Abstract
Background:
During an oral glucose tolerance test (OGTT), morphological features of the glucose curve (monophasic curve, glucose peak >30mins and 1-hr glucose ≥155mg/dL) maybe associated with higher prediabetes risk, but their reproducibility and predictive ability in adolescents with obesity are unknown.
Design/Methods:
Nondiabetic adolescent girls with obesity underwent a multiple-sample OGTT at baseline (n=93), 6-weeks (n=83), and 1-year (n=72). Short-term reproducibility (baseline to 6-weeks) and the predictive ability for prediabetes (baseline to 1-year) for each feature were compared to standard fasting and 2-hr OGTT diagnostic criteria.
Results:
There was fair/moderate short-term reproducibility (κ<0.5) for all morphological features. At 1-year, compared to standard OGTT criteria, the ROC-AUCs for glucose peak >30 mins, 1-hr ≥155mg/dL or a combination of the 2 criteria were comparable (all P>0.05), but the monophasic curve had the lowest ROC-AUC (P<0.001).
Conclusion:
In adolescent girls with obesity, glucose peak > glucose ≥155mg/dL had similar reproducibility and 1-year predictive ability for prediabetes compared to standard OGTT criteria. The shortened 1-hr OGTT may provide diagnostic equivalence for prediabetes risk with the additional advantage of a less time-consuming risk assessment.
Keywords: glucose tolerance, obesity, reproducibility, prediabetes, adolescence, OGTT
INTRODUCTION
Prediabetes is a clinical state of altered glucose metabolism, characterized by increasing insulin resistance and declining beta cell function, that significantly increases the risk for the development of type 2 diabetes (T2DM). According to thresholds established by the American Diabetes Association, prediabetes may be defined using fasting or 2-hr glucose concentrations during an oral glucose tolerance test (OGTT) or percentage of glycosylated hemoglobin (HbA1c) (1). Yet, the prevalence of prediabetes in youth is highly variable and dependent upon which of these three metrics are used for diagnosis (2). In up to 25% of youth who develop T2DM, the fasting, 2-hr glucose concentration or HbA1c may be below the prediabetes diagnostic thresholds 2 years prior to diabetes diagnosis (3, 4). The need to improve risk stratification is further amplified in adolescent girls who have high rates of developing T2DM and may benefit from targeted intensive intervention programs (5).
Assessing the morphological characteristics of the glucose curve during an OGTT has shown promise for improving prediabetes risk evaluation (6–9). Specifically, three main morphological features of the glucose curve are associated with increased risk for prediabetes in adults: time to glucose peak >30mins, 1-hr glucose concentration ≥155 mg/dL, and the monophasic curve shape (6, 10–12). However, the utility of these morphological features for predicting prediabetes in youth is still unclear. Although some studies examined the ability of curve shape to predict prediabetes in youth, only a few have evaluated the time to glucose peak parameter or 1-hr glucose as potential alternatives (11, 13–16). Evaluating the glucose peak and 1-hr glucose thresholds are intriguing because these parameters are relatively simple to quantify for clinical use and could be obtained during a shortened 1-hr OGTT. Moreover, using a 1-hr vs. 2-hr OGTT has been linked to high discrimination for diabetes-related complication, and has the potential to identify at-risk individuals early, potentially improving clinical efficiency and patient satisfaction (9, 17).
However, the reliability and diagnostic ability of these three morphological parameters (monophasic curve, glucose peak >30mins, and 1-hr glucose ≥155 mg/dL) have not been directly compared in youth and their use may not be generalizable because the reproducibility of each parameter may be quite variable (18). Differences in the macronutrient composition of meals in the days preceding the OGTT are known to alter glycemic response and inter- and intra-individual variability for these OGTT features are not well characterized (19, 20). Therefore, if these morphological parameters are to be considered as robust screening tools, the degree to which intra-individual variability affects diagnostic accuracy must be ascertained. In this secondary analysis of data from adolescent girls with obesity who were at-risk for development of type 2 diabetes, we compared the reproducibility and diagnostic accuracy of these three morphological features of the OGTT glucose curve over a 6-week period. Additionally, we determined the predictive ability of the morphological features, characterized at baseline, for diagnosing prediabetes 1-year later.
