Abstract
Bone metastasis is a major cause of prostate cancer (PCa) mortality. Although docetaxel chemotherapy initially extends patients’ survival, in most cases PCa becomes chemoresistant and eventually progresses without a cure. In this study, we developed a novel small-molecule compound BKM1972, which exhibited potent in vitro cytotoxicity in PCa and other cancer cells regardless of their differences in chemo-responsiveness. Mechanistic studies demonstrated that BKM1972 effectively inhibited the expression of anti-apoptotic protein survivin and membrane-bound efflux pump ATP binding cassette B 1 (ABCB1, p-glycoprotein), presumably via signal transducer and activator of transcription 3 (Stat3). BKM1972 was well tolerated in mice and as a monotherapy, significantly inhibited the intraosseous growth of chemosensitive and chemoresistant PCa cells. These results indicate that BKM1972 is a promising small-molecule lead to treat PCa bone metastasis and overcome docetaxel resistance.
Keywords: Prostate cancer, bone metastasis, chemoresistance, small-molecule therapy, preclinical model
1. Introduction
The American Cancer Society estimates that 164,690 new cases of prostate cancer (PCa) will be diagnosed and 29,430 patients will die in 2018 [1]. More than 85% of PCa patients show evidence of skeletal metastasis at autopsy, with a median survival between 30 to 35 months [2, 3]. As a first-line standard of care for PCa bone metastasis, docetaxel chemotherapy initially extends the overall survival of patients by approximately 3~4 months [4]. Nonetheless, in most cases PCa becomes chemoresistant and eventually progresses without a cure. Clearly, it is imperative to develop novel targeted therapy to overcome docetaxel chemoresistance and treat PCa bone metastasis.
Survivin is the smallest member of the inhibitor of apoptosis (IAP) family and expressed in virtually all solid tumors, thereby positioning itself as a genuine “oncofetal” protein and a prominent target for cancer drug development. Survivin overexpression has been frequently associated with high Gleason scores, poor clinical outcome and therapeutic resistance in PCa [5–10]. Previously, we provided the first evidence indicating a positive correlation between survivin expression and clinical PCa bone metastasis, an observation supported by other studies [10–12]. We further showed that inhibition of survivin by small molecules or natural compounds could sensitize PCa cells to docetaxel treatment, resulting a synergistic suppression of tumor growth in mouse skeletons [13, 14]. These results indicate that targeting survivin is a promising strategy to overcome therapeutic resistance and treat PCa bone metastasis. However, although several survivin inhibitors have entered clinical trials in PCa patients, these agents only demonstrated very limited survival benefits [15, 16], highlighting an unmet need to develop more effective survivin inhibitors for clinical testing.
Upregulation of ATP-binding cassette (ABC) transporters is a major mechanism of drug resistance in most cancer types. ABC sub-family B member 1 (ABCB1), also known as multidrug resistance 1 (MDR1) or p-glycoprotein, mediates energy-dependent drug efflux and is highly expressed in chemoresistant cancer cells [17, 18]. A direct correlation has been identified between ABCB1 expression and PCa grade and prostate specific antigen (PSA) levels, indicating a significant role of ABCB1 in PCa progression [19]. In various experimental models of acquired chemoresistance, ABCB1 is frequently identified as one of top upregulated genes. Consistently, suppression of ABCB1 expression and/or activity effectively reverses chemoresistance in these cellular models [20, 21].
We have developed several bradykinin antagonist mimetics (BKM) and demonstrated their anticancer activities in the experimental models of lung cancer, ovarian cancer, glioblastoma and PCa [11, 14, 22–25]. Herein, we report that BKM1972, a novel BKM compound with an aminobisphosphonate moiety, exhibits unique activities against the in vitro and in vivo growth of bone metastatic PCa cells. Mechanistically, BKM1972 effectively inhibits the expression of survivin and ABCB1, thereby inducing apoptosis in both chemosensitive and chemoresistant PCa cells.
2. Materials and Methods
2.1. Chemical Synthesis
BKM1972 (Bcpa-Bip-AMDP(OEt)4, C38H44Cl2N2O8P2; 790 Dalton; refer to Abbreviations) was synthesized using a two-step procedure as we described previously [26] (Fig. S1). BKM1972 was purified by preparative reversed-phase high-performance liquid chromatography (RP-HPLC). The purity (≥ 99.0%) of final products was cross-checked by analytical RP-HPLC and liquid chromatography-mass spectrometry.
2.2. Cell Culture
Human PCa cell lines ARCaPM, C4–2 and luciferase-tagged C4–2-Luc [27–29] were provided by Dr. Leland WK Chung (Cedars-Sinai Medical Center) in 2004, C4–2B cells and its docetaxel-resistant derivative C4–2B-TaxR subline [20] were provided by Dr. Allen C. Gao (University of California Davis) in 2016. ARCaPM and C4–2 cells were routinely maintained in T-medium (Life Technologies, Carlsbad, CA) with 5% fetal bovine serum (FBS; Atlanta Biologicals, Atlanta, GA). C4–2-Luc cells were cultured in T-medium supplemented with 5% FBS and 400 μg/ml G418. C4–2B-TaxR cells were routinely maintained in RPMI1640 medium supplemented with 10% FBS and 100 nM docetaxel (LC Laboratories, Woburn, MA). The final concentration of docetaxel in C4–2B-TaxR cultures was reduced to 5 nM before experimental assays. CWR22Rv1 cells were provided by Dr. Jin-Tang Dong (Emory University) in 2016, and maintained in RPMI1640 2% L-glutamine (Thermo Fisher Scientific., Waltham, MA) supplemented with 10% FBS, 1.5 g/L sodium bicarbonate, 4.5 g/L glucose, 10 mM HEPES and 10 mM sodium pyruvate. Multidrug-resistant cell lines KB-C-1, KB-V-1 and chemosensitive lines KB-3–1 cell and KB-85 were provided by Dr. Zhuo G Chen (Emory University) in 2010 and routinely maintained as described in [30]. All cell lines were authenticated by the providers and were tested negative for mycoplasma. All cells were passaged for less than 3 months before renewal from frozen, early-passage stocks.
2.3. Cell Viability Assay
The in vitro cytotoxicity of BKM1972 in the NCI-60 panel of human cancer cell lines was evaluated at the National Cancer Institute Developmental Therapeutics Program (Rockville, Maryland) following the procedures described at https://dtp.cancer.gov/discovery_development/nci-60/methodology.htm. CellTiter 96 AQueous non-radioactive cell proliferation assay kit (Promega, Madison, WI) or cell counting kit-8 (CCK-8, Dojindo Molecular Technologies, Inc., Rockville, MD) were used in cell viability assays following the manufacturers’ instructions. Dimethyl sulfoxide (DMSO) was purchased from Sigma-Aldrich (St. Louis, MO) and used as the vehicle control in all in vitro and in vivo experiments. The half minimal inhibitory concentration (IC50) of specified agent was calculated with SigmaPlot program (Systat Software Inc., San Jose, CA).
2.4. Apoptosis and Cell Cycle Analyses
For apoptosis assay, PCa cells treated with DMSO or BKM1972 were stained with an APC Annexin V Apoptosis Detection Kit (BioLegend, San Diego, CA) following the manufacturer’s instruction. For cell cycle assay, PCa cells were stained with propidium iodide (20 μg/ml, Sigma-Aldrich) at 37°C for 2h. Apoptosis and cell cycle were measured by flow cytometry with FACSCanto II flow cytometer (BD Biosciences, Bedford, MA) and results were analyzed using FlowJo software (Tree Star, Inc., Ashland, OR).
2.5. Cellular Uptake of Fluorescent Dye-Conjugated Paclitaxel
PCa cells pre-treated with DMSO or BKM1972 were incubated with paclitaxel-Oregon™ green 488 (Life Technologies) at 37°C for different times, then washed three times with cold PBS and resuspended. Drug accumulation in live cells was recorded with a confocal microscope or flow cytometry.
