Abstract
Sleep has been universally conserved across animal species. The basic functions of sleep remain unclear, but insufficient sleep impairs memory acquisition and retention in both vertebrates and invertebrates. Sleep is also a homeostatic process that is influenced not only by the amount of time awake, but also by neural activity and plasticity. Due to the breadth and precision of available genetic tools, the fruit fly has become a powerful model system to understand sleep regulation and function. Importantly, these tools enable the dissection of memory-encoding circuits at the level of individual neurons, and have allowed the development of genetic tools to induce sleep on-demand. This review describes recent investigations of the role for sleep in memory using Drosophila and current hypotheses of sleep’s functions for supporting plasticity, learning, and memory.
Introduction
Sleep is vital for cognition, but the basic functions of sleep in the brain remain poorly understood. While consequences of sleep loss degrade systems throughout the body, cognitive deficits, including learning and memory impairments, are among the earliest to occur (For reviews, see [1-3]). Although neural architecture and physiology differ between vertebrates and invertebrates, the properties and functions of sleep are tightly conserved across evolution. Sleep-like rest was first described in a cockroaches roughly 35 years ago [4], and sleep has since been characterized in a variety of other invertebrates, including insects [5-7], nematodes [8], and mollusks [9-11]. In all of these species, sleep matches the behavioral criteria originally used to describe mammalian sleep patterns: quiescence, reversibility, postural change, decreased arousability, and homeostatic regulation [12,13]. Importantly, many drugs and genetic lesions influence sleep similarly in humans and invertebrates [5,14-17], providing strong evidence for broadly conserved mechanisms of sleep regulation and function.
Because sleep has been universally conserved across animal species, examining the functions of sleep in relatively small, simple invertebrate brains will likely provide a better understanding of why sleep is required for all animals. Sleep’s role in supporting learning and memory appears to be an evolutionarily ancient function – insufficient sleep degrades memory similarly in animals ranging from humans to invertebrates, including Drosophila melanogaster and Aplysia californica [3,18]. Because a role for sleep in memory has been broadly conserved across evolution, it is likely that sleep fulfills a basic function that promotes the consolidation of recent memories and maintains the capacity for new memory acquisition. To date, however, a mechanistic understanding of sleep’s functions in memory remain incomplete. Examining sleep’s effects within memory-encoding circuits of insect brains, therefore, will likely uncover functions of sleep that may be generalized across species. Due to a combination of genetic accessibility and a relatively simple nervous system, the fruit fly, Drosophila melanogaster, has become the focus of many investigations into sleep regulation and function.
Plastic regulation of sleep
Sleep is a homeostatic process that is influenced not only by the amount of time awake, but also by neural activity and plasticity. In vertebrates, slow wave activity during sleep is elevated in cortical areas that have been recently active or plastic [19-21], suggesting that sleep can be regulated in a use-dependent manner. Similarly, several studies have found an elevated need for sleep in invertebrates following novel experiences that drive plasticity. Housing Drosophila in an enriched social environment for several days, for instance, drives synaptic elaboration in visual and olfactory circuits [22-25]. After this social experience, flies sleep more than socially isolated siblings for up to three days [26]. The effect of waking experience on sleep scales with the size of the fly’s social group, and is eliminated by disrupting visual or olfactory perception or by mutating genes necessary for synaptic plasticity [24,26]. Similar increases in sleep are also temporarily observed after associative training protocols that produce protein synthesis-dependent long-term memories (LTM) [26]. Studies of honeybees have also identified changes in sleep time and architecture based on an individual’s social role in the hive [27,28]. Together, these findings suggest that insects provide models to understand why elevated neural plasticity/activity increase the need for sleep and how sleep might be altering recently plastic synapses.
While the function of sleep after novel experience is not clear, one recent hypothesis has raised the possibility that sleep acts as a mechanism for homeostatic synaptic scaling [29,30]. Waking increases the abundance of synaptic proteins [31], likely reflecting synaptic expansion and growth to encode newly formed associations. One function of sleep may be the homeostatic weakening or removal of these expanded connections to prevent synaptic saturation [29,30]. In support of this hypothesis, the overall abundance of several synaptic proteins in fly brain homogenates is elevated after sleep deprivation and reduced after a night of sleep [31]. Subsequent studies have also found that extended waking increases the size or number of pre-synaptic active zones from several cell types, including Mushroom body-intrinsic Kenyon cells, large-field visual interneurons in the medulla, and circadian ventral lateral neurons [24,25]. Similarly, recovery sleep following enriched social experience is required to reduce synaptic size and number back to baseline levels [24,25]. Recent publications have also found trends for increased synapse strength [32], synapse size [33], or dendritic spine retention [34] in rodent cortex following sleep deprivation, suggesting that similar scaling may occur in flies and mammals.