METHODS
Participants and Study Design
This was a secondary analysis of a randomized controlled trial of cognitive behavioral therapy (CBT) vs. health education in adolescent girls (age 14.8±1.6 years, range: 12–17 years) who had overweight/obesity (BMI≥85th percentile) at study entry (21). Youth with a first- or second-degree relative with type 2 diabetes and mild or moderate depressive symptoms were initially enrolled into a 6-week randomized controlled trial of depression-focused cognitive behavioral therapy (CBT) vs. health education to study the effects on mood and insulin resistance (NCT 01425905) and followed for a 1-year period. The results of the primary outcome analysis showing no between-group difference for the total sample in change in whole-body insulin sensitivity index after 6-weeks were already reported (21); given no effect of CBT, reproducibility of OGTT features could be studied using these data. During the year-long study, there was no specific dietary or lifestyle intervention.
Participants had three multiple-sample OGTTs performed at baseline, 6-weeks and 1-year after randomization. All girls were admitted to the NIH Hatfield Clinical Research Center (CC) after an 8–10-hr fast. Plasma samples to measure glucose and insulin concentrations were obtained at 0, 30, 60, 90, and 120 minutes (21). Participants were combined into a single group for this secondary analysis because there were no differences in age, BMI, glucose or insulin concentrations between intervention arms (21). Supplementary Figure 1 illustrates the flow of participants who underwent OGTT in the study: 83 girls had complete OGTT glucose and insulin data at baseline and at 6-weeks; 72 girls had complete data at baseline and 1-year. The study was approved by the Institutional Review Board of the National Institute of Child Health and Human Development and all parents and participants gave written informed consent and assent.
Definitions
Using standard OGTT criteria, prediabetes was defined as fasting glucose ≥100 mg/dL and <126 mg/dL, and/or 2-hr glucose ≥140 mg/dL and <200 mg/dL and normal glucose tolerance (NGT) as fasting glucose <100mg/dL and 2-hr glucose <140mg/dL (22).
Morphological Features
Participants were categorized into dichotomous classification variables according to the following parameters:
1-hr glucose: <155 or ≥155mg/dL (6);
Glucose peak: at 30 minutes or > 30 minutes (8);
Glucose curve shape: Monophasic curve if the glucose increased to a maximum between 30 and 90 minutes followed by a decrease at 120 minutes, or biphasic if the curve had a peak at 30 or 60 minutes followed by a nadir and second peak by 120 minutes (23);
COMBO: Combination of dichotomous variables 1 and 2 (having either a 1-hr glucose ≥155 mg/dL or peak glucose >30 minutes versus neither).
Prior to shape classification, the upward or downward change in glucose between time points was defined as a glucose difference of >4 mg/dL. This value was based upon the upper limit of the coefficient of variation of glucose samples run at the NIH CC laboratory (10).
Analyses and calculations
Plasma insulin concentrations were determined using an immunochemiluminometric assay from Diagnostic Product Corporation (Los Angeles, CA, USA) (21). Glucose concentrations were measured in serum using an enzymatic hexokinase assay on the Hitachi 917 analyzer (Roche Diagnostics Indianapolis, IN, USA) (21). Hemoglobin A1c was determined by HPLC - D10 instrument (BioRad Laboratories, Hercules, CA). Indices of insulin secretion and sensitivity during the OGTT were calculated by the Matsuda index and insulinogenic index, respectively (24, 25). Due to sample hemolysis, insulinogenic and Matsuda index could not be calculated in 15 and 33 OGTTs respectively; insulinogenic index (n=6 at screening and 6-week and n=3 at 1-year follow-up) and Matsuda index (n=10 at screening, n=18 at 6-weeks and n=5 at 1-year follow-up).
Statistical Analyses
Data are presented as mean±SD unless otherwise stated. Participant characteristics at baseline to 1-year follow-up were compared using pairwise comparison of means and the Bonferroni test. Reproducibility analyses for the morphological features were compared at baseline and 6-weeks with the Cohen’s κ statistic (26). Briefly, the κ coefficient is the observed agreement minus the agreement expected by chance, divided by perfect agreement minus the agreement by chance (27). Equation: where a is the actual observed agreement and e is the probability of chance agreement (26). The κ coefficients were interpreted by conventional standards (0.01–0.20 slight agreement, 0.21–0.40 fair agreement, 0.41–0.60, moderate agreement, 0.61–0.80 substantial agreement, 0.81–0.99 almost perfect agreement) (26).