2.6. Western Blot Analysis
Total cell lysates were prepared using radioimmunoprecipitation (RIPA) buffer (Santa Cruz Biotechnology). Nuclear proteins were prepared using NucBuster Protein Extraction Kit (Novagen, Darmstadt, Germany). Western blot analyses were performed following standard procedures. Antibodies used in Western blotting are listed in Table S1.
2.7. Polymerase Chain Reaction (PCR) Analysis
Total RNA was isolated using Qiagen RNeasy Kit (Valencia, CA). cDNA was synthesized using SuperScript® III First-Strand Synthesis System (Life Technologies). Regular PCR was performed as we described previously [11]. Real-time PCR was performed with Stratagene Mx3005P system (Agilent technologies) using a Brilliant® SYBR® Green QPCR Master Mix (Stratagene, Santa Clara, CA). The primers used for PCR reactions are listed in Table S2.
2.8. Reporter Assay
PCa cells were seeded at a density of 1 × 105 cells per well in 24-well plates 24 h before transfection. Human survivin reporters (pSurvivin-Luc1430 and pSurvivin-Luc230; kindly provided by Dr. Allen Gao) [13] or ABCB1 reporter (pMDR1–221 and pMDR1–1202; Addgene) [31] were transfected with pRL-TK (internal control; Promega) using Lipofectamine 2000 (Invitrogen) following the manufacturer’s instructions. Luciferase activities were measured at the indicated times using a Dual-Luciferase reporter assay system (Promega). Relative luciferase units were expressed as firefly luciferase intensity normalized to Renilla luciferase activity.
2.9. Repeat-Dose Toxicity
All animal procedures were performed in compliance with Emory University and Augusta University Institutional Animal Care and Use Committee (IACUC) and National Institutes of Health guidelines. Male athymic nude mice (5-week old, Envigo RMS, Inc, Indianapolis, IN) were randomly divided into 3 groups and received intraperitoneal injection of vehicle control (100% DMSO) or BKM1972 at two doses (10 mg/kg and 20 mg/kg), three times per week, for 3 weeks. Body weights were recorded twice per week, animal behaviors were closely observed. At the end point, major organs were examined for any abnormality.
2.10. Intratibial Xenograft Model
A total of 2×106 C4–2-Luc or C4–2B-TaxR cells were inoculated into the bilateral tibia of male athymic nude mouse (5-week old). Following the confirmation of tumor formation by rising PSA levels in mouse sera, mice were randomized into different groups and treated with vehicle control or BKM1972 at the indicated doses, three times per week, via intraperitoneal route. Docetaxel treatment in the C4–2B-TaxR model was administered via intraperitoneal injection at the dosage of 2.5 mg/kg, once per week. Mice were weighed twice per week, and tumor growth in bilateral tibia was followed by serum PSA using an enzyme-linked immunosorbent assay (ELISA) kit from United Biotech, Inc (Mountain View, CA). At the end point, X-ray radiography was performed using MX-20 System (Faxitron, Tucson, Arizona). Tumor-bearing tibias were harvested and fixed in 10% neutralized formalin for future analyses. Haematoxylin and eosin (H&E) and tartrate resistant acid phosphatase (TRAP) studies were performed following standard procedures.
2.11. Statistical Analysis
All in vitro data represent three or more experiments. The unpaired t-test was used to determine the significance of differences between any two groups. Two-way ANOVA was used to test the overall difference between different treatment groups during the whole study period. Prism 7.03 program (GraphPad Software Inc., La Jolla, CA) was used to perform the statistical analyses. p < 0.05 was considered as statistically significant.
3. Results
3.1. BKM1972 is a novel survivin inhibitor and exhibits potent cytotoxicity in human cancer cells
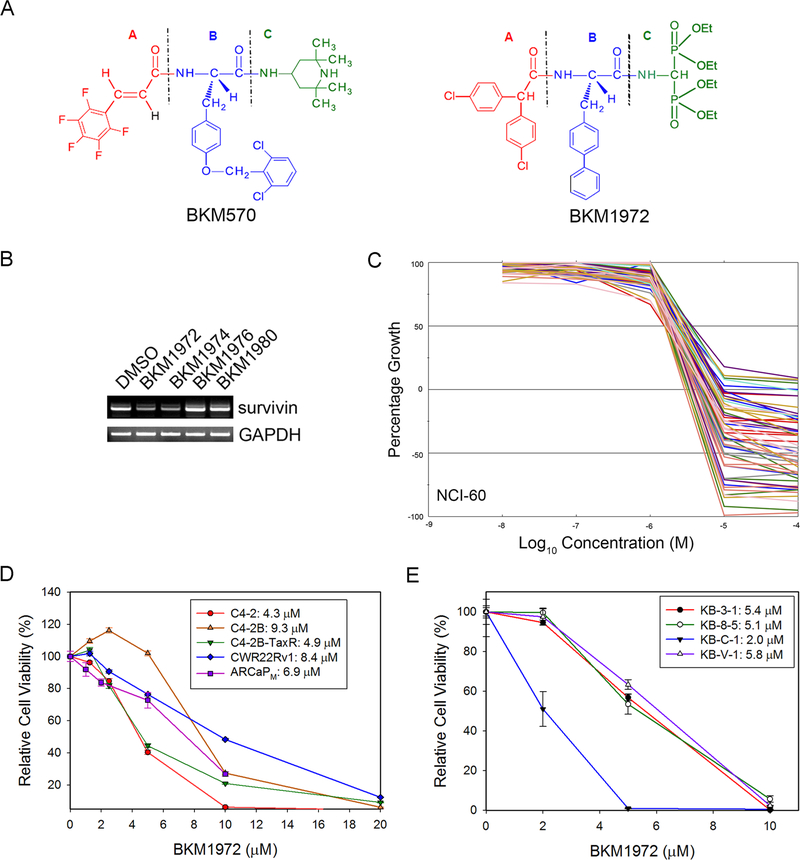
Given the prominent targeting potential of survivin in bone metastatic PCa, we have developed several novel survivin inhibitors and investigated their anticancer activities in experimental models. These small molecules are chemical analogs of BKM570, our first-generation BKM compound with a 3-component “A-B-C” structure, where the “B” component is a tyrosine residue [2,3,4,5,6-pentaflurocinnamoyl-(O-2,6-dichlorobenzyl)-tyrosine, or F5c-OC2Y] (Fig. 1A, left). Our previous studies indicated that the F5c-OC2Y moiety is indispensable to the anticancer activity of BKM570 in lung cancer and ovarian cancer cells [22, 24, 32]. By replacing the (“A” component) in BKM570 with an amino-bisphosphonate moiety, we generated a group of new BKM compounds with the intention of increasing their selective deposit in bone tissues (Fig. 1A, right). These compounds were screened for their capabilities of suppressing survivin expression in PCa cells, as exemplified in Fig. 1B. BKM1972 was identified as a potent inhibitor of survivin transcription.
Figure 1. BKM1972 suppresses survivin expression and inhibits the in vitro viability of human cancer cell lines.
(A) Chemical structures of BKM570 and its analog BKM1972. Note both compounds have a 3-moiety “A-B-C” core structure. (B) PCR analysis of the effect of several BKM570 analogs on survivin mRNA expression in C4–2 cells (5 μM, 24 h). (C) A summary of the in vitro cytotoxicity of BKM1972 in the NCI-60 panel (48 h). GI50 is the concentration that causes 50% growth inhibition, relative to the vehicle control. (D) MTS assay of the in vitro cytotoxicity of BKM1972 in established PCa cell lines (72 h). (E) MTS assay of the in vitro cytotoxicity of BKM1972 in lineage-derived KB cells (72 h).
