Abstract
Cortical circuits are particularly sensitive to incoming sensory information during well-defined intervals of postnatal development called “critical periods.” The critical period for cortical plasticity closes in adults, thus restricting the brain’s ability to indiscriminately store new sensory information. For example, children acquire language in an exposure-based manner, whereas learning language in adulthood requires more effort and attention. It has been suggested that pairing sounds with the activation of neuromodulatory circuits involved in attention reopens this critical period. Here, we review two critical period hypotheses related to neuromodulation: cortical disinhibition and thalamic adenosine. We posit that these mechanisms co-regulate the critical period for auditory cortical plasticity. We also discuss ways to reopen this period and rejuvenate cortical plasticity in adults.
Introduction
Learning and memory involve the acquisition and storage of sensory information. These cognitive processes occur throughout life. However, children and adults learn differently: children learn faster and more efficiently than do adolescents and adults. These observations triggered the idea of “critical periods,” which are optimal periods of development when brain circuits acquire and store information [1–5]. One example of this concept is language acquisition. Learning a second language after puberty is much more conscious and labored than the automatic acquisition occurring in young children as a result of mere passive exposure [5;6].
Acoustic information in the form of auditory patterns is most likely represented in the primary auditory cortex (ACx) [7]. Similar to other sensory cortices, the ACx is topographically organized [8;9]: neurons with different preferred sound frequencies are spatially distributed in the ACx, forming tonotopic maps [10]. This information is inherited from tonotopic organization of the cochlea and delivered to the ACx through thalamocortical (TC) projections emanating from the auditory thalamus [11]. In animals, the ACx is plastic throughout the lifespan. This plasticity is observed at the level of tonotopic maps, as individual neurons’ responses shift to reinforced sound frequencies [12]. Plasticity in sensory cortices is believed to be the cellular correlate of perceptual learning [13–16] (however, see [17;18]).
Auditory plasticity is thought to optimize neural circuits for processing species-specific vocalizations, including language in humans [5;19]. As with language acquisition in humans, ACx plasticity differs between young and adult animals. In rodent pups, passive sound exposure induces ACx plasticity [20–23] but only for a few days after hearing onset [postnatal day (P)11– P15]. In adults, passive sound exposure is substantially less effective in inducing ACx plasticity [20–22;24].
These studies and those of other sensory cortices demonstrate the existence of critical periods for cortical plasticity [24;25]. Most concepts on the mechanisms of cortical plasticity originated from visual cortex studies [26]. Although in some cases the assumption of similarity between cortices holds true, ACx plasticity is distinct from visual cortex plasticity due to physiological and structural differences [27]. Herein, we review recent advances in cortical plasticity research in rodents, particularly from the perspective of the primary ACx.
Neuromodulation of ACx plasticity
The concept of a critical period for ACx plasticity implies that developmental events impede adults from learning in a passive mode. Studies of adult ACx plasticity commonly report the requirements of attention and alertness. ACx plasticity is well documented in mature animals when pairing specific sounds with associative cues (e.g., reward or punishment) [14;16;28–31].
Attention, vigilance, and alertness are mediated, at least in part, by neuromodulatory drive to the neocortex, which receives cholinergic, noradrenergic, and dopaminergic inputs from the nucleus basalis (NB), locus coeruleus (LC), and ventral tegmental area (VTA), respectively [32–35]. Aversive or rewarding stimuli activate these networks and promote the release of transmitters onto downstream regions [36–40]. Such stimuli activate NB cholinergic neurons with high speed and precision [37]. In adult rodents, pairing sounds with neuromodulatory circuit activation causes robust associative ACx plasticity [12;32;41] and heightened auditory perception [40;42], despite closure of the critical period.
In young pups, the ACx learns from the surrounding acoustic milieu: in this exposure-based model of plasticity [43], neuromodulation is less necessary. However, the ability of the adult ACx to learn from sensory exposure alone is curbed, making it difficult for that information to alter neuronal circuits. The adult ACx becomes an “associative learner” (i.e., circuits are modified when sensory information is behaviorally relevant). In this reinforcement-based mode of plasticity [43], neuromodulator-mediated attention and alertness augment sensory information and store that which applies to important tasks or experiences [44].
Developmental events that control critical periods most likely are not fully engaged during the critical period, but once implemented, they close the critical period by restricting cortical plasticity. Removing these restrictions may extend the critical period and rejuvenate cortical plasticity in adults, as suggested for deleting chondroitin sulfate proteoglycans or Nogo receptors in the visual cortex [45;46] or Icam5 in the ACx [20]. Although these molecules control the critical period, whether they engage the same mechanisms as neuromodulators to rejuvenate adult cortical plasticity remains unknown. Here we discuss two theories of the critical period for ACx plasticity that are mechanistically connected to neuromodulation––the long-standing theory of “cortical disinhibition” and a recent theory of “thalamic adenosine.”