Prediabetes, diagnosed by standard OGTT fasting and 2-hr glucose criteria, was designated the reference variable or gold-standard at each time point (baseline, 6-weeks and 1-year). Receiver operating curves (ROC) were computed for the dichotomous variables for each morphological feature (classification variables). The areas under the ROC (ROC-AUC) for each morphological feature were compared at baseline and at 6-weeks with the Mann-Whitney U test (for correlated curves). To determine the ability of baseline dichotomous variables to predict prediabetes at 1-year, the equality of ROC-AUCs of each morphological feature assigned at baseline (classification variable) was tested against the gold standard ROC-AUC (prediabetes diagnosed by standard OGTT at baseline), using Bonferroni corrections for multiple comparisons. P-values <0.05 were considered statistically significant. All analyses were performed with STATA, v 15.1 (College Station, TX USA).
RESULTS
Participant characteristics
Table 1 illustrates the demographic and metabolic characteristics for participants at baseline, 6-weeks, and 1-year. HbA1c was modestly higher after 1-year (P=0.03). There were no other significant differences in participants characteristics between baseline and 6-weeks (data not shown) or baseline and 1-year (all P-values >0.30, Table 1).
Table 1:
Participant Characteristics
| Baseline (n=93) |
6-weeks (n=83) |
1-year (n=72) |
P-value Baseline vs. 1-year |
|
|---|---|---|---|---|
| Age (years) | 14.4±1.6 | 14.8±1.6 | 15.5±3.4 | <0.01 |
| Race, n (%) | - | |||
| Black | 58 (62%) | 51 (61%) | 45 (63%) | |
| White | 15 (18%) | 13 (16%) | 11 (15%) | |
| Asian | 3 (3%) | 3 (4%) | 3 (4%) | |
| Mixed race | 17 (18%) | 16 (19%) | 13 (18%) | |
| BMI (kg/m2) | 32.6±6.5 | - | 33.9±9.9 | 0.31 |
| Systolic BP (mmHg)* | 118.5±9.5 | - | - | - |
| Diastolic BP (mmHg)* | 64.6±8.3 | - | - | - |
| Fasting glucose (mg/dL) | 89.0±7.5 | 88.6±7.7 | 88.2±7.1 | 0.52 |
| 2-hr glucose (mg/dL) | 103.3±21.4 | 104.6±20.5 | 107.1±22.6 | 0.26 |
| Fasting insulin (μU/mL)# | 20.3 (13.8–27.7) | 20.5 (15.2–27.9) | 18.8 (12.9–33.8) | 0.85 |
| Pre-diabetes, n (%) | 12 (13) | 12 (14) | 7 (13) | 0.53 |
| HbA1c (%) | 5.3±0.4 | 5.3±0.4 | 5.4±0.4 | 0.03 |
| Insulinogenic index | 4.2 (2.5–6.6) | 4.1 (2.9–6.9) | 3.6 (2.1–6.2) | 0.77 |
| Matsuda index | 2.3 (1.5–3.2) | 2.2 (1.4–3.0) | 2.7 (1.3–4.0) | 0.99 |
Data are mean±SD, n (%) or median (25th-75th percentile). Paired t-tests and Fisher exact tests were used to compare characteristics at baseline to 1-year. BMI: body mass index; HbA1c: glycosylated hemoglobin A1c. (*n=81, #n=91)
Reproducibility and diagnostic accuracy at 6-weeks
Between baseline and 6-weeks, κ coefficient was ≤0.48 for the morphological features of the OGTT (Table 2). The percentage of youth with prediabetes (12%) was the same at baseline and 6-weeks, (P=0.76, Table 1). Six girls diagnosed with prediabetes at baseline were reclassified as NGT at 6-weeks, while 8 girls who were NGT at baseline were reclassified as prediabetes at 6-weeks. The ROC-AUCs of OGTT morphological features were not significantly different when compared at baseline or at 6-weeks (P≥0.21, Table 2).
