovery to a stress stimulus.31 Importantly, the DEHP dose shown to influence autonomic regulation was ~20-fold smaller than the DEHP daily intake during hemodialysis. BPA exposure has also been shown to adversely affect cardiac electrical conduction in a concentration-dependent manner32. Using an excised whole heart model, BPA was shown to slow AV conduction, slow epicardial conduction velocity and lengthen action potential duration17. Accordingly, BPA has been shown to bind directly to and block sodium channels17 and voltage-gated calcium channels33, and activate potassium channels34, resulting in cellular hyperpolarization and decreased cardiac excitability which could lead to bradycardia, sinoatrial block, AV block, asystole, or reentrant ventricular tachyarrhythmias. Elevated exposure to BPA and DEHP chemical contaminants may partially explain a higher than expected rate of bradyarrhythmias35 in dialysis patients. Such a correlation has also been established in animal models of chronic kidney disease, in which animals have an elevated incidence of bradycardia and SCD.36
Figure 3.
Monolayers of neonatal rat cardiomyocytes display a marked decrease in conduction velocity after 3-day treatment with 50μg/ml DEHP. Individual frames from fast camera system show fast, homogenous wavefronts in control (A) versus slow, fractionated propagation in samples treated with 50μg/ml DEHP for three days (B). Note that the sequential frames in the upper and lower rows are 20ms apart, versus 80 ms for the DEHP-treated sample. A time stamp was placed on the left lower corner of the image. A ten-fold decrease in apparent conduction velocity was observed in the DEHP-treated samples. (C). Recordings of calcium transients are shown as relative units of Fluo-4 fluorescence (n=8). Reproduced with permission from26.
Figure 4.
Exaggerated drop in mean arterial blood pressure (MAP) in response to chlorisondamine (12 mg/kg), indicating increased sympathetic tone in DEHP-treated animals. *P < 0.05. Reproduced with permission from31.
Abnormal electrophysiological substrate in end-stage renal disease
ESRD patients are characterized by the presence of kidney-disease-specific abnormal electrophysiological (EP) substrate, which is responsible for an increased risk of SCD.37 Fluctuations of electrolytes and fluid, a pro-inflammatory state, left ventricular hypertrophy with diastolic dysfunction, intradialytic hypotension, and repetitive myocardial injury from uremia and/or dialysis-induced myocardial ‘stunning’38 are known risk factors of SCD that are specific to dialysis patients. Unfortunately, as treatment of kidney disease in ESRD patients is only partially successful, currently there is no effective intervention that would be able to reverse an already developed (due to kidney disease and known cardiovascular risk factors) EP substrate in ESRD. In Figure 5, we summarize the current understanding of SCD mechanisms in ESRD that are likely involved in the development of an abnormal EP substrate. We also refer readers to several excellent recent reviews on this topic.10, 12
Figure 5.
Hypothesis of interaction between electrophysiological substrate in ESRD patients and BPA/DEHP exposure in incident dialysis, resulting in increased risk of SCD. This scheme was illustrated using the Motifolio PPT Drawing Toolkits (Motifolio Inc, MD, USA). Schematic drawing of dialysis is adapted from www.kidney.org.au.