To obtain an unbiased evaluation of the anticancer activity of BKM1972, we submitted the compound to the National Cancer Institute Developmental Therapeutics Program. As shown in Fig. 1C and Supplementary Information (Fig. S2-S4), BKM1972 induced growth inhibition and cell death across a broad spectrum of human cancer cells in the NCI-60 panel, with cytotoxicities comparable with common chemotherapy drugs such as cisplatin, etoposide and doxorubicin [33]. In vitro analyses (Fig. 1D) further showed that BKM1972 effectively inhibited the viability of multiple PCa cell lines, including castration-resistant C4–2, C4–2B, CWR22RV1 and ARCaPM cells [27–29]. Intriguingly, BKM1972 had lower IC50 value (4.9 μM) in C4–2B-TaxR cells, a C4–2B derivative that is highly resistant to docetaxel treatment [20], than in the parental C4–2B cells (IC50 = 9.3 μM). These results indicated that BKM1972 is a potent inhibitor of both chemosensitive and chemoresistant PCa cells. To test this notion, we further examined the in vitro cytotoxicity of BKM1972 in a panel of human cancer cell lines widely used in the study of chemoresistance, i.e., the chemosensitive KB-3–1 line and its lineage-derived, chemoresistant lines KB-8–5, KB-C-1 and KB-V1, which is more resistant to paclitaxel treatment than KB-3–1 by 16-, 117- and 4,000-fold, respectively [34, 35]. The IC50 of BKM1972 in these cell lines was determined as 5.4 μM (KB-3–1), 5.1 μM (KB-8–5), 2.0 μM (KB-C-1) and 5.8 μM (KB-V-1), respectively (Fig. 1E). Taken together, these data indicated that BKM1972 is a potent inhibitor of chemoresistant cancer cells.
3.2. BKM1972 inhibits survivin expression via signal transducer and activator of transcription 3 (Stat3) signaling
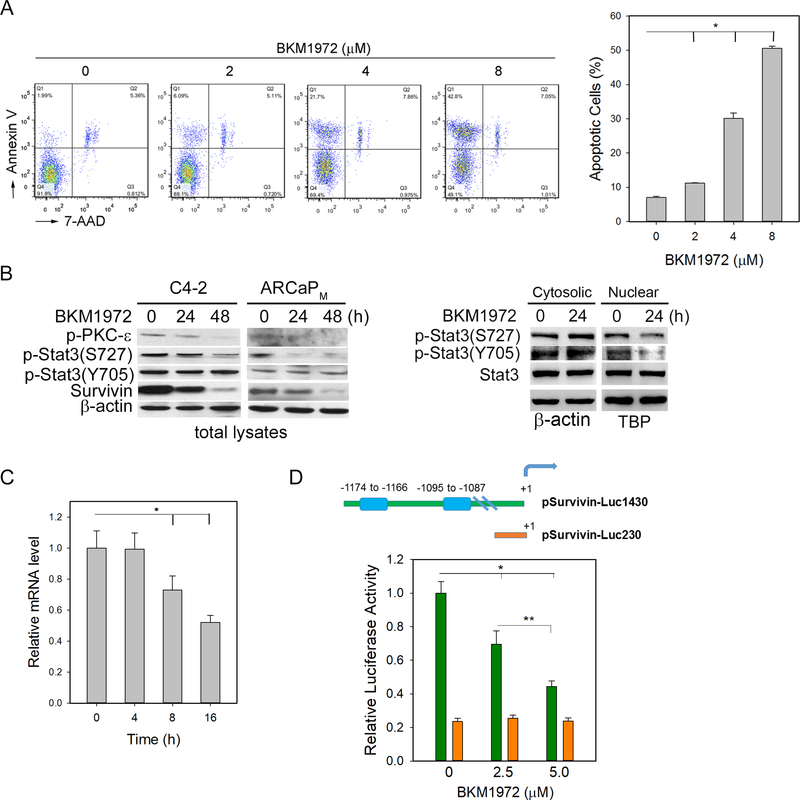
Flow cytometry showed that BKM1972 increased the surface expression of Annexin V in C4–2 cells in a dose-dependent manner (Fig. 2A), suggesting that BKM1972 inhibited cell viability by activating apoptosis. We further examined the effect of BKM1972 on the expression of survivin and its potential upstream regulators, including Stat3 [36], in the total protein lysates from C4–2 and ARCaPM cells (Fig. 2B, left; Fig. S5). Western blot analyses showed that BKM1972 inhibited protein expression of survivin and phosphorylated Stat3 (p-Stat3) at serine 727 (S727). In comparison, the changes in p-Stat3 at tyrosine 705 (Y705) in the total protein lysates were less significant. Phosphorylated protein kinase C-ε (p-PKC-ε), a known Stat3-interacting protein that activates p-Stat3(S727) [37, 38], was reduced upon BKM1972 treatment.
Figure 2. BKM1972 inhibits Stat3 signaling and suppresses survivin transcription in PCa cells.
(A) Flow cytometry assay of Annexin V expression in C4–2 cells treated with varying concentrations of BKM1972 (72 h). *: p < 0.001. (B) Left: protein expression of p-PKC-ɛ(S729), p-Stat3, Stat3 and survivin in C4–2 and ARCaPM cells treated with 5 μM BKM1972 for the indicated times. β-actin was used as the loading control; Right: Expression of p-Stat3 and Stat3 in the cytosolic and nuclear lysates from C4–2 cells treated with BKM1972 (5 μM). TATAbinding protein (TBP) was used as the loading control for nuclear proteins. (C) PCR analysis of survivin mRNA levels in C4–2 cells treated with BKM1972 (5 μM) at the indicated times. *, **: p < 0.05. (D) Upper: Human survivin reporter pSurvivin-Luc1430 contains two putative Stat3-binding cis-elements (blue bars), which are absent in the deletion construct pSurvivin-Luc230. The transcriptional start site was as +1; Bottom: Relative luciferase activity of pSurvivin-Luc1430 and pSurvivin-Luc230 in C4–2 cells treated with BKM1972 at the indicated concentrations (48 h). *: p < 0.01.
Nuclear presence of p-Stat3 is indicative of Stat3-dependent transcription activation [39, 40]. Western blot analyses showed that BKM1972 reduced the nuclear expression of both p-Stat3(S727) and p-Stat3(Y705) in C4–2 cells within 24 h. Consistently, there was a slight increase in the cytosolic expression of both phosphorylated forms of Stat3 protein (Fig. 2B, right). These results confirmed the inhibitory effect of BKM1972 on Stat3 activation in PCa cells.
Real-time PCR analyses showed that BKM1972 treatment resulted in a rapid reduction of survivin mRNA (Fig. 2C). Interestingly, BKM1972 significantly reduced the luciferase activity of pSurvivin-Luc1430 reporter that contains a 1,430-bp region of human survivin promoter [41], but not of pSurvivin-Luc230 that does not contain two putative Stat3 cis-elements located between −1,174 to −1,166 nt and between −1,095 to −1,087 nt (Fig. 2D). These results indicated that BKM1972 may inhibit survivin transcription through a Stat3-dependent mechanism.