Cortical disinhibition
Because the theory of cortical disinhibition has been reviewed extensively [32;47;48], we will present only its main points and recent developments. Glutamatergic and GABAergic neuronal activity in the neocortex sets the appropriate ratio of excitation to inhibition, known as “E:I balance.” The notion that GABAergic interneurons restrain learning has been comprehensively investigated [32;38]. Initially, delayed maturation of inhibitory neurons during the critical period was proposed, implying that the E:I balance is not established early in life and cortical plasticity could occur [47]. However, in the P17–P24 rat ACx, in vivo whole-cell recordings showed that excitation and inhibition are balanced and tuned to the same frequencies [49]. Another study demonstrated a fully established E:I balance in thalamorecipient layer (L) 4 neurons of the rat ACx as early as P12 [50]. Recording from neurons in multiple cortical layers, another study demonstratedthat immediately after the onset of hearing (P12), inhibition is strong but more poorly tuned than excitation. By P21, E:I balance is achieved as excitation and inhibition tuning become highly correlated [51]. Discrepancies among these studies may stem from variability of E:I balance between neurons at different cortical layers, even within the same animal [51]. Together, these works suggest that during the critical period, the E:I balance is achieved, at least for thalamorecipient L4 neurons.
The E:I balance can be transiently disrupted by repeated pairing of a tone with electrical stimulation of cholinergic neurons in the NB [52]. In adult rats, immediately after the pairing protocol, neurons shift their tuning toward the pairing tone. This is accompanied by a rapid reduction in inhibition, which alters the E:I balance. This reorganization lasts for approximately 2 h even after a brief pairing and may prime the ACx for plasticity. NB neurons are activated by foot-shocks and acetylcholine release onto L1 interneurons in the ACx [53]. Nicotinic receptor–dependent depolarization of L1 inhibitory interneurons occurring within 50–60 ms of the shock inhibits parvalbumin (PV)–positive L2/3 interneurons and subsequently disinhibits excitatory neurons. This disinhibition mechanism likely underlies ACx plasticity associated with fear learning [48;53]. These studies point to L1 inhibitory neurons as a possible hub of sensory cortical disinhibition. Of L1 interneurons, vasoactive intestinal peptide (VIP)–positive (L1/VIP) interneurons may assert disinhibitory control through PV- and somatostatin (SOM)–positive interneurons in deeper cortical layers [54] (Figure 1). Interestingly, L1/VIP neurons in the ACx and visual cortex are activated in response to various salient stimuli (e.g., air puffs, water reward [54], or locomotion [55]).
Figure 1. Circuit mechanisms of cortical disinhibition.
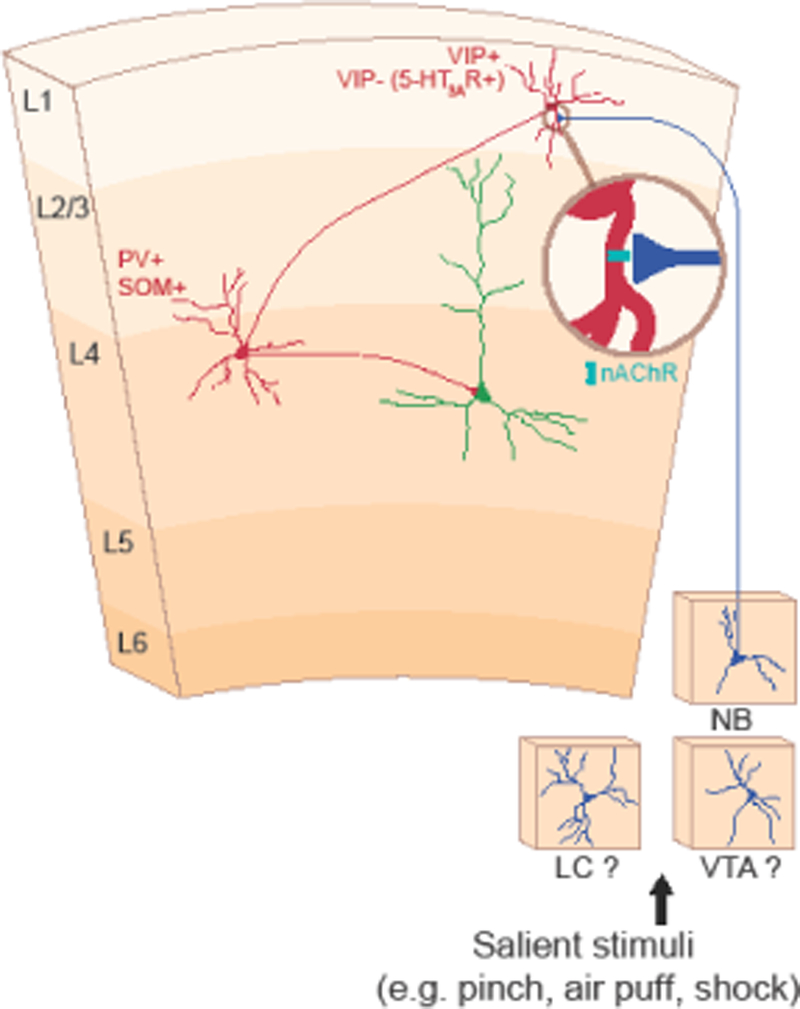
In layer 1 (L1) of the auditory cortex, vasoactive intestinal peptide positive (VIP+) and VIP– neurons that express the 5-HT3A receptor (5-HT3AR+) disinhibit downstream pyramidal neurons by inhibiting parvalbumin (PV+) or somatostatin (SOM+) neurons. Salient stimuli such as air puffs and shock activate neuromodulatory inputs, such as acetylcholine from the nucleus basalis (NB) that impinge upon L1 VIP+ or VIP– neurons. These inputs signal through nicotinic acetylcholine receptors (nAChR) to drive this disinhibitory circuit. Future work is required to determine whether other neuromodulatory inputs such as norepinephrine from the locus coeruleus (LC) and dopamine from the ventral tegmental area (VTA) also activate this circuit. Red denotes inhibitory neurons; green denotes excitatory neurons; and blue denotes neuromodulatory inputs. This figure is adapted from [48;53;54;57].