Although these studies are consistent with a role for sleep in homeostatic synaptic downscaling, the processes by which synapses are both tagged for pruning and weakened/removed have not been dissected. It is currently unknown, for example, which particular synapses are most likely to be pruned during sleep, or how a homeostatic set point for synapse number or strength might be maintained. The answers to these questions may lie in mechanisms that underlie homeostatic control of firing rate [35,36], neural excitability [37-39], and synaptic scaling [39-46]. Studies using rodents have begun to elegantly examine electrophysiological mechanisms of neural homeostasis across wake and sleep [36,47], and complementary investigations using the fly may also unravel the underlying molecular machinery.
The data described above support a role for sleep in homeostatic synapse downscaling, but plastic changes during sleep may vary across circuits or cell types. Sleep depriving young Drosophila during the first day after eclosion, for example, can cause long-lasting deficits in courtship and memory [48,49]. In flies, as in other species, sleep is elevated early in life as neural circuits undergo high levels of developmental plasticity and refinement [5,49-52]. During the initial days after eclosion, male flies require sleep for the expansion of olfactory glomeruli in the antennal lobe that process social pheromone cues [49]. This result parallels observations of ocular dominance plasticity in the visual cortex of cats and mice, in which sleep is required for neurons in primary visual cortex to weaken inputs from a closed eye and expand inputs from an open eye [52,53]. In the case of ocular dominance plasticity, sleep promotes the strengthening of inputs from an open eye to visual cortex via NMDA-dependent signaling mechanisms [53]. The sleep-dependent expansion of synapses in developing Drosophila olfactory glomeruli and mammalian visual cortex suggests that plasticity during sleep is not unidirectional, but the factors that determine how sleep alters synapses in different circuits or different stages of development have not been established. To address these issues, additional studies will be required to examine which rules govern synaptic scaling in different circuits or cell types during sleep, and to identify the molecular mechanisms that might modulate synaptic size or number during sleep.
Sleep and learning in the Mushroom body
Short-term memory deficits have been observed in wild-type flies that have been previously sleep deprived [54-57] or exhibit spontaneously fragmented sleep [55], in short-sleeping hyperkinetic [58] and crossveinless-c mutant flies [56], and in flies genetically selected for low sleep time [59]. Conversely, sleep is altered in a variety of mutants and neurodegenerative disease models that influence learning and memory [60,61] and promoting sleep in many of these genotypes is sufficient to improve learning [62,63]. Together, these findings point to a vital role for sleep before learning in maintaining the capacity to acquire new memories.
Mushroom bodies (MBs) are an associative neuropil vital for the acquisition and storage of olfactory memories (See Figure 1 for schematic) [64,65]. The logic that underlies memory encoding in the MB has been the focus of intense investigation, and recently created Drosophila genetic tools have allowed the dissection of MB circuits at the level of individual neurons [66,67]. Drosophila form olfactory memories within the Mushroom bodies, an associative center that receives synaptic input from second-order olfactory projection neurons. MB-intrinsic Kenyon cells (KCs) encode olfactory cue information and can be divided into three subsets that project to distinct axonal lobes (α/β, α’/β’, and γ) [68]. Each of these lobes can be further subdivided into individual zones that provide output to individual MB output neurons (MBONs) and receive reinforcement signals from distinct types of dopaminergic neurons [67]. New olfactory memories are encoded in the MB when reinforcing dopamine signals modify the strength of synapses between active KCs and individual MBONs that encode either attraction or aversion [66,69,70] (See Figure 1B). Sleep is required for MBs to encode and retain new memories in several associative assays [55-57] (See Table 1). Two factors are most likely to contribute to learning and memory deficits following sleep loss: excessive synaptic potentiation (see description of the synaptic homeostasis hypothesis above), and dysregulated reinforcement from dopaminergic neurons in the MB. As shown by Seugnet et al [55], transcript levels of the dopaminergic receptor Dop1R1 are reduced after sleep loss. Expression of this receptor in the MB is required for associative learning [71], and learning impairments can be overcome in sleep-deprived flies either by pharmacologically increasing dopamine signaling or by overexpressing Dop1R1 in MB-intrinsic KCs [55]. Together, these data suggest that prolonged waking may degrade MB sensitivity to dopaminergic release that signals reinforcement. Sleep may, therefore, allow Drosophila KCs to re-sensitize to dopaminergic inputs by enforcing a period of low activity in both KCs and dopaminergic neurons [72,73]. Neuroimaging studies have also found that sleep deprivation reduces available D2/D3 receptors in the human striatum, suggesting that similar effects may be conserved in mammals [74,75].
Figure 1.