3.3. BKM1972 inhibits ABCB1 expression and enhances drug uptake in chemoresistant PCa cells
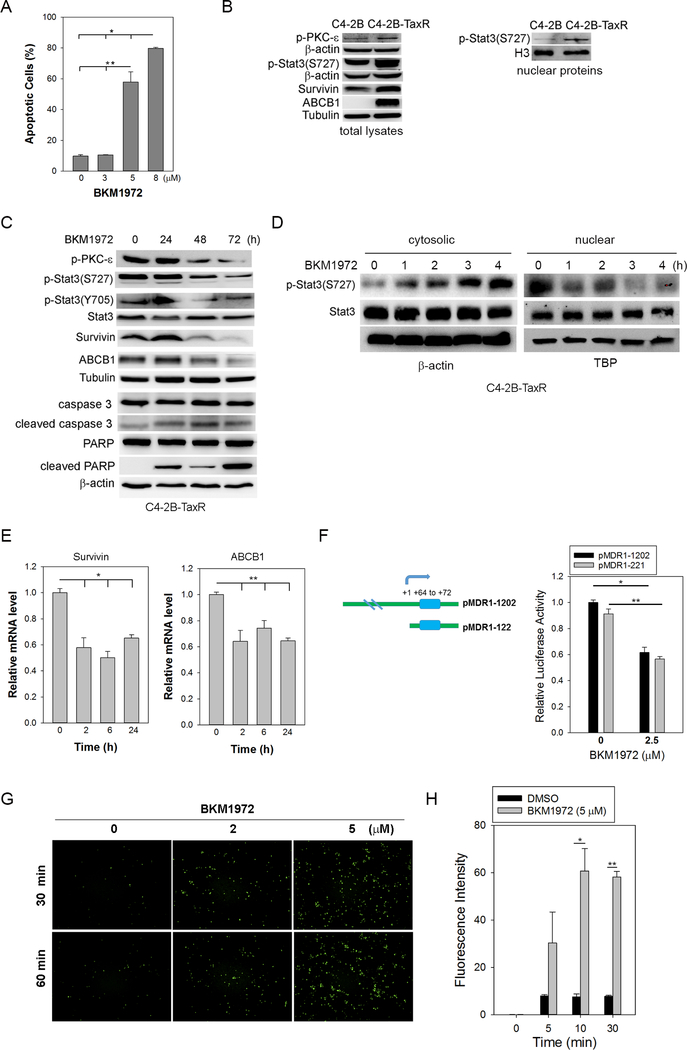
A unique feature of BKM1972 is that the compound exhibited high cytotoxicity in chemoresistant cancer cells, such as C4–2B-TaxR, KB-8–5, KB-C-1 and KB-V1 (Fig. 1D, 1E). Flow cytometry analyses showed that BKM1972 effectively induced apoptosis in a dose-dependent manner in C4–2B-TaxR cells (Fig. 3A, Fig. S6). Consistent with previous reports [20, 21, 42], C4–2B-TaxR cells expressed significantly higher level of ABCB1 protein compared to parental C4–2B cells. Interestingly, expression of survivin, p-Stat3(S727) and p-PKC-ɛ(S729) was markedly increased in the total protein lysates of C4–2B-TaxR cells (Fig. 3B, left). Western blot analysis also revealed a significantly higher level of nuclear expression of p-Stat3(S727) in C4–2B-TaxR cells (Fig. 3B, right), indicating the activation of Stat3 signaling in chemoresistant PCa cells.
Figure 3. LG1980 inhibits the expression of survivin and ABCB1 and induces apoptosis in chemoresistant PCa cells.
(A) Flow cytometry assay of Annexin V expression in C4–2B-TaxR cells treated with varying concentrations of BKM1972 (72 h). *, **: p < 0.01. (B) Left: protein expression of p-PKC-ε(Ser729), p-Stat3(Ser727), survivin and ABCB1 in C4–2B-TaxR and parental C4–2B cells. Α-tubulin and β-actin were used as the loading controls; Right: Western blot analyses of nuclear expression of p-Stat3(S727) in C4–2B and C4–2B-TaxR cells. H3 histone was used as the loading control for nuclear proteins. (C) Western blot analyses of C4–2B-TaxR cells treated with BKM1972 (5 μM) for the indicated times. (D) Western blot analyses of the cytosolic and nuclear proteins in C4–2B-TaxR cells treated with BKM1972 (5 μM) for the indicated times. (E) PCR analysis of mRNA expression of survivin and ABCB1 in C4–2B-TaxR cells treated with BKM1972 (5 μM) for the indicated times. *, **: p < 0.05. (F) Left: The sketch map of putative Stat3 binding motif (blue bar) within human ABCB1 promoter. The transcriptional start site was as +1; Right: Relative luciferase activity of ABCB1 reporters (pMDR1–221 and pMDR1–1202) in C4–2B-TaxR cells treated with varying concentrations of BKM1972 (48 h). *, **: p < 0.01. (G) Fluorescence microscopy images of cellular uptake of Oregon Green 488-paclitaxel in C4–2B-TaxR cells. Cells were first treated with varying concentrations of BKM1972 for 72 h prior to paclitaxel incubation for the indicated times. (H) Flow cytometry assay of Oregon Green 488-paclitaxel in C4–2B-TaxR cells pre-treated with BKM1972 (5 μM) for 72 h prior to paclitaxel incubation for the indicated times. *, **: p < 0.05.
BKM1972 treatment in C4–2B-TaxR cells resulted in significant reductions in the expression of p-PKC-ɛ(S729), p-Stat3(S727), survivin and ABCB1, and induced the cleavage of caspase-3 and poly ADP ribose polymerase (PARP) (Fig. 3C). We further determined the expression of total Stat3 and p-Stat3(S727) in the cytosolic and nuclear lysates of C4–2B-TaxR cells following short-term BKM1972 treatment. As shown in Fig. 3D, there was a rapid decrease in the nuclear expression of p-Stat3(S727) and a simultaneous increase in its cytosolic expression. In comparison, there was no significant change in total Stat3 expression in either cytosolic or nuclear portions. These results indicated that BKM1972 inhibited the nuclear translocation of p-Stat3(S727), thereby suppressing Stat3-dependent transcription.
At mRNA levels, BKM1972 rapidly suppressed the expression of both survivin and ABCB1 (Fig. 3E). Previously, it has been reported that Stat3 binds the DNA sequence at +64 ~ +72 region of human ABCB1 promoter and activates ABCB1 transcription (Fig. 3F, left) [43]. To confirm whether BKM1972 affected ABCB1 transcription, C4–2B-TaxR cells were transfected with a full-length human ABCB1 reporter containing the promoter sequence from −1,202 to +118 (pMDR1–1202) or its deletion construct pMDR1–221 [31], respectively, and further treated with BKM1972. As shown in Fig. 3F, BKM1972 effectively inhibited the luciferase activity of both reporters, indicating that the inhibitory effect of BKM1972 on ABCB1 expression may mainly occur at the transcriptional levels, presumably via the suppression of Stat3 signaling.
Since BKM1972 suppressed ABCB1 expression, we determined the in vitro uptake of chemotherapeutics in BKM1972-treated C4–2B-TaxR cells. Fluorescence microscopy found that BKM1972 treatment resulted in a rapid (within 30 min) accumulation of paclitaxel. In comparison, there was no obvious paclitaxel uptake until 60 min in the control cells (Fig. 3G). Flow cytometry further confirmed a significant increase of Oregon green 488-conjugated paclitaxel in BKM1972-treated C4–2B-TaxR cells (Fig. 3H). These results indicated that BKM1972 facilitated the uptake of chemotherapeutics in chemoresistant PCa cells.
3.4. In vivo toxicity of BKM1972
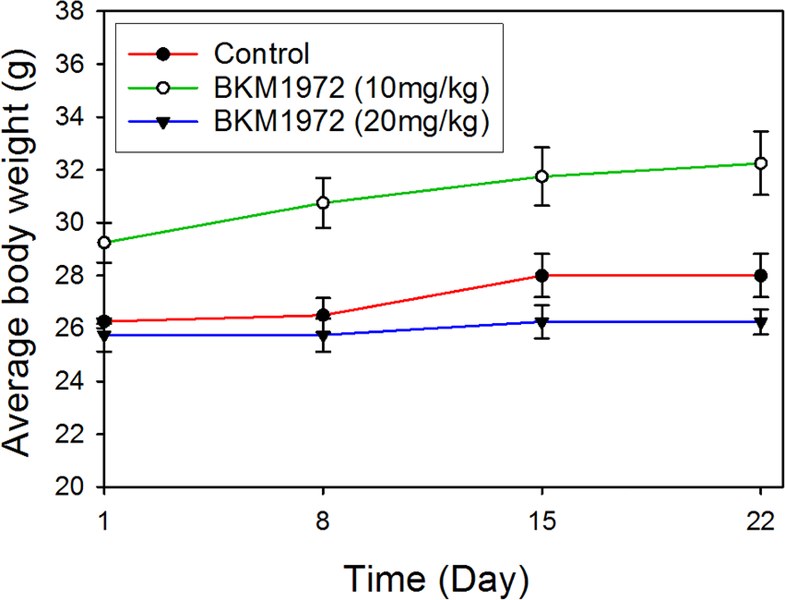
BKM1972 exhibited in vitro cytotoxicity in RWPE-1, an immortalized prostate epithelial cell line [44], with an IC50 of 6.7 μM (Fig. S7). To evaluate the repeat-dose sub-chronic toxicity of BKM1972, healthy athymic nude mice were treated with two doses of BKM1972 (10 mg/kg, 20 mg/kg) or vehicle control via intraperitoneal injection, three times per week, for 3 weeks. At the endpoint, the average body weight gain in each group was 6.6% (control), 10.3% (5 mg/kg BKM1972) and 1.9% (10 mg/kg BKM1972), respectively (Fig. 4). Ex vivo examination of major organs did not observe obvious abnormalities. These results indicated that BKM1972 did not have significant side effects in mice.