Can we extend the critical period into adulthood by manipulating cortical disinhibitory circuits? Deletion of Lynx1, an endogenous inhibitor of nicotinic receptors that has higher expression in adult mice than pups, extends cortical plasticity in the visual cortex to P60 [56]. Lynx1 is expressed in L1 interneurons, which also express 5-HT3A receptors (but not VIP) [57]. These L1/5-HT3A interneurons receive cholinergic inputs that activate α4 nicotinic receptors and disinhibit the ACx through PV-positive L4 neurons (Figure 1). Silencing L1/5-HT3A interneurons abolishes critical period plasticity in acute slices from pups [57].
These studies suggest that cortical disinhibition helps control the critical period of ACx plasticity. However, it is not sufficient to achieve input specificity, an important feature of ACx plasticity [12]. For instance, a salient unconditioned stimulus such as foot-shock achieves cortical disinhibition by activating L1 neurons broadly throughout the ACx and visual cortex [53]. Moreover, the E:I balance of L4 neurons in the ACx shows little difference during and after the critical period [50]. Therefore, cortical disinhibition can be seen as a widespread permissive gate that can be transiently (within milliseconds [37]) achieved by activating a neuromodulatory system with salient stimuli to briefly and reliably broadcast a unified signal to large brain areas to allow modification by concomitantly presented input [52]. Under this condition, response specificity depends on information delivered by excitatory inputs [48]. This also implies that the excitatory inputs to the ACx should be plastic during the critical period but not afterward. The TC projections are the primary candidates that satisfy these parameters.
Thalamic adenosine
Whereas the cortical disinhibition hypothesis is based on polysynaptic functioning of neural circuitry in the neocortex, the thalamic adenosine hypothesis stems from monosynaptic mechanisms of plasticity at the TC synapses. TC projections canonically synapse onto excitatory L4 neurons in sensory cortices [58;59]. These glutamatergic projections functionally differ from other glutamatergic (e.g., corticocortical) projections. First, TC projections undergo short-term depression in response to two or more stimuli delivered in quick succession, whereas most glutamatergic projections undergo facilitation [60;61]. Second, TC projections are plastic during the critical period, but unlike other excitatory synapses, they abruptly lose this plasticity with age [44;62].
In acute brain slices from mouse pups (younger than P15) but not from adults (older than P15),TC long-term depression and potentiation (LTD/LTP) can be induced by trains of stimuli applied to thalamic afferents. In adults, TC LTD/LTP in the ACx do not disappear but become gated [63;64]. This gating is mediated by thalamic adenosine [44], which is produced by ecto-5’-nucleotidase (Nt5e) and signals through adenosine A1 receptors (A1Rs) on thalamic afferents (Figure 2). A1R signaling reduces glutamate release from presynaptic terminals, thus making TC synapses prone to depression not facilitation [65]. Deleting or pharmacologically blocking A1Rs or Nt5e unmasks TC LTD/LTP in the adult ACx [63;64]. Adult TC LTD/LTP are expressed through postsynaptically localized group 1 metabotropic glutamate receptors (mGluR1s), unlike NMDA receptor–dependent synaptic plasticity, which is characteristic of TC synapses in other sensory cortices [62] (however, NMDA receptors have been previously implicated in the TC critical period in the ACx [66]). Adult TC LTD/LTP are unmasked when thalamic activity is paired with stimulation of cholinergic projections from the NB. Unlike cortical disinhibition described above, which operates through nicotinic receptors, unmasking adult TC synaptic plasticity in the ACx requires M1 muscarinic receptors (mAChRs) [63;64]. The activation of mAChRs may inhibit adenosine production or signaling through presynaptic thalamic A1Rs [44] (Figure 2). Inhibition of the adenosine machinery enables sustained glutamate release, which activates postsynaptic mGluR1s to induce TC LTD/LTP in adults [63;64].
Figure 2. Adenosine gating of thalamocortical long-term synaptic plasticity.