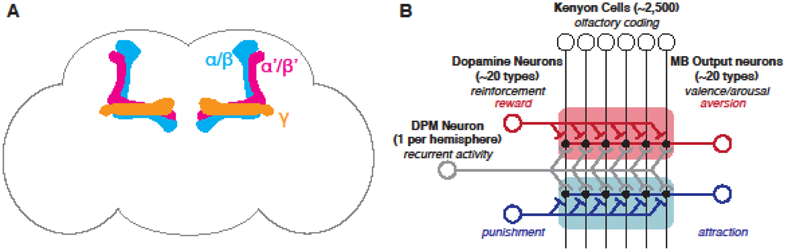
Organization of Mushroom body circuit that encodes olfactory memories and modulates sleep
(A)Cartoon of MB lobes formed by axons of MB-intrinsic Kenyon cells (KCs). KC axons are subdivided into α/β lobe shown in blue, α’/β’ in magenta, and γ in orange. (Illustration adapted from [89])
(B)Schematic of MB circuitry. Axons of odor-coding KCs can be subdivided into tiled zones that are each innervated by a single type of dopaminergic reinforcement neuron and provide output to a single MB output neuron (MBONs). Olfactory memories are encoded within the strength of synapses between KCs and MBONs. Modulatory DPM neurons project throughout the MB lobes and their recurrent activation after learning is required for consolidation of recent memories. (Based on findings from [67,69,81]).
Table 1.
Summary of behavioral assays used to test memory deficits associated with sleep loss. Learning/short-term memory loss was typically tested in flies sleep-deprived for one night prior to learning, while the role for sleep in long-term memory consolidation was tested using sleep deprivation that occurred after training.
| Short-term Memory | Long-term Memory | |
|---|---|---|
| Aversive Phototactic Suppression | Impaired by SD before training Seugnet et al., 2008 | No LTM in this assay |
| Courtship Conditioning | No published data | Impaired by SD after training Ganguly-Fitzgerald et al., 2006 |
| Aversive Olfactory Conditioning | Impaired by SD before training Li et al., 2009 | Impaired by SD after training Li et al., 2009 |
| Aversive Taste Memory | Impaired by SD before training Seidner et al., 2015 | LTM not characterized in this assay |
| Heat-box Spatial Memory | Impaired in short-sleeping hk mutants Bushey et al., 2007 | No LTM in this assay |
MB circuitry not only encodes memories, but also influences sleep regulation. Manipulating activity in distinct subpopulations of MB-intrinsic KCs or MBONs can strongly either increase or decrease sleep [76-78]. Dissection of this circuitry suggests that wake- and sleep-promoting circuits within the MB may be organized in parallel, with wake-promoting KCs exciting wake-promoting MBONs and sleep-promoting KCs exciting sleep-promoting MBONs [78]. Sleep-promoting KCs and MBONs exhibit increased activity in sleep-deprived flies [78], indicating that they may provide a representation of homeostatic sleep need. It is important to note that the MBONs that modulate sleep may also encode aspects of olfactory valence; wake-promoting MBONs are also activated by odors with a negative valence, and sleep-promoting MBONs are responsive to attractive odors [66,78]. Future studies will be required to untangle whether the coding of sleep/wake and positive/negative valence within these MB circuits are linked, or if each signal can be independently relayed to downstream effectors.
Sleep and Memory Consolidation
Sleep is also required after learning to consolidate recent associations into long-lasting memories. Initial studies examining the function of post-training sleep found that sleep was significantly increased following a spaced training protocol for Courtship conditioning, an associative assay during which male flies learn to suppress their courtship behaviors following unsuccessful mating attempts [26]. Sleep deprivation during the first several hours after training prevented memory consolidation, and sleep was not changed after training in flies with genetic defects that impair memory consolidation [24,26]. These studies suggested that sleep was necessary for LTM formation in MB-dependent assays (See Table 1), raising the possibility that acutely promoting sleep after training may be sufficient to enhance memory consolidation.
Drosophila provides a unique model for examining the beneficial effects of sleep – genetic screening has identified a cluster of neurons projecting into the dorsal fan-shaped body (dFB) that can be acutely activated to induce sleep on-demand [79]. To test the role of sleep in promoting memory consolidation, male flies experienced a single episode of Courtship training, then sleep was induced via dFB activation. While the single training episode was not sufficient to elicit LTM in control flies, the same protocol resulted in robust LTM when sleep was induced via dFB activation immediately following training [79]. Because this LTM enhancement was eliminated when flies were sleep deprived during dFB activation, it is likely that increased sleep after learning may actively promote the consolidation of recently acquired memories. Similar impairments in consolidation with sleep loss and consolidation enhancements with sleep induction have also been observed using classical olfactory conditioning [54,73], suggesting that the beneficial role for sleep in memory consolidation may be shared across MB-dependent assays.