Recent data suggest that that bradyarrhythmic SCD is likely the most frequent type of SCD in ESRD.11, 12 Genetic predisposition can affect the risk of SCD. The TBX3 gene has been implicated in the development of central conduction system, which can potentially explain the development of bradyarrhythmias and ventricular conduction abnormalities, under certain exposures.39 In a genomewide association study, TBX3 was associated with electrocardiographic spatial QRS-T angle.39 Spatial QRS-T angle was also associated with SCD in incident hemodialysis patients.40 Wide spatial QRS-T angle can act as a marker of abnormal EP substrate in ESRD,40 and the general population.41
Creative Concept Hypothesis: Pre-existing abnormal EP substrate interacts with plastic chemical exposure in incident dialysis, which increases risk of SCD in genetically predisposed ESRD patients
Several clinical observations point to a possible interaction between BPA and DEHP exposure in ESRD patients and abnormal EP substrate, resulting in increased CV mortality and SCD (Figure 5): First, the risk of SCD in dialysis patients falls substantially after successful transplantation.42 Second, the use of hemodialysis catheters is associated with both a larger DEHP exposure and a higher risk of SCD, as compared to the use of arteriovenous fistulas.43, 44 The shift in electrolytes, fluid, and blood pressure after dialysis do not fully explain the increased risk of SCD in these patients.43, 45 Hemodialysis access complications themselves also fail to explain this higher risk of SCD.46 Third, faster blood flow in dialysis (e.g. less BPA/DEHP leaching) is associated with lower mortality.47 Fourth, there is an association between the hemodialysis schedule and SCD rate. In addition to a well-known increase in mortality after the long interdialytic interval, SCD is more common within 6 hours after the end of a hemodialysis session48-50. This timing coincides with peak blood levels of DEHP and BPA.51-55 Lastly, DEHP exposure is higher during hemodialysis, as compared to peritoneal dialysis56. Consistently, the pattern of mortality is different, too (Figures 1–2). Patients on hemodialysis have the highest mortality in the first year, lowest mortality in the second year, and monotonically increasing mortality after that. Conversely, peritoneal dialysis is associated with monotonically increasing risk of death, with the most moderate risk in the first year of dialysis. The difference in mortality patterns might be explained by an interaction of DEHP/BPA exposure (effect modifier) with pre-existing abnormal EP substrate, likely responsible for the highest mortality in patients with both risk factors (high DEHP/BPA exposure + abnormal EP substrate).
Importantly, BPA/DEHP exposure may be a modifiable risk factor of SCD in dialysis. Unfortunately, despite epidemiological associations and experimental research findings, there is currently no restriction on the use of phthalate and bisphenol chemicals in the manufacturing of medical devices in the United States. Alternatives to BPA and DEHP do exist and could potentially be used as replacements for dialysis procedures57. Unfortunately, the lack of government regulation and minimally available alternatives (and higher pricing or those alternatives that do exist) have impeded the clinical adoption of these alternative materials. Replacement of BPA- and DEHP- leaching plastics may reduce morbidity and mortality of ESRD patients on dialysis, and other patients, undergoing invasive interventions with prolonged exposure to plastics (e.g. cardiac surgery). Prospective clinical studies and randomized controlled trials are needed to test our hypothesis.
Acknowledgments
Funding Sources: This research was supported in part by the National Institute of Health #1R01HL118277 (LGT) #R01HL139472, #R00ES023477 (NGP).
Footnotes
Conflict of interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2003;41:1–12. [DOI] [PubMed] [Google Scholar]
- 2.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, Ckd EPI. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Foley RN. Clinical epidemiology of cardiac disease in dialysis patients: left ventricular hypertrophy, ischemic heart disease, and cardiac failure. Semin.Dial. 2003;16:111–117. [DOI] [PubMed] [Google Scholar]
- 4.Keith DS, Nichols GA, Gullion CM, Brown JB, Smith DH. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med 2004;164:659–663. [DOI] [PubMed] [Google Scholar]
- 5.United States Renal Data System. 2017; https://www.usrds.org Accessed 07/21/2018, 2018.