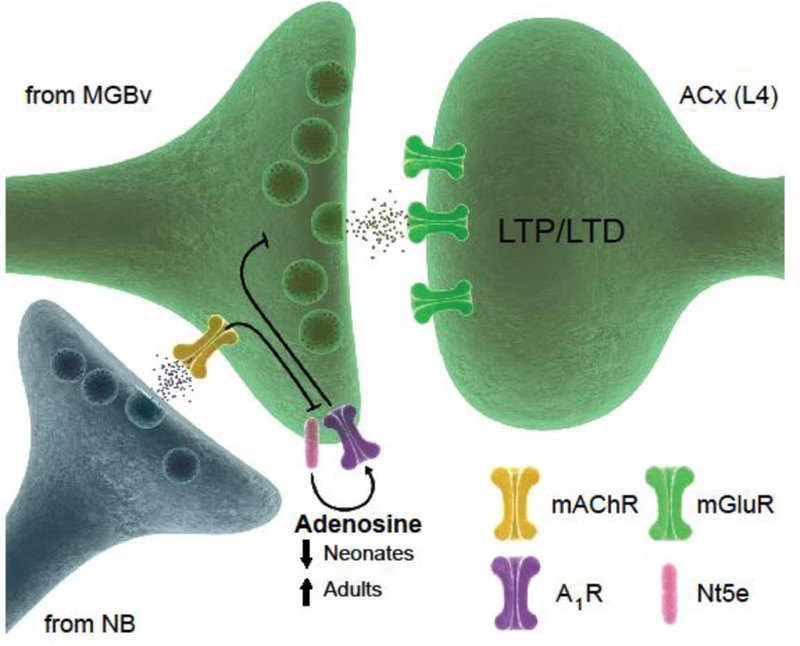
Adenosine signaling through presynaptic A1 receptors (A1Rs) prevents the expression of thalamocortical long-term potentiation and depression (LTP/LTD) in layer 4 (L4) of the auditory cortex (ACx) and closes the critical period. Acetylcholine (ACh) released from nucleus basalis (NB) neuronsactivates muscarinic M1 receptors (mAChRs) on thalamic terminals from the ventral part of the medial geniculate body (MGBv), which inhibit the production of adenosine by inhibiting ecto-5’-nucleotidase (Nt5e) or A1R signaling. We hypothesize that mAChRs and Nt5e are localized on presynaptic thalamic terminals, but this machinery may be located elsewhere, such as on neighboring astrocytes. mGluR: metabotropic group glutamate receptor.This figure is adapted from [44;67].
Consistent with its role in TC synaptic plasticity, thalamic adenosine is central to closing the critical period for ACx plasticity in vivo. Nt5e and adenosine levels are lower in pups than in adults [67]. Nt5e deletion in adults prevents increased adenosine levels; lower juvenile levels are maintained. Deletion or knockdown of Nt5e or A1Rs in the thalamus or pharmacological inhibition of A1Rs extends the critical period into adulthood. A passive tone exposure of adult mice with deficient Nt5e or A1Rs but not of wild-type mice expands ACx areas specifically responsive to this tone. Furthermore, knocking down A1Rs in only the auditory thalamus enables ACx plasticity in response to passive sound exposure even in elderly (P300) mice. Consistent with TC LTD/LTP mechanisms, ACx plasticity is blocked by mGluR1 inhibition, and A1R activation in pups prematurely closes the critical period for ACx plasticity [67].
Taken together, adenosine signaling in the thalamus gates the critical period for plasticity in the adult ACx, and cholinergic projections from the NB remove this gate by activating mAChRs. Whether other neuromodulators act on ACx plasticity through thalamic adenosine remains unknown.
Concluding remarks and future directions
Here we describe two hypotheses of the critical period for cortical plasticity in sensory cortices. Both proposed mechanisms mediate neuromodulatory projections, which facilitate cortical plasticity in adults. We describe cortical disinhibition and thalamic adenosine hypotheses from the perspective of the ACx, but whether these mechanisms operate in other sensory cortices remains unknown. It should be noted that we have presented a simplified view of cortical plasticity. Other mechanisms and neural circuits outside the neuromodulatory context are almost certainly involved [25;68].
Do these cortical and thalamic mechanisms work independently or in concert? Do they sequentially or concurrently control the critical period? Evidence supports that either mechanism is sufficient to extend plasticity into adulthood. For instance, manipulating L1 neuronal activity or inhibiting thalamic adenosine extends the critical period [57;67]. Whether these mechanisms contribute equally to the critical period is debatable. If the E:I balance is established from the onset of hearing, we would argue that low adenosine levels in the thalamus are more important in keeping the critical period open.
In adults, these two hypotheses may be complementary (Figure 3). Because the thalamic adenosine mechanism is based on TC LTP/LTD, it provides long-term enhancement or weakening of a specific tonotopically defined input to the ACx, whereas cortical disinhibition provides a transient, nonselective, permissive gate. These two mechanisms also could be interconnected. For instance, full expression of TC LTP in adult slices requires that cortical GABAergic and thalamic adenosine signaling be inhibited [64]. Furthermore, L1 neurons receive a noncanonical TC input [57], suggesting that they are regulated by thalamic adenosine and act as a cellular hub for regulating adult cortical plasticity. Cholinergic projections influence L1 interneurons through nicotinic receptors and thalamic adenosine through muscarinic receptors. The importance of these points of convergence warrants future investigation.
Figure 3. Overarching hypothesis of critical period regulation.