To date, however, the consolidation mechanisms that occur during sleep have not been clearly described. It is known, however, that memory consolidation requires sleep and the recurrent activation of modulatory neurons within the MB during the few hours after training [26,73,80]. Synaptic release from the dorsal paired medial neurons (DPMs), which consist of 1 neuron in each hemisphere that innervate all MB lobes (See Figure 1B for schematic), is not required during memory acquisition or retrieval, but silencing DPM release during the hours after learning impairs LTM consolidation [80,81]. Similarly, slow oscillatory activity in dopaminergic MB neurons following learning is also correlated with memory consolidation [82]. While it is not known whether recurrent DPM activity during consolidation coincides with sleep, DPM activation has been shown to drive robust sleep increases [83]. While these studies suggest that DPM activation may gate memory consolidation by modulating sleep, no studies have yet confirmed whether DPM activity is required to promote sleep during consolidation. Recurrent activity of MB circuits during sleep may also contribute to the consolidation of recent memories in insects other than Drosophila. In an elegant study by Zwaka et al. (2015), honeybees were trained to associate a novel odor with a sugar reward. Bees that were re-exposed to the same conditioned odor during subsequent sleep episodes retained a stronger appetitive memory than bees that were exposed to the conditioned odor during post-training waking [84]. This study closely parallels human studies that used contextual odor cues during slow wave sleep to reactivate hippocampal circuits and increase declarative memory retention [85], suggesting that similar patterns of recurrent activation might support memory consolidation in insects and vertebrates.
Conclusion
Sleep is a physiological state that we share with all animals, including insects like the fruit fly. Because learning and memory impairments are closely shared from humans to Drosophila, the genetic accessibility and relative simple nervous system of the fly provide an ideal model to uncover the fundamental mechanisms of sleep function. While evidence suggests that sleep may play a role in synaptic scaling and calibrating the strength of dopamingergic reinforcement signals, the precise functions of sleep and their underlying mechanisms are still poorly understood. With recently developed tools to precisely dissect and control MB circuits at the level of single cells [67] and to control the timing of sleep in Drosophila [57,79,86-88], current and future research will have a precision to examine sleep’s role in plasticity that is unavailable in other species. Importantly, these studies open the possibility of studying not only the detrimental consequences of sleep loss, but also the benefits of promoting sleep [62].
Highlights.
Insufficient sleep impairs the acquisition and consolidation of memories in the Drosophila Mushroom bodies
Sleep may benefit memory-encoding circuits by homeostatically scaling synaptic connections
Genetic tools to control sleep timing in Drosophila may provide unique opportunities to study the cognitive benefits of sleep
Acknowledgements
JD is supported by a Career Development Award from the Human Frontiers Science Program (CDA-00026/2017-C), a Klingenstein-Simons Fellowship in Neuroscience, and NIH/NINDS grant R01 NS105967.
Footnotes
Conflict of interest statement
No conflicts to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Diekelmann S, Born J: The memory function of sleep. Nat. Rev. Neurosci 2010, 11:114–126. [DOI] [PubMed] [Google Scholar]
- 2.Goel N, Rao H, Durmer JS, Dinges DF: Neurocognitive consequences of sleep deprivation. Semin Neurol 2009, 29:320–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dissel S, Melnattur K, Shaw PJ: Sleep, Performance, and Memory in Flies. Curr Sleep Med Rep 2015, 1:47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tobler I: Effect of forced locomotion on the rest-activity cycle of the cockroach. Behav. Brain Res 1983, 8:351–360. [DOI] [PubMed] [Google Scholar]
- •5.Shaw PJ, Cirelli C, Greenspan RJ, Tononi G: Correlates of sleep and waking in Drosophila melanogaster. Science 2000, 287:1834–1837. [DOI] [PubMed] [Google Scholar]
- •6.Hendricks JC, Finn SM, Panckeri KA, Chavkin J, Williams JA, Sehgal A, Pack AI: Rest in Drosophila is a sleep-like state. Neuron 2000, 25:129–138. [DOI] [PubMed] [Google Scholar]; References 5 & 6 provided the initial behavioral characterizations of sleep in Drosophila.