- 6.Cheung AK, Sarnak MJ, Yan G, Berkoben M, Heyka R, Kaufman A, Lewis J, Rocco M, Toto R, Windus D, Ornt D, Levey AS. Cardiac diseases in maintenance hemodialysis patients: results of the HEMO Study. Kidney Int. 2004;65:2380–2389. [DOI] [PubMed] [Google Scholar]
- 7.Parekh RS, Plantinga LC, Kao WH, Meoni LA, Jaar BG, Fink NE, Powe NR, Coresh J, Klag MJ. The association of sudden cardiac death with inflammation and other traditional risk factors. Kidney Int 2008;74:1335–1342. [DOI] [PubMed] [Google Scholar]
- 8.Shamseddin MK, Parfrey PS. Sudden cardiac death in chronic kidney disease: epidemiology and prevention. Nat Rev Nephrol 2011;7:145–154. [DOI] [PubMed] [Google Scholar]
- 9.Banerjee D Sudden cardiac death in haemodialysis: clinical epidemiology and mechanisms. J Electrocardiol 2016. [DOI] [PubMed] [Google Scholar]
- 10.Makar MS, Pun PH. Sudden Cardiac Death Among Hemodialysis Patients. Am J Kidney Dis 2017;69:684–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roy-Chaudhury P, Tumlin JA, Koplan BA, et al. Primary outcomes of the Monitoring in Dialysis Study indicate that clinically significant arrhythmias are common in hemodialysis patients and related to dialytic cycle. Kidney Int 2018;93:941–951. [DOI] [PubMed] [Google Scholar]
- 12.Turakhia MP, Blankestijn PJ, Carrero JJ, et al. Chronic kidney disease and arrhythmias: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Eur Heart J 2018;39:2314–2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foley RN. Epidemiology and Risk Factors for Early Mortality After Dialysis Initiation. Seminars in Nephrology 2017;37:114–119. [DOI] [PubMed] [Google Scholar]
- 14.Chertow GM, Johansen KL, Lew N, Lazarus JM, Lowrie EG. Vintage, nutritional status, and survival in hemodialysis patients. Kidney Int 2000;57:1176–1181. [DOI] [PubMed] [Google Scholar]
- 15.Avram MM, Mittman N, Fein PA, Agahiu S, Hartman W, Chattopadhyay N, Matza B. Dialysis vintage, body composition, and survival in peritoneal dialysis patients. Adv Perit Dial 2012;28:144–147. [PubMed] [Google Scholar]
- 16.Ramesh S, Zalucky A, Hemmelgarn BR, Roberts DJ, Ahmed SB, Wilton SB, Jun M. Incidence of sudden cardiac death in adults with end-stage renal disease: a systematic review and metaanalysis. BMC Nephrol 2016;17–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Posnack NG. The adverse cardiac effects of Di(2-ethylhexyl)phthalate and Bisphenol A. Cardiovascular toxicology 2014;14:339–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faouzi MA, Dine T, Gressier B, Kambia K, Luyckx M, Pagniez D, Brunet C, Cazin M, Belabed A, Cazin JC. Exposure of hemodialysis patients to di-2-ethylhexyl phthalate. Int J Pharm 1999;180:113–121. [DOI] [PubMed] [Google Scholar]
- 19.Sampson J, de Korte D. DEHP-plasticised PVC: relevance to blood services. Transfus Med 2011;21:73–83. [DOI] [PubMed] [Google Scholar]
- 20.Bosch-Panadero E, Mas S, Sanchez-Ospina D, Camarero V, Pérez-Gómez MV, Saez-Calero I, Abaigar P, Ortiz A, Egido J, González-Parra E. The Choice of Hemodialysis Membrane Affects Bisphenol A Levels in Blood. J Am Soc Nephrol 2016;27:1566–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lopez-Cervantes J, Paseiro-Losada P. Determination of bisphenol A in, and its migration from, PVC stretch film used for food packaging. Food Addit Contam 2003;20:596–606. [DOI] [PubMed] [Google Scholar]
- 22.Pollack GM, Buchanan JF, Slaughter RL, Kohli RK, Shen DD. Circulating concentrations of di(2-ethylhexyl) phthalate and its de-esterified phthalic acid products following plasticizer exposure in patients receiving hemodialysis. Toxicol Appl Pharm 1985;79:257–267. [DOI] [PubMed] [Google Scholar]
- 23.Plonait SL, Nau H, Maier RF, Wittfoht W, Obladen M. Exposure of newborn infants to di-(2- ethylhexyl)-phthalate and 2-ethylhexanoic acid following exchange transfusion with polyvinylchloride catheters. Transfusion 1993;33:598–605. [DOI] [PubMed] [Google Scholar]