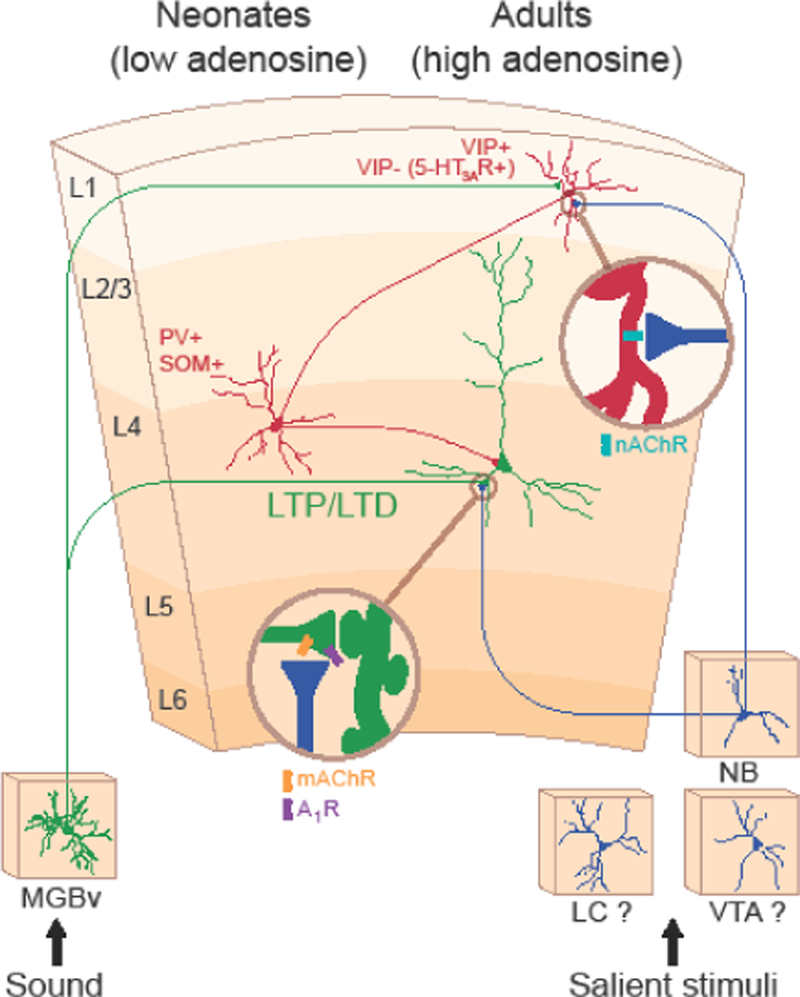
The circuit mechanism of cortical disinhibition and the synaptic mechanism of thalamocortical (TC) LTP/LTD regulation by adenosine may work cooperatively to mediate neuromodulatory inputs to the auditory cortex to manage cortical map plasticity and timing of the critical period. We hypothesize that refinement of tonotopic inputs from the auditory thalamus [ventral part of the medial geniculate body (MGBv)] is regulated at TC projections by adenosine signaling, whereas cortical disinhibition acts as a nonselective gate. Several questions remain about how (if at all) these combined mechanisms interact and whether other neuromodulators, such as dopamine from the ventral tegmental area (VTA) and norepinephrine from the locus coeruleus (LC) are involved.
One of the most important tasks neuroscientists are facing is to determine the behavioral relevance of ACx plasticity. On one hand, there is support for the idea that ACx plasticity underlies auditory learning and memory [12]. Furthermore, we know that disabling thalamic adenosine signaling improves tone discrimination in mice [67]. On the other hand, ACx plasticity is not always correlated with improved auditory performance [17;18;69]. For instance, ACx plasticity in rats is sufficient to improve perceptual learning, but long-term improvement in tone-discrimination persists even when ACx plasticity fades [15]. To rectify these inconsistencies, it is imperative to assign the above-described and other mechanisms of ACx plasticity to different aspects of auditory behavior. It is also imperative that we identify the behavioral consequences of prolonging the critical periods of cortical plasticity. What is the biological purpose of the critical periods in sensory systems? What would happen to sensory performance if adults could retain the cortical plasticity of their youth? These are intriguing questions for future research.
Highlights.
Critical (sensitive) period for cortical plasticity can be extended into adulthood
Neuromodulators reopen the critical period for cortical plasticity in adults
Neuromodulators operate through cortical disinhibition and thalamic adenosine
Cortical disinhibition is mediated by layer 1 interneurons
Thalamic adenosine production and A1 receptor signaling gate the critical period
Acknowledgements
We thank Vani Shanker and Angela MacArthur for editing the manuscript and Joshua Stokes for preparing the figures. We apologize to the colleagues whose work in the field could not be cited due to space restrictions. This work was supported, in part, by the National Institutes of Health (R01 MH097742 and R01 DC012833) and by ALSAC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
S.S.Z and J.A.B. filed a patent on the use of adenosine inhibition for improving learning (PCT/US2016/018377).
References and recommended reading
Papers of particular interest and published within the period of review have been highlighted as
follows:
• of special interest
•• of outstanding interest
•Froemke, 2015: This extensive review highlights the importance of cortical E:I balance on synaptic plasticity and the influence of various neuromodulatory systems on cortical plasticity.
•Hangya et al, 2015: This study was the first to record from optogenetically identified cholinergic neurons in the basal forebrain in vivo during an auditory attention task. The authors found that cholinergic neuronal firing is correlated with reward and punishment signals. Using computational modeling, they demonstrated that the magnitude of cholinergic response is greater when the signals are unexpected. Because similar effects were shown in two nuclei of the basal forebrain, these findings suggest that cholinergic nuclei send a broad and unified signal to the cortex.