- 7.Klein BA, Stiegler M, Klein A, Tautz J: Mapping sleeping bees within their nest: spatial and temporal analysis of worker honey bee sleep. PLoS ONE 2014, 9:e102316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raizen DM, Zimmerman JE, Maycock MH, Ta UD, You Y-J, Sundaram MV, Pack AI: Lethargus is a Caenorhabditis elegans sleep-like state. Nature 2008, 451:569–572. [DOI] [PubMed] [Google Scholar]
- 9.Vorster APA, Krishnan HC, Cirelli C, Lyons LC: Characterization of sleep in Aplysia californica. Sleep 2014, 37:1453–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frank MG, Waldrop RH, Dumoulin M, Aton SJ, Boal JG: A preliminary analysis of sleep-like states in the cuttlefish Sepia officinalis. PLoS ONE 2012, 7:e38125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown ER, Piscopo S, De Stefano R, Giuditta A: Brain and behavioural evidence for rest-activity cycles in Octopus vulgaris. Behav. Brain Res 2006, 172:355–359. [DOI] [PubMed] [Google Scholar]
- 12.Campbell SS, Tobler I: Animal sleep: a review of sleep duration across phylogeny. Neurosci Biobehav Rev 1984, 8:269–300. [DOI] [PubMed] [Google Scholar]
- 13.Piéron H: Le problème physiologique du sommeil. Masson; 1913. [Google Scholar]
- 14.Cirelli C, Bushey D, Hill S, Huber R, Kreber R, Ganetzky B, Tononi G: Reduced sleep in Drosophila Shaker mutants. Nature 2005, 434:1087–1092. [DOI] [PubMed] [Google Scholar]
- 15.Douglas CL, Vyazovskiy V, Southard T, Chiu S-Y, Messing A, Tononi G, Cirelli C: Sleep in Kcna2 knockout mice. BMC Biol. 2007, 5:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foltenyi K, Greenspan RJ, Newport JW: Activation of EGFR and ERK by rhomboid signaling regulates the consolidation and maintenance of sleep in Drosophila. Nat. Neurosci 2007, 10:1160–1167. [DOI] [PubMed] [Google Scholar]
- 17.Kushikata T, Fang J, Chen Z, Wang Y, Krueger JM: Epidermal growth factor enhances spontaneous sleep in rabbits. Am. J. Physiol 1998, 275:R509–14. [DOI] [PubMed] [Google Scholar]
- 18.Krishnan HC, Gandour CE, Ramos JL, Wrinkle MC, Sanchez-Pacheco JJ, Lyons LC: Acute Sleep Deprivation Blocks Short- and Long-Term Operant Memory in Aplysia. Sleep 2016, 39:2161–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huber R, Ghilardi MF, Massimini M, Ferrarelli F, Riedner BA, Peterson MJ, Tononi G: Arm immobilization causes cortical plastic changes and locally decreases sleep slow wave activity. Nat. Neurosci 2006, 9:1169–1176. [DOI] [PubMed] [Google Scholar]
- 20.Huber R, Ghilardi MF, Massimini M, Tononi G: Local sleep and learning. Nature 2004, 430:78–81. [DOI] [PubMed] [Google Scholar]
- 21.Huber R, Esser SK, Ferrarelli F, Massimini M, Peterson MJ, Tononi G: TMS-induced cortical potentiation during wakefulness locally increases slow wave activity during sleep. PLoS ONE 2007, 2:6276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Technau GM: Fiber number in the mushroom bodies of adult Drosophila melanogaster depends on age, sex and experience. Journal of Neurogenetics 1984, 1:113–126. [DOI] [PubMed] [Google Scholar]
- 23.Barth M, Heisenberg M: Vision affects mushroom bodies and central complex in Drosophila melanogaster. Learn. Mem 1997, 4:219–229. [DOI] [PubMed] [Google Scholar]
- ••24.Donlea JM, Ramanan N, Shaw PJ: Use-dependent plasticity in clock neurons regulates sleep need in Drosophila. Science 2009, 324:105–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••25.Bushey D, Tononi G, Cirelli C: Sleep and synaptic homeostasis: structural evidence in Drosophila. Science 2011, 332:1576–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]; References 24 & 25 provide evidence for homeostatic synapse pruning during sleep in the Drosophila brain.