Table 2:
Diagnostic accuracy and reproducibility of OGTT features for prediabetes.
| Baseline (n=83) | 6-weeks (n=83) | Reproducibility | ||||||
|---|---|---|---|---|---|---|---|---|
| OGTT Feature | Sensitivity | Specificity | ROC-AUC (95% CI) |
Sensitivity | Specificity | ROC-AUC (95% CI) |
Kappa | 95% CI |
| Morphological features | ||||||||
| Glucose peak >30 mins | 0.70 | 0.71 | 0.71 (0.55 – 0.86) |
0.58 | 0.62 | 0.60 (0.45 – 0.76) |
0.23 | 0.02–0.44 |
| Monophasic curve | 0.60 | 0.53 | 0.57 (0.40 – 0.74) |
0.58 | 0.48 | 0.53 (0.37 – 0.69) |
0.23 | 0.02–0.43 |
| 1-hr glucose≥155mg/dL | 0.40 | 0.95 | 0.67 (0.51 – 0.83) |
0.33 | 0.98 | 0.66 (0.52 – 0.79) |
0.42 | 0.07–0.77 |
| COMBO | 0.66 | 0.75 | 0.71 (0.56–0.85) |
0.41 | 0.73 | 0.57 (0.42–0.72) |
0.46 | 0.30–0.61 |
| Gold-standard criteria | ||||||||
| Fasting glucose ≥100 mg/dL | 0.38 | 0.04–0.72 | ||||||
| 2-hr glucose ≥140 mg/dL | 0.28 | −0.08–0.64 | ||||||
OGTT: oral glucose tolerance test; COMBO: glucose peak>30mins and/or 1-hr glucose ≥155mg/dL.
Predictive ability of baseline OGTT parameters at 1-year follow-up
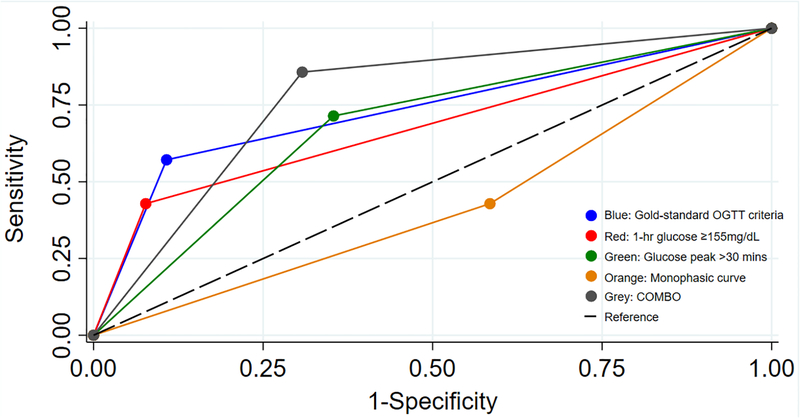
Among the 72 girls examined at 1-year follow-up, 11 had prediabetes at baseline (15%) and 7 (10%) had prediabetes at 1-year. From baseline to 1-year, 4 girls maintained a prediabetes diagnosis; the diagnosis of prediabetes resolved in 7 girls; and 3 girls were newly diagnosed with prediabetes at 1-year. Figure 1. Illustrates the ROC-AUC of OGTT parameters classified at baseline to predict prediabetes at 1-year. Compared to the gold-standard OGTT criteria the area under the ROC curve (ROC-AUC: 0.73, 95% CI: 0.53–0.93) was significantly lower for monophasic curve variable (0.42, 95% CI: 0.22–0.63, P<0.001) but not different for 1-hr glucose ≥155mg/dL (0.67, 0.48–0.88), glucose peak >30mins (0.68, 0.49–0.87) or COMBO (0.77, 0.62–0.93). There was no difference between ROC-AUC for glucose peak, 1-hr glucose, and COMBO parameters (P=0.39).
Figure 1. Predictive ability of morphological features for prediabetes at 1-year follow-up.
Receiver operating characteristic (ROC-AUC) curves; blue: gold-standard OGTT criteria (fasting glucose ≥100mg/dL and/or 2-hr glucose ≥140mg/dL,), red: 1-hr glucose ≥155mg/dL, green: glucose peak>30 minutes, orange: monophasic curve, grey: COMBO (glucose peak >30mins and/or 1-hr glucose ≥155mg/dL), black dashed: reference. Compared to the gold-standard OGTT criteria, the monophasic curve had the lowest ROC AUC (P<0.001). The ROC-AUC for the glucose peak, 1-hr glucose, and COMBO parameters were comparable to gold-standard OGTT criteria (P=0.39).