••Martins and Froemke, 2015: This study addresses the question of how neuromodulation influences the downstream processing of sensory signals. Using in vivo whole-cell and cell-attached recordings, the authors demonstrated that LC projections undergo synaptic plasticity in response to salient auditory stimuli, which induces a long-lasting shift in the tuning curve and best frequency of ACx receptive fields. Finally, they found that cholinergic signaling downregulates phasic inhibition of the cortex, thereby linking neuromodulation, cortical E:I balance, and receptive-field plasticity.
•Letzkus et al, 2015: This article provides an exhaustive review of the importance of inhibition (and thus disinhibition) in neural circuits, with a primary focus on auditory fear conditioning. It also highlights the role of L1 inhibitory interneurons in processing unconditioned environmental stimuli.
••Pi et al, 2013: Using a combination of in vitro and in vivo optogenetics and single-cell recordings, the authors investigated the functional role of L1/VIP neurons in the ACx. Here, a novel inhibitory microcircuit between L1/VIP neurons and SOM neurons was identified, and the activity of L1/VIP neurons was implicated in the performance of an auditory-discrimination task.
••Takesian et al, 2018: This study identified a distinct cortical disinhibitory microcircuit involving L1/5-HT3AR neurons and L4 PV+ interneurons. Using a combination of slice electrophysiology, Brainbow technology, and in vivo chemogenetics, the authors identify convergent neuromodulatory and thalamic inputs onto L1/5-HT3AR interneurons that tune pyramidal cell responses in downstream layers. Removing L1 inhibitory influence on PV+ interneurons disinhibits pyramidal cell firing and reopens the critical period for plasticity.
••Blundon et al, 2017: This study demonstrated that inhibiting the production or signaling of adenosine in the thalamus allows ACx plasticity to occur past the closure (over 300 days) of the critical period and enhances auditory discrimination in adults. This paper uses in vivo imaging of individual neurons to demonstrate that age-dependent adenosine signaling gates the critical period for ACx plasticity.
References
- 1.Friederici AD, Wartenburger I: Language and brain. Wiley.Interdiscip.Rev Cogn Sci 2010, 1:150–159. [DOI] [PubMed] [Google Scholar]
- 2.Kuhl PK: Brain mechanisms in early language acquisition. Neuron 2010, 67:713–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Werker JF, Hensch TK: Critical periods in speech perception: new directions. Annu.Rev.Psychol 2015, 66:173–196. [DOI] [PubMed] [Google Scholar]
- 4.Friederici AD: The cortical language circuit: from auditory perception to sentence comprehension. Trends.Cogn.Sci 2012, 16:262–268. [DOI] [PubMed] [Google Scholar]
- 5.Doupe AJ, Kuhl PK: Birdsong and human speech: common themes and mechanisms. Annu.Rev.Neurosci 1999, 22:567–631. [DOI] [PubMed] [Google Scholar]
- 6.Lenneberg EH: Biological foundations of language New York: John Wiley and Sons; 1967. [Google Scholar]
- 7.Nelken I, Fishbach A, Las L, Ulanovsky N, Farkas D: Primary auditory cortex of cats: feature detection or something else? Biol.Cybern 2003, 89:397–406. [DOI] [PubMed] [Google Scholar]
- 8.Kanold PO, Nelken I, Polley DB: Local versus global scales of organization in auditory cortex. Trends.Neurosci 2014, 37:502–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schreiner CE, Winer JA: Auditory cortex mapmaking: principles, projections, and plasticity. Neuron 2007, 56:356–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaas JH: The Evolution of Auditory Cortex: The Core Areas. In The Auditory Cortex Edited by Winer JA, Schreiner CE. Berlin, Heidelberg, New York: Springer; 2011:407428. [Google Scholar]
- 11.Hackett TA, Barkat TR, O’Brien BM, Hensch TK, Polley DB: Linking topography to tonotopy in the mouse auditory thalamocortical circuit. J.Neurosci 2011, 31:29832995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weinberger NM: Specific long-term memory traces in primary auditory cortex. Nat.Rev.Neurosci 2004, 5:279–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bieszczad KM, Weinberger NM: Representational gain in cortical area underlies increase of memory strength. Proc.Natl.Acad.Sci.U.S.A 2010, 107:3793–3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Polley DB, Steinberg EE, Merzenich MM: Perceptual learning directs auditory cortical map reorganization through top-down influences. J.Neurosci 2006, 26:4970–4982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reed A, Riley J, Carraway R, Carrasco A, Perez C, Jakkamsetti V, Kilgard MP: Cortical map plasticity improves learning but is not necessary for improved performance. Neuron 2011, 70:121–131. [DOI] [PubMed] [Google Scholar]
- 16.Rutkowski RG, Weinberger NM: Encoding of learned importance of sound by magnitude of representational area in primary auditory cortex. Proc.Natl.Acad.Sci.U.S.A 2005, 102:13664–13669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohl FW, Scheich H: Learning-induced plasticity in animal and human auditory cortex. Curr.Opin.Neurobiol 2005, 15:470–477. [DOI] [PubMed] [Google Scholar]