- 26.Ganguly-Fitzgerald I, Donlea J, Shaw PJ: Waking experience affects sleep need in Drosophila. Science 2006, 313:1775–1781. [DOI] [PubMed] [Google Scholar]
- 27.Eban-Rothschild AD, Bloch G: Differences in the sleep architecture of forager and young honeybees (Apis mellifera). - PubMed - NCBI. J. Exp. Biol 2008, 211:2408–2416. [DOI] [PubMed] [Google Scholar]
- 28.Klein BA, Olzsowy KM, Klein A, Saunders KM, Seeley TD: Caste-dependent sleep of worker honey bees. J. Exp. Biol 2008, 211:3028–3040. [DOI] [PubMed] [Google Scholar]
- 29.Tononi G, Cirelli C: Sleep and synaptic homeostasis: a hypothesis. Brain Res. Bull 2003, 62:143–150. [DOI] [PubMed] [Google Scholar]
- 30.Tononi G, Cirelli C: Sleep and the price of plasticity: from synaptic and cellular homeostasis to memory consolidation and integration. Neuron 2014, 81:12–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gilestro GF, Tononi G, Cirelli C: Widespread changes in synaptic markers as a function of sleep and wakefulness in Drosophila. Science 2009, 324:109–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diering GH, Nirujogi RS, Roth RH, Worley PF, Pandey A, Huganir RL: Homer1a drives homeostatic scaling-down of excitatory synapses during sleep. Science 2017, 355:511–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Vivo L, Bellesi M, Marshall W, Bushong EA, Ellisman MH, Tononi G, Cirelli C: Ultrastructural evidence for synaptic scaling across the wake/sleep cycle. Science 2017, 355:507–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li W, Ma L, Yang G, Gan W-B: REM sleep selectively prunes and maintains new synapses in development and learning. Nat. Neurosci 2017, 20:427–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hengen KB, Lambo ME, Van Hooser SD, Katz DB, Turrigiano GG: Firing rate homeostasis in visual cortex of freely behaving rodents. Neuron 2013, 80:335–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hengen KB, Torrado Pacheco A, McGregor JN, Van Hooser SD, Turrigiano GG: Neuronal Firing Rate Homeostasis Is Inhibited by Sleep and Promoted by Wake. Cell 2016, 165:180–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Joseph A, Turrigiano GG: All for One But Not One for All: Excitatory Synaptic Scaling and Intrinsic Excitability Are Coregulated by CaMKIV, Whereas Inhibitory Synaptic Scaling Is Under Independent Control. J. Neurosci 2017, 37:6778–6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lambo ME, Turrigiano GG: Synaptic and intrinsic homeostatic mechanisms cooperate to increase L2/3 pyramidal neuron excitability during a late phase of critical period plasticity. J. Neurosci 2013, 33:8810–8819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Turrigiano G: Too many cooks? Intrinsic and synaptic homeostatic mechanisms in cortical circuit refinement. Annu. Rev. Neurosci 2011, 34:89–103. [DOI] [PubMed] [Google Scholar]
- 40.Haghighi AP, McCabe BD, Fetter RD, Palmer JE, Hom S, Goodman CS: Retrograde control of synaptic transmission by postsynaptic CaMKII at the Drosophila neuromuscular junction. Neuron 2003, 39:255–267. [DOI] [PubMed] [Google Scholar]
- 41.Frank CA, Kennedy MJ, Goold CP, Marek KW, Davis GW: Mechanisms underlying the rapid induction and sustained expression of synaptic homeostasis. Neuron 2006, 52:663–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goold CP, Davis GW: The BMP ligand Gbb gates the expression of synaptic homeostasis independent of synaptic growth control. Neuron 2007, 56:109–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dickman DK, Davis GW: The schizophrenia susceptibility gene dysbindin controls synaptic homeostasis. Science 2009, 326:1127–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bergquist S, Dickman DK, Davis GW: A hierarchy of cell intrinsic and target-derived homeostatic signaling. Neuron 2010, 66:220–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Turrigiano GG: The self-tuning neuron: synaptic scaling of excitatory synapses. Cell 2008, 135:422–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wierenga CJ, Ibata K, Turrigiano GG: Postsynaptic expression of homeostatic plasticity at neocortical synapses. J. Neurosci 2005, 25:2895–2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aton SJ, Suresh A, Broussard C, Frank MG: Sleep promotes cortical response potentiation following visual experience. Sleep 2014, 37:1163–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seugnet L, Suzuki Y, Donlea JM, Gottschalk L, Shaw PJ: Sleep deprivation during early-adult development results in long-lasting learning deficits in adult Drosophila. Sleep 2011, 34:137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••49.Kayser MS, Yue Z, Sehgal A: A critical period of sleep for development of courtship circuitry and behavior in Drosophila. Science 2014, 344:269–274. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study describes the detrimental effects of sleep deprivation soon after eclosion on olfactory circuit expansion and later courtship behavior.
- 50.Roffwarg HP, Muzio JN, Dement WC: Ontogenetic development of the human sleep-dream cycle. Science 1966, 152:604–619. [DOI] [PubMed] [Google Scholar]
- 51.Jouvet-Mounier D, Astic L, Lacote D: Ontogenesis of the states of sleep in rat, cat, and guinea pig during the first postnatal month. Dev Psychobiol 1970, 2:216–239. [DOI] [PubMed] [Google Scholar]
- 52.Frank MG, Issa NP, Stryker MP: Sleep enhances plasticity in the developing visual cortex. Neuron 2001, 30:275–287. [DOI] [PubMed] [Google Scholar]
- 53.Aton SJ, Seibt J, Dumoulin M, Jha SK, Steinmetz N, Coleman T, Naidoo N, Frank MG: Mechanisms of sleep-dependent consolidation of cortical plasticity. Neuron 2009, 61:454–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li X, Yu F, Guo A: Sleep deprivation specifically impairs short-term olfactory memory in Drosophila. Sleep 2009, 32:1417–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••55.Seugnet L, Suzuki Y, Vine L, Gottschalk L, Shaw PJ: D1 receptor activation in the mushroom bodies rescues sleep-loss-induced learning impairments in Drosophila. Curr. Biol 2008, 18:1110–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]; This manuscript provided an initial characterization of associative learning deficits following sleep loss in the fly, and demonstrated that elevating dopaminergic signaling could protect learning after sleep loss.