Figure 4.
Average body weights of athymic nude mice treated with BKM1972 (10 mg/kg or 20 mg/kg) or vehicle control, via intraperitoneal injection, three times per week, for 3 weeks (n = 5 per group).
3.5. BKM1972 inhibits the intratibial growth of C4–2 tumors
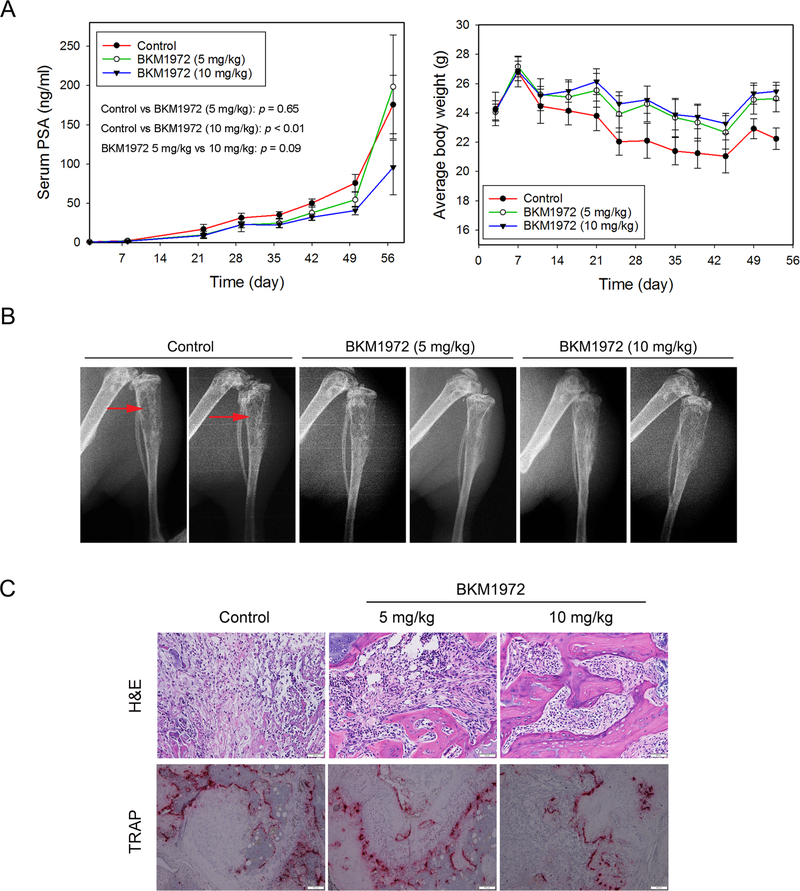
We evaluated the in vivo efficacy of BKM1972 in athymic nude mice bearing intratibial C4–2 tumors, which represent chemotherapy-naïve, bone metastatic and castration-resistant PCa [45]. Mice were treated with two escalating doses of BKM1972 (5 mg/kg and 10 mg/kg) or vehicle control respectively, via the intraperitoneal route, three times per week, for 8 weeks. Serum PSA level was monitored as the indicator of tumor growth in mouse bones. At the endpoint, the average PSA level of each group was determined as 175.6 ± 37.1 ng/ml (control), 198.3 ± 66.1 ng/ml (5 mg/kg BKM1972) and 95.7 ± 34.9 ng/ml (10 mg/kg BKM1972). Compared with the control, BKM1972 at 5 mg/kg effectively suppressed C4–2 tumor growth until day 50 (p = 0.015), but the statistical difference between the two groups disappeared on day 57 (p = 0.65). There was a significant difference in PSA values between the control group and 10 mg/kg BKM1972 group (p < 0.01) (Fig. 5A, left). BKM1972 treatment at both doses was associated with slight gains in average body weights, 3.8% in the 5 mg/kg group and 5.5% in the 10 mg/kg group. In comparison, there was a net loss (−8.4%) in the average body weight in the control group (Fig. 5A, right). X-ray radiography and histology demonstrated improved bone structure in mice treated with both doses of BKM1972 (Fig. 5B, 5C). Osteoclast activity determined by TRAP staining revealed that compared to the control group or 5 mg/kg BKM1972-treated tissues, there were fewer TRAP-positive cells in the bone tumor tissues treated with 10 mg/kg BKM1972 (Fig. 5C). These results demonstrated that BKM1972 inhibited the skeletal growth of C4–2 tumors in mice and potentially suppressed tumor-associated bone turnover.
Figure 5. BKM1972 inhibits the intratibial growth of C4–2 tumors in athymic nude mice.
(A) Left: Serum PSA levels of C4–2 tumor-bearing mice treated with vehicle control (n = 5), 5 mg/kg BKM1972 (n = 5) or 10 mg/kg BKM1972 (n = 6) for 8 weeks. Two-way ANOVA was used for all pairwise comparisons; Right: Average body weights of C4–2 tumor-bearing mice treated with vehicle or BKM1972 (5 mg/kg or 10 mg/kg). (B) X-ray radiography of tumor-bearing bones from different treatment groups. Red arrows indicate tumor-induced osteolytic lesions. (C) H&E and TRAP staining of C4–2 bone tumors from different treatment groups. Scale bar is 50 μM for H&E and 100 μM for TRAP respectively.
3.6. BKM1972 inhibits the intratibial growth of C4–2B-TaxR tumors
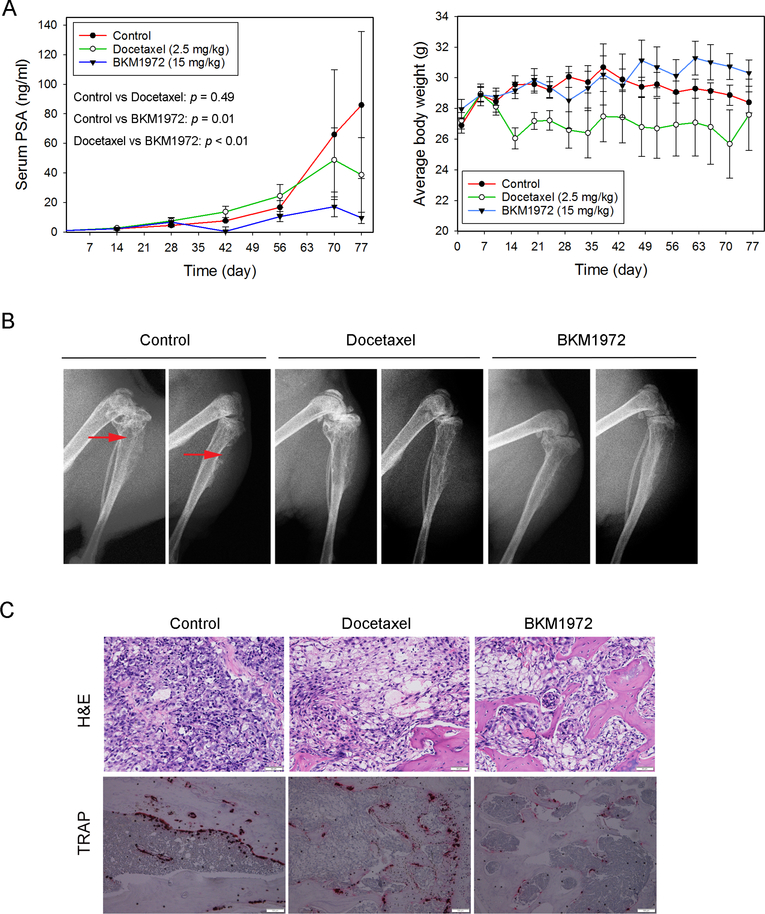
The in vivo efficacy of BKM1972 against chemoresistant PCa was evaluated in the intratibial xenografts of C4–2B-TaxR. Athymic nude mice were treated with vehicle control, docetaxel (2.5 mg/kg, once per week) or BKM1972 (15 mg/kg, three times per week) via intraperitoneal route, respectively, for 11 weeks. Compared with the control group, BKM1972 significantly retarded tumor growth (p = 0.01), as demonstrated by the reduction in average PSA levels (86.0 ± 49.7 ng/ml in the control group vs 9.7 ± 3.8 ng/ml in the BKM1972 group). In contrast, there was no statistical difference (p = 0.49) in the PSA levels between the control group and docetaxel-treated mice (38.8 ± 25.2 ng/ml) (Fig. 6A, left). All mice gained body weights at the endpoints, with an increase of 5.6% (control), 1.4% (docetaxel) and 8.4% (BKM1972) respectively (Fig. 6A, right). X-ray radiography and histology showed that BKM1972 treatment was associated with reduced osteolytic lesions in tumor-bearing tibias (Fig. 6B, 6C). Compared with the control or docetaxel-treated groups, there were fewer TRAP-positive cells in the BKM1972-treated bone tumor tissues (Fig. 6C). These results indicated that as a monotherapy, BKM1972 is efficacious in suppressing the skeletal growth of chemoresistant PCa.