- 18.Yotsumoto Y, Watanabe T, Sasaki Y: Different dynamics of performance and brain activation in the time course of perceptual learning. Neuron 2008, 57:827–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buonomano DV, Merzenich MM: Cortical plasticity: from synapses to maps. Annu.Rev.Neurosci 1998, 21:149–186. [DOI] [PubMed] [Google Scholar]
- 20.Barkat TR, Polley DB, Hensch TK: critical period for auditory thalamocortical connectivity. Nat.Neurosci 2011, 14:1189–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Villers-Sidani E, Chang EF, Bao S, Merzenich MM: Critical period window for spectral tuning defined in the primary auditory cortex (A1) in the rat. J.Neurosci 2007, 27:180–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Insanally MN, Kover H, Kim H, Bao S: Feature-dependent sensitive periods in the development of complex sound representation. J.Neurosci 2009, 29:5456–5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang LI, Bao S, Merzenich MM: Persistent and specific influences of early acoustic environments on primary auditory cortex. Nat.Neurosci 2001, 4:1123–1130. [DOI] [PubMed] [Google Scholar]
- 24.de Villers-Sidani E, Merzenich M : Lifelong plasticity in the rat auditory cortex: basic mechanisms and role of sensory experience. Prog.Brain Res 2011, 191:119–131. [DOI] [PubMed] [Google Scholar]
- 25.Kral A: Auditory critical periods: a review from system’s perspective. Neuroscience 2013, 247:117–133. [DOI] [PubMed] [Google Scholar]
- 26.Wiesel T, Hubel D: Singe-cell responses in striate coretx of kittens deprived of vision in one eye. J.Neurophysiol 1963, 26:1003–1017. [DOI] [PubMed] [Google Scholar]
- 27.King AJ, Nelken I: Unraveling the principles of auditory cortical processing: can we learn from the visual system? Nat.Neurosci 2009, 12:698–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bakin JS, Weinberger NM: Classical conditioning induces CS-specific receptive field plasticity in the auditory cortex of the guinea pig. Brain Res 1990, 536:271–286. [DOI] [PubMed] [Google Scholar]
- 29.Blake DT, Heiser MA, Caywood M, Merzenich MM: Experience-dependent adult cortical plasticity requires cognitive association between sensation and reward. Neuron 2006, 52:371–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ji W, Suga N: Development of reorganization of the auditory cortex caused by fear conditioning: effect of atropine. J.Neurophysiol 2003, 90:1904–1909. [DOI] [PubMed] [Google Scholar]
- 31.Keuroghlian AS, Knudsen EI: Adaptive auditory plasticity in developing and adult animals. Prog.Neurobiol 2007, 82:109–121. [DOI] [PubMed] [Google Scholar]
- 32.Froemke RC: Plasticity of cortical excitatory-inhibitory balance. Annu.Rev.Neurosci 2015, 38:195–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weinberger NM: Auditory associative memory and representational plasticity in the primary auditory cortex. Hear.Res 2007, 229:54–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sara SJ: The locus coeruleus and noradrenergic modulation of cognition. Nat.Rev.Neurosci 2009, 10:211–223. [DOI] [PubMed] [Google Scholar]
- 35.Gu Q: Neuromodulatory transmitter systems in the cortex and their role in cortical plasticity. Neuroscience 2002, 111:815–835. [DOI] [PubMed] [Google Scholar]
- 36.Bao S, Chan VT, Merzenich MM: Cortical remodelling induced by activity of ventral tegmental dopamine neurons. Nature 2001, 412:79–83. [DOI] [PubMed] [Google Scholar]
- 37.Hangya B, Ranade SP, Lorenc M, Kepecs A: Central Cholinergic Neurons Are Rapidly Recruited by Reinforcement Feedback. Cell 2015, 162:1155–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kilgard MP, Merzenich MM: Cortical map reorganization enabled by nucleus basalis activity. Science 1998, 279:1714–1718. [DOI] [PubMed] [Google Scholar]
- 39.Manunta Y, Edeline JM: Noradrenergic induction of selective plasticity in the frequency tuning of auditory cortex neurons. J Neurophysiol 2004, 92:1445–1463. [DOI] [PubMed] [Google Scholar]
- 40.Martins AR, Froemke RC: Coordinated forms of noradrenergic plasticity in the locus coeruleus and primary auditory cortex. Nat.Neurosci 2015, 18:1483–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Engineer ND, Riley JR, Seale J , Vrana WA, Shetake JA, Sudanagunta SP, Borland MS, Kilgard MP: Reversing pathological neural activity using targeted plasticity. Nature 2011, 470:101–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Froemke RC, Carcea I, Barker AJ, Yuan K, Seybold BA, Martins AR, Zaika N, Bernstein H, Wachs M, Levis A, Polley DB, Merzenich MM, Schreiner CE: Long-term modification of cortical synapses improves sensory perception. Nat.Neurosci 2013, 16:79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Popescu MV, Polley DB: Monaural deprivation disrupts development of binaural selectivity in auditory midbrain and cortex. Neuron 2010, 65:718–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blundon JA, Zakharenko SS: Presynaptic gating of postsynaptic synaptic plasticity: a plasticity filter in the adult auditory cortex. Neuroscientist 2013, 19:465–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett JW, Maffei L: Reactivation of ocular dominance plasticity in the adult visual cortex. Science 2002, 298:1248–1251. [DOI] [PubMed] [Google Scholar]