- 56.Donlea JM, Pimentel D, Miesenböck G: Neuronal machinery of sleep homeostasis in Drosophila. Neuron 2014, 81:860–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Seidner G, Robinson JE, Wu M, Worden K, Masek P, Roberts SW, Keene AC, Joiner WJ: Identification of Neurons with a Privileged Role in Sleep Homeostasis in Drosophila melanogaster. Curr. Biol 2015, 25:2928–2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bushey D, Huber R, Tononi G, Cirelli C: Drosophila Hyperkinetic mutants have reduced sleep and impaired memory. J. Neurosci 2007, 27:5384–5393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Seugnet L, Suzuki Y, Thimgan MS, Donlea J, Gimbel SI, Gottschalk L, Duntley SP, Shaw PJ: Identifying sleep regulatory genes using a Drosophila model of insomnia. J. Neurosci 2009, 29:7148–7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hendricks JC, Williams JA, Panckeri K, Kirk D, Tello M, Yin JC, Sehgal A: A non-circadian role for cAMP signaling and CREB activity in Drosophila rest homeostasis. Nat. Neurosci 2001, 4:1108–1115. [DOI] [PubMed] [Google Scholar]
- 61.Tabuchi M, Lone SR, Liu S, Liu Q, Zhang J, Spira AP, Wu MN: Sleep interacts with aβ to modulate intrinsic neuronal excitability. Curr. Biol 2015, 25:702–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dissel S, Angadi V, Kirszenblat L, Suzuki Y, Donlea J, Klose M, Koch Z, English D, Winsky-Sommerer R, van Swinderen B, et al. : Sleep restores behavioral plasticity to Drosophila mutants. Curr. Biol 2015, 25:1270–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dissel S, Klose M, Donlea J, Cao L, English D, Winsky-Sommerer R, van Swinderen B, Shaw PJ: Enhanced sleep reverses memory deficits and underlying pathology in drosophila models of Alzheimer's disease. Neurobiology of Sleep and Circadian Rhythms 2017, 2:15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Heisenberg M, Borst A, Wagner S, Byers D: Drosophila mushroom body mutants are deficient in olfactory learning. Journal of Neurogenetics 1985, 2:1–30. [DOI] [PubMed] [Google Scholar]
- 65.McBride SM, Giuliani G, Choi C, Krause P, Correale D, Watson K, Baker G, Siwicki KK: Mushroom body ablation impairs short-term memory and long-term memory of courtship conditioning in Drosophila melanogaster. Neuron 1999, 24:967–977. [DOI] [PubMed] [Google Scholar]
- 66.Aso Y, Sitaraman D, Ichinose T, Kaun KR, Vogt K, Belliart-Guérin G, Plaçais P-Y, Robie AA, Yamagata N, Schnaitmann C, et al. : Mushroom body output neurons encode valence and guide memory-based action selection in Drosophila. Elife 2014, 3:e0458o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aso Y, Hattori D, Yu Y, Johnston RM, Iyer NA, Ngo T-TB, Dionne H, Abbott LF, Axel R, Tanimoto H, et al. : The neuronal architecture of the mushroom body provides a logic for associative learning. Elife 2014, 3:e04577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Crittenden JR, Skoulakis EM, Han K-A, Kalderon D, Davis RL: Tripartite mushroom body architecture revealed by antigenic markers. Learn. Mem 1998, 5:38–51. [PMC free article] [PubMed] [Google Scholar]
- 69.Owald D, Felsenberg J, Talbot CB, Das G, Perisse E, Huetteroth W, Waddell S: Activity of defined mushroom body output neurons underlies learned olfactory behavior in Drosophila. Neuron 2015, 86:417–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hige T, Aso Y, Modi MN, Rubin GM, Turner GC: Heterosynaptic Plasticity Underlies Aversive Olfactory Learning in Drosophila. Neuron 2015, 88:985–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim Y-C, Lee H-G, Han K-A: D1 dopamine receptor dDA1 is required in the mushroom body neurons for aversive and appetitive learning in Drosophila. J. Neurosci 2007, 27:7640–7647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bushey D, Tononi G, Cirelli C: Sleep- and wake-dependent changes in neuronal activity and reactivity demonstrated in fly neurons using in vivo calcium imaging. Proc. Natl. Acad. Sci. U.S.A 2015, 112:4785–4790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Berry JA, Cervantes-Sandoval I, Chakraborty M, Davis RL: Sleep Facilitates