Figure 6. BKM1972 inhibits the intratibial growth of C4–2B-TaxR tumors in athymic nude mice.
(A) Left: Serum PSA levels of C4–2B-TaxR tumor-bearing mice treated with vehicle control (n = 5), docetaxel (2.5 mg/kg, n = 7) or BKM1972 (15 mg/kg, n = 7) for 11 weeks. Two-way ANOVA was used for all pairwise comparisons; Right: Average body weights of C4–2B-TaxR tumor-bearing mice treated with vehicle, docetaxel or BKM1972. (B) X-ray radiography of tumor-bearing bones from different treatment groups. Red arrows: osteolytic lesions. (C) H&E and TRAP staining of C4–2B-TaxR bone tumors from different treatment groups. Scale bar is 50 μM for H&E and 100 μM for TRAP respectively.
4. Discussion
Docetaxel chemotherapy was hailed as a breakthrough in PCa management and approved in 2004 as the first-line treatment for metastatic castration-resistant PCa. Recent randomized trials have shown additional therapeutic benefits with docetaxel in newly diagnosed patients with high risk, high volume disease. In the STAMPEDE trial, the inclusion of docetaxel to standard hormonal therapy (with or without radiotherapy) extended the overall survival by 10 months in patients with hormone-naïve advanced PCa [46]. A similar survival benefit (13.6 months) was observed in the CHAARTED study [47]. These promising results indicated that docetaxel will remain a standard of care for advanced PCa. Unfortunately, however, in most cases PCa develops extreme resistance and continues to progress, which has no cure. Therefore, there is an urgent need to design new therapies overcoming docetaxel resistance and treating bone metastasis.
Overexpression of survivin has been associated with clinical PCa bone metastasis [11, 12]. In previous studies, we observed an increase in survivin expression following docetaxel treatment, and inhibition of survivin antagonized this inductive effect of docetaxel and sensitized PCa cells to chemotherapy [13, 14]. These results indicate that survivin-targeted strategy could be a promising approach to overcome docetaxel chemoresistance and treat PCa bone metastasis. Using a “molecular hybridization” strategy, we have designed a new generation BKM compounds that incorporate the anticancer moiety of BKM570, i.e., F5c-OC2Y, and AMDP(OEt)4, a tetraethyl ester derivative of bisphosphonate [26]. Here, we described the in vitro and in vivo activities of BKM1972, which was initially identified as a potential survivin inhibitor. Mechanistic studies further found that BKM1972 also suppressed the expression of ABCB1, a major membrane-bound transporter responsible for the efflux of a broad range of chemotherapeutics drugs. Significantly, BKM1972 effectively induced apoptosis in chemoresistant cancer cells with similar or higher potency compared with that in chemosensitive cells, and exhibited potent in vivo efficacy against the skeletal growth of both chemoresponsive and chemoresistant PCa in mouse models. These unique biological features position BKM1972 as a lead compound for the treatment of bone metastatic and chemoresistant PCa.
Constitutive activation of Stat3 signaling has been correlated to pathologic stage and Gleason score in PCa [48, 49]. Human survivin promoter contains several Stat3-binding cis-elements [36], and Stat3 activation increases ABCB1 transcription and multidrug resistance [43, 50, 51]. Interestingly, chemoresistant C4–2B-TaxR cells express higher levels of p-Stat3(S727) and p- PKCε(S729), a direct upstream activator of Stat3 serine phosphorylation [37, 38, 52], indicating that the activation of PKCε-Stat3 signaling may be an underlying mechanism for the upregulation of survivin and ABCB1 and acquired docetaxel resistance. Our results further showed that BKM1972 effectively suppressed the expression of p-PKCε(S729) and p-Stat3(S727), suggesting that BKM1972 may inhibit the expression of survivin and ABCB1 through a PKCε-Stat3-dependent mechanism, thereby promoting apoptosis in both chemosensitive and chemoresistant PCa cells.
BKM1972 was well tolerated in mice and exhibited excellent safety profile. With the demonstration of its in vivo efficacy against bone metastatic PCa in preclinical models, we will further investigate the pharmacological properties of BKM1972 as a drug candidate, including pharmacokinetics, absorption, distribution, metabolism and excretion. BKM1972 exhibited potent cytotoxicity in the NCI-60 panel, and effectively induced apoptosis in human cancer cells regardless of their difference in chemotherapeutic responsiveness. These results indicate that BKM1972 can be developed as a monotherapy to overcome chemoresistance in PCa and other cancers.
Supplementary Material
Highlights.
BKM1972 exhibits potent cytotoxicity in the NCI-60 human cancer cell panel
BKM1972 induces apoptosis in both chemosensitive and chemoresistant prostate cancer cells
BKM1972 inhibits the expression of survivin and ABCB1
BKM1972 inhibits the skeletal growth of PCa and overcomes docetaxel resistance in mice
Acknowledgements
This work was supported by the National Cancer Institute grants 1R41CA186498-01A1, 1R41CA206725-01A1 and 1R41CA217491-01A1, Georgia Research Alliance (Atlanta, GA) VentureLab Award, Georgia Cancer Center Startup Fund (DW), National Natural Science Foundation of China grant 81401759 (YC). We thank the National Cancer Institute for the NCI-60 assay and the Pathology Core Research Laboratory at University of Alabama at Birmingham for technical assistance in bone specimen preparation, histology and TRAP staining.