- 24.Frederiksen H, Skakkebaek NE, Andersson AM. Metabolism of phthalates in humans. Mol Nutr Food Res 2007;51:899–911. [DOI] [PubMed] [Google Scholar]
- 25.Rubin RJ, Jaeger RJ. Some pharmacologic and toxicologic effects of di-2-ethylhexyl phthalate (DEHP) and other plasticizers. Environ Health Perspect 1973;3:53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gillum N, Karabekian Z, Swift LM, Brown RP, Kay MW, Sarvazyan N. Clinically relevant concentrations of di (2-ethylhexyl) phthalate (DEHP) uncouple cardiac syncytium. Toxicol Appl Pharm 2009;236:25–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aronson CE, Serlick ER, Preti G. Effects of di-2-ethylhexyl phthalate on the isolated perfused rat heart. Toxicol Appl Pharm 1978;44:155–169. [DOI] [PubMed] [Google Scholar]
- 28.Posnack NG, Lee NH, Brown R, Sarvazyan N. Gene expression profiling of DEHP-treated cardiomyocytes reveals potential causes of phthalate arrhythmogenicity. Toxicology 2011;279:54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Posnack NG, Swift LM, Kay MW, Lee NH, Sarvazyan N. Phthalate exposure changes the metabolic profile of cardiac muscle cells. Environ Health Perspect 2012;120:1243–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rock G, Labow RS, Franklin C, Burnett R, Tocchi M. Hypotension and cardiac arrest in rats after infusion of mono(2-ethylhexyl) phthalate (MEHP), a contaminant of stored blood. N Engl J Med 1987;316:1218–1219. [DOI] [PubMed] [Google Scholar]
- 31.Jaimes R 3rd, Swiercz A, Sherman M, Muselimyan N, Marvar PJ, Posnack NG. Plastics and cardiovascular health: phthalates may disrupt heart rate variability and cardiovascular reactivity. Am J Physiol Heart Circ Physiol 2017;313:H1044–h1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Posnack NG, Jaimes R 3rd, Asfour H, Swift LM, Wengrowski AM, Sarvazyan N, Kay MW. Bisphenol A exposure and cardiac electrical conduction in excised rat hearts. Environ Health Perspect 2014;122:384–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deutschmann A, Hans M, Meyer R, Haberlein H, Swandulla D. Bisphenol A inhibits voltage-activated Ca(2+) channels in vitro: mechanisms and structural requirements. Mol Pharmacol 2013;83:501–511. [DOI] [PubMed] [Google Scholar]
- 34.Asano S, Tune JD, Dick GM. Bisphenol A activates Maxi-K (K(Ca)1.1) channels in coronary smooth muscle. Br J Pharmacol 2010;160:160–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wong MCG, Kalman JM, Pedagogos E, Toussaint N, Vohra JK, Sparks PB, Sanders P, Kistler PM, Halloran K, Lee G, Joseph SA, Morton JB. Bradycardia and Asystole Is the Predominant Mechanism of Sudden Cardiac Death in Patients With Chronic Kidney Disease. J Am Coll Cardiol 2015;65:1263–1265. [DOI] [PubMed] [Google Scholar]
- 36.Zhao Y, Chen NX, Shirazi JT, Shen C, Lin SF, Fishbein MC, Moe SM, Chen PS. Subcutaneous nerve activity and mechanisms of sudden death in a rat model of chronic kidney disease. Heart Rhythm 2016;13:1105–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Waks JW, Tereshchenko LG, Parekh RS. Electrocardiographic predictors of mortality and sudden cardiac death in patients with end stage renal disease on hemodialysis. J Electrocardiol 2016;49:848–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burton JO, Jefferies HJ, Selby NM, McIntyre CW. Hemodialysis-induced cardiac injury: determinants and associated outcomes. Clin J Am Soc Nephrol 2009;4:914–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tereshchenko LG, Sotoodehnia N, Sitlani CM, et al. Genome-Wide Associations of Global Electrical Heterogeneity ECG Phenotype: The ARIC (Atherosclerosis Risk in Communities) Study and CHS (Cardiovascular Health Study). J Am Heart Assoc 2018;7:e008160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tereshchenko LG, Kim ED, Oehler A, et al. Electrophysiologic Substrate and Risk of Mortality in Incident Hemodialysis. J Am Soc Nephrol 2016;27:3413–3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Waks JW, Sitlani CM, Soliman EZ, et al. Global Electric Heterogeneity Risk Score for Prediction of Sudden Cardiac Death in the General Population: The Atherosclerosis Risk in Communities (ARIC) and Cardiovascular Health (CHS) Studies. Circulation 2016;133:2222–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Collins AJ, Foley RN, Chavers B, et al. ‘United States Renal Data System 2011 Annual Data Report: Atlas of chronic kidney disease & end-stage renal disease in the United States. Am J Kidney Dis. 2012;59:A7, e1–A7,420. [DOI] [PubMed] [Google Scholar]