DISCUSSION
The era of personalized medicine has ushered in a search for novel biomarkers that can optimally predict prediabetes, especially in at-risk youth for whom lengthy and/or costly diagnostic testing may be challenging in the outpatient setting. The current investigation in adolescent girls at-risk for T2DM directly compared the reproducibility and diagnostic accuracy of three morphological parameters of the glucose curve and evaluated the utility of using parameters obtained during a 1-hr vs. 2-hr OGTT as risk prediction tools. We found fair to moderate reproducibility of all three parameters with good diagnostic accuracy of each variable over a 6-week period. Most importantly, we demonstrated that the reproducibility of morphological features, derived from a 1-hr OGTT (1-hr glucose ≥155mg/dL and glucose peak>30mins), was comparable to the gold-standard 2-hr glucose threshold (Table 2). Further, use of these two dichotomous variables characterized during the baseline OGTT had similar discrimination compared to the standard 2-hr glucose threshold for predicting prediabetes at 1-year follow-up (Figure 1). These findings are of potential clinical importance because they suggest that obtaining a 1-hr OGTT, with blood glucose sampling at 0, 30, and 60 minutes, has similar diagnostic accuracy to a 2-hr OGTT for predicting prediabetes, and thus, could be a shorter, clinically acceptable alternative for assessing the risk of progression to prediabetes.
Though the 2-hr OGTT is well-established for the diagnosis of T2DM, more recent analyses have highlighted poor intraindividual reproducibility and decreased reliability for prediabetes diagnosis in youth (28, 29) as well as reduced discriminatory ability in pregnant and post-menopausal women (30–32). Another study found differences in OGTT results were related to sex and/or variations in body size (tall/short) (33). Therefore, alternative strategies to improve diagnostic accuracy for prediabetes are needed. By itself, an elevated 1-hr glucose concentration is an emerging biomarker with similar or higher discrimination compared to the 2-hr glucose threshold for diabetes prediction (9, 15). The 1-hr glucose ≥155 mg/dL was comparable to the 2-hr threshold ≥140mg/dL for predicting the development of diabetic complications and mortality in adults (34). In this study, we confirmed the high diagnostic specificity (95%) of the 1-hr glucose classification variable (Table 2). However, the 1-hr glucose threshold had low diagnostic sensitivity (40%) and when used alone would increase the risk of false negative diagnoses. Similarly, the diagnostic accuracy of time to glucose peak was comparable to 1-hr glucose at baseline and 6-weeks, but when used alone would increase the number of false positive results (Table 2).
Employing two or more predictive biomarkers is an attractive and feasible option that may have superior predictive ability (35). In this study, we showed that the combination of two parameters (COMBO), obtained within a 1-hr OGTT may be a viable approach and was associated with a ROC-AUC of 0.77 (Table 2). The advantages of using 1-hr glucose and time to glucose peak are two-fold. First, these parameters correlate with detailed measures of insulin resistance and response and reflect the pathophysiologic response of increasing insulin resistance and declining beta cell function associated with prediabetes (6, 8). Second, their use would provide diagnostic equivalence for prediabetes risk with the additional advantage of a less time-consuming risk assessment. While the additional timepoints could marginally increase cost, the shortened diagnostic procedure time has the potential to improve patient-provider engagement, decrease youth’s stress associated with waiting on the 2-hr blood draw, and could improve workflow and patient/ parent satisfaction (36).
Importantly, we also identified the monophasic curve shape an unreliable biomarker with the lowest diagnostic sensitivity and specificity at 6-weeks and 1-year compared to all other morphological features. Though previous studies in adolescents with obesity found an association of monophasic curve (compared to the biphasic) with increased insulin resistance (11), more recent data in youth and adults suggest that the monophasic curve has high false positive rates (8, 16). When curve shape is directly compared to the time to glucose peak parameter, the mono/ biphasic curve variable mischaracterized prediabetes status in >50% of cases (8, 16) (37). In keeping with these analyses, this study confirms that the monophasic curve shape was associated with lowest reproducibility and modest diagnostic accuracy compared to both glucose peak and 1-hr glucose parameters. These findings, coupled with high intra-individual variability or the monophasic curve argue against using curve shape alone as a reliable biomarker for prediabetes in adults or children (38).