Abbreviations
- AMDP(OEt)4
1-aminomethylenebisphosphonic acid tetraethyl ester
- Atmp
4-amino-2,2,6,6-tetramethylpiperidine
- Bcpa
bis(4-Chlorophenyl)acetyl
- Bip
β-(4-biphenylyl)alanyl
- F5c
2,3,4,5,6-pentaflurocinnamoyl
- OC2Y
(O-2,6-dichlorobenzyl)tyrosyl
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Siegel RL, Miller KD, Jemal A, Cancer statistics, 2018, CA Cancer J Clin, 68 (2018) 7–30. [DOI] [PubMed] [Google Scholar]
- [2].Bubendorf L, Schopfer A, Wagner U, Sauter G, Moch H, Willi N, Gasser TC, Mihatsch MJ, Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients, Hum Pathol, 31 (2000) 578–583. [DOI] [PubMed] [Google Scholar]
- [3].Jacobs SC, Spread of prostatic cancer to bone, Urology, 21 (1983) 337–344. [DOI] [PubMed] [Google Scholar]
- [4].Pienta KJ, Smith DC, Advances in prostate cancer chemotherapy: a new era begins, CA Cancer J Clin, 55 (2005) 300–318; quiz 323–305. [DOI] [PubMed] [Google Scholar]
- [5].Kishi H, Igawa M, Kikuno N, Yoshino T, Urakami S, Shiina H, Expression of the survivin gene in prostate cancer: correlation with clinicopathological characteristics, proliferative activity and apoptosis, J Urol, 171 (2004) 1855–1860. [DOI] [PubMed] [Google Scholar]
- [6].Shariat SF, Lotan Y, Saboorian H, Khoddami SM, Roehrborn CG, Slawin KM, Ashfaq R, Survivin expression is associated with features of biologically aggressive prostate carcinoma, Cancer, 100 (2004) 751–757. [DOI] [PubMed] [Google Scholar]
- [7].Nomura T, Yamasaki M, Nomura Y, Mimata H, Expression of the inhibitors of apoptosis proteins in cisplatin-resistant prostate cancer cells, Oncol Rep, 14 (2005) 993–997. [PubMed] [Google Scholar]
- [8].Zhang M, Latham DE, Delaney MA, Chakravarti A, Survivin mediates resistance to antiandrogen therapy in prostate cancer, Oncogene, 24 (2005) 2474–2482. [DOI] [PubMed] [Google Scholar]
- [9].Zhang M, Ho A, Hammond EH, Suzuki Y, Bermudez RS, Lee RJ, Pilepich M, Shipley WU, Sandler H, Khor LY, Pollack A, Chakravarti A, Prognostic value of survivin in locally advanced prostate cancer: study based on RTOG 8610, Int J Radiat Oncol Biol Phys, 73 (2009) 1033–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Koike H, Sekine Y, Kamiya M, Nakazato H, Suzuki K, Gene expression of survivin and its spliced isoforms associated with proliferation and aggressive phenotypes of prostate cancer, Urology, 72 (2008) 1229–1233. [DOI] [PubMed] [Google Scholar]
- [11].Seo SI, Gera L, Zhau HE, Qian WP, Iqbal S, Johnson NA, Zhang S, Zayzafoon M, Stewart J, Wang R, Chung LW, Wu D, BKM1740, an acyl-tyrosine bisphosphonate amide derivative, inhibits the bone metastatic growth of human prostate cancer cells by inducing apoptosis, Clin Cancer Res, 14 (2008) 6198–6206. [DOI] [PubMed] [Google Scholar]
- [12].Akfirat C, Zhang X, Ventura A, Berel D, Colangelo ME, Miranti CK, Krajewska M, Reed JC, Higano CS, True LD, Vessella RL, Morrissey C, Knudsen BS, Tumour cell survival mechanisms in lethal metastatic prostate cancer differ between bone and soft tissue metastases, J Pathol, 230 (2013) 291–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wang Y, Zhang S, Iqbal S, Chen Z, Wang X, Wang YA, Liu D, Bai K, Ritenour C, Kucuk O, Wu D, Pomegranate extract inhibits the bone metastatic growth of human prostate cancer cells and enhances the in vivo efficacy of docetaxel chemotherapy, Prostate, (2013). [DOI] [PubMed] [Google Scholar]
- [14].Zhang S, Gera L, Mamouni K, Li X, Chen Z, Kucuk O, Wu D, Inhibition of skeletal growth of human prostate cancer by the combination of docetaxel and BKM1644: an aminobisphosphonate derivative, Oncotarget, 7 (2016) 27489–27498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wiechno P, Somer BG, Mellado B, Chlosta PL, Cervera Grau JM, Castellano D, Reuter C, Stockle M, Kamradt J, Pikiel J, Duran I, Wedel S, Callies S, Andre V, Hurt K, Brown J, Lahn M, Heinrich B, A randomised phase 2 study combining LY2181308 sodium (survivin antisense oligonucleotide) with first-line docetaxel/prednisone in patients with castration-resistant prostate cancer, Eur Urol, 65 (2014) 516–520. [DOI] [PubMed] [Google Scholar]
- [16].Kelly RJ, Thomas A, Rajan A, Chun G, Lopez-Chavez A, Szabo E, Spencer S, Carter CA, Guha U, Khozin S, Poondru S, Van Sant C, Keating A, Steinberg SM, Figg W, Giaccone G, A phase I/II study of sepantronium bromide (YM155, survivin suppressor) with paclitaxel and carboplatin in patients with advanced non-small-cell lung cancer, Ann Oncol, 24 (2013) 2601–2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Huang Y, Anderle P, Bussey KJ, Barbacioru C, Shankavaram U, Dai Z, Reinhold WC, Papp A, Weinstein JN, Sadee W, Membrane transporters and channels: role of the transportome in cancer chemosensitivity and chemoresistance, Cancer Res, 64 (2004) 4294–4301. [DOI] [PubMed] [Google Scholar]
- [18].Pan ST, Li ZL, He ZX, Qiu JX, Zhou SF, Molecular mechanisms for tumour resistance to chemotherapy, Clin Exp Pharmacol Physiol, 43 (2016) 723–737. [DOI] [PubMed] [Google Scholar]
- [19].Bhangal G, Halford S, Wang J, Roylance R, Shah R, Waxman J, Expression of the multidrug resistance gene in human prostate cancer, Urol Oncol, 5 (2000) 118–121. [DOI] [PubMed] [Google Scholar]
- [20].Zhu Y, Liu C, Nadiminty N, Lou W, Tummala R, Evans CP, Gao AC, Inhibition of ABCB1 expression overcomes acquired docetaxel resistance in prostate cancer, Mol Cancer Ther, 12 (2013) 1829–1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zhu Y, Liu C, Armstrong C, Lou W, Sandher A, Gao AC, Antiandrogens Inhibit ABCB1 Efflux and ATPase Activity and Reverse Docetaxel Resistance in Advanced Prostate Cancer, Clin Cancer Res, 21 (2015) 4133–4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Stewart JM, Gera L, Chan DC, Bunn PA Jr., York EJ, Simkeviciene V, Helfrich B, Bradykinin-related compounds as new drugs for cancer and inflammation, Can J Physiol Pharmacol, 80 (2002) 275–280. [DOI] [PubMed] [Google Scholar]
- [23].Stewart JM, Gera L, Chan DC, York EJ, Simkeviciene V, Bunn PA Jr., Taraseviciene-Stewart L, Combination cancer chemotherapy with one compound: pluripotent bradykinin antagonists, Peptides, 26 (2005) 1288–1291. [DOI] [PubMed] [Google Scholar]
- [24].Jutras S, Bachvarova M, Keita M, Bascands JL, Mes-Masson AM, Stewart JM, Gera L, Bachvarov D, Strong cytotoxic effect of the bradykinin antagonist BKM-570 in ovarian cancer cells--analysis of the molecular mechanisms of its antiproliferative action, FEBS J, 277 (2010) 5146–5160. [DOI] [PubMed] [Google Scholar]
- [25].Avdieiev S, Gera L, Havrylyuk D, Hodges RS, Lesyk R, Ribrag V, Vassetzky Y, Kavsan V, Bradykinin antagonists and thiazolidinone derivatives as new potential anti-cancer compounds, Bioorg Med Chem, 22 (2014) 3815–3823. [DOI] [PubMed] [Google Scholar]
- 26.[] Gera L, Stewart J, Chung LW, Wu D, Compositions and methods for treating bone cancer. US Patent Publication: US 2010/0144678, 2010.