- 43.de Bie MK, van Dam B, Gaasbeek A, van Buren M, van Erven L, Bax JJ, Schalij MJ, Rabelink TJ, Jukema JW. The current status of interventions aiming at reducing sudden cardiac death in dialysis patients. Eur Heart J 2009;30:1559–1564. [DOI] [PubMed] [Google Scholar]
- 44.Karnik JA, Young BS, Lew NL, Herget M, Dubinsky C, Lazarus JM, Chertow GM. Cardiac arrest and sudden death in dialysis units. Kidney Int 2001;60:350–357. [DOI] [PubMed] [Google Scholar]
- 45.Whitman IR, Feldman HI, Deo R. CKD and sudden cardiac death: epidemiology, mechanisms, and therapeutic approaches. J Am Soc Nephrol 2012;23:1929–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ravani P, Quinn R, Oliver M, Robinson B, Pisoni R, Pannu N, MacRae J, Manns B, Hemmelgarn B, James M, Tonelli M, Gillespie B. Examining the Association between Hemodialysis Access Type and Mortality: The Role of Access Complications. Clin J Am Soc Nephrol 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Floege J, Gillespie IA, Kronenberg F, Anker SD, Gioni I, Richards S, Pisoni RL, Robinson BM, Marcelli D, Froissart M, Eckardt KU. Development and validation of a predictive mortality risk score from a European hemodialysis cohort. Kidney Int 2015;87:996–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bleyer AJ, Hartman J, Brannon PC, Reeves-Daniel A, Satko SG, Russell G. Characteristics of sudden death in hemodialysis patients. Kidney Int. 2006;69:2268–2273. [DOI] [PubMed] [Google Scholar]
- 49.Foley RN, Gilbertson DT, Murray T, Collins AJ. Long interdialytic interval and mortality among patients receiving hemodialysis. N Engl J Med 2011;365:1099–1107. [DOI] [PubMed] [Google Scholar]
- 50.Perl J, Chan CT. Timing of sudden death relative to the hemodialysis procedure. Nat Clin Pract Nephrol 2006;2:668–669. [DOI] [PubMed] [Google Scholar]
- 51.Koch HM, Preuss R, Angerer J. Di(2-ethylhexyl)phthalate (DEHP): human metabolism and internal exposure-- an update and latest results. Int J Androl 2006;29:155–165; discussion 1810-155. [DOI] [PubMed] [Google Scholar]
- 52.Kessler W, Numtip W, Volkel W, Seckin E, Csanady GA, Putz C, Klein D, Fromme H, Filser JG. Kinetics of di(2-ethylhexyl) phthalate (DEHP) and mono(2-ethylhexyl) phthalate in blood and of DEHP metabolites in urine of male volunteers after single ingestion of ring-deuterated DEHP. Toxicol Appl Pharmacol 2012;264:284–291. [DOI] [PubMed] [Google Scholar]
- 53.Koch HM, Angerer J, Drexler H, Eckstein R, Weisbach V. Di(2-ethylhexyl)phthalate (DEHP) exposure of voluntary plasma and platelet donors. Int J Hyg Environ Health 2005;208:489–498. [DOI] [PubMed] [Google Scholar]
- 54.Volkel W, Colnot T, Csanady GA, Filser JG, Dekant W. Metabolism and kinetics of bisphenol a in humans at low doses following oral administration. Chem Res Toxicol 2002;15:1281–1287. [DOI] [PubMed] [Google Scholar]
- 55.Shin BS, Kim CH, Jun YS, Kim DH, Lee BM, Yoon CH, Park EH, Lee KC, Han SY, Park KL, Kim HS, Yoo SD. Physiologically based pharmacokinetics of bisphenol A. J Toxicol Environ Health A 2004;67:1971–1985. [DOI] [PubMed] [Google Scholar]
- 56.Nassberger L, Arbin A, Ostelius J. Exposure of patients to phthalates from polyvinyl chloride tubes and bags during dialysis. Nephron 1987;45:286–290. [DOI] [PubMed] [Google Scholar]
- 57.Bernard L, Decaudin B, Lecoeur M, Richard D, Bourdeaux D, Cueff R, Sautou V, Armed Study G. Analytical methods for the determination of DEHP plasticizer alternatives present in medical devices: a review. Talanta 2014;129:39–54. [DOI] [PubMed] [Google Scholar]