Despite the advantages of this longitudinal assessment and serial OGTTs in youth at high risk for T2DM, a few study limitations are noteworthy. First, this is a secondary analysis in youth who were participating in a randomized controlled behavioral trial; thus, results of reproducibility over the 6-week period could have been altered by intervention arm or variations in chronic physical activity that were not measured. Nevertheless, the intervention did not affect insulin resistance or glucose tolerance status in the entire cohort, and thus the study presented a good opportunity to assess the complexity of the glucose curve over the short and long-term (21). Second, the study was relatively small and therefore limited by potential sampling bias. Third, the Cohen’s κ statistic may underestimate the observed agreement because prediabetes was uncommon (occurring only in <15% of cases) even in this small cohort selected to be at-risk for T2DM. Lastly, this was a trial of predominantly white and black adolescent girls with depressive symptoms who were considered at-risk for the development of T2DM and these findings may not be generalizable to all youth. It is important to note that although the 1-hr OGTT is a promising diagnostic tool, a complete clinical history and physical examination remains the hallmark of diabetes risk stratification and additional testing would be needed to assess indices of insulin resistance and β-cell function.
In conclusion, an elevated 1-hr glucose and time to glucose peak greater than 30 mins, obtained during a 1-hr OGTT, had moderate reproducibility and comparable discriminative ability to established fasting and 2-hr glucose thresholds for predicting prediabetes at 1-year. Using these morphological parameters of the glucose curve as biomarkers for prediabetes risk stratification may be an alternative strategy to the standard 2-hr OGTT glucose thresholds. Future studies are warranted to confirm whether using the morphological features obtained during a 1-hr OGTT may be cost-effective and efficacious among all youth at risk for prediabetes.
Supplementary Material
ACKNOWLEDGEMENTS:
JAY is a Commissioned Officer in the United States Public Health Service (PHS). The opinions and assertions expressed herein are those of the authors and are not to be construed as reflecting the views of the PHS or the Department of Health and Human Services. The study was designed by JAY, LBS, and STC. Data were collected by LBS and JAY. Statistical analyses were conducted by KK and STC, who wrote the first draft of the manuscript. All authors provided critical review of the manuscript and had final approval of the submitted and published versions.
Footnotes
CONFLICTS OF INTEREST: The authors have no conflicts of interest to disclose. This work was supported by the Intramural Research Programs of NICHD ZIA-HD-00641 (to JAY) and NIDDK Z99-DK-999999 (to STC), with supplemental funding from the NIH Office of Behavioral and Social Sciences Research (to JAY) and K99/R00HD069516 (to LBS).
References
- 1.Association AD. Cardiovascular Disease and Risk Management: Standards of Medical Care in Diabetes. Diabetes care. 2018;41(Suppl 1):S86–s104. [DOI] [PubMed] [Google Scholar]
- 2.Haemer MA, Grow HM, Fernandez C, Lukasiewicz GJ, Rhodes ET, Shaffer LA, et al. Addressing Prediabetes in Childhood Obesity Treatment Programs: Support from Research and Current Practice. Childhood Obesity. 2014;10(4):292–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brufani C, Tura A, Bedogni G, Luciano R, Sbrignadello S, Fintini D, et al. Inside out the Ragbag of Glucose Intolerance in Obese Adolescents. Hormone research in paediatrics. 2017;87(5):287–94. [DOI] [PubMed] [Google Scholar]
- 4.Nowicka P, Santoro N, Liu H, Lartaud D, Shaw MM, Goldberg R, et al. Utility of hemoglobin A(1c) for diagnosing prediabetes and diabetes in obese children and adolescents. Diabetes care. 2011;34(6):1306–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weiss R, Santoro N, Giannini C, Galderisi A, Umano GR, and Caprio S. Prediabetes in youths: mechanisms and biomarkers. The Lancet Child & Adolescent Health. 2017;1(3):240–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bianchi C, Miccoli R, Trombetta M, Giorgino F, Frontoni S, Faloia E, et al. Elevated 1-hour postload plasma glucose levels identify subjects with normal glucose tolerance but impaired beta-cell function, insulin resistance, and worse cardiovascular risk profile: the GENFIEV study. J Clin Endocrinol Metab. 2013;98(5):2100–5. [DOI] [PubMed] [Google Scholar]