Memory by Blocking Dopamine Neuron-Mediated Forgetting. Cell 2015, 161:1656–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Volkow ND, Tomasi D, Wang GJ, Telang F, Fowler JS, Logan J, Benveniste H, Kim R, Thanos PK, Ferre S: Evidence That Sleep Deprivation Downregulates Dopamine D2R in Ventral Striatum in the Human Brain. Journal of Neuroscience 2012, 32:6711–6717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tomasi D, Wang GJ, Volkow ND: Association between striatal dopamine D2/D3 receptors and brain activation during visual attention: effects of sleep deprivation. Transl Psychiatry 2016, 6:e828–e828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pitman JL, McGill JJ, Keegan KP, Allada R: A dynamic role for the mushroom bodies in promoting sleep in Drosophila. Nature 2006, 441:753–756. [DOI] [PubMed] [Google Scholar]
- 77.Joiner WJ, Crocker A, White BH, Sehgal A: Sleep in Drosophila is regulated by adult mushroom bodies. Nature 2006, 441:757–760. [DOI] [PubMed] [Google Scholar]
- •78.Sitaraman D, Aso Y, Jin X, Chen N, Felix M, Rubin GM, Nitabach MN: Propagation of Homeostatic Sleep Signals by Segregated Synaptic Microcircuits of the Drosophila Mushroom Body. Curr. Biol 2015, 25:2915–2927. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article dissects MB subcircuits that promote sleep or wake upon activation using behavioral screening and calcium imaging.
- •79.Donlea JM, Thimgan MS, Suzuki Y, Gottschalk L, Shaw PJ: Inducing sleep by remote control facilitates memory consolidation in Drosophila. Science 2011, 332:1571–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article characterizes the sleep-inducing effects of dFB activation, and demonstrates that promoting sleep after learning can enhance memory consolidation
- 80.Keene AC, Stratmann M, Keller A, Perrat PN, Vosshall LB, Waddell S: Diverse odor-conditioned memories require uniquely timed dorsal paired medial neuron output. Neuron 2004, 44:521–533. [DOI] [PubMed] [Google Scholar]
- 81.Keene AC, Krashes MJ, Leung B, Bernard JA, Waddell S: Drosophila dorsal paired medial neurons provide a general mechanism for memory consolidation. Curr. Biol 2006, 16:1524–1530. [DOI] [PubMed] [Google Scholar]
- 82.Plaçais P-Y, Trannoy S, Isabel G, Aso Y, Siwanowicz I, Belliart-Guérin G, Vernier P, Birman S, Tanimoto H, Preat T: Slow oscillations in two pairs of dopaminergic neurons gate long-term memory formation in Drosophila. Nat. Neurosci 2012, 15:592–599· [DOI] [PubMed] [Google Scholar]
- •83.Haynes PR, Christmann BL, Griffith LC: A single pair of neurons links sleep to memory consolidation in Drosophila melanogaster. Elife 2015, 4:R774. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article describes a role for DPM activation in promoting sleep, providing a possible link between memory consolidation and the regulation of sleep after plastic events.
- •84.Zwaka H, Bartels R, Gora J, Franck V, Culo A, Götsch M, Menzel R: Context odor presentation during sleep enhances memory in honeybees. Curr. Biol 2015, 25:2869–2874. [DOI] [PubMed] [Google Scholar]; This study provides evidence that memory reactivation during sleep promotes consolidation in the insect Mushroom body.
- 85.Rasch B, Büchel C, Gais S, Born J: Odor cues during slow-wave sleep prompt declarative memory consolidation. Science 2007, 315:1426–1429. [DOI] [PubMed] [Google Scholar]
- 86.Pimentel D, Donlea JM, Talbot CB, Song SM, Thurston AJF, Miesenböck G: Operation of a homeostatic sleep switch. Nature 2016, 536:333–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu S, Liu Q, Tabuchi M, Wu MN: Sleep Drive Is Encoded by Neural Plastic Changes in a Dedicated Circuit. Cell 2016, 165:1347–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Donlea JM, Pimentel D, Talbot CB, Kempf A, Omoto JJ, Hartenstein V, Miesenböck G: Recurrent Circuitry for Balancing Sleep Need and Sleep. Neuron 2018, 97:378–389.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Donlea JM: Neuronal and molecular mechanisms of sleep homeostasis. Curr Opin Insect Sci 2017, doi: 10.1016/j.cois.2017.09.008. [DOI] [PubMed] [Google Scholar]