- 46.McGee AW, Yang Y, Fischer QS, Daw NW, Strittmatter SM: Experience-driven plasticity of visual cortex limited by myelin and Nogo receptor. Science 2005, 309:2222–2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hensch TK: Critical period plasticity in local cortical circuits. Nat.Rev.Neurosci 2005, 6:877–888. [DOI] [PubMed] [Google Scholar]
- 48.Letzkus JJ, Wolff SB, Luthi A: Disinhibition, a Circuit Mechanism for Associative Learning and Memory. Neuron 2015, 88:264–276. [DOI] [PubMed] [Google Scholar]
- 49.Wehr M, Zador AM: Balanced inhibition underlies tuning and sharpens spike timing in auditory cortex. Nature 2003, 426:442–446. [DOI] [PubMed] [Google Scholar]
- 50.Sun YJ, Wu GK, Liu BH, Li P, Zhou M, Xiao Z, Tao HW, Zhang LI: Fine-tuning of prebalanced excitation and inhibition during auditory cortical development. Nature 2010, 465:927–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dorrn AL, Yuan K, Barker AJ, Schreiner CE, Froemke RC: Developmental sensory experience balances cortical excitation and inhibition. Nature 2010, 465:932–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Froemke RC, Merzenich MM, Schreiner CE: A synaptic memory trace for cortical receptive field plasticity. Nature 2007, 450:425–429. [DOI] [PubMed] [Google Scholar]
- 53.Letzkus JJ, Wolff SB, Meyer EM, Tovote P, Courtin J, Herry C, Luthi A: A disinhibitory microcircuit for associative fear learning in the auditory cortex. Nature 2011, 480:331–335. [DOI] [PubMed] [Google Scholar]
- 54.Pi HJ, Hangya B, Kvitsiani D, Sanders JI, Huang ZJ, Kepecs A: Cortical interneurons that specialize in disinhibitory control. Nature 2013, 503:521–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fu Y, Tucciarone JM, Espinosa JS, Sheng N, Darcy DP, Nicoll RA, Huang ZJ, Stryker MP: A cortical circuit for gain control by behavioral state. Cell 2014, 156:1139–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Morishita H, Miwa JM, Heintz N, Hensch TK: Lynx1, a cholinergic brake, limits plasticity in adult visual cortex. Science 2010, 330:1238–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takesian AE, Bogart LJ, Lichtman JW, Hensch TK: Inhibitory circuit gating of auditory critical-period plasticity. Nat.Neurosci 2018, 21:218–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee CC, Winer JA: Connections of cat auditory cortex: I. Thalamocortical system. J.Comp.Neurol 2008, 507:1879–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nahmani M, Erisir A: VGluT2 immunochemistry identifies thalamocortical terminals in layer 4 of adult and developing visual cortex. J Comp Neurol 2005, 484:458–473. [DOI] [PubMed] [Google Scholar]
- 60.Bayazitov IT, Westmoreland JJ, Zakharenko SS: Forward suppression in the auditory cortex is caused by the Ca(v)3.1 calcium channel-mediated switch from bursting to tonic firing at thalamocortical projections. J.Neurosci 2013, 33:18940–18950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Beierlein M, Connors BW: Short-term dynamics of thalamocortical and intracortical synapses onto layer 6 neurons in neocortex. J.Neurophysiol 2002, 88:1924–1932. ACCEPTED [DOI] [PubMed] [Google Scholar]
- 62.Daw MI, Scott HL, Isaac JT: Developmental synaptic plasticity at the thalamocortical input to barrel cortex: mechanisms and roles. Mol.Cell Neurosci 2007, 34:493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Blundon JA, Bayazitov IT, Zakharenko SS: Presynaptic gating of postsynaptically expressed plasticity at mature thalamocortical synapses. J.Neurosci 2011, 31:16012–16025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chun S, Bayazitov IT, Blundon JA, Zakharenko SS: Thalamocortical long-term potentiation becomes gated after the early critical period in the auditory cortex. J.Neurosci 2013, 33:7345–7357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dunwiddie TV, Masino SA: The role and regulation of adenosine in the central nervous system. Annu.Rev.Neurosci 2001, 24:31–55. [DOI] [PubMed] [Google Scholar]
- 66.Sun H, Takesian AE, Wang TT, Lippman-Bell JJ, Hensch TK, Jensen FE: Early Seizures Prematurely Unsilence Auditory Synapses to Disrupt Thalamocortical Critical Period Plasticity. Cell Rep 2018, 23:2533–2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Blundon JA, Roy NC, Teubner BJW, Yu J, Eom TY, Sample KJ, Pani A, Smeyne RJ, Han SB, Kerekes RA, Rose DC, Hackett TA, Vuppala PK, Freeman BB III, Zakharenko SS: Restoring auditory cortex plasticity in adult mice by restricting thalamic adenosine signalling. Science 2017, 356:1352–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kral A, Yusuf PA, Land R: Higher-order auditory areas in congenital deafness: Top- down interactions and corticocortical decoupling. Hear.Res 2017, 343:50–63. [DOI] [PubMed] [Google Scholar]
- 69.Ohl FW, Scheich H: Fallacies in behavioural interpretation of auditory cortex plasticity. Nature Reviews Neuroscience 2004, 5:972. [Google Scholar]