- 7.Kramer CK, Ye C, Hanley AJ, Connelly PW, Sermer M, Zinman B, et al. Delayed timing of post-challenge peak blood glucose predicts declining beta cell function and worsening glucose tolerance over time: insight from the first year postpartum. Diabetologia. 2015;58(6):1354–62. [DOI] [PubMed] [Google Scholar]
- 8.Chung ST, Ha J, Onuzuruike AU, Kasturi K, Galvan-De La Cruz M, Bingham BA, et al. Time to glucose peak during an oral glucose tolerance test identifies prediabetes risk. Clinical endocrinology. 2017;87(5):484–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bergman M, Chetrit A, Roth J, Jagannathan R, Sevick M, and Dankner R. One-hour post-load plasma glucose level during the OGTT predicts dysglycemia: Observations from the 24year follow-up of the Israel Study of Glucose Intolerance, Obesity and Hypertension. Diabetes research and clinical practice. 2016;120:221–8. [DOI] [PubMed] [Google Scholar]
- 10.Chung STGD-LCM, Kasturi K, Bingham BA, Onuzuruike A, Baker RL, Utumatwishima JN, Mabundo LS, Ricks M, Ha J, Sherman AS, Sumner AE. Time to Glucose Peak During an Oral Glucose Tolerance Test Identifies High Risk for Prediabetes: Results from a Multiethnic Study. Endocrine Society, 2017. 2017; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim J, Michaliszyn S, Nasr A, Lee S, Tfayli H, Hannon T, et al. The Shape of the Glucose Response Curve During an Oral Glucose Tolerance Test Heralds Biomarkers of Type 2 Diabetes Risk in Obese Youth. Diabetes care. 2016;39(8):1431–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdul-Ghani MA, Lyssenko V, Tuomi T, Defronzo RA, and Groop L. The shape of plasma glucose concentration curve during OGTT predicts future risk of type 2 diabetes. Diabetes/metabolism research and reviews. 2010;26(4):280–6. [DOI] [PubMed] [Google Scholar]
- 13.Yin C, Zhang H, Xiao Y, and Liu W. Shape of glucose curve can be used as a predictor for screening prediabetes in obese children. Acta Paediatr. 2014;103(5):e199–205. [DOI] [PubMed] [Google Scholar]
- 14.Kim J, Coletta D, Mandarino L, and Shaibi G. Glucose response curve and type 2 diabetes risk in Latino adolescents. Diabetes care. 2012;35(9):1925–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marcovecchio ML, Bagordo M, Marisi E, de Giorgis T, Chiavaroli V, Chiarelli F, et al. One-hour post-load plasma glucose levels associated with decreased insulin sensitivity and secretion and early makers of cardiometabolic risk. Journal of endocrinological investigation. 2017;40(7):771–8. [DOI] [PubMed] [Google Scholar]
- 16.Cree-Green M, Xie D, Rahat H, Garcia-Reyes Y, Bergman BC, Scherzinger A, et al. Oral glucose tolerance test glucose peak time is most predictive of pre-diabetes and hepatic steatosis in obese girls. Journal of the Endocrine Society. 2018: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pareek M, Bhatt DL, Nielsen ML, Jagannathan R, Eriksson KF, Nilsson PM, et al. Enhanced Predictive Capability of a 1-Hour Oral Glucose Tolerance Test: A Prospective Population-Based Cohort Study. Diabetes care. 2018;41(1):171–7. [DOI] [PubMed] [Google Scholar]
- 18.Kramer CK, Vuksan V, Choi H, Zinman B, and Retnakaran R. Emerging parameters of the insulin and glucose response on the oral glucose tolerance test: reproducibility and implications for glucose homeostasis in individuals with and without diabetes. Diabetes research and clinical practice. 2014;105(1):88–95. [DOI] [PubMed] [Google Scholar]
- 19.Meng H, Matthan NR, Ausman LM, and Lichtenstein AH. Effect of prior meal macronutrient composition on postprandial glycemic responses and glycemic index and glycemic load value determinations. The American journal of clinical nutrition. 2017;106(5):1246–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hales CN, and Randle PJ. Effects of low-carbohydrate diet and diabetes mellitus on plasma concentrations of glucose, non-esterified fatty acid, and insulin during oral glucose-tolerance tests. Lancet. 1963;1(7285):790–4. [DOI] [PubMed] [Google Scholar]