- [27].Wu HC, Hsieh JT, Gleave ME, Brown NM, Pathak S, Chung LW, Derivation of androgen-independent human LNCaP prostatic cancer cell sublines: role of bone stromal cells, Int J Cancer, 57 (1994) 406–412. [DOI] [PubMed] [Google Scholar]
- [28].Wu TT, Sikes RA, Cui Q, Thalmann GN, Kao C, Murphy CF, Yang H, Zhau HE, Balian G, Chung LW, Establishing human prostate cancer cell xenografts in bone: induction of osteoblastic reaction by prostate-specific antigen-producing tumors in athymic and SCID/bg mice using LNCaP and lineage-derived metastatic sublines, Int J Cancer, 77 (1998) 887–894. [DOI] [PubMed] [Google Scholar]
- [29].Thalmann GN, Sikes RA, Wu TT, Degeorges A, Chang SM, Ozen M, Pathak S, Chung LW, LNCaP progression model of human prostate cancer: androgen-independence and osseous metastasis, Prostate, 44 (2000) 91–103 Jul 101;144(102). [DOI] [PubMed] [Google Scholar]
- [30].Shen DW, Fojo A, Chin JE, Roninson IB, Richert N, Pastan I, Gottesman MM, Human multidrug-resistant cell lines: increased mdr1 expression can precede gene amplification, Science, 232 (1986) 643–645. [DOI] [PubMed] [Google Scholar]
- [31].Jin S, Scotto KW, Transcriptional regulation of the MDR1 gene by histone acetyltransferase and deacetylase is mediated by NF-Y, Mol Cell Biol, 18 (1998) 4377–4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Gera L, Chan DC, Helfrich B, Bunn PAJ, York EJ, Stewart JM, Bradykinin-related compounds having anti-cancer activity in vivo superior to Cisplatin and SU5416, in “Peptides 2000”, Martinez J, and Fehrentz J-A, Eds. EDK, Paris, France, (2001) 637–638. [Google Scholar]
- [33].Kreis W, Budman DR, Calabro A, Unique synergism or antagonism of combinations of chemotherapeutic and hormonal agents in human prostate cancer cell lines, Br J Urol, 79 (1997) 196–202. [DOI] [PubMed] [Google Scholar]
- [34].Shen DW, Cardarelli C, Hwang J, Cornwell M, Richert N, Ishii S, Pastan I, Gottesman MM, Multiple drug-resistant human KB carcinoma cells independently selected for high-level resistance to colchicine, adriamycin, or vinblastine show changes in expression of specific proteins, J Biol Chem, 261 (1986) 7762–7770. [PubMed] [Google Scholar]
- [35].Wang X, Beitler JJ, Wang H, Lee MJ, Huang W, Koenig L, Nannapaneni S, Amin AR, Bonner M, Shin HJ, Chen ZG, Arbiser JL, Shin DM, Honokiol enhances paclitaxel efficacy in multi-drug resistant human cancer model through the induction of apoptosis, PLoS One, 9 (2014) e86369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Gritsko T, Williams A, Turkson J, Kaneko S, Bowman T, Huang M, Nam S, Eweis I, Diaz N, Sullivan D, Yoder S, Enkemann S, Eschrich S, Lee JH, Beam CA, Cheng J, Minton S, Muro-Cacho CA, Jove R, Persistent activation of stat3 signaling induces survivin gene expression and confers resistance to apoptosis in human breast cancer cells, Clin Cancer Res, 12 (2006) 11–19. [DOI] [PubMed] [Google Scholar]
- [37].Aziz MH, Manoharan HT, Church DR, Dreckschmidt NE, Zhong W, Oberley TD, Wilding G, Verma AK, Protein kinase Cepsilon interacts with signal transducers and activators of transcription 3 (Stat3), phosphorylates Stat3Ser727, and regulates its constitutive activation in prostate cancer, Cancer Res, 67 (2007) 8828–8838. [DOI] [PubMed] [Google Scholar]
- [38].Aziz MH, Manoharan HT, Sand JM, Verma AK, Protein kinase Cepsilon interacts with Stat3 and regulates its activation that is essential for the development of skin cancer, Mol Carcinog, 46 (2007) 646–653. [DOI] [PubMed] [Google Scholar]
- [39].Nielsen M, Svejgaard A, Skov S, Odum N, Interleukin-2 induces tyrosine phosphorylation and nuclear translocation of stat3 in human T lymphocytes, Eur J Immunol, 24 (1994) 3082–3086. [DOI] [PubMed] [Google Scholar]
- [40].Zhang X, Blenis J, Li HC, Schindler C, Chen-Kiang S, Requirement of serine phosphorylation for formation of STAT-promoter complexes, Science, 267 (1995) 1990–1994. [DOI] [PubMed] [Google Scholar]
- [41].Li F, Altieri DC, Transcriptional analysis of human survivin gene expression, Biochem J, 344 Pt 2 (1999) 305–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Liu C, Zhu Y, Lou W, Nadiminty N, Chen X, Zhou Q, Shi XB, deVere White RW, Gao AC, Functional p53 determines docetaxel sensitivity in prostate cancer cells, Prostate, 73 (2013) 418–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Zhang X, Xiao W, Wang L, Tian Z, Zhang J, Deactivation of signal transducer and activator of transcription 3 reverses chemotherapeutics resistance of leukemia cells via downregulating P-gp, PLoS One, 6 (2011) e20965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Bello D, Webber MM, Kleinman HK, Wartinger DD, Rhim JS, Androgen responsive adult human prostatic epithelial cell lines immortalized by human papillomavirus 18, Carcinogenesis, 18 (1997) 1215–1223. [DOI] [PubMed] [Google Scholar]
- [45].Thalmann GN, Anezinis PE, Chang SM, Zhau HE, Kim EE, Hopwood VL, Pathak S, von Eschenbach AC, Chung LW, Androgen-independent cancer progression and bone metastasis in the LNCaP model of human prostate cancer, Cancer Res, 54 (1994) 2577–2581. [PubMed] [Google Scholar]
- [46].James ND, Sydes MR, Clarke NW, Mason MD, Dearnaley DP, Spears MR, Ritchie AW, Parker CC, Russell JM, Attard G, de Bono J, Cross W, Jones RJ, Thalmann G, Amos C, Matheson D, Millman R, Alzouebi M, Beesley S, Birtle AJ, Brock S, Cathomas R, Chakraborti P, Chowdhury S, Cook A, Elliott T, Gale J, Gibbs S, Graham JD, Hetherington J, Hughes R, Laing R, McKinna F, McLaren DB, O’Sullivan JM, Parikh O, Peedell C, Protheroe A, Robinson AJ, Srihari N, Srinivasan R, Staffurth J, Sundar S, Tolan S, Tsang D, Wagstaff J, Parmar MK, investigators S, Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial, Lancet, 387 (2016) 1163–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Sweeney CJ, Chen YH, Carducci M, Liu G, Jarrard DF, Eisenberger M, Wong YN, Hahn N, Kohli M, Cooney MM, Dreicer R, Vogelzang NJ, Picus J, Shevrin D, Hussain M, Garcia JA, DiPaola RS, Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer, N Engl J Med, 373 (2015) 737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Mora LB, Buettner R, Seigne J, Diaz J, Ahmad N, Garcia R, Bowman T, Falcone R, Fairclough R, Cantor A, Muro-Cacho C, Livingston S, Karras J, Pow-Sang J, Jove R, Constitutive activation of Stat3 in human prostate tumors and cell lines: direct inhibition of Stat3 signaling induces apoptosis of prostate cancer cells, Cancer Res, 62 (2002) 6659–6666. [PubMed] [Google Scholar]
- [49].Horinaga M, Okita H, Nakashima J, Kanao K, Sakamoto M, Murai M, Clinical and pathologic significance of activation of signal transducer and activator of transcription 3 in prostate cancer, Urology, 66 (2005) 671–675. [DOI] [PubMed] [Google Scholar]
- [50].Bourguignon LY, Peyrollier K, Xia W, Gilad E, Hyaluronan-CD44 interaction activates stem cell marker Nanog, Stat-3-mediated MDR1 gene expression, and ankyrin-regulated multidrug efflux in breast and ovarian tumor cells, J Biol Chem, 283 (2008) 17635–17651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Wang H, Huang C, Zhao L, Zhang H, Yang JM, Luo P, Zhan BX, Pan Q, Li J, Wang BL, Histone deacetylase inhibitors regulate P-gp expression in colorectal cancer via transcriptional activation and mRNA stabilization, Oncotarget, 7 (2016) 49848–49858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Aziz MH, Manoharan HT, Verma AK, Protein kinase C epsilon, which sensitizes skin to sun’s UV radiation-induced cutaneous damage and development of squamous cell carcinomas, associates with Stat3, Cancer Res, 67 (2007) 1385–1394. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.