- 21.Shomaker LB, Kelly NR, Pickworth CK, Cassidy OL, Radin RM, Shank LM, et al. A Randomized Controlled Trial to Prevent Depression and Ameliorate Insulin Resistance in Adolescent Girls At-Risk for Type 2 Diabetes. Annals of behavioral medicine : a publication of the Society of Behavioral Medicine. 2016;50(5):762–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Association AD. Standards of Medical Care in Diabetes. Diabetes care. 2017;40 (Suppl. 1):S13. [Google Scholar]
- 23.Tschritter O, Fritsche A, Shirkavand F, Machicao F, Haring H, and Stumvoll M. Assessing the shape of the glucose curve during an oral glucose tolerance test. Diabetes care. 2003;26(4):1026–33. [DOI] [PubMed] [Google Scholar]
- 24.Matsuda M, and DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462–70. [DOI] [PubMed] [Google Scholar]
- 25.Ferrannini E, Gastaldelli A, Miyazaki Y, Matsuda M, Mari A, and DeFronzo RA. beta-Cell function in subjects spanning the range from normal glucose tolerance to overt diabetes: a new analysis. J Clin Endocrinol Metab. 2005;90(1):493–500. [DOI] [PubMed] [Google Scholar]
- 26.McHugh ML. Interrater reliability: the kappa statistic. Biochemia Medica. 2012;22(3):276–82. [PMC free article] [PubMed] [Google Scholar]
- 27.Jacob C A Coefficient of Agreement for Nominal Scales. Educational and Psychological Measurement. 1960;20(1):37–46. [Google Scholar]
- 28.Chen ME, Aguirre RS, and Hannon TS. Methods for Measuring Risk for Type 2 Diabetes in Youth: the Oral Glucose Tolerance Test (OGTT). Current diabetes reports. 2018;18(8):51. [DOI] [PubMed] [Google Scholar]
- 29.Libman IM, Barinas-Mitchell E, Bartucci A, Robertson R, and Arslanian S. Reproducibility of the oral glucose tolerance test in overweight children. J Clin Endocrinol Metab. 2008;93(11):4231–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bonongwe P, Lindow SW, and Coetzee EJ. Reproducibility of a 75G oral glucose tolerance test in pregnant women. J Perinat Med. 2015;43(3):333–8. [DOI] [PubMed] [Google Scholar]
- 31.Munang YN, Noubiap JJ, Danwang C, Sama JD, Azabji-Kenfack M, Mbanya JC, et al. Reproducibility of the 75 g oral glucose tolerance test for the diagnosis of gestational diabetes mellitus in a sub-Saharan African population. BMC Res Notes. 2017;10(1):622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van de Velde FP, Dierickx A, Depypere H, Delanghe JR, Kaufman JM, and Lapauw B. Reproducibility and least significant differences of oral glucose tolerance test-derived parameters in a postmenopausal population without diabetes. Diabetes Metab. 2017;43(5):484–7. [DOI] [PubMed] [Google Scholar]
- 33.Faerch K, Pacini G, Nolan JJ, Hansen T, Tura A, and Vistisen D. Impact of glucose tolerance status, sex, and body size on glucose absorption patterns during OGTTs. Diabetes care. 2013;36(11):3691–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paddock E, Looker HC, Piaggi P, Knowler WC, Krakoff J, and Chang DC. One-Hour Plasma Glucose Compared With 2-Hour Plasma Glucose in Relation to Diabetic Retinopathy in American Indians. Diabetes care. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sumner AE, Duong MT, Aldana PC, Ricks M, Tulloch-Reid MK, Lozier JN, et al. A1C Combined With Glycated Albumin Improves Detection of Prediabetes in Africans: The Africans in America Study. Diabetes Care. 2016;39(2):271–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mazaheri Habibi MR, Abadi FM, Tabesh H, Vakili-Arki H, Abu-Hanna A, and Eslami S. Evaluation of patient satisfaction of the status of appointment scheduling systems in outpatient clinics: Identifying patients’ needs. J Adv Pharm Technol Res. 2018;9(2):51–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tran S, Kramer CK, Zinman B, Choi H, and Retnakaran R. Effect of chronic liraglutide therapy and its withdrawal on time to postchallenge peak glucose in type 2 diabetes. American journal of physiology Endocrinology and metabolism. 2018;314(3):E287–E95. [DOI] [PubMed] [Google Scholar]
- 38.Manco M, Nolfe G, Pataky Z, Monti L, Porcellati F, Gabriel R, et al. Shape of the OGTT glucose curve and risk of impaired glucose metabolism in the EGIR-RISC cohort. Metabolism: clinical and experimental. 2017;70:42–